Abstract
Subtle semantic deficits can be observed in Alzheimer’s disease (AD) patients even in the early stages of the illness. In this work, we tested the hypothesis that the semantic control network is deregulated in mild AD patients. We assessed the integrity of the semantic control system using resting-state functional magnetic resonance imaging in a cohort of patients with mild AD (n = 38; mean mini-mental state examination = 20.5) and in a group of age-matched healthy controls (n = 19). Voxel-wise analysis spatially constrained in the left fronto-temporal semantic control network identified two regions with altered functional connectivity (FC) in AD patients, specifically in the pars opercularis (POp, BA44) and in the posterior middle temporal gyrus (pMTG, BA21). Using whole-brain seed-based analysis, we demonstrated that these two regions have altered FC even beyond the semantic control network. In particular, the pMTG displayed a wide-distributed pattern of lower connectivity to several brain regions involved in language-semantic processing, along with a possibly compensatory higher connectivity to the Wernicke’s area. We conclude that in mild AD brain regions belonging to the semantic control network are abnormally connected not only within the network, but also to other areas known to be critical for language processing.
Keywords: Alzheimer’s disease, semantic control network, posterior middle temporal gyrus, inferior frontal gyrus, resting-state fMRI, voxel-wise functional connectivity
INTRODUCTION
The hallmark of Alzheimer’s disease (AD) has been long recognized to be a profound deficit in episodic memory. However, language dysfunctions with a semantic basis are also observed in AD patients (Kempler, 1995) even at predementia stages (Mickes et al., 2007; Amieva et al., 2008; Taler and Phillips, 2008). The early pattern of language deterioration in AD is quite specific and characterized by a predominant semantic impairment with a relatively sparing of other language features, such as syntax or phonology (Kirshner, 2012). The most evident symptom is a word-finding difficulty which can be observed in spontaneous speech (Nicholas et al., 1985) as well as in language standardized tests (e.g., confrontation naming and verbal fluency; Henry et al., 2004). While the neural substrates underlying episodic memory impairment in AD have been extensively studied, the role of semantic memory still remains poorly investigated. PET studies indicated that frontal and lateral temporal regions are implicated in semantic deterioration in AD (Zahn et al., 2004; Teipel et al., 2006; Nelissen et al., 2007; Melrose et al., 2009), yet the question arises of which component of semantic cognition is being affected by the pathology.
Semantic cognition is theorized to include the storage of conceptual knowledge and the process by which such knowledge is manipulated in a time, context, and task appropriate fashion (Tulving, 1987). These two components (i.e., storage and control) are thought to be subserved by two distinct but interactive neuronal systems (Jefferies, 2013; Ralph et al., 2017). The conceptual knowledge is controlled by a wide-distributed network composed of sensorimotor and verbal-related areas that store object-specific features and lexical information (Damasio et al., 2004; Martin, 2007), along with an amodal hub, located in the bilateral anterior temporal lobe, acting as a convergence zone (Patterson et al., 2007). On the contrary, the semantic control is supported by a left-lateralized fronto-temporal and possibly parietal network (Jefferies and Ralph, 2006; Noonan et al., 2010). In particular, the left inferior frontal gyrus (pars opercularis and triangularis), and the left posterior middle temporal gyrus (pMTG) are considered the most relevant regions in the semantic control network (Whitney et al., 2011a,b; Jefferies, 2013; Krieger-Redwood and Jefferies, 2014). However, other regions have been implicated in semantic control, including the dorsal angular gyrus (Noonan et al., 2013) and the posterior cingulate cortex (Binder et al., 2009), although their exact role needs to be clarified.
Neuropsychological research about the nature of semantic deficits in AD has provided conflicting results, pointing to either a degraded conceptual knowledge (Hodges et al., 1992; Garrard et al., 2005; Lin et al., 2014) or a deregulated control/access to this information (Bayles et al., 1991; Nebes and Halligan, 1996). The apparent inconsistency among neuropsychological studies could be related to disease severity (Bayles et al., 1991; Duong et al., 2006). This notion is supported by a recent study that examined how different stages of the pathology affect distinct components of semantic cognition (Corbett et al., 2012). Specifically, in the mild stage (mean mini-mental state examination, MMSE ~ 20) patients showed impairments distinctive of deregulated control of semantic information, whereas in the severe stage (mean MMSE ~ 10) this problem, besides getting worse, became compounded by degradation of semantic representations (Corbett et al., 2012). The pathophysiological counterpart of the reported pattern of semantic impairment in AD is expected to be a functional alteration in the semantic control system, which should be visible early in the disease progression. To the best of our knowledge, such hypothesis has not been tested yet.
Here we adopted resting-state functional magnetic resonance imaging (rsfMRI), based on the blood-oxygen-level-dependent (BOLD) signal, to examine, for the first time, the functional connectivity (FC) integrity of the semantic control network in patients with AD at mild stage. Compared to task-based fMRI, the resting-state design has the advantage of not relying on any task choice. This feature is particularly important for dementia subjects in which uncontrolled familiarity of stimulus concepts and/or task demands might represent critical, and not manageable, confounds (Bayles et al., 1991). Our main hypothesis was that mild AD patients are characterized by altered FC within the semantic control network. Secondly, we hypothesized that such alteration correlates with language impairment (verbal fluency and confrontation naming). Finally, given the continuous interplay between the semantic control and the wide-distributed representation network, we expected that affected regions in the semantic control network would present FC abnormalities in other semantic-related regions.
EXPERIMENTAL PROCEDURES
Subjects
A cohort of 38 right-handed patients with probable AD-typical were recruited for the current study. The diagnosis of probable AD was performed according to the clinical criteria of the National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA, McKhann et al., 2011). To be included, patients had to meet the Diagnostic and Statistical Manual of Mental Disorders (DSM-V) criteria (American Psychiatric Association, 2013) for the diagnosis of major neurocognitive disorders due to AD. An expert neurologist (M.B.) in each recruited patient reviewed carefully the clinical history, the cognitive profile and the conventional MRI scan and excluded the vascular signs and symptoms associated typically with vascular dementia. Nineteen right-handed healthy elderly individuals were also recruited and served as healthy controls (HC). All healthy subjects reported scores within the range of normality at the Mini Mental State Examination (MMSE, Italian cut-off >23.8; Folstein et al., 1975; Magni et al., 1996). Major systemic, psychiatric, vascular and other neurological illnesses were carefully investigated and excluded in all recruited subjects. The study was carried out in accordance with a protocol approved by the Ethics Committee of Santa Lucia Foundation. All recruited subjects gave written informed consent in accordance with the Declaration of Helsinki and European Union regulations.
Neuropsychological assessment
All participants underwent a neuropsychological battery covering several cognitive domains, which included: (1) verbal episodic long-term memory: 15-Word List (Immediate and 15-min Delayed recall; Carlesimo et al., 1996); Short Story Test (Immediate and 20-min Delayed recall; Carlesimo et al., 2002); (2) visuo-spatial long-term memory: Complex Rey’s Figure (Immediate and 20-min Delayed recall; Carlesimo et al., 2002); (3) short-term memory: Digit span and the Corsi Block Tapping task (Monaco et al., 2013); (4) executive functions: Phonological Word Fluency (Carlesimo et al., 1996) and Modified Card Sorting Test (Nocentini et al., 2002); (5) language: Naming objects subtest of the BADA (“Batteria per l’Analisi dei Deficit Afasici”, Italian for “Battery for the analysis of aphasic deficits”; Miceli, 1994); (6) reasoning: Raven’s Coloured Progressive Matrices (Carlesimo et al., 1996); (7) constructional praxis: copy of simple drawings with and without landmarks (Carlesimo et al., 1996) and copy of Complex Rey’s Figure (Carlesimo et al., 2002).
Data acquisition
Data were acquired on a 3 T MRI system (Magnetom Allegra, Siemens, Erlangen, Germany). All subjects underwent a resting-state fMRI scan using an echo planar imaging (EPI) sequence with the following parameters: TR = 2080 ms, TE = 30 ms, 32 axial slices parallel to AC-PC plane, matrix = 64 × 64, in plane resolution = 3 × 3 mm2, slice thickness = 2.5 mm, 50% skip, flip angle = 70°. The slices were positioned starting from the vertex and covering the whole cerebrum. The cerebellum did not consistently fall in the field of view of each acquired subject, thus, it was excluded from any subsequent analysis. Resting scans lasted for 7 min and 20 s for a total of 220 volumes during which subjects were instructed to keep their eyes closed, to not think of anything in particular and to refrain from falling asleep. Immediately after the scan, subjects were interrogated and asked for compliance. No subject showed any behavior sign suggestive of sleeping. A T1-weighted three-dimensional modified driven equilibrium Fourier transform scan (MDEFT, Deichmann et al., 2004) was acquired for each subject for anatomical localization purposes and for gray matter (GM) volumetry; the parameters were as follows: TR = 1338 ms, TE = 2.4 ms, TI = 910 ms, flip angle = 15°, matrix = 256 × 224 × 176, FOV = 256 × 224 mm2, slice thickness = 1 mm, total scan time = 12 min. Fluid attenuated inversion recovery (FLAIR) images (TR = 8170 ms, TE = 96 ms, TI = 2100 ms) were also acquired from all subjects to exclude the presence of remarkable signs suggestive of cerebro-vascular disease. No subject was considered affected by cerebro-vascular pathology based on previously described criteria (Serra et al., 2010).
Data preprocessing
Functional and structural MRI data were preprocessed using CONN 15.b: functional connectivity toolbox (Whitfield-Gabrieli and Nieto-Castanon, 2012; http://www.nitrc.org/projects/conn). For each subject, the first four volumes of the EPI time series were discarded to allow for signal and scanner stabilization. Realignment and slice-time correction were implemented to compensate for head movements and slice-dependent time shifts, respectively. Additionally, to reduce the movement-related residual variance induced by the susceptibility-by-movements interaction effect, the unwarp algorithm was applied (Andersson et al., 2001). Then, functional volumes were spatially normalized into Montreal Neurological Institute (MNI) coordinates (voxel size: 2 × 2 × 2 mm3) using as source image the EPI mean volume obtained from the realignment step. Unless otherwise specified, normalized images were smoothed applying an 8 × 8 × 8 mm3 full width at half maximum (FWHM) Gaussian Kernel. Separately, the T1 weighted high-resolution volumes were segmented and normalized to MNI space to obtain GM, White Matter (WM) and Cerebral Spinal Fluid (CSF) tissue probability maps. WM and CSF segments were used for the removal of confounding effects from fMRI data as detailed below. Additionally, GM segments were post-processed as previously described (Mascali et al., 2015) in order to obtain, for each subject, the GM volume (GMV) to be used as covariate of no interest in statistical group comparisons.
Additional preprocessing steps were applied to functional data for mitigating the effect of various spurious sources of variance. These included despiking and application of a 0.008–0.09-Hz band-pass temporal filter simultaneously to the regression out, via a general linear model (GLM), of the following confounds: (1) a linear trend; (2) the six parameters of realignment and their first derivatives; (3) the first five eigenvectors of the PCA decomposition of the EPI time series separately averaged over WM and CSF, following the aCompCor noise removal approach (Behzadi et al., 2007); (4) the outlier volumes detected using the Artifact Detection Tools (ART: www.nitrc.org/projects/artifact_detect/). The simultaneous filter/regression was achieved by bandpass filtering both the fMRI time series and the confounds, prior to the regression in the GLM. This approach slightly outperforms the more common regression followed by filter (Hallquist et al., 2013).
To assess the amount of head motion during functional scans we computed the framewise displacement (FD) as defined in (Power et al., 2012).
Functional connectivity
The residual BOLD time series resulting from the preprocessing were used to compute two different types of FC metrics, namely, a voxel-wise and a seed-based metric.
First, a spatially constrained version of the weighted Global Brain Connectivity (wGBC; Cole et al., 2010; Mascali et al., 2015) was computed to investigate the voxel-wise FC of the semantic control network. wGBC can be employed to quantify the average FC of every voxel to all other voxels within the brain or in a specific mask. To differentiate between the whole-brain versus the spatially constrained version of this quantity, we refer to the masked version, here adopted, as weighted Regional Brain Connectivity (wRBC). Mathematically, given the BOLD time course of the i-th voxel in the mask, the wRBC of that voxel is defined as the weighted average of the Pearson correlation, r, computed for every other time course in the mask:
where the weighting function is the z-Fisher transformation, . As in the original wGBC definition, the wRBC encompasses both positive and negative correlation values. The chosen mask was composed of key regions of the semantic control network (Jefferies, 2013) extracted from the Harvard-Oxford probabilistic atlas (Desikan et al., 2006). The included regions were the left inferior frontal gyrus (pars opercularis and triangularis) and the left pMTG (posterior and temporooccipital parts), which have been consistently implicated in semantic control (Indefrey and Levelt, 2004; Whitney et al., 2011a,b; Krieger-Redwood and Jefferies, 2014). We limited the calculation to this network because the wGBC (i.e., the whole-brain version) has an intrinsically poor sensitivity if the effect is localized (due to mutual correlations among functionally heterogeneous voxels) and it is also prone to include the effect of possible compensatory mechanisms. For the same reason, the limitation to the most relevant regions of the semantic control network improves the specificity of the wRBC metric. The computation was performed using the time efficient algorithm of 3dTcorrMap (AFNI package; Cox, 1996). To avoid partial volume errors, for this metric the spatial smoothing was restrained into the relevant mask using the 3dBlurInMask function (AFNI package; Cox, 1996), with a FWHM Gaussian kernel of 4 × 4 × 4 mm3.
Secondly, the seed-based metric (Biswal et al., 1995) was adopted to further investigate the changes in FC highlighted by the wRBC metric. Specifically, clusters of altered wRBC inside the semantic control network were used as seed regions to assess their FC with the rest of the brain. To preserve the spatial specificity of seed regions, seed time courses were extracted from unsmoothed data. As for wRBC, a z-Fisher transformation was applied to seed-based FC to attain normality.
Statistical analysis
For each investigated parameter, statistical comparisons were performed adopting a 2 × 2 factorial design with group and gender as factors. Although the gender effect was not the focus of the present work, the inclusion in the model of such factor allowed for testing possible interaction effects between group and sex, which might be expected given previous reports of sex influence on language processing (for example, Shaywitz et al., 1995). In all statistical models, age, years of formal education and GMV were used as nuisance covariates. Within the factorial design, between-group comparisons, for either FC measure (i.e., wRBC and seed-based FC), were performed using two-sample t-tests, while group-level seed-based FC maps were obtained with one-sample t-tests. The interaction effect between group and sex was tested via f-tests.
Statistical threshold was set to p < 0.05 after correction for multiple comparisons via Monte Carlo simulations (3dClustSim, AFNI package; Cox, 1996). Simulations were run with a mixed model (Gaussian plus mono-exponential) for the estimation of the spatial autocorrelation function from residuals of statistical models. The corrected threshold corresponds to a single voxel level of p < 0.001 with a minimum cluster size depending on the estimated smoothness and on the number of tested voxels (see results section for details). The corrected threshold is expected to be much lower for the wRBC than for the whole-brain analysis given the marked difference in size of the tested volumes (i.e., the semantic control network vs the whole brain).
Correlations between pathology-associated altered FC and neuropsychological tests were assessed by means of a GLM, controlling for the effect of age, sex, years of formal education and GMV.
RESULTS
Demographic characteristics and neuropsychological assessment of participants
As reported in Table 1, there was no significant age difference between groups. Conversely, there were significant differences in years of formal education, gender distribution, MMSE score and total GMV between patients and controls. As selected by design, AD patients were in the mild stage of the pathology (MMSE = 20.5 ± 3.6, mean ± sd). AD patients performed significantly worse than controls in each administered cognitive test (see Table 2).
Table 1.
Principal demographical and clinical characteristics of studied subjects
| AD | HC | P-value | |
|---|---|---|---|
| N | 38 | 19 | |
| Age (years) | 72.2 ± 7.8 | 68.5 ± 6.8 | 0.089b |
| Education (years) | 9.8 ± 4.4 | 12.1 ± 3.1 | 0.056b |
| Gender (M/F) | 10/28 | 13/6 | 0.002a |
| MMSE score | 20.5 ± 3.6 | 28.8 ± 1.2 | <0.001b |
| Gray matter volume (dl) | 5.39 ± 0.53 | 6.30 ± 0.73 | <0.001b |
Data presented as mean ± SD. AD, Alzheimer’s disease; HC, healthy control; MMSE, Mini Mental State Examination.
The p-value was obtained by chi-square test.
The p-value was obtained by t-test.
Table 2.
Neuropsychological assessment of studied subjects
| Cognitive domain | Neuropsychological test | Mean (SD) scores
|
|
|---|---|---|---|
| AD | HC | ||
| Verbal episodic long-term memory |
15-Words List Immediate recall (cut-off ≥ 28.5) |
15.8 (7.2)* | 39.8 (11.4) |
| Delayed recall (cut-off ≥ 4.6) Short story test |
2.3 (5.5)* | 8.3 (2.3) | |
| Immediate recall (cut-off ≥ 3.1) | 3.3 (6.2) | 6.1 (1.4) | |
| Delayed recall (cut-off ≥ 2.6) | 1.3 (2.0)* | 6.0 (1.3) | |
| Visuo-spatial episodic long-term memory |
Complex Rey’s Figure: Immediate recall (cut-off ≥ 6.4) |
2.1 (2.9)* | 14.3 (6.6) |
| Delayed recall (cut-off ≥ 6.3) | 1.9 (2.7)* | 13.1 (5.9) | |
| Verbal short-term memory | Digit span forward (cut-off ≥ 3.7) | 15.1 (1.9)* | 16.3 (1.3) |
| Visuo-spatial short-term memory | Corsi span forward (cut-off ≥ 3.5) | 3.1 (1.5)* | 4.9 (0.9) |
| Executive functions | Phonological Word Fluency (cut-off ≥ 17.3) | 20.6 (11.0)* | 37.6 (8.7) |
| Modified Card Sorting Test Criteria achieved (cut-off ≥ 4.2) | 1.6 (0.9)* | 5.9 (0.3) | |
| Language | Naming of objects (cut-off ≥ 22) | 24.1 (5.7)* | 29.1 (0.8) |
| Reasoning | Raven’s Coloured Progressive Matrices (cut-off ≥ 18.9) | 18.8 (7.9)* | 30.4 (3.9) |
| Constructional praxis | Copy of drawings (cut-off ≥ 7.1) | 6.3 (3.9)* | 10.6 (1.3) |
| Mini Mental State Examination (cut-off ≥ 61.8) | 50.4 (23.0)* | 69.3 (1.0) | |
| Copy of Complex Rey’s Figure (cut-off ≥ 23.7) | 18.0 (13.1)* | 31.0 (4.3) | |
One-way ANOVA p < 0.05.
Head motion
The two groups did not significantly differ in the amount of head motion during functional scans as assessed by the average FD (p = 0.09; two-sample t-test: t = −1.7, df = 55). The average FD in the two groups was 0.192 ± 0.090 mm and 0.152 ± 0.067 mm for AD and HC, respectively.
Functional connectivity results
Fig. 1 shows that wRBC within the semantic control network was significantly reduced in AD patients compared to healthy subjects (p < 0.05, corrected. 3dClustSim parameters: single-voxel p < 0.001; cluster size threshold depending of residual smoothness, 10 voxels; mask size, ~3.6 · 103 voxels). Two distinct clusters of reduced wRBC were found. One cluster was located in the left posterior middle temporal gyrus (pMTG; MNI coordinates = [−54, −30, −2] mm; t = 4.7; volume = 13 voxels; Brodmann area (BA) 21), the other one was located in the pars opercularis of the left frontal gyrus (POp; MNI coordinates = [−56, 10, 6] mm; t = 4.4; volume = 34 voxels; BA44). No significant interaction effect between group and sex was found (no voxel survived the single-voxel threshold; f-value <12.2).
Fig. 1.
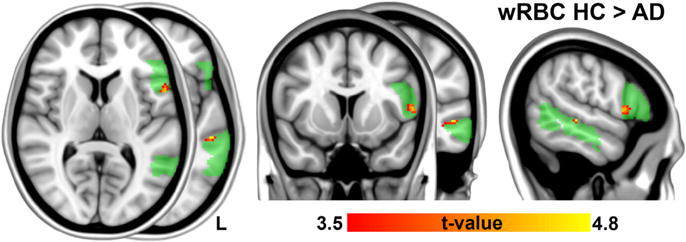
Pathology-associated differences in weighted Regional Brain Connectivity (wRBC). Patients with AD compared to HC revealed a reduction of wRBC within the semantic control network (highlighted in green). Color-coded t-statistic map shows a pattern of significantly reduced voxel-wise connectivity in the left pars opercularis (POp; peak MNIcoord: −56, 10, 8) and in the left posterior middle temporal gyrus (pMTG; peak MNIcoord: −56, −30, −2). The result was obtained via a two-sample, two-tailed t-test (|t| > 3.5, df = 50, p < 0.05, corrected. 3dClustSim parameters: single-voxel p < 0.001; cluster size threshold: 10 voxels; mask size: ~3.6 · 103 voxels). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The FC reductions highlighted by wRBC analysis were further investigated using a seed-based approach to assess possible connectivity abnormalities outside the semantic control network. The pattern of FC between each seed region and the rest of the brain is shown in Fig. 2 for patients and controls (p < 0.05, corrected. 3dClustSim parameters: single-voxel p < 0.001; mask size, ~2.2 · 105 voxels; cluster size threshold depending on residual smoothness, 222 and 104 for POp and pMTG, respectively). In both groups the POp cluster was connected to the orbito-frontal and pre-frontal cortex bilaterally, and to the left parietal cortex. The HC group was also connected to the left temporal cortex. Conversely, when considering the pMTG cluster as seed region, the HC group showed a widespread pattern of connectivity involving bilateral frontal and temporal regions and the left parietal cortex, while AD patients showed a more restricted pattern confined to the bilateral temporal lobes.
Fig. 2.
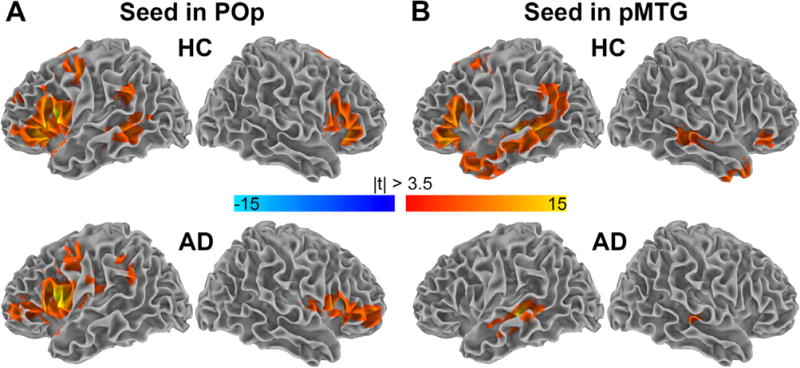
Group-level, seed-based FC from cluster in POp and in pMTG to the rest of the brain. Color-coded t-static maps showing significant group-level, seed-based FC from clusters of altered wRBC (Fig. 1). Panel (A) shows the pattern of FC between the pars opercularis (POp) cluster and the rest of the brain, which was similar in patients with AD and HC, with the only exception of temporal regions which were not significantly connected in the AD group. Panel (B) shows the pattern of FC between the posterior middle temporal gyrus (pMTG) cluster and the rest of the brain. HC revealed a widespread pattern of connectivity in the frontal, parietal and temporal regions. The pattern is largely consistent with the one arising from a region previously implicated in sentence comprehension according to a lesion study (compare B in HC with Fig. 5 in Turken and Dronkers, 2011). In contrast, AD patients showed a more restricted connectivity pattern limited to the temporal lobes. Result were obtained via one-sample, two-tailed t-test (|t| > 3.5, df = 50, p < 0.05, corrected. 3dClustSim parameters: single-voxel p < 0.001; mask size, ~2.2 · 105 voxels; cluster size threshold, 222 and 104 for POp and pMTG, respectively). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
When comparing patients and controls, specific patterns of altered FC were identified for POp and pMTG clusters as shown in Fig. 3A, B, respectively (p < 0.05, corrected as above). Patients showed reduced FC to the POp cluster in brain regions mainly localized within the semantic control network used for wRBC computation, with the only exception of the bilateral superior frontal gyrus. Conversely, regions of reduced FC to the pMTG cluster extended beyond the semantic control network, including several left-lateralized brain regions (i.e., inferior frontal and anterior temporal regions, the superior frontal gyrus and the angular gyrus). The pMTG cluster was also disconnected from the right hemisphere in the temporal pole. In addition, increased connectivity to the pMTG cluster was found within the Wernicke’s area (i.e., the left planum temporale and in the left parietal operculum) of AD patients compared to HC. Detailed cluster information is reported in Table 3. No significant interaction effect between group and sex was found (p > 0.5 and p > 0.2, for POp and pMTG, respectively, corrected as above. f-value <12.2).
Fig. 3.
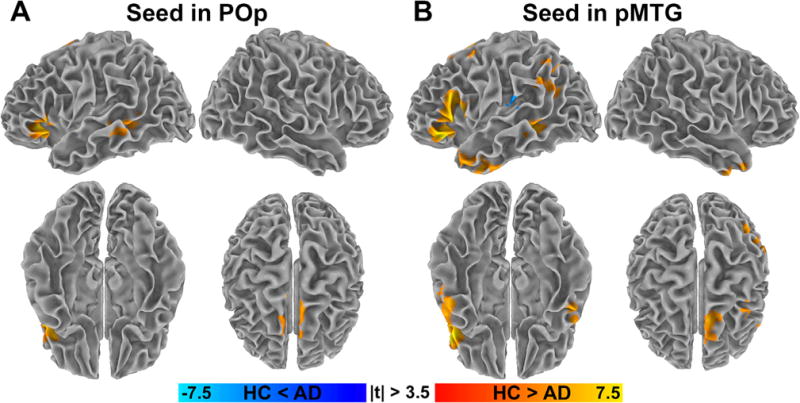
Between-group differences in seed-based FC from cluster in POp and in pMTG to the rest of the brain. Color-coded t-static maps showing significant between-group differences of seed-based FC from clusters of altered wRBC (Fig. 1). (A) Seed located in the POp cluster. (B) Seed located in the pMTG cluster. The majority of the regions showing FC alterations belongs to the verbal-semantic network according to the meta-analysis from Binder et al. (compare B to Fig. 7A in Binder et al., 2009). Result were obtained via two-sample, two-tailed t-test (|t| > 3.5, df = 50, p < 0.05, corrected. 3dClustSim parameters: single-voxel p < 0.001; mask size, around 2.2 · 105 voxels; cluster size threshold, 222 and 104 for POp and pMTG, respectively). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Table 3.
Pathology-associated differences in functional connectivity
| Seed | Brain Regions | Side | Vol (voxels) | MNI coordinates
|
Peak t-value |
||
|---|---|---|---|---|---|---|---|
| X | y | z | |||||
| POp | AD patients < HC | ||||||
| Frontal Orbital cortex, Temporal Pole, Pars Triangularis and Opercularis, Frontal Operculum cortex | L | 1014 | −40 | 26 | −4 | 6.68 | |
| Superior Frontal gyrus, Supplementary motor cortex | B | 617 | 0 | 0 | 70 | 4.77 | |
| Posterior Middle and Superior Temporal gyrus, Temporooccipital Middle temporal gyrus | L | 378 | −62 | −34 | −2 | 5.06 | |
| pMTG | AD patients < HC | ||||||
| Pars Opercularis, Temporal Pole, Pars Triangularis, Frontal Orbital cortex, anterior Inferior and anterior Middle Temporal gyrus | L | 2415 | −48 | 26 | −12 | 7.75 | |
| Superior Frontal gyrus, Supplementary Motor cortex | L | 562 | −12 | 14 | 66 | 5.61 | |
| Posterior and temporooccipital Middle Temporal gyrus | L | 425 | −54 | −42 | −6 | 5.55 | |
| Angular gyrus, posterior Supramarginal gyrus | L | 303 | −48 | −56 | 26 | 4.58 | |
| Temporal Pole, anterior Inferior Temporal gyrus | R | 241 | 50 | 16 | −36 | 5.33 | |
| Superior Lateral Occipital cortex, Angular gyrus, Supramarginal gyrus | L | 184 | −38 | −62 | 44 | 5.01 | |
| Middle Frontal gyrus | L | 111 | −40 | 4 | 60 | 4.76 | |
| AD patients > HC | |||||||
| Planum temporale, Parietal Operculum Cortex, Superior Temporal gyrus. | L | 111 | −64 | −26 | 14 | −4.10 | |
Regions showing significant pathology-associated differences in seed-based functional connectivity with seed in pars opercularis and posterior middle temporal gyrus clusters (two-sample t-tests). Brain regions are sorted relative to their volume contribution inside the cluster. B, bilateral; L, left; R, right.
Finally, there was no significant correlation between FC outcomes and neuropsychological tests (p > 0.05).
DISCUSSION
Using resting-state fMRI, here we aimed at characterizing the brain functional correlates of semantic impairment in AD. We thus focused on identifying functional abnormalities in language-semantic-related regions during the mild stage of the illness. The main result was a reduction of FC in brain areas critically involved in language/semantic processing in AD patients compared to controls.
Altered connectivity within the semantic control network
Based on the neuropsychological-supported hypothesis of early deterioration of the semantic control system in AD (Corbett et al., 2012), we assessed the internal connectivity of the semantic control network adopting a constrained voxel-wise FC metric, namely a modified version of wGBC (Cole et al., 2010) that we refer to as wRBC. Restricting the computation to the semantic control network, we were able to increase the sensitivity of the metric, allowing the detection of even subtle changes in FC.
In agreement with our main hypothesis, we observed pathology-associated reductions of wRBC in the semantic control network (Fig. 1). Degraded connectivity in this network is consistent with the specific pattern of semantic impairment previously reported in the mild stage of the disease (Corbett et al., 2012). Indeed, the poor performance of mild AD patients in semantic tasks has been related to a failure of controlling or shaping semantic knowledge in a task-appropriate fashion, rather than to a degraded conceptual knowledge. The wRBC reductions in the semantic control network were restricted to two small, but highly significant, clusters, one located in the pars opercularis (referred to as POp cluster) and another in the pMTG (referred to as pMTG cluster). The seed-based analysis, utilizing as seed regions the POp and the pMTG clusters, revealed a common pattern of functional disconnection between the two regions inside the semantic control network (Fig. 3). Such mutual disconnection between the POp and the pMTG clusters strongly indicates that the reduction of wRBC was primarily due to a specific fronto-temporal functional disconnection rather than to an overall weakening of the semantic control network. In previous studies on healthy subjects, the importance of the coupling between frontal and temporal regions for semantic control, specifically for lexical/semantic retrieval, has been proven by transcranial magnetic stimulation (TMS). Indeed, TMS applied separately to both regions (i.e., inferior frontal and pMTG) was shown to disrupt executively demanding semantic judgments (Whitney et al., 2011b) and to affect lexical retrieval (Krieger-Redwood and Jefferies, 2014).
Contrary to our secondary hypothesis, we did not observe any significant correlation between wRBC and language neuropsychological tests, including phonological word fluency and object naming (p > 0.05), although AD patients performed significantly worse than HC (Table 2). However, a caveat must be considered regarding the extent to which our neuropsychological tests tap the semantic control system. Although both tests (phonemic fluency and confrontation naming) are designed to involve several facets of the language/semantic cognition, including the semantic control, they might be not sufficiently specific to grasp the exact function subserved by the two FC impaired regions reported here. More focused neuropsychological tests, involving, for example, the effect of cue and miscue in name retrieval to modulate the involvement of the semantic control system, might give further insight in the reported pattern of functional disconnection.
Functional alteration beyond the semantic control network
Consistent with our initial expectations, the two identified regions, POp and pMTG, showed also reduced connectivity beyond the semantic control network, in several other language-semantic-related areas. In particular, when considering the left POp cluster as seed region, AD patients revealed functional disconnection within the left orbito-frontal and superior frontal gyrus (bilaterally), and in the posterior part of the left superior and middle temporal gyrus. In the “dual stream” model of language comprehension and production (Hickok and Poeppel, 2004, 2007), these areas are part of the “dorsal stream”, which is considered as a key structure for the articulatory (motor) representation of the language as well as for the processing of complex syntactic structures. Conversely, when considering the left pMTG cluster as seed region, a more complex pattern of functional disconnection was observed. Indeed, the pMTG showed reduced connectivity in the majority of the regions observed for the POp cluster (i.e., in the dorsal stream) but also in the anterior temporal lobe (bilaterally) and left angular gyrus. These areas belong to the “ventral stream” and are implicated in semantic processing (Hickok and Poeppel, 2004, 2007). Notably, the pattern of abnormal FC arising from the pMTG cluster involved the majority of the regions implicated in verbal-semantic processing according to a meta-analysis of 120 neuroimaging studies (Binder et al., 2009). Moreover, all these brain regions are anatomically connected by several WM tracts, which are traditionally considered to be implicated in language processing (Catani et al., 2005; Dick et al., 2014).
The functional disconnection arising from the cluster in pMTG was found to be more spatially widespread and significant than that arising from the cluster in POp (see Table 3). Moreover, most of the affected regions in the POp connectivity were also compromised in the connectivity arising from the pMTG, while the opposite was not the case. Together, these results suggest that the pMTG plays a key role in generating the patterns of functional disconnection in mild AD. The left pMTG is thought to have a pivotal role in language-semantic processing. A growing body of evidence has supported such notion, including the already cited meta-analyses of functional imaging studies focusing on verbal-semantic processing (Binder et al., 2009), meta-analyses focusing on language comprehension and production (Price, 2010; Indefrey, 2011), studies focusing on the N400 (an event-related potential associated with lexical and semantic processing; for a review see Lau et al., 2008), lesion analyses involved in language comprehension (Hart and Gordon, 1990; Dronkers et al., 2004) and name retrieval (Baldo et al., 2013). For example, Dronkers and colleagues have shown that lesions in the left pMTG produced language comprehension impairments even for the most simple sentences, supporting the notion that the left pMTG holds up the function to tie concepts to their corresponding lexical representations (Dronkers et al., 2004). Remarkably, a later study focusing on the FC of this region in healthy subjects showed a pattern of connectivity largely consistent with the one we reported here in HC (Fig. 2B), indicating the consistency between our pMTG region and the one from the lesion study (Turken and Dronkers, 2011). In this context, it is tempting to argue that the functional disconnection arising from the pMTG here reported might underpin the semantic impairment in AD patients specifically by affecting the conceptually driven access to lexical representations. Lexical selection, the following step in word retrieval after lexical activation, would be affected only consequently. Indeed, the frontal regions, which have been implicated in lexical selection (Lau et al., 2008; Piai et al., 2014), displayed lower connectivity to pMTG in AD patients compared to HC. Of course, such interpretation requires further support from more specific neuropsychological tests and possibly from the integration with other experimental procedures, such as TMS.
We also observed an increase of FC between the pMTG cluster and the left planum temporale and parietal operculum in AD patients. Both these regions fall within the Wernicke’s area. The left planum temporale has been previously implicated in phonological memory, playing an important role in speech perception and production (Buchsbaum and D’Esposito, 2008; Pillay et al., 2014). Indeed, the planum temporale has been found frequently activated across phonological studies and has been proposed at the base of an audio-motor loop for phonological processing (Vigneau et al., 2006, 2011). The reported increase in FC might be interpreted as a compensatory mechanism to overcome an impaired conceptually driven access to the mental lexicon. The increase in FC between a region involved in phonemic processing (i.e., planum temporale) and a region involved in lexical/semantic processing (i.e., pMTG), might explain previous results showing less impaired phonemic fluency than category fluency in AD patients (Henry et al., 2004; Clark et al., 2009). However, we did not find correlations between the increased FC in AD patients and the phonemic fluency nor the confrontation naming test. Nonetheless, in older adults, the GM volume of the left planum temporale has been found to be inversely correlated with reaction time, but not with the total score, on the Boston Naming test (Obler et al., 2010). Thus, it is possible that the increased connectivity might affect reaction times rather than scores. More detailed neuropsychological tests and longitudinal data are needed to clarify the origin of increased connectivity in this region.
POp and pMTG in the progress of AD pathology
The notion that the pMTG, not the POp, might be at the origin of the reported disconnection pattern is supported by the characteristic pathway of neuropathological degeneration across the brain. While the amyloid depositions have been found of limited significance in staging the pathology, the neurofibrillary tangles have shown a characterized distribution pattern across the disease progression (Braak and Braak, 1991; Delacourte et al., 1999). First found in transentorhinal cortex, neurofibrillary degeneration expands in medial temporal lobe and in lateral temporal regions starting from inferior regions until reaching superior regions. Only in later stages, other associative cortices, including the inferior frontal regions, are found to be compromised by neurofibrillary degeneration (Delacourte et al., 1999). Indeed, in post-mortem AD patients GM atrophy was found in tempo-parietal regions, while inferior frontal regions (Broca’s region, specifically BA44, BA45 and 47) were found almost free of atrophy (Harasty et al., 1999). Consistently, AD patients are characterized by fluent speech, not compatible with the fragmented speech distinctive of the dissolution of Broca’s regions (Kempler, 1995).
Limitations of the study
The major limitation of the present study is represented by an insufficient battery of neuropsychological tests for assessing semantic cognition. Indeed, despite the abnormal connectivity inside the semantic control network was in agreement with a previous neuropsychological study (Corbett et al., 2012), we failed to report any significant correlation between the altered connectivity and the performance of AD patients. Future studies, with a detailed assessment of semantic performance, are required to determine the cognitive correlates of the reported abnormal connectivity.
Another caveat regards the a priori definition of the semantic control network where the wRBC was computed. Despite a growing body of evidence has supported the participation of the left inferior frontal and left posterior temporal regions (for a review see, Ralph et al., 2017), the precise boundaries of the network have still to be determined. Although we substantially mitigate the issue adopting a voxelwise FC metric (i.e., wRBC), the inclusion in the mask of regions that do not participate in semantic processing, as well as the exclusion of regions that do participate in the processing, might have lowered the sensitivity of the approach. It is worth mentioning that the seed-based analysis, revealing functional disconnections in mild AD, is not constrained and thus does not suffer from this limitation.
CONCLUSIONS
In the present work, we demonstrated abnormal FC inside the semantic control network of AD patients at the mild stage. This finding is consistent with previous neuropsychological results supporting an impaired control of semantic knowledge underpinning the semantic impairment in the mild stage of the disease. In addition, FC deteriorations extended beyond the semantic control network, involving several regions critically involved in the language-semantic processing. To our knowledge, this is the first fMRI study that specifically assessed modifications of connectivity in brain regions supporting language-semantic processing in AD. Future studies with more detailed neuropsychological assessment are warranted to clarify the precise involvement of the reported regions in the disease.
Acknowledgments
Funding: the present work was supported by the Italian Ministry for Education, University and Research (Ministero dell’Istruzione, dell’Università e della Ricerca, MIUR) under the grant “Progetto premiale NETFUN: NETwork FUNzionali cerebrali studiati con NMR” (Functional brain networks studied by NMR). Research reported in this publication was also supported by Regione Lazio, grant PAMINA (to F.G.) and by the National Institutes of Health, award number R01DK099137 (to S.M.). This project has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skodowska-Curie grant agreement No 691110 (MICROBRADAM). M.D.N. is supported by the European Union’s Horizon 2020 research and innovation programme under the Marie Skodowska-Curie grant agreement No 701635. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding bodies.
Abbreviations
- AD
Alzheimer’s disease
- BOLD
blood-oxygen-level-dependent
- CSF
Cerebral Spinal Fluid
- EPI
echo planar imaging
- FC
functional connectivity
- FD
framewise displacement
- fMRI
functional magnetic resonance imaging
- FWHM
full width at half maximum
- GLM
general linear model
- GM
gray matter
- GMV
gray matter volume
- HC
healthy controls
- MMSE
mean mini-mental state examination
- MNI
Montreal Neurological Institute
- pMTG
posterior middle temporal gyrus
- POp
pars opercularis
- TMS
transcranial magnetic stimulation
- wGBC
weighted Global Brain Connectivity
- WM
White Matter
- wRBC
weighted Regional Brain Connectivity
Footnotes
Author contributions statement: DM, MDN, MB, BM and FG designed research. LS and MB performed experiments. DM and LS analyzed data. DM, MDN, LS, SM, MB and FG interpreted results of experiments. DM wrote the main manuscript text and prepared figures. DM, MDN, LS, SM and FG edited and revised manuscript. All authors reviewed and approved the manuscript.
DISCLOSURE/CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- Association AP. Diagnostic and statistical manual of mental disorders (DSM-5®) American Psychiatric Pub; 2013. [DOI] [PubMed] [Google Scholar]
- Amieva H, Le Goff M, Millet X, Orgogozo JM, Pérès K, Barberger-Gateau P, Jacqmin-Gadda H, Dartigues JF. Prodromal Alzheimer’s disease: successive emergence of the clinical symptoms. Ann Neurol. 2008;64:492–498. doi: 10.1002/ana.21509. [DOI] [PubMed] [Google Scholar]
- Andersson JL, Hutton C, Ashburner J, Turner R, Friston K. Modeling geometric deformations in EPI time series. NeuroImage. 2001;13:903–919. doi: 10.1006/nimg.2001.0746. [DOI] [PubMed] [Google Scholar]
- Baldo JV, Arévalo A, Patterson JP, Dronkers NF. Grey and white matter correlates of picture naming: evidence from a voxel-based lesion analysis of the Boston Naming Test. Cortex. 2013;49:658–667. doi: 10.1016/j.cortex.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayles KA, Tomoeda CK, Kaszniak AW, Trosset MW. Alzheimer’s disease effects on semantic memory: loss of structure or impaired processing? J Cogn Neurosci. 1991;3:166–182. doi: 10.1162/jocn.1991.3.2.166. [DOI] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage. 2007;37:90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex. 2009;19:2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Buchsbaum BR, D’Esposito M. The search for the phonological store: from loop to convolution. J Cogn Neurosci. 2008;20:762–778. doi: 10.1162/jocn.2008.20501. [DOI] [PubMed] [Google Scholar]
- Carlesimo GA, Caltagirone C, Gainotti G. The Mental Deterioration Battery: normative data, diagnostic reliability and qualitative analyses of cognitive impairment. The Group for the Standardization of the Mental Deterioration Battery. Eur Neurol. 1996;36:378–384. doi: 10.1159/000117297. [DOI] [PubMed] [Google Scholar]
- Carlesimo GA, Buccione I, Fadda L, Graceffa A, Mauri M, Lorusso S, Bevilacqua G, Caltagirone C. Standardizzazione di due test di memoria per uso clinico: Breve Racconto e Figura di Rey. Nuova Rivista di Neurologia. 2002;12:1–13. [Google Scholar]
- Catani M, Jones DK, et al. Perisylvian language networks of the human brain. Ann Neurol. 2005;57:8–16. doi: 10.1002/ana.20319. [DOI] [PubMed] [Google Scholar]
- Clark LJ, Gatz M, Zheng L, Chen Y-L, McCleary C, Mack WJ. Longitudinal verbal fluency in normal aging, preclinical, and prevalent Alzheimer’s disease. Am J Alzheimers Dis Other Demen. 2009;24:461–468. doi: 10.1177/1533317509345154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Pathak S, Schneider W. Identifying the brain’s most globally connected regions. NeuroImage. 2010;49:3132–3148. doi: 10.1016/j.neuroimage.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Corbett F, Jefferies E, Burns A, Ralph MAL. Unpicking the semantic impairment in Alzheimer’s disease: qualitative changes with disease severity. Behav Neurol. 2012;25:23–34. doi: 10.3233/BEN-2012-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Damasio H, Tranel D, Grabowski T, Adolphs R, Damasio A. Neural systems behind word and concept retrieval. Cognition. 2004;92:179–229. doi: 10.1016/j.cognition.2002.07.001. [DOI] [PubMed] [Google Scholar]
- Deichmann R, Schwarzbauer C, Turner R. Optimisation of the 3D MDEFT sequence for anatomical brain imaging: technical implications at 1.5 and 3 T. NeuroImage. 2004;21:757–767. doi: 10.1016/j.neuroimage.2003.09.062. [DOI] [PubMed] [Google Scholar]
- Delacourte A, David JP, Sergeant N, Buee L, Wattez A, Vermersch P, Ghozali F, Fallet-Bianco C, Pasquier F, Lebert F, et al. The biochemical pathway of neurofibrillary degeneration in aging and Alzheimer’s disease. Neurology. 1999;52:1158–1158. doi: 10.1212/wnl.52.6.1158. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Dick AS, Bernal B, Tremblay P. The language connectome new pathways, new concepts. The Neuroscientist. 2014;20:453–467. doi: 10.1177/1073858413513502. [DOI] [PubMed] [Google Scholar]
- Dronkers NF, Wilkins DP, Van Valin JRD, Redfern BB, Jaeger JJ. Lesion analysis of the brain areas involved in language comprehension. Cognition. 2004;92:145–177. doi: 10.1016/j.cognition.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Duong A, Whitehead V, Hanratty K, Chertkow H. The nature of lexico-semantic processing deficits in mild cognitive impairment. Neuropsychologia. 2006;44:1928–1935. doi: 10.1016/j.neuropsychologia.2006.01.034. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Garrard P, Lambon Ralph MA, Patterson K, Pratt KH, Hodges JR. Semantic feature knowledge and picture naming in dementia of Alzheimer’s type: a new approach. Brain Lang. 2005;93:79–94. doi: 10.1016/j.bandl.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Hallquist MN, Hwang K, Luna B. The nuisance of nuisance regression: spectral misspecification in a common approach to resting-state fMRI preprocessing reintroduces noise and obscures functional connectivity. NeuroImage. 2013;82:208–225. doi: 10.1016/j.neuroimage.2013.05.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harasty JA, Halliday GM, Kril JJ, Code C. Specific temporoparietal gyral atrophy reflects the pattern of language dissolution in Alzheimer’s disease. Brain. 1999;122(Pt 4):675–686. doi: 10.1093/brain/122.4.675. [DOI] [PubMed] [Google Scholar]
- Hart J, Gordon B. Delineation of single-word semantic comprehension deficits in aphasia, with anatomical correlation. Ann Neurol. 1990;27:226–231. doi: 10.1002/ana.410270303. [DOI] [PubMed] [Google Scholar]
- Henry JD, Crawford JR, Phillips LH. Verbal fluency performance in dementia of the Alzheimer’s type: a meta-analysis. Neuropsychologia. 2004;42:1212–1222. doi: 10.1016/j.neuropsychologia.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. Dorsal and ventral streams: a framework for understanding aspects of the functional anatomy of language. Cognition. 2004;92:67–99. doi: 10.1016/j.cognition.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. The cortical organization of speech processing. Nat Rev Neurosci. 2007;8:393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Salmon DP, Butters N. Semantic memory impairment in Alzheimer’s disease: failure of access or degraded knowledge? Neuropsychologia. 1992;30:301–314. doi: 10.1016/0028-3932(92)90104-t. [DOI] [PubMed] [Google Scholar]
- Indefrey P. The spatial and temporal signatures of word production components: a critical update. Front Psychol. 2011;2:255–255. doi: 10.3389/fpsyg.2011.00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indefrey P, Levelt WJM. The spatial and temporal signatures of word production components. Cognition. 2004;92:101–144. doi: 10.1016/j.cognition.2002.06.001. [DOI] [PubMed] [Google Scholar]
- Jefferies E. The neural basis of semantic cognition: converging evidence from neuropsychology, neuroimaging and TMS. Cortex. 2013;49:611–625. doi: 10.1016/j.cortex.2012.10.008. [DOI] [PubMed] [Google Scholar]
- Jefferies E, Ralph MAL. Semantic impairment in stroke aphasia versus semantic dementia: a case-series comparison. Brain. 2006;129:2132–2147. doi: 10.1093/brain/awl153. [DOI] [PubMed] [Google Scholar]
- Kempler D. Language changes in dementia of the Alzheimer type. Dementia and Communication: Research and Clinical Implications. 1995:98–114. [Google Scholar]
- Kirshner HS. Primary progressive aphasia and Alzheimer’s disease: brief history, recent evidence. Curr Neurol Neurosci Rep. 2012;12:709–714. doi: 10.1007/s11910-012-0307-2. [DOI] [PubMed] [Google Scholar]
- Krieger-Redwood K, Jefferies E. TMS interferes with lexical-semantic retrieval in left inferior frontal gyrus and posterior middle temporal gyrus: Evidence from cyclical picture naming. Neuropsychologia. 2014;64C:24–32. doi: 10.1016/j.neuropsychologia.2014.09.014. [DOI] [PubMed] [Google Scholar]
- Lau EF, Phillips C, Poeppel D. A cortical network for semantics:(de) constructing the N400. Nat Rev Neurosci. 2008;9:920–933. doi: 10.1038/nrn2532. [DOI] [PubMed] [Google Scholar]
- Lin C-Y, Chen T-B, Lin K-N, Yeh Y-C, Chen W-T, Wang K-S, Wang P-N. Confrontation naming errors in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2014;37:86–94. doi: 10.1159/000354359. [DOI] [PubMed] [Google Scholar]
- Magni E, Binetti G, Padovani A, Cappa SF, Bianchetti A, Trabucchi M. The Mini-Mental State Examination in Alzheimer’s disease and multi-infarct dementia. Int Psychogeriatr. 1996;8:127–134. doi: 10.1017/s1041610296002529. [DOI] [PubMed] [Google Scholar]
- Martin A. The representation of object concepts in the brain. Annu Rev Psychol. 2007;58:25–45. doi: 10.1146/annurev.psych.57.102904.190143. [DOI] [PubMed] [Google Scholar]
- Mascali D, DiNuzzo M, Gili T, Moraschi M, Fratini M, Maraviglia B, Serra L, Bozzali M, Giove F. Intrinsic patterns of coupling between correlation and amplitude of low-frequency fMRI fluctuations are disrupted in degenerative dementia mainly due to functional disconnection. PLoS ONE. 2015;10:e0120988–e0120988. doi: 10.1371/journal.pone.0120988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack JCR, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melrose RJ, Campa OM, Harwood DG, Osato S, Mandelkern MA, Sultzer DL. The neural correlates of naming and fluency deficits in Alzheimer’s disease: an FDG-PET study. Int J Geriatr Psychiatry. 2009;24:885–893. doi: 10.1002/gps.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miceli G. Batteria per l’analisi dei deficit afasici BADA: Servizio di neuropsicologia. Cuore: Università cattolica del S; 1994. [Google Scholar]
- Mickes L, Wixted JT, Fennema-Notestine C, Galasko D, Bondi MW, Thal LJ, Salmon DP. Progressive impairment on neuropsychological tasks in a longitudinal study of preclinical Alzheimer’s disease. Neuropsychology. 2007;21:696–705. doi: 10.1037/0894-4105.21.6.696. [DOI] [PubMed] [Google Scholar]
- Monaco M, Costa A, Caltagirone C, Carlesimo GA. Forward and backward span for verbal and visuo-spatial data: standardization and normative data from an Italian adult population. Neurol Sci. 2013;34:749–754. doi: 10.1007/s10072-012-1130-x. [DOI] [PubMed] [Google Scholar]
- Nebes RD, Halligan EM. Sentence context influences the interpretation of word meaning by Alzheimer patients. Brain Lang. 1996;54:233–245. doi: 10.1006/brln.1996.0073. [DOI] [PubMed] [Google Scholar]
- Nelissen N, Vandenbulcke M, Fannes K, Verbruggen A, Peeters R, Dupont P, Van Laere K, Bormans G, Vandenberghe R. Abeta amyloid deposition in the language system and how the brain responds. Brain. 2007;130:2055–2069. doi: 10.1093/brain/awm133. [DOI] [PubMed] [Google Scholar]
- Nicholas M, Obler LK, Albert ML, Helm-Estabrooks N. Empty speech in Alzheimer’s disease and fluent aphasia. J Speech Hear Res. 1985;28:405–410. doi: 10.1044/jshr.2803.405. [DOI] [PubMed] [Google Scholar]
- Nocentini U, Di Vincenzo S, Panella M, Pasqualetti P, Caltagirone C. La valutazione delle funzioni esecutive nella pratica neuropsicologica: dal Modified Card Sorting Test al Modified Card Sorting Test: Roma Version. Dati di standardizzazione. Nuova Rivista di Neurologia. 2002;12:14–24. [Google Scholar]
- Noonan KA, Jefferies E, Corbett F, Lambon Ralph MA. Elucidating the nature of deregulated semantic cognition in semantic aphasia: evidence for the roles of prefrontal and temporo-parietal cortices. J Cogn Neurosci. 2010;22:1597–1613. doi: 10.1162/jocn.2009.21289. [DOI] [PubMed] [Google Scholar]
- Noonan KA, Jefferies E, Visser M, Lambon Ralph MA. Going beyond inferior prefrontal involvement in semantic control: evidence for the additional contribution of dorsal angular gyrus and posterior middle temporal cortex. J Cogn Neurosci. 2013;25:1824–1850. doi: 10.1162/jocn_a_00442. [DOI] [PubMed] [Google Scholar]
- Obler LK, Rykhlevskaia E, Schnyer D, Clark-Cotton MR, Spiro rA, Hyun J, Kim DS, Goral M, Albert ML. Bilateral brain regions associated with naming in older adults. Brain Lang. 2010;113:113–123. doi: 10.1016/j.bandl.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson K, Nestor PJ, Rogers TT. Where do you know what you know? The representation of semantic knowledge in the human brain. Nat Rev Neurosci. 2007;8:976–987. doi: 10.1038/nrn2277. [DOI] [PubMed] [Google Scholar]
- Piai V, Roelofs A, Jensen O, Schoffelen J-M, Bonnefond M. Distinct patterns of brain activity characterise lexical activation and competition in spoken word production. PLoS ONE. 2014;9:e88674–e88674. doi: 10.1371/journal.pone.0088674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillay SB, Stengel BC, Humphries C, Book DS, Binder JR. Cerebral localization of impaired phonological retrieval during rhyme judgment. Ann Neurol. 2014;76:738–746. doi: 10.1002/ana.24266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ. The anatomy of language: a review of 100 fMRI studies published in 2009. Ann N Y Acad Sci. 2010;1191:62–88. doi: 10.1111/j.1749-6632.2010.05444.x. [DOI] [PubMed] [Google Scholar]
- Ralph MAL, Jefferies E, Patterson K, Rogers TT. The neural and computational bases of semantic cognition. Nat Rev Neurosci. 2017;18:42–55. doi: 10.1038/nrn.2016.150. [DOI] [PubMed] [Google Scholar]
- Serra L, Cercignani M, Lenzi D, Perri R, Fadda L, Caltagirone C, Macaluso E, Bozzali M. Grey and white matter changes at different stages of Alzheimer’s disease. J Alzheimers Dis. 2010;19:147–159. doi: 10.3233/JAD-2010-1223. [DOI] [PubMed] [Google Scholar]
- Shaywitz BA, Shaywitz SE, Pugh KR, Constable RT, et al. Sex differences in the functional organization of the brain for language. Nature. 1995;373:607–607. doi: 10.1038/373607a0. [DOI] [PubMed] [Google Scholar]
- Taler V, Phillips NA. Language performance in Alzheimer’s disease and mild cognitive impairment: a comparative review. J Clin Exp Neuropsychol. 2008;30:501–556. doi: 10.1080/13803390701550128. [DOI] [PubMed] [Google Scholar]
- Teipel SJ, Willoch F, Ishii K, Bürger K, Drzezga A, Engel R, Bartenstein P, Möller HJ, Schwaiger M, Hampel H. Resting state glucose utilization and the CERAD cognitive battery in patients with Alzheimer’s disease. Neurobiol Aging. 2006;27:681–690. doi: 10.1016/j.neurobiolaging.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Tulving E. Multiple memory systems and consciousness. Hum Neurobiol. 1987;6:67–80. [PubMed] [Google Scholar]
- Turken U, Dronkers NF. The neural architecture of the language comprehension network: converging evidence from lesion and connectivity analyses. Front Syst Neurosci. 2011;5:1–1. doi: 10.3389/fnsys.2011.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneau M, Beaucousin V, Hervé PY, Duffau H, Crivello F, Houdé O, Mazoyer B, Tzourio-Mazoyer N. Meta-analyzing left hemisphere language areas: phonology, semantics, and sentence processing. NeuroImage. 2006;30:1414–1432. doi: 10.1016/j.neuroimage.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Vigneau M, Beaucousin V, Hervé P-Y, Jobard G, Petit L, Crivello F, Mellet E, Zago L, Mazoyer B, Tzourio-Mazoyer N. What is right-hemisphere contribution to phonological, lexico-semantic, and sentence processing?: Insights from a meta-analysis. NeuroImage. 2011;54:577–593. doi: 10.1016/j.neuroimage.2010.07.036. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2:125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- Whitney C, Jefferies E, Kircher T. Heterogeneity of the left temporal lobe in semantic representation and control: priming multiple versus single meanings of ambiguous words. Cereb Cortex. 2011a;21:831–844. doi: 10.1093/cercor/bhq148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney C, Kirk M, O’Sullivan J, Lambon Ralph MA, Jefferies E. The neural organization of semantic control: TMS evidence for a distributed network in left inferior frontal and posterior middle temporal gyrus. Cereb Cortex. 2011b;21:1066–1075. doi: 10.1093/cercor/bhq180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn R, Juengling F, Bubrowski P, Jost E, Dykierek P, Talazko J, Huell M. Hemispheric asymmetries of hypometabolism associated with semantic memory impairment in Alzheimer’s disease: a study using positron emission tomography with fluorodeoxyglucose-F18. Psychiatry Res. 2004;132:159–172. doi: 10.1016/j.pscychresns.2004.07.006. [DOI] [PubMed] [Google Scholar]


