Abstract
Treponema pallidum subspecies pallidum (T. pallidum) causes syphilis via sexual exposure or vertical transmission during pregnancy. T. pallidum is renowned for its invasiveness and immune-evasiveness; its clinical manifestations result from local inflammatory responses to replicating spirochetes and often imitate those of other diseases. The spirochete has a long latent period during which patients have no signs or symptoms, but can remain infectious. Despite the availability of simple diagnostic tests and the effectiveness of treatment with a single dose of long-acting penicillin, syphilis is re-emerging as a global public health problem, particularly among men who have sex with men (MSM) in high-income and middle-income countries. Syphilis also causes several hundred thousand stillbirths and neonatal deaths every year in developing nations. Although several low-income countries have achieved WHO targets for the elimination of congenital syphilis, an alarming increase of syphilis in HIV-infected MSM serves as a strong reminder of the tenacity of T. pallidum as a pathogen. Strong advocacy and community involvement is needed to ensure that syphilis is given high priority on the global health agenda. More investment in research is needed on the interaction between HIV and syphilis in MSM, as well as into improved diagnostics, a better test of cure, intensified public health measures and, ultimately, a vaccine.
Introduction
Syphilis is a sexually and vertically transmitted infection (STI) caused by the spirochaete Treponema pallidum subspecies pallidum (order Spirochaetales) (Fig. 1). Three other organisms within this genus are causes of nonvenereal or endemic treponematoses. T. pallidum subspecies pertenue is the causative agent of yaws, T. pallidum subspecies endemicum causes endemic (non-venereal) syphilis and T. carateum causes pinta. These pathogens are morphologically and antigenically indistinguishable. They can, however, be differentiated by their age of acquisition, principal mode of transmission, clinical manifestations, capacity for invasion of the central nervous system and placenta, and genomic sequences, although the accuracy of these differences remains a subject of debate1. Analyses based on the mutation rates of genomic sequences suggest that the causative agents of yaws and venereal syphilis diverged several thousand years ago from a common progenitor originating in Africa2. These estimates argue against the so-called Columbian hypothesis — the notion that shipmates of Christopher Columbus imported a newly evolved spirochete causing venereal syphilis from the New World into Western Europe in the late 15th century3.
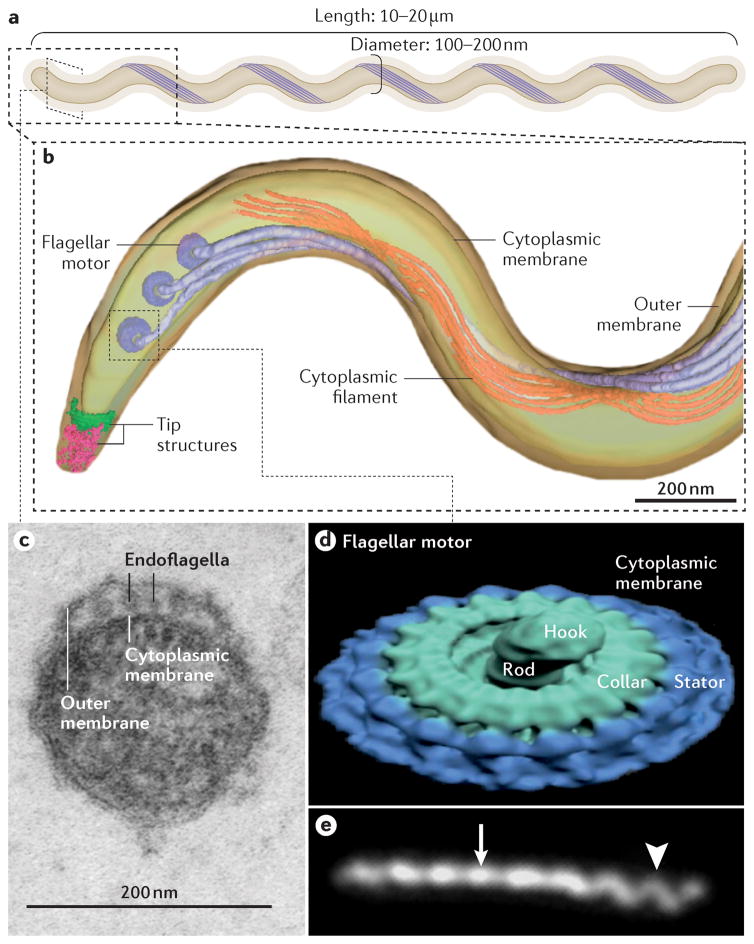
Figure 1. Treponema pallidum.
A | Like all spirochetes, T. pallidum consists of a protoplasmic cylinder and cytoplasmic membrane bounded by a thin peptidoglycan sacculus and outer membrane239,240. Usually described as spiral-shaped, T. pallidum is actually a thin planar wave similar to Borrelia burgdorferi, the agent of Lyme borreliosis239. The bacterium replicates slowly and poorly tolerates desiccation, elevated temperatures and high oxygen tensions55. B | Periplasmic flagellar filaments, a defining morphological feature of spirochetes, originate from nanomotors situated at each pole and wind around the cylinder atop the peptidoglycan, overlapping at mid-cell. Force exerted by the rigid filaments against the elastic peptidoglycan deforms the sacculus to create the flat wave morphology of the spirochete100. Panel B used with permission from Ref.239 C | Ultra-thin section of T. pallidum showing the outer and cytoplasmic membranes and flagellar filaments (endoflagella) within the periplasmic space9. D | Surface rendering of a flagellar motor based on cryoelectron tomograms. Panel D used permission from Ref.240. E | Darkfield micrograph showing the flat-wave morphology of Tpallidum. The arrow and arrowhead indicate segments that are oriented 90° from each other. The different appearances of the helical wave at 90° to the viewer can be explained only by a flat wave morphology; a corkscrew would appear the same from any angle. Panel E used permission from Ref 239.
T. pallidum is an obligate human pathogen renowned for its invasiveness and immunoevasiveness4–7; clinical manifestations result from the local inflammatory response elicited by spirochetes replicating within tissues8–10. Infected individuals typically follow a disease course divided into primary, secondary, latent and tertiary stages over a period of ≥10 years. Different guidelines define early latency as starting 1–2 years after exposure. Typically, ‘early syphilis’ refers to infections that can be transmitted sexually (including primary, secondary and early latent infections) and is synonymous with active (infectious) syphilis; the WHO defines ‘early syphilis’ as infection of <2 years duration11, whereas the guidelines from the United States12 and Europe13 define it as infection <1 year in duration. These differences in definition can affect interpretation of results and in therapeutic regimens used in some circumstances.
Owing to its varied and often subtle manifestations that can mimic other infections, syphilis has earned the names of the Great Imitator or Great Mimicker14. Patients with primary syphilis present with a single ulcer (chancre) or multiple lesions on the genitals or other body sites involved in sexual contact and regional lymphadenopathy ~3 weeks post-infection; these are typically painless and resolve spontaneously. Resolution of primary lesions is followed 6–8 weeks later by secondary manifestations, which can include fever, headache and a maculopapular rash on the flank, shoulders, arm, chest or back and that often involves the palms of the hands and soles of the feet. As signs and symptoms subside, patients enter a latent phase, which can last many years. A patient in the first 1–2 years of latency are still considered infectious owing to a 25% risk of secondary syphilis-like relapses15. Historical literature suggests that 15–40% of untreated individuals will develop tertiary syphilis, which can manifest as destructive cardiac or neurological conditions, severe skin or visceral lesions (gummas) or bony involvement9. More-recent data suggest tertiary syphilis may be less common today, perhaps owing to wide use of antibiotics. Numerous case reports and small series suggest that HIV infection predisposes to neuro-ophthalmological complications in those with syphilis16. Importantly, neurosyphilis is typically described as a late manifestation but can occur in early syphilis. Indeed, T. pallidum can be frequently identified in the cerebral spinal fluid (CSF) of patients with early disease9,15,17. However, the majority of patients with early syphilis who have CSF abnormalities do not demonstrate central nervous system symptoms and do not require therapy for neurosyphilis12. Symptomatic manifestations of neurosyphilis include chronic meningitis, meningovascular stroke-like syndromes and manifestations common in the neurological forms of tertiary syphilis (namely, tabes dorsalis and general paresis, a progressive dementia mimicking a variety of psychotic syndromes)9.
Sexual transmission of syphilis occurs during the first 1–2 years after exposure (that is, during primary, secondary and early latent stages of infection) 9. The risk of mother-to-child transmission (MTCT) is highest in primary and secondary stages, followed by early latent syphilis. However, transmission risk continues during the first 4 years after exposure, after which vertical transmission risk declines over time18. The rate of fetal infection depends on stage of maternal infection, with approximately 30% of pregnancies resulting in fetal death in utero, stillbirth (late second and third trimester fetal death) or death shortly after delivery19–21. Infants born to infected mothers are often preterm, of low birth weight or with clinical signs that mimic neonatal sepsis (that is, poor feeding, lethargy, rash, jaundice, hepatosplenomegaly and anaemia).
Given that T. pallidum has a relatively long generation time of 30–33 hours22, long-acting penicillin preparations such as benzathine penicillin G is the preferred therapy for most patients with syphilis. Since the 1940s (when penicillin became widely available), syphilis prevalence continued decline in regions able to appropriately test and treat the infection. However, syphilis outbreaks continue to occur throughout the world. In particular, with declining AIDS-related mortality related to effective HIV treatment over the past two decades, syphilis has re-emerged in urban settings among men who have sex with men (MSM). High-income and middle-income countries have observed rises in syphilis case rates as well as increased case rates of neurosyphilis (such as ocular syphilis) and, in some countries, congenital syphilis. In low income countries where syphilis prevalence remains high, MTCT of syphilis continues to be the most common cause of STI-related mortality outside of HIV23,24, with perinatal deaths owing to untreated syphilis exceeding those of HIV or malaria25. Syphilis is now the second leading cause of preventable stillbirths worldwide, following malaria25.
Syphilis should be an ideal disease for elimination as it has no known animal reservoir, can usually be diagnosed with simple inexpensive tests and can be cured9,16. Nevertheless, syphilis remains a continuing public health challenge globally26. In this Primer, we describe recent discoveries that have improved our understanding of the biological and genetic structure of the pathogen, novel diagnostic tests and testing approaches that can improve disease detection, as well as current, evidence-based management recommendations. We also draw attention to the call for global elimination of MTCT of syphilis and HIV and recent success in elimination in in low and middle income countries (LMICs), particularly through fundamental public health strategies such as ensuring quality antenatal care that includes testing for syphilis early in pregnancy and providing prompt treatment of women and their partners. We also report on the rising numbers of syphilis cases in MSM, ongoing work supporting improved interventions against syphilis in marginalized populations and, ultimately, development of an effective vaccine.
Epidemiology
According to the most recent estimation of the WHO, approximately 17.7 million individuals 15–49 years of age globally had syphilis in 2012, with an estimated 5.6 million new cases every year27 (Fig. 2). The estimated prevalence and incidence of syphilis varied substantially by region or country, with the highest prevalence in Africa and >60% of new cases occurring in LMICs27. The greatest burden of maternal syphilis occurs in Africa, representing >60% of the global estimate23,24.
Figure 2. Incidence of syphilis worldwide.
The WHO estimates of incident cases of syphilis by region in 2012 are shown for the different geographical regions. Data from Ref.27
Prevalence and incidence
In LMICs, heterosexual spread of syphilis has declined in the general population but remains problematic in some high-risk sub-populations, such as female sex workers (FSWs) and their male clients. A recent study of FSWs in Johannesburg, South Africa, showed that 21% of participating women had antibodies that suggested past or current infection and 3% had active (infectious) infection28. Another study of FSWs in 14 zones in Sudan showed high seroprevalence (median 4.1%), with the highest value of 8.9% in the eastern zone of the country29. A large study of >1,000 FSWs in Kampala, Uganda, showed 21% were seropositive for syphilis and 10% had active infection30. Studies in emerging economies, such as China, indicate that syphilis is increasing among ‘mobile men with money’31. Although syphilis case rates are low in the general population in China, syphilis prevalence is ~5% among FSWs and 3% among their male clients31,32. Risk of infection varies among FSWs working in different venues, with the highest prevalence (~10%) among street-based FSWs and lower prevalence (~2%) among venue-based FSWs33.
By contrast, higher-income countries have had declining syphilis prevalence among heterosexual men and women. However, a resurgence of syphilis that disproportionately affects MSM has been noted. Syphilis is associated with high-risk sexual behaviours and substantially increased HIV transmission and acquisition. Indeed, the numbers and rates of reported cases of syphilis among MSM in the United States and Western Europe have been increasing since 1998 (Ref.34). In 2015, the case rates for primary and secondary syphilis among MSM (309 per 100,000) in the United States were 221-times the rate for women (1.4 per 100,000) and 106 times the rate for heterosexual men (2.9 per 100,000)35. In Canada, compared with reported cases in the general male population, the incidence of syphilis was >300-times greater among HIV-positive MSM36. Syphilis infection has been associated with certain behavioural and other factors, including incarceration; multiple or anonymous sex partners; sexual activity connected with illicit drug use; seeking sex partners through the internet and other high-risk sexual network dynamics37–41. Risk factors for syphilis are frequently overlapping40. Reports of unusual presentations and rapid progression of syphilis in patients with concurrent HIV infection has led to the hypothesis that infection with or treatment for HIV alters the natural history of syphilis42.
MTCT
Adverse birth outcomes caused by fetal exposure to syphilis are preventable if women are screened for syphilis and treated before the end of the second trimester of pregnancy21. However, MTCT of syphilis continued to cause such perinatal and infant mortality that, in 2007, the WHO and partners launched a global initiative to eliminate it as a public health problem43–45. At the time of the campaign launch, an estimated 1.4 million pregnant women had active syphilis infections, of whom 80% had attended at least one antenatal visit — suggesting missed opportunities for testing and treatment23. At that time, untreated maternal syphilis infection was estimated to have resulted in >500,000 adverse pregnancy outcomes, including more than 300,000 perinatal deaths (stillbirths and early neonatal deaths).
Syphilis testing and treatment during pregnancy is highly effective and was included in the Lives Saved Tools of effective maternal–child health interventions46. Furthermore, studies have shown that prenatal syphilis screening, treatment support testing and treatment during pregnancy are highly cost-effective in most countries regardless of prevalence or availability of resources, and can even be cost-saving in LMICs with syphilis prevalence ≥3% in pregnant women47–50. In China, where syphilis and HIV prevalence in pregnant women is low but rising, integration of prenatal syphilis and HIV screening was found to be highly cost-effective51.
Since 2007, an increasing number of countries have implemented regional and national initiatives to prevent MTCT of syphilis52, improving guidance documents, using point-of-care (POC) tests as a means of improving access to testing and treatment and integrating behavioural and medical interventions into HIV prevention and control programmes53. By 2012, these efforts had contributed to a reduction in global adverse pregnancy outcomes due to MTCT of syphilis to 350,000, including 210,000 perinatal deaths, and decreased the rates of maternal and congenital syphilis decreased by 38% and 39%, respectively23,24. In 2015, Cuba became the first country to be validated for having achieved elimination of MTCT of HIV and syphilis54. Subsequently, Thailand, Belarus and four United Kingdom Overseas Territories (Bermuda, the Cayman Islands, Montserrat and Antigua) was validated for elimination of MTCT of HIV and syphilis, Moldova was validated for elimination of MTCT of syphilis, and Armenia was validated for elimination of MTCT of HIV. However, these gains were mostly in Asia and the Americas — maternal prevalence in Africa has remained largely unchanged23,24.
Mechanisms/pathophysiology
Although a local inflammatory response elicited by spirochetes is thought to be the root cause of all clinical manifestations of syphilis9, the mechanisms that cause tissue damage, as well as the host defences that eventually gain a measure of control over the bacterium, are ill defined. The recalcitrance of T. pallidum to in vitro culture and the consequent inability to harness genetic techniques to delineate its virulence determinants remains the primary obstacle to progress55. Additionally, the fragility and low protein content of its outer membrane have confounded efforts to characterize surface-exposed molecules56,57. Finally, facile murine models to dissect the host response and the components of protective immunity are also lacking58. Outbred rabbits are essential for isolating T. pallidum strains from clinical specimens59 and routine propagation in the laboratory60. Because rabbits are highly susceptible to T. pallidum infection, develop lesions grossly and histopathologically resembling chancres following intradermal inoculation and generate antibody responses similar to those in humans, the rabbit is the model of choice for studying endogenous and exogenous protective immunity61,62. However, the rabbit model poorly recapitulates many clinical and immunological facets of human disease63. Not surprisingly, even in the post-genomics era, our understanding of pathogenic mechanisms in syphilis lags well behind other common bacterial diseases63.
Molecular Features
The morphological features of T. pallidum are described in Figure 1. Because of its double-membrane structure, the spirochete is often described as a Gram-negative bacterium. However, this analogy is phylogenetically, biochemically and ultrastructurally inaccurate63,64. The T. pallidum outer membrane lacks lipopolysaccharide65 and has a markedly different phospholipid composition than the outer membranes of typical Gram-negative bacteria66. Although T. pallidum expresses abundant lipoproteins, these molecules reside predominantly below the surface5,63,67. Accordingly, this paucity of surface-exposed pathogen-associated molecular patterns (PAMPs) enables the spirochete to avoid triggering host innate surveillance mechanisms, facilitating local replication and early dissemination. Its limited surface antigenicity promotes evasion of adaptive immune responses (that is, antibodies), facilitating persistence5,56,68,69. Collectively, these attributes have earned T. pallidum its designation as ‘the stealth pathogen’63,69. Understanding events unfolding at the host–pathogen interface requires a detailed knowledge of the T. pallidum repertoire of surface-exposed proteins. However, characterization of the protein constituents of the outer membrane has been, and continues to be, daunting8,55,57,63.
Lipoproteins
In the 1980s, investigators screened E. coli recombinant libraries with syphilitic sera and murine monoclonal antibodies based upon the unproven (and, as it turned out, immunologically incorrect) assumption that immunoreactive proteins ought to be surface-exposed in T. pallidum57. Biochemical and genetic analyses subsequently revealed that most of the antigens identified by these screens are lipoproteins70–72 tethered by their N-terminal lipids to the cytoplasmic membrane (hence, the protein moieties are in the periplasmic space)67,73–75. However, convincing evidence now shows that the spirochete displays small amounts of lipoproteins on its surface that have the potential to enhance infectivity (Fig. 3). For example, TP0751 (also known as pallilysin) is a laminin-binding lipoprotein and zinc-dependent metalloproteinase capable of degrading clots and extracellular matrix76–78. Although expressed by T. pallidum in minute quantities, surface exposure of TP0751 has been demonstrated by knock-in experiments in Borrelia burgdorferi (the spirochete that causes Lyme borreliosis79), and the cultivatable commensal treponeme T. phagedenis80, opsonophagocytosis assays in T. pallidum77 and, most recently, protection of immunized rabbits against dissemination of spirochetes following intradermal challenge81. The X-ray structure of TP0751, demonstrating an unusual lipocalin fold, should inform efforts to clarify its multi-functionality79. Additionally, the lipoprotein Tpp17 (also known as TP0435) has been shown to be at least partially surface-exposed and can function as a cytadhesin82. The structurally characterized lipoprotein TP0453 attaches to the inner leaflet of the outer membrane via its N-terminal lipids and two amphipathic helices within its protein moiety83.
Figure 3. Molecular architecture of the cell envelope of Treponema pallidum.
Shown in the outer membrane are TP0751 (as known as pallilysin)79,81 and Tpp17 (also known as TP0435)82,241 — two surface-exposed lipoproteins; TP0453, a lipoprotein attached to the inner leaflet of the outer membrane83; BamA (also known as TP0326)84,94; a full-length T. pallidum repeat (Tpr) attached by its N-terminal portion to the peptidoglycan93,94; and a generic β-barrel that represents other non-Tpr outer-membrane proteins identified by computational mining of the T. pallidum genome112. Substrates and nutrients present in high concentration in the extracellular milieu (such as, glucose) traverse the outer membrane through porins, such as TprC. At the cytoplasmic membrane, prototypic ABC-like transporters (such as RfuABCD, a riboflavin transporter) use a periplasmic substrate-binding protein (SBP), usually lipoproteins, and components with transmembrane and ATP-binding domains to bind nutrients that have traversed the outer membrane for transport across the cytoplasmic membrane. The energy coupling factor (ECF)-type ABC transporters use a transmembrane ligand-binding protein in place of a separate periplasmic SBP for binding of ligands (BioMNY is thought to transport biotin)242. Symporter permeases (for example, TP0265) use the chemiosmotic or electrochemical gradient across the cytoplasmic membrane to drive substrate transport243. The tripartite ATP-independent periplasmic (TRAP)-type transporters also use transmembrane electrochemical gradients to drive substrate transport; the periplasmic component protein TatT (also known as TP0956) likely associates with the SBP TatP (also known as TP0957) that binds ligands (perhaps hydrophobic molecules, such as long chain fatty acids), uptake of which is probably is facilitated by the permease TatQ-M (also known as TP0958) 244,245. Figure adapted from Ref.63 with permission.
BamA
With the publication of the T. pallidum genome in 1998 (Ref.65), only one protein with sequence relatedness to an outer membrane protein of Gram-negative bacteria was identified: TP0326 (also known as β-barrel assembly machinery A; BamA)84,85. BamA has a dual domain architecture consisting of a 16-stranded, outer membrane-inserted, C-terminal β-barrel and five tandem polypeptide transport-associated (POTRA) repeats within the periplasm84,85. The opening of the channel is covered by a ‘dome’ comprising three extracellular loops, one of which contains an opsonic target that is sequence variable among T. pallidum strains85. BamA is the essential central component of the molecular machine that catalyses insertion of newly exported outer membrane proteins to the outer membrane86.
Tpr proteins
The T. pallidum repeat (Tpr) proteins, a 12-member paralogous family with sequence homology to the major outer sheath protein of the oral commensal T. denticola, were also identified by the T. pallidum genomic sequence65. Of these, TprK (TP0897) has received the greatest attention because of its presumed role in immune evasion by the spirochete87,88; it has been shown to undergo antigenic variation in seven regions believed to be extracellular loops harbouring B-cell epitopes89–92. DNA sequence cassettes that correspond to V-region sequences in an area of the T. pallidum chromosome located away from the tprK gene have been proposed to serve as unidirectional donor sites for the generation of variable regions by nonreciprocal gene conversion89. Two other Tpr proteins, TprC and TprI, have met stringent experimental criteria for rare outer membrane proteins. They form trimeric β-barrels when refolded in vitro, cause large increases in permeability upon insertion into liposomes and are surface-exposed opsonic targets in T. pallidum93,94. Unlike classic porins, for which the entire polypeptide forms a β-barrel, TprC and TprI are bipartite. As with BamA, the C-terminal domain forms the surface-exposed β-barrel, whereas the N-terminal half anchors the barrel to the peptidoglycan sacculus. These results collectively imply that Tprs serve as functional orthologs of Gram-negative porins, using variations in substrate specificities of their channel-forming β-barrels, probably along with differential expression, to import the spirochete’s nutritional requirements into the periplasmic space from blood and body fluids95,96. These proteins also furnish a topological template for efforts to understand how antibody responses to Tprs promote bacterial clearance.
Biosynthetic machinery
T. pallidum has evolved to dispense with a vast amount of the biosynthetic machinery found in other bacterial pathogens55,63–65. To compensate for its loss of biosynthetic capacity, the spirochete maintains a complex assortment of ABC transporters and symporters (totalling ~5% of its 1.14 MB circular genome) to transfer the broad spectrum of molecules essential for viability from periplasm to cytosol (Fig. 3). T. pallidum relies on an optimized conventional glycolytic pathway as its primary means for generating ATP. By dispensing with oxidative phosphorylation, the spirochete has no need for cytochromes and the iron required to synthesize them. Accordingly, the spirochete maintains a complex, yet parsimonious, assortment of ABC transporters and symporters (totalling ~5% of its 1.14 MB circular genome) to transfer essential molecules from the periplasmic space to the cytosol (Fig. 3). Whereas many pathogens have highly redundant systems for uptake of transition metals across the cytoplasmic membrane, T. pallidum accomplishes this task with just two ABC transporters (Tro, which imports zinc, manganese and iron, and Znu, which is zinc-specific). A small, but powerful arsenal of enzymes neutralize superoxides and peroxides to fend off host responses to infection. Lastly, the spirochete possesses novel and surprisingly intricate mechanisms ostensibly to redirect transcription and fine-tune metabolism in response to environmental cues and nutrient flux63.
Transmission and dissemination
Transmission of venereal syphilis occurs during sexual contact with an actively infected partner; exudate containing as few as 10 organisms can transmit disease8,68. Spirochetes directly penetrate mucous membranes or enter through abrasions in skin, which is less heavily keratinized in peri-genital and peri-anal areas than skin elsewhere8,68. To establish infection, T. pallidum must adhere to epithelial cells and extracellular matrix components; in vitro binding studies suggest that fibronectin and laminin are key substrates for these interaction76,97–99. Once below the epithelium, organisms multiply locally and begin to disseminate through the lymphatics and bloodstream. Spirochetes penetrate extracellular matrix and intercellular junctions by ‘stop and go’ movements that coordinate adherence with motility, powered by front-to-back undulating waves generated by flagellar rotation and presumably assisted by the proteolytic activity of TP0751 77,100. Ex vivo studies using cultured human umbilical vein endothelial cells (Fig. 4A) suggest that spirochetes invade tissues using direct motility to negotiate their way through intercellular junctions, so-called inter-junctional penetration7,101. The infection rapidly becomes systemic9,16,100. Profuse spirochetes within the epidermis and superficial dermis in secondary syphilitic lesions (Fig. 4B) enable tiny abrasions created during sexual activity to transmit infection10,102. Penetration of the blood–brain barrier, occurring in as many as 40% of individuals with untreated early syphilis, can cause devastating neurological complications9,16.
Figure 4. Treponema pallidum invasion.
A | Transmission electron micrograph of T. pallidum (arrowheads) penetrating the junctions between cultured umbilical vein endothelial cells. ‘Inter-junctional invasion’ following attachment to vascular endothelium is thought to provide T. pallidum access to tissue parenchyma during haematogenous dissemination. Reprinted with permission from Reference101. B | Immunohistochemical staining (using commercial anti-T. pallidum antibodies) of a secondary syphilis skin lesion reveals abundant spirochetes embedded within a mixed cellular inflammatory infiltrate in the papillary dermis. The inflammatory response elicited by spirochetes replicating in tissues is widely thought to be the cause of clinical manifestations in all stages of syphilis. Reprinted with permission from10. C | Human syphilitic serum (HSS) dramatically enhances opsonophagocytosis of T. pallidum by purified human peripheral blood monocytes compared with D | normal human serum (NHS). Arrowheads indicate treponemes being degraded within phagolysosomes.
Adaptive immune response and inflammation
Although the paucity of PAMPs in the T. pallidum outer membrane enables the bacterium to replicate locally and undergo repeated bouts of dissemination, pathogen sensing in the host is eventually triggered. The organisms are taken up by dendritic cells103, which then traffic to draining lymph nodes to present cognate treponemal antigens to naive B cells and T cells. The production of opsonic antibodies markedly enhances the uptake and degradation of spirochetes by phagocytes (Fig. 4C,D), liberating lipopeptides and other PAMPs for binding to Toll-like receptors lining the interior of the phagosome and antigenic peptides for presentation to locally recruited T cells62,104,105. Activated lesional T cells secrete IFN-γ, promoting clearance by macrophages, but also bolstering the production of tissue-damaging cytokines, such as tumor necrosis factor (TNF) and IL-6 10,106,107. Immunohistochemical analysis has identified CD4+ and CD8+ T cells10,106,108,109, natural killer cells10 and activated macrophages in early syphilis lesions10,109. Perivascular infiltration of lymphocytes, histiocytes (phagocytic cells in connective tissues) and plasma cells with endothelial cell swelling and proliferation are characteristic histopathological findings in all stages of syphilis and can progress to frank endarteritis obliterans (leading to occlusion of arteries and severe clinical manifestations, such as the stroke syndromes of meningovascular syphilis)9,110.
Antibody avoidance
T. pallidum is widely regarded as an extracellular bacterium61. Thus, a question of paramount importance is why, unlike ‘classic’ extracellular pathogens, syphilis spirochetes not only fail to be cleared rapidly but can replicate and circulate in the midst of a prolific antibody response8,68,69. Immunolabelling, opsonophagocytosis, and complement-dependent neutralization assays have shown that T. pallidum populations consist of antibody-binding and non-binding subpopulations; the minority of organisms that bind antibodies do so in minute amounts and with delayed kinetics10,111–114. Accordingly, one can envision a scenario whereby nonbinders replenish the spirochetes that bind and are cleared63.
Understanding the basis for the heterogeneity of T. pallidum’s surface antigenicity is critical to unravelling its strategy for antibody avoidance. The picture emerging from our evolving concepts of the spirochete’s molecular architecture is multi-factorial and likely involves copious production of antibodies against subsurface lipoprotein ‘decoys’57,110; poor target availability owing to low copy numbers of outer membrane proteins and surface-exposed lipoproteins67,77,82,84,93; in the case of bipartite outer membrane proteins, limited production of antibodies against surface-exposed epitopes along with skewed production of antibodies against periplasmic domain84,93; organism-to-organism variation in the levels of expression of outer membrane proteins and outer surface lipoproteins through a variety of mechanisms, including phase variation82,92,115,116; and, in the case of TprK, antigenic variation as a result of intra-genomic recombination89,92,117. Additionally, the ability of motile spirochetes to ‘outrun’ infiltrating phagocytes and reach sequestered locations, including the epidermis, could be an under-appreciated aspect of immune evasion10,102. As infection proceeds, the antibody repertoire possibly broadens and intensifies to the point where the antigen-poor surface of the spirochete is overwhelmed and its capacity for antigenic variation is exhausted, ushering in the asymptomatic period called latency. Once in the latent state, the organism can survive for years in untreated individuals, establishing niduses of inflammation in skin, bones, the thoracic aorta, the posterior uveal tract and the central nervous system, that set the stage for recrudescent disease — collectively referred to as tertiary syphilis. How immune containment mechanisms decline and enable the balance to shift back in favour of the pathogen in tertiary syphilis is inclear9, although a hyper-intense cellular response to the spirochete is generally believed to be the cause of the highly destructive lesions of tertiary syphilis9. Numerous case reports and small series suggest that HIV infection predisposes to neuro-ophthalmological complications in those with syphilis16. Cardiovascular syphilis, typically involving the aortic arch and leading to aneurysmal dilatation, usually occurs 10–30 years after the initial infection9.
Congenital infection
Although MTCT of syphilis can occur at the time of delivery, the overwhelming majority of cases are caused by to in utero transmission. Studies have shown spirochetes in placenta and umbilical cord samples, supporting transplacental passage of the organism to the fetus, as early as 9–10 weeks of gestation118. Although fetal syphilis infection was not thought to occur prior to the second trimester, the fetus can indeed be infected very early in pregnancy but may be unable to mount a characteristic immune response until development of the embryonic immune system at 18–20 weeks gestation.
Transmission risk is directly related to stage of syphilis in the pregnant woman (that is, the extent and duration of fetal exposure to spirochetes). Small case series have found highest MTCT risk in primary and secondary stages, during which transmission probability may be ≥80%. In latent (asymptomatic) infections during pregnancy, probability of transmission to the fetus is highest during the first 4 years after infection, after which risk declines18,45. Systematic reviews assessing women with predominantly asymptomatic infections are consistent in showing that delayed or lack of adequate treatment results in stillbirth, early neonatal death, prematurity, low birth weight or congenital infection in infants (more than half of syphilis-exposed pregnancies); syphilitic stillbirth was the most commonly observed adverse outcomes in syphilis-exposed pregnancies21,45,119.
Diagnosis, screening and prevention
Syphilis has varied and often subtle manifestations that make clinical diagnosis difficult and lead to many infections being unrecognized. The classically painless lesions of primary syphilis can be missed, especially in hidden sites of exposure such as the cervix or rectum. The rash (Fig. 5) and other symptoms of secondary syphilis can be faint or mistaken for other conditions. A syphilis diagnosis is often based on a suggestive clinical history and supportive laboratory9,16 (that is, serodiagnostic) tests. Serological testing has become the most common means to diagnose syphilis whether in people with symptoms of syphilis, or in those who have no symptoms but are detected through screening. A limitation of all syphilis serological tests is their inability to distinguish between infection with T. pallidum subsp. pallidum and the non-venereal T. pallidum subspecies that cause yaws, pinta or bejel.
Figure 5. Clinical presentation of primary, secondary and congenital syphilis.
A | Primary chancre. B | Primary chancre with rash of secondary syphilis. C | Secondary syphilis in a pregnant woman, who has palmar rash. D | Secondary syphilis as palmar rash. E | 3-month old baby with congenital syphilis, showing hepatosplenomegaly and desquamating rash. The child also presented with nasal discharge. F | Typical palmar desquamating rash in baby with congenital syphilis.
Ensuring accuracy and reliability of syphilis testing is important, especially in nonspecialized laboratories where most patients are tested120. Syphilis-specific quality assurance strategies include training of technologists on specific techniques, internal quality control systems; test evaluation; and interassay standardization of commercially available test kits on a regular basis37,120. It is especially important to provide adequate training and regular external quality assessment or proficiency testing with corrective action to ensure the quality of tests and testing for health care providers who perform rapid tests in clinic based or outreach settings121–124. Because many parts of the world lack laboratory capacity for accurate diagnosis, the requirement for laboratory testing has greatly constrained syphilis control and congenital syphilis elimination. However, development of inexpensive rapid tests that can be performed at the POC has greatly increased access to prenatal screening and diagnosis, even in low-resourced and remote settings.
Definitive diagnosis by direct detection
The choice of method for diagnosing syphilis depends on the stage of disease and the clinical presentation125. In patients presenting with primary syphilitic ulcers, condyloma lata (genital lesions of secondary syphilis) or lesions of congenital syphilis, direct detection methods — which include darkfield microscopy, fluorescent antibody staining, immunohistochemistry and PCR — may be used to make a microbiological diagnosis. However, with the exception of PCR, these methods are insensitive and require fresh lesions from which swab or biopsy material can be collected as well as well-experienced technologists (Table 1).
Table 1.
Direct detection methods for Treponema pallidum
| Method | Sample | Advantages | Disadvantages |
|---|---|---|---|
| Darkfield microscopy | Fresh (<20 minutes) sample from chancres or erosive cutaneous lesions of primary, secondary or congenital syphilis |
|
|
| Direct fluorescent antibody staining for T. pallidum | Sample from chancres or erosive cutaneous lesions of primary, secondary or congenital syphilis |
|
|
| Immunohistochemistry | Skin, mucosal or tissue lesions performed on fixed paraffin embedded tissues using commercially available treponemal antibody reagents |
|
|
| PCR | Skin, mucosal or tissue lesions; not recommended for blood or CSF as few organisms present |
|
|
Microscopy had been used for direct detection and diagnosis since 1920, but is now used infrequently. A 2014 survey of national reference and large clinical laboratories in Latin America and the Caribbean found that only two of 69 participating facilities, of which half were reference laboratories, still performed darkfield or direct fluorescent antibody staining for T. pallidum (DFA-TP)126. The most recent European guidelines recommended against DFA-TP testing in clinical settings, and the reagents are no longer available13. PCR techniques are increasingly used; however, there is as yet no commercially available or internationally approved test for T. pallidum13. Species-specific and subspecies-specific T. pallidum PCR testing is a developing technology that is still primarily available in research laboratories127,128, although these tests are anticipated to be more widely available in the near future. A systematic review and meta-analysis concluded that T. pallidum PCR was more efficient for confirmation than to exclude syphilis diagnosis in lesions129. Recent research indicates that this technology may be helpful for the diagnosis of neurosyphilis by the detection of T. pallidum DNA in the cerebrospinal fluid (CSF) of patients with syphilis, particularly among HIV-infected individuals130,131.
Diagnosis using serology
Serodiagnostic tests are the only means for screening asymptomatic individuals and are the most commonly used methods to diagnose patients presenting with signs and symptoms suggestive of syphilis. Serodiagnostic tests for syphilis can be broadly categorized into non-treponemal tests (NTTs) and treponemal tests (TTs).
NTTs
NTTs measure immunoglobulins (IgM and IgG) produced in response to lipoidal material released from the bacterium and/or dying host cells. The most commonly used NTTs — the Rapid Plasma Reagin (RPR) test, the Toluidine Red Unheated Serum Test (TRUST) and the Venereal Disease Research Laboratory (VDRL) test — are flocculation (precipitation) tests that detect antibodies to a suspension of lecithin (including phosphatidylcholine and phosphatidylethanolamine), cholesterol and cardiolipin. NTTs are useful in detecting active syphilis. However, because they do not become positive until 10–15 days after onset of the primary lesion, 25–30% of primary syphilis cases may be missed (Fig. 6)132,133. Although relatively simple and inexpensive, NTTs must be performed manually on serum, and rely on subjective interpretation (Table 2). These tests also require trained laboratory personnel and specialized reagents and equipment and, therefore, do not fulfil the ASSURED (Affordable, Sensitive, Specific, User-friendly, Rapid and robust, equipment-free and Deliverable to those who need them) criteria for tests that can be used at the point of care 134.
Figure 6. Serological response to primary and secondary syphilis.
Diagnosis of syphilis can be made by measuring a patient’s serological response to infection. IgM antibodies against Treponema pallidum proteins are the first to appear, followed a few weeks later by IgG antibodies. Both IgM and IgG antibodies can be measured using treponemal tests such as the T. pallidum Haemagglutination Assay (TPHA), T. pallidum Particle Assay (TPPA), Fluorescent Treponemal Antibody Absorption assay (FTA-ABS), enzyme immunoassays (EIA) and Chemilluminescent immunoassays (CIA). IgM and IgG antibodies against proteins that are not specific to T. pallidum (non-treponemal antibodies) can be detected using the Rapid Plasma Reagin (RPR), Venereal Disease Research Laboratory (VDRL) or toluidine red unheated serum (TRUST) tests and usually appear 2–3 week after treponemal antibodies. With effective treatment (which is arbitrarily shown here at 6 months), the non-treponemal antibody levels decline whereas the treponemal antibodies remain high for many years. In ~20% of patients, non-trepnemal antibodies persist 6 months after treatment; these individuals are labelled as having a serofast status. Despite repeated treatment, ~11% of patients remain serofast187. Here, we show early syphilis (including primary, secondary and early latent infections; infectious syphilis) and late syphilis (including late latent and tertiary infections) as being ≤1 year in duration and >1 year in duration, respectively, in line with US and European guidelines. However, the WHO guidelines place this demarcation at 2 years. Beyond primary and secondary syphilis, the pattern of serological response over time is less well defined and is accordingly not shown.
Table 2.
Serological tests for Treponema pallidum
| Method | Sample | Advantages | Disadvantages |
|---|---|---|---|
| Nontreponemal tests (NTTs) | |||
| Venereal Disease Research Laboratory (VDRL) slide test | Serum, plasma or CSF |
|
|
| Rapid Plasma Reagin (RPR) or Toluidine Unheated Serum Test (TRUST) | Serum or plasma |
|
|
| Treponemal tests (TTs) | |||
| Fluorescent Treponemal Antibody Absorbed (FTA-ABS) test | Serum or plasma, CSF |
|
|
| T. pallidum Particle Agglutination (TPPA) | Serum or plasma |
|
|
| T. pallidum Haemagglutination (TPHA) and micro-haemagglutination assay (MHA-Tp) | Serum or plasma |
|
|
| Treponemal Enzyme Immunoassay (EIA) | Serum |
|
|
| Chemiluminescence Immunoassay (CIA) | Serum |
|
|
| Rapid tests | |||
| Treponemal | Whole blood, plasma or serum |
|
|
| Treponemal/nontreponemal test | Whole blood, plasma or serum |
|
|
| Dual syphilis/HIV tests | Whole blood, plasma or serum |
|
|
The data on sensitivity and specificity of each test with respect to disease stage are reported in the WHO Manual on Laboratory Diagnosis of Sexually Transmitted Infections, including HIV 252.
Without treatment, titres peak at 1–2 years after infection and remain positive even in late disease (usually at a low titre). After treatment, titres generally decline and in most immunocompetent individuals become nonreactive within 6 months. However, up to 20% of syphilis-infected individuals have a persistently reactive (albeit low-titre) NTT even after treatment, possibly related to a less robust pro-inflammatory immune response135. These patients are labelled as having a serofast status and are observed more commonly with treatment for late latent than early syphilis37,136. Biologic false positive results can occur in ~2–5% of the population, regardless of the NTT test used— although the proportion is difficult to estimate with certainty because it is influenced by the population studied137. These low-titre reactions might be of limited duration if related to acute factors (such as febrile illness, immunization or pregnancy) or longer duration if related to chronic conditions (such as autoimmune diseases, hepatitis C infection or leprosy) 136,138. By contrast, false-negative results can occur in sera with very high titres (such as those with secondary syphilis) that are not diluted before testing, a phenomenon known as a Prozone effect. Pre-dilution of sera re-establishes the concentration needed for optimal antibody–antigen interaction and avoids this problem.
TTs
In contrast to NTTs, TTs detect antibodies directed against T. pallidum proteins and are theoretically highly specific. However, as most syphilis-infected individuals develop treponemal antibodies that persist throughout life, TTs cannot be used to distinguish an active from a past or previously treated infection and are not useful in evaluating treatment efficacy. TTs are used as confirmatory assays following a positive NTT result.
TTs become positive 6–14 days after the primary chancre appears (~5 weeks after infection) and, therefore, may be useful to detect early syphilis missed by NTT testing. These tests are usually laboratory based and include the fluorescent treponemal antibody absorbed (FTA-ABS) test, the microhaemagglutination assay for antibodies to T. pallidum (MHA-TP), the T. pallidum passive particle agglutination (TPPA) and T. pallidum haemagglutination (TPHA) assays (Table 2). These tests also require trained personnel in a laboratory setting, are more expensive and technically more complex than NTTs and involve specialized reagents and equipment. For these reasons, in the developing world, laboratory-based TTs are not widely available in primary care settings, hence limiting their utility as confirmatory assays for NTTs.
In recent years, TTs using recombinant T. pallidum antigens in enzyme and chemiluminescence immunoassay (EIA and CIA, respectively) formats have been commercialized. These assays are useful for large-scale screening as they are automated or semi-automated and, because they are read spectrophotometrically, are not subjective13,139–142. In higher income countries, many health care institutions depend upon high throughput screening and have adopted ‘reverse’ algorithms that screen with an automated treponemal EIA or CIA and confirm with a NTT rather than the opposite, traditional approach (Fig. 7). Few studies as yet have addressed the accuracy of these ‘reverse testing’ algorithms 40,143. The traditional and reverse approaches should theoretically produce the same result. However, the reverse alogorithm results in the detection of early syphilis cases (TT-positive, NTT-negative) that would not detected by the conventional approach144. As this pattern of serological reactivity occurs in very early primary syphilis, in previously treated disease and late infection, considerable attention should be given to a thorough physical examination of the patient, previous history and recent sexual risk before initiating any treatment and partner notification activities.
Figure 7. Screening algorithms for syphilis.
A | The traditional algorithm begins with a qualitative non-treponemal test (NTT) that is confirmed with a treponemal test (TT). This algorithm has a high positive predictive value when both tests are reactive, although early primary and previously treated infections can be missed owing to the lower sensitivity of NTTs136. Importantly, this algorithm is less costly than reverse screening algorithms, and does not require highly specialized laboratory equipment, but is limited by subjective interpretation of the technologist. Additionally, false negative NTT results can arise from the prozone effect (when there is an excess of antibody). Finally, because the traditional algorithm is not always followed by a confirmatory TT, previously treated, early untreated and late latent cases can be missed and biologically false-positive cases can be overtreated. B | The reverse screening algorithm uses a TT with recombinant T. pallidum antigens in enzyme immunoassay (EIA) or chemiluminescence immunoassay (CIA) formats that, when reactive, is followed by an NTT. This approach is associated with higher initial setup costs and ongoing operational costs than the traditional algorithm, but the algorithm permits treatment of 99% of syphilis cases, compared to the traditional algorithm in a low-prevalence setting246. Also, because TTs are not flocculation assays, false negative tests due to the Prozone effect do not occur. However, in high-risk populations, screening with a TT can result in a high rate of positive results due to previously treated infections, leading to increased clinician workload needed to review cases and determine appropriate management. Some guidelines recommend further evaluation of reactive TT with a quantitative NTT and, if results of the latter are nonreactive, a second (different) TT to help resolve the discordant results143,247,248. The European Centre for Disease Prevention and Control uses a variation of this approach: a reactive TT immunoassay is followed by a second (different) TT of any kind (that is, not followed by an NTT)249. Ideally, a positive TT should be supplemented by another TT or an NTT. However, in most developing countries, and in particular given the serious consequences of syphilis in pregnancy, treatment is recommended in a patient with a positive TT result.
Rapid tests
POC rapid TTs are a recent technology that enable onsite screening and treatment, and are particularly useful in settings with limited laboratory capacity. Rapid syphilis use a finger-prick whole blood sample and are are typically immuno-chromatographic strip-based TT assays that can be stored at room temperature, require no equipment and minimal training, and give a result in <20 minutes145 (Table 2). Various rapid tests have been evaluated in a range of clinical and community settings and shown to fulfil the ASSURED criteria134,146–154. Like other TTs, most POC diagnostics have the limitation of being unable to distinguish between recent and previously treated syphilis infection and, therefore, could lead to overtreatment. Ideally, patients found to have a reactive POC TT would be further evaluated with an NTT to support management decisions; however, this is often not possible in settings with limited laboratory capacity as is the case in many antenatal care clinics and outreach programmes for high-risk populations. POC rapid tests play an important part when delayed diagnosis is problematic, such as in pregnant women in whom delayed or no treatment poses significant risks to the fetus that far outweigh the risks of overtreatment for the mother45,155. In non-pregnant individuals, treatment is recommended in those who test positive if they have no prior history of treatment, and to refer those with a prior history to have an NTT11.
At least one test has been developed that enables simultaneous detection of non-treponemal and treponemal antibodies in a single POC device156–158. Additionally, dual syphilis/HIV rapid tests are available to screen for HIV and treponemal antibodies using a single lateral flow immunochromatographic strip. These are an increasingly important tool in the global elimination of MTCT of HIV and syphilis in settings in which laboratory capacity is limited159.
Tests useful in special situations
Neurosyphilis
The diagnosis of neurosyphilis is challenging. The CSF is frequently abnormal in patients with neurosyphilis with both pleocytosis (lymphocyte accumulation) and a raised protein concentration. The Venereal Disease Research Laboratory (VDRL) assay performed on CSF is considered the gold standard for specificity but is recognized to have limited sensitivity160,161. Other CSF tests, including serological assays — such as the Rapid Plasma Reagin (RPR)162, FTA-ABS163 and TPHA164 — and molecular assays including PCR165 have all been assessed for CSF and have variable specificity and sensitivity for the diagnosis of neurosyphilis. Difficulties in interpretation of CSF pleocytosis in individuals co-infected with HIV and syphilis challenge the evaluation of the relationship between the two diseases. CSF pleocytosis occurs in individuals with either infection alone37,165; thus, discerning the cause of pleocytosis in co-infected individuals is not always possible.
Congenital syphilis
Diagnosis of congenital syphilis in exposed, asymptomatic infants is another area in testing can be improved. Because maternal nontreponemal and treponemal IgG antibodies can be transferred from mother to child, treponemal testing of infant serum is difficult to interpret and is not recommended37. An infant with a reactive RPR or VDRL serum titre that is ≥4-times than those of the mother is highly suggestive of congenital syphilis, but its absence does not exclude the diagnosis. A clinical examination, reactive infant CSF VDRL, abnormal complete blood count or liver function tests or suggestive long-bone radiographs (that, for example, show retarded ossification or dislocation of epiphyses and radiolucencies, especially of long bones) can support a diagnosis of congenital syphilis. Use of IgM immunoblots is controversial owing to limited availability of tests and inconclusive data thus far on sensitivity; their use in diagnosing congenital syphilis is recommended in some guidelines11,13 but not others37. Maternal syphilis infection is highly correlated with fetal loss, therefore, evaluation of a stillborn infant should include evaluation of maternal tests for syphilis11.
Screening
The wide availability of effective treatment and resulting decline in syphilis prevalence has led to low yield of screening in low prevalence settings; thus, screening in low-risk adults (for example, premarital adults or those admitted to hospital) has been abandoned in most places. However, systematic reviews provide convincing evidence in favour of syphilis screening in pregnant women13,166, adults and adolescents at increased risk for infection13,40 and individuals donating blood, blood products or solid organs 13,167–169. Several countries also recommend syphilis testing in people with unexplained sudden visual loss, deafness or meningitis as these may be manifestations of early neurosyphilis13,37.
Prenatal screening
Syphilis screening is universally recommended for pregnant women, regardless of previous exposure, because of the high risk of MTCT during pregnancy and the availability of a highly effective preventive intervention against adverse pregnancy outcomes11,37,41,46. Global normative authorities and most national guidelines recommend syphilis screening at the first prenatal visit, ideally during the first trimester11,37,41,170. Some countries recommend that women at high risk have repeat screening in the third trimester and again at delivery to identify new infections37. Women should be tested in each pregnancy, even if they have tested negatively in a previous pregnancy. When access to prenatal care is not optimal or laboratory capacity is limited, rapid tests have been found beneficial in detecting and treating syphilis in pregnancy148. Guidelines recommend that, post-delivery, neonates should not be discharged from the health facility unless the serological status of the mother had been determined at least once during pregnancy and preferably again at delivery11,37.
The importance of universal syphilis screening in pregnancy to prevent perinatal and infant morbidity and mortality is highlighted in the current WHO global initiative to eliminate congenital syphilis43,44 and justified by the continuing high global burden of congenital syphilis, availability of an effective and affordable preventive intervention and wider availability of low cost POC rapid tests that can be used when laboratory capacity is lacking23,43,44,46,145. A systematic review of studies (most of which were conducted in low-income countries) reporting on antenatal programmes initiating or expanding syphilis screening, compared with various local control conditions, found that enhanced screening reduced syphilis-associated adverse birth outcomes by >50% 171. Integration of syphilis testing with other prenatal interventions, including HIV testing, has been shown to be cost-effective across settings, even when syphilis prevalence is low48–51. Strategies that enhance screening coverage, such as increased use of POC rapid testing and integrating syphilis and HIV screening, will further support global elimination of congenital syphilis145,172–174.
Screening at-risk populations
Increased risk for infection can be related to personal or partner behaviours leading to syphilis infection or living in a community with high syphilis prevalence37,40. In many countries, syphilis testing is recommended for all attendees at STI or sexual health centres and as part of integrated services targeted to high-risk groups (such as HIV testing centres or drug treatment centres)13,37. The optimal screening interval for individuals at increased risk for infection is not well established; however, some guidelines suggest that MSM or people with HIV may benefit from more frequent screening than others at risk for syphilis (for example, 3 monthly rather than annual screening) 37,40,175,176.
At-risk communities are often marginalized from care and experience discrimination and stigma when using traditional STI services177. Innovations in promoting uptake of testing and developing user-friendly services are important in the control of syphilis in these communities to reduce transmission. Social entrepreneurship and crowdsourcing approaches have been shown as innovative approaches to improve HIV and syphilis testing coverage rates and accelerate linkage to care, two fundamental elements within the cascade of STI service delivery178,179. Evaluation studies of other interventions, such as pre-exposure prophylaxis (PrEP) for syphilis, are also underway180. One option in the future might be to simultaneously administer PrEP for syphilis and HIV181.
Blood bank screening
Although syphilis was among the first identified infectious risks for blood donation and s transmission through blood has been documented182–184, reports of transfusion-transmitted syphilis have become exceedingly rare over the past 60 years as increasingly more countries adopt donor selection processes, universal serological screening of donors and use of refrigerated products rather than fresh blood components183,185. Survival of T. pallidum in different blood components has been shown to vary according to storage conditions, with fresh blood or blood components stored for <5 days more infectious than blood stored for longer periods183. Syphilis screening of blood, blood components or solid organs remains a recommendation in many countries13,169. Occasional cases of transfusion-transmitted syphilis are still reported in settings with high syphilis prevalence, particularly with transfusion of fresh blood167.
Prevention
There is as yet no vaccine against syphilis, and the most effective mode of prevention is prompt treatment to avoid continued transmission of the disease sexually or vertically from mother-to-child, and treatment of all sex partners to avoid reinfection. Other prevention modalities against venereal transmission of syphilis are latex condom use, male circumcision and avoiding sex with infected partners37. Treatment of exposed sex partners is important to avoid reinfection37.
Management
Important factors in managing syphilis are early detection, prompt treatment with an effective antibiotic regimen and treatment of sex partners of a person with infectious syphilis (primary, secondary or early latent infections). The WHO guidelines11 (Box 1) and European guidelines13 for the management of early syphilis in adults are the same. The CDC guidelines do not offer procaine penicillin as a treatment, but are otherwise identical12. Patients with late syphilis are no longer infectious. Thus, the objective of treatment is to prevent complications in persons who are asymptomatic (that is, have late latent syphilis) or arrest their development if the patient has manifestations of tertiary disease. Treatment of late syphilis requires longer courses of antimicrobial therapy than early disease.
Box 1. WHO guidelines for the treatment of syphilis.
Early syphilis
Intramuscular benzathine penicillin G (single dose)
Or intramuscular procaine penicillin (daily doses for 10–14 days)
If penicillin-based treatment cannot be used, oral doxycycline (twice daily doses for 10–14 days)* or intramuscular ceftriaxone (daily doses for 10–14 days)
Late syphilis
Intramuscular benzathine penicillin G (weekly doses for 3 weeks)
Or intramuscular procaine penicillin (daily doses for 20 days)
If penicillin-based treatment cannot be used, oral doxycycline (daily doses for 30 days)*
Congenital syphilis
Intravenous aqueous benzyl penicillin six hourly (for 10–15 days)
Or intramuscular procaine penicillin daily (for 10–15 days)
*Contraindicated in pregnancy. From Ref.11
Penicillin
Penicillin has been the mainstay of treatment for syphilis since it first became widely available in the late 1940s. Although its efficacy was never demonstrated in a randomized controlled trial, it was clearly far superior to all previous treatments, and T. pallidum resistance to penicillin has never been reported. As T. pallidum divides slower than most bacteria, it is necessary to maintain penicillin levels in the blood above the minimum inhibitory concentration (MIC) for at least 10 days; this can be achieved by giving a single intramuscular injection of long-acting benzathine penicillin G (which benefits from not requiring patient adherence to a prolonged drug regimen). The first-line treatments for early syphilis recommended by the CDC and European (authored by the International Union Against Sexually Transmitted Infections) guidelines are very similar12,13 as are recommendations for treatment of exposed sex partners. Patients with late syphilis, or with syphilis of unknown duration, should receive longer courses of treatment (Box 1). Those with symptoms suggestive of neurosyphilis or ocular involvement should undergo lumbar puncture to confirm or rule out the presence of neurosyphilis, which requires more intensive treatment. However, CDC and European guidelines define latent syphilis as occurring beginning at 1 year after infection, whereas the WHO defines latent syphilis to occur beginning at 2 years, resulting in some differences in management; that is, longer treatment duration is required for some patients in the United States and Europe.
Given that confirmation or exclusion of the presence of viable T. pallidum after treatment is not possible, treatment efficacy is measured indirectly using serology. Cure is usually defined as reversion to negative or a fourfold reduction in titre of an NTT. However, as noted earlier, a minority of patients remain seropositive, with a less than four-fold reduction in NTT titre, in spite of almost certainly having been cured and with no evidence of progressive disease — the so-called serofast state186. The management of these patients depends on taking a careful sexual history to exclude the possibility of reinfection, which can be challenging as patients may not recognize new infections. The serofast state more commonly occurs in patients with late syphilis and low NTT titres and in HIV-positive patients who are not on anti-retroviral treatment187. Because few data are available on long-term clinical outcomes in serofast patients, CDC guidelines recommend continuing clinical follow up and retreatment if follow up cannot be ensured12.
Second-line treatments
Patients who are allergic to penicillin should be treated with doxycycline or ceftriaxone (though allergy to cephalosporins is more common in those who are allergic to penicillin) with repeat NTT serology as follow up. Doxycycline is contraindicated in pregnancy. Two treatment trials of early syphilis in Africa showed that a single oral dose of azithromycin was equivalent to benzathine penicillin G188,189. Unfortunately, strains of T. pallidum with a mutation that confers resistance to azithromycin and other macrolide antibiotics are common in the United States, Europe, China and Australia190–194. A study in HIV-positive patients with syphilis showed that azithromycin to prevent opportunistic infections led to better serological outcomes195. The WHO recommends the use of azithromycin for the treatment of syphilis only in settings where the prevalence of macrolide-resistant T. pallidum is known to be very low.
HIV co-infection
In patients with early syphilis, a raised CSF cell count and protein are found more frequently in the CSF of patients with HIV infection than in HIV-uninfected patients, and there is some evidence that early symptomatic neurosyphilis is more common in HIV-positive patients196,197. As single-dose benzathine penicillin G does not reliably lead to treponemicidal levels in the CSF, some experts have suggested that HIV co-infected patients with early syphilis should receive enhanced treatment 198. However, a randomized controlled trial (n= 541) showed no significant difference in clinical outcomes between patients receiving standard or enhanced treatment15. Notably, the 101 HIV-infected patients enrolled in the trial responded less well serologically, but due to loss to follow up the study was underpowered to detect a two-fold difference in standard versus enhanced treatment in HIV co-infected patients. Furthermore, a large (n=573) prospective observational study in Taiwan found no difference between single-dose benzathine penicillin G and enhanced treatment in a per-protocol analysis199. However, using a last-observed-carried-forward analysis to account for missing data, the authors concluded that 67.1% of those who received one dose responded serologically compared with 74.8%who received the enhanced treatment, a statistically significant difference (P=0.044) 199. Finally, a retrospective study (n= 478) showed no difference in serological response at 13 months between those receiving single-dose benzathine penicillin G and enhanced treatment 200. Given the inconclusive results of these studies, many clinicians continue to offer enhanced therapy to HIV co-infected patients with early syphilis.
Treatment in pregnancy
Adverse pregnancy outcomes are common in women with syphilis45,119. A study in Tanzania found that, of women with latent syphilis who had RPR titres ≥1:8, 25% delivered a stillborn, and 33% a live but preterm infant21. A second study showed that adverse pregnancy outcomes due to syphilis can be prevented with a single dose of benzathine penicillin G given before 28 weeks’ gestation201 and that, in this setting, in which 5–6% of pregnant women had syphilis, this was one of the most cost-effective interventions available in terms of cost per disability-adjusted life year saved202.
Penicillin is the only antibiotic known to be effective in treating syphilis in pregnancy and preventing adverse birth outcomes. Since doxycycline is contraindicated in pregnancy, and macrolides such as azithromycin and erythromycin do not cross the placenta well, there are few alternatives to penicillin for the treatment of pregnant women with syphilis who are allergic to penicillin. The CDC recommends desensitization for those who are allergic to penicillin12.
Congenital syphilis
The WHO recommends that infants with suspected congenital syphilis, including infants who are born to syphilis-seropositive mothers who were not treated with penicillin >30 days before delivery, should be treated with aqueous benzyl penicillin or procaine penicillin (Box 1). All syphilis-exposed infants, including infants without signs or symptoms at birth, should be followed closely, ideally with NTT titres. Titres should decline by 3 months of age and be nonreactive by 6 months12. TTs are not useful in infants due to persistent maternal antibody.
Neurosyphilis and ocular syphilis
CNS involvement can occur during any stage of syphilis, but there is no evidence supporting a need to deviate from recommended syphilis regimens without presence of clinical neurological findings (such as ophthalmical or auditory symptoms, cranial nerve palsies, cognitive dysfunction, motor or sensory deficits, or signs of meningitis or stroke)203. With symptoms and tests indicating neurosyphilis, or any suggestion of ocular syphilis regardless of CSF testing, more-intensive treatment is recommended. For example, the CDC recommends that adults with neurosyphilis or ocular syphilis should be treated with high-dose intravenous aqueous crystalline, or intramuscular procaine penicillin plus probenecid, for 10–14 days204.
Quality of life
Historical reports dating from the 15th century indicate that syphilis was perceived as a dangerous infection, and a source of public alarm via fear of contagion and dread of its manifestations and anxiety around its highly toxic ‘cures’ (heavy metal therapy with mercury, arsenicals or bismuth)205–207. Case reports through the 19th century as well as modern re-evaluations of skeletal remains support the fact that the disease could cause severe physical stigmata, with individuals having disfiguring rashes; non-healing ulcerations; painful bony lesions that often involved destruction of the nose and palate; visceral involvement; dementia and other incapacitating neurological complications; and early death208. Stigmatization associated with syphilis was also evident, with symptomatic patients quarantined to specialized hospitals, and affected people hiding their symptoms — perhaps fearing societal shunning or the dubiously effective treatment regimens even more than the disease209. Reductions in syphilis prevalence were documented after the introduction of penicillin210 and since that time, the most virulent manifestations of the disease have almost vanished, and today it is rare to find a patient with tertiary disease211. Nevertheless, continuing reports emphasize that complications of late syphilis, particularly those involving the eyes, CNS and cardiovascular system, can cause lifelong disability and even death9. For example, case numbers of ocular syphilis have increased with rising syphilis incidence rates in many communities212, with delayed treatment associated with permanently diminished visual acuity213. It is essential, therefore, that caregivers be cognizant of the need to screen at-risk patients for latent infection and administer therapy if previous treatment has not been documented.
Few modern studies have addressed quality of life in men and women with syphilis, whether in social, psychological or economic contexts. One study (n= 250) showed only a minor effect on patient-reported quality of life at time of treatment, and essentially no effect 1 month after treatment214. The currently high case rates of syphilis infection and reinfection among MSM in urban centres throughout the world may lend support to the notion that syphilis in the modern era poses limited impact on quality of life as long as it is detected and treated. However, partner notification studies suggest STI diagnoses can lead to significant social stigma, intense embarrassment, and fear of retaliation, domestic violence or loss of relationship177. Public health experts have posited that syphilis is the source of more stigma than other STI diagnoses, although this is difficult to measure with certainty because STI programmes tend to focus contact tracing efforts more strongly on syphilis than other curable STIs owing to its serious consequences 215. In one study measuring the level of shame associated with several stigmatizing skin diseases, patients assigned greatest shame to syphilis — more than to AIDS, other STIs or several disfiguring skin conditions216.
Untreated maternal syphilis results in severe adverse perinatal outcomes, most prominently stillbirth, in at least half of affected pregnancies45. While MTCT of syphilis is clearly linked to lack of prenatal care, WHO data indicate that globally, whether in wealthy or poor nations, most adverse pregnancy outcomes caused by maternal syphilis are in women who attended prenatal care but were not adequately tested or treated24. This suggests other factors, such as weak health systems, gender inequality, lack of political will to support quality STI and reproductive health services, or other structural influences associated with lack of screening might be at play217. An increasing literature supports that, as for infant loss, a stillbirth can lead to poor mental and other health outcomes for both parents and the wider family, even extending to health care providers. For example, experiencing a stillbirth has been linked to ‘unspoken grief’ and a variety of psychosocial consequences such as depression, blame, shame, social isolation, problems in future pregnancies and relationship dissolution218–220. In Haiti, pregnancy loss associated with syphilis (which had a maternal prevalence of 6%) is so common that a myth about a werewolf sucking the blood out of the unborn fetus has developed to help women with their loss and suffering221. Economic research suggests a stillbirth results in substantial direct and indirect costs and can sometimes require more resources than a livebirth219.
Outlook
With syphilis continuing to be the leading cause of preventable stillbirths in the developing world and re-emerging as a public health threat in developed nations, particularly in HIV co-infected MSM, the demand for improved diagnostics, prevention strategies and treatments is growing. Here, we describe the most pressing issues and propose a call to action (Box 2).
Box 2. Major challenges and a call to action wish list.
Eliminate mother-to-child transmission of syphilis
Requires political commitment
Prenatal syphilis screening to be integrated into mother-to-child elimination programmes for HIV or as a component of an essential diagnostic package for prenatal care
Develop point-of-care tests with data connectivity or data transmission capability to facilitate automated surveillance and to improve the efficiency of health systems
Address HIV and syphilis co-infection in MSM
Requires research into potential synergies between the two infections
Implement scientific and community involvement to reach at-risk populations
Integrate programmes for HIV, syphilis, hepatitis and other STIs
Develop tests for active infection, neurosyphilis and congenital syphilis
Development of biomarkers for test development
Development of network of clinical sites for rapid validation of new tests
Develop new oral drugs to prevent transmission to fetus and to sexual partners
Provide incentives for drug discovery programmes
Provide incentives to evaluate drug combinations
Develop vaccines
Requires research to better understand pathogenesis
Requires research to identify vaccine targets and methods for validation
Elimination of MTCT of syphilis
The WHO campaign to eradicate yaws, which treated >50 million people with penicillin and reduced the number of cases by at ≥95% worldwide between 1952 and 1964, was ultimately unsuccessful. What can we learn from this heroic failure? The yaws eradication campaign was based on clinical examination and serological testing to determine prevalence by community, and mass treatment or selective mass treatment (cases and contacts) of communities with penicillin, depending on prevalence. Unfortunately, as the prevalence of yaws fell, it was no longer perceived as an important public health problem worthy of an expensive vertical programme; resources were diverted to other programmes, yaws was forgotten, and it came re-emerged222. To some extent the same is true of syphilis; once penicillin became available, its incidence and prevalence declined in many parts of the world, and it was no longer seen as a public health priority. Although screening of all pregnant women for syphilis has continued to be recommended in most countries, coverage has been low in many regions; for example, WHO estimates that approximately 50% of antenatal clinic attenders in Africa are not currently screened for syphilis24. This low coverage has resulted in a high burden of entirely preventable stillbirths and neonatal deaths23. Exacerbating this situation, the WHO has received reports of stock outs and shortages of injectable benzathine penicillin G in multiple countries, many with a high burden of maternal and congenital syphilis. In collaboration with international partners, the WHO has spearheaded an initiative to assess global supply, current and projected demand, and production capacity for benzathine penicillin G223.
Strong advocacy will be needed to ensure that the control and elimination of syphilis is given a high priority on the global health agenda. Policy makers and funders need to be made aware that syphilis is a leading cause of preventable stillbirths and neonatal death, that these deaths can be prevented with a single dose of penicillin given to the mother before 28 weeks gestation, and that this is one of the most cost-effective health interventions available51,202. Perhaps with this awareness and political will, syphilis MTCT elimination programmes — which have failed to progress in the past 10 years224 — will witness the success of the MTCT HIV programmes in Africa. Other developments are occurring that are forging change. For example, the availability of POC tests has led to increased coverage of antenatal screening and treatment for syphilis in many settings148, and the WHO campaign for the elimination of MTCT of HIV and syphilis has increased the visibility of syphilis on the global health agenda. In 2014, the WHO target for the elimination of MTCT of syphilis was ≤50 cases of congenital syphilis per 100,000 live births. The process targets are antenatal care coverage (at least one visit) of ≥95% of pregnant women; coverage of syphilis testing ≥95% of pregnant women; and treatment of ≥95% of seropositive pregnant woment225. Additionally, the WHO has conducted a systematic review of the performance of dual HIV-syphilis rapid tests and issued an information note on testing algorithms for dual HIV-syphilis tests226.
The huge reduction in the number of HIV-positive infants in Africa in recent years, a more difficult undertaking than reducing MTCT of syphilis, is proof of concept that congenital syphilis elimination is achievable. Given that Cuba, Thailand, Belarus, Moldova and Armenia have eliminated MTCT of HIV, syphilis or both, elimination can be achieved with political will and a well-organized health care system. Indeed, inclusion of syphilis and HIV screening with tests for anaemia, diabetes and pre-eclampsia as a package of essential diagnostics for prenatal care should be implemented as a minimum standard to ensure safe and healthy pregnancies worldwide.
POC testing has greatly increased access to screening for pregnant women, and has the potential to increase access to screening for high-risk groups such as MSM and FSWs through outreach programmes. However, the quality of testing must be assured given these tests are conducted outside the laboratory. Strategies to ensure reliability of POC tests include use of electronic readers227 and microfluidic assays powered by smart phones228 for real-time monitoring of progress229, and routine provision of proficiency testing panels121,122. For example, one study in the Amazon region of Brazil showed that proficiency panels consisting of dried serum tubes that were assessed by each healthcare worked could be used to monitor the performance of healthcare workers in remote settings123.
HIV and syphilis co-infection in MSM
In developed countries, the incidence of syphilis in MSM is several hundred times higher than in the general population. Furthermore, the incidence continues to increase as condom use has fallen with increasing use of pre-exposure prophylactic anti-retroviral medications for HIV42,230. Indeed, with wider HIV treatment coverage in recent years and HIV no longer considered a ‘death sentence’, there has been a decline in safe sex practices and more risk-taking behaviours231. However, the alarming increase in incidence of syphilis, compared to other STIs, in HIV-infected MSM cannot be explained by behavioural factors alone. The frequent co-infection of HIV and syphilis in MSM in many countries have led researchers and policy makers to consider the hypothesis that treatment for HIV may be a double-edged sword that contributed to increased susceptibility to syphilis through impairment of the innate or acquired immunity to T. pallidum 42,232.
Accordingly, research is urgently needed to understand the underlying causes of this twin epidemic. The involvement of the MSM community is critical in the design and implementation of innovative approaches to promote the uptake of testing and linkage to care, particularly as this community is still stigmatized and marginalized from care in many societies. Although self-testing for HIV and hepatitis C virus infection is now possible using highly sensitive and specific oral tests that are commercially available, syphilis does not elicit sufficient antibody for an oral test. Thus, implementation science is needed to integrate and optimize the delivery of a package of HIV, syphilis, hepatitis and other STI screening and treatment strategies and partner notification systems for MSM in different cultural, socioeconomic and political settings.
Better diagnostic tests
Research is needed to identify biomarkers that can more accurately distinguish between past, treated and active syphilis requiring treatment, identify patients who have become reinfected, and provide a test of cure. Using current serological tools, a high proportion of patients remain serofast after treatment in some settings, and the optimal management of these individuals is uncertain. Additionally, more-accurate diagnostic tests are needed to confirm the diagnosis of congenital syphilis, as serological tests based on IgG antibodies cannot distinguish between infected infants and those with passively acquired maternal antibodies. IgM tests can be highly sensitive in symptomatic infants but have suboptimal sensitivity in infants who are infected but not symptomatic at birth12.
The diagnosis of neurosyphilis also remains a challenge, particularly in HIV co-infected patients, in whom a raised CSF protein or cell count does not necessarily indicate that the patient has neurosyphilis. Promisingly, a POC rapid test has been adapted for the diagnosis of neurosyphilis using CSF233; performance of this test is better in cell-free specimens, requiring the use of a centrifuge. Another promising assay might be measurement of macrophage migration factor (MIF); CSF levels of MIF alone was shown to have a sensitivity of 74.42% and a specificity of 67.74% for the diagnosis of neurosyphilis in one study (n=43)234. By integrating all CSF parameters (pleocytosis, increased protein and MIF), the sensitivity and specificity would be improved to 100% by parallel testing. Additionally, assay of B cell attractant chemokine CXCL13 in the CSF could be used to distinguish the pleoctysosis caused by HIV from that due to neurosyphilis in HIV-infected patients235.
Better use of existing drugs
With penicillin, many countries still struggle with the fear of injections on the part of patients and the management of anaphylactic shock on the part of the health care providers. Oral regimens that are safe in pregnancy and effective in preventing the transmission of syphilis to the fetus are urgently needed. Furthermore, macrolide resistance is correlated with treatment failure in patients with primary syphilis191, lending further urgency to the need to find alternative oral therapies. Incentives for a drug discovery programme for syphilis needs to be established and, in the meantime, evaluation of existing drug combinations might be useful as alternative to reduce the threat of development of resistance.
Vaccine development
Human challenge studies have shown that people with late latent syphilis are resistant to symptomatic reinfection with heterologous strains of T. pallidum, and protective immunity has been induced in rabbits by repeated inoculation with γ-irradiated T. pallidum236,237.Accordingly, it should be possible to develop protective vaccines. However, research on virulence determinants of T. pallidum, and our understanding of protective immunity against it, have been hindered by our inability to culture the bacteria in vitro. To overcome this limitation, genome sequencing of T. pallidum directly from clinical samples is now possible92,238 This advance should enable understanding of strain variation on a global scale, and help to identify outer membrane proteins and other surface antigens as possible vaccine candidates81. A recent study showed that immunization of rabbits with the lipoprotein TP071 prevented dissemination of T. pallidum and, hence, has become a promising vaccine candidate81. Integration of potential vaccine targets with diagnostic targets in discovery programmes also hold promise in accelerating progress towards improved tools for control, prevention and ultimately the elimination of this disease.
Acknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the US Centers for Disease Control and Prevention.
Footnotes
Author contributions
Introduction (R.W.P. and D.M.); Epidemiology (D.M. and X.-S.C.); Mechanisms/pathophysiology (J.D.R.); Diagnosis, screening and prevention (R.W.P., M.L.K., X.-S.C. and A.S.B.); Management (D.M.); Quality of life (M.K. and A.S.B.); Outlook (all authors); overview of the Primer (R.W.P.).
Competing interests
J.D.R. receives royalties for licensing of syphilis diagnostics reagents. The other authors declare no competing interests.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Giacani L, Lukehart SA. The Endemic Treponematoses. Clin Microbiol Rev. 2014;27:89–115. doi: 10.1128/CMR.00070-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smajs D, Norris SJ, Weinstock GM. Genetic diversity in Treponema pallidum: implications for pathogenesis, evolution and molecular diagnostics of syphilis and yaws. Infect Genet Evol. 2012;12:191–202. doi: 10.1016/j.meegid.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Melo FL, de Mello JCM, Fraga AM, Nunes K, Eggers S. Syphilis at the crossroad of phylogenetics and paleopathology. PLoS Negl Trop Dis. 2010;4:e575. doi: 10.1371/journal.pntd.0000575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.PENN CW. Avoidance of Host Defences by Treponema pallidum in Situ and on Extraction from Infected Rabbit Testes. Microbiology. 1981;126:69–75. doi: 10.1099/00221287-126-1-69. [DOI] [PubMed] [Google Scholar]
- 5.Stamm LV, Hodinka RL, Wyrick PB, Bassford PJ. Changes in the cell surface properties of Treponema pallidum that occur during in vitro incubation of freshly extracted organisms. Infect Immun. 1987;55:2255–61. doi: 10.1128/iai.55.9.2255-2261.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salazar JC, Rathi A, Michael NL, Radolf JD, Jagodzinski LL. Assessment of the kinetics of Treponema pallidum dissemination into blood and tissues in experimental syphilis by real-time quantitative PCR. Infect Immun. 2007;75:2954–8. doi: 10.1128/IAI.00090-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas DD, et al. Treponema pallidum invades intercellular junctions of endothelial cell monolayers. Proc Natl Acad Sci U S A. 1988;85:3608–12. doi: 10.1073/pnas.85.10.3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.LaFond RE, Lukehart SA. Biological Basis for Syphilis. Clin Microbiol Rev. 2006;19:29–49. doi: 10.1128/CMR.19.1.29-49.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Radolf JD, Tramont EC, Salazar JC. In: Mandell, Douglas and Bennett’s Principles and Practice of Infectious Diseases. Bennett JE, Dolin R, Blaser MJ, editors. 2014. pp. 2684–2709. [Google Scholar]
- 10.Cruz AR, et al. Immune Evasion and Recognition of the Syphilis Spirochete in Blood and Skin of Secondary Syphilis Patients: Two Immunologically Distinct Compartments. PLoS Negl Trop Dis. 2012;6:e1717. doi: 10.1371/journal.pntd.0001717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO. WHO guidelines for the treatment of Treponema pallidum (syphilis) [online] 2016 http://www.who.int/reproductivehealth/publications/rtis/syphilis-treatment-guidelines/en/ [PubMed]
- 12.Workowski KA, Bolan GA Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recommen Rep. 2015;64:1–137. doi: 10.1093/cid/civ771. [DOI] [PubMed] [Google Scholar]
- 13.Janier M, et al. 2014 European guideline on the management of syphilis. J Eur Acad Dermatology Venereol. 2014;28:1581–1593. doi: 10.1111/jdv.12734. [DOI] [PubMed] [Google Scholar]
- 14.Peeling RW, Hook EW. The pathogenesis of syphilis: the Great Mimicker, revisited. J Pathol. 2005;208:224–232. doi: 10.1002/path.1903. [DOI] [PubMed] [Google Scholar]
- 15.Rolfs RT, et al. A Randomized Trial of Enhanced Therapy for Early Syphilis in Patients with and without Human Immunodeficiency Virus Infection. N Engl J Med. 1997;337:307–314. doi: 10.1056/NEJM199707313370504. [DOI] [PubMed] [Google Scholar]
- 16.Golden MR, Marra CM, Holmes KK. Update on Syphilis. JAMA. 2003;290:1510. doi: 10.1001/jama.290.11.1510. [DOI] [PubMed] [Google Scholar]
- 17.Lukehart SA, et al. Invasion of the central nervous system by Treponema pallidum: implications for diagnosis and treatment. Ann Intern Med. 1988;109:855–62. doi: 10.7326/0003-4819-109-11-855. [DOI] [PubMed] [Google Scholar]
- 18.Shaffi T, Radolf JD, Sanchez PJ, Schulz KF, Murphy FK. In: Sexually Transmitted Diseases. 4. Holmes KK, et al., editors. 2008. pp. 1577–1609. [Google Scholar]
- 19.Sánchez PJ, et al. Evaluation of molecular methodologies and rabbit infectivity testing for the diagnosis of congenital syphilis and neonatal central nervous system invasion by Treponema pallidum. J Infect Dis. 1993;167:148–57. doi: 10.1093/infdis/167.1.148. [DOI] [PubMed] [Google Scholar]
- 20.FIUMARA NJ. Congenital syphilis in Massachusetts. N Engl J Med. 1951;245:634–40. doi: 10.1056/NEJM195110252451702. [DOI] [PubMed] [Google Scholar]
- 21.Watson-Jones D, et al. Syphilis in Pregnancy in Tanzania. I. Impact of Maternal Syphilis on Outcome of Pregnancy. J Infect Dis. 2002;186:940–947. doi: 10.1086/342952. A comprehensive and well designed study that showed the outcomes of syphilis in pregnancy. [DOI] [PubMed] [Google Scholar]
- 22.MAGNUSON HJ, EAGLE H, FLEISCHMAN R. The minimal infectious inoculum of Spirochaeta pallida (Nichols strain) and a consideration of its rate of multiplication in vivo. Am J Syph Gonorrhea Vener Dis. 1948;32:1–18. [PubMed] [Google Scholar]
- 23.Newman L, et al. Global Estimates of Syphilis in Pregnancy and Associated Adverse Outcomes: Analysis of Multinational Antenatal Surveillance Data. PLoS Med. 2013;10:e1001396. doi: 10.1371/journal.pmed.1001396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wijesooriya NS, et al. Global burden of maternal and congenital syphilis in 2008 and 2012: a health systems modelling study. Lancet Glob Heal. 2016;4:e525–e533. doi: 10.1016/S2214-109X(16)30135-8. This paper is one of three WHO studies that have provided the backbone of our data on the global burden of syphlis; it provided updated global estimates in pregnant women and of adverse pregnancy outcomes 5 years into the global programme for congenital syphilis elimination (that is, monitoring progress) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lawn JE, et al. Stillbirths: rates, risk factors, and acceleration towards 2030. Lancet (London, England) 2016;387:587–603. doi: 10.1016/S0140-6736(15)00837-5. The first paper that showed that syphilis has emerged as the leading cause of preventable stillbirths. [DOI] [PubMed] [Google Scholar]
- 26.Hook EW, Peeling RW. Syphilis Control — A Continuing Challenge. N Engl J Med. 2004;351:122–124. doi: 10.1056/NEJMp048126. [DOI] [PubMed] [Google Scholar]
- 27.Newman L, et al. Global Estimates of the Prevalence and Incidence of Four Curable Sexually Transmitted Infections in 2012 Based on Systematic Review and Global Reporting. PLoS One. 2015;10:e0143304. doi: 10.1371/journal.pone.0143304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Black V, et al. Field evaluation of Standard Diagnostics’ Bioline HIV/Syphilis Duo test among female sex workers in Johannesburg, South Africa. Sex Transm Infect. 2016;92:495–498. doi: 10.1136/sextrans-2015-052474. [DOI] [PubMed] [Google Scholar]
- 29.Elhadi M, et al. Integrated bio-behavioural HIV surveillance surveys among female sex workers in Sudan, 2011–2012. Sex Transm Infect. 2013;89(Suppl):17–22. doi: 10.1136/sextrans-2013-051097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vandepitte J, et al. HIV and other sexually transmitted infections in a cohort of women involved in high-risk sexual behavior in Kampala, Uganda. Sex Transm Dis. 2011;38:316–23. [PMC free article] [PubMed] [Google Scholar]
- 31.McLaughlin MM, et al. Sexually Transmitted Infections among Heterosexual Male Clients of Female Sex Workers in China: A Systematic Review and Meta-Analysis. PLoS One. 2013;8:e71394. doi: 10.1371/journal.pone.0071394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Su S, et al. Sustained high prevalence of viral hepatitis and sexually transmissible infections among female sex workers in China: a systematic review and meta-analysis. BMC Infect Dis. 2015;16 doi: 10.1186/s12879-015-1322-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen X-S, et al. Prevalence of syphilis infection in different tiers of female sex workers in China: implications for surveillance and interventions. BMC Infect Dis. 2012;12 doi: 10.1186/1471-2334-12-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abara WE, Hess KL, Neblett Fanfair R, Bernstein KT, Paz-Bailey G. Syphilis Trends among Men Who Have Sex with Men in the United States and Western Europe: A Systematic Review of Trend Studies Published between 2004 and 2015. PLoS One. 2016;11:e0159309. doi: 10.1371/journal.pone.0159309. A good review that descrobed the alarming increase of syphilis in MSM in the developed world. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Voux A, et al. State-Specific Rates of Primary and Secondary Syphilis Among Men Who Have Sex with Men - United States, 2015. MMWR Morb Mortal Wkly Rep. 2017;66:349–354. doi: 10.15585/mmwr.mm6613a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burchell AN, et al. High incidence of diagnosis with syphilis co-infection among men who have sex with men in an HIV cohort in Ontario, Canada. BMC Infect Dis. 2015;15 doi: 10.1186/s12879-015-1098-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention. Syphilis Treatment and Care, in 2015 STD Treatment Guidelines – Syphilis [online] 2015 https://www.cdc.gov/std/syphilis/treatment.htm.
- 38.Brodsky JL, et al. Syphilis Outbreak at a California Men’s Prison, 2007–2008: Propagation by Lapses in Clinical Management, Case Management, and Public Health Surveillance. J Correct Heal Care. 2012;19:54–64. doi: 10.1177/1078345812458088. [DOI] [PubMed] [Google Scholar]
- 39.Van Wagoner NJ, Harbison HS, Drewry J, Turnipseed E, Hook EW. Characteristics of Women Reporting Multiple Recent Sex Partners Presenting to a Sexually Transmitted Disease Clinic for Care. Sex Transm Dis. 2011;38:210–215. doi: 10.1097/OLQ.0b013e3181f6fe42. [DOI] [PubMed] [Google Scholar]
- 40.Bibbins-Domingo K, et al. Screening for Syphilis Infection in Nonpregnant Adults and Adolescents. JAMA. 2016;315:2321. doi: 10.1001/jama.2016.5824. [DOI] [PubMed] [Google Scholar]
- 41.European Centre for Disease Prevention and Control. Antenatal screening for HIV, hepatitis B, syphilis and rubella susceptibility in the EU/EEA [online] 2017 http://ecdc.europa.eu/en/publications/Publications/antenatal-screening-sci-advice-2017.pdf.
- 42.Rekart ML, et al. A double-edged sword: does highly active antiretroviral therapy contribute to syphilis incidence by impairing immunity to Treponema pallidum? Sex Transm Infect. 2017 doi: 10.1136/sextrans-2016-052870. sextrans-2016-052870. An interesting hypothesis that sought to explain the twin epidemics of HIV and syphilis in MSM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmid GP, Stoner BP, Hawkes S, Broutet N. The Need and Plan for Global Elimination of Congenital Syphilis. Sex Transm Dis. 2007;34:S5–S10. doi: 10.1097/01.olq.0000261456.09797.1b. [DOI] [PubMed] [Google Scholar]
- 44.WHO. The global elimination of congenital syphilis: rationale and strategy for action [online] 2007 http://www.who.int/reproductivehealth/publications/rtis/9789241595858/en/
- 45.Gomez GB, et al. Untreated maternal syphilis and adverse outcomes of pregnancy: a systematic review and meta-analysis. Bull World Health Organ. 2013;91:217–226. doi: 10.2471/BLT.12.107623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blencowe H, Cousens S, Kamb M, Berman S, Lawn JE. Lives Saved Tool supplement detection and treatment of syphilis in pregnancy to reduce syphilis related stillbirths and neonatal mortality. BMC Public Health. 2011;11:S9. doi: 10.1186/1471-2458-11-S3-S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.WHO. Investment case for eliminating mother-to-child transmission of syphilis [online] 2012 http://apps.who.int/iris/bitstream/10665/75480/1/9789241504348_eng.pdf?ua=1.
- 48.Kahn JG, et al. The Cost and Cost-Effectiveness of Scaling up Screening and Treatment of Syphilis in Pregnancy: A Model. PLoS One. 2014;9:e87510. doi: 10.1371/journal.pone.0087510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kuznik A, Lamorde M, Nyabigambo A, Manabe YC. Antenatal Syphilis Screening Using Point-of-Care Testing in Sub-Saharan African Countries: A Cost-Effectiveness Analysis. PLoS Med. 2013;10:e1001545. doi: 10.1371/journal.pmed.1001545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kuznik A, Muhumuza C, Komakech H, Marques EMR, Lamorde M. Antenatal Syphilis Screening Using Point-Of-Care Testing in Low- and Middle-Income Countries in Asia and Latin America: A Cost-Effectiveness Analysis. PLoS One. 2015;10:e0127379. doi: 10.1371/journal.pone.0127379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Owusu-Edusei K, et al. Cost-effectiveness of Integrated Routine Offering of Prenatal HIV and Syphilis Screening in China. Sex Transm Dis. 2014;41:103–110. doi: 10.1097/OLQ.0000000000000085. [DOI] [PubMed] [Google Scholar]
- 52.Pan American Health Organization. Regional initiative for the elimination of mother-to-child transmission of HIV and congenital syphilis in Latin America and the Caribbean. Concept document for the Caribbean [online] http://www.paho.org/derechoalaSSR.
- 53.Chen X, Yin Y, Wang Q, Wang B. Historical perspective of syphilis in the past 60 years in China: eliminated, forgotten, on the return. Chin Med J (Engl) 2013;126:2774–9. Many lessons can be learnt from this account of the history of syphilis in China, which was once eliminated but has now come back with a vengeance. [PubMed] [Google Scholar]
- 54.Taylor M, et al. Elimination of mother-to-child transmission of HIV and Syphilis (EMTCT): Process, progress, and program integration. PLoS Med. 2017;14:e1002329. doi: 10.1371/journal.pmed.1002329. This paper described a success story of syphilis control and elimination, and highlighted how continuing global efforts can achieve an AIDS-free and syphilis-free generation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Norris SJ, Cox DL, Weinstock GM. Biology of Treponema pallidum: correlation of functional activities with genome sequence data. J Mol Microbiol Biotechnol. 2001;3:37–62. [PubMed] [Google Scholar]
- 56.PENN CW, COCKAYNE A, BAILEY MJ. The Outer Membrane of Treponema pallidum: Biological Significance and Biochemical Properties. Microbiology. 1985;131:2349–2357. doi: 10.1099/00221287-131-9-2349. [DOI] [PubMed] [Google Scholar]
- 57.Radolf JD. Treponema pallidum and the quest for outer membrane proteins. Mol Microbiol. 1995;16:1067–1073. doi: 10.1111/j.1365-2958.1995.tb02332.x. [DOI] [PubMed] [Google Scholar]
- 58.Silver AC, et al. MyD88 Deficiency Markedly Worsens Tissue Inflammation and Bacterial Clearance in Mice Infected with Treponema pallidum, the Agent of Syphilis. PLoS One. 2013;8:e71388. doi: 10.1371/journal.pone.0071388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Turner TB, Hardy PH, Newman B. Infectivity tests in syphilis. Sex Transm Infect. 1969;45:183–195. [PubMed] [Google Scholar]
- 60.Lukehart SA, Marra CM. Current Protocols in Microbiology. John Wiley & Sons, Inc; 2007. [DOI] [Google Scholar]
- 61.Sell S, Norris SJ. The biology, pathology, and immunology of syphilis. Int Rev Exp Pathol. 1983;24:203–76. [PubMed] [Google Scholar]
- 62.Lukehart SA. Scientific Monogamy: Thirty Years Dancing with the Same Bug. Sex Transm Dis. 2008;35:2–7. doi: 10.1097/OLQ.0b013e318162c4f2. [DOI] [PubMed] [Google Scholar]
- 63.Radolf JD, et al. Treponema pallidum, the syphilis spirochete: making a living as a stealth pathogen. Nat Rev Microbiol. 2016;14:744–759. doi: 10.1038/nrmicro.2016.141. The review provided a very informative summary of the interaction of T. pallidum and its human hosts. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Paster BJ, Dewhirst F. In: Pathogenic Treponema: Molecular and Cellular Biology. Radolf JD, Lukehart SA, editors. 2006. pp. 9–18. [Google Scholar]
- 65.Fraser CM. Complete Genome Sequence of Treponema pallidum, the Syphilis Spirochete. Science (80-) 1998;281:375–388. doi: 10.1126/science.281.5375.375. [DOI] [PubMed] [Google Scholar]
- 66.Radolf JD, et al. Characterization of outer membranes isolated from Treponema pallidum, the syphilis spirochete. Infect Immun. 1995;63:4244–52. doi: 10.1128/iai.63.11.4244-4252.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cox DL, Akins DR, Porcella SF, Norgard MV, Radolf JD. Treponema pallidum in gel microdroplets: a novel strategy for investigation of treponemal molecular architecture. Mol Microbiol. 1995;15:1151–1164. doi: 10.1111/j.1365-2958.1995.tb02288.x. [DOI] [PubMed] [Google Scholar]
- 68.Radolf JD, Hazlett KRO, Lukehart SA. In: Pathogenic Treponemes: Cellular and Molecular Biology. Radolf JD, Lukehart SA, editors. 2006. pp. 197–236. [Google Scholar]
- 69.Salazar JC, Hazlett KRO, Radolf JD. The immune response to infection with Treponema pallidum, the stealth pathogen. Microbes Infect. 2002;4:1133–1140. doi: 10.1016/s1286-4579(02)01638-6. [DOI] [PubMed] [Google Scholar]
- 70.Chamberlain NR, Brandt ME, Erwin AL, Radolf JD, Norgard MV. Major integral membrane protein immunogens of Treponema pallidum are proteolipids. Infect Immun. 1989;57:2872–7. doi: 10.1128/iai.57.9.2872-2877.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Purcell BK, Swancutt MA, Radolf JD. Lipid modification of the 15 kilo Dalton major membrane immunogen of Treponema pallidum. Mol Microbiol. 1990;4:1371–1379. doi: 10.1111/j.1365-2958.1990.tb00716.x. [DOI] [PubMed] [Google Scholar]
- 72.Akins DR, Purcell BK, Mitra MM, Norgard MV, Radolf JD. Lipid modification of the 17-kilodalton membrane immunogen of Treponema pallidum determines macrophage activation as well as amphiphilicity. Infect Immun. 1993;61:1202–10. doi: 10.1128/iai.61.4.1202-1210.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Radolf JD, Chamberlain NR, Clausell A, Norgard MV. Identification and localization of integral membrane proteins of virulent Treponema pallidum subsp pallidum by phase partitioning with the nonionic detergent triton X-114. Infect Immun. 1988;56:490–8. doi: 10.1128/iai.56.2.490-498.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cox DL, Chang P, McDowall AW, Radolf JD. The outer membrane, not a coat of host proteins, limits antigenicity of virulent Treponema pallidum. Infect Immun. 1992;60:1076–83. doi: 10.1128/iai.60.3.1076-1083.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Deka RK, et al. Crystal Structure of the Tp34 (TP0971) Lipoprotein of Treponema pallidum: IMPLICATIONS OF ITS METAL-BOUND STATE AND AFFINITY FOR HUMAN LACTOFERRIN. J Biol Chem. 2006;282:5944–5958. doi: 10.1074/jbc.M610215200. [DOI] [PubMed] [Google Scholar]
- 76.Cameron CE, Brouwer NL, Tisch LM, Kuroiwa JMY. Defining the Interaction of the Treponema pallidum Adhesin Tp0751 with Laminin. Infect Immun. 2005;73:7485–7494. doi: 10.1128/IAI.73.11.7485-7494.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Houston S, Hof R, Honeyman L, Hassler J, Cameron CE. Activation and Proteolytic Activity of the Treponema pallidum Metalloprotease, Pallilysin. PLoS Pathog. 2012;8:e1002822. doi: 10.1371/journal.ppat.1002822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Houston S, et al. The multifunctional role of the pallilysin-associatedTreponema pallidumprotein, Tp0750, in promoting fibrinolysis and extracellular matrix component degradation. Mol Microbiol. 2014;91:618–634. doi: 10.1111/mmi.12482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Parker ML, et al. The Structure of Treponema pallidum Tp0751 (Pallilysin) Reveals a Non-canonical Lipocalin Fold That Mediates Adhesion to Extracellular Matrix Components and Interactions with Host Cells. PLOS Pathog. 2016;12:e1005919. doi: 10.1371/journal.ppat.1005919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cameron CE, et al. Heterologous Expression of the Treponema pallidum Laminin-Binding Adhesin Tp0751 in the Culturable Spirochete Treponema phagedenis. J Bacteriol. 2008;190:2565–2571. doi: 10.1128/JB.01537-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lithgow KV, et al. A defined syphilis vaccine candidate inhibits dissemination of Treponema pallidum subspecies pallidum. Nat Commun. 2017;8:14273. doi: 10.1038/ncomms14273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chan K, et al. Treponema pallidum Lipoprotein TP0435 Expressed in Borrelia burgdorferi Produces Multiple Surface/Periplasmic Isoforms and mediates Adherence. Sci Rep. 2016;6:25593. doi: 10.1038/srep25593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Luthra A, et al. The Transition from Closed to Open Conformation of Treponema pallidum Outer Membrane-associated Lipoprotein TP0453 Involves Membrane Sensing and Integration by Two Amphipathic Helices. J Biol Chem. 2011;286:41656–41668. doi: 10.1074/jbc.M111.305284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Desrosiers DC, et al. TP0326, a Treponema pallidum β-barrel assembly machinery A (BamA) orthologue and rare outer membrane protein. Mol Microbiol. 2011;80:1496–1515. doi: 10.1111/j.1365-2958.2011.07662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Luthra A, et al. A Homology Model Reveals Novel Structural Features and an Immunodominant Surface Loop/Opsonic Target in the Treponema pallidum BamA Ortholog TP_0326. J Bacteriol. 2015;197:1906–1920. doi: 10.1128/JB.00086-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rollauer SE, Sooreshjani MA, Noinaj N, Buchanan SK. Outer membrane protein biogenesis in Gram-negative bacteria. Philos Trans R Soc B Biol Sci. 2015;370:20150023. doi: 10.1098/rstb.2015.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Centurion-Lara A, et al. Treponema pallidum Major Sheath Protein Homologue Tpr K Is a Target of Opsonic Antibody and the Protective Immune Response. J Exp Med. 1999;189:647–656. doi: 10.1084/jem.189.4.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hazlett KRO, et al. The Tprk Protein ofTreponema pallidumIs Periplasmic and Is Not a Target of Opsonic Antibody or Protective Immunity. J Exp Med. 2001;193:1015–1026. doi: 10.1084/jem.193.9.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Centurion-Lara A, et al. Gene conversion: a mechanism for generation of heterogeneity in the tprK gene of Treponema pallidum during infection. Mol Microbiol. 2004;52:1579–1596. doi: 10.1111/j.1365-2958.2004.04086.x. [DOI] [PubMed] [Google Scholar]
- 90.LaFond RE, Centurion-Lara A, Godornes C, Van Voorhis WC, Lukehart SA. TprK Sequence Diversity Accumulates during Infection of Rabbits with Treponema pallidum subsp pallidum Nichols Strain. Infect Immun. 2006;74:1896–1906. doi: 10.1128/IAI.74.3.1896-1906.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Giacani L, et al. Antigenic Variation in Treponema pallidum: TprK Sequence Diversity Accumulates in Response to Immune Pressure during Experimental Syphilis. J Immunol. 2010;184:3822–3829. doi: 10.4049/jimmunol.0902788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pinto M, et al. Genome-scale analysis of the non-cultivable Treponema pallidum reveals extensive within-patient genetic variation. Nat Microbiol. 2016;2:16190. doi: 10.1038/nmicrobiol.2016.190. [DOI] [PubMed] [Google Scholar]
- 93.Anand A, et al. TprC/D (Tp0117/131), a Trimeric, Pore-Forming Rare Outer Membrane Protein of Treponema pallidum, Has a Bipartite Domain Structure. J Bacteriol. 2012;194:2321–2333. doi: 10.1128/JB.00101-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Anand A, et al. Bipartite Topology ofTreponema pallidumRepeat Proteins C/D and I. J Biol Chem. 2015;290:12313–12331. doi: 10.1074/jbc.M114.629188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Giacani L, et al. TP0262 is a modulator of promoter activity oftprSubfamily II genes ofTreponema pallidumssp.pallidum. Mol Microbiol. 2009;72:1087–1099. doi: 10.1111/j.1365-2958.2009.06712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Radolf JD, Desrosiers DC. Treponema pallidum, the stealth pathogen, changes, but how? Mol Microbiol. 2009;72:1081–1086. doi: 10.1111/j.1365-2958.2009.06711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Brinkman MB, et al. A Novel Treponema pallidum Antigen, TP0136, Is an Outer Membrane Protein That Binds Human Fibronectin. Infect Immun. 2008;76:1848–1857. doi: 10.1128/IAI.01424-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cameron CE, Brown EL, Kuroiwa JMY, Schnapp LM, Brouwer NL. Treponema pallidum Fibronectin-Binding Proteins. J Bacteriol. 2004;186:7019–7022. doi: 10.1128/JB.186.20.7019-7022.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ke W, Molini BJ, Lukehart SA, Giacani L. Treponema pallidum subsp pallidum TP0136 Protein Is Heterogeneous among Isolates and Binds Cellular and Plasma Fibronectin via its NH2-Terminal End. PLoS Negl Trop Dis. 2015;9:e0003662. doi: 10.1371/journal.pntd.0003662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Harman M, Vig DK, Radolf JD, Wolgemuth CW. Viscous Dynamics of Lyme Disease and Syphilis Spirochetes Reveal Flagellar Torque and Drag. Biophys J. 2013;105:2273–2280. doi: 10.1016/j.bpj.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Riley BS, Oppenheimer-Marks N, Hansen EJ, Radolf JD, Norgard MV. Virulent Treponema pallidum activates human vascular endothelial cells. J Infect Dis. 1992;165:484–93. doi: 10.1093/infdis/165.3.484. [DOI] [PubMed] [Google Scholar]
- 102.Quatresooz P, Piérard GE. Skin Homing of Treponema pallidum in Early Syphilis. Appl Immunohistochem Mol Morphol. 2009;17:47–50. doi: 10.1097/PAI.0b013e3181788186. [DOI] [PubMed] [Google Scholar]
- 103.Bouis DA, Popova TG, Takashima A, Norgard MV. Dendritic cells phagocytose and are activated by Treponema pallidum. Infect Immun. 2001;69:518–28. doi: 10.1128/IAI.69.1.518-528.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Moore MW, et al. Phagocytosis of Borrelia burgdorferi and Treponema pallidum Potentiates Innate Immune Activation and Induces Gamma Interferon Production. Infect Immun. 2007;75:2046–2062. doi: 10.1128/IAI.01666-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Salazar JC, et al. Treponema pallidumElicits Innate and Adaptive Cellular Immune Responses in Skin and Blood during Secondary Syphilis: A Flow-Cytometric Analysis. J Infect Dis. 2007;195:879–887. doi: 10.1086/511822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Stary G, et al. Host Defense Mechanisms in Secondary Syphilitic Lesions. Am J Pathol. 2010;177:2421–2432. doi: 10.2353/ajpath.2010.100277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Van Voorhis WC, et al. Primary and Secondary Syphilis Lesions Contain mRNA for Thl Cytokines. J Infect Dis. 1996;173:491–495. doi: 10.1093/infdis/173.2.491. [DOI] [PubMed] [Google Scholar]
- 108.van Voorhis WC, Barrett LK, Nasio JM, Plummer FA, Lukehart SA. Lesions of primary and secondary syphilis contain activated cytolytic T cells. Infect Immun. 1996;64:1048–50. doi: 10.1128/iai.64.3.1048-1050.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.McBroom RL, et al. Secondary Syphilis in Persons Infected With and Not Infected With HIV-1. Am J Dermatopathol. 1999;21:432. doi: 10.1097/00000372-199910000-00005. [DOI] [PubMed] [Google Scholar]
- 110.Radolf JD, Lukehart SA. In: Pathogenic Treponemes: Cellular and Molecular Biology. Radolf JD, Lukehart SA, editors. 2006. pp. 285–322. [Google Scholar]
- 111.Lukehart SAL, Shaffer JM, Zander SAB. A Subpopulation of Treponema pallidum Is Resistant to Phagocytosis: Possible Mechanism of Persistence. J Infect Dis. 1992;166:1449–1453. doi: 10.1093/infdis/166.6.1449. [DOI] [PubMed] [Google Scholar]
- 112.Cox DL, et al. Surface Immunolabeling and Consensus Computational Framework To Identify Candidate Rare Outer Membrane Proteins of Treponema pallidum. Infect Immun. 2010;78:5178–5194. doi: 10.1128/IAI.00834-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.NELSON RA, MAYER MM. Immobilization of Treponema pallidum in vitro by antibody produced in syphilitic infection. J Exp Med. 1949;89:369–93. doi: 10.1084/jem.89.4.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bishop NH, Miller JN. Humoral immunity in experimental syphilis. II The relationship of neutralizing factors in immune serum to acquired resistance. J Immunol. 1976;117:197–207. [PubMed] [Google Scholar]
- 115.Giacani L, Lukehart S, Centurion-Lara A. Length of guanosine homopolymeric repeats modulates promoter activity of subfamily IItprgenes ofTreponema pallidumssp.pallidum. FEMS Immunol Med Microbiol. 2007;51:289–301. doi: 10.1111/j.1574-695X.2007.00303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Giacani L, et al. Transcription of TP0126, Treponema pallidum Putative OmpW Homolog, Is Regulated by the Length of a Homopolymeric Guanosine Repeat. Infect Immun. 2015;83:2275–2289. doi: 10.1128/IAI.00360-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Deitsch KW, Lukehart SA, Stringer JR. Common strategies for antigenic variation by bacterial, fungal and protozoan pathogens. Nat Rev Microbiol. 2009;7:493–503. doi: 10.1038/nrmicro2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Harter C, Benirschke K. Fetal syphilis in the first trimester. Am J Obstet Gynecol. 1976;124:705–11. doi: 10.1016/s0002-9378(16)33340-3. [DOI] [PubMed] [Google Scholar]
- 119.Qin J, et al. Reported Estimates of Adverse Pregnancy Outcomes among Women with and without Syphilis: A Systematic Review and Meta-Analysis. PLoS One. 2014;9:e102203. doi: 10.1371/journal.pone.0102203. Together with Ref. 45, these two large systematic reviews estimated the extent of adverse pregnancy outcomes caused by untreated maternal syphilis, and provided our best evidence on adverse pregnancy outcomes in syphilis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Muller I, et al. Is Serological Testing a Reliable Tool in Laboratory Diagnosis of Syphilis? Meta-Analysis of Eight External Quality Control Surveys Performed by the German Infection Serology Proficiency Testing Program. J Clin Microbiol. 2006;44:1335–1341. doi: 10.1128/JCM.44.4.1335-1341.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Parekh BS, et al. Dried tube specimens: A simple and cost-effective method for preparation of HIV proficiency testing panels and quality control materials for use in resource-limited settings. J Virol Methods. 2010;163:295–300. doi: 10.1016/j.jviromet.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 122.Beber AMB, Sabidó M, Vieira JMR, Bazzo ML, Benzaken AS. External quality assessment in the voluntary counseling and testing centers in the Brazilian Amazon using dried tube specimens: results of an effectiveness evaluation. Rev Soc Bras Med Trop. 2015;48:87–97. doi: 10.1590/0037-8682-0106-2014. [DOI] [PubMed] [Google Scholar]
- 123.Benzaken AS, et al. External quality assurance with dried tube specimens (DTS) for point-of-care syphilis and HIV tests: experience in an indigenous populations screening programme in the Brazilian Amazon. Sex Transm Infect. 2013;90:14–18. doi: 10.1136/sextrans-2013-051181. [DOI] [PubMed] [Google Scholar]
- 124.Smit PW, et al. The development and validation of dried blood spots for external quality assurance of syphilis serology. BMC Infect Dis. 2013;13 doi: 10.1186/1471-2334-13-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sparling PF. In: Sexually Transmitted Diseases. 3. Holmes KK, Sparling PF, Mardh PA, et al., editors. 1999. pp. 473–478. [Google Scholar]
- 126.Pan American Health Organization and World Health Organization. Syphilis Testing Practices in the Americas Region: Results of the 2014 Survey [online] 2016 http://www2.paho.org/hq/index.php?option=com_docman&task=doc_view&Itemid=270&gid=37676&lang=en.
- 127.Chi KH, et al. Molecular Differentiation of Treponema pallidum Subspecies in Skin Ulceration Clinically Suspected as Yaws in Vanuatu Using Real-Time Multiplex PCR and Serological Methods. Am J Trop Med Hyg. 2014;92:134–138. doi: 10.4269/ajtmh.14-0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Liu H, Rodes B, Chen CY, Steiner B. New Tests for Syphilis: Rational Design of a PCR Method for Detection of Treponema pallidum in Clinical Specimens Using Unique Regions of the DNA Polymerase I Gene. J Clin Microbiol. 2001;39:1941–1946. doi: 10.1128/JCM.39.5.1941-1946.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gayet-Ageron A, Lautenschlager S, Ninet B, Perneger TV, Combescure C. Sensitivity, specificity and likelihood ratios of PCR in the diagnosis of syphilis: a systematic review and meta-analysis. Sex Transm Infect. 2013;89:251–6. doi: 10.1136/sextrans-2012-050622. [DOI] [PubMed] [Google Scholar]
- 130.Castro R, et al. Detection of Treponema pallidum Sp Pallidum DNA in Cerebrospinal Fluid (CSF) by Two PCR Techniques. J Clin Lab Anal. 2016;30:628–32. doi: 10.1002/jcla.21913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fraga D, et al. Detection of Treponema pallidum by Semi-Nested PCR in the Cerebrospinal Fluid of Asymptomatic HIV-Infected Patients with Latent Syphilis. Clin Lab. 2014;60 doi: 10.7754/clin.lab.2014.140605. [DOI] [PubMed] [Google Scholar]
- 132.Creegan L, et al. An evaluation of the relative sensitivities of the venereal disease research laboratory test and the Treponema pallidum particle agglutination test among patients diagnosed with primary syphilis. Sex Transm Dis. 2007;34:1016–1018. [PubMed] [Google Scholar]
- 133.WENDE RD, MUDD RL, KNOX JM, HOLDER WR. The VDRL Slide Test in 322 Cases of Darkfield Positive Primary Syphilis. South Med J. 1971;64:633. doi: 10.1097/00007611-197105000-00030. [DOI] [PubMed] [Google Scholar]
- 134.Peeling RW, Holmes KK, Mabey D, Ronald A. Rapid tests for sexually transmitted infections (STIs): the way forward. Sex Transm Infect. 2006;82:v1–v6. doi: 10.1136/sti.2006.024265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pastuszczak M, et al. Robust pro-inflammatory immune response is associated with serological cure in patients with syphilis: an observational study. Sex Transm Infect. 2016;93:11–14. doi: 10.1136/sextrans-2016-052681. [DOI] [PubMed] [Google Scholar]
- 136.Larsen SA, Johnson RE. Diagnostic tests in Manual of Tests for Syphilis. (9) 1998 [online], https://www.cdc.gov/std/syphilis/manual-1998/chapt1.pdf.
- 137.Sparling PF. Diagnosis and Treatment of Syphilis. N Engl J Med. 1971;284:642–653. doi: 10.1056/NEJM197103252841205. [DOI] [PubMed] [Google Scholar]
- 138.Fiumara NJ. Posttreatment Serological Response of Biologic False-Positive Reactors. JAMA J Am Med Assoc. 1982;247:817. [PubMed] [Google Scholar]
- 139.Donkers A, Levy HR, Letens-van Vliet A. Syphilis detection using the Siemens ADVIA Centaur Syphilis treponemal assay. Clin Chim Acta. 2014;433:84–87. doi: 10.1016/j.cca.2014.01.041. [DOI] [PubMed] [Google Scholar]
- 140.Gomez E, Jespersen DJ, Harring JA, Binnicker MJ. Evaluation of the Bio-Rad BioPlex 2200 Syphilis Multiplex Flow Immunoassay for the Detection of IgM- and IgG-Class Antitreponemal Antibodies. Clin Vaccine Immunol. 2010;17:966–968. doi: 10.1128/CVI.00086-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sambri V, et al. Western Immunoblotting with Five Treponema pallidum Recombinant Antigens for Serologic Diagnosis of Syphilis. Clin Vaccine Immunol. 2001;8:534–539. doi: 10.1128/CDLI.8.3.534-539.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wong EH, et al. Evaluation of an IgM/IgG Sensitive Enzyme Immunoassay and the Utility of Index Values for the Screening of Syphilis Infection in a High-Risk Population. Sex Transm Dis. 2011;1 doi: 10.1097/olq.0b013e318205491a. [DOI] [PubMed] [Google Scholar]
- 143.Centers for Disease Control and Prevention (CDC) Discordant results from reverse sequence syphilis screening--five laboratories, United States, 2006–2010. MMWR Morb Mortal Wkly Rep. 2011;60:133–7. [PubMed] [Google Scholar]
- 144.Centers for Disease Control and Prevention (CDC) Syphilis testing algorithms using treponemal tests for initial screening--four laboratories, New York City, 2005–2006. MMWR Morb Mortal Wkly Rep. 2008;57:872–5. [PubMed] [Google Scholar]
- 145.Peeling RW, Ye H. Diagnostic tools for preventing and managing maternal and congenital syphilis: an overview. Bull World Health Organ. 2004;82:439–46. [PMC free article] [PubMed] [Google Scholar]
- 146.Benzaken AS, et al. Field performance of a rapid point-of-care diagnostic test for antenatal syphilis screening in the Amazon region, Brazil. Int J STD AIDS. 2011;22:15–18. doi: 10.1258/ijsa.2010.010145. [DOI] [PubMed] [Google Scholar]
- 147.Mabey D, et al. Prospective, multi-centre clinic-based evaluation of four rapid diagnostic tests for syphilis. Sex Transm Infect. 2006;82:v13–v16. doi: 10.1136/sti.2006.022467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mabey DC, et al. Point-of-Care Tests to Strengthen Health Systems and Save Newborn Lives: The Case of Syphilis. PLoS Med. 2012;9:e1001233. doi: 10.1371/journal.pmed.1001233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Siedner M, Zapitz V, Ishida M, De La Roca R, Klausner JD. Performance of Rapid Syphilis Tests in Venous and Fingerstick Whole Blood Specimens. Sex Transm Dis. 2004;31:557–560. doi: 10.1097/01.olq.0000137903.48413.5e. [DOI] [PubMed] [Google Scholar]
- 150.Tinajeros F, et al. Diagnostic accuracy of a point-of-care syphilis test when used among pregnant women in Bolivia. Sex Transm Infect. 2006;82:v17–v21. doi: 10.1136/sti.2006.022640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.West B. Performance of the rapid plasma reagin and the rapid syphilis screening tests in the diagnosis of syphilis in field conditions in rural Africa. Sex Transm Infect. 2002;78:282–285. doi: 10.1136/sti.78.4.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gianino MM, et al. Performance and costs of a rapid syphilis test in an urban population at high risk for sexually transmitted infections. J Prev Med Hyg. 2007;48:118–22. [PubMed] [Google Scholar]
- 153.Hernández-Trejo M, Hernández-Prado B, Uribe-Salas F, Juárez-Figueroa L, Conde-González CJ. Maternal and congenital syphilis in two Mexican hospitals: evaluation of a rapid diagnostic test. Rev Invest Clin. 58:119–25. [PubMed] [Google Scholar]
- 154.Lien TX, et al. Evaluation of rapid diagnostic tests for the detection of human immunodeficiency virus types 1 and 2, hepatitis B surface antigen, and syphilis in Ho Chi Minh City, Vietnam. Am J Trop Med Hyg. 2000;62:301–309. doi: 10.4269/ajtmh.2000.62.301. [DOI] [PubMed] [Google Scholar]
- 155.Galvao TF, et al. Safety of Benzathine Penicillin for Preventing Congenital Syphilis: A Systematic Review. PLoS One. 2013;8:e56463. doi: 10.1371/journal.pone.0056463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Castro AR, et al. Novel Point-of-Care Test for Simultaneous Detection of Nontreponemal and Treponemal Antibodies in Patients with Syphilis. J Clin Microbiol. 2010;48:4615–4619. doi: 10.1128/JCM.00624-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yin YP, et al. A Dual Point-of-Care Test Shows Good Performance in Simultaneously Detecting Nontreponemal and Treponemal Antibodies in Patients With Syphilis: A Multisite Evaluation Study in China. Clin Infect Dis. 2012;56:659–665. doi: 10.1093/cid/cis928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Causer LM, et al. An Evaluation of a Novel Dual Treponemal/Nontreponemal Point-of-Care Test for Syphilis as a Tool to Distinguish Active From Past Treated Infection. Clin Infect Dis. 2015;61:184–191. doi: 10.1093/cid/civ243. [DOI] [PubMed] [Google Scholar]
- 159.Gliddon HD, et al. A systematic review and meta-analysis of studies evaluating the performance and operational characteristics of dual point-of-care tests for HIV and syphilis. Sex Transm Infect. 2017 doi: 10.1136/sextrans-2016-053069. sextrans-2016-053069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ghanem KG, Workowski KA. Management of adult syphilis. Clin Infect Dis. 2011;53(Suppl 3):S110–28. doi: 10.1093/cid/cir701. [DOI] [PubMed] [Google Scholar]
- 161.Kingston M, et al. UK national guidelines on the management of syphilis 2015. Int J STD AIDS. 2016;27:421–46. doi: 10.1177/0956462415624059. [DOI] [PubMed] [Google Scholar]
- 162.Marra CM, et al. The rapid plasma reagin test cannot replace the venereal disease research laboratory test for neurosyphilis diagnosis. Sex Transm Dis. 2012;39:453–7. doi: 10.1097/OLQ.0b013e31824b1cde. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Marra CM, Tantalo LC, Maxwell CL, Dougherty K, Wood B. Alternative cerebrospinal fluid tests to diagnose neurosyphilis in HIV-infected individuals. Neurology. 2004;63:85–8. doi: 10.1212/01.wnl.0000131902.69113.34. [DOI] [PubMed] [Google Scholar]
- 164.Marra CM, Maxwell CL, Dunaway SB, Sahi SK, Tantalo LC. Cerebrospinal Fluid Treponema pallidum Particle Agglutination Assay for Neurosyphilis Diagnosis. J Clin Microbiol. 2017;55:1865–1870. doi: 10.1128/JCM.00310-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Marra CM, et al. Cerebrospinal Fluid Abnormalities in Patients with Syphilis: Association with Clinical and Laboratory Features. J Infect Dis. 2004;189:369–376. doi: 10.1086/381227. [DOI] [PubMed] [Google Scholar]
- 166.Meyers D, et al. USPSTF recommendations for STI screening. Am Fam Physician. 2008;77:819–24. [PubMed] [Google Scholar]
- 167.Owusu-Ofori A, Parry C, Bates I. Transfusion-transmitted Syphilis in Teaching Hospital, Ghana. Emerg Infect Dis. 2011;17 doi: 10.3201/eid1711.110985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.FDA. Requirements for blood and blood components intended for transfusion or for further manufacturing use. Federal Register, Rules and Regulations [online] 2015 https://www.gpo.gov/fdsys/pkg/FR-2015-05-22/pdf/2015-12228.pdf. [PubMed]
- 169.Tapko JB, Toure B, Sambo LG. Status of blood safety in the WHO African region: Report of the 2010 survey [online] 2014 http://www.afro.who.int/index.php?option=com_docman&task=doc_download&gid=9135&Itemid=2593.
- 170.Hossain M, Broutet N, Hawkes S. The Elimination of Congenital Syphilis: A Comparison of the Proposed World Health Organization Action Plan for the Elimination of Congenital Syphilis With Existing National Maternal and Congenital Syphilis Policies. Sex Transm Dis. 2007;34:S22–S30. doi: 10.1097/01.olq.0000261049.84824.40. [DOI] [PubMed] [Google Scholar]
- 171.Hawkes S, Matin N, Broutet N, Low N. Effectiveness of interventions to improve screening for syphilis in pregnancy: a systematic review and meta-analysis. Lancet Infect Dis. 2011;11:684–691. doi: 10.1016/S1473-3099(11)70104-9. The systematic review of 10 studies showed that interventions to improve the coverage and effect of screening programmes for antenatal syphilis could reduce the syphilis-attributable incidence of stillbirth and perinatal death by 50%. [DOI] [PubMed] [Google Scholar]
- 172.Swartzendruber A, Steiner RJ, Adler MR, Kamb ML, Newman LM. Introduction of rapid syphilis testing in antenatal care: A systematic review of the impact on HIV and syphilis testing uptake and coverage. Int J Gynecol Obstet. 2015;130:S15–S21. doi: 10.1016/j.ijgo.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Valderrama J, Zacarías F, Mazin R. Sífilis materna y sífilis congénita en América Latina: un problema grave de solución sencilla. Rev Panam Salud Pública. 2004;16 doi: 10.1590/s1020-49892004000900012. [DOI] [PubMed] [Google Scholar]
- 174.Pan American Health Organization. Update 2014: Elimination of Mother-to-Child Transmission of HIV and Syphilis in the Americas [online] 2014 http://iris.paho.org/xmlui/handle/123456789/31357.
- 175.Cantor AG, Pappas M, Daeges M, Nelson HD. Screening for Syphilis. JAMA. 2016;315:2328. doi: 10.1001/jama.2016.4114. [DOI] [PubMed] [Google Scholar]
- 176.Tuite A, Fisman D. Go big or go home: impact of screening coverage on syphilis infection dynamics. Sex Transm Infect. 2015;92:49–54. doi: 10.1136/sextrans-2014-052001. [DOI] [PubMed] [Google Scholar]
- 177.Reed JL, et al. Adolescent Patient Preferences Surrounding Partner Notification and Treatment for Sexually Transmitted Infections. Acad Emerg Med. 2014;22:61–66. doi: 10.1111/acem.12557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Tucker JD, et al. Organizational characteristics of HIV/syphilis testing services for men who have sex with men in South China: a social entrepreneurship analysis and implications for creating sustainable service models. BMC Infect Dis. 2014;14 doi: 10.1186/s12879-014-0601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Tucker JD, Fenton KA, Peckham R, Peeling RW. Social Entrepreneurship for Sexual Health (SESH): A New Approach for Enabling Delivery of Sexual Health Services among Most-at-Risk Populations. PLoS Med. 2012;9:e1001266. doi: 10.1371/journal.pmed.1001266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Dubourg G, Raoult D. The challenges of preexposure prophylaxis for bacterial sexually transmitted infections. Clin Microbiol Infect. 2016;22:753–756. doi: 10.1016/j.cmi.2016.08.022. [DOI] [PubMed] [Google Scholar]
- 181.Molina JM, et al. On-Demand Preexposure Prophylaxis in Men at High Risk for HIV-1 Infection. N Engl J Med. 2015;373:2237–2246. doi: 10.1056/NEJMoa1506273. [DOI] [PubMed] [Google Scholar]
- 182.NIH. Infectious Disease Testing for Blood Transfusions. JAMA. 1995;274:1374. doi: 10.1001/jama.1995.03530170054032. [DOI] [PubMed] [Google Scholar]
- 183.Gardella C, Marfin AA, Kahn RH, Swint E, Markowitz LE. Persons with Early Syphilis Identified through Blood or Plasma Donation Screening in the United States. J Infect Dis. 2002;185:545–549. doi: 10.1086/338829. [DOI] [PubMed] [Google Scholar]
- 184.Marfin AA, et al. Amplification of the DNA polymerase I gene of Treponema pallidum from whole blood of persons with syphilis. Diagn Microbiol Infect Dis. 2001;40:163–6. doi: 10.1016/s0732-8893(01)00275-9. [DOI] [PubMed] [Google Scholar]
- 185.Chambers RW, Foley HT, Schmidt PJ. Transmission of Syphilis by Fresh Blood Components. Transfusion. 1969;9:32–34. doi: 10.1111/j.1537-2995.1969.tb04909.x. [DOI] [PubMed] [Google Scholar]
- 186.Seña AC, et al. A systematic review of syphilis serological treatment outcomes in HIV-infected and HIV-uninfected persons: rethinking the significance of serological non-responsiveness and the serofast state after therapy. BMC Infect Dis. 2015;15:479. doi: 10.1186/s12879-015-1209-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Sena AC, et al. Predictors of Serological Cure and Serofast State After Treatment in HIV-Negative Persons With Early Syphilis. Clin Infect Dis. 2011;53:1092–1099. doi: 10.1093/cid/cir671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Riedner G, et al. Single-dose azithromycin versus penicillin G benzathine for the treatment of early syphilis. N Engl J Med. 2005;353:1236–44. doi: 10.1056/NEJMoa044284. [DOI] [PubMed] [Google Scholar]
- 189.Hook EW, III, et al. A Phase III Equivalence Trial of Azithromycin versus Benzathine Penicillin for Treatment of Early Syphilis. J Infect Dis. 2010;201:1729–1735. doi: 10.1086/652239. [DOI] [PubMed] [Google Scholar]
- 190.Lukehart SA, et al. Macrolide Resistance inTreponema pallidumin the United States and Ireland. N Engl J Med. 2004;351:154–158. doi: 10.1056/NEJMoa040216. [DOI] [PubMed] [Google Scholar]
- 191.Zhou P, et al. Azithromycin Treatment Failure Among Primary and Secondary Syphilis Patients in Shanghai. Sex Transm Dis. 2010;37:726–729. doi: 10.1097/OLQ.0b013e3181e2c753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Read P, et al. Azithromycin-Resistant Syphilis-Causing Strains in Sydney, Australia: Prevalence and Risk Factors. J Clin Microbiol. 2014;52:2776–2781. doi: 10.1128/JCM.00301-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Stamm LV. Global challenge of antibiotic-resistant Treponema pallidum. Antimicrob Agents Chemother. 2010;54:583–9. doi: 10.1128/AAC.01095-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Grillová L, et al. Molecular typing of Treponema pallidum in the Czech Republic during 2011 to 2013: increased prevalence of identified genotypes and of isolates with macrolide resistance. J Clin Microbiol. 2014;52:3693–700. doi: 10.1128/JCM.01292-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Ghanem KG, et al. Antiretroviral Therapy Is Associated with Reduced Serologic Failure Rates for Syphilis among HIV-Infected Patients. Clin Infect Dis. 2008;47:258–265. doi: 10.1086/589295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ghanem KG, et al. Neurosyphilis in a clinical cohort of HIV-1-infected patients. AIDS. 2008;22:1145–1151. doi: 10.1097/QAD.0b013e32830184df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Tuddenham S, Ghanem KG. Emerging trends and persistent challenges in the management of adult syphilis. BMC Infect Dis. 2015;15 doi: 10.1186/s12879-015-1028-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Zetola NM, Klausner JD. Syphilis and HIV infection: an update. Clin Infect Dis. 2007;44:1222–8. doi: 10.1086/513427. [DOI] [PubMed] [Google Scholar]
- 199.Yang CJ, et al. One Dose versus Three Weekly Doses of Benzathine Penicillin G for Patients Co-Infected with HIV and Early Syphilis: A Multicenter, Prospective Observational Study. PLoS One. 2014;9:e109667. doi: 10.1371/journal.pone.0109667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Ganesan A, et al. A Single Dose of Benzathine Penicillin G Is as Effective as Multiple Doses of Benzathine Penicillin G for the Treatment of HIV-Infected Persons With Early Syphilis. Clin Infect Dis. 2014;60:653–660. doi: 10.1093/cid/ciu888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Watson-Jones D, et al. Syphilis in Pregnancy in Tanzania. II The Effectiveness of Antenatal Syphilis Screening and Single-Dose Benzathine Penicillin Treatment for the Prevention of Adverse Pregnancy Outcomes. J Infect Dis. 2002;186:948–957. doi: 10.1086/342951. [DOI] [PubMed] [Google Scholar]
- 202.Terris-Prestholt F. Is antenatal syphilis screening still cost effective in sub-Saharan Africa. Sex Transm Infect. 2003;79:375–381. doi: 10.1136/sti.79.5.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Singh AE, Romanowski B. Syphilis: review with emphasis on clinical, epidemiologic, and some biologic features. Clin Microbiol Rev. 1999;12:187–209. doi: 10.1128/cmr.12.2.187. An excellent review that covered all aspects of syphilis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Centers for Disease Control and Prevention. 2015 Sexually Transmitted Diseases Treatment Guidelines: Syphilis [online] 2015 https://www.cdc.gov/std/tg2015/syphilis.htm.
- 205.Harman NB. Staying the Plague. 1927. pp. 33–44. [Google Scholar]
- 206.Frith J. Syphilis – its early history and treatment until penicillin and the debate on its origins. J Mil Veterens Heal. 2012;20:49–28. [Google Scholar]
- 207.Sartin JS, Perry HO. From mercury to malaria to penicillin: The history of the treatment of syphilis at the Mayo Clinic—1916–1955. J Am Acad Dermatol. 1995;32:255–261. doi: 10.1016/0190-9622(95)90136-1. [DOI] [PubMed] [Google Scholar]
- 208.Lopes C, Powell ML, Santos AL. Syphilis and cirrhosis: a lethal combination in a XIX century individual identified from the Medical Schools Collection at the University of Coimbra (Portugal) Mem Inst Oswaldo Cruz. 2010;105:1050–1053. doi: 10.1590/s0074-02762010000800016. [DOI] [PubMed] [Google Scholar]
- 209.Bjarne B. Radesyken (‘Wicked Disease’) – a Norwegian tragedy. Tidsskr Nor Laegeforen. 2003;24:2557–8. [PubMed] [Google Scholar]
- 210.Centers for Disease Control and Prevention. Sexually Transmitted Diseases Surveillance 2015 [online] 2015 https://www.cdc.gov/std/stats15/
- 211.Ropper AH. Two Centuries of Neurology and Psychiatry in theJournal. N Engl J Med. 2012;367:58–65. doi: 10.1056/NEJMra1104781. [DOI] [PubMed] [Google Scholar]
- 212.Oliver SE, et al. Ocular Syphilis — Eight Jurisdictions, United States, 2014–2015. MMWR Morb Mortal Wkly Rep. 2016;65:1185–1188. doi: 10.15585/mmwr.mm6543a2. [DOI] [PubMed] [Google Scholar]
- 213.Moradi A, et al. Clinical Features and Incidence Rates of Ocular Complications in Patients With Ocular Syphilis. Am J Ophthalmol. 2015;159:334–343.e1. doi: 10.1016/j.ajo.2014.10.030. [DOI] [PubMed] [Google Scholar]
- 214.Andreyev SV, Setko NP, Voronina LG. Assessment of quality of life of patients with syphilis. Prakt Med Infect Dis. 2014;7:1. [Google Scholar]
- 215.Ferreira A, Young T, Mathews C, Zunza M, Low N. Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Rzepa T, Jakubowicz O, Witmanowski H, Żaba R. Disease-induced level of shame in patients with acne, psoriasis and syphilis. Adv Dermatology Allergol. 2013;4:233–236. doi: 10.5114/pdia.2013.37033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Wu D, Hawkes S. Eliminating mother-to-child transmission of syphilis: the need for more consistent political commitment. Public Heal Emerg. 2016;1:41. [Google Scholar]
- 218.Burden C, et al. From grief, guilt pain and stigma to hope and pride – a systematic review and meta-analysis of mixed-method research of the psychosocial impact of stillbirth. BMC Pregnancy Childbirth. 2016;16 doi: 10.1186/s12884-016-0800-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Heazell AEP, et al. Stillbirths: economic and psychosocial consequences. Lancet. 2016;387:604–616. doi: 10.1016/S0140-6736(15)00836-3. [DOI] [PubMed] [Google Scholar]
- 220.Kiguli J, et al. Stillbirths in sub-Saharan Africa: unspoken grief. Lancet (London, England) 2016;387:e16–8. doi: 10.1016/S0140-6736(15)01171-X. [DOI] [PubMed] [Google Scholar]
- 221.Fitzgerald DW, Behets FMTF. A piece of my mind. Beyond folklore. JAMA. 2002;288:2791–2. doi: 10.1001/jama.288.22.2791. [DOI] [PubMed] [Google Scholar]
- 222.Meheus A, Antal GM. The endemic treponematoses: not yet eradicated. World Health Stat Q. 1992;45:228–37. [PubMed] [Google Scholar]
- 223.Taylor MM, et al. Estimating Benzathine Penicillin Need for the Treatment of Pregnant Women Diagnosed with Syphilis during Antenatal Care in High-Morbidity Countries. PLoS One. 2016;11:e0159483. doi: 10.1371/journal.pone.0159483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Peeling RW, Mabey D. Celebrating the decline in syphilis in pregnancy: a sobering reminder of what’s left to do. Lancet Glob Heal. 2016;4:e503–4. doi: 10.1016/S2214-109X(16)30154-1. [DOI] [PubMed] [Google Scholar]
- 225.World Health Organization. Global Guidance on Criteria and Processes for Validation: Elimination of Mother-to-Child Transmission of HIV and Syphilis [online] 2014 http://apps.who.int/iris/bitstream/10665/112858/1/9789241505888_eng.pdf.
- 226.World Health Organization. WHO information note on the use of dual HIV/Syphilis rapid diagnostic tests (RDT) [online] 2017 http://www.who.int/reproductivehealth/publications/rtis/dual-hiv-syphilis-diagnostic-tests/en/
- 227.Wedderburn CJ, Murtagh M, Toskin I, Peeling RW. Using electronic readers to monitor progress toward elimination of mother-to-child transmission of HIV and syphilis: An opinion piece. Int J Gynecol Obstet. 2015;130:S81–S83. doi: 10.1016/j.ijgo.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 228.Guo T, Patnaik R, Kuhlmann K, Rai AJ, Sia SK. Smartphone dongle for simultaneous measurement of hemoglobin concentration and detection of HIV antibodies. Lab Chip. 2015;15:3514–3520. doi: 10.1039/c5lc00609k. [DOI] [PubMed] [Google Scholar]
- 229.Peeling RW. Diagnostics in a digital age: an opportunity to strengthen health systems and improve health outcomes. Int Health. 2015;7:384–9. doi: 10.1093/inthealth/ihv062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Hook EW. Syphilis. Lancet (London, England) 2017;389:1550–1557. doi: 10.1016/S0140-6736(16)32411-4. [DOI] [PubMed] [Google Scholar]
- 231.Lewnard JA, Berrang-Ford L. Internet-based partner selection and risk for unprotected anal intercourse in sexual encounters among men who have sex with men: a meta-analysis of observational studies. Sex Transm Infect. 2014;90:290–6. doi: 10.1136/sextrans-2013-051332. [DOI] [PubMed] [Google Scholar]
- 232.Tuddenham S, Shah M, Ghanem KG. Syphilis and HIV: Is HAART at the heart of this epidemic? Sex Transm Infect. 2017;93:311–312. doi: 10.1136/sextrans-2016-052940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Ho EL, Tantalo LC, Jones T, Sahi SK, Marra CM. Point-of-care treponemal tests for neurosyphilis diagnosis. Sex Transm Dis. 2015;42:48–52. doi: 10.1097/OLQ.0000000000000222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Lin LR, et al. Macrophage migration inhibitory factor as a novel cerebrospinal fluid marker for neurosyphilis among HIV-negative patients. Clin Chim Acta. 2016;463:103–108. doi: 10.1016/j.cca.2016.10.018. [DOI] [PubMed] [Google Scholar]
- 235.Marra CM, Tantalo LC, Sahi SK, Maxwell CL, Lukehart SA. CXCL13 as a Cerebrospinal Fluid Marker for Neurosyphilis in HIV-Infected Patients With Syphilis. Sex Transm Dis. 2010;1 doi: 10.1097/OLQ.0b013e3181d877a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Cameron CE, Lukehart SA. Current status of syphilis vaccine development: need, challenges, prospects. Vaccine. 2014;32:1602–9. doi: 10.1016/j.vaccine.2013.09.053. This review described the progress in vaccine development for syphilis and all the challenges that need to be overcome. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Miller JN. Immunity in experimental syphilis. VI Successful vaccination of rabbits with Treponema pallidum, Nichols strain, attenuated by -irradiation. J Immunol. 1973;110:1206–15. [PubMed] [Google Scholar]
- 238.Arora N, et al. Origin of modern syphilis and emergence of a pandemic Treponema pallidum cluster. Nat Microbiol. 2016;2:16245. doi: 10.1038/nmicrobiol.2016.245. [DOI] [PubMed] [Google Scholar]
- 239.Izard J, et al. Cryo-Electron Tomography Elucidates the Molecular Architecture of Treponema pallidum, the Syphilis Spirochete. J Bacteriol. 2009;191:7566–7580. doi: 10.1128/JB.01031-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Liu J, et al. Cellular Architecture of Treponema pallidum: Novel Flagellum, Periplasmic Cone, and Cell Envelope as Revealed by Cryo Electron Tomography. J Mol Biol. 2010;403:546–561. doi: 10.1016/j.jmb.2010.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Brautigam CA, Deka RK, Liu WZ, Norgard MV. Insights into the potential function and membrane organization of the TP0435 (Tp17) lipoprotein fromTreponema pallidumderived from structural and biophysical analyses. Protein Sci. 2014;24:11–19. doi: 10.1002/pro.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Erkens GB, Majsnerowska M, ter Beek J, Slotboom DJ. Energy Coupling Factor-Type ABC Transporters for Vitamin Uptake in Prokaryotes. Biochemistry. 2012;51:4390–4396. doi: 10.1021/bi300504v. [DOI] [PubMed] [Google Scholar]
- 243.Boudker O, Ryan RM, Yernool D, Shimamoto K, Gouaux E. Coupling substrate and ion binding to extracellular gate of a sodium-dependent aspartate transporter. Nature. 2007;445:387–393. doi: 10.1038/nature05455. [DOI] [PubMed] [Google Scholar]
- 244.Brautigam CA, Deka RK, Schuck P, Tomchick DR, Norgard MV. Structural and Thermodynamic Characterization of the Interaction between Two Periplasmic Treponema pallidum Lipoproteins that are Components of a TPR-Protein-Associated TRAP Transporter (TPAT) J Mol Biol. 2012;420:70–86. doi: 10.1016/j.jmb.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Deka RK, et al. Structural, Bioinformatic, and In Vivo Analyses of Two Treponema pallidum Lipoproteins Reveal a Unique TRAP Transporter. J Mol Biol. 2012;416:678–696. doi: 10.1016/j.jmb.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Park IU, et al. Screening for syphilis with the treponemal immunoassay: analysis of discordant serology results and implications for clinical management. J Infect Dis. 2011;204:1297–304. doi: 10.1093/infdis/jir524. [DOI] [PubMed] [Google Scholar]
- 247.Binnicker MJ, Yao JD, Cockerill FR. Non-Treponemal Serologic Tests: A Supplemental, Not Confirmatory Testing Approach. Clin Infect Dis. 2010;52:274–275. doi: 10.1093/cid/ciq127. [DOI] [PubMed] [Google Scholar]
- 248.Lipinsky D, Schreiber L, Kopel V, Shainberg B. Validation of Reverse Sequence Screening for Syphilis. J Clin Microbiol. 2012;50:1501. doi: 10.1128/JCM.06286-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Tong ML, et al. Analysis of 3 algorithms for syphilis serodiagnosis and implications for clinical management. Clin Infect Dis. 2014;58:1116–24. doi: 10.1093/cid/ciu087. [DOI] [PubMed] [Google Scholar]
- 250.Tucker JD, et al. Accelerating worldwide syphilis screening through rapid testing: a systematic review. Lancet Infect Dis. 2010;10:381–386. doi: 10.1016/S1473-3099(10)70092-X. [DOI] [PubMed] [Google Scholar]
- 251.Jafari Y, et al. Are Treponema pallidum Specific Rapid and Point-of-Care Tests for Syphilis Accurate Enough for Screening in Resource Limited Settings? Evidence from a Meta-Analysis. PLoS One. 2013;8:e54695. doi: 10.1371/journal.pone.0054695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.WHO. Laboratory diagnosis of sexually transmitted infections, including human immunodeficiency virus [online] 2013 http://who.int/reproductivehealth/publications/rtis/9789241505840/en/