Abstract
Evolution of complex behaviors in higher vertebrates and primates require the development of sophisticated neuronal circuitry and the expansion of brain surface area to accommodate the vast number of neuronal and glial populations. To achieve these goals, the neocortex in primates and the cerebellum in amniotes have developed specialized types of basal progenitors to aid the folding of their cortices. In the cerebellum, Bergmann glia constitute such a basal progenitor population, having a distinctive morphology and playing critical role in cerebellar corticogenesis. Here we review recent studies on the induction of Bergmann glia and their crucial role in mediating folding of the cerebellar cortex. These studies uncover a key function of FGF-ERK-ETV signaling cascade in the transformation of Bergmann glia from radial glia in the ventricular zone. Remarkably, in the neocortex, the same signaling axis operates to facilitate the transformation of ventricular radial glia into basal radial glia, a Bergmann glia-like basal progenitor population, which have been implicated in the establishment of neocortical gyri. These new findings draw a striking similarity in the function and ontogeny of the two basal progenitor populations born in distinct brain compartments.
Introduction
Bergmann glia (BG), also called Golgi epithelial cells, are specialized, unipolar glial cells featuring cell bodies situated in the Purkinje cell layer and radial fibers passing through the molecular layer [1–3]. BG precursors are derived from radial glia that reside in the cerebellar ventricular zone. During their derivation process, BG precursors maintain basal processes and retract their apical processes, then relocate their cell bodies toward the cortex [4, 5] (Fig 1A). Each BG extends two to six fibers, arranging in palisade pattern, to the subpial basement membrane [6](Fig 1B). The BG radial fibers aid the migration of neurons and the elongation of dendrites and axons [3, 7]. In the mature cerebellum, BG actively participate in the information processing of the cerebellum. They also maintain structural integrity and synaptic connections in the cerebellum [1, 5, 8]. After induction at E13.5 in mouse embryos [4], BG precursors continue to proliferate at least until the second postnatal weeks [9–11]. In the adult cerebellum, BG express numerous stem cell markers such as Sox1 and Sox2, and they may constitute the adult neural stem cells [12–14]. For excellent reviews of BG development and their role in the mature cerebellum, readers can refer to these references [3, 5]. Here, we focus on discussing the novel understanding of BG genesis and their function in the foliation of the cerebellar cortex. Our discussion also cast these new findings in the context of the evolution of the neocortical basal progenitors and the neocortical gyrification process.
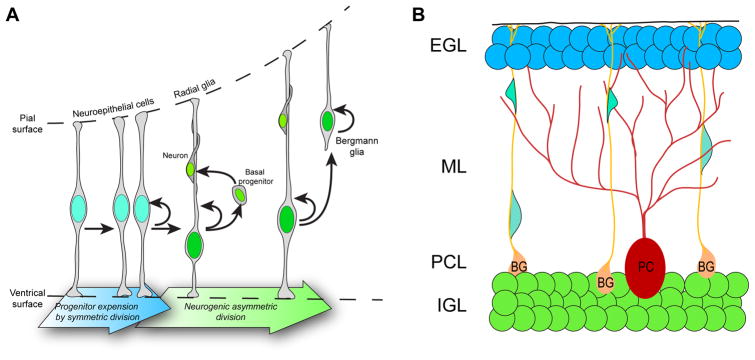
Figure 1.
(A) Progression of neurogenesis and the birth of BG precursors in the cerebellum during embryonic development. The arrows represent, either, a transformation, differentiation or cell division event. Neurogenesis in the cerebellum is a multi-step process. Neuroepithelial progenitors have direct contacts with the ventricular and pial surfaces and undergo symmetric cell division to expand the number of progenitors. As development progresses, neuroepithelial progenitors transform into radial glia cells, which still retain their pial and ventricular contacts and starts to generate basal progenitors that will directly give rise to neurons. Some of the radial glia also start to lose its ventricular processes and give rive to another type of basal progenitor, BG precursors, which retain their apical processes and serve specialized functions in the cerebellum. (B) Configuration of different neuronal and glial populations in the perinatal cerebellum. Granule neuron progenitors from the external granular layer (EGL, in grey shades) migrate along BG basal fibers towards the internal granular layer (IGL), their mature location in the cerebellum. BG – Bergmann glia; ML- molecular layer; PC – Purkinje cells; PCL – Purkinje cell layer.
The mammalian cerebellum
The cerebellum is well known for its sensorimotor processing function. Emerging evidence indicates that the cerebellum is also involved in higher cognition. Accordingly, cerebellar pathology and dysfunction are linked to many debilitating neurodevelopmental diseases, including autism spectrum disorder [15–19]. In this regard, there is a resurging interest in studying the development and the novel cognitive role of the cerebellum.
The adult cerebellar cortex is a trilaminar structure. Purkinje neurons and BG somata comprise the middle layer, sandwiched between an internal granule layer and an outer molecular layer. The internal granule layer consists of mature granule neurons, while the molecular layer contains interneurons, granule cell axons, Purkinje dendrites, and BG radial fibers [20, 21]. Cerebellar cell types arise from two principal germinal regions of the embryonic cerebellum. The anterior rhombic lip, located at the dorsal region of the hindbrain, gives rise to glutamatergic neurons, including cerebellar nuclear neurons and granule neurons. The ventricular zone produces GABAergic Purkinje neurons, GABAergic interneurons and various glial cell types [22]. Because of its relatively simple logic in cytogenesis, the cerebellum has been serving as an excellent experimental paradigm to study neurogenesis and gliogenesis.
Similar to the gyri in the neocortex, the amniote cerebella undergo stereotypic folding of their cortex resulting in the establishment of an elaborate set of folia. The formation of these extensive folds in the cerebellar cortex correlates with the evolution of increasingly complex behaviors in animals [23–25]. From sharks to primates, the cerebellum and neocortex grow regularly and disproportionately to the rest of the brain, with the extent of gyrification reflecting the size of these structures [26]. These observations suggest that convolution of the cerebral and cerebellar cortices represent an evolutionary adaptation to accommodate more complex functions and behaviors.
Ptpn11 is essential for Bergmann glia induction
Perturbations of signaling pathways, including Notch [27–31], Erbb [32–34], thyroid hormone [35], integrin [36–38], Pten [39], sonic hedgehog [40, 41], Wnt/β-catenin [42, 43], and FGF [44–46], result in abnormal number and/or morphology of BG. The mechanism that control the induction of BG precursors, or the transformation process from radial glia to BG precursors, was still unclear until more recent studies revealing an essential function of Ptpn11.
Ptpn11 (protein tyrosine phosphatase non-receptor type 11, also known as Shp2) belongs to a family of protein tyrosine phosphatases that modulate diverse signaling. Mutations in the human PTPN11 gene result in various developmental syndromes and cancers [47, 48]. In the neocortex, Ptpn11 deletion altered the extracellular signal-regulated protein kinase (ERK) and Stat3 signaling pathways, leading to an imbalanced genesis of neurons and glia [49, 50]. Deletion of Ptpn11 at embryonic stage (E)10.5 using a Nestin-Cre (Nestin;Ptpn11CKO) resulted in a disorganization of BG fibers and an abnormal lamination of the cerebellar cortex [51]. Based on in vitro data, the authors concluded that the cerebellar phenotype was attributed to a cell-autonomous requirement of Ptpn11 in granule cell precursors (GCP). However, a subsequent study showed that specific removal of Ptpn11 from GCP does not alter layering of the cerebellar cortex [52]. By contrast, deletion of Ptpn11 from the cerebellar progenitors using En1-Cre (En1;Ptpn11CKO) from an earlier embryonic stage (E8.5) resulted in similar, but more severe, defects in the cerebellar cortex than those found in Nestincre;Ptpn11CKO mice [51, 52]. Cell labeling, marker gene analysis, and genome-wide transcriptome profiling demonstrated that Ptpn11 deletion blocked the induction of BG precursors, whereas the generation of Purkinje neurons, interneurons, and granule neurons were less affected [52, 53]. Interestingly, the astrocytes in the granular layer, but not those in the white matter, were missing in En1;Ptpn11CKO cerebella [52]. This suggests that the granule layer astrocytes and BG may be derived from a different lineage from the white matter astrocytes. The En1;Ptpn11CKO mice represent the first characterized mouse mutation that completely blocks the induction of BG precursors.
The Ptpn11-controlled FGF-ERK-ETV axis is important for BG formation
It was found that Ptpn11 deletion affected ERK, but not AKT, signaling pathway [52]. The authors showed that robust phosphorylated ERK immunoreactivity was detected in the ventricular zone as well as the radial fibers of BG precursors in the wildtype mouse cerebellum [52]. Expression of a constitutively active MEK1 (MEK1DD), which phosphorylates ERK independently of extracellular signals, rescued BG formation in the En1;Ptpn11CKO mice [52, 53]. These observations demonstrate the importance of Ptpn11 in the induction of BG precursors through ERK signaling.
During cerebellar development, multiple FGF ligands are expressed at defined developmental stages and in distinct cerebellar regions [54]. Transcripts of Fgfr1 and Fgfr2, which code for FGF receptors, are first present in the ventricular zone, and later in the Purkinje cell layer where the cell bodies of BG reside [45, 54]. Single, double, and triple deletions of Fgfr1, Fgfr2, and Fgfr3 resulted in progressively more severe defect in the generation of BG precursors [44–46]. In fact, deletion of Fgfr1, Fgfr2, and Fgfr3 from the cerebellum results in a nearly complete loss of BG similar to En1;Ptpn11CKO [44, 53]. These findings suggest that Ptpn11 mediates the FGF-ERK signaling in the induction of BG precursors.
Transcription factors Etv4 and Etv5, which are known targets and mediators of FGF [55, 56], are highly expressed from early embryonic stages through perinatal stages in the cerebellum [52, 54]. Initially, Etv5 is expressed in the whole cerebellar anlage and later gradually restricted to BG precursors; presenting strong evidence of a key functional role of FGF signaling during the induction phase and the subsequent development of BG precursors. Deletion of Ptpn11 resulted in the loss of Etv4 and Etv5 expression, whereas ectopic expression of Mek1DD restored their transcription [53]. Finally, forced expression of Etv4 or Etv5 rescued the formation of BG in the En1;Ptpn11CKO cerebella [53]. Altogether, these observations demonstrate that the FGF-ERK-ETV axis is important for the induction of BG precursors.
Bergmann glia are essential for the folding of the cerebellar surfaces
An elegant study described the formation of the so-called anchoring centers in the cerebellar cortex that will become the base of each fissure [57]. Although the authors determined that granule cell precursors were the primary drivers of the location and timing of fissure formation, coordinated changes in the Purkinje cell layer and BG fibers were observed at the onset of the forming anchoring centers [57]. Numerous studies suggest that the interaction between BG and the basement membrane is important for cerebellar foliation [31, 36, 38, 39, 58–63]. Examining postnatal En1cre;Ptpn11CKO mice uncovers that their cerebella failed to form any visible folia and displayed a smooth surface morphology [52]. Interestingly, the inward converging movement of granule cell precursors persists in the absence of BG in the En1;Ptpn11CKO cerebellum, leading to the accumulation of granule cell precursors immediately beneath the external granular layer [52]. This demonstrates that granule cell precursors invagination alone is insufficient to cause folding of the Purkinje cell layer and the pial basement membrane. Importantly, rescuing BG formation by reactivating the MEK/ERK pathway restores both the formation and organization of cerebellar folia [52]. These findings demonstrate that BG are essential for cerebellar foliation, likely by coordinating the invagination of granule cell precursors with that of Purkinje cell layer and the pial membrane (Fig 2).
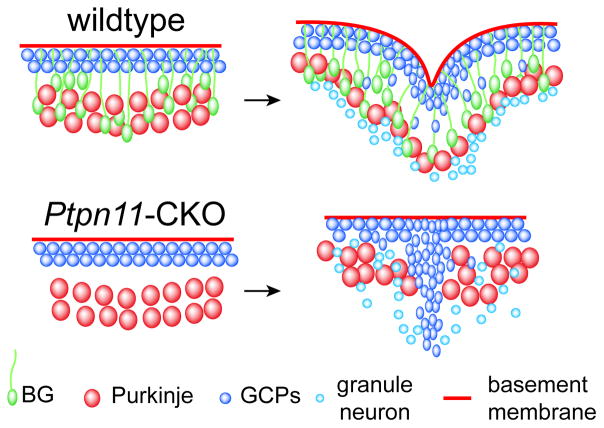
Figure 2.
Arrangement of Purkinje neurons and granule neurons before and during fissure formation in wildtype and En1;Ptpn11CKO mouse cerebella. In wildtype, the presence of BG anchors on the basement membrane helps pull in the EGL and couple the inward movement of granule neurons with a corresponding rearrangement of the soma of Purkinje neurons. In En1;Ptpn11CKO, failed induction of BG precursors and hence lack of BG anchors lead to uncoupling of the invasion of granule neurons with the inward displacement of the basement membrane and Purkinje neurons.
Neocortical basal radial glia and cerebellar Bergmann glial precursors bear similar gene signatures
In the neocortex, radial glia can generate neurons either directly by asymmetric divisions or via an intermediate progenitor cell lineage normally occupying the subventricular zone [64]. More recent studies have discovered additional basal progenitors residing in the subventricular zone aside from the already known intermediate progenitor cells [65–67]. Bearing similarities to the genesis of BG precursors, this novel basal progenitor population, called basal radial glia (bRG) or outer radial glia, selectively loses their apical processes and move their soma to the outer subventricular zone at mid-neurogenesis [65–68]. Remarkably, bRG are abundantly present in the gyrencephalic cortices [65–67], but are relatively rare in lissencephalic cortices, such as the mouse [43, 69]. It has thus been speculated that bRG expansion is responsible for the emergence of convolutions in the neocortex [67, 70–77].
Given their high similarity in cytogenesis and their potential roles in cortical folding, the transcriptomic profiles of human bRG and mouse BG precursors were studied [53]. By exploring the published single-cell RNA-sequencing datasets [78, 79], Heng et al. found that over 50% of the bRG markers were coexpressed in BG precursors. The authors also identified a panel of BG candidate genes by extensive RNA-sequencing analysis and gene co-expression network analysis [53]. Multiple statistical model analyses demonstrated that this BG candidate gene list bore significant similarity with that compiled from the bRG [53]. These data further demonstrate that BG and bRG not only share functional similarity in cortical folding and stem cell property, but also a highly similar transcriptomic signature.
The FGF-ERK-ETV signaling axis is involved in basal radial glia formation
From the consensus gene list compiled for bRG and ventricular zone radial glia in the human cortex, a number of early response genes for ERK signaling are identified [53]. A systematic comparison of multiple available datasets of human and mouse neocortices reveals that classical FGF targets (Spry and Etv genes) as well as ERK response genes are expressed at significantly higher levels in the human than the mouse neocortex, especially in cortical radial glia [53, 80]. Immunostaining confirmed that pERK signaling was low in the ventricular zone of the mouse cortex but readily detectable in the human embryonic neocortical tissue sections [53]. Ectopic expression of FgfR1K656E (a constitutively active FGFR1), MEK1DD, or Etv4 induce bRG-like cells in the mouse cortex expressing markers such as Hopx, Sox2, Pax6, Tnc, Slc1a3 and Ptprz1 [53, 79, 80]. The induced cells are capable of self-renewal and neuronal differentiation under both in vivo and in vitro conditions [53]. These data support a model that posits a common mechanism regulating the formation of BG precursors and bRG. Such a mechanism could have co-evolved under common selection forces in different brain compartments during the speciation of amniote and primate species.
Perspective
The above findings have demonstrated that BG play an important role in cerebellar corticogenesis. Several questions, however, remain to be addressed.
First, how does the FGF-ERK-ETV signaling axis control the transformation process from radial glia into BG and bRG? It has been shown that the ERK pathway determines the mitotic spindle orientation of epithelial cells [81]. A number of studies show that increasing the proportion of horizontal divisions, in which the cleavage furrow is parallel to the ventricular surface, contributes to the generation of bRG in both the human and the mouse cortices [68, 69, 82]. Our preliminary data suggested that the loss of Ptpn11 altered mitotic spinal orientation in the cerebellar radial glia at E14.5 (unpublished data by Leung and Li). Further studies are warranted to determine if ERK signaling controls the generation of BG precursors and bRG by regulating the spindle orientation.
Secondly, how do BG orchestrate the folding of the cerebellar cortex? Heng et al. shows that the expression of Mek1DD specifically expands bRG but fails to induce folding of the mouse neocortex [53]. This finding is in agreement with the notion that an abundance of bRG is insufficient for gyrencephaly [83, 84]. Therefore, expansion of other basal progenitors, together with that for bRG, may be necessary for the successful folding of the neocortex. A notable parallel can be found in the cerebellum where both the granule cell precursors and BG play critical roles in cerebellar foliation. Understanding how BG orchestrate cerebellar corticogenesis will provide new insight into the evolution of a convoluted neocortex.
Finally, how is BG proliferation regulated? Like bRG in the human cortex, BG precursors express genes related to extracellular matrix production and receptors for growth factor signaling that are important for stem cell maintenance [53]. It is important to determine if and how BG create a self-sustaining niche that supports their proliferation, particularly in coordination with the enlargement of the granule cell pool during cerebellar foliation. This research will help us determine how BG and bRG drive brain fold formation and how brain fold formation relates to the development of complex sensorimotor and cognitive function found in mammalian species. Future research is warranted towards determining whether the appearance of BG is associated with the folding of the cerebellum in other mammalian species, and whether the abnormal formation of bRG due to malfunctions of the FGF-ERK-ETV genetic cascade contributes to human congenital conditions that affect the folding and the function of the neocortex.
Acknowledgments
We thank Dr. John Wizeman for critical proofreading of our manuscript. This work was supported by a grant from the National Institutes of Health (R01MH094914) to J. Li.
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
References
- 1.Cui W, Allen ND, Skynner M, Gusterson B, Clark AJ. Inducible ablation of astrocytes shows that these cells are required for neuronal survival in the adult brain. Glia. 2001;34:272–82. doi: 10.1002/glia.1061. [DOI] [PubMed] [Google Scholar]
- 2.Reeber SL, Arancillo M, Sillitoe RV. Bergmann Glia are Patterned into Topographic Molecular Zones in the Developing and Adult Mouse Cerebellum. Cerebellum. 2014 doi: 10.1007/s12311-014-0571-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buffo A, Rossi F. Origin, lineage and function of cerebellar glia. Prog Neurobiol. 2013;109:42–63. doi: 10.1016/j.pneurobio.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Yuasa S. Bergmann glial development in the mouse cerebellum as revealed by tenascin expression. Anat Embryol (Berl) 1996;194:223–34. doi: 10.1007/BF00187133. [DOI] [PubMed] [Google Scholar]
- 5.Yamada K, Watanabe M. Cytodifferentiation of Bergmann glia and its relationship with Purkinje cells. Anat Sci Int. 2002;77:94–108. doi: 10.1046/j.0022-7722.2002.00021.x. [DOI] [PubMed] [Google Scholar]
- 6.de Blas AL. Monoclonal antibodies to specific astroglial and neuronal antigens reveal the cytoarchitecture of the Bergmann glia fibers in the cerebellum. J Neurosci. 1984;4:265–73. doi: 10.1523/JNEUROSCI.04-01-00265.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bellamy TC. Interactions between Purkinje neurones and Bergmann glia. Cerebellum. 2006;5:116–26. doi: 10.1080/14734220600724569. [DOI] [PubMed] [Google Scholar]
- 8.Iino M, Goto K, Kakegawa W, Okado H, Sudo M, Ishiuchi S, Miwa A, Takayasu Y, Saito I, Tsuzuki K, et al. Glia-synapse interaction through Ca2+-permeable AMPA receptors in Bergmann glia. Science. 2001;292:926–9. doi: 10.1126/science.1058827. [DOI] [PubMed] [Google Scholar]
- 9.Parmigiani E, Leto K, Rolando C, Figueres-Onate M, Lopez-Mascaraque L, Buffo A, Rossi F. Heterogeneity and Bipotency of Astroglial-Like Cerebellar Progenitors along the Interneuron and Glial Lineages. J Neurosci. 2015;35:7388–402. doi: 10.1523/JNEUROSCI.5255-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shiga T, Ichikawa M, Hirata Y. Spatial and temporal pattern of postnatal proliferation of Bergmann glial cells in rat cerebellum: an autoradiographic study. Anat Embryol (Berl) 1983;167:203–11. doi: 10.1007/BF00298511. [DOI] [PubMed] [Google Scholar]
- 11.Das GD, Lammert GL, McAllister JP. Contact guidance and migratory cells in the developing cerebellum. Brain Res. 1974;69:13–29. doi: 10.1016/0006-8993(74)90366-7. [DOI] [PubMed] [Google Scholar]
- 12.Alcock J, Lowe J, England T, Bath P, Sottile V. Expression of Sox1, Sox2 and Sox9 is maintained in adult human cerebellar cortex. Neurosci Lett. 2009;450:114–6. doi: 10.1016/j.neulet.2008.11.047. [DOI] [PubMed] [Google Scholar]
- 13.Koirala S, Corfas G. Identification of novel glial genes by single-cell transcriptional profiling of Bergmann glial cells from mouse cerebellum. PLoS One. 2010;5:e9198. doi: 10.1371/journal.pone.0009198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sottile V, Li M, Scotting PJ. Stem cell marker expression in the Bergmann glia population of the adult mouse brain. Brain Res. 2006;1099:8–17. doi: 10.1016/j.brainres.2006.04.127. [DOI] [PubMed] [Google Scholar]
- 15.Hampson DR, Blatt GJ. Autism spectrum disorders and neuropathology of the cerebellum. Front Neurosci. 2015;9:420. doi: 10.3389/fnins.2015.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D’Mello AM, Stoodley CJ. Cerebro-cerebellar circuits in autism spectrum disorder. Front Neurosci. 2015;9:408. doi: 10.3389/fnins.2015.00408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mosconi MW, Wang Z, Schmitt LM, Tsai P, Sweeney JA. The role of cerebellar circuitry alterations in the pathophysiology of autism spectrum disorders. Front Neurosci. 2015;9:296. doi: 10.3389/fnins.2015.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reeber SL, Otis TS, Sillitoe RV. New roles for the cerebellum in health and disease. Front Syst Neurosci. 2013;7:83. doi: 10.3389/fnsys.2013.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marien P, van Dun K, Verhoeven J. Cerebellum and apraxia. Cerebellum. 2015;14:39–42. doi: 10.1007/s12311-014-0620-1. [DOI] [PubMed] [Google Scholar]
- 20.Hatten ME. Central nervous system neuronal migration. Annu Rev Neurosci. 1999;22:511–39. doi: 10.1146/annurev.neuro.22.1.511. [DOI] [PubMed] [Google Scholar]
- 21.Wang VY, Zoghbi HY. Genetic regulation of cerebellar development. Nat Rev Neurosci. 2001;2:484–91. doi: 10.1038/35081558. [DOI] [PubMed] [Google Scholar]
- 22.Leto K, Arancillo M, Becker EB, Buffo A, Chiang C, Ding B, Dobyns WB, Dusart I, Haldipur P, Hatten ME, et al. Consensus Paper: Cerebellar Development. Cerebellum. 2016;15:789–828. doi: 10.1007/s12311-015-0724-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hall ZJ, Street SE, Healy SD. The evolution of cerebellum structure correlates with nest complexity. Biol Lett. 2013;9:20130687. doi: 10.1098/rsbl.2013.0687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iwaniuk AN, Hurd PL, Wylie DR. Comparative morphology of the avian cerebellum: I. Degree of foliation. Brain Behav Evol. 2006;68:45–62. doi: 10.1159/000093530. [DOI] [PubMed] [Google Scholar]
- 25.Lisney TJ, Yopak KE, Montgomery JC, Collin SP. Variation in brain organization and cerebellar foliation in chondrichthyans: batoids. Brain Behav Evol. 2008;72:262–82. doi: 10.1159/000171489. [DOI] [PubMed] [Google Scholar]
- 26.Yopak KE, Lisney TJ, Darlington RB, Collin SP, Montgomery JC, Finlay BL. A conserved pattern of brain scaling from sharks to primates. Proc Natl Acad Sci U S A. 2010;107:12946–51. doi: 10.1073/pnas.1002195107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eiraku M, Tohgo A, Ono K, Kaneko M, Fujishima K, Hirano T, Kengaku M. DNER acts as a neuron-specific Notch ligand during Bergmann glial development. Nat Neurosci. 2005;8:873–80. doi: 10.1038/nn1492. [DOI] [PubMed] [Google Scholar]
- 28.Hiraoka Y, Komine O, Nagaoka M, Bai N, Hozumi K, Tanaka K. Delta-like 1 regulates Bergmann glial monolayer formation during cerebellar development. Mol Brain. 2013;6:25. doi: 10.1186/1756-6606-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Komine O, Nagaoka M, Watase K, Gutmann DH, Tanigaki K, Honjo T, Radtke F, Saito T, Chiba S, Tanaka K. The monolayer formation of Bergmann glial cells is regulated by Notch/RBP-J signaling. Dev Biol. 2007;311:238–50. doi: 10.1016/j.ydbio.2007.08.042. [DOI] [PubMed] [Google Scholar]
- 30.Kuang Y, Liu Q, Shu X, Zhang C, Huang N, Li J, Jiang M, Li H. Dicer1 and MiR-9 are required for proper Notch1 signaling and the Bergmann glial phenotype in the developing mouse cerebellum. Glia. 2012;60:1734–46. doi: 10.1002/glia.22392. [DOI] [PubMed] [Google Scholar]
- 31.Weller M, Krautler N, Mantei N, Suter U, Taylor V. Jagged1 ablation results in cerebellar granule cell migration defects and depletion of Bergmann glia. Dev Neurosci. 2006;28:70–80. doi: 10.1159/000090754. [DOI] [PubMed] [Google Scholar]
- 32.Patten BA, Peyrin JM, Weinmaster G, Corfas G. Sequential signaling through Notch1 and erbB receptors mediates radial glia differentiation. J Neurosci. 2003;23:6132–40. doi: 10.1523/JNEUROSCI.23-14-06132.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sathyamurthy A, Yin DM, Barik A, Shen C, Bean JC, Figueiredo D, She JX, Xiong WC, Mei L. ERBB3-mediated regulation of Bergmann glia proliferation in cerebellar lamination. Development. 2015;142:522–32. doi: 10.1242/dev.115931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rio C, Rieff HI, Qi P, Khurana TS, Corfas G. Neuregulin and erbB receptors play a critical role in neuronal migration. Neuron. 1997;19:39–50. doi: 10.1016/s0896-6273(00)80346-3. [DOI] [PubMed] [Google Scholar]
- 35.Fauquier T, Chatonnet F, Picou F, Richard S, Fossat N, Aguilera N, Lamonerie T, Flamant F. Purkinje cells and Bergmann glia are primary targets of the TRalpha1 thyroid hormone receptor during mouse cerebellum postnatal development. Development. 2014;141:166–75. doi: 10.1242/dev.103226. [DOI] [PubMed] [Google Scholar]
- 36.Belvindrah R, Nalbant P, Ding S, Wu C, Bokoch GM, Muller U. Integrin-linked kinase regulates Bergmann glial differentiation during cerebellar development. Mol Cell Neurosci. 2006;33:109–25. doi: 10.1016/j.mcn.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 37.Frick A, Grammel D, Schmidt F, Poschl J, Priller M, Pagella P, von Bueren AO, Peraud A, Tonn JC, Herms J, et al. Proper cerebellar development requires expression of beta1-integrin in Bergmann glia, but not in granule neurons. Glia. 2012;60:820–32. doi: 10.1002/glia.22314. [DOI] [PubMed] [Google Scholar]
- 38.Qiu Z, Cang Y, Goff SP. Abl family tyrosine kinases are essential for basement membrane integrity and cortical lamination in the cerebellum. J Neurosci. 2010;30:14430–9. doi: 10.1523/JNEUROSCI.2861-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yue Q, Groszer M, Gil JS, Berk AJ, Messing A, Wu H, Liu X. PTEN deletion in Bergmann glia leads to premature differentiation and affects laminar organization. Development. 2005;132:3281–91. doi: 10.1242/dev.01891. [DOI] [PubMed] [Google Scholar]
- 40.Dahmane N, Ruiz i Altaba A. Sonic hedgehog regulates the growth and patterning of the cerebellum. Development. 1999;126:3089–100. doi: 10.1242/dev.126.14.3089. [DOI] [PubMed] [Google Scholar]
- 41.Mecklenburg N, Martinez-Lopez JE, Moreno-Bravo JA, Perez-Balaguer A, Puelles E, Martinez S. Growth and differentiation factor 10 (Gdf10) is involved in Bergmann glial cell development under Shh regulation. Glia. 2014;62:1713–23. doi: 10.1002/glia.22710. [DOI] [PubMed] [Google Scholar]
- 42.Wen J, Yang HB, Zhou B, Lou HF, Duan S. beta-Catenin is critical for cerebellar foliation and lamination. PLoS One. 2013;8:e64451. doi: 10.1371/journal.pone.0064451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang X, Tsai JW, LaMonica B, Kriegstein AR. A new subtype of progenitor cell in the mouse embryonic neocortex. Nat Neurosci. 2011;14:555–61. doi: 10.1038/nn.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin Y, Chen L, Lin C, Luo Y, Tsai RY, Wang F. Neuron-derived FGF9 is essential for scaffold formation of Bergmann radial fibers and migration of granule neurons in the cerebellum. Dev Biol. 2009;329:44–54. doi: 10.1016/j.ydbio.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meier F, Giesert F, Delic S, Faus-Kessler T, Matheus F, Simeone A, Holter SM, Kuhn R, Weisenhorn DM, Wurst W, et al. FGF/FGFR2 signaling regulates the generation and correct positioning of Bergmann glia cells in the developing mouse cerebellum. PLoS One. 2014;9:e101124. doi: 10.1371/journal.pone.0101124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith KM, Maragnoli ME, Phull PM, Tran KM, Choubey L, Vaccarino FM. Fgfr1 inactivation in the mouse telencephalon results in impaired maturation of interneurons expressing parvalbumin. PLoS One. 2014;9:e103696. doi: 10.1371/journal.pone.0103696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grossmann KS, Rosario M, Birchmeier C, Birchmeier W. The tyrosine phosphatase Shp2 in development and cancer. Adv Cancer Res. 2010;106:53–89. doi: 10.1016/S0065-230X(10)06002-1. [DOI] [PubMed] [Google Scholar]
- 48.Feng GS. Shp2-mediated molecular signaling in control of embryonic stem cell self-renewal and differentiation. Cell Res. 2007;17:37–41. doi: 10.1038/sj.cr.7310140. [DOI] [PubMed] [Google Scholar]
- 49.Gauthier AS, Furstoss O, Araki T, Chan R, Neel BG, Kaplan DR, Miller FD. Control of CNS cell-fate decisions by SHP-2 and its dysregulation in Noonan syndrome. Neuron. 2007;54:245–62. doi: 10.1016/j.neuron.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ke Y, Zhang EE, Hagihara K, Wu D, Pang Y, Klein R, Curran T, Ranscht B, Feng GS. Deletion of Shp2 in the brain leads to defective proliferation and differentiation in neural stem cells and early postnatal lethality. Mol Cell Biol. 2007;27:6706–17. doi: 10.1128/MCB.01225-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hagihara K, Zhang EE, Ke YH, Liu G, Liu JJ, Rao Y, Feng GS. Shp2 acts downstream of SDF-1alpha/CXCR4 in guiding granule cell migration during cerebellar development. Dev Biol. 2009;334:276–84. doi: 10.1016/j.ydbio.2009.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li K, Leung AW, Guo Q, Yang W, Li JY. Shp2-dependent ERK signaling is essential for induction of Bergmann glia and foliation of the cerebellum. J Neurosci. 2014;34:922–31. doi: 10.1523/JNEUROSCI.3476-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heng X, Guo Q, Leung AW, Li JY. Analogous mechanism regulating formation of neocortical basal radial glia and cerebellar Bergmann glia. Elife. 2017:6. doi: 10.7554/eLife.23253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yaguchi Y, Yu T, Ahmed MU, Berry M, Mason I, Basson MA. Fibroblast growth factor (FGF) gene expression in the developing cerebellum suggests multiple roles for FGF signaling during cerebellar morphogenesis and development. Dev Dyn. 2009;238:2058–72. doi: 10.1002/dvdy.22013. [DOI] [PubMed] [Google Scholar]
- 55.Mao J, McGlinn E, Huang P, Tabin CJ, McMahon AP. Fgf-dependent Etv4/5 activity is required for posterior restriction of Sonic Hedgehog and promoting outgrowth of the vertebrate limb. Dev Cell. 2009;16:600–6. doi: 10.1016/j.devcel.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang Z, Verheyden JM, Hassell JA, Sun X. FGF-regulated Etv genes are essential for repressing Shh expression in mouse limb buds. Dev Cell. 2009;16:607–13. doi: 10.1016/j.devcel.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sudarov A, Joyner AL. Cerebellum morphogenesis: the foliation pattern is orchestrated by multi-cellular anchoring centers. Neural Dev. 2007;2:26. doi: 10.1186/1749-8104-2-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ma S, Kwon HJ, Huang Z. Ric-8a, a guanine nucleotide exchange factor for heterotrimeric G proteins, regulates bergmann glia-basement membrane adhesion during cerebellar foliation. J Neurosci. 2012;32:14979–93. doi: 10.1523/JNEUROSCI.1282-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mills J, Niewmierzycka A, Oloumi A, Rico B, St-Arnaud R, Mackenzie IR, Mawji NM, Wilson J, Reichardt LF, Dedhar S. Critical role of integrin-linked kinase in granule cell precursor proliferation and cerebellar development. J Neurosci. 2006;26:830–40. doi: 10.1523/JNEUROSCI.1852-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qu Q, Smith FI. Neuronal migration defects in cerebellum of the Largemyd mouse are associated with disruptions in Bergmann glia organization and delayed migration of granule neurons. Cerebellum. 2005;4:261–70. doi: 10.1080/14734220500358351. [DOI] [PubMed] [Google Scholar]
- 61.Kaartinen V, Gonzalez-Gomez I, Voncken JW, Haataja L, Faure E, Nagy A, Groffen J, Heisterkamp N. Abnormal function of astroglia lacking Abr and Bcr RacGAPs. Development. 2001;128:4217–27. doi: 10.1242/dev.128.21.4217. [DOI] [PubMed] [Google Scholar]
- 62.Delaney CL, Brenner M, Messing A. Conditional ablation of cerebellar astrocytes in postnatal transgenic mice. J Neurosci. 1996;16:6908–18. doi: 10.1523/JNEUROSCI.16-21-06908.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hoser M, Baader SL, Bosl MR, Ihmer A, Wegner M, Sock E. Prolonged glial expression of Sox4 in the CNS leads to architectural cerebellar defects and ataxia. J Neurosci. 2007;27:5495–505. doi: 10.1523/JNEUROSCI.1384-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gotz M, Huttner WB. The cell biology of neurogenesis. Nat Rev Mol Cell Biol. 2005;6:777–88. doi: 10.1038/nrm1739. [DOI] [PubMed] [Google Scholar]
- 65.Fietz SA, Kelava I, Vogt J, Wilsch-Brauninger M, Stenzel D, Fish JL, Corbeil D, Riehn A, Distler W, Nitsch R, et al. OSVZ progenitors of human and ferret neocortex are epithelial-like and expand by integrin signaling. Nat Neurosci. 2010;13:690–9. doi: 10.1038/nn.2553. [DOI] [PubMed] [Google Scholar]
- 66.Hansen DV, Lui JH, Parker PR, Kriegstein AR. Neurogenic radial glia in the outer subventricular zone of human neocortex. Nature. 2010;464:554–61. doi: 10.1038/nature08845. [DOI] [PubMed] [Google Scholar]
- 67.Reillo I, de Juan Romero C, Garcia-Cabezas MA, Borrell V. A role for intermediate radial glia in the tangential expansion of the mammalian cerebral cortex. Cereb Cortex. 2011;21:1674–94. doi: 10.1093/cercor/bhq238. [DOI] [PubMed] [Google Scholar]
- 68.Martinez-Martinez MA, De Juan Romero C, Fernandez V, Cardenas A, Gotz M, Borrell V. A restricted period for formation of outer subventricular zone defined by Cdh1 and Trnp1 levels. Nat Commun. 2016;7:11812. doi: 10.1038/ncomms11812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shitamukai A, Konno D, Matsuzaki F. Oblique radial glial divisions in the developing mouse neocortex induce self-renewing progenitors outside the germinal zone that resemble primate outer subventricular zone progenitors. J Neurosci. 2011;31:3683–95. doi: 10.1523/JNEUROSCI.4773-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Borrell V, Gotz M. Role of radial glial cells in cerebral cortex folding. Curr Opin Neurobiol. 2014;27:39–46. doi: 10.1016/j.conb.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 71.Dehay C, Kennedy H, Kosik KS. The outer subventricular zone and primate-specific cortical complexification. Neuron. 2015;85:683–94. doi: 10.1016/j.neuron.2014.12.060. [DOI] [PubMed] [Google Scholar]
- 72.Fernandez V, Llinares-Benadero C, Borrell V. Cerebral cortex expansion and folding: what have we learned? EMBO J. 2016;35:1021–44. doi: 10.15252/embj.201593701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fietz SA, Huttner WB. Cortical progenitor expansion, self-renewal and neurogenesis-a polarized perspective. Curr Opin Neurobiol. 2011;21:23–35. doi: 10.1016/j.conb.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 74.Geschwind DH, Rakic P. Cortical evolution: judge the brain by its cover. Neuron. 2013;80:633–47. doi: 10.1016/j.neuron.2013.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lui JH, Nowakowski TJ, Pollen AA, Javaherian A, Kriegstein AR, Oldham MC. Radial glia require PDGFD-PDGFRbeta signalling in human but not mouse neocortex. Nature. 2014;515:264–8. doi: 10.1038/nature13973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nonaka-Kinoshita M, Reillo I, Artegiani B, Martinez-Martinez MA, Nelson M, Borrell V, Calegari F. Regulation of cerebral cortex size and folding by expansion of basal progenitors. EMBO J. 2013;32:1817–28. doi: 10.1038/emboj.2013.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Reillo I, Borrell V. Germinal zones in the developing cerebral cortex of ferret: ontogeny, cell cycle kinetics, and diversity of progenitors. Cereb Cortex. 2012;22:2039–54. doi: 10.1093/cercor/bhr284. [DOI] [PubMed] [Google Scholar]
- 78.Pollen AA, Nowakowski TJ, Chen J, Retallack H, Sandoval-Espinosa C, Nicholas CR, Shuga J, Liu SJ, Oldham MC, Diaz A, et al. Molecular identity of human outer radial glia during cortical development. Cell. 2015;163:55–67. doi: 10.1016/j.cell.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Thomsen ER, Mich JK, Yao Z, Hodge RD, Doyle AM, Jang S, Shehata SI, Nelson AM, Shapovalova NV, Levi BP, et al. Fixed single-cell transcriptomic characterization of human radial glial diversity. Nat Methods. 2016;13:87–93. doi: 10.1038/nmeth.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pollen AA, Nowakowski TJ, Shuga J, Wang X, Leyrat AA, Lui JH, Li N, Szpankowski L, Fowler B, Chen P, et al. Low-coverage single-cell mRNA sequencing reveals cellular heterogeneity and activated signaling pathways in developing cerebral cortex. Nat Biotechnol. 2014;32:1053–8. doi: 10.1038/nbt.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tang N, Marshall WF, McMahon M, Metzger RJ, Martin GR. Control of mitotic spindle angle by the RAS-regulated ERK1/2 pathway determines lung tube shape. Science. 2011;333:342–5. doi: 10.1126/science.1204831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.LaMonica BE, Lui JH, Hansen DV, Kriegstein AR. Mitotic spindle orientation predicts outer radial glial cell generation in human neocortex. Nat Commun. 2013;4:1665. doi: 10.1038/ncomms2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hevner RF, Haydar TF. The (not necessarily) convoluted role of basal radial glia in cortical neurogenesis. Cereb Cortex. 2012;22:465–8. doi: 10.1093/cercor/bhr336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kelava I, Reillo I, Murayama AY, Kalinka AT, Stenzel D, Tomancak P, Matsuzaki F, Lebrand C, Sasaki E, Schwamborn JC, et al. Abundant occurrence of basal radial glia in the subventricular zone of embryonic neocortex of a lissencephalic primate, the common marmoset Callithrix jacchus. Cereb Cortex. 2012;22:469–81. doi: 10.1093/cercor/bhr301. [DOI] [PMC free article] [PubMed] [Google Scholar]