Abstract
Background
Islet transplantation is an effective therapy for selected patients with type 1 diabetes with labile glycemic control and hypoglycemic unawareness, but donor organs are limited. Islet xenotransplantation using porcine islets will potentially solve this problem. Although successful proof of concept studies using clinically-inapplicable anti-CD154 monoclonal antibody (mAb) in pig-to-non-human primate (NHP) islet xenotransplantation have been demonstrated by several groups worldwide, potentially clinically-applicable anti-CD40 (2C10R4) mAb-based studies have not been reported.
Methods
Nine streptozotocin (STZ)-induced diabetic rhesus monkeys were transplanted with adult porcine islets isolated from designated-pathogen free (DPF) miniature pigs. They were treated with anti-CD40 mAb-based immunosuppressive regimen and were divided into 3 groups: anti-CD40 only group (n=2), belatacept group (anti-CD40 mAb+belatacept, n=2), and tacrolimus group (anti-CD40 mAb+tacrolimus, n=5). All monkeys received anti-thymocyte globulin (ATG), cobra venom factor (CVF), adalimumab, and sirolimus. Blood glucose levels (BGL) and serum porcine C-peptide concentrations were measured. Humoral and cellular immune responses were assessed by ELISA and ELISPOT, respectively. Liver biopsy and subsequent immunohistochemistry were conducted.
Results
All animals restored normoglycemia immediately after porcine islet transplantation and finished the follow-up without any severe adverse effects except for one animal (R092). Most animals maintained their body weight. Median survival, as defined by a serum porcine C-peptide concentration of >0.15ng/ml, were 31, 27 and 60 days for anti-CD40 only, belatacept, and tacrolimus groups, respectively. Anti-αGal IgG levels in serum and the number of interferon-γ secreting T cells in peripheral blood mononuclear cells did not increase in most animals.
Conclusion
These results showed that anti-CD40 mAb combined with tacrolimus was effective in prolonging porcine islet graft survival, but anti-CD40 mAb was not as effective as anti-CD154 mAb in terms of preventing early islet loss.
Introduction
Pancreatic islet transplantation, rather than any advanced medical treatment including closed-loop insulin pump (1), has been recently demonstrated to be a more effective therapy for patients who suffer from severe hypoglycemic unawareness. However, a shortage of deceased human pancreata restricts this promising therapy to only a handful of patients. Xenotransplantation using porcine islets could potentially solve this problem.
Several research groups, including our own, have demonstrated that porcine islets can restore and maintain normoglycemia in diabetic NHPs for >6 months using an anti-CD154 mAb-based immunosuppressive regimen (2). Because this mAb cannot be used in the clinics due to its thromboembolic complications (3), an anti-CD154 mAb-sparing immunosuppressive regimen is urgently needed. Although two groups independently demonstrated the prolonged survival of porcine embryonic pancreatic primordia or porcine islets using an immunosuppressive regimen that did not include anti-CD154 mAb (4, 5), the FTY720 used in the former study has been reported to be ineffective in organ transplantation (6) and anti-CD40 mAb (Chi220) in the latter study had a significant depleting effect on B cells, thus limiting its use as immunosuppression maintenance drug.
Anti-CD40 mAb (2C10R4) is a chimeric form of mouse Fab and rhesus IgG4 Fc fragments, and its major action mechanism is to block CD40-CD154 molecular interaction (7). Importantly, recent studies demonstrated that this mAb was effective in preventing rejection of porcine organs, including the heart and kidney, in NHPs (8, 9). However, pig-to-NHP islet xenotransplantation using a 2C10R4-based immunosuppressive regimen has not been reported yet. Here, we present pre-clinical results of pig-to-NHP islet xenotransplantation using this mAb-based immunosuppressive regimen.
Materials and Methods
Animals
A total of 9 rhesus monkeys (Macaca mulatta), 4–12 years of age, were used in this study. After being imported from China, the quarantine process of 1 month concluded that all subjects were in good general condition. Then, the monkeys were acclimated to our animal facility at least for 6 months. The monkeys were maintained in single-housed cages and had daily access to food (2050 Teklad Global 20% Protein Primate Diet, Harlan Laboratories; fresh fruit, local market) and unlimited access to water. The room maintained 24 ± 4°C and a relative humidity of 50 ± 10%, with an artificial light-dark cycle of 12:12 (7:00 AM onset) and with 13–18 air changes per hour. R092 died on day 59 after islet transplantation because of severe thrombocytopenia and anemia. All procedures that affected handling and care of animals were in compliance with the guidelines set forth in the Guide for the Care and Use of Laboratory Animals prepared by the Institute of Laboratory Animal Resources and published by the National Institutes of Health (NIH Publication No. 86-23, revised 2011), and approved by the Seoul National University Institutional Animal Care and Use Committee (IACUC no. 15-0297-S1A0). The islet donor pigs (Seoul National University [SNU] miniature pigs) were bred and maintained in a barrier-sustained and specific pathogen-free facility. Among 17 SNU miniature pigs in this study, 9 were female (sow, n=4; gilt, n=5). The median age of 17 pigs was 38.47 months (range, 24 to 69).
Porcine islet isolation
Total pancreatectomies from SNU miniature pigs were performed under sterile conditions. The surgical procedure was performed identically throughout the study period and is described elsewhere (10, 11). After transportation, islet isolation was performed using the modified Ricordi method that has been previously described (11). The harvested pancreas was distended intraductally using a preservation solution containing CIzyme™ collagenase and CIzyme™ BP protease (VitaCyte, Indianapolis, IN, USA).
Islet quality measurement
The final islet quality was measured by dithizone staining, FACS analysis, and mouse bioassay as described in the previous report (12). The results showed that they were >90% pure, >75% viable and had >60% diabetes correction capacity in marginal islet mass transplantation in diabetic NOD/SCID mice.
Induction of diabetes in NHPs
Monkeys were fasted overnight and were pre-hydrated with normal saline (0.9% NaCl, 40–60 ml/kg/day i.v.) via a tether system for 12 h before STZ (Sigma Aldrich, St. Louis, MO, USA) administration to reduce adverse nephrotoxic effects. Butorphanol or metoclopramide was also administered to prevent vomiting caused by STZ. A high dose of STZ (110 mg/kg) was diluted with 10 ml of normal saline and given i.v. within 5 min. Complete diabetes induction was confirmed by persistent hyperglycemia (>300 mg/dl), <1 ng/ml of fasting C-peptide levels and absence of C-peptide responses in IVGTT and AST as previously reported (13).
Islet transplantation into NHPs
All monkeys were fasted for 12 h before surgery. A laparotomy was performed, and the jejunal arch was exposed to infuse the islets. A 24- or 22-gauge catheter was inserted through the jejunal vein and advanced towards the portal vein. The porcine islets were infused under gravity pressure over 8–12 min. After infusion, the vessel was ligated with a 5-0 Prolene suture. Ketamine (50 μg/kg/min) and lidocaine (0.6 mg/kg/hr) were continuously infused for 3 days. Meloxicam (0.1 mg/kg, i.v. SID) was injected for 3 days and butorphanol (0.05 mg/kg, i.v.) was injected when necessary for post-operative analgesic regimen. After surgery, the tether system was applied for continuous fluid therapy and infusion of low-dose dextrose, if necessary.
Immunosuppressive therapy
Immunosuppression was induced with anti-CD40 mAb (2C10R4, NIH NHP Reagent Resource), sirolimus (Rapamune®, Wyeth), and ATG (Thymoglobulin®, Genzyme) (Table 1). Anti-CD40 mAb (20∼50 mg/kg) was infused i.v. on days -4, 0, 4, 7, 10, 14 of the transplantation, weekly for three months, and biweekly thereafter. Sirolimus was orally administered daily to achieve stable trough levels (3∼8 ng/ml). ATG (5 mg/ml) was administered i.v. on days -3 and -1. Cobra venom factor (CVF) (100 U/kg, Quidel, San Diego, CA) was administered i.v. on day -1 of the transplant to deplete the complement. TNF-α neutralizing mAb, adalimumab (Humira®, Abbott Laboratories, Queensborough, UK) was administered subcutaneously 2∼3 h before islet infusion at a dose of 5 mg/kg. Belatacept (20 mg/kg, Nulojix®, Bristol-Myers Squibb Korea, Seoul, Korea) was i.v. administered at days -2, 0, 3, 7, and then weekly. Tacrolimus (Advagraf®, Astellas Pharma Korea, Seoul, Korea) was orally administered daily from day -3 up to 56 (R92; 56, R015; 22, R087; 25, R008; 15, R131; 18) to achieve stable trough levels (3∼6 ng/ml). The target trough levels were sustained by adjusting dosages of tacrolimus (0.5-2.5 mg/kg/day) and sirolimus (0.08-0.3 mg/kg/day) after confirming serum concentration by liquid chromatography-tandem mass spectrometry.
Table 1. Immunosuppressive regimens and graft outcomes in the three experimental groups.
| Group | Monkey ID and Sex | Common treatment (ATG, CVF, Adalimumab, Sirolimus/methylprednisolone) | Body weight (kg) | Transplanted Islet mass (IEQ/kg) | Number of pigs used | Insulin independence day | Graft survival# |
|---|---|---|---|---|---|---|---|
| Anti-CD40 only group | R086, F | Anti-CD40 mAb | 5.3 | 100,000 | 1 | 4 | 20 |
| R088, F | 5.4 | 77,490 | 1 | 5 | 41 | ||
|
| |||||||
| Belatacept group | R091, F | Anti-CD40 mAb, Belatacept | 5.3 | 100,000 | 1 | 25 | 41 |
| R095, M | 4.8 | 100,000 | 2 | 8 | 13 | ||
|
| |||||||
| Tacrolimus group | R092, M | Anti-CD40 mAb, Tacrolimus | 6.2 | 100,000 | 3 | >60* | >60* |
| R015, F | 6.3 | 100,000 | 2 | 266 | >320 | ||
| R087, F | 5.6 | 100,000 | 2 | 10 | 39 | ||
| R008, F | 4.7 | 87,230 | 2 | 14 | >92 | ||
| R131, F | 5.3 | 100,000 | 3 | 3 | >50 | ||
R092 expired on day 60 after transplantation by severe thrombocytopenia and anemia.
Graft survival day; this was defined as the day on which the serum porcine C-peptide fell <0.15 ng/ml, as measured by ELISA.
C-peptide measurement
For measuring serum C-peptide concentrations, blood samples were collected in a serum separating tube. Blood samples were centrifuged at 3,000 rpm for 20 min at 4°C, and the separated serum was stored frozen at -80 °C until further use. Monkey and porcine serum C-peptide concentrations were determined by an immunoradiometric assay (Insulin IRMA Kit, Diasource, Belgium) and porcine C-peptide ELISA assay kits (Mercodia, Uppsala, Sweden) according to the manufacturer's instructions.
Biopsy and Immunohistochemistry
When the fasting BGL increased to 200 mg/dl after porcine islet transplantation, liver biopsy was performed in all animals except R086. Under general anesthesia, the monkey was placed in the supine position. The abdominal wall was incised from the xyphoid process to the umbilicus. The margin of the central lobe of the liver was gently grasped and excised about 10 mm distal to the margin (wedge biopsy, 1×1 cm). Hemorrhage from the biopsy site was controlled with electrocautery and absorbable hemostat (SURGICEL®, Ethicon Inc., NJ, USA). Routine abdominal wall closure was performed. Meloxicam (0.1 mg/kg, SID) was injected for 3 days for post-operative analgesia. Physical activity and food consumption returned to normal within 3 days in all cases. Biopsy samples from the liver were fixed with 10% neutral buffered formalin and then embedded in paraffin. Embedded tissues were sectioned (4 μm) using a microtome. Sections were immunostained, as described previously (12).
Enzyme-linked immunosorbent spot (ELISPOT) assay
ELISPOT analysis was performed by the method previously described (14). Briefly, the frequencies of interferon (IFN) γ-secreting antigen-specific T cells in the peripheral blood of NHPs were measured using an ELISPOT kit (Mabtech, Nacka Strand, Sweden). The resulting spots were enumerated by a computer-assisted ELISPOT Reader System (AID, Strassberg, Germany).
Measurement of anti-αGal IgG levels
The plasma concentrations of anti-αGal IgG were determined weekly using an enzyme-linked immunosorbent assay (ELISA) as previously described (15).
Statistical analysis
The statistical software GRAPHPAD PRISM5 (GraphPad Software, La Jolla, CA) was used for log-rank Mantel Cox test.
Results
Overview
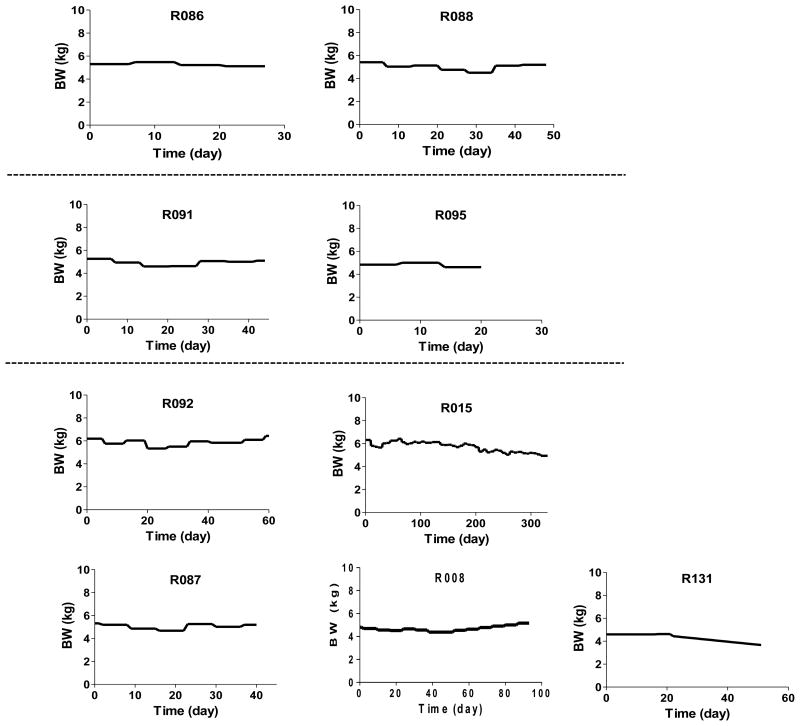
In this study, 9 rhesus monkeys were used as porcine islet recipients. They were divided into three experimental groups based on the immunosuppressive regimen used (Table 1). Each animal was rendered diabetic by STZ and was transplanted with median 96,080 (range; 77,490∼100,000) IEQ/kg of porcine islets isolated from 1∼3 DPF SNU pigs depending on the islet yield. All animals reestablished normoglycemia immediately after porcine islet transplantation and finished the follow-up without severe adverse effects except for R092. Importantly, most animals maintained the body weight throughout the study period (Figure 1).
Figure 1. Changes in body weight of all animals.
Body weight (BW) was weekly measured during the follow-up period.
The regimen harnessing high dose of anti-CD40 mAb combined with Tacrolimus was effective in prolongation of graft survival
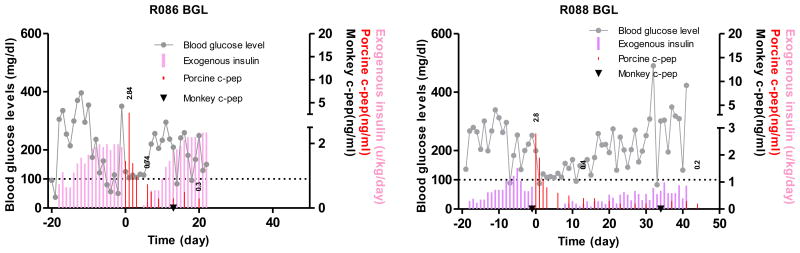
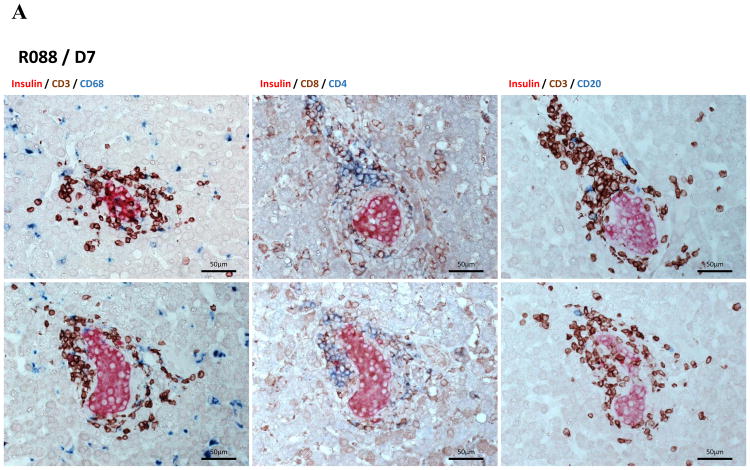
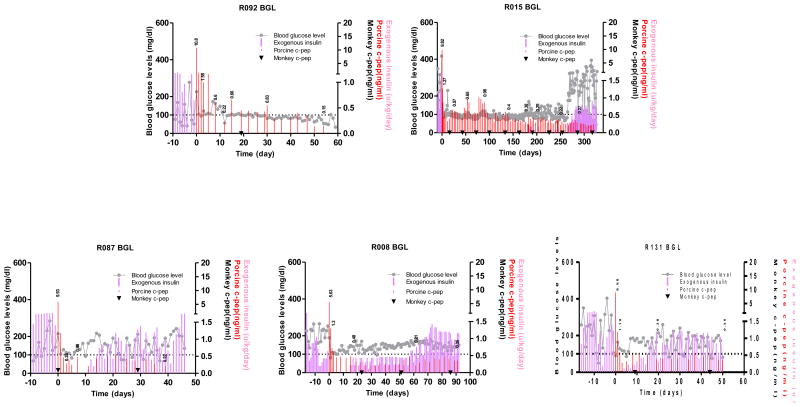
Because we have shown that anti-CD154 mAb-based immunosuppression could lead to long-term porcine islet graft survivals in a reproducible manner, the first step was to simply replace anti-CD154 mAb (20 mg/kg) with similar doses of anti-CD40 mAb while all other immunosuppressants (ATG, CVF, adalimumab, sirolimus) were administered in the same way to that in our previous report (12). For R086, 20 mg/kg of anti-CD40 was administered. Upon islet transplantation, normoglycemia was restored promptly and maintained for 5 days when the fasting BGL rose to >180 mg/dl (Figure 2). For R088, the dosage of anti-CD40 was raised to 30 mg/kg and the fasting BGL was maintained between 80 and 150 mg/dl for 2 weeks with less than 0.3 U/kg/day of exogenous insulin injection (Figure 2). Immunohistochemical staining of biopsied liver on 7 days after transplantation showed infiltration of CD4+ and CD8+ T cells mostly in peri-islet space and some inside the islets (Figure 3). These data suggested that anti-CD40 was effective in delaying the graft rejection and the regimen could be improved by the addition of controlling T cell activation either through increasing the dosage of anti-CD40 or add another co-stimulation blockade.
Figure 2. Blood glucose control by the transplanted porcine islets using anti-CD40 mAb-based immunosuppression.
Fasting BGL and porcine and monkey C-peptide were measured in two monkeys that were intraportally transplanted with adult porcine islets. R086 and R088 monkeys were insulin-independent for 4 and 5 days, respectively. Grey line: fasting BGL, red bar: porcine C-peptide, filled inverted triangle (▼): monkey C-peptide, pink bar: exogenous insulin. The values above red bar are porcine C-peptide.
Figure 3. Immunohistochemical staining of liver biopsies.
Liver was biopsied from R088 on 7 days after transplantation. Liver samples were analysed by immunohistochemical staining using anti-insulin, CD3, CD4, CD8, CD20, CD68 antibodies with appropriate secondary antibodies to reveal porcine β-cells, T cells, CD4+ T cells, CD8+ T cells, B cells, and macrophages, respectively. Some islets showed well-preserved architecture with peripheral T cells and some macrophages, but most islets were destroyed by T cells.
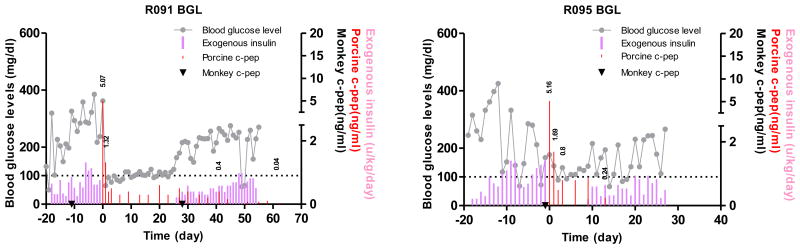
Instead of increasing the dosage of anti-CD40, belatacept, a higher affinity version of CTLA-4Ig, shown to be effective in preventing the immune response when combined with anti-CD40 mAb in murine and NHP models (16, 17), was added to the anti-CD40 mAb-based regimen. The porcine islet grafts in two recipients (R091, and R095) survived 41 and 13 days after the islet transplantation and they maintained insulin-independence for 25 and 8 days, respectively (Figure 4). These results demonstrated that the addition of a second co-stimulation blockade agent, belatacept, had only marginal effect on porcine islet graft prolongation.
Figure 4. Blood glucose control by the transplanted porcine islets using anti-CD40 mAb+belatacept-based immunosuppression.
Fasting BGL and porcine and monkey C-peptide were measured in two monkeys that were intraportally transplanted with adult porcine islets. R091 and R095 monkeys were insulin-independent for 25 and 8 days, respectively. Grey line: fasting BGL, red bar: porcine C-peptide, filled inverted triangle (▼): monkey C-peptide, pink bar: exogenous insulin. The values above red bar are porcine C-peptide.
Because the most prominent cell type found around the porcine islets was the T cells, a calcineurin inhibitor (CNI) tacrolimus was incorporated in replacement of belatacept and the dosage of anti-CD40 was raised to 50 mg/kg. One out of five animals (R015) maintained normoglycemia for over 266 days after transplantation by now. R092 maintained normoglycemia for 60 days but it was expired by severe thrombocytopenia and anemia which might be associated with immunosuppression and subsequent viral reactivation. The monitoring of the Rhesus CMV in peripheral blood by RT-PCR and Ab titer as shown in Supplementary Table 1 implied that the copy number did not seem to be high enough to cause severe clinical symptoms. For this reason, although the transplantation and subsequent immunosuppression could not be ruled out as potential cause of thrombocytopenia and anemia, this animal was included in data analysis. The other three animals (R087, R008, and R131) lost insulin-independence on 10, 14, and 3 days, respectively (Figure 5). Importantly, last three animals (R087, R008, and R131) showed partial porcine islet graft function as defined by porcine C-peptide concentration of >0.15 ng/ml in the serum for 39, >92, >50 days after transplantation, respectively, suggesting that the CNI incorporation and increase of the dosage of anti-CD40 mAb are effective for the prolongation of islet graft survival.
Figure 5. Blood glucose control by the transplanted porcine islets using anti-CD40 mAb+tacrolimus-based immunosuppression.
Fasting BGL and porcine and monkey C-peptide were measured in five monkeys that were intraportally transplanted with adult porcine islets. R092, R015, R087, R008, and R131 monkeys were insulin-independent for 60, 266, 10, 14, and 3 days, respectively. Grey line: fasting BGL, red bar: porcine C-peptide, filled inverted triangle (▼): monkey C-peptide, pink bar: exogenous insulin. The values above red bar are porcine C-peptide.
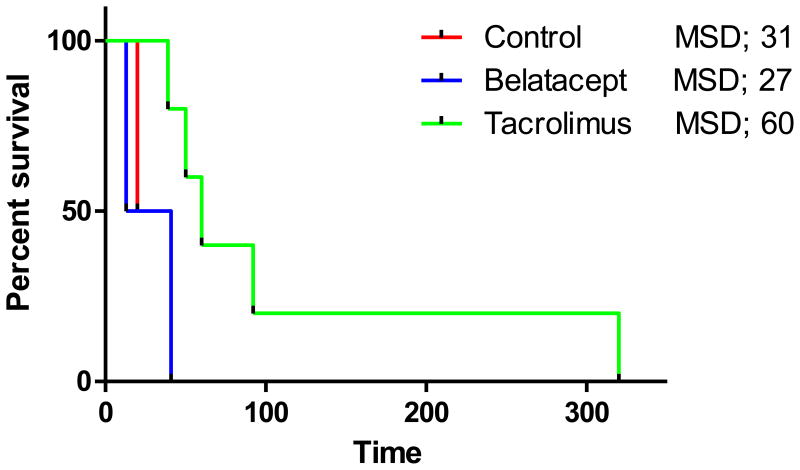
Finally, taken together in the 9 animals, based on graft survival, but not insulin-independence, 50 mg/kg of anti-CD40 mAb combined with tacrolimus prolonged porcine islet graft survival from median survival day (MSD) of 31 in anti-CD40 only group to a MSD 60 in the tacrolimus group, though the difference did not reach statistical significance (log-rank Mantel-Cox test, p=0.09) (Figure 6).
Figure 6. Kaplan-Meir survival curves for the three experimental groups.
Common treatment of the three groups included ATG, CVF, adalimumab, and sirolimus. Control, Belatacept, and Tacrolimus groups were administered with anti-CD40 mAb, anti-CD40 mAb+belatacept, and anti-CD40 mAb+tacrolimus, respectively. Comparisons of median survival day (MSD) were analysed by log-rank Mantel-Cox test.
Control of humoral and cellular immune responses
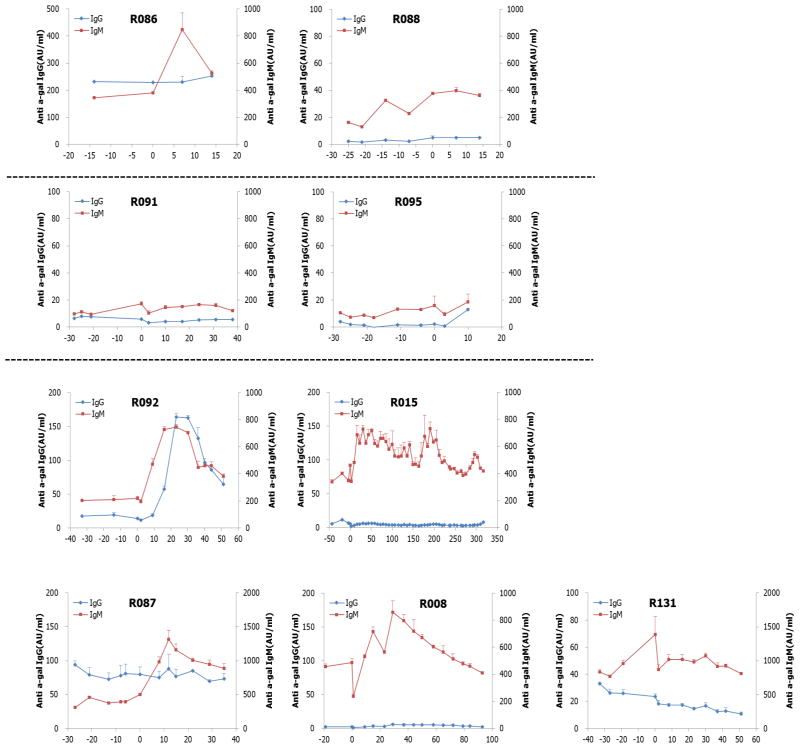
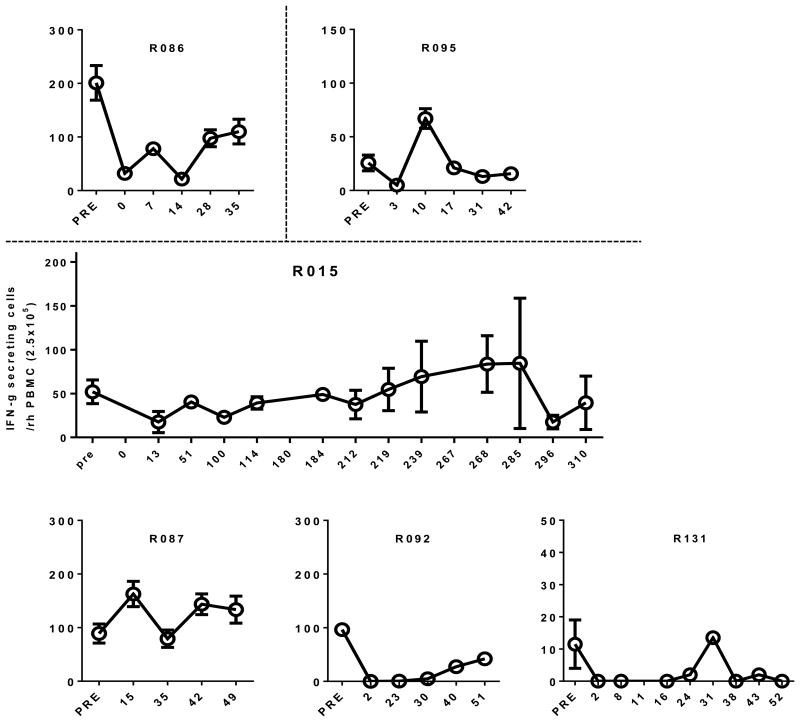
In addition to monitoring of BGL and histology of the biopsied liver, humoral and cellular responses to porcine antigens were examined by ELISA and ELISPOT, respectively, in all recipient animals. Importantly, anti-αGal IgG levels were controlled within the range equal or lower to the extent of pre-transplant period in most animals except R092 whereas anti-αGal IgM levels varied depending on the individual animal (Figure 7). Also, the number of IFN γ-secreting cells among peripheral blood mononuclear cells (PBMC) against porcine antigen (splenocytes) was not significantly increased in most animals. For example, the number was 54±11.7 cells in pre-transplant period, but 31±12.0 during graft-survival period (up to 180 days after transplantation) in one representative animal (R015) (p<0.05, Figure 8). These results suggested that anti-CD40 mAb was effective for controlling humoral and cellular immune responses to porcine antigen.
Figure 7. Humoral immune responses after transplantation.
The levels of anti-αGal IgG and IgM in serum from all animals were measured by ELISA. They remained equal or lower than those in pre-transplant period in most animals.
Figure 8. Cellular immune responses after transplantation.
The number of IFN γ-secreting cells in PBMC was measured by ELISPOT analysis in 6 animals. The number of these cells in graft survival period remained significantly lower than that in pre-transplant period, but it gradually increased as the BGL deteriorated with time.
Discussion
In this study, following pig-to-NHP islet xenotransplantation, anti-CD40 mAb prolonged the graft survival. The addition of tacrolimus delayed graft rejection. Although one monkey out of 5 rhesus monkeys in the tacrolimus group showed long-term porcine islet graft survival (>6 months, >320 d after transplantation in R015), we were unable to obtain consistent and reproducible results with any of the immunosuppressive regimens compared with the anti-CD154 mAb-based regimen in our previous report (12). In some cases, the graft rejection within 1 week after transplantation occurred.
Several research groups recently reported that successful long-term survival of the porcine organs in NHPs such as heterotopic heart in baboon and kidney and cornea in rhesus monkeys using the same anti-CD40 mAb (7, 8). It is curious that the outcome of our study was different from those of other solid organ xenotransplantation studies. We think that the success of porcine islets in NHPs depends on overcoming the initial instant blood-mediated inflammatory reaction (IBMIR) and subsequent strong T cell responses to the porcine islets (18, 19). Although tacrolimus in addition to anti-CD40 antibody was effective for prolonging survival of the porcine islets, it could not achieve long term insulin independence as had been shown with anti-CD154 antibody-based regimen in our previous study (12). To assess the islet loss in early period after transplantation, we measured the porcine C-peptide release within 4 hrs and the ratio of porcine C-peptide to fasting blood glucose (Cp/G ratio) during early period after transplantation and compared them between anti-CD154 mAb- and anti-CD40 mAb-based regimen groups. As shown in Supplementary Figure 1, the difference in the amount of porcine C-peptide release within 4 hrs between 2 groups was negligible. However, the difference in the Cp/G ratio between two groups was evident. When the anti-CD40 mAb based regimen group was further divided into short term graft survival (rejected within 2 weeks, n=7) and long term survival (survived longer than 2 weeks, n=2) groups, the Cp/G ratio of anti-CD40 short term survival group was significantly lower than that of anti-CD154 group. When we measured serum IL-6 which as a pro-inflammatory cytokine could contribute to early islet loss, the level of IL-6 in anti-CD40 group was significantly higher than that in anti-CD154 group as shown in Supplementary Figure 2. These results suggested that the anti-CD40 mAb-based regimen would be less effective in protecting the islet mass in peri-transplant period than anti-CD154 mAb-based regimen, although the data of porcine C-peptide release within 4 hrs did not support this notion. We believe that incorporation of the better way to control loss of islet mass in peri-transplant period could achieve better result with anti-CD40 mAb-based regimen. Importantly, our data on humoral and cellular immune responses supported that anti-CD40 antibody is effective for preventing B cell and T cell responses to porcine antigens as revealed by little increment in the levels of anti-αGal IgG and IFN γ-secreting T cells in most animals.
There are several limitations to this study. The first is the unfeasibility of full replacement of all immunosuppressive drugs to clinically applicable forms. CVF, which was used to deplete complement C3 and thus alleviate IBMIR, cannot be used in the clinics because of its immunogenicity (20). However, recombinant human factor H could be used instead of CVF, as we have demonstrated previously (21). The second is that a significant amount of porcine islets was used (100,000 IEQ/kg). The translation of compatible islet mass in the clinical trial may require tens of pigs for a 70 kg adult. However, considering the 2∼5-fold higher insulin requirement to maintain normoglycemia in NHPs compared to humans, the amount of porcine islets that will be placed into diabetic patients would be significantly less than that used in NHPs. Also, if various genetically engineered pigs, e.g. GTKO and CD46 transgenic pigs are available to us, the number of pigs required and the extent of immunosuppression will be significantly reduced (22). The third is lack of direct methods to accurately assess the extent of IBMIR reflecting early islet loss. In our previous study, porcine C-peptide release within 4 hrs after islet transplantation and the ratio of porcine C-peptide to the fasting BGL were used for the assessment of the loss of islet mass in peri-transplant period and were nicely fitted to the graft survival duration (12). However, in current study, porcine C-peptide release within 4 hrs did not correlate with the graft survival. The fourth is inconsistency of anti-CD40 mAb dosing in early experiments in the current study (for R086, R088, R091, R095; 30 mg/kg vs. for R092, R015, R087, R008, R131; 50 mg/kg), which might underestimate the potency of anti-CD40 mAb alone or in combination with belatacept.
In conclusion, we have demonstrated that anti-CD40 mAb combined with tacrolimus prolonged porcine islet graft survival in NHPs, but further studies to preserve porcine islet mass during initial period after transplantation, which could be controlled by anti-CD154 but not by anti-CD40, are required.
Supplementary Material
Acknowledgments
This work was supported by a grant from the Korea Healthcare Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry for Health and Welfare, Republic of Korea (Grant No. HI13C0954). Anti-CD40 antibody used in these studies was provided by the NIH Nonhuman Primate Reagent Resource (U24 AI126683 and R24 OD10976). The authors would like to thank S. K. Park, J. W. Choi, and S. Y. Kim for isolating porcine islets; H. Y. Nam for conducting FACS and ELISPOT analyses; W. Y. Jung, M. S. Kim, G. E. Lee, J. E. Kim for caring for all NHPs.
Abbreviations
- AST
arginine stimulation test
- CVF
Cobra venom factor
- IBMIR
Instant blood-mediated inflammatory reaction
- IVGTT
Intravenous glucose tolerance test
- mAb
monoclonal antibody
- NHP
Nonhuman primate
- SNU
Seoul National University
- STZ
Streptozotocin
Footnotes
Contributions of authors: J. S. Shin, J. M. Kim, B. H. Min, and I. H. Yoon contributed to data acquisition, analysis and interpretation of the data. J. S. Kim, H. J. Kim, Y. H. Kim, S. J. Kang, J. Kim and E.S. Hwang contributed to data acquisition. H. J. Kang contributed to data acquisition, analysis and interpretation. D. G. Lim, S. J. Kim, J. Ha, W. B. Park and C. G. Park contributed to conception and design. J. S. Shin and C. G. Park contributed to writing and C. G. Park is responsible for the final approval of the manuscript.
References
- 1.Hering BJ, Clarke WR, Bridges ND, Eggerman TL, Alejandro R, Bellin MD, et al. Phase 3 Trial of Transplantation of Human Islets in Type 1 Diabetes Complicated by Severe Hypoglycemia. Diabetes care. 2016;39:1230–1240. doi: 10.2337/dc15-1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park CG, Bottino R, Hawthorne WJ. Current status of islet xenotransplantation. Int J Surg. 2015;23:261–266. doi: 10.1016/j.ijsu.2015.07.703. [DOI] [PubMed] [Google Scholar]
- 3.Kawai T, Andrews D, Colvin RB, Sachs DH, Cosimi AB. Thromboembolic complications after treatment with monoclonal antibody against CD40 ligand. Nature medicine. 2000;6:114. doi: 10.1038/72162. [DOI] [PubMed] [Google Scholar]
- 4.Hecht G, Eventov-Friedman S, Rosen C, Shezen E, Tchorsh D, Aronovich A, et al. Embryonic pig pancreatic tissue for the treatment of diabetes in a nonhuman primate model. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:8659–8664. doi: 10.1073/pnas.0812253106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thompson P, Cardona K, Russell M, Badell IR, Shaffer V, Korbutt G, et al. CD40-specific costimulation blockade enhances neonatal porcine islet survival in nonhuman primates. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2011;11:947–957. doi: 10.1111/j.1600-6143.2011.03509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Budde K, Schutz M, Glander P, Peters H, Waiser J, Liefeldt L, et al. FTY720 (fingolimod) in renal transplantation. Clinical transplantation. 2006;20(Suppl 17):17–24. doi: 10.1111/j.1399-0012.2006.00596.x. [DOI] [PubMed] [Google Scholar]
- 7.Lowe M, Badell IR, Thompson P, Martin B, Leopardi F, Strobert E, et al. A novel monoclonal antibody to CD40 prolongs islet allograft survival. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2012;12:2079–2087. doi: 10.1111/j.1600-6143.2012.04054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohiuddin MM, Singh AK, Corcoran PC, Thomas ML, III, Clark T, Lewis BG, et al. Chimeric 2C10R4 anti-CD40 antibody therapy is critical for long-term survival of GTKO.hCD46.hTBM pig-to-primate cardiac xenograft. Nature communications. 2016;7:11138. doi: 10.1038/ncomms11138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iwase H, Liu H, Wijkstrom M, Zhou H, Singh J, Hara H, et al. Pig kidney graft survival in a baboon for 136 days: longest life-supporting organ graft survival to date. Xenotransplantation. 2015;22:302–309. doi: 10.1111/xen.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim HI, Lee SY, Jin SM, Kim KS, Yu JE, Yeom SC, et al. Parameters for successful pig islet isolation as determined using 68 specific-pathogen-free miniature pigs. Xenotransplantation. 2009;16:11–18. doi: 10.1111/j.1399-3089.2008.00504.x. [DOI] [PubMed] [Google Scholar]
- 11.Jin SM, Kim KS, Lee SY, Gong CH, Park SK, Shin JS, et al. The sequential combination of a JNK inhibitor and simvastatin protects porcine islets from peritransplant apoptosis and inflammation. Cell transplantation. 2011;20:1139–1151. doi: 10.3727/096368910X550170. [DOI] [PubMed] [Google Scholar]
- 12.Shin JS, Kim JM, Kim JS, Min BH, Kim YH, Kim HJ, et al. Long-term control of diabetes in immunosuppressed nonhuman primates (NHP) by the transplantation of adult porcine islets. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2015;15:2837–2850. doi: 10.1111/ajt.13345. [DOI] [PubMed] [Google Scholar]
- 13.Kim JM, Shin JS, Min BH, Kim HJ, Kim JS, Yoon IH, et al. Induction, management, and complications of streptozotocin-induced diabetes mellitus in rhesus monkeys. Xenotransplantation. 2016;23:472–478. doi: 10.1111/xen.12266. [DOI] [PubMed] [Google Scholar]
- 14.Kim HJ, Yoon IH, Min BH, Kim YH, Shin JS, Kim JM, et al. Porcine antigen-specific IFN-gamma ELISpot as a potentially valuable tool for monitoring cellular immune responses in pig-to-non-human primate islet xenotransplantation. Xenotransplantation. 2016;23:310–319. doi: 10.1111/xen.12248. [DOI] [PubMed] [Google Scholar]
- 15.Kang HJ, Lee H, Park EM, Kim JM, Shin JS, Kim JS, et al. Increase in anti-Gal IgM level is associated with early graft failure in intraportal porcine islet xenotransplantation. Ann Lab Med. 2015;35:611–617. doi: 10.3343/alm.2015.35.6.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilson CR, Milas Z, Gangappa S, Hollenbaugh D, Pearson TC, Ford ML, et al. Anti-CD40 monoclonal antibody synergizes with CTLA4-Ig in promoting long-term graft survival in murine models of transplantation. Journal of immunology. 2009;183:1625–1635. doi: 10.4049/jimmunol.0900339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Page A, Srinivasan S, Singh K, Russell M, Hamby K, Deane T, et al. CD40 blockade combines with CTLA4Ig and sirolimus to produce mixed chimerism in an MHC-defined rhesus macaque transplant model. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2012;12:115–125. doi: 10.1111/j.1600-6143.2011.03737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scalea J, Hanecamp I, Robson SC, Yamada K. T-cell-mediated immunological barriers to xenotransplantation. Xenotransplantation. 2012;19:23–30. doi: 10.1111/j.1399-3089.2011.00687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van DER Windt DJ, Bottino R, Casu A, Campanile N, Cooper DK. Rapid loss of intraportally transplanted islets: an overview of pathophysiology and preventive strategies. Xenotransplantation. 2007;14:288–297. doi: 10.1111/j.1399-3089.2007.00419.x. [DOI] [PubMed] [Google Scholar]
- 20.Cochrane CG, Muller-Eberhard HJ, Aikin BS. Depletion of plasma complement in vivo by a protein of cobra venom: its effect on various immunologic reactions. Journal of immunology. 1970;105:55–69. [PubMed] [Google Scholar]
- 21.Kang HJ, Lee H, Ha JM, Lee JI, Shin JS, Kim KY, et al. The role of the alternative complement pathway in early graft loss after intraportal porcine islet xenotransplantation. Transplantation. 2014;97:999–1008. doi: 10.1097/TP.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 22.Ekser B, Ezzelarab M, Hara H, Van DER Windt DJ, Wijkstrom M, Bottino R, et al. Clinical xenotransplantation: the next medical revolution? Lancet. 2012;379:672–683. doi: 10.1016/S0140-6736(11)61091-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.