Abstract
We have discovered that the P/Q-type voltage-gated Ca2+ channel (VGCC) gene, CACNA1A, encodes both the α1A (Cav2.1) subunit and a newly recognized transcription factor, α1ACT, by means of a novel internal ribosomal entry site (IRES) within the α1A c-terminal coding region. α1ACT, when mutated with an expansion of the polyglutamine tract in the c terminus, gives rise to spinocerebellar ataxia type 6 (SCA6). Because silencing of the entire CACNA1A gene would result in the loss of the essential Cav2.1 channel, the IRES controlling α1ACT expression is an excellent target for selective silencing of α1ACT as a therapeutic intervention for SCA6. We performed a high-throughput screen of FDA-approved small molecules using a dual luciferase reporter system and identified10 hits able to selectively inhibit the IRES. We identified four main candidates that showed selective suppression of α1ACT relative to α1A in HEK cells expressing a native CACNA1A vector. We also pursued another avenue of molecular intervention through miRNA silencing. We studied three human miRNAs (miRNA-711, −3191-5p, −4786) that would potentially bind to sequences within the CACNA1A IRES region, based on an miRNA prediction program. Only miRNA-3191-5p was found to selectively inhibit the translation of α1ACT in cells. We developed a hyperacute model of SCA6 in mice by injecting a pathogenic form of the IRES-mediated α1ACT (AAV9-α1ACTQ33). Finally, we tested the effectiveness of the miRNA therapy by co-expressing either control miRNA or miRNA-3191-5p and found that miRNA-3191-5p decreased the levels of α1ACTQ33 and prevented the hyperacute disease in mice. These studies provide the proof of principle that a therapy directed at selectively preventing α1ACT expression could be used to treat SCA6.
Introduction
CACNA1A and SCA6
Spinocerebellar ataxia type 6 (SCA6) is a late-onset progressive neurodegenerative disease characterized by progressive ataxia and incoordination together with cerebellar atrophy and selective Purkinje cell degeneration [1]. The mutation in this autosomal dominant disease consists of an expanded polyglutamine (polyQ)-encoding CAG repeat in the voltage-gated calcium channel (VGCC) gene CACNA1A [2]. SCA6 mutation does not abolish gene function, because genetic deletion of the CACNA1A gene results in neonatal lethality while hypomorph models such as Leaner and KIKO are viable but present with motor abnormalities [3, 6]. This gene encodes two proteins, the 250 kDa α1A, the main pore-forming subunit of P/Q Cav2.1 channel [1], and a 75 kDa protein, α1ACT, which encompasses the C-terminal portion of α1A. Although both proteins contain polyQ tract, the function of P/Q channels bearing the expanded polyQ does not appear to be directly disturbed [4, 5].
The α1ACT protein encoded by CACNA1A and bearing the polyQ tract is translocated to nuclei, suggesting that the native protein has a role in gene expression. This also suggests that the polyQ-expanded form of this protein is responsible for the pathogenesis in SCA6 [6, 7]. In support of this our group has shown that α1ACT functions as a transcription factor that regulates several genes, such as BTG1, GRN, and PMCA2 in cultured neuronal cells (in vitro) and cerebellar tissue (in vivo) [6]. Additionally, wildtype α1ACT (Q11) promotes nerve growth factor (NGF)-mediated neurite outgrowth in differentiating PC12 cells, improves parallel fiber connections onto Purkinje cells, and partially rescues the phenotype of CACNA1A knockout mice [6]. Conversely, SCA6-associated α1ACT (Q33) mediates cell death in vitro, and causes gait abnormalities and cerebellar molecular layer thinning in mice [6]. Therefore, while genetic suppression of the CACNA1A gene has been shown to have lethal consequences, selective inhibition of α1ACT may be a viable therapy in SCA6.
IRES-mediated translation in SCA6
We have recently shown that α1ACT is translated from an intragenic internal ribosome entry site (IRES) within the coding region of CACNA1A [6]. Eukaryotic mRNAs are predominantly translated using a cap-dependent (i.e. 7-methyl-guanosine) mode of initiation. However these transcripts are increasingly recognized as having alternate modes of initiation, including via IRES initiation, the pathway employed by viruses [8]. Viral IRESs are capable, through their secondary or tertiary structure, of recruiting and forming a complex with ribosomes, eukaryotic initiation factors (eIFs) and other IRES-transacting factors (ITAFs) to selectively initiate translation of viral proteins [8]. Similarly, many eukaryotic mRNAs have IRESs in the 5′ untranslated region (UTR) that are active, particularly during cell stress and cell proliferation, when cap-dependent translation may be restricted, and are sites for a secondary method of translating the primary protein [9].
A less recognized phenomenon in mammalian gene expression acts via inter- and intragenic cellular IRESs, in which one transcript mediates the translation of two or more distinct polypeptides through independent regulation of each translation site [9]. In the case of CACNA1A, the calcium channel subunit α1A is translated canonically by cap-dependent translation, while α1ACT translation is directed in a cap-independent manner through an approximately 1kb IRES present within the 3′ end of the coding sequence of CACNA1A gene [6]. In silico RNA folding simulation of the putative IRES reveals a complex hairpin structure, while mass spectrometry showed that the N terminal amino acid start site of α1ACT was methionine codon 1960 of the α1A subunit [6]. We confirmed that this CACNA1A sequence possessed IRES activity using a dual luciferase reporter (pRCT1014TF) bearing the Renilla luciferase (RL, followed by a stop codon) and Firefly luciferase (FL) coding regions in a single transcript flanking the CACNA1A IRES, or a control reporter (pRF) lacking the intervening CACNA1A sequence. Normally, only the Renilla luciferase bioluminescence can be detected when the control vector is expressed in a cell line. In cells transfected with pRCT1014TF, ratio of FL to RL were increased 20–40-fold relative to that in cells transfected with pRF. This confirmed that this 1014 nucleotide sequence in the CACNA1A transcript was capable of eliciting expression of the α1ACT protein in a cap-independent manner.
miRNA inhibition of α1ACT
Many miRNAs act to block gene expression by binding to mRNA at specific sites and targeting the transcript for RNAase-mediated degradation through the RISC complex [14]. A smaller number of miRNAs also bind mRNA but act by direct translational blockade without recruiting the RISC complex, and without degrading the target mRNA. Based on the miRNA_Targets program we chose three human miRNAs (miRNA-711, −3191-5p, −4786) that were predicted to bind to sequences within the CACNA1A IRES region [15]. To test their effect on the IRES we used the CACNA1A IRES dual luciferase reporter assay we used to identify the IRES region. We found that all three candidates showed more than 50% decrease in the ratio of FL to RL when compared to the negative control miRNA [15]. Subsequently, we tested the effect of each miRNA on the expression of either of the two CACNA1A proteins expressed from the native α1A transcript using HEK293 cells stably expressing α1AQ11 and α1ACTQ11. We performed western blots on cells transfected 48 hours previously with one of the three miRNAs. While miRNA-711 and miRNA-4786 showed a decrease in both the 250 kDa α1A subunit and the 75 kDa α1ACT proteins, miRNA-3191-5p selectively inhibited only the IRES-mediated α1ACT [15], suggesting that two miRNAs elicited degradation of the α1A mRNA, while miRNA-3191-5p acted by the mRNA-sparing translational blockade pathway. In support of this we found that α1A mRNA expression was reduced by miRNA-711 and miRNA-4786, while miRNA-3191-5p was not reduced [15]. This suggested that miRNA-3191-5p acts to inhibit the CACNA1A IRES specifically at the translational level. miRNAs that degrade their target transcripts act by binding to the Argonaut 2 protein (Ago2), to form the RISC complex [14]. Interestingly, in follow-up studies we found that miRNA-3191-5p acts by recruiting instead Argonaute 4 (Ago4), an Argonaute protein lacking a catalytic domain. This interaction appeared to inhibit the interactions between eIFAII, eIFGII, and the IRES, a finding that may explain the selective effect on translation [15].
Results
Small molecule targeting of the CACNA1A IRES
Since the pathogenesis of SCA6 is attributable to the mutant properties of polyQ-expanded α1ACT, while the α1A subunit appears essential for life, one possible therapeutic strategy for SCA6 is to develop a means to selectively inhibit expression of α1ACT by targeting of the IRES-mediated translation of this protein, in a manner that spares expression of the α1A subunit. In parallel with our efforts to develop miRNA inhibitors, we have conducted an investigation into the use of small molecules to selectively inhibit the IRES. To achieve this we performed a high-throughput small molecule screening using the same dual luciferase reporter assay we employed previously. We tested the hypothesis that a small molecule inhibitor could be identified that selectively interfered with the IRES.
We developed a screening assay using a HEK 293 cell line stably expressing either the dual luciferase reporter (pRCT1014TF) or the control reporter (pRF) lacking the intervening CACNA1A sequence to test for the ability of compounds in a small molecule library to block IRES activity. First, we engineered the neomycin resistance marker into the mammalian dual luciferase expression vectors pRF and pRCT1014TF (npRF and npRCT1014TF). The HEK293 cell lines stably expressing dual luciferase with or without CACNA1A IRES were established by transiently transfecting the npRF and npRCT1014TF with neomycin in plasmid DNAs.
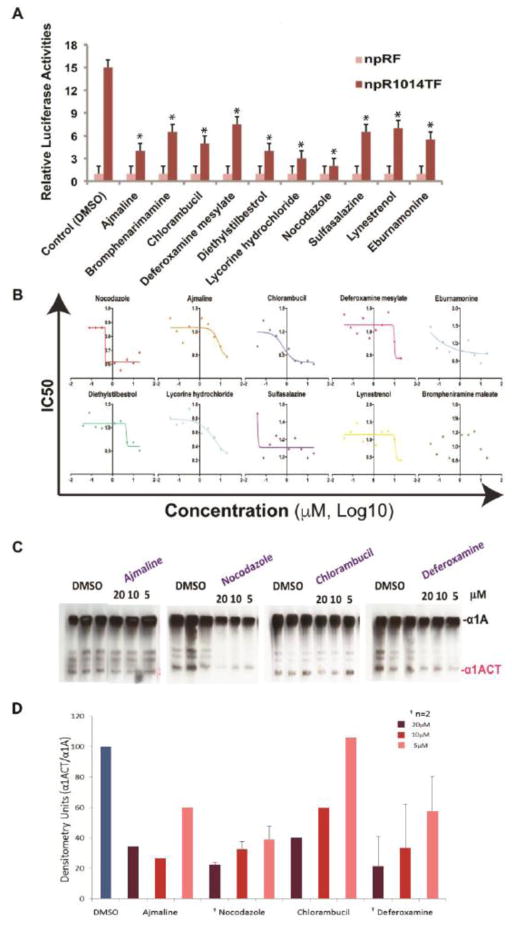
We then screened approximately 1120 FDA-approved drugs from the Prestwick Library for their ability to block IRES activity using an automated high through small compound platform in Cellular Screening Center Core (CSC)at the University of Chicago. We found 10 preliminary hits based on a decrease of at least 50% in the ratio of FL to RL in the npRCT1014TF expressing cell line without a corresponding decrease in RL and the ratio of FL to RL in the control pRF when compared to the vehicle control of DMSO (Figure 1A). The 10 compounds were then tested over a range of concentrations to determine the IC50 (Figure 1B). Subsequently we tested these drugs in cultured cells expressing full length, FLAG-tagged α1A and α1ACT for their ability to reduce expression of the 75kDa α1ACT protein and spare the larger α1A polypeptide at three concentrations (5, 10, and 20 μM). We found four primary candidates, ajmaline, nocodazole, chlorambucil, and deferoxamine, that were effective at selectively inhibiting α1ACT (Figure 1C, 1D). Ajmaline is a class I antiarrhythmic drug with potent sodium channel blocking effect [10]. Nocodazole suppresses microtubule dynamics and arrests cells at the G2- or M-phase [11]. Chlorambucil is a chemotherapy drug for leukemia and lymphoma that induces apoptosis by cross-linking DNA, thereby promoting DNA damage [12]. Deferoxamine is an iron-chelating agent used primarily to treat iron toxicity [13]. These drugs are being further tested for their ability to protect cultured cells from the toxic effects of polyQ-expanded α1ACT as well as for any potential side effects in vivo. The mechanism by which these drugs selectively inhibit the CACNA1A IRES has not been elucidated. Further investigation into this mechanism of action may give insight into structural elements and specific ITAFs used to make α1ACT, and point to other agents with a higher therapeutic index.
Figure 1. High-throughput screening for small molecule inhibitors of IRES-mediated α1ACT.
A) Small molecule high-throughput screening of 1120 compounds revealed 10 hits that decrease the ratio of Firefly luciferase to Renilla luciferase by of at least 50% in the npRCT1014TF expressing cell line, without a corresponding decrease in the control npRF when compared to the DMSO vehicle control. * indicates p<0.01 between DMSO npRCT1014TF and npRCT1014TF with drug treatments.
B) IC50 of each of 10 hits in npRCT1014TF stable HEK293 cell lines.
C) Western blot analysis showing the effects of ajmaline, nocodazole, chlorambucil, and deferoxamine (5, 10, 20 uM) on the expression α1ACT relative to α1A.
D) Densitometry analysis of representative western blots showing the effects of the drugs on the expression α1ACT relative to α1A.
Discussion
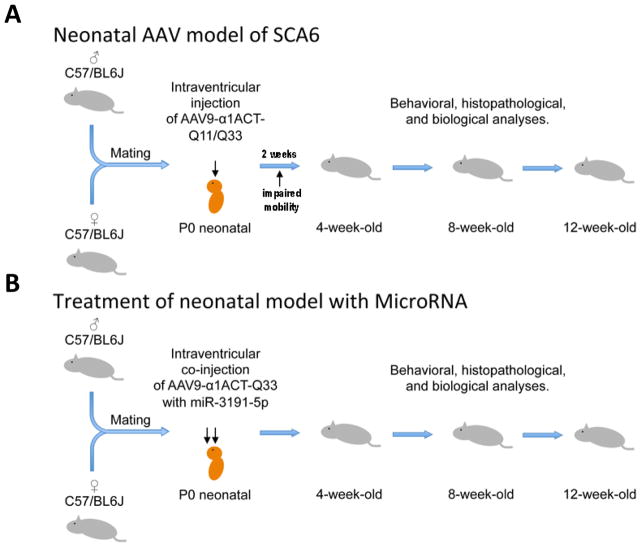
Selective inhibition of polyQ-expanded α1ACT provides an opportunity for therapeutic intervention in patients with SCA6. To test whether miRNA-3191-5p could be used for that purpose, we developed an acute, adeno-associated virus (AAV)-mediated SCA6 model. We used intraventricular injection of a recombinant AAV9 that was engineered to express either α1ACTQ11 or α1ACTQ33 (AAV9-α1ACTQ11/Q33) under the control of the CACNA1A IRES into control C57BL/6J mice at P0 [15] (Figure 2). This somatic gene transfer system allowed for expression of α1ACT that could be detected in the hippocampus, cerebral cortex, and cerebellum [15]. Mice receiving the AAV9- α1ACTQ33 had detectable motor abnormalities at 2 weeks of age, while those injected with AAV9- α1ACTQ11 did not appear different from control. At four, eight and twelve weeks post-injection behavioral and histological assessments revealed that the α1ACTQ33 mice had impaired mobility, and histological study showed thinning of the molecular layer and decreased numbers of Purkinje cells, compared to α1ACTQ11 or GFP control mice [15]. Subsequently we developed a second, therapeutic recombinant AAV9 vector that was designed to express either miRNA-3191-5p or control miRNA. We tested whether miR-3191 could prevent the hyperacute SCA6 disease by simultaneous injection of either AAV9-miR-3191 or AAV9 control miRNA with the AAV9-α1ACTQ33 vector. We found that AAV9-miR-3191 blocked the expression of α1ACTQ33 protein, but spared the α1A, as expected. More importantly, miR-3191 delivered this way prevented the development of the motor and morphological abnormalities, while the control miRNA-expressing AAV had no effect [15]. These studies provided the proof of principle that a therapy directed at selectively preventing α1ACT expression could be used to treat SCA6 [15]. Our recent study on small molecule inhibition of α1ACT provides a parallel avenue for treatment and in vivo studies would elucidate the translational effectiveness of such drugs. In the future, options for selectively targeting the CACNA1A IRES may include, in addition, more specific small molecule inhibitors or antisense oligonucleotides (ASOs). ASOs have recently been employed in the clinic with encouraging success in treating Duchenne muscular dystrophy (DMD) and spinal muscular atrophy (SMA) [16–18].
Figure 2. Scheme for the development of AAV9-α1ACTQ33 hyperacute model of SCA6 in mice and the subsequent treatment with miRNA-3191.
A) Wildtype control C57BL/6J were intraventricular injected at P0 with recombinant AAV9 (adeno-associated virus) which expressed either α1ACTQ11 or α1ACTQ33 (AAV9- α1ACTQ11/Q33) under the control of the CACNA1A IRES.
B) Wildtype control C57BL/6J were intraventricular injected at P0 with both recombinant AAV9 (adeno-associated virus), which expressed α1ACTQ33 (AAV9- α1ACTQ33) under the control of the CACNA1A IRES and recombinant AAV9 that expressed miR-3191-5p.
References
- 1.Solodkin A, Gomez CM. Spinocerebellar ataxia type 6. Handb Clin Neurol. 2012;103:461–73. doi: 10.1016/B978-0-444-51892-7.00029-2. [DOI] [PubMed] [Google Scholar]
- 2.Zhuchenko O, Bailey J, Bonnen P, et al. Autosomal dominant cerebellar ataxia (SCA6) associated with small polyglutamine expansions in the alpha1A-voltage-dependent calcium channel. Nat Genet. 1997;15:62–9. doi: 10.1038/ng0197-62. [DOI] [PubMed] [Google Scholar]
- 3.Jun K, Piedras-Renteria ES, Smith SM, et al. Ablation of P/Q-type Ca(2+) channel currents, altered synaptic transmission, and progressive ataxia in mice lacking the alpha(1A)-subunit. Proc Natl Acad Sci USA. 1999;21:15245–50. doi: 10.1073/pnas.96.26.15245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watase K, Barrett CF, Miyazaki T, et al. Spinocerebellar ataxia type 6 knockin mice develop a progressive neuronal dysfunction with age-dependent accumulation of mutant CaV2.1 channels. Proc Natl Acad Sci USA. 2008;105:11987–92. doi: 10.1073/pnas.0804350105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saegusa H, Wakamori M, Matsuda Y, et al. Properties of human Cav2.1 channel with a spinocerebellar ataxia type 6 mutation expressed in Purkinje cells. Mol Cell Neurosci. 2007;34:261–70. doi: 10.1016/j.mcn.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Du X, Wang J, Zhu H, et al. A second cistron in the CACNA1A gene encodes a transcription factor that mediates cerebellar development and SCA6. Cell. 2013;154:118–133. doi: 10.1016/j.cell.2013.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kordasiewicz HB, Thompson RM, Clark HB, et al. C-termini of P/Q-type Ca2+ channel alpha1A subunits translocate to nuclei and promote polyglutamine-mediated toxicity. Hum Mol Genet. 2006;15:1587–99. doi: 10.1093/hmg/ddl080. [DOI] [PubMed] [Google Scholar]
- 8.Martinez-Salas E, Pineiro D, Fernandez N. Alternative Mechanisms to Initiate Translation in Eukaryotic mRNAs. Comp. Funct. Genomics. 2012 doi: 10.1155/2012/391546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karginov TA, Pastor Hejazi DP, Semler BL, et al. Mammalian Polycistronic mRNAs and Disease. Trends Genet. 2017;33:129–142. doi: 10.1016/j.tig.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rolf S, Bruns HJ, Wichter T, et al. The ajmaline challenge in Brugada syndrome: Diagnostic impact, safety, and recommended protocol. Eur Heart J. 2003;24:1085–86. doi: 10.1016/s0195-668x(03)00195-7. [DOI] [PubMed] [Google Scholar]
- 11.Vasquez RJ, Howell B, Yvon AM, et al. Nanomolar concentrations of nocodazole alter microtubule dynamic instability in vivo and in vitro. Mol Biol Cell. 1997;8:973–85. doi: 10.1091/mbc.8.6.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Begleiter A, Mowat M, Israels LG, et al. Chlorambucil in Chronic Lymphocytic Leukemia: Mechanism of Action. Leuk Lymphoma. 1996;3:187–201. doi: 10.3109/10428199609054821. [DOI] [PubMed] [Google Scholar]
- 13.Porter JB, Rafique R, Srichairatanakool S, et al. Recent Insights into Interactions of Deferoxamine with Cellular and Plasma Iron Pools: Implications for Clinical Use. Ann N Y Acad Sci. 2005;1054:155–68. doi: 10.1196/annals.1345.018. [DOI] [PubMed] [Google Scholar]
- 14.MacFarlane LA, Murphy PR. MicroRNA: Biogenesis, Function and Role in Cancer. Curr Genomics. 2010;11:537–61. doi: 10.2174/138920210793175895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miyazaki Y, Du X, Muramatsu S, et al. An miRNA-mediated therapy for SCA6 blocks IRES-driven translation of the CACNA1A second cistron. Sci Transl Med. 2016;8:347–94. doi: 10.1126/scitranslmed.aaf5660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim KRQ, Maruyama R, Tokota T. Eteplirsen in the treatment of Duchenne muscular dystrophy. Drug Des Devel Ther. 2017;11:533–45. doi: 10.2147/DDDT.S97635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiriboga CA, Swoboda KJ, Darras BT, et al. Results from a phase 1 study of nusinersen (ISIS-SMN(Rx)) in children with spinal muscular atrophy. Neurology. 2016;8:890–7. doi: 10.1212/WNL.0000000000002445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Finkel RS, Chiriboga CA, Vajsar J, et al. Treatment of infantile-onset spinal muscular atrophy with nusinersen: a phase 2, open-label, dose-escalation study. Lancet. 2016;388:3017–26. doi: 10.1016/S0140-6736(16)31408-8. [DOI] [PubMed] [Google Scholar]