Abstract
The maintenance of balance and posture is a result of the collaborative efforts of vestibular, proprioceptive, and visual sensory inputs, but a fourth neural input, audition, may also improve balance. Here, we tested the hypothesis that auditory inputs function as environmental spatial landmarks whose effectiveness depends on sound localization ability during ambulation. Eight blindfolded normal young subjects performed the Fukuda-Unterberger test in three auditory conditions: silence, white noise played through headphones (head-referenced condition), and white noise played through a loudspeaker placed directly in front at 135 centimeters away from the ear at ear height (earth-referenced condition). For the earth-referenced condition, an additional experiment was performed where the effect of moving the speaker azimuthal position to 45, 90, 135, and 180 degrees was tested. Subjects performed significantly better in the earth-referenced condition than in the head-referenced or silent conditions. Performance progressively decreased over the range from 0° to 135° but all subjects then improved slightly at the 180° compared to the 135° condition. These results suggest that presence of sound dramatically improves the ability to ambulate when vision is limited, but that sound sources must be located in the external environment in order to improve balance. This supports the hypothesis that they act by providing spatial landmarks against which head and body movement and orientation may be compared and corrected. Balance improvement in the azimuthal plane mirrors sensitivity to sound movement at similar positions, indicating that similar auditory mechanisms may underlie both processes. These results may help optimize the use of auditory cues to improve balance in particular patient populations.
Keywords: Audition, Balance, Fukuda, Spatial, Orientation, Localization
Introduction
Visual, vestibular, and proprioceptive input are considered the critical sensory inputs for maintaining balance. Visual cues act as external, earth-referenced landmarks allowing the position and motion of the head in space to be measured, vestibular inputs provide internal, head-referenced signals indicating similar information, and the proprioceptive system binds the orientation of the body parts relative to each other, to the head, and to the surrounding supportive substrate. These cues are merged and compared to provide feedback during body motion, allowing corrective actions to be taken to maintain balance.
The possibility of a fourth important contributor to balance, auditory input, has by comparison been relatively ignored. A small but increasing body of evidence, however, indicates that the presence of an external auditory source or sources may also contribute to maintaining balance. An early study tested this hypothesis by examining static postural stability in a group of congenitally blind and sighted subjects [1]. They mounted two speakers, each located 5 cm lateral to the subject’s ears. Standing on a force plate in the dark, their subjects showed less motion of the center of pressure in the presence of the sound cues compared to silence.
Further experiments have generally confirmed this finding, although not unanimously. In one study, static postural stability, as measured by the motion of a subject’s center of pressure on a force plate, was greater in a silent audio booth than in a clinic room, although the conclusions were limited by somewhat inconsistent results [2]. A similar result was found in a group of older adults who were found to be more stable when wearing their hearing aids than without amplification [3]. However, another recent article found no difference in sway between subjects listening to music through headphones versus hearing ambient noise in an untreated room [4].
Maintaining static postural stability while standing requires monitoring and adjusting to the position and orientation of the body to minimize its movement in space. Preserving dynamic balance during ambulation adds complexity, because one must anticipate and compensate for the body’s planned direction and amount of motion through space rather than simply minimizing it. Along these lines, a common clinical observation among patients with vestibular loss is that they may complain most about difficulty with navigating in a straight line and less about postural stability while standing still.
Given the promising findings in a static situation, it seems reasonable to hypothesize that auditory stimuli could also be important in optimizing the ability to ambulate. Earlier work has asked normal subjects to march in place in the dark either in silence or in the presence of an auditory cue (a nearby loudspeaker or metronome) [5,6]. Audition was found to improve the error in subject heading during ambulation, as measured by the degree to which each subject turned during the task [5,6].
However, the amount of improvement under various relevant auditory conditions, and the reason for this variability, is not fully explored. Knowing this would elucidate the fundamental mechanism for the benefit of auditory inputs, and guide the development of auditory environments or augmentative devices that might improve balance using auditory cues. In this study, we tested two hypotheses: first, that auditory stimuli function as spatial landmarks, analogous to elements of a visual scene, to improve balance and orientation during ambulation; and second, that specific characteristics of the sound source, such as its location relative to the subject, influences its ability to provide meaningful spatial cues.
Methods
This study was completed with the approval of the appropriate Institutional Review Board. Subjects were required to have normal hearing (defined as pure-tone average (PTA) of no less than 25 dB at 0.5, 1, and 2 kHz) and PTA in the worse ear of no less than 10 dB below the better ear’s level (Model 10D, Beltone, Chicago, Illinois), have normal or corrected-to-normal vision on a standard Snellen chart, and speak English. Exclusion criteria were inability to complete the experiment, history of degenerative neurologic disease, stroke, or spinal stenosis, or current use of balance-altering medication. Eight people participated; 6 male and 2 female subjects (mean age = 21; age range = 18–26). No subject had worse than a 12 dB pure-tone average in the poorer ear.
Dynamic balance was assessed using the Fukuda-Unterberger stepping test [7]. Subjects were required to walk 50 steps in place, arms outstretched and shoulder-width apart with the eyes closed. The 0 degree azimuth was defined as the initial direction faced by the subjects, with the positive direction measured clockwise. All subjects wore a blindfold and shoes. Error in heading direction at the end of the test was measured using a goniometer.
Auditory input was provided by a speaker with a frequency response of 0.1–22 kHz (model R1, YC Cable, Ontario, CA) providing a broadband white noise stimulus that was generated by MATLAB (bandlimited over 0–4 kHz). The speaker was positioned at ear level, 185 cm from the center of the subject’s head. The sound intensity was measured to be 65 dB SPL re 20 uPa. All testing was performed in a quiet carpeted conference room without additional soundproofing.
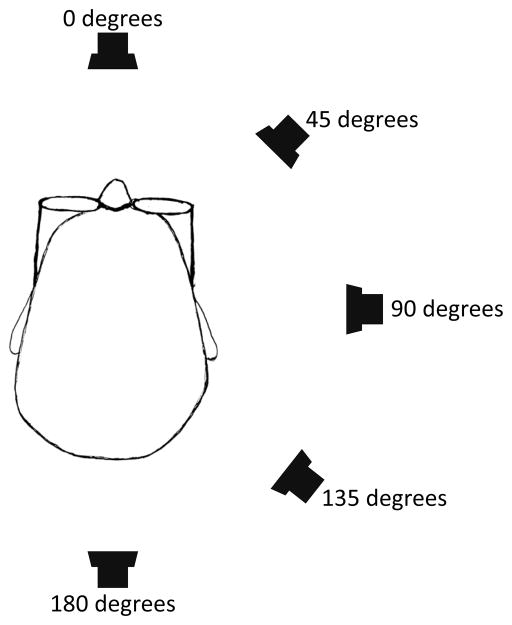
Subjects performed testing under a total of seven different conditions. The “silent” condition used no speaker and prevented subjects from gaining ambient auditory input by using noise-canceling ear plugs, (−32 dB NRR, Hearos Ear Plugs, Aliso Viejo, CA) and, in addition, commercial circumaural ear muffs (−30 dB NRR). The “head-referenced” condition presented white noise (bandlimited at 0–4 kHz and derived from the same sound file as used for the external speaker) through in-the-ear speakers (Philips, SHS3200/37) to provide an auditory experience that did not provide any spatial information. In the “earth-referenced” condition, the auditory source was placed directly in front of the patient (defined as 0 degrees in the azimuthal plane). Subjects were instructed to adjust the volume of the in-the-ear speakers to match the volume of the external sound source subjectively. In a separate experiment, for the “earth-referenced” condition, results at 0 degrees were compared to performance in four additional locations at 45, 90, 135, and 180 degrees along a semicircle in the azimuthal plane from directly forward, to over the right shoulder, to behind the head (subjects were tested only on one side to reduce the duration of the experiment) (Fig. 1).
Figure 1.
Bird’s eye view of the speaker locations.
Trial conditions were randomized. Each condition was repeated three times and the median value of the three trials was used in data analysis. After completion of each trial, subjects were guided away from their ending position in a large arc back to the starting point to prevent them from having any feedback on performance on the previous trial.
The independent variable for the first experiment was the auditory condition (silence, head-referenced, and earth-referenced) and for the second was the position of the sound source relative to the subject. The outcome was measured as the angular error, or absolute value of the difference in degrees between the starting orientation and the final orientation (the direction the subject was facing). Given the relatively small number of participants, a normal distribution could not be assumed and nonparametric statistics were required. These were performed using Friedman’s test with Dunn’s correction for multiple pairwise comparisons (GraphPad Prism 7.00).
Results
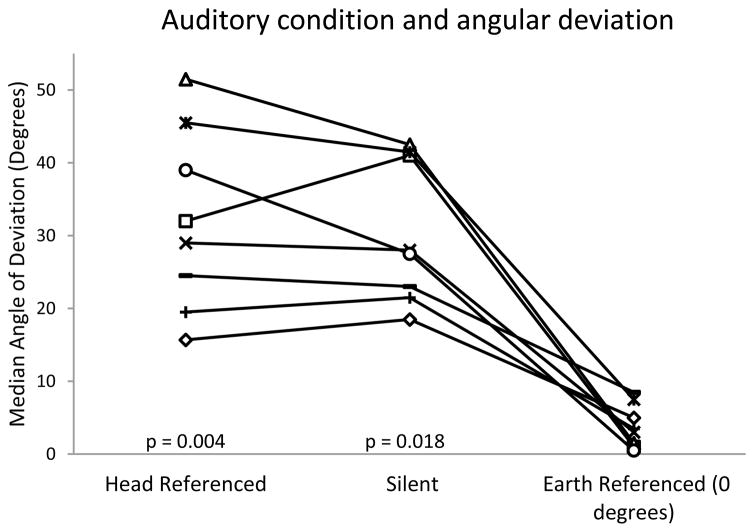
The comparison between the silent, head-referenced, and earth-referenced conditions was performed with the earth-referenced sound source at the 0 deg position. All subjects performed worse in the silent and head-referenced conditions than in the earth-referenced condition (Fig. 2). The mean angular deviation was 31.6 deg ± 11.5 deg in the silent condition, 28.7 deg ± 10.8 deg in the head-referenced condition, and 3.8 deg ± 3.1 deg in the 0 deg earth-referenced condition. The angular deviations in the silent and head-referenced conditions were not significantly different from each other (p>0.999), but the angular deviations in both the silent and head-referenced conditions were significantly worse than the 0 deg earth-referenced condition (p=0.018, and p=0.004 respectively). These corresponded to a large effect size between the silent and earth-referenced conditions (0.630) and between the head-referenced and earth-referenced conditions (0.631).
Figure 2.
Effect of sound condition on performance. Y-axis represents error in degrees during Fukuda-Unterberger stepping test. Left column: sound provided through headphones (head-referenced). Middle column: silence. Right column: external sound provided at zero degree azimuth (earth-referenced). P values represent comparisons to the earth referenced condition.
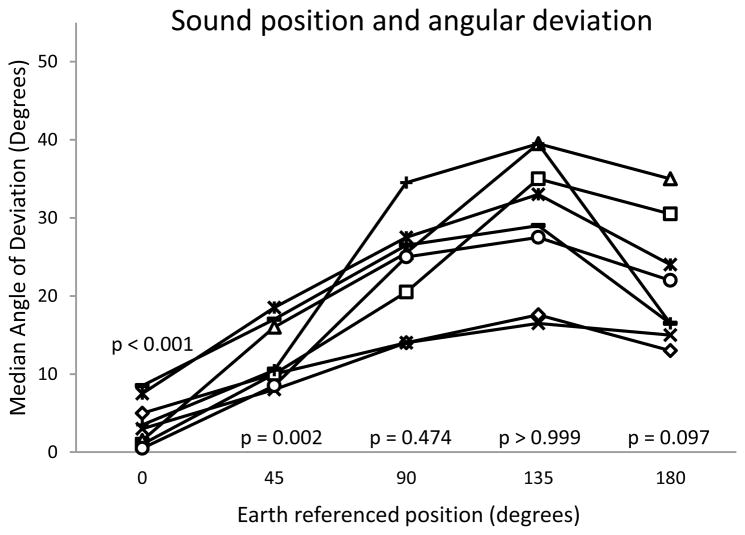
Performance in the earth-referenced condition decreased steady with increasing azimuthal angle from 0 deg to 135 deg before improving slightly at 180 degrees (Fig. 3). The mean error at 45 deg angle was 12.3 deg ± 5.7 deg, at 90 deg was 23.4 deg ± 10.2 deg, at 135 deg was 29.7 deg ± 12.9 deg, and at 180 deg was 21.6 deg ± 10.3 deg. Performance at 0 and 45 degrees was significantly better than in silence, but at 90, 135, and 180 degrees was not (p<0.001, p=0.002, p=0.474, p>0.999, p=0.097 respectively).
Figure 3.
Effect of sound location on ambulatory angle of deviation. X-axis: location of external sound source, with zero degrees being directly in front of the patient, 90 degrees directly over the right shoulder, and 180 degrees directly behind the patient. In all cases, the sound source was 185 cm from the subject. Y-axis represents error in degrees during Fukuda-Unterberger stepping test. P values represent comparisons to the silent condition.
Discussion
Our results show that providing an external sound source improves performance only when the source can act as a spatial reference landmark in the environment, and found that this effect is highly dependent on its location. The results also provide indirect evidence that the improvement in performance is related to sound localization ability, suggesting novel mechanisms for training paradigms improving balance.
The Fukuda-Unterberger test used here is an advantageous method for examining the effect of sound on ambulation because it provides a relevant measure (ability to maintain a desired orientation) and an easily defined outcome variable (angular deviation). Similar techniques have been used in two previous reports. One found that a sound placed at zero degrees azimuth reduced the angular error during a Fukuda-Unterberger test [5], and another group also found that linear displacement was less [6]. Neither, however, compared a sound presented in the subject’s spatial environment versus without spatial cues through headphones as done here, leaving open the possibility that simply the presence of the sound itself, rather than its placement in the environment, could be important for balance. Here, we added an important control of head-referenced sound (provided through headphones) and found that sound improves balance only when serving as a spatial environmental cue. Although a previous report came to the opposite conclusion, the omnidirectional environmental sound stimulus used there was not salient enough to function effectively as a spatial landmark [4].
Two other recent articles have used different outcome variables. In the first, three people with vestibular loss walked, eyes closed, along an instrumented track on the floor, with the sound source comprising a bank of empty but rotating treadmills to the side of and paralleling their direction of travel [8]. They showed improved gait velocity when environmental auditory cues were available. A second report using body-worn inertial sensors, in contrast, was unable to find a consistent improvement among a set of hearing-impaired older people wearing hearing aids or cochlear implants while walking in a straight line [9]. Given the previous robust results, it may be that the inertial sensors were simply insensitive to important changes in gait: for example, while sensors can measure velocity or acceleration, their ability to identify changes in direction as measured here is limited.
In contrast to the results regarding ambulation presented here, the vast majority of work exploring the effect of audition on balance has used measures of static posture (such as sway velocity) as outcome variables [1,2,5,10,11,12]. While these have generally shown some improvement in balance, the difference has been modest at best and some papers have either found no improvement or a decrease in some subjects [4,10,13]. The consistently robust responses found here, as well as in other studies of ambulation [5,6,8], suggest that sound may be a more important modulator of dynamic rather than static balance. As most falls occur during movement [14], this only increases the importance of the current findings.
Conventional sound localization experiments ask, “where is the sound source relative to me?” The method by which this localization is performed is well known: the brain triangulates using the difference in arrival time of a sound and its relative amplitude between the ears [15]. Spectral cues offer an additional cue determined by the head-related transfer function (HRTF), which is a filtering of the acoustic signal based on the unique shape of the pinna, head, and torso and the location of the sound source [16].
Here, the brain was required to perform the inverse calculation: “where am I relative to the sound source?” or more accurately, “how am I moving relative to the sound source?” The brain’s ability to determine this is characterized by the “minimum audible movement angle” (MAMA), or threshold for perceiving that a sound source is moving relative to the head [17,18]. Just like the effect of external auditory cues on balance, the MAMA worsens with increasing azimuthal location of the sound source away from the frontal plane (from less than 2 deg/sec in front up to 10 deg/sec at 40 deg to the side) [19,20], consistent with them depending on the same mechanisms and bolstering the argument that external sound sources could improve ambulation performance by acting as spatial landmarks. The MAMA determined by the position of a sound source may determine the upper limit of that sound’s ability to improve dynamic balance during ambulation. Although the effect of hearing loss on the MAMA is not known, presumably people in whom sound localization is impaired (such as those with single-sided deafness) would enjoy less balance-related benefit from external auditory sources than normal-hearing listeners.
Several studies have shown that training can improve the MAMA [18]. To the extent that the benefit of sound on balance is determined by the MAMA, this leads to the intriguing idea that improving the MAMA might also improve postural stability. This may form the basis for the development of novel therapies for imbalance.
In patients with other deficits of balance-related sensory cues, the contribution of audition is likely to increase because the loss of other sensory inputs would cause reweighting toward more salient cues [21,22]. Auditory input should thus probably be considered along with vestibular, visual, and proprioceptive signals in sensory reweighting experiments, and recognized as a possible confounder. At the same time, auditory input is useless without being able to combine it accurately with proprioceptive inputs from the neck, trunk, and legs, vestibular inputs, and efference copy to be effectively coded into a body centered framework necessary to guide ambulation [23,24,25]. Significant defects in these systems would presumably lead to decreased effectiveness of auditory cues, and might explain subjects who are exceptions to the general finding that audition is most beneficial to balance in people with the greatest baseline instability [10,11].
Despite the small sample set, our findings are consistent across all subjects suggesting broad clinical implications. A recent study that tested balance of experienced older bilateral hearing-aid users (standing with eyes closed in the presence of a 65 dB sound source located 1 m in front of the head) found that all subjects demonstrated significantly better balance in the sound-aided than in the unaided condition [3]. Nonetheless, among older adults with hearing loss who could benefit from hearing aids, only about one third wear them [26]. Such findings highlight the importance of considering hearing loss and/or the use of hearing aids when implementing balance rehabilitation. It is possible that people who tend to fall in the home or at night (when hearing aids are not in use) could be, in part, victims of the loss of auditory input for orientation.
These data are also significant for determining the proper conditions for performing the Fukuda-Unterberger test. This test has been used previously as a test for unilateral vestibular loss, with the subject tending to turn to the weaker side. Unfortunately, this test shows poor sensitivity and specificity [27]. Traditionally, this test has been performed in a clinical testing room without any particular attention paid to the quality of the acoustic environment. The results here confirm that the presence and location of sound sources can have a significant effect on results, and if salient enough the presence of sound may cause a false negative result to the test. Performing this test in silence (probably with ear muffs in place) may improve the clinical reliability of results [6].
Highlights.
Measured ability to maintain direction of gait under varying auditory conditions.
Performance was best in the presence of sound.
Sound served as a spatial environmental landmark, not an alerting stimulus.
Bilateral hearing improves gait, dependent on position of stimulus.
Training for sound localization may be shown to improve gait.
Acknowledgments
The authors gratefully acknowledge the assistance of Rosalie Uchanski, Madelyn Stevens, and Nicholas N-Y Chang. This work was supported by the National Institutes of Health K08 DC006869
Footnotes
Conflict of Interest Statement
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Easton RD, Greene AJ, DiZio P, Lackner JR. Auditory cues for orientation and postural control in sighted and congenitally blind people. Exp Brain Res. 1998;118:541–50. doi: 10.1007/s002210050310. [DOI] [PubMed] [Google Scholar]
- 2.Kanegaonkar RG, Amin K, Clarke M. The contribution of hearing to normal balance. J Laryngol Otol. 2012;126:984–8. doi: 10.1017/S002221511200179X. [DOI] [PubMed] [Google Scholar]
- 3.Rumalla K, Karim AM, Hullar TE. The effect of hearing aids on postural stability. Laryngoscope. 2015;125:720–3. doi: 10.1002/lary.24974. [DOI] [PubMed] [Google Scholar]
- 4.Palm H-G, Strobel J, Achatz G, von Luebken F, Friemert B. The role and interaction of visual and auditory afferents in postural stability. Gait Posture. 2009;30:328–33. doi: 10.1016/j.gaitpost.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 5.Zhong X, Yost WA. Relationship between postural stability and spatial hearing. J Am Acad Audiol. 2013;24:782–8. doi: 10.3766/jaaa.24.9.3. [DOI] [PubMed] [Google Scholar]
- 6.Munnings A, Chisnall B, Oji S, Whittaker M, Kanegaonkar R. Environmental factors that affect the Fukuda stepping test in normal participants. J Laryngol Otol. 2015;129:450–453. doi: 10.1017/S0022215115000560. [DOI] [PubMed] [Google Scholar]
- 7.Fukuda T. The stepping test: two phases of the labyrinthine reflex. Acta Otolaryngol (Stockh) 1959;50:95–108. doi: 10.3109/00016485909129172. [DOI] [PubMed] [Google Scholar]
- 8.Shayman CS, Earhart GM, Hullar TE. Improvements in gait with hearing aids and cochlear implants. Otol Neurotol. 2017;38:484–486. doi: 10.1097/MAO.0000000000001360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weaver TS, Shayman TS, Hullar TE. The effect of hearing aids and cochlear implants on balance during gait. Otol Neurotol. 2017;38:1327–1332. doi: 10.1097/MAO.0000000000001551. [DOI] [PubMed] [Google Scholar]
- 10.Stevens MN, Baudhuin JE, Hullar TE Washington University Cochlear Implant Study Group. Short-term risk of falling after cochlear implantation. Audiol Neurootol. 2014;19:370–377. doi: 10.1159/000363214. [DOI] [PubMed] [Google Scholar]
- 11.Vitkovic J, Le C, Lee SL, Clark RA. The contribution of hearing and hearing loss to balance control. Audiol Neurootol. 2016;21:195–202. doi: 10.1159/000445100. [DOI] [PubMed] [Google Scholar]
- 12.Gandemer L, Parseihian G, Kronland-Martinet R, Bourdin C. Spatial cues provided by sound improve postural stabilization: Evidence of a spatial auditory map? Front Neurosci. 2017;11:357. doi: 10.3389/fnins.2017.00357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gago MF, Fernandes V, Ferreira J, Yelshyna D, Silva HD, Rodrigues ML, Rocha L, Bicho E, Sousa N. Role of the visual and auditory systems in postural stability in Alzheimer’s disease. J Alzheimers Dis. 2015;46:441–9. doi: 10.3233/JAD-150131. [DOI] [PubMed] [Google Scholar]
- 14.Blake AJ, Morgan K, Bendall MJ, et al. Falls by elderly people at home: Prevalence and associated factors. Age Ageing. 1988;17:365–372. doi: 10.1093/ageing/17.6.365. [DOI] [PubMed] [Google Scholar]
- 15.Makous JC, Middlebrooks JC. Two-dimensional sound localization by human listeners. J Acoust Soc Am. 1990;87:2188–2200. doi: 10.1121/1.399186. [DOI] [PubMed] [Google Scholar]
- 16.Carlile S, Martin R, McAnally K. Spectral information in sound localization. Int Rev Neurobiol. 2005;70:399–434. doi: 10.1016/S0074-7742(05)70012-X. [DOI] [PubMed] [Google Scholar]
- 17.Chandler DW, Grantham DW. Minimum audible movement angle in the horizontal plane as a function of stimulus frequency and bandwidth, source azimuth, and velocity. J Acoust Soc Am. 1992;91:1624–36. doi: 10.1121/1.402443. [DOI] [PubMed] [Google Scholar]
- 18.Carlile S, Leung J. The perception of auditory motion. Trends Hear. 2016;20:1–19. doi: 10.1177/2331216516644254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grantham DW. Detection and discrimination of simulated motion of auditory targets in the horizontal plane. J Acoust Soc Am. 1986;79:1939–1949. doi: 10.1121/1.393201. [DOI] [PubMed] [Google Scholar]
- 20.Strybel TZ, Manligas CL, Perrott DR. Minimum audible movement angle as a function of the azimuth and elevation of the source. Hum Factors. 1992;34:267–75. doi: 10.1177/001872089203400302. [DOI] [PubMed] [Google Scholar]
- 21.Assländer L, Peterka RJ. Sensory reweighting dynamics in human postural control. J Neurophysiol. 2014;112:525–542. doi: 10.1152/jn.00669.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hwang S, Agada P, Kiemel T, Jeka JJ. Dynamic reweighting of three modalities for sensor fusion. PLoS One. 2014;31:e88123. doi: 10.1371/journal.pone.0088132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goossens HH, van Opstal AJ. Influence of head position on the spatial representation of acoustic targets. J Neurophysiol. 1999;81:2720–2736. doi: 10.1152/jn.1999.81.6.2720. [DOI] [PubMed] [Google Scholar]
- 24.DeZio P, Held R, Lackner JR, Shinn-Cunningham B, Durlach N. Gravitoinertial force magnitude and direction influence head-centric auditory localization. J Neurophysiol. 2001;85:2455–2460. doi: 10.1152/jn.2001.85.6.2455. [DOI] [PubMed] [Google Scholar]
- 25.Lewald J, Karnath HO. Vestibular influence on human auditory space perception. J Neurophysiol. 2000;14:1107–1111. doi: 10.1152/jn.2000.84.2.1107. [DOI] [PubMed] [Google Scholar]
- 26.Bainbridge KE, Ramachandran V. Hearing aid use among older U.S. adults: The national health and nutrition examination survey, 2005–2006 and 2009–2010. Ear Hear. 2014;35:289–94. doi: 10.1097/01.aud.0000441036.40169.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Honaker JA, Boismier TE, Shepard NP, Shepard NT. Fukuda stepping test: sensitivity and specificity. J Am Acad Audiol. 2009;20:311–314. doi: 10.3766/jaaa.20.5.4. [DOI] [PubMed] [Google Scholar]