Abstract
The basolateral amygdala (BLA) controls numerous behaviors, like anxiety and reward seeking, via the activity of glutamatergic principal neurons. These BLA neurons receive excitatory inputs primarily via two major anatomical pathways – the external capsule (EC), which contains afferents from lateral cortical structures, and the stria terminalis (ST), containing synapses from more midline brain structures. Chronic intermittent ethanol (CIE) exposure/withdrawal produces distinct alterations in these pathways. Specifically, 10 days of CIE (via vapor inhalation) increases presynaptic function at ST synapses and postsynaptic function at EC synapses. Given that 10-day CIE/withdrawal also increases anxiety-like behavior, we sought to examine the development of these alterations at these inputs using an exposure time-course in both male and female rats. Specifically, using 3, 7, and 10 days CIE exposure, we found that all three durations increase anxiety-like behavior in the elevated plus maze. At BLA synapses, increased presynaptic function at ST inputs required shorter exposure durations relative to post-synaptic alterations at EC inputs in both sexes. But, synaptic alterations in females required longer ethanol exposures compared to males. These data suggest that presynaptic alteration at ST-BLA afferents is an early neuroadaptation during repeated ethanol exposures. And, the similar patterns of presynaptic-then-postsynaptic facilitation across the sexes suggest the former may be required for the latter. These cooperative interactions may contribute to the increased anxiety-like behavior that is observed following CIE-induced withdrawal and may provide novel therapeutic targets to reverse withdrawal-induced anxiety.
Keywords: Basolateral amygdala, anxiety, paired-pulse ratio, strontium substitution, sex differences, ethanol dependence
Introduction
Anxiety disorders and alcohol use disorders (AUDs) frequently co-occur and the clinical significance of this relationship has been reported in epidemiological studies for decades (Ross, 1995; Merikangas et al., 1998; Kushner et al., 2000; Burns and Teesson, 2002; Grant et al., 2015). Interestingly, the relationship between anxiety disorders and AUDs is more strongly associated with alcohol dependence than with alcohol abuse (Kushner et al., 2000; Hasin et al., 2007). This is likely because alcohol dependent individuals who suddenly stop or drastically reduce their drinking experience a wide range of physical (e.g., heightened respiration, blood pressure, seizures, delirium tremors) and psychological (e.g., anxiety, dysphoria, agitation) symptoms (Finn and Crabbe, 1997; Becker, 2000). The anxiety that emerges from alcohol withdrawal is so severe that people often relapse and self-medicate with alcohol to seek relief from their symptoms (Schellekens et al., 2015; Driessen et al., 2001). Therefore, withdrawal-induced anxiety symptoms associated with terminating long-term alcohol exposure are strong contributing factors for relapse in alcohol-dependent individuals. Similar to humans, animals show increased anxiety-like behavior during withdrawal, which may likewise contribute to the enhanced alcohol consumption observed during this time (Valdez et al., 2002).
One commonly used and well validated animal model of producing alcohol (ethanol) dependence is via vapor inhalation (Goldstein and Pal, 1971). This model consistently produces a dependence-like phenotype and yields behaviors (e.g., increased anxiety, enhanced ethanol consumption) that are frequently cited as markers of ethanol withdrawal in the rodent literature (Finn and Crabbe, 1997; Kliethermes et al., 2004; O’Dell et al., 2004;). In addition to overt behavioral signs that emerge following chronic ethanol vapor exposure, neurophysiological adaptations also occur in the lateral/basolateral amygdala (BLA), a brain region that is an integral component of the fear/anxiety circuit (Janak and Tye, 2015; Davis et al., 1994; Phillips and LeDoux, 1992).
The amygdala receives sensory information through multiple projections with major pathways arriving via the external capsule (‘lateral’ inputs in a coronal slice) and the stria terminalis (‘medial’ inputs) (Rainnie et al., 1991; Davis et al., 1994; Bauer et al., 2002). This information is first processed in the BLA, and is then relayed to downstream brain regions, ultimately resulting in a physiological/psychological response (e.g., anxiety) (Davis et al., 1994; Janak and Tye, 2015). The BLA is comprised primarily of pyramidal-shaped glutamatergic projection neurons and non-pyramidal-shaped GABAergic interneurons, and manipulating activity of these neurons dramatically alters anxiety-like behavior in rodents (Sanders and Shekhar, 1995a, 1995b, Sajdyk and Shekhar, 1997a, 1997b). There is also evidence demonstrating that the BLA may be involved with the anxiogenic effects of ethanol observed during withdrawal (Läck et al., 2007; 2008). Our laboratory has also shown that alcohol dependence/withdrawal modulates glutamatergic synaptic transmission onto BLA projection neurons in an input-dependent manner (Christian et al., 2012, 2013). Specifically, 24h after 10 days of chronic intermittent ethanol (CIE) vapor exposure, glutamatergic afferents arriving along the external capsule/lateral pathway express postsynaptic alterations characterized by increased AMPA receptor function that correlate with increased receptor phosphorylation and trafficking. These effects contrast with glutamatergic afferents arriving via the stria terminalis/medial pathway which express presynaptic adaptations represented by increased glutamate release probability, increased synaptic glutamate concentrations, a larger pool of readily releasable vesicles, and decreased failure rates at these terminals.
Our laboratory and others demonstrated that withdrawal from alcohol produces increases in anxiety-like behavior, and this may be associated with adaptations in glutamatergic synaptic transmission occurring in the BLA during dependence. However, the time-course of these behavioral and neurophysiological alterations are unknown. This is significant because in the fear conditioning literature, a temporal relationship exists between pre- and post-synaptic plasticity. More specifically, presynaptic activation of the stria terminalis inputs facilitates postsynaptic long-term potentiation at external capsule synapses, which may be important for fear learning (Cho et al., 2012; Fonseca, 2013). It is possible that ethanol dependence and withdrawal may also differentially modulate presynaptic facilitation at stria inputs and postsynaptic plasticity expressed at the external capsule in time-specific ways.
Sex differences to a variety of ethanol-related behaviors have been reported in both preclinical and clinical studies (Devaud and Chadda, 2001; Nolen-Hoeksema, 2004; Devaud et al., 2006; Morales et al., 2015; Jury et al., 2017). For example, our laboratory has recently shown that while dependence produced by 10 days of CIE exposure increased ethanol consumption in males (as has been reported numerous times by others (Woolley et al., 1997; Carnicella et al., 2008; Simms et al., 2008; Meyer et al., 2013; Kimbrough et al., 2017), female ethanol drinking remains unaffected (Butler et al., 2014; Rosenwasser et al., 2014; Morales et al., 2015). Despite behavioral evidence demonstrating differences between males and females in alcohol use and sensitivity, we and few others have examined neurophysiological changes that may emerge following ethanol dependence in females. Therefore, the current series of experiments examined the time course of ethanol adaptations that occur presynaptically from stria terminalis afferents and postsynaptically via external capsule afferents onto BLA principal neurons after various durations of CIE vapor exposure and 24h withdrawal in male and female Sprague-Dawley rats. These data will provide further characterization of synaptic adaptations that occur on BLA principal neurons after various CIE exposures that likely contributes to anxiety-like behavior during withdrawal, which may ultimately lead to relapse.
Experimental Procedures
Animals
Five week old male and female Sprague-Dawley rats were obtained from Envigo (Indianapolis, IN) and were given unlimited access to standard rat chow and water throughout the experimental procedure. Upon arrival, rats were pair-housed and maintained on a reverse 12:12 h light dark cycle (lights on at 9 PM). All animal care procedures were in accordance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the Wake Forest Animal Care and Use Committee.
Chronic intermittent ethanol (CIE) vapor exposure
Pair-housed rats were exposed to chronic intermittent ethanol (CIE) vapor for 3, 7, or 10 days, using standard procedures from our laboratory (Läck et al., 2007; Christian et al., 2012; Morales et al., 2015). Briefly, home cages were placed in larger, custom-built Plexiglas chambers (Triad Plastics, Winston-Salem, NC), and at the beginning of the light cycle (9pm EST), ethanol vapor was pumped into the chambers and maintained at 15-20 mg/L throughout the exposure for 12 h day. Air-exposed control animals were similarly housed, except they received room-air only while in the chambers. Animals were weighed daily; and, tail blood samples were collected once during the CIE exposure to monitor blood ethanol concentrations (BECs) and adjust ethanol vapor levels as necessary (Table 1). Blood ethanol concentrations were determined using a standard, commercially-available alcohol dehydrogenase/NADH enzymatic assay (Diagnostic Chemicals Limited, Oxford CT). At arrival, body weights (in grams ±SEM) for males and females were 99.15±1.15 and 86.25±0.57, respectively. After 3, 7, and 10 days of CIE exposure, males weighed 183.91±2.59, 173.21±1.18, and 161.89±2.89, while air-exposed males weighed 219.05±5.01. After 3, 7, and 10 days of CIE exposure, females weighed 138.85±5.01, 139.5±3.44, and 135.43±5.66, while air-exposed females weighed 148.94±3.48.
Table 1.
Mean ± SEM For Blood Ethanol Concentrations during Each CIE Exposurea
| CIE Duration | 3 Days | 7 Days | 10 Days |
|---|---|---|---|
| Males | 231.6 ± 6.3 | 191.4 ± 9.9 | 259.5 ± 12.2 |
| Females | 247.5 ± 17.6 | 205.7 ± 22.9 | 238.6 ± 12.1 |
Units are mg/dL. See Methods for details.
Elevated Plus Maze
To validate our CIE vapor exposure as a model of ethanol dependence, we assessed anxiety-like behavior during withdrawal using the Elevated Plus Maze (EPM) apparatus. Rats were placed onto the central junction of the EPM facing an open arm for the 5 min test. Open arms were dimly lit (~40 lux). Anxiety-like behavior and general locomotion were measured from time spent and entries into each arm. Beam breaks were collected by a computer equipped with MED-PC (Med Associates) connected to the plus maze. Between animals, the apparatus was cleaned with warm water and mild soap, and then thoroughly dried.
Estrous Cycle determination
To determine estrous cycle in a separate group of females, samples were collected via vaginal lavage. At the same time each morning (9 am, EST), female rats were gently restrained and a clean pipette filled with warm, sterile saline (200μL) was inserted at the tip of the vaginal opening. The pipette was flushed 2-3 times and a sample was collected and placed onto a clean microscope slide. Cells were visualized and identified using a light microscope, and determination of estrous cycle stage was based on the cells present (Marcondes et al., 2002; Caligioni, 2009; McLean et al., 2012).
Electrophysiology
Slice preparation
Animals were anesthetized with isoflurane prior to decapitation according to an approved Wake Forest University Health Sciences IACUC protocol. Brains were rapidly removed and incubated in an ice-cold sucrose-modified artificial cerebral spinal fluid (aCSF) solution containing (in mM): 180 Sucrose, 30 NaCl, 4.5 KCl, 1 MgCl2•6H20, 26 NaHCO3, 1.2 NaH2PO4, 10 glucose, 0.10 ketamine. Coronal amygdala slices (400μM) were prepared using a VT1200/S vibrating blade microtome (Leica, Buffalo Grove, IL) and incubated for ≥1 h in room temperature, oxygenated, standard aCSF solution containing (in mM): 126 NaCl, 3 KCl, 1.25 NaH2PO4, 2 MgSO4, 26 NaHCO3, 10 glucose, and 2 CaCl2 prior to recordings. All chemicals were obtained from Sigma-Aldrich (St. Louis, MO) or Tocris (Ellisville, Missouri).
Whole-cell patch-clamp recording
Methods for whole-cell voltage clamp recordings were similar to those reported previously from our laboratory (Christian et al., 2012, 2013; Gioia et al., 2017). Coronal slices containing the BLA were transferred to a submersion-type recording chamber that was continuously perfused with room temperature aCSF (2 ml/min). Glutamatergic responses were recorded from a membrane holding potential of −70mV using electrodes filled with an intracellular solution containing (in mM): 145 CsOH, 10 EGTA, 5 NaCl, 1 MgCl, 10 HEPES, 4 Mg-ATP, 0.4 Na-GTP, pH ~7.25 with gluconic acid, osmolarity between 285-295 mmol/kg. Glutamatergic synaptic currents were pharmacologically isolated using the GABAA antagonist, picrotoxin (100 μM) in the bath aCSF. Synaptic responses were electrically evoked every 30s using concentric bipolar stimulating electrodes (FHC Inc, Bowdoin, ME) placed either within the internal capsule (stria terminalis) or within the external capsule along the lateral boarder of the BLA. Stimulus intensities were submaximal and normalized to elicit synaptic responses with amplitudes less than 200pA. Previous work has shown these stimulation intensities produce distinct responses from anatomically unique inputs (Christian et al., 2013) and prevent response contamination by local circuits. Data were collected via an Axopatch 700B amplifier (Molecular Devices, Foster City, CA) and later analyzed using pClamp software (Molecular Devices, Foster City, CA). Presumptive principal neurons were included based on their electrophysiological characteristics of high membrane capacitance (>100 pF) and low access resistance (≤25 MΩ) (Washburn and Moises, 1992). Cells that did not meet these criteria were excluded from analysis.
Paired-pulse ratio
Two electrical stimuli were evoked at the stria terminalis or external capsule at an inter-stimulus interval of 50 msec, with this short interval traditionally viewed as an indicator of presynaptic release probability (Schulz et al., 1994; Fioravante and Regehr, 2011). The paired-pulse ratio (PPR) was conservatively calculated using the evoked EPSC amplitudes as: ([Peak 2 amplitude - Peak 1 amplitude]/Peak 1 amplitude).
Strontium Substitution
As a measure of postsynaptic function, a strontium (Sr2+) substitution method was used whereby extracellular calcium (2mM) was partially replaced by strontium (2mM). This technique allows for the measurement of asynchronous EPSCs (aEPSCs) (Choi and Lovinger, 1997; Christian et al., 2012), with changes in amplitude providing a measure of postsynaptic efficacy and changes in frequency providing a measure of calcium-independent presynaptic function. As described previously by our laboratory (Christian et al., 2012), a bipolar stimulating electrode was placed at the external capsule where an electrical stimulation was applied every 30 seconds. Only responses beginning 50 ms post-stimulation artifact were included during a 400 ms window. aEPSC amplitude and frequency were analyzed with MiniAnalysis software (Decatur, GA).
Statistics
All data analyses were conducted using GraphPad Prism 5.0. Data were analyzed via analysis of variance (ANOVA), with Bonferroni post-hoc tests used to determine the locus of effect. Chi-square tests were conducted on categorical data. Significance was set at p<0.05. Data are presented as mean ± SEM throughout the text and figures.
Results
All durations of chronic intermittent ethanol exposure increased anxiety-like behavior in males and females during withdrawal
To behaviorally validate our CIE model of ethanol dependence, rats were tested for anxiety-like behavior on the elevated plus maze 24h into withdrawal after 3, 7, or 10 days of CIE. Percent open arm time (OAT: open arm time/[open arm time + closed arm time]x100)) and percent open arm entries (OAE: open arm entries/[open arm entries + closed arm entries]x100)) were calculated and used as indices of anxiety-like behavior (Pellow et al., 1985) on the elevated plus maze. The two-way ANOVA of %OAT revealed a main effect of exposure [F(3,59)=11.87,p<0.001] and sex [F(1,59)=8.02,p<0.01] but, importantly, no interaction [F(3,59]=0.78,p>0.05] (Figure 1, left). Bonferroni post-hoc tests demonstrated that all CIE durations decreased time spent in the open arms, regardless of sex. The two-way ANOVA of %OAE likewise revealed a main effect of exposure only [F(3,59)=6.34,p<0.001] (Figure 1, middle); however, Bonferroni post-hoc tests demonstrated that males entered the open arms significantly less after 3 and 7 days whereas this effect was only observed in females after 10 days of CIE. Closed arm entries, a proxy for general locomotor activity, were significantly lower in females compared to males [F(1,59)=6.12,p<0.05]; but there was no significant effect of treatment [F(3,59)=2.08,p>0.05] and no significant sex × treatment interaction [F(3,59)=0.21,p>0.05] (Figure 1, right).
Fig. 1.
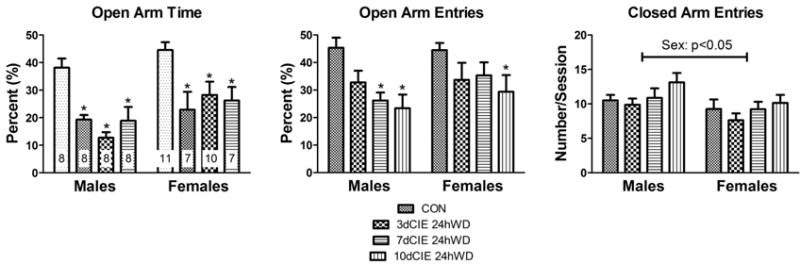
All durations of chronic intermittent ethanol exposure (3, 7, or 10 day) increased anxiety-like behavior in male and female rats during withdrawal (24h) on the elevated plus maze. Percent (%) open arm time (left, ‘n’ for these experiments is indicated within each column) and percent (%) open arm entries (right) for male and female rats. * denotes a significant difference with a 2-way ANOVA and Bonferroni’s post-hoc test (p<0.001) between CIE duration and control (CON).
Withdrawal increases glutamate release probability at medial inputs after fewer ethanol exposures in males
To examine the presynaptic effects of CIE exposure on medial inputs, a two-way ANOVA of the paired-pulse ratio was conducted and revealed a main effect of exposure [F(3,48)=14.92, p<0.001]. Bonferroni post-hoc tests revealed that withdrawal from all CIE durations decreased the paired-pulse ratio, demonstrating an increase in glutamate release probability in males. However, a decrease in the paired-pulse ratio was only observed after 7 and 10 days of CIE exposure in females, suggesting that females may be less sensitive to presynaptic alterations in glutamate release probability than males (Figure 2). To confirm that these presynaptic effects are specific for medial inputs as we have previously seen (Christian et al., 2012), a separate group of male and female rats underwent 10 days of CIE exposure, and paired-pulse ratios were collected from electrical stimulation at the external capsule 24h into withdrawal. As expected, the two-way ANOVA of paired-pulse ratios at the external capsule revealed no significant main effects or interactions in males or females (Figure 3).
Fig. 2.
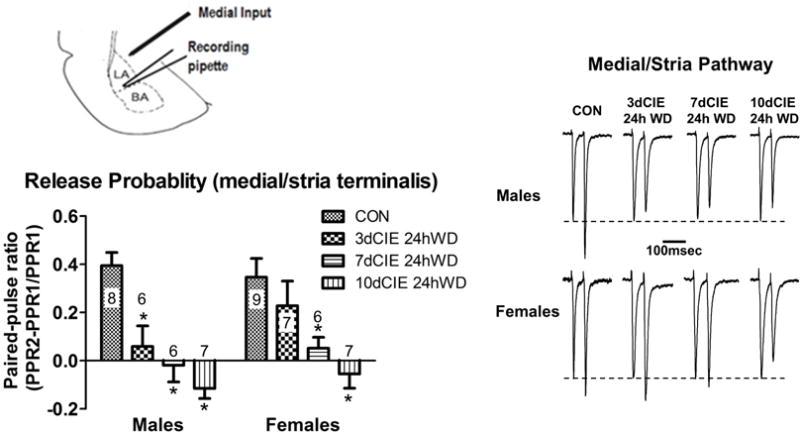
Chronic intermittent ethanol and withdrawal increased glutamate release probability at medial/stria terminalis inputs after fewer exposures in males. Diagram showing the recording pipette in the lateral/basolateral amygdala and the stimulating electrode at the medial input pathway (top left). Summary of paired-pulse ratio data for the treatment groups and sexes (lower left; ‘n’ for each group indicates number of neurons). * denotes a significant difference with a 2-way ANOVA and Bonferroni’s post-hoc test (p<0.001) between CIE duration and control (CON). Inter-stimulus interval of 50 msec. Representative traces for each CIE duration in males and females (right). Inter-stimulus interval of 50msec.
Fig. 3.
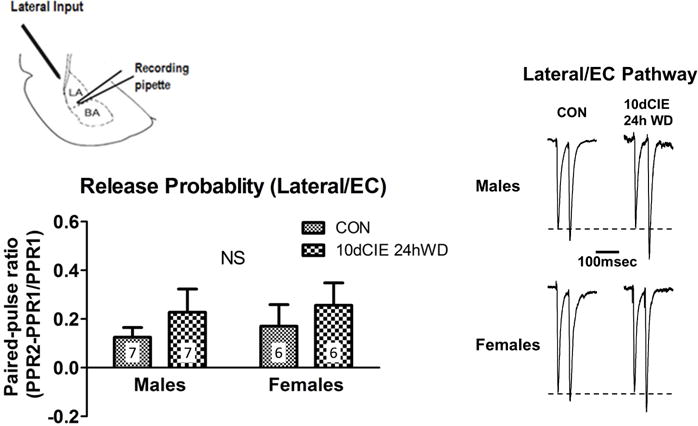
Chronic intermittent ethanol and withdrawal did not alter glutamate release probability at lateral/external capsule inputs in male or female rats. Diagram showing the recording pipette in the lateral/basolateral amygdala and the stimulating electrode at the lateral input (top left). Summary of paired-pulse ratios from the different treatment groups and sexes (lower left; ‘n’ for each group indicates the number of neurons). NS = non-significant (p=0.92), with a 2-way ANOVA. Representative traces in males and females (right). Inter-stimulus interval of 50 msec.
Withdrawal from chronic intermittent ethanol exposure increases asynchronous EPSC amplitude at external capsule inputs after longer durations in males and females
To explore the post-synaptic effects of withdrawal from CIE exposure at external capsule inputs, a two-way ANOVA for amplitude of aEPSCs was conducted and revealed significant main effects of exposure [F(3,47)=24.24, p<0.01] and sex [F(1,47)=16.69,p<0.001] (Figure 4). Bonferroni post-hoc tests revealed that, in males, 7 and 10 days of CIE increased aEPSCs at external capsule inputs whereas this effect emerged in females only after 10 days of CIE. These increases in amplitude are suggestive of post-synaptic alterations that are duration-dependent in males and females. These data also support previous findings demonstrating that presynaptic activation of medial (i.e., stria terminalis) inputs can facilitate alterations at external capsule synapses (Cho et al., 2012).
Fig. 4.
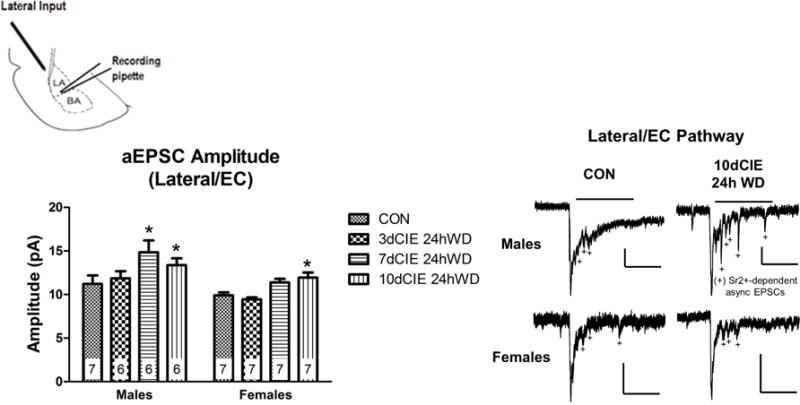
Chronic intermittent ethanol and withdrawal increased asynchronous EPSC amplitude at lateral/external capsule inputs after longer durations in males and females. Diagram showing the recording pipette in the lateral/basolateral amygdala and the stimulating electrode at the lateral input (top left). Summary of aEPSC amplitude data across the treatment groups and sexes. Numbers represents the ‘n’ (neurons) for each group. Representative traces in males and females (right). Scalar bars denote 250msec for all traces and either 50pA or 25pA (male 10dCIE 24h WD only). Bars above the traces indicate the 400msec window used to analyze aEPSC amplitude and frequency (see Methods). * denotes a significant difference with a 2-way ANOVA and Bonferroni’s post-hoc test (p<0.01) between CIE duration and control (CON).
Longer durations of chronic intermittent ethanol exposure disrupts estrous cycle in females
To determine the impact of chronic intermittent ethanol exposure on estrous cycle, a group of females was exposed to 10 days of CIE exposure or air and vaginal smears were collected at three different time points: 1) Baseline: before CIE exposure; 2) Day 4: middle of CIE exposure; Day 10: final day of CIE exposure. The chi-square analysis revealed a strong trend (χ2(2)=5.677, p=0.059) at the 10 day CIE time point. These data suggest that CIE exposure disrupts estrous cycle, with more CIE-exposed females tending to be in diestrus I and II compared to air-exposed females (Figure 5).
Fig. 5.
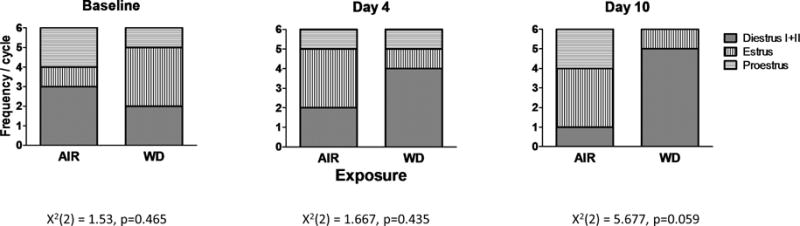
Longer durations of chronic intermittent ethanol exposure disrupted estrous cycle in females. Proportion of females in Diestrus I+II, Estrus, and Proestrus during baseline (χ2, p=0.465), Day 4 (χ2, p=0.435), and Day 10 (χ2, p=0.059) of the 10 day CIE exposure (n=6 animals).
Discussion
The current experiments examined the temporal relationships between pre- and post-synaptic alterations at medial and lateral afferents onto BLA principal neurons during/following chronic intermittent ethanol vapor exposure. Our main findings are that 1) pre-synaptic facilitation requires fewer ethanol exposures compared to post-synaptic alterations independent of sex and, 2) female synapses require longer ethanol exposures than do male synapses. These findings together suggest that withdrawal-induced increases in glutamate release at medial afferents may be necessary for the development of post-synaptic alterations at lateral afferents.
Anxiogenic effects of ethanol withdrawal
Our laboratory has previously shown that withdrawal following 10 days of CIE exposure is sufficient to increase anxiety-like behavior (Läck et al., 2007, 2008; Morales et al., 2015). However, it was not clear that shorter CIE durations would produce similar phenotypes. The results from the elevated plus maze data show that males and females express enhanced anxiety-like behavior during withdrawal from all CIE durations tested (Figure 1). These data complement previous reports utilizing longer CIE vapor exposures (e.g., weeks to months) to produce ethanol dependence (Valdez et al., 2002; Kliethermes et al., 2004; O’Dell et al., 2004; Finn et al., 2007), and provides further support for our shorter CIE vapor exposure to yield similar behavioral effects. We did not observe sex differences in anxiety-like behavior following shorter CIE exposures, which is consistent with previous studies using longer exposures (Devaud et al., 1999; Morales et al., 2015). However, there are other reports showing that males generally demonstrate greater levels of anxiety-like behavior than females (Gatch and Lai, 2001; Tanchuck-Nipper et al., 2015; Jury et al., 2017). One factor that may contribute to discrepant findings in anxiety-like behavior during ethanol withdrawal among laboratories may be differences in BECS. In the current experiments, we maintained similar BECs between males and females during the development of ethanol dependence for all CIE durations examined (Table I), which may have produced similar outcomes on the elevated plus maze. An additional contributing factor may be related to sleep disturbances given we exposed animals to ethanol during their light cycle, a time when rodents typically sleep.
Sleep deprivation, like chronic ethanol exposure, enhances circulating corticosterone levels suggesting similar activation of the HPA-axis (da Silva Rocha-Lopes et al. 2017; Suchecki et al. 2002). However, despite this activation, socialization during sleep deprivation modulates anxiety-like behavior such that individually-housed, sleep-deprived animals express greater levels of anxiety measured on the plus maze while socially-housed/deprived animals express less (Suckecki et al. 2002). It is noteworthy that our animals were group-housed during the ethanol exposure. In support of this, sleep deprivation decreases glutamatergic synaptic transmission and long-term potentiation at hippocampal synapses (Ravassard et al. 2009; McDermott et al. 2006) while chronic ethanol exposure increases similar measures at BLA glutamatergic synapses (this work). This suggests that sleep deprivation may not have dramatically influenced outcomes associated with chronic ethanol exposure.
Interestingly, while males and females showed increased anxiety-like behavior on the elevated plus maze, there were no changes in the paired-pulse ratio in females after a 3 day CIE exposure. This suggests that other synapses were altered in these animals which resulted in behavioral changes during withdrawal. As discussed earlier, in addition to excitatory neurotransmission, the BLA is comprised of GABAergic interneurons, which when either systems are altered, can change anxiety-like behaviors (Sanders and Shekhar, 1995a, 1995b, Sajdyk and Shekhar, 1997a, 1997b). Chronic ethanol exposure and withdrawal has also been shown to alter GABAA expression and function (Papadeas et al., 2001; Mccool et al., 2003; Isoardi et al., 2007; Diaz et al., 2011). Therefore, while the present series of experiments only examined glutamate neurotransmission, it is likely that alterations in glutamate and GABAA neurotransmission likely produced the increased anxiety-like behaviors observed following CIE exposure, particularly in females.
Timing-dependent hetereosynaptic plasticity during withdrawal from chronic intermittent ethanol exposure
The results from the current experiments show that presynaptic alterations of medial (stria terminalis) glutamatergic inputs require fewer intermittent ethanol exposures than postsynaptic adaptations of lateral (external capsule) inputs. In males, for example, 3, 7, and 10 day exposures all decreased the paired-pulse ratio of the stria terminalis inputs which suggests increased glutamate release probability after only a few days of intermittent ethanol. In females however, increased glutamate release probability required exposures of at least 7 days. This suggests that the different sexes are differentially sensitive to the presynaptic effects of intermittent ethanol. Importantly, there was no effect of any CIE duration on the paired-pulse ratio at external capsule inputs in either sex. Additionally, we have previously shown that there were no changes in post-synaptic response amplitudes at stria terminalis/medial BLA inputs during withdrawal when using a strontium substitution (Christian et al., 2013). Altogether, the current data supports and extends our previous findings showing that presynaptic effects are specific to the medial input pathway (Christian et al., 2012, 2013).
While we have recently shown that postsynaptic alterations during withdrawal from 10 days of CIE exposure occur at the external capsule (Christian et al., 2012), the current work suggests these develop only after 7 days of CIE in males, and after 10 days in females. The initial development of presynaptic facilitation followed by postsynaptic potentiation at these independent synapses is similar to data suggesting that presynaptic activation of stria terminalis inputs facilitates postsynaptic long-term potentiation at external capsule synapses (Cho et al., 2012). These cooperative interactions between medial presynaptic glutamate release and postsynaptic function at lateral inputs may be an important neurophysiological mechanism governing fear-conditioned behaviors. Likewise, our results strongly suggest that withdrawal from chronic intermittent ethanol exposure initiates the increased presynaptic function at medial (stria terminalis) inputs and establishes a permissive state for the increased postsynaptic function at lateral (external capsule) inputs, which may drive anxiety-like behavior during withdrawal.
It is worth noting that in the current experiments, pre- and post-synaptic adaptations were observed during acute (24h) withdrawal. Our laboratory has recently collected unpublished data demonstrating that the persistence of presynaptic changes is dependent on CIE exposure duration. For instance, after a single 3d CIE exposure, the presynaptic facilitation seen during 24h withdrawal reverses to air-exposed control levels by 72h. However, presynaptic facilitation persists for at least one week after a 6 day CIE exposure, and for at least forty days after a 10 day CIE exposure. While we have not yet similarly examined the persistence of post-synaptic changes, our preliminary unpublished data does suggest that longer CIE exposures (e.g., 10 days), do produce long-lasting neurophysiological effects. These persistent changes in synaptic transmission may manifest in the enduring behavioral changes (e.g., increased anxiety and alcohol intake) that occur during withdrawal from chronic alcohol use.
Finally, although we did not examine intrinsic excitability of BLA neurons following chronic ethanol/withdrawal, there is evidence showing that both exposure to stress and chronic ethanol alters intrinsic excitability in several brain regions (Ibbotson et al., 1997; Hendricson et al., 2007; Nimitvilai et al., 2016). For instance, an early-life stress model in juvenile/adolescent rats resulted in increased intrinsic excitability of BLA pyramidal neurons (Rau et al., 2015), just as chronic ethanol has been shown to do in the dorsal raphe (Lowery-Gionta et al., 2015), inferior olive (Welsh et al., 2011), and the prefrontal cortex and extended amygdala (Pleil et al., 2015). However, it still unknown if alterations in intrinsic excitability occurs prior to or in parallel with synaptic facilitation following chronic ethanol/withdrawal, and this is an important question that should be addressed in future studies. Regardless, it is likely a coordinated effort between intrinsic properties of BLA neurons coupled with changes in synaptic transmission following chronic ethanol exposure that drives anxiety-like behaviors during ethanol withdrawal.
Sex differences in neurophysiological alterations during withdrawal from chronic intermittent ethanol
Female rats required a longer duration of CIE exposure in order to express increases in glutamate release probability from stria inputs compared to males. The postsynaptic alterations expressed at external capsule inputs likewise were expressed following longer ethanol exposures in females. These results suggest that female synapses are less sensitive to chronic ethanol exposure/withdrawal compared with males. These data are consistent with the robust literature illustrating that the brain is a target for sex steroid function as well as the large evidence for sex differences in the development, progression, withdrawal, relapse, and treatment phases of alcohol dependence (Erol and Karpyak, 2015; Grant et al., 2015).
Interestingly, sex differences in alcohol consumption and drinking patterns emerge during late adolescence (Spear, 2000; Young et al., 2002), which strongly indicates that sexual maturation may be an important factor in early development of alcohol abuse. Transient (“activational”) effects of sex steroids directly modulate a variety of alcohol-related behaviors such as drinking (Lakoza and Barkov, 1980; Purohit et al., 1998; Martin et al., 1999; Apter and Eriksson, 2003; Vetter-O’Hagen et al., 2011; Sherrill et al., 2011; Vetter-O’Hagen, 2012), aggression (Lugo et al., 2006) and withdrawal-induced anxiety (Sharma et al., 2007). In addition, sex steroids and their derivatives directly modulate the physiological responses of neurotransmitter receptors. For instance, the progesterone derivative, pregnenolone, and 17β- estradiol alter excitatory transmission in the brain (Wu et al., 1991; Womble et al., 2002; Kim et al., 2006) and also modulate numerous behavioral effects of alcohol (Jung et al., 2005; Pierucci-Lagha et al., 2006; Rezvani and Levin, 2014). Estrogen is also neuroprotective in numerous in vivo and in vitro models of ischemic brain damage (Simpkins et al., 1997; Green and Simpkins, 2000) and dementia (Woolley et al., 1997). Importantly, 17β-estradiol exerts neural and behavioral protective effects during ethanol withdrawal (Jung et al., 2005). Although we did not directly determine hormonal status in females during our studies, we did collect vaginal smears to determine the impact of CIE on estrous cycle. Estradiol levels are low during diestrus, peak during protestrus, and begin to decline during estrus (Caligioni, 2009). If estrous cycle, and consequently estradiol levels, is protective during ethanol withdrawal, we would expect that females with disrupted estrous cycles/low estradiol levels would show similar changes in synaptic neurotransmission as males whereas glutamate release probability in females with “normal” estrous cycles/high estradiol levels would not be altered after CIE. This hypothesis is supported by our data demonstrating that 1) females required longer CIE exposures to produce synaptic alterations, and 2) estrous cycle was impacted only after the longest CIE exposure (Figure 5, right panel; p=0.059).
In addition to direct, transient (“activational”) interactions between sex steroids and alcohol, permanent “organizational” functions of sex steroids also contribute to sex differences in response to alcohol. During prenatal and pubertal periods for example, sex hormones control the differentiation of sexually dimorphic brain structures. And, sex steroids have been shown to alter synaptic organization of neuronal circuits, growth of axons and dendrites, and the number of synapses (Arnold, 2009). These effects extend beyond brain regions related to sexual behavior. For example, in the human literature, men have larger amygdala volume and density (Ruigrok et al., 2014)—with this difference emerging across adolescence (Giedd et al., 1996; Schumann, 2004). However, basolateral amygdala dendritic complexity (length, number of branches, spine density) develops similarly across adolescence in both males and females, suggesting a similar remodeling of excitatory synapses between sexes (Koss et al., 2014). Neuron and glial numbers (Rubinow and Juraska, 2009) are likewise not different between the sexes. Regardless, the literature on estrogen receptor and/or estradiol’s effects on BLA synaptic physiology are extremely limited. For example, estrogen receptor β knockout female mice showed increases in anxiety-like behavior coupled with a lower threshold for LTP induction in BLA neurons compared to wild-type controls (Krezel et al., 2001). Bath application of estrogen (17β-estradiol) can also dramatically reduce EPSP amplitudes measured from BLA principal neurons, suggesting acute modulation of glutamatergic neurotransmission (Womble et al., 2002). A study using ovariectomized females, found that local infusion of estradiol into the BLA was able to reverse stress-induced anxiety-like behavior in the open field and elevated plus maze. Furthermore, these authors found that infusion of estradiol was also able to reverse stress-induced glutamate receptor upregulation, partially through GPR30, a novel estrogen membrane receptor, whose expression was significantly increased following both stressors (Tian et al., 2013). Overall, the literature suggests that females may display a decrease in sensitivity to chronic ethanol’s effects during withdrawal due to activational and organizational effects of sex steroids. Future studies should address the limitations regarding sex differences in the current experiments by collecting samples for hormone level determination and correlate that with changes in synaptic neurophysiology and behavior.
The chronic intermittent ethanol exposure paradigm used in the current experiments started during mid-adolescence, a unique developmental period, particularly in response to a variety of ethanol-related behaviors (Spear, 2000; Spear and Varlinskaya, 2010). For instance, human and rodent models show that alcohol consumption is high during adolescence (Vetter et al., 2007; García-Burgos et al., 2009; Johnston et al., 2016), a pattern of drinking that may be due to their insensitivity to several adverse effects associated with ethanol (Schramm-Sapyta et al., 2009). Although the data are mixed, there is some evidence to suggest that adolescents may be less sensitive than adults to the anxiogenic effects associated with ethanol withdrawal (Doremus et al., 2003; Doremus-Fitzwater and Spear, 2007; Wills et al., 2008; Morris et al., 2010), and that exposure to ethanol during adolescence may result in later higher ethanol intake (Siciliano and Smith, 2001; Strong et al., 2010). Therefore, while adult comparison groups were not included in the current experiments, it is likely that chronic ethanol exposure during this time period may have resulted in strong presynaptic alterations that are unique to the adolescent period and may be related to ethanol drinking. Future studies should include an adult comparison group to address this important area of research directly.
The basolateral amygdala is a critical component of the fear/anxiety circuit and of the anxiety that emerges during withdrawal from chronic ethanol exposure. The current experiments demonstrate that distinct afferents to the BLA undergo alterations during ethanol withdrawal in a time-dependent manner. These data strongly parallel postsynaptic LTP induction produced by fear conditioning that is dependent on stria terminalis presynaptic glutamate release. These findings suggest that ethanol dependence differentially engages pre- and postsynaptic function in a cooperative manner, which may help facilitate anxiety-like behavior during withdrawal. In addition, we observed sex-dependent responses in synaptic function, with females requiring longer durations of CIE exposure to demonstrate alterations in pre- and postsynaptic function than males. It is possible that sex steroid influences—in particular, estrogen—may have served to protect females from changes in excitatory neurotransmission that occurred in males at shorter durations.
Acknowledgments
This work was supported by the National Institutes of Health [grants R01AA014445, F32AA024949, T32AA007565].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflict of interest.
References
- Apter SJ, Eriksson CJP. The effect of alcohol on testosterone concentrations in alcohol-preferring and non-preferring rat lines. Alcohol Clin Exp Res. 2003;27:1190–1193. doi: 10.1097/01.ALC.0000075832.83254.81. [DOI] [PubMed] [Google Scholar]
- Arnold AP. The organizational-activational hypothesis as the foundation for a unified theory of sexual differentiation of all mammalian tissues. Horm Behav. 2009;55:570–578. doi: 10.1016/j.yhbeh.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer EP, Schafe GE, LeDoux JE. NMDA receptors and L-type voltage-gated calcium channels contribute to long-term potentiation and different components of fear memory formation in the lateral amygdala. J Neurosci. 2002;22:5239–5249. doi: 10.1523/JNEUROSCI.22-12-05239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker HC. Animal models of alcohol withdrawal. Alcohol Res Health. 2000;24:105–113. [PMC free article] [PubMed] [Google Scholar]
- Burns L, Teesson M. Alcohol use disorders comorbid with anxiety, depression and drug use disorders. Findings from the Australian National Survey of Mental Health and Well Being. Drug Alcohol Depend. 2002;68:299–307. doi: 10.1016/s0376-8716(02)00220-x. [DOI] [PubMed] [Google Scholar]
- Butler TR, Carter E, Weiner JL. Adolescent Social Isolation Does Not Lead to Persistent Increases in Anxiety- Like Behavior or Ethanol Intake in Female Long-Evans Rats. Alcohol Clin Exp Res. 2014;38:2199–2207. doi: 10.1111/acer.12476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caligioni C. Assessing reproductive status/stages in mice. Curr Protoc Neurosci. 2009 doi: 10.1002/0471142301.nsa04is48. Appendix 4I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnicella S, Kharazia V, Jeanblanc J, Janak PH, Ron D. GDNF is a fast-acting potent inhibitor of alcohol consumption and relapse. Proc Natl Acad Sci. 2008;105:8114–8119. doi: 10.1073/pnas.0711755105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho J-H, Bayazitov I, Meloni E, Myers K, Carlezon W, Jr, Zakharenko S, Bolshakov V. Coactivation of thalamic and cortical pathways induces input timing-dependent plasticity in the amygdala. Nat Neurosci. 2012;15:113–122. doi: 10.1038/nn.2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S, Lovinger DM. Decreased frequency but not amplitude of quantal synaptic responses associated with expression of corticostriatal long-term depression. J Neurosci. 1997;17:8613–8620. doi: 10.1523/JNEUROSCI.17-21-08613.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian DT, Alexander NJ, Diaz MR, McCool BA. Thalamic glutamatergic afferents into the rat basolateral amygdala exhibit increased presynaptic glutamate function following withdrawal from chronic intermittent ethanol. Neuropharmacology. 2013;65:134–142. doi: 10.1016/j.neuropharm.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian DT, Alexander NJ, Diaz MR, Robinson S, McCool BA. Chronic intermittent ethanol and withdrawal differentially modulate basolateral amygdala AMPA-type glutamate receptor function and trafficking. Neuropharmacology. 2012;62:2429–2438. doi: 10.1016/j.neuropharm.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva Rocha-Lopes J, Machado RB, Suchecki D. Chronic REM sleep restriction in juvenile male rats induces anxiety-like behavior and alters monoamine systems in the amygdala and hippocampus. Mol Neurobiol. 2017 doi: 10.1007/s12035-017-0541-3. [DOI] [PubMed] [Google Scholar]
- Davis M, Rainnie D, Cassell M. Neurotransmission in the rat amygdala related to fear and anxiety. Trends Neurosci. 1994;17:208–214. doi: 10.1016/0166-2236(94)90106-6. [DOI] [PubMed] [Google Scholar]
- Devaud LL, Chadda R. Sex differences in rats in the development of and recovery from ethanol dependence assessed by changes in seizure susceptibility. Alcohol Clin Exp Res. 2001;25:1689–1696. [PubMed] [Google Scholar]
- Devaud LL, Matthews DB, Morrow AL. Gender impacts behavioral and neurochemical adaptations in ethanol- dependent rats. Pharmacol Biochem Behav. 1999;64:841–849. doi: 10.1016/s0091-3057(99)00164-1. [DOI] [PubMed] [Google Scholar]
- Devaud LL, Risinger FO, Selvage D. Impact of the hormonal milieu on the neurobiology of alcohol dependence and withdrawal. J Gen Psychol. 2006;133:337–356. doi: 10.3200/GENP.133.4.337-356. [DOI] [PubMed] [Google Scholar]
- Diaz MR, Christian DT, Anderson NJ, Mccool BA. Chronic ethanol and withdrawal differentially modulate lateral/basolateral amygdala paracapsular and local GABAergic synapses. J Pharmacol Exp Ther. 2011;337:162–170. doi: 10.1124/jpet.110.177121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Spear LP. Developmental differences in acute ethanol withdrawal in adolescent and adult rats. Alcohol Clin Exp Res. 2007;31:1516–1527. doi: 10.1111/j.1530-0277.2007.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Varlinskaya EI, Spear LP. Anxiogenic effects during withdrawal from acute ethanol in adolescent and adult rats. Pharmacol Biochem Behav. 2003;75:411–418. doi: 10.1016/s0091-3057(03)00134-5. [DOI] [PubMed] [Google Scholar]
- Driessen M, Meier S, Hill A, Wetterling T, Lange W, Junghanns K. The course of anxiety, depression and drinking behaviors after completed detoxification in alcoholics without comorbid anxiety and depressive disorders. Alcohol Alcohol. 2001;36:249–255. doi: 10.1093/alcalc/36.3.249. [DOI] [PubMed] [Google Scholar]
- Erol A, Karpyak VM. Sex and gender-related differences in alcohol use and its consequences: Contemporary knowledge and future research considerations. Drug Alcohol Depend. 2015;156:1–13. doi: 10.1016/j.drugalcdep.2015.08.023. [DOI] [PubMed] [Google Scholar]
- Finn DA, Crabbe JC. Exploring alcohol withdrawal syndrome. Alcohol Health Res World. 1997;21:149–156. [PMC free article] [PubMed] [Google Scholar]
- Finn DA, Snelling C, Fretwell AM, Tanchuck MA, Underwood L, Cole M, Crabbe JC, Roberts AJ. Increased drinking during withdrawal from intermittent ethanol exposure is blocked by the CRF receptor antagonist D-Phe-CRF(12-41) Alcohol Clin Exp Res. 2007;31:939–949. doi: 10.1111/j.1530-0277.2007.00379.x. [DOI] [PubMed] [Google Scholar]
- Fioravante D, Regehr WG. Short-term forms of presynaptic plasticity. Curr Opin Neurobiol. 2011;21:269–274. doi: 10.1016/j.conb.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca R. Asymmetrical synaptic cooperation between cortical and thalamic inputs to the amygdala. Neuropsychopharmacology. 2013;38:2675–2687. doi: 10.1038/npp.2013.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Burgos D, González F, Manrique T, Gallo M. Patterns of ethanol intake in preadolescent, adolescent, and adult wistar rats under acquisition, maintenance, and relapse-like conditions. Alcohol Clin Exp Res. 2009;33:722–728. doi: 10.1111/j.1530-0277.2008.00889.x. [DOI] [PubMed] [Google Scholar]
- Gatch MB, Lai H. Animal models ofthe anxiogenic effects of ethanol withdrawal. Drug Dev Res. 2001;54:95–115. [Google Scholar]
- Giedd J, Vaituzis C, Hamburger S, Lange N, Rajapakse JC, Kaysen D, Vauss YC, Rapoport JL. Quantitative MRI of the temoral lobe, amydala, and hippocampus in normal human development: Ages 4-18 Years. J Comp Neurol. 1996;366:223–230. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Gioia DA, Alexander N, McCool BA. Ethanol mediated inhibition of synaptic vesicle recycling at amygdala glutamate synapses is dependent upon Munc13-2. Front Neurosci. 2017;11:1–11. doi: 10.3389/fnins.2017.00424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DB, Pal N. Alcohol dependence produced in mice by inhalation of ethanol: grading the withdrawal reaction. Science. 1971;172:288–290. doi: 10.1126/science.172.3980.288. [DOI] [PubMed] [Google Scholar]
- Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, Pickering RP, Ruan WJ, Smith SM, Huang B, Hasin DS. Epidemiology of DSM-5 Alcohol Use Disorder. JAMA Psychiatry. 2015;72:757. doi: 10.1001/jamapsychiatry.2015.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green PS, Simpkins JW. Neuroprotective effects of estrogens: Potential mechanisms of action. Int J Dev Neurosci. 2000;18:347–358. doi: 10.1016/s0736-5748(00)00017-4. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2007;64:830–842. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- Hendricson AW, Maldve RE, Salinas AG, Theile JW, Zhang TA, Diaz LM, Morrisett RA. Aberrant synaptic activation of N-Methyl- D -aspartate receptors underlies ethanol withdrawal hyperexcitability. J Pharmacol Exp Ther. 2007;321:60–72. doi: 10.1124/jpet.106.111419. [DOI] [PubMed] [Google Scholar]
- Ibbotson T, Field MJ, Boden PR. Effect of chronic ethanol treatment in vivo on excitability in mouse cortical neurones in vitro. Br J Pharmacol. 1997;122:956–962. doi: 10.1038/sj.bjp.0701471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isoardi NA, Bertotto ME, Martijena ID, Molina VA, Carrer HF. Lack of feedback inhibition on rat basolateral amygdala following stress or withdrawal from sedative-hypnotic drugs. Eur J Neurosci. 2007;26:1036–1044. doi: 10.1111/j.1460-9568.2007.05714.x. [DOI] [PubMed] [Google Scholar]
- Janak PH, Tye KM. From circuits to behaviour in the amygdala. Nature. 2015;517:284–292. doi: 10.1038/nature14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Miech RA, Bachman JG, Schulenberg JE. Demographic subgroup trends among adolescents in the use of various licit and illicit drugs, 1975-2015. Ann Arbor, MI: Institute for Social Research, The University of Michigan; 2016. (Monitoring the Future Occasion Paper No. 86). [Google Scholar]
- Jung ME, Gatch MB, Simpkins JW. Estrogen neuroprotection against the neurotoxic effects of ethanol withdrawal: potential mechanisms. ExpBiolMed (Maywood) 2005;230:8–22. doi: 10.1177/153537020523000102. [DOI] [PubMed] [Google Scholar]
- Jury NJ, DiBerto JF, Kash TL, Holmes A. Sex differences in the behavioral sequelae of chronic ethanol exposure. Alcohol. 2017;58:53–60. doi: 10.1016/j.alcohol.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MT, Soussou W, Gholmieh G, Ahuja A, Tanguay A, Berger TW, Brinton RD. 17β-estradiol potentiates field excitatory postsynaptic potentials within each subfield of the hippocampus with greatest potentiation of the associational/commissural afferents of CA3. Neuroscience. 2006;141:391–406. doi: 10.1016/j.neuroscience.2006.03.075. [DOI] [PubMed] [Google Scholar]
- Kimbrough A, Kim S, Cole M, Brennan M, George O. Intermittent access to ethanol drinking facilitates the transition to excessive drinking after chronic intermittent ethanol vapor exposure. Alcohol Clin Exp Res. 2017;41:1502–1509. doi: 10.1111/acer.13434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliethermes CL, Cronise K, Crabbe JC. Anxiety-like behavior in mice in two apparatuses during withdrawal from chronic ethanol vapor inhalation. Alcohol Clin Exp Res. 2004;28:1012–1019. doi: 10.1097/01.alc.0000131976.40428.8f. [DOI] [PubMed] [Google Scholar]
- Koss WA, Belden CE, Hristov AD, Juraska JM. Dendritic remodeling in the adolescent medial prefrontal cortex and the basolateral amygdala of male and female rats. Synapse. 2014;68:61–72. doi: 10.1002/syn.21716. [DOI] [PubMed] [Google Scholar]
- Krezel W, Dupont S, Krust A, Chambon P, Chapman PF. Increased anxiety and synaptic plasticity in estrogen receptor β-deficient mice. Proc Natl Acad Sci U S A. 2001;98 doi: 10.1073/pnas.221451898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner MG, Abrams K, Borchardt C. The relationship between anxiety disorders and alcohol use disorders: a review of major perspectives and findings. Clin Psychol Rev. 2000;20:149–171. doi: 10.1016/s0272-7358(99)00027-6. [DOI] [PubMed] [Google Scholar]
- Läck AK, Ariwodola OJ, Chappell AM, Weiner JL, McCool BA. Ethanol inhibition of kainate receptor-mediated excitatory neurotransmission in the rat basolateral nucleus of the amygdala. Neuropharmacology. 2008;55:661–668. doi: 10.1016/j.neuropharm.2008.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Läck AK, Diaz MR, Chappell A, DuBois DW, McCool BA. Chronic ethanol and withdrawal differentially modulate pre- and postsynaptic function at glutamatergic synapses in rat basolateral amygdala. JNeurophysiol. 2007;98:3185–3196. doi: 10.1152/jn.00189.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakoza GN, Barkov NK. The role of testosterone in the development of experimental alcoholism. Bull Narc. 1980;32:41–48. [PubMed] [Google Scholar]
- Lowery-Gionta EG, Marcinkiewcz CA, Kash TL. Functional alterations in the dorsal raphe nucleus following acute and chronic ethanol exposure. Neuropsychopharmacology. 2015;40:590–600. doi: 10.1038/npp.2014.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugo JN, Marino MD, Gass JT, Wilson MA, Kelly SJ. Ethanol exposure during development reduces resident aggression and testosterone in rats. Physiol Behav. 2006;87:330–337. doi: 10.1016/j.physbeh.2005.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcondes FK, Bianchi F, Tanno A. Determination of the estrous cycle phases of rats: some helpful considerations. Brazilian J Biol. 2002;62:609–614. doi: 10.1590/s1519-69842002000400008. [DOI] [PubMed] [Google Scholar]
- Martin CA, Mainous AG, Curry T, Martin D. Alcohol use in adolescent females: correlates with estradiol and testosterone. Am J Addict. 1999;8:9–14. doi: 10.1080/105504999306036. [DOI] [PubMed] [Google Scholar]
- McCool BA, Frye GD, Pulido MD, Botting SK. Effects of chronic ethanol consumption on rat GABAA and strychnine- sensitive glycine receptors expressed by lateral/basolateral amygdala neurons. Brain Res. 2003;963:165–177. doi: 10.1016/s0006-8993(02)03966-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott CM, Hardy MN, Bazan NG, Magee JC. Sleep deprivation-induced alterations in excitatory synaptic transmission in the CA1 region of the hippocampus. J Physiol. 2006;570(pt. 3):553–565. doi: 10.1113/jphysiol.2005.093781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean AC, Valenzuela N, Fai S, Bennett SAL. Performing vaginal lavage, crystal violet staining, and vaginal cytological evaluation for mouse estrous cycle staging identification. J Vis Exp. 2012:4–9. doi: 10.3791/4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas K, Mehta R, Molnar BE, Walters EE, Swendsen JD, Aguilar-Gaziola S, Bijl R, Borges G, Caraveo-Anduaga JJ, Dewit DJ, Kolody B, Vega WA, Wittchen H-U, Kessler RC. Comorbidity of substance use disorders with mood and anxiety disorders: Results of the international consortium in psychiatric epidemiology. Addict Behav. 1998;23:893–907. doi: 10.1016/s0306-4603(98)00076-8. [DOI] [PubMed] [Google Scholar]
- Meyer EM, Long V, Fanselow MS, Spigelman I. Stress increases voluntary alcohol intake, but does not alter established drinking habits in a rat model of posttraumatic stress disorder. Alcohol Clin Exp Res. 2013;37:566–574. doi: 10.1111/acer.12012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M, McGinnis MM, Mccool BA. Chronic ethanol exposure increases voluntary home cage intake in adult male, but not female, Long-Evans rats. Pharmacol Biochem Behav. 2015;139:67–76. doi: 10.1016/j.pbb.2015.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SA, Kelso ML, Liput DJ, Marshall SA, Nixon K. Similar withdrawal severity in adolescents and adults in a rat model of alcohol dependence. Alcohol. 2010;44:89–98. doi: 10.1016/j.alcohol.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimitvilai S, You C, Arora DS, McElvain MA, Vandegrift BJ, Brodie MS, Woodward JJ. Differential effects of toluene and ethanol on dopaminergic neurons of the ventral tegmental area. Front Neurosci. 2016;10:1–12. doi: 10.3389/fnins.2016.00434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. Gender differences in risk factors and consequences for alcohol use and problems. Clin Psychol Rev. 2004;24:981–1010. doi: 10.1016/j.cpr.2004.08.003. [DOI] [PubMed] [Google Scholar]
- O’Dell LE, Roberts AJ, Smith RT, Koob GF. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcohol Clin Exp Res. 2004;28:1676–1682. doi: 10.1097/01.alc.0000145781.11923.4e. [DOI] [PubMed] [Google Scholar]
- Papadeas S, Grobin AC, Morrow AL. Chronic ethanol consumption differentially alters GABA(A) receptor alpha1 and alpha4 subunit peptide expression and GABA(A) receptor-mediated 36 Cl(-) uptake in mesocorticolimbic regions of rat brain. Alcohol Clin Exp Res. 2001;25:1270–5. [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Pierucci-Lagha A, Covault J, Feinn R, Khisti RT, Morrow AL, Marx CE, Shampine LJ, Kranzler HR. Subjective effects and changes in steroid hormone concentrations in humans following acute consumption of alcohol. Psychopharmacology (Berl) 2006;186:451–461. doi: 10.1007/s00213-005-0231-0. [DOI] [PubMed] [Google Scholar]
- Pleil KE, Lowery-Gionta EG, Crowley NA, Li C, Marcinkiewcz CA, Rose JH, McCall NM, Maldonado-Devincci AM, Morrow AL, Jones SR, Kash TL. Effects of chronic ethanol exposure on neuronal function in the prefrontal cortex and extended amygdala. Neuropharmacology. 2015;99:735–749. doi: 10.1016/j.neuropharm.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purohit V, Whelan E, Kava R. Moderate alcohol consumption and estrogen levels in postmenopausal women: a review. Alcohol Clin Exp Res. 1998;22:994–997. doi: 10.1111/j.1530-0277.1998.tb03694.x. [DOI] [PubMed] [Google Scholar]
- Rainnie DG, Asprodini EK, Shinnick-Gallagher P. Excitatory transmission in the basolateral amygdala. J Neurophysiol. 1991;66:986–998. doi: 10.1152/jn.1991.66.3.986. [DOI] [PubMed] [Google Scholar]
- Rau AR, Chappell AM, Butler TR, Ariwodola OJ, Weiner JL. Increased basolateral amygdala pyramidal cell excitability may contribute to the anxiogenic phenotype induced by chronic early-life stress. J Neurosci. 2015;35:9730–9740. doi: 10.1523/JNEUROSCI.0384-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravassard P, Pachoud B, Comte JC, Mejia-Perez C, Scoté-Blachon C, Gay N, Claustrat B, Touret M, Luppi PH, Salin PA. Paradoxical (REM) sleep deprivation casues a large and rapidly reversible decrease in long-term potentiation, synaptic transmission, glutamate receptor protein levels, and ERK/MAPK activation in the dorsal hippocampus. Sleep. 2009;32:227–240. doi: 10.1093/sleep/32.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezvani AH, Levin ED. Assessment of pregnenolone effects on alcohol intake and preference in male alcohol preferring (P) rats. Eur J Pharmacol. 2014;740:53–57 A. doi: 10.1016/j.ejphar.2014.07.003. [DOI] [PubMed] [Google Scholar]
- Rosenwasser AM, McCulley WD, Fecteau M. Circadian activity rhythms and voluntary ethanol intake in male and female ethanol-preferring rats: Effects of long-term ethanol access. Alcohol. 2014;48:647–655. doi: 10.1016/j.alcohol.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross HE. DSM-III-R alcohol abuse and dependence and psychiatric comorbidity in Ontario: results from the Mental Health Supplement to the Ontario Health Survey. Drug Alcohol Depend. 1995;39:111–128. doi: 10.1016/0376-8716(95)01150-w. [DOI] [PubMed] [Google Scholar]
- Rubinow MJ, Juraska JM. Neuron and glia numbers in the basolateral nucleus of the amygdala from preweaning through old age in male and female rats: A stereological study. J Comp Neurol. 2009;512:717–725. doi: 10.1002/cne.21924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruigrok ANV, Salimi-Khorshidi G, Lai M-C, Baron-Cohen S, Lombardo MV, Tait RJ, Suckling J. A meta-analysis of sex differences in human brain structure. Neurosci Biobehav Rev. 2014;39:34–50. doi: 10.1016/j.neubiorev.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajdyk TJ, Shekhar A. Excitatory amino acid receptors in the basolateral amygdala regulate anxiety responses in the social interaction test. Brain Res. 1997a;764:262–264. doi: 10.1016/s0006-8993(97)00594-5. [DOI] [PubMed] [Google Scholar]
- Sajdyk TJ, Shekhar A. Excitatory amino acid receptor antagonists block the cardiovascular and anxiety responses elicited by gamma-aminobutyric acidA receptor blockade in the basolateral amygdala of rats. J Pharmacol Exp Ther. 1997b;283:969–977. [PubMed] [Google Scholar]
- Sanders SK, Shekhar A. Regulation of Anxiety by GABA A Receptors in the Rat Amygdala. Pharmacol Biochem Behav. 1995a;52:701–706. doi: 10.1016/0091-3057(95)00153-n. [DOI] [PubMed] [Google Scholar]
- Sanders SK, Shekhar A. Anxiolytic effects of chlordiazepoxide blocked by injection of GABAA and benzodiazepine receptor antagonists in the region of the anterior basolateral amygdala of rats. Biol Psychiatry. 1995b;37:473–476. doi: 10.1016/0006-3223(94)00183-4. [DOI] [PubMed] [Google Scholar]
- Schellekens AF, de Jong CA, Buitelar JK, Verkes RJ. Co-morbid anxiety disorders predic early relapse after inpatient alcohol treatment. Eur Psychiatry. 2015;30:128–136. doi: 10.1016/j.eurpsy.2013.08.006. [DOI] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Walker QD, Caster JM, Levin ED, Kuhn CM. Are adolescents more vulnerable to drug addiction than adults? Evidence from animal models. Psychopharmacology (Berl) 2009;206:1–21. doi: 10.1007/s00213-009-1585-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz PE, Cook EP, Johnston D. Changes in paired-pulse facilitation suggest presynaptic involvement in long-term potentiation. J Neurosci. 1994;14:5325–5337. doi: 10.1523/JNEUROSCI.14-09-05325.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann CM. The amygdala is enlarged in children but not adolescents with autism; the hippocampus is enlarged at all ages. J Neurosci. 2004;24:6392–6401. doi: 10.1523/JNEUROSCI.1297-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrill LK, Koss WA, Foreman ES, Gulley JM. The effects of pre-pubertal gonadectomy and binge-like ethanol exposure during adolescence on ethanol drinking in adult male and female rats. Behav Brain Res. 2011;216:569–575. doi: 10.1016/j.bbr.2010.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siciliano D, Smith RF. Periadolescent alcohol alters adult behavioral characteristics in the rat. Physiol Behav. 2001;74:637–643. doi: 10.1016/s0031-9384(01)00623-0. [DOI] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, Bartlett SE. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res. 2008;32:1816–1823. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpkins JW, Rajakumar G, Zhang YQ, Simpkins CE, Greenwald D, Yu CJ, Bodor N, Day a L. Estrogens may reduce mortality and ischemic damage caused by middle cerebral artery occlusion in the female rat. J Neurosurg. 1997;87:724–730. doi: 10.3171/jns.1997.87.5.0724. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP, Varlinskaya EI. Sensitivity to ethanol and other hedonic stimuli in an animal model of adolescence: Implications for prevention science? Dev Psychobiol. 2010;52:236–243. doi: 10.1002/dev.20457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong MN, Yoneyama N, Fretwell AM, Snelling C, Tanchuck MA, Finn DA. “Binge” drinking experience in adolescent mice shows sex differences and elevated ethanol intake in adulthood. Horm Behav. 2010;58:82–90. doi: 10.1016/j.yhbeh.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchecki D, Tiba PA, Tufik S. Hormonal and behavioral responses of paradoxical sleep-deprived rats to the elevated plus maze. J Neuroendocrinol. 2002;14:549–554. doi: 10.1046/j.1365-2826.2002.00812.x. [DOI] [PubMed] [Google Scholar]
- Tanchuck-Nipper MA, Ford MM, Hertzberg A, Beadles-Bohling A, Cozzoli DK, Finn DA. Sex differences in ethanol’s anxiolytic effect and chronic ethanol withdrawal severity in mice with a null mutation of the 5α-reductase Type 1 gene. Behav Genet. 2015;45:354–367. doi: 10.1007/s10519-014-9691-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Z, Wang Y, Zhang N, Yan Guo Y, Feng B, Bing Liu S, Gao Zhao M. Estrogen receptor GPR30 exerts anxiolytic effects by maintaining the balance between GABAergic and glutamatergic transmission in the basolateral amygdala of ovariectomized mice after stress. Psychoneuroendocrinology. 2013;38:2218–2233. doi: 10.1016/j.psyneuen.2013.04.011. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP, Koob GF. Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: regulation by corticotropin-releasing factor. Alcohol Clin Exp Res. 2002;26:1494–1501. doi: 10.1097/01.ALC.0000033120.51856.F0. [DOI] [PubMed] [Google Scholar]
- Vetter-O’Hagen CS. The role of puberty and gonadal hormones in age and sex differences in ethanol intake, ethanol sensitivity and responses to novelty in Sprague-Dawley rats. Diss Abstr Int Sect B Sci Eng. 2012;72:6436. [Google Scholar]
- Vetter-O’Hagen CS, Sanders KW, Spear LP. Evidence for suppressant effects of testosterone on sex-typical ethanol intake in male Sprague-Dawley rats. Behav Brain Res. 2011;224:403–407. doi: 10.1016/j.bbr.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter CS, Doremus-Fitzwater TL, Spear LP. Time course of elevated ethanol intake in adolescent relative to adult rats under continuous, voluntary-access conditions. Alcohol Clin Exp Res. 2007;31:1159–1168. doi: 10.1111/j.1530-0277.2007.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn MS, Moises HC. Electrophysiological and morphological properties of rat basolateral amygdaloid neurons in vitro. J Neurosci. 1992;12:4066–4079. doi: 10.1523/JNEUROSCI.12-10-04066.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh JP, Han VZ, Rossi DJ, Mohr C, Odagiri M, Daunais JB, Grant KA. Bidirectional plasticity in the primate inferior olive induced by chronic ethanol intoxication and sustained abstinence. Proc Natl Acad Sci U S A. 2011;108:10314–10319. doi: 10.1073/pnas.1017079108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills TA, Knapp DJ, Overstreet DH, Breese GR. Differential dietary ethanol intake and blood ethanol levels in adolescent and adult rats: Effects on anxiety-like behavior and seizure thresholds. Alcohol Clin Exp Res. 2008;32:1350–1360. doi: 10.1111/j.1530-0277.2008.00709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womble MD, Andrew JA, Crook JJ. 17Beta-Estradiol reduces excitatory postsynaptice potentional (EPSP) amplutide in rat basolateral amygdala neurons. Neurosci Lett. 2002;331:83–86. doi: 10.1016/s0304-3940(02)00871-6. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Weiland NG, McEwen BS, Schwartzkroin PA. Estradiol increases the sensitivity of hippocampal CA1 pyramidal cells to NMDA receptor-mediated synaptic input: correlation with dendritic spine density. J Neurosci. 1997;17:1848–59. doi: 10.1523/JNEUROSCI.17-05-01848.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F-S, Gibbs TT, Farb DH. Pregnenolone Sulfate: A Positive Allosteric Modulator at the N-Methyl-D-aspartate Receptor. Mol Pharmacol. 1991;40:333–336. [PubMed] [Google Scholar]
- Young SE, Corley RP, Stallings MC, Rhee SH, Crowley TJ, Hewitt JK. Substance use, abuse and dependence in adolescence: Prevalence, symptom profiles and correlates. Drug Alcohol Depend. 2002;68:309–322. doi: 10.1016/s0376-8716(02)00225-9. [DOI] [PubMed] [Google Scholar]


