Abstract
Background
People with PD are at high risk of developing cognitive impairment and dementia. Cross-sectional studies have identified candidate biomarkers associated with cognitive decline. However, longitudinal studies on this topic are rarer, and few have investigated the use of biomarker panels encompassing multiple modalities. The objective of this study was to find baseline predictors of cognitive decline in longitudinally followed, nondemented Parkinson’s disease patients.
Methods
We performed a prospective cohort study of 100 PD patients with a median disease duration of 6.4 years. All participants were nondemented at baseline. We examined 16 baseline biomarkers from clinical, genetic, biochemical, and MRI-based imaging modalities for their association with longitudinal cognitive decline for up to 8 years. We investigated biomarkers individually, as well as in a multivariate linear mixed-effects model encompassing multimodal biomarkers, with change in the Mattis Dementia Rating Scale-2 over time as the primary outcome. Annual consensus process-derived cognitive diagnosis was used for Cox proportional hazards modeling of risk for cognitive decline.
Results
In multivariate analysis, the presence of the APOE E4 allele, thought disorder, and an Alzheimer’s disease pattern of brain atrophy (spatial pattern of abnormality for recognition of early Alzheimer’s disease index) best predicted cognitive decline, with APOE E4 genotype exerting the greatest effect. The presence of the APOE E4 allele was associated with a 3.5 times higher risk of worsening cognitive diagnosis over time (HR, 3.53; 95% CI, 1.52–8.24; P < 0.05). The APOE genotype effect was not specific to any Mattis Dementia Rating Scale-2 domain.
Conclusions
Our results confirm the importance of Alzheimer’s disease biomarkers as risk factors for cognitive decline in established Parkinson’s disease.
Keywords: Parkinson’s, dementia, APOE, SPARE-AD, hallucination
Parkinson’s disease (PD) is the second most common neurodegenerative disease, affecting an estimated 1% of the world population older than age 60 years.1,2 Many PD patients will develop cognitive impairment during the course of disease. Indeed, as many as 83% of PD patients developed dementia (a condition known as PD dementia) in one 20-year follow-up study3; mild cognitive impairment (MCI) — defined as cognitive impairment in the absence of a significant effect on activities of daily living — affects 20%–30% of PD patients4 and increases the risk of developing dementia.5
Biomarkers are defined as characteristics that can be objectively measured and evaluated as indicators of normal biological processes, pathogenic processes, or pharmacological responses to therapeutic intervention.6 Prognostic biomarkers may inform the understanding of the antecedent pathophysiology of cognitive decline in PD. Furthermore, identifying those at highest risk of cognitive decline can augment future clinical trial enrollment and guide clinical care.7–9 Although existing clinical, biochemical, genetic, and imaging-based biomarkers have been correlated with various aspects of cognition in PD,10–17 few studies have examined multiple biomarkers, particularly multiple biomarkers from different modalities, in a prospective longitudinal setting.18 Examining multiple biomarkers may allow for improved predictive power above single tests alone. Moreover, markers on the same biological pathway may demonstrate correlated expression, suggesting that the relationships among markers are worthy of investigation to best design a parsimonious predictive panel.
Various patient characteristics and clinical measurements are reported to associate with cognitive dysfunction in PD. These include advanced age,19,20 male sex,20,21 depression,11 motor severity,11,22 disease duration,19 thought disorder or hallucinations,22 and postural instability.19
Biochemical markers that have been associated with dementia in PD include higher cerebrospinal fluid (CSF) total tau and phosphorylated tau10,23 and lower amyloid- β42 (Aβ).23–26 In addition, lower plasma epidermal growth factor, a factor in dopamine neuronal survival,27,28 is also a predictor of cognitive decline in PD.15,29
Genetic markers associated with dementia in PD include apolipoprotein E (APOE)13 and catechol-O-methyltransferase (COMT)14 genotypes, microtubule-associated protein-tau (MAPT)14 haplotypes, and the presence of mutations, as well as a common polymorphism in the glucocerebrosidase (GBA) gene.30,31
Finally, among several proposed imaging-based biomarkers,32,33 the pattern of cortical atrophy captured in the spatial pattern of abnormality for recognition of early Alzheimer’s disease (SPARE-AD) volumetric imaging scale has been shown to be predictive of cognitive decline in PD.17
In this study, we prospectively enrolled 100 PD patients without dementia and followed them for up to 8 years to ascertain the natural progression of cognitive impairment in a longitudinal cohort. A dense data set of 16 clinical, genetic, biochemical, and radiographic biomarkers that have been previously reported to associate with cognition in PD were evaluated in this cohort at baseline, with the goal of understanding relationships among biomarkers and their ability to predict longitudinal cognitive decline.
Methods
Please see Supplementary Methods for additional details.
Participants
Patients 50 years and older diagnosed with PD based on the UK Brain Bank criteria34 were prospectively enrolled at the University of Pennsylvania (UPenn) Udall Center of Excellence for Parkinson’s Disease Research (Philadelphia, PA). For this study, consecutively enrolled subjects with at least 1 year of follow-up were included, whereas subjects with a consensus process-derived diagnosis of dementia at enrollment were excluded. All baseline clinical, genetic, biochemical, and imaging biomarkers were predesignated at the outset of the study in 2012 to avoid biases with post hoc selection of markers to evaluate in analysis. A small subset of patients previously consecutively enrolled into the UPenn Udall Center and followed (2006 through 2012) had qualifying MRI scans, CSF and plasma samples, and DNA available at the study outset. These individuals were also included, and their biomarkers measured with the main cohort.
Standard Protocol and Informed Consent
This study was approved by the University of Pennsylvania Institutional Review Board. Informed consent was obtained at study enrollment.
Assessments
Clinical and neuropsychological assessments were administered by trained research staff. At the baseline visit, clinical information was obtained, and neuropsychological testing was performed. All baseline biomarkers were collected within 1 year of the baseline visit. Neuropsychological assessments were administered annually for the first 4 years and biennially thereafter.
Motor severity was assessed by the Unified Parkinson’s Disease Rating Scale (UPDRS) Part III35 and modified Hoehn and Yahr scales.36 Levodopa equivalent daily dose (LEDD) for all PD medications was determined for each subject.37 The Geriatric Depression Scale-15 item, a validated measure of depression in PD,38 was administered to each participant at baseline. The tremor/postural instability gait disorder (PIGD) ratio was calculated as previously described39 and was calculated by dividing the average tremor score by the average PIGD score. The UPDRS Part I question on thought disorders was used to assess the presence and severity of hallucinations.35 The responses to this question are graded as 0 = none, 1 = vivid dreams, 2 = hallucinations with insight retained, 3 = hallucinations without insight, and 4 = persistent hallucinations, delusions, or psychosis. Thought disorder is defined as any nonzero value on this scale. Candidate biomarkers were nominated from the literature on cognitive decline in PD, including genetic, biochemical, and neuroimaging biomarkers. Additional details on biomarker collection are found in the Supplementary Methods.
Neuropsychological Evaluation
The Mattis Dementia Rating Scale-2 (DRS) was used to assess global cognitive performance and age-adjusted scores were used for analysis.40 A cognitive diagnosis (cognitively normal, MCI, or dementia) for each participant was determined annually/biennially by expert clinical consensus at the UPenn Udall Center, as previously described.20
Statistical Analysis
All statistical analysis was performed in R (http://www.r-project.org); R-scripts can be found in the Supplementary Methods. Descriptive statistics were calculated for baseline demographic, clinical, genetic, biochemical, and imaging data using available data. MAPT haplotypes were grouped into H1/H1 versus other haplotypes based on previously-reported genotype-phenotype associations,41 and APOE genotypes were grouped into those with 1 or more E4 alleles versus those without any E4 alleles. Kaplan-Meier analysis was used to estimate the probability of progression to cognitive impairment, defined as any change in cognitive diagnosis (ie, cognitively normal to MCI or dementia or MCI to dementia). Missing data were multiply imputed using the “mi” package in R.42 Results using imputed values were compared with results excluding missing values.
To assess relationships between biomarkers, pairwise Spearman correlation coefficients were calculated among continuous biomarkers and categorical biomarkers with at least 5 categories.
Linear mixed-effects models43 were used to test for associations between each biomarker and age-adjusted DRS score over time, with covariates of age and sex. This approach controls for the variable follow-up time and variable intervals between follow-up visits. A random intercept was included in the mixed-effects model to account for correlations among repeated measures. As the purpose of the bivariate model was to nominate biomarkers into the multivariate model selection, we did not apply a multiple testing correction. Each biomarker found to be significant in a bivariate model screen was then incorporated in a multivariate linear mixed-effects model using standardized predictors covarying for age and sex; best-fitted model selection was done by comparing the Akaike information criterion (AIC) of all possible combinations of biomarkers. Additional analyses incorporating disease duration or education as covariates were also performed where indicated in the text.
The independent effect of the APOE genotype on cognitive decline was further assessed using Cox’s proportional hazards regression model, determining hazard ratios for time to change in cognitive diagnosis. Finally, linear mixed-effects models were used to determine the effect of the APOE genotype on change in individual, standardized DRS domain scores, covarying for age and sex. Statistical tests were 2-sided, and alpha was set at 0.05.
Results
Cognitive Decline in Longitudinal Follow-Up
A total of 100 consecutive PD subjects (69% male) were prospectively enrolled in this study between December 2006 and May 2016 from a single clinical center. Median age at PD symptom onset was 58 years (interquartile range [IQR], 52–63 years) and at study enrollment was 65 years (IQR, 61–70 years). At baseline, 67% of the patient cohort were cognitively normal, and 33% had MCI. Ten subjects died during the study: 7 of these subjects were diagnosed with dementia prior to death, 1 was diagnosed with MCI, and 2 were cognitively normal. Sixteen biomarkers were investigated in the cohort; of the 1600 possible data points, 32 (2%) were missing. Baseline biomarker summaries are shown in Table 1.
TABLE 1.
Summary of baseline biomarkers
| Biomarker Clinical | Disease duration (years), median (IQR) | 6.4 (4.0–10.75) | |
| UPDRS Part III, median (IQR) | 19 (15–26) | ||
| MODHY (count) | I | 6 | |
| II | 35 | ||
| III | 56 | ||
| IV | 1 | ||
| LEDD, median (IQR) | 555 (369–803) | ||
| GDS, median (IQR) | 2.0 (0.25–4) | ||
| Tremor/PIGD ratio, median (IQR) | 0.6 (0.2–1.3) | ||
| UPDRS Part I — thought disorder | 0 | 51 | |
| 1 | 39 | ||
| 2 | 3 | ||
| 3 | 1 | ||
| Genetic | APOE E4 allele (count) | 0 | 70 |
| 1 | 30 | ||
| 2 | 0 | ||
| MAPT | H1/H1 | 75 | |
| H2/− | 25 | ||
| GBA mutant | No | 95 | |
| Yes | 4 (E326K:1, L444P:2, N370S:1) | ||
| COMT Met allele (count) | 0 | 21 | |
| 1 | 54 | ||
| 2 | 24 | ||
| Biochemical | CSF Aβ42, median (IQR) | 283.5 (231–331) | |
| CSF t-tau, median (IQR) | 38.53 (31.75–51) | ||
| CSF p-tau, median (IQR) | 19 (14–26) | ||
| Plasma EGF, median (IQR) | 22.52 (9.59–62.28) | ||
| Imaging | SPARE-AD score, median (IQR) | 0.159 (−0.668–0.583) |
IQR, interquartile range; UPDRS, Unified Parkinson’s Disease Rating Scale; MODHY, Modified Hoehn & Yahr; LEDD, levodopa-equivalent daily dose; GDS, geriatric depression scale; PIGD, postural instability gait disorder; APOE4, apolipoprotein E4; MAPT, microtubule-associated protein tau; GBA, glucocerebrosidase; COMT, catechol-O-methyltransferase; Aβ 42, amyloid-β1–42 peptide; EGF, epidermal growth factor; SPARE-AD, spatial pattern of abnormality for recognition of early Alzheimer’s disease. Biochemical markers are reported in pg/mL.
The cohort was followed for up to 8 years, with a mean ± SD follow-up time of 2.9 ± 2.1 years (Figure 1a). A change in cognitive diagnosis, as previously defined, occurred in 22% of the cohort. The estimated cumulative rate of cognitive decline based on Kaplan-Meier analysis is shown in Figure 1b, and the estimated cumulative rate of progression to dementia is shown in Figure 1c.
FIG. 1.
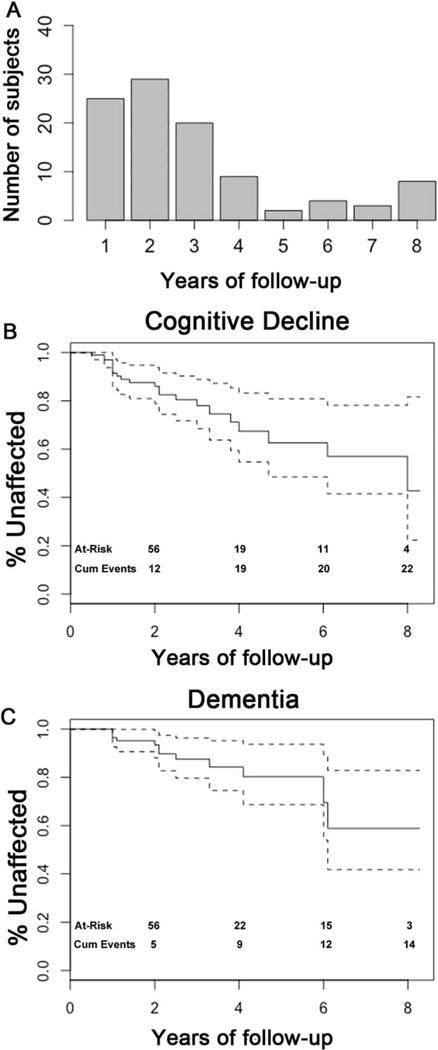
Summary of cognitive outcomes. Subjects (N = 100) were evaluated yearly for up to 8 years (A). Cognitive decline (B) and dementia (C) were diagnosed by clinician consensus, and Kaplan-Meier analyses for preservation of cognition are shown. Dashed lines represent 95% CI.
Lack of Internal Correlation Among Biomarkers
To investigate the degree of internal correlation among biomarkers assessed, Spearman correlation coefficients were calculated for pairs of markers. Confirming our previous report in a cross-sectional analysis of a partial data set44 from this cohort, markers did not show a high degree of internal correlation (Figure 2).
FIG. 2.
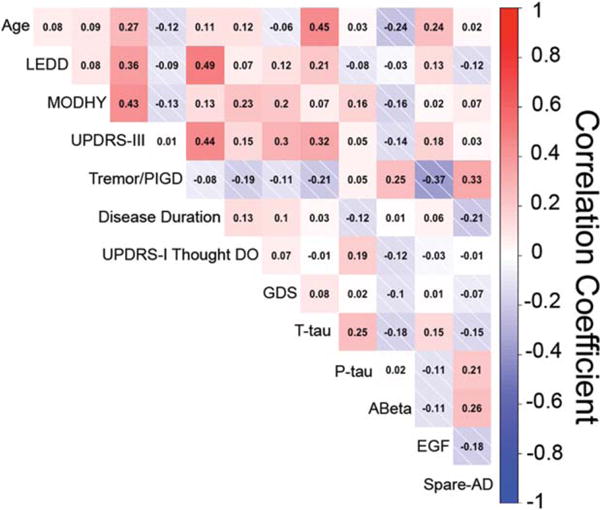
Pairwise Spearman correlation coefficients are shown for all pairs of continuous biomarkers as well as categorical biomarkers with at least 5 categories. Shades of red indicate positive correlation while shades of blue indicate negative correlation. Biomarkers are not highly correlated.
Predictors of Longitudinal Cognitive Decline
Linear mixed-effects models adjusting for age and sex were used to examine the association of individual biomarkers with change in cognitive performance, as captured in the age-adjusted DRS. Six individual markers from 4 modalities were associated with cognitive decline — LEDD, thought disorder as measured by the UPDRS Part I, APOE E4 genotype, COMT genotype, lower CSF Aβ levels, and higher SPARE-AD score (Supplementary Table 1).
Individual biomarkers associating with cognitive decline were then evaluated together in a multivariate linear mixed-effects model adjusting for age and sex. Model selection was performed by comparing AIC values of all possible combinations of biomarkers. The best-fitted model predicting subsequent cognitive decline obtained in this manner included 3 biomarkers: (1) thought disorder, (2) APOE E4 allele, and (3) the SPARE-AD score (Table 2). Additional analyses including either education or disease duration as covariates in the multivariate mixed-effects model did not affect our results.
TABLE 2.
Best-fitted model for predicting longitudinal cognitive decline
| Biomarker × time | Change in DRS (standardized) | 95% CI | P |
|---|---|---|---|
| UPDRS Part I Thought Disorder | −0.507 | (−0.867 to −0.145) | 0.01a |
| APOE E4 allele | −0.758 | (−1.127 to −0.388) | < 0.01a |
| SPARE-AD score | −0.323 | (−0.743 to 0.095) | 0.13 |
Linear mixed-effects model with covariates of age and sex. Results are shown as standardized β-coefficients representing the difference in rate of change in the age-adjusted dementia rating scale for 1 standardized unit increase in the predictor.
P < 0.05. DRS, Dementia Rating Scale; APOE, apolipoprotein E; SPARE-AD, spatial pattern of abnormality for recognition of early Alzheimer’s disease.
APOE Genotype as Predictor of Cognitive Decline
In the multimodal model the largest effect size was seen for APOE genotype, prompting us to evaluate this biomarker further. In Cox proportional hazards models, the presence of 1 APOE E4 allele was associated with a 3.5 times higher risk of worsening in cognitive diagnosis (HR, 3.54; 95% CI, 1.517–8.241; P = 0.003; Figure 3a).
FIG. 3.
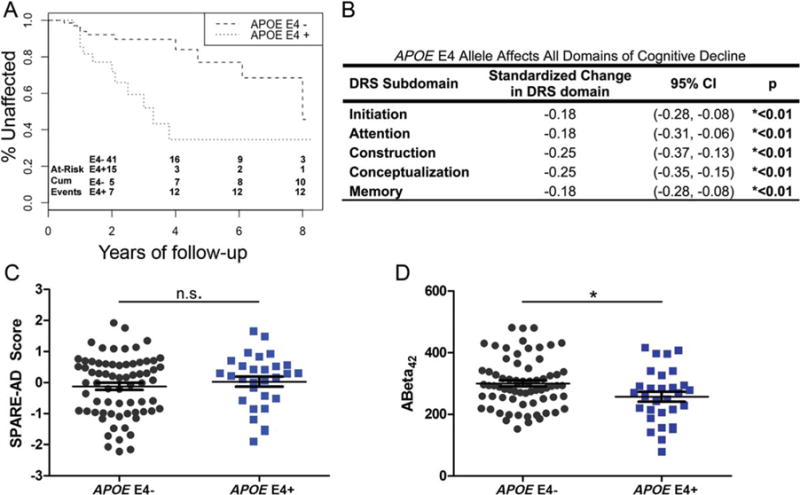
APOE E4 carriers have higher risk of early cognitive decline (HR = 3.5357, 95% CI = 1.517–8.241, p = 0.003, A), across all cognitive domains (B). SPARE-AD scores do not differ between groups (C), although CSF ABeta42 is lower in APOE E4 carriers (D, * = p<0.05). [Color figure can be viewed at wileyonlinelibrary.com]
Because APOE genotype is a risk factor for Alzheimer’s disease (AD), in which memory deficits are diagnostic, we next investigated whether APOE genotype effects were confined to particular cognitive domains of the DRS. We investigated the individual DRS domains of initiation, attention, construction, conceptualization, and memory. As shown in Figure 3b, the presence of the APOE E4 allele was associated with significantly greater decline in all domains, with the greatest effects seen in construction and conceptualization.
Finally, because APOE genotype, SPARE-AD score, and measures of CSF Aβ have all been linked to AD pathophysiological pathways, we investigated the effect of APOE genotype on the other 2 markers in our cohort. As shown in Figure 3c, although subjects who possessed an APOE E4 allele did not differ in SPARE-AD score from subjects without an APOE E4 allele, significant differences were detected in CSF Aβ measures, with E4 carriers having lower CSF Aβ measures. These differences persisted after adjustment for age and sex.
Discussion
In this study, we evaluated a set of 16 biomarkers from clinical, genetic, biochemical, and imaging modalities in a deeply characterized longitudinal cohort of 100 PD patients followed for up to 8 years. These 16 biomarkers were chosen a priori as part of the study aims, based on previous reports suggesting an association with cognition in PD. We found that 6 of the biomarkers were associated with cognitive decline in our cohort; the best-fitted multivariate linear mixed-effects model incorporated 3 of these biomarkers (thought disorder, SPARE-AD brain atrophy score, and APOE genotype), demonstrating the synergy afforded by investigating biomarkers from different modalities. Among these biomarkers, APOE genotype exerted the strongest effect, with APOE E4 allele carriers showing significantly greater cognitive decline in all cognitive domains.
Our findings corroborate and extend several previous reports. For example, the association between thought disorder or hallucinations and subsequent cognitive decline was first reported in an 8-year follow-up study demonstrating an increased risk of dementia in those with hallucinations (OR, 3.1; 95% CI, 1.6–6.2)45 and has subsequently been replicated in other longitudinal studies as well.21,46 In addition, baseline CSF Aβ and SPARE-AD score were both shown to predict subsequent cognitive decline in our study, although only SPARE-AD score was incorporated into the final multivariate model. CSF Aβ is a well-established biomarker of AD,47 and lower baseline CSF Aβ was predictive of cognitive decline in multiple longitudinal cohort studies of nondemented PD subjects.26,48,49 The SPARE-AD score is derived from a pattern of cortical atrophy seen in AD50,51 and has previously been shown to predict cognitive decline in both AD52 and in a cohort of 59 nondemented PD patients.17 Finally, the APOE E4 allele is an established risk factor for AD as well as reduced cognitive function in healthy adults.53,54 Although there are some exceptions,55–57 the majority of studies in PD58–60 also suggest a modest effect of APOE genotype on cognition in this disease. This is corroborated in meta-analyses demonstrating odds ratios of 1.661 and 1.7456 for dementia among PD patients carrying the APOE E4 allele. Overall, these studies demonstrate a modest effect of APOE genotype on cognitive outcome; however, they rely primarily on cross-sectional data.
Longitudinal studies investigating the effect of APOE genotype on cognitive decline in PD are rarer. However, in the CamPaIGN cohort of 107 subjects followed for 5 years, APOE E4 was not found to associate with cognitive decline, as measured by the Mini-Mental State Examination score or incidence of dementia,56 and a study of 64 PD patients who were followed for an average of 9.6 years also did not demonstrate an association between APOE and development or progression of dementia or time to dementia.62 These studies contrast with our prior reports of a significantly greater decline in DRS scores among APOE E4 carriers in 212 PD patients followed for an average of 2.04 years,13 and from the findings of the Parkinson’s Progression Marker Initiative (PPMI) study, in which APOE E4 carriers among 390 early-stage PD patients followed for 2 years showed significantly greater decline in Montreal Cognitive Assessment scores over time.49 The PPMI cohort enrolled subjects at an earlier phase of disease, demonstrating that in both the early and middle stages of disease, APOE genotype predicts more rapid cognitive decline. As the negative studies were smaller, used different instruments for assessment of PD cognition, and employed different statistical methods, they may have been underpowered to detect true differences in cognitive outcome among carriers of different APOE genotypes. In this respect, our finding that APOE E4 carriers are more likely to decline in all cognitive domains, versus only in the memory domain, suggests that global cognitive tests that cover nonmemory domains more extensively may be more sensitive to detection of the effect of APOE E4.
Beyond confirming previously reported predictors of cognitive decline in PD, our study examined many predictors from multiple modalities in relation to each other, an approach that is rarely used and may be important both for the development of clinically useful panels9 and for insight into underlying biological pathways. We found, for example, that although CSF Aβ levels and SPARE-AD scores are only modestly correlated (r = 0.26) and that both are independently associated with cognitive decline in PD, the best-fitted multivariate model omits CSF Aβ levels. In contrast, APOE genotype and SPARE-AD score, although both believed to reflect an underlying AD-like process, are both incorporated into the best-fitted multivariate model, suggesting independent contributions to predictive power. Indeed, when we directly examined SPARE-AD scores and CSF Aβ measures in our cohort, we found that individuals with an APOE E4 allele showed significant differences in CSF Aβ measures only, compared with individuals without an APOE E4 allele. Taken together, our results suggest that in our cohort APOE genotype effects and CSF Aβ measures may reflect a shared mechanistic process similar to the so-called amyloid cascade seen in AD, whereas SPARE-AD score may reflect a distinct pathogenic process that may or may not be AD pathology–driven. In this respect, it is worth noting that neuropathological features characteristic of AD are found in a large number of PD patients in multiple autopsy series, including a recent study of more than 200 autopsy-confirmed PD cases with 77% showing concomitant amyloid plaques and neurofibrillary tangles.63 Thus, it is likely that many of our cohort subjects, although nondemented at baseline, might have concomitant AD pathology. However, that the APOE genotype effect extends across cognitive domains, with no particular favoring of the memory domain, raises the question of whether APOE genotype effects in PD patients entirely mirror effects seen in AD patients.
Some biomarkers did not demonstrate an association with cognitive decline in our cohort. Education was not a significant independent predictor of change in DRS-2 in our mixed-effects model analysis. This finding may reflect the high degree of education in our cohort (average of 16 years of education, with SD of 2.3 years); it is also consistent with a recent longitudinal study of cognitive reserve in PD showing that although performance on cognitive testing may be affected by educational attainment, the ultimate development of dementia is unaffected.64 In addition, disease duration at enrollment was not an independent predictor of cognitive decline in our cohort (Supplemental Table 1). Moreover, when either education or disease duration was added as a covariate in the final multivariate mixed-effects model, results were unchanged, with no differences in the significance and minimal changes in the effect size, of individual biomarkers’ association with cognitive decline. These findings underline the relative importance of, in particular, the AD-related biomarkers of SPARE-AD score and APOE genotype in predicting future cognitive decline in even a somewhat heterogeneous population of midstage PD patients.
Several limitations of our study should be acknowledged. First, our cohort had a mean follow-up time of 2.9 years, with a range from 1 to 8 years. Because of this limitation, we used mixed linear models, which partially address this variability. Second, subjects in our cohort were enrolled on average 6 years into disease, so our results may not extend to an early PD cohort. However, the advantage of this type of cohort is that it may more accurately reflect the real-life patient population in a movement disorders clinic. Third, we used a single item on the UPDRS to cover the clinical characteristic of thought disorders at baseline; as a consequence, we are limited in our knowledge of what type of thought disorder or psychosis shows the strongest effect, and future work in this area may be informative. Finally, our study only encompassed 100 nondemented PD patients and as such may have been underpowered to detect smaller effects. The low number of GBA mutations found in this cohort is a particular example of this limitation. However, in this case, we chose depth (extensive characterization across multiple modalities in a longitudinally followed cohort) over breadth (larger numbers of less well-characterized individuals), as our objective was to understand which of the previously-reported biomarkers associated with cognition in PD might have the greatest effects.
In conclusion, among previously reported biomarkers associated with cognition in PD, we found that the presence of thought disorder, an AD-like regional pattern of atrophy, and the presence of the APOE E4 genotype most effectively predicted future cognitive decline in a multivariate analysis of longitudinally followed PD patients. Moreover, carriers of APOE E4 alleles were more likely to decline in all cognitive subdomains tested.
Supplementary Material
Acknowledgments
The authors acknowledge our patients for their generous participation in this study, Travis Unger for technical support, Lior Rennert for assistance with statistics, and the clinical research associates at the University of Pennsylvania Parkinson’s Disease and Movement Disorders Center.
Funding agencies: Funding for this study was from the NIH (P50NS053488).
Footnotes
Supporting Data
Additional Supporting Information may be found in the online version of this article at the publisher’s website
Relevant conflicts of interest/financial disclosures: None.
References
- 1.Guttmacher AE, Collins FS, Nussbaum RL, Ellis CE. Alzheimer’s disease and Parkinson’s disease. N Engl J Med. 2003;348(14):1356–1364. doi: 10.1056/NEJM2003ra020003. [DOI] [PubMed] [Google Scholar]
- 2.de Lau LM, Breteler MM. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006;5(6):525–535. doi: 10.1016/S1474-4422(06)70471-9. [DOI] [PubMed] [Google Scholar]
- 3.Hely MA, Reid WGJ, Adena MA, Halliday GM, Morris JGL. The Sydney Multicenter Study of Parkinson’s disease: the inevitability of dementia at 20 years. Mov Disord. 2008;23(6):837–844. doi: 10.1002/mds.21956. [DOI] [PubMed] [Google Scholar]
- 4.Litvan I, Aarsland D, Adler CH, et al. MDS task force on mild cognitive impairment in Parkinson’s disease: Critical review of PD-MCI. Mov Disord. 2011;26(10):1814–1824. doi: 10.1002/mds.23823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pedersen KF, Larsen JP, Tysnes O-B, Alves G. Prognosis of mild cognitive impairment in early Parkinson disease: the Norwegian Park West study. JAMA Neurol. 2013;70(5):580–586. doi: 10.1001/jamaneurol.2013.2110. [DOI] [PubMed] [Google Scholar]
- 6.Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69(3):89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- 7.Rosenthal LS, Drake D, Alcalay RN, et al. The NINDS Parkinson’s disease biomarkers program. Mov Disord. 2016;31(6):915–923. doi: 10.1002/mds.26438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The Parkinson Progression Marker Initiative (PPMI) Prog Neurobiol. 2011;95(4):629–635. doi: 10.1016/j.pneurobio.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mollenhauer B, Rochester L, Chen-Plotkin A, Brooks D. What can biomarkers tell us about cognition in Parkinson’s disease? Mov Disord. 2014;29(5):622–633. doi: 10.1002/mds.25846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Compta Y, Martí MJ, Ibarretxe-Bilbao N, et al. Cerebrospinal tau, phospho-tau, and beta-amyloid and neuropsychological functions in Parkinson’s disease. Mov Disord. 2009;24(15):2203–2210. doi: 10.1002/mds.22594. [DOI] [PubMed] [Google Scholar]
- 11.Marder K, Tang MX, Cote L, Stern Y, Mayeux R. The frequency and associated risk factors for dementia in patients with Parkinson’s disease. Arch Neurol. 1995;52(7):695–701. doi: 10.1001/archneur.1995.00540310069018. [DOI] [PubMed] [Google Scholar]
- 12.Compta Y, Valente T, Saura J, et al. Correlates of cerebrospinal fluid levels of oligomeric- and total-α-synuclein in premotor, motor and dementia stages of Parkinson’s disease. J Neurol. 2015;262(2):294–306. doi: 10.1007/s00415-014-7560-z. [DOI] [PubMed] [Google Scholar]
- 13.Morley JF, Xie SX, Hurtig HI, et al. Genetic influences on cognitive decline in Parkinson’s disease. Mov Disord. 2012;27(4):512–518. doi: 10.1002/mds.24946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams-Gray CH, Evans JR, Goris A, et al. The distinct cognitive syndromes of Parkinson’s disease: 5 year follow-up of the CamPaIGN cohort. Brain. 2009;132(11):2958–2969. doi: 10.1093/brain/awp245. [DOI] [PubMed] [Google Scholar]
- 15.Lim NS, Swanson CR, Cherng H-R, et al. Plasma EGF and cognitive decline in Parkinson’s disease and Alzheimer’s disease. Ann Clin Transl Neurol. 2016;3(5):346–355. doi: 10.1002/acn3.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neumann J, Bras J, Deas E, et al. Glucocerebrosidase mutations in clinical and pathologically proven Parkinson’s disease. Brain. 2009;132(7):1783–1794. doi: 10.1093/brain/awp044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weintraub D, Dietz N, Duda JE, et al. Alzheimer’s disease pattern of brain atrophy predicts cognitive decline in Parkinson’s disease. Brain. 2012;135(1):170–180. doi: 10.1093/brain/awr277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Compta Y, Pereira JB, Ríos J, et al. Combined dementia-risk biomarkers in Parkinson’s disease: A prospective longitudinal study. Parkinsonism Relat Disord. 2013;19(8):717–724. doi: 10.1016/j.parkreldis.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 19.Williams-Gray CH, Foltynie T, Brayne CEG, Robbins TW, Barker RA. Evolution of cognitive dysfunction in an incident Parkinson’s disease cohort. Brain. 2007;130(7):1787–1798. doi: 10.1093/brain/awm111. [DOI] [PubMed] [Google Scholar]
- 20.Pigott K, Rick J, Xie SX, et al. Longitudinal study of normal cognition in Parkinson disease. Neurology. 2015;85(15):1276–1282. doi: 10.1212/WNL.0000000000002001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uc EY, McDermott MP, Marder KS, et al. Incidence of and risk factors for cognitive impairment in an early parkinson disease clinical trial cohort. Neurology. 2009;73(18):1469–1477. doi: 10.1212/WNL.0b013e3181bf992f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aarsland D, Andersen K, Larsen JP, Lolk A, Nielsen H, Kragh-Sørensen P. Risk of dementia in Parkinson’s disease: a community-based, prospective study. Neurology. 2001;56(6):730–736. doi: 10.1212/wnl.56.6.730. [DOI] [PubMed] [Google Scholar]
- 23.Mollenhauer B, Trenkwalder C, von Ahsen N, et al. Beta-amlyoid 1–42 and tau-protein in cerebrospinal fluid of patients with Parkinson’s disease dementia. Dement Geriatr Cogn Disord. 2006;22(3):200–208. doi: 10.1159/000094871. [DOI] [PubMed] [Google Scholar]
- 24.Bäckström DC, Eriksson Domellöf M, Linder J, et al. Cerebrospinal Fluid Patterns and the Risk of Future Dementia in Early, Incident Parkinson Disease. JAMA Neurol. 2015;72(10):1175. doi: 10.1001/jamaneurol.2015.1449. [DOI] [PubMed] [Google Scholar]
- 25.Montine TJ, Shi M, Quinn JF, et al. CSF Aβ 42 and tau in Parkinson’s disease with cognitive impairment. Mov Disord. 2010;25(15):2682–2685. doi: 10.1002/mds.23287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siderowf A, Xie SX, Hurtig H, et al. CSF amyloid β 1–42 predicts cognitive decline in Parkinson disease. Neurology. 2010;75(12):1055–1061. doi: 10.1212/WNL.0b013e3181f39a78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iwakura Y, Piao YS, Mizuno M, et al. Influences of dopaminergic lesion on epidermal growth factor-ErbB signals in Parkinson’s disease and its model: nurotrophic implication in nigrostriatal neurons. J Neurochem. 2005;93(4):974–983. doi: 10.1111/j.1471-4159.2005.03073.x. [DOI] [PubMed] [Google Scholar]
- 28.Inoue H, Lin L, Lee X, et al. Inhibition of the leucine-rich repeat protein LINGO-1 enhances survival, structure, and function of dopaminergic neurons in Parkinson’s disease models. Proc Natl Acad Sci U S A. 2007;104(36):14430–14435. doi: 10.1073/pnas.0700901104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen-Plotkin AS, Hu WT, Siderowf A, et al. Plasma epidermal growth factor levels predict cognitive decline in Parkinson disease. Ann Neurol. 2011;69(4):655–663. doi: 10.1002/ana.22271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chahine LM, Qiang J, Ashbridge E, et al. Clinical and biochemical differences in patients having Parkinson disease with vs without GBA mutations. JAMA Neurol. 2013;70(7):852–858. doi: 10.1001/jamaneurol.2013.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davis MY, Johnson CO, Leverenz JB, et al. Association of GBA Mutations and the E326K Polymorphism With Motor and Cognitive Progression in Parkinson Disease. JAMA Neurol. 2016;73(10):1217. doi: 10.1001/jamaneurol.2016.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weintraub D, Doshi J, Koka D, et al. Neurodegeneration across stages of cognitive decline in Parkinson disease. Arch Neurol. 2011;68(12):1562–1568. doi: 10.1001/archneurol.2011.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hanganu A, Bedetti C, Degroot C, et al. Mild cognitive impairment is linked with faster rate of cortical thinning in patients with Parkinson’s disease longitudinally. Brain. 2014;137(4):1120–129. doi: 10.1093/brain/awu036. [DOI] [PubMed] [Google Scholar]
- 34.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55(3):181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fahn S, Elton R. Unified Parkinson’s Disease Rating Scale. Recent Developments in Parkinson’s Disease. 1987:153–163. [Google Scholar]
- 36.Goetz CG, Poewe W, Rascol O, et al. Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: Status and recommendations. Mov Disord. 2004;19(9):1020–1028. doi: 10.1002/mds.20213. [DOI] [PubMed] [Google Scholar]
- 37.Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord. 2010;25(15):2649–2653. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- 38.Goodarzi Z, Mrklas KJ, Roberts DJ, Jette N, Pringsheim T, Holroyd-Leduc J. Detecting depression in Parkinson disease. Neurology. 2016;87(4):426–437. doi: 10.1212/WNL.0000000000002898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jankovic J, McDermott M, Carter J, et al. Variable expression of Parkinson’s disease: a base-line analysis of the DATATOP cohort. The Parkinson Study Group. Neurology. 1990;40(10):1529–1534. doi: 10.1212/wnl.40.10.1529. [DOI] [PubMed] [Google Scholar]
- 40.Lucas JA, Smith GE, Bohac DL, et al. Normative data for the Mattis Dementia Rating Scale. J Clin Exp Neuropsychol. 1998;20(4):536–547. doi: 10.1076/jcen.20.4.536.1469. [DOI] [PubMed] [Google Scholar]
- 41.Goris A, Williams-Gray CH, Clark GR, et al. Tau and α-synuclein in susceptibility to, and dementia in, Parkinson’s disease. Ann Neurol. 2007;62(2):145–153. doi: 10.1002/ana.21192. [DOI] [PubMed] [Google Scholar]
- 42.Su Y-S, Gelman A, Hill J, Yajima M. Multiple imputation with diagnostics (mi) in R: Opening Windows into the Black Box. J Stat Softw. 2011;45(2):1–31. [Google Scholar]
- 43.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38(4):963–974. [PubMed] [Google Scholar]
- 44.Berlyand Y, Weintraub D, Xie SX, et al. An Alzheimer’s disease-derived biomarker signature identifies Parkinson’s disease patients with dementia. PLoS One. 2016;11(1):e0147319. doi: 10.1371/journal.pone.0147319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aarsland D, Andersen K, Larsen JP, et al. Prevalence and characteristics of dementia in Parkinson disease. Arch Neurol. 2003;60(3):387. doi: 10.1001/archneur.60.3.387. [DOI] [PubMed] [Google Scholar]
- 46.Anang JBM, Gagnon J-F, Bertrand J-A, et al. Predictors of dementia in Parkinson disease: A prospective cohort study. Neurology. 2014;83(14):1253–1260. doi: 10.1212/WNL.0000000000000842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blennow K, Hampel H, Weiner M, Zetterberg H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat Rev Neurol. 2010;6(3):131–144. doi: 10.1038/nrneurol.2010.4. [DOI] [PubMed] [Google Scholar]
- 48.Terrelonge M, Marder KS, Weintraub D, Alcalay RN. CSF β-amyloid 1–42 predicts progression to cognitive impairment in newly diagnosed Parkinson disease. J Mol Neurosci. 2016;58(1):88–92. doi: 10.1007/s12031-015-0647-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schrag A, Siddiqui UF, Anastasiou Z, Weintraub D, Schott JM. Clinical variables and biomarkers in prediction of cognitive impairment in patients with newly diagnosed Parkinson’s disease: a cohort study. Lancet Neurol. 2017;16(1):66–75. doi: 10.1016/S1474-4422(16)30328-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fan Y, Batmanghelich N, Clark CM, Davatzikos C, Alzheimer’s Disease Neuroimaging Initiative, ADN Spatial patterns of brain atrophy in MCI patients, identified via high-dimensional pattern classification, predict subsequent cognitive decline. Neuroimage. 2008;39(4):1731–1743. doi: 10.1016/j.neuroimage.2007.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Davatzikos C, Fan Y, Wu X, Shen D, Resnick SM. Detection of prodromal Alzheimer’s disease via pattern classification of magnetic resonance imaging. Neurobiol Aging. 2008;29(4):514–523. doi: 10.1016/j.neurobiolaging.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Davatzikos C, Xu F, An Y, Fan Y, Resnick SM. Longitudinal progression of Alzheimer’s-like patterns of atrophy in normal older adults: the SPARE-AD index. Brain. 2009;132(8):2026–2035. doi: 10.1093/brain/awp091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Farrer LA, Cupples LA, Haines JL, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 278(16):1349–1356. [PubMed] [Google Scholar]
- 54.Small BJ, Rosnick CB, Fratiglioni L, Bäckman L. Apolipoprotein E and cognitive performance: a meta-analysis. Psychol Aging. 2004;19(4):592–600. doi: 10.1037/0882-7974.19.4.592. [DOI] [PubMed] [Google Scholar]
- 55.Marder K, Maestre G, Cote L, et al. The apolipoprotein epsilon 4 allele in Parkinson’s disease with and without dementia. Neurology. 1994;44(7):1330–1331. doi: 10.1212/wnl.44.7.1330. [DOI] [PubMed] [Google Scholar]
- 56.Williams-Gray CH, Goris A, Saiki M, et al. Apolipoprotein E genotype as a risk factor for susceptibility to and dementia in Parkinson’s disease. J Neurol. 2009;256(3):493–498. doi: 10.1007/s00415-009-0119-8. [DOI] [PubMed] [Google Scholar]
- 57.Ezquerra M, Campdelacreu J, Gaig C, et al. Lack of association of APOE and tau polymorphisms with dementia in Parkinson’s disease. Neurosci Lett. 2008;448(1):20–23. doi: 10.1016/j.neulet.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 58.Parsian A, Racette B, Goldsmith LJ, Perlmutter JS. Parkinson’s disease and apolipoprotein E: possible association with dementia but not age at onset. Genomics. 2002;79(3):458–461. doi: 10.1006/geno.2002.6707. [DOI] [PubMed] [Google Scholar]
- 59.Pankratz N, Byder L, Halter C, et al. Presence of an APOE4 allele results in significantly earlier onset of Parkinson’s disease and a higher risk with dementia. Mov Disord. 2006;21(1):45–49. doi: 10.1002/mds.20663. [DOI] [PubMed] [Google Scholar]
- 60.Tsuang D, Leverenz JB, Lopez OL, et al. APOE ∈4 Increases Risk for Dementia in Pure Synucleinopathies. JAMA Neurol. 2013;70(2):223. doi: 10.1001/jamaneurol.2013.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang X, Chen P, Kaufer DI, et al. Apolipoprotein E and dementia in Parkinson disease. Arch Neurol. 2006;63(2):189. doi: 10.1001/archneur.63.2.189. [DOI] [PubMed] [Google Scholar]
- 62.Kurz MW, Dekomien G, Nilsen OB, Larsen JP, Aarsland D, Alves G. APOE alleles in Parkinson disease and their relationship to cognitive decline: a population-based, longitudinal study. J Geriatr Psychiatry Neurol. 2009;22(3):166–170. doi: 10.1177/0891988709332945. [DOI] [PubMed] [Google Scholar]
- 63.Irwin DJ, Grossman M, Weintraub D, et al. Neuropathological and genetic correlates of survival and dementia onset in synucleinopathies: a retrospective analysis. Lancet Neurol. 2017;16(1):55–65. doi: 10.1016/S1474-4422(16)30291-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hindle JV, Hurt CS, Burn DJ, et al. The effects of cognitive reserve and lifestyle on cognition and dementia in Parkinson’s disease—a longitudinal cohort study. Int J Geriatr Psychiatry. 2016;31(1):13–23. doi: 10.1002/gps.4284. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


