Abstract
Cutaneous wound healing occurs in distinct yet overlapping steps with the end goal of reforming a stratified epithelium to restore epidermal barrier function. A key component of this process is re-epithelialization, which involves the proliferation and migration of epidermal keratinocytes surrounding the wound. This spatiotemporally controlled process resembles aspects of the epithelial-to-mesenchymal transition (EMT) process and is thus proposed to involve a partial EMT. Here we review current literature on the cellular and molecular changes that occur during, and the known or potential regulatory factors of cutaneous wound re-epithelialization and EMT to highlight their similarities and differences. We also discuss possible future directions towards better understanding the underlying regulatory mechanisms with implications for developing new therapeutics to improve wound repair in humans.
Keywords: EMT, wound healing, skin, epidermis, re-epithelialization
Cutaneous wound healing
The mammalian epidermis is a stratified epithelium with proliferative stem/progenitor cells residing in the basal layer maintaining epidermal homeostasis and fueling repair/regeneration (Hsu et al., 2014). Cutaneous wounding presents a unique challenge whereby the epidermis must alter its proliferative, migratory, and differentiating dynamics to re-establish a functional permeability barrier. The overall process of adult wound healing occurs in multiple distinct but overlapping steps (Shaw and Martin, 2009; Eming et al., 2014). Almost immediately following wounding, inflammation occurs characterized by a coagulation cascade to prevent any further blood loss through formation of a fibrin clot and recruitment of immune cells to the wound site to eliminate potential infections. Signals from keratinocytes, platelets, and other immune cells trigger major changes in both the epidermis and dermis. Multiple events including fibroblast proliferation and extracellular matrix (ECM) remodeling occur in the dermis with a goal of replacing the fibrin clot with granulation tissue. Angiogenesis occurs in the wound granulation tissue, presumably due to increased metabolic needs of the repairing tissue. Re-epithelialization is characterized by the migration and proliferation of the epidermal cells over granulation tissue. Multiple distinct populations of epithelial stem cells contribute to re-epithelialization: those in the hair follicle bulge participate in the healing process transiently, whereas those in the interfollicular epidermis and isthmus/junctional zone participate in long term to generate new epidermis (Arwert et al., 2012; Plikus et al., 2012). Simultaneous and important to the re-epithelialization process is the contraction of the wound, which is aided by fibroblasts and myofibroblasts in the dermis with contractile abilities. Wound healing ends with a resolution phase, where the two migrating fronts of keratinocytes make contact with one another, halting migration and regenerating a stratified epithelium, and where remodeling and restructuring of the ECM occurs leading to scar formation. With the wound clear of debris and infections, a mass removal of immune cells (and fibroblasts) occurs either by apoptosis or returning to blood vessels. The current review will focus on existing literature that implicates the existence and regulation of a partial EMT in the re-epithelialization process of wound healing.
Epithelial-to-mesenchymal transition (EMT)
It has been long recognized that epithelial cells possess a range of inherent plasticity including the ability to become mesenchymal cells. The EMT process is known to produce migratory mesenchymal cell types, such as mesoderm and neural crest, during embryogenesis (Thiery et al., 2009). EMT is also extensively studied in cancer, as it is believed to play a crucial role in cancer invasion, metastasis, and chemoresistance (see recent comprehensive reviews on EMT that discuss advances in these areas (Lamouille et al., 2014; Nieto et al., 2016)). Originally thought of as a transformation, implying a unidirectional and committed switch, EMT is now considered a transition implying a transient and reversible process (Lamouille et al., 2014; Nieto et al., 2016). The reverse process of EMT is termed mesenchymal-to-epithelial transition (MET).
During the process of EMT, epithelial cells undergo cytoskeleton rearrangement, lose their cell-cell junctions and apical-basal polarity, change their interaction with the ECM, and acquire mesenchymal features including enhanced motility and invasiveness (Thiery et al., 2009; Lim and Thiery, 2012; Lamouille et al., 2014; Nieto et al., 2016) (Table 1). To facilitate such cellular changes, EMTing cells alter their gene expression program, such as downregulating the expression of epithelial junctional components and upregulating the expression of genes involved in promoting cytoskeletal changes and adhesion to mesenchymal cells (Lamouille et al., 2014) (Table 1). The extent of these cellular and molecular changes differs depending on cell/tissue type and on the extent of EMT.
Table 1.
Comparison of cellular and molecular changes between cutaneous wound re-epithelialization and EMT.
| EMT | Wound re-epithelialization | References | |
|---|---|---|---|
| Cell-cell adhesions | Destabilization of adherens junctions; downregulating E-cadherin; upregulating N-cadherin and NCAM; dissolution of apical tight junctions and desmosomes | Reduced desmosomal adhesion; reduced E-cadherin |
Beaudry et al., 2010 Coulombe et al., 1997 Garrod et al., 2005 Kuwahara et al., 2001 |
| Cell-matrix adhesions | Downregulation of α6β4; requirement for α3β1; increased α5β1, αvβ6, α1β1 and α2β1; increased MMPs | Redistribution of α2β1, α3β1, and α6β4; activated expression of α5β1, αvβ6, α9β1, and αvβ5; increased MMPs |
Arnoux et al., 2005 Lamouille et al., 2014 |
| Intermediate filaments | Decreased cytokeratin; increased vimentin | Altered cytokeratin; increased vimentin? |
Arnoux et al., 2005 Lamouille et al., 2014 |
| Mode of migration | Single cell migration or collective migration | Collective migration |
Arnoux et al., 2005 Lim et al., 2012 Nieto et al., 2016 Park et al., 2017 |
| Growth factors and signaling cascades | TGF-β, EGF, FGF, HGF, Wnt, Hh, Notch | TGF-β, EGF, FGF, HGF, KGF, Wnt, Hh, Notch |
Arnoux et al., 2005 Bielefeld et al., 2013 Eming et al., 2014 Lamouille et al., 2014 |
| EMT-TFs | (+) Snail, Slug, Zeb1, Zeb2, Twist (−) Grhl2, Ovol1/2 |
Slug |
Arnoux et al., 2005 Nieto et al., 2016 (1) Savagner et al., 1997 Shirley et al., 2010 |
(+) and (−) indicate positive and negative regulation of EMT, respectively.
Generally, EMT-related studies have examined the expression of epithelial (i.e. E-cadherin) and mesenchymal (i.e. N-cadherin or vimentin) markers to define the process, whereas definitive experimental evidence for true mesenchymal state as the end point is lacking in numerous cases where the EMT term is used. In such cases, epithelial-to-mesenchymal-like-epithelial transition might be a more accurate term, but could generate additional confusion in an already controversial field. Instead, EMT has been most recently described as a “continuum” where metastable epithelial cells can exhibit different states along the EMT spectrum between the epithelial ‘E” state and mesenchymal “M” state (Nieto et al., 2016). Intermediate states, known as “EM” states where cells exhibit partial E and M features, have been observed both experimentally and in mathematical modeling. Partial EMT has been used to describe a number of processes in contexts such as development, fibrosis, cancer, and wound healing (Nieto et al., 2016). EMT “continuum” or partial EMT still implies mesenchymal state as the obligatory destination of the process if it was to reach completion. An alternative, although purely hypothetical, scenario is that multiple destination states are possible. That said, broadening the underlying definition of EMT would accommodate a wide array of observed variations of epithelial plasticity in both developmental and pathological contexts.
EMT is induced by a variety of signaling molecules, and is regulated by a number of transcription factors, microRNAs, as well as epigenetic factors (extensively reviewed in (Lamouille et al., 2014)). Growth factors and signaling cascades that induce EMT, some in a tissue- and context-dependent manner, include transforming growth factor beta (TGF-β), epidermal growth factor (EGF), fibroblast growth factor (FGF), hepatocyte growth factor (HGF), Wnt, Hedgehog (Hh), and Notch (Table 1). Transcriptional regulators include both EMT-promoting transcription factors, such as Snai1 (Snai1), Slug (Snai2), Zeb1, Zeb2, and Twist, and EMT-inhibiting transcription factors, such as Grhl2, Ovol1, and Ovol2 (Nieto et al., 2016) (Table 1).
Recently, we have shown that loss of Ovol1/Ovol2 results in developing mouse epidermal cells undergoing morphological, behavioral, and molecular changes reminiscent of EMT (Lee et al., 2014). These cells fail to execute a proper epidermal differentiation program, and functional rescue experiments suggest a causal relationship between the EMT-like phenotype and the terminal differentiation defect. This necessity to suppress EMT-like events during epidermal morphogenesis implicates the possible existence of partial EMT in embryonic epidermis. The notion that partial EMT occurs in adult epidermal wound healing to facilitate the migration of epidermal cells during re-epithelialization was proposed in 2005, and has been widely accepted (Arnoux et al., 2005; Nieto et al., 2016). However, whether this notion has received strong experimental support or remains an attractive hypothesis warrants a closer look.
Morphological changes and cellular dynamics during wound re-epithelialization
Various organisms have been used as experimental models to elucidate and characterize the morphological and cellular changes that allow for the process of wound re-epithelialization during embryogenesis or adulthood. Variations in mechanisms have been identified and appear to be organism-, developmental stage-, and epithelial tissue-dependent, but collectively add to a better understanding that has the potential to be applied to improving wound healing in humans.
Lessons from lower organisms and embryos
Studies in model organisms such as fly and chick embryos have provided insights into the role of the actin-based machinery in wound closure. Live imaging studies in fly embryos coupled with small GTPase perturbations underscore the formation of a “purse string” by the actin cable to help generate the necessary contractive forces, as well as suggest the existence of redundant mechanisms and highlight the importance of actin-based filopodia and lamellipodia for “kitting” of the epithelial cells at the terminal stages of wound repair (Wood et al., 2002). Interestingly, the “purse string” mechanism seems to be specific to embryonic stages, whereas adult flies have a lamellae-specific mechanism that involves epidermal polyploidization and cell fusion (Razzell et al., 2011; Losick et al., 2013). Experiments carried out in chick embryos also illustrated how actin cables generate a contractile “purse string” around the wound, as opposed to adult wounds where cells migrate by lamellipodia (Martin and Lewis, 1992).
Zebrafish has also been used as a model system for studying cutaneous wound healing, and its healing process in adult skin shares similar steps as that of mammals except for the formation of an external fibrin clot (Richardson et al., 2013). Adult zebrafish heal their wounds with minimal scarring, despite the presence of a strong inflammatory response. Although the process of re-epithelialization in adult zebrafish has yet to be meticulously dissected, it is worth noting that the rate of re-epithelialization appears to be very rapid (Richardson et al., 2013).
Wound healing in mouse embryos is distinctly different from that in adult animals, particularly in that embryonic wounds heal perfectly without scarring (McCluskey and Martin, 1995). Embryonic day 16 is the latest stage where mice can heal without visible scars (Ferguson and O’Kane, 2004). A possible explanation for regeneration in embryos vs. scar formation in adults is the absence of inflammation during embryonic wound healing in mice (Redd et al., 2004). This said, recent studies have shown that when wounds are sufficiently large, proper regeneration including the formation of hair follicles can occur in the center of wounds in several mammalian models such mice, rabbits, and even humans (Ito et al., 2007; Plikus et al., 2012; Plikus et al., 2017).
Re-epithelialization during mammalian skin wound healing
Wound re-epithelialization in adult mammals involves collective migration, proliferation, and differentiation of keratinocytes around and/or within the damaged site (Shaw and Martin, 2009). A combination of in vitro and in vivo studies has been used to characterize the re-epithelialization events. The in vitro methods include standard scratch assays with primary keratinocyte or epidermal cell lines. Previous in vivo evidence on wound healing studies in mice had been limited to static histological, immunostaining, and electron microscopic images, limiting our understanding of the spatiotemporal dynamics of cell proliferation and migration during re-epithelialization. What was clear though is that migration of epidermal cells is restricted to the region that is proximal to the injury site whereas cells distal from the injury site proliferate (Coulombe, 1997; Arnoux et al., 2005) (Figure 1). A recent study pioneered the use of intravital imaging of wound re-epithelialization in live mice to examine its spatiotemporal cellular dynamics (Park et al., 2017). This work not only re-enforced the accepted notion of spatially separated migratory and proliferative zones, but also discovered the existence of a so-called mixed zone where migration and proliferation co-exist. Additionally, this work highlights several important points related to epidermal cell migration in the healing wounds: 1) both basal and differentiating suprabasal cells migrate towards the wound in a spatially organized fashion; 2) the rate of local migration correlates with the rate of upward differentiation of migrating epidermal cells; 3) cell migration and elongation predict the directionality of cell divisions towards the wound center.
Figure 1. EMT-associated cellular and molecular events during mammalian cutaneous wound re-epithelialization.
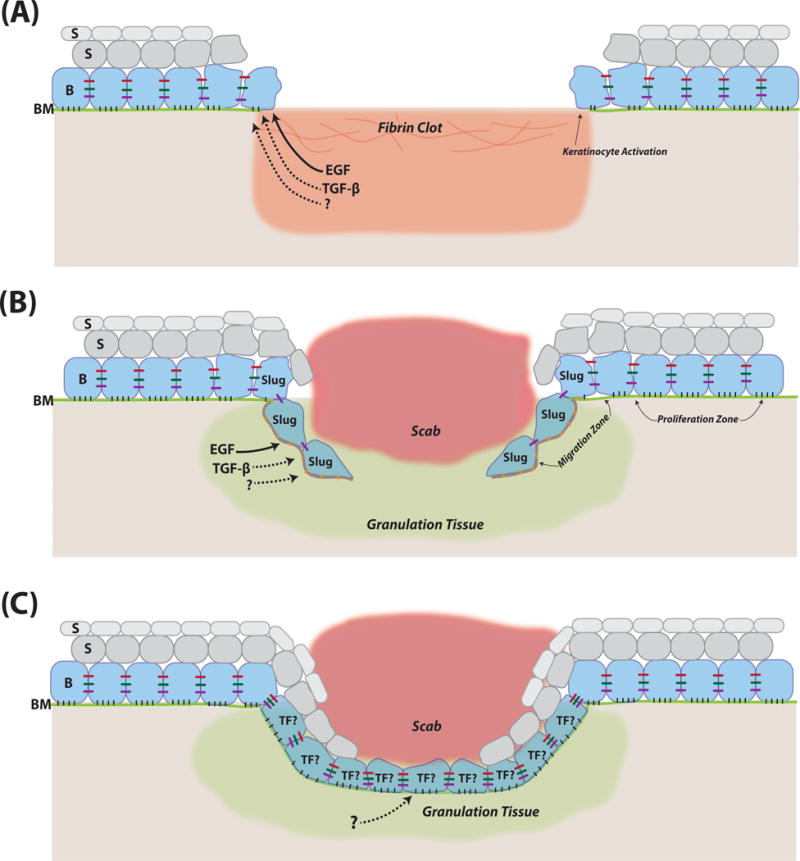
(A) Diagram of wound margins shortly after injury. (B) Diagram showing migrating epidermal fronts. (C) Diagram of wound neoepidermis at the resolution stage. B, basal cells. S, suprabasal cells. BM, basement membrane (green). TF denotes putative transcription factors that are important for maintenance of epithelial identity and/or resumption of a full epithelial state. Red, green, and purple bars between cells in the basal layer represent tight junctions, adherens junctions, and desmosomes, respectively. Black and orange bars between basal cells and basement membrane represent distinct cell-matrix interactions in wound periphery and migrating front. Solid and dashed arrows originating from the growth factors represent their known and potential roles, respectively, in inducing EMT-like changes of wound keratinocytes. Note that components in the diagrams are not drawn to scale.
In both in vitro and in vivo experiments, a preparatory phase is found to exist prior to the onset of migration towards wound center, whereby neighboring keratinocytes are alerted to the trauma and undergo an activation process characterized by molecular, morphological, cytoskeletal, and adhesive changes (Grinnell, 1992; Coulombe, 1997; Arnoux et al., 2005). Some of these changes resemble those that occur during EMT, leading to the prevailing proposal that wound re-epithelialization is a partial EMT process (Arnoux et al., 2005). Below we discuss the cellular and molecular changes during re-epithelialization that bear relevance to classical EMT (Table 1), focusing primarily on evidence from in vivo studies.
Directly around the wound, cell ‘ruffling’ was initially used to describe the morphological changes at the early stages in human wounds (Odland and Ross, 1968). More specifically, cells change their shape from being polarized cuboidal to being more flattened and elongated with extended cytoplasmic projections (Figure 1A–B). These cell shape changes are preceded and/or accompanied by alterations in gene expression including upregulation of hyperproliferation-associated keratin 6 (K6) and K16, retraction of keratin filaments (which normally associates with desmosomes and hemidesmosomes) from the cell periphery, as well as major reorganization of the actin cytoskeletal network (which normally associates with adherens junctions) (Coulombe, 1997; Coulombe, 2003; Arnoux et al., 2005). Cell-cell adhesions are altered, characterized by reduced desmosomal adhesion between cells as well as the reduced presence of adherens junction components such as E-cadherin, leading to appearance of intercellular gaps (Coulombe, 1997; Arnoux et al., 2005; Garrod et al., 2005; Nunan et al., 2015) (Figure 1B). Failure to downregulate desmosomal adhesion, as in mice where protein kinase C α is deficient, is associated with delayed wound healing (Thomason et al., 2012). Downregulation of adherens junctions and tight junctions, but not desmosomes, has been shown to be mediated by ephrin-B-EphB signaling, as epidermal-specific knockout of both ephrin-B1 and ephrin-B2 results in impaired wound closure, characterized by persistent adherens junctions between cells in the migrating front (Nunan et al., 2015). Cell-matrix adhesions are also altered to facilitate migration from a normally collagen/laminin-rich basement membrane to and through a fibronectin/tenascin-rich provisional matrix of the clot (Shaw and Martin, 2009; Nunan et al., 2015) (Figure 1A–B). Specific changes include redistribution of α2β1, α3β1, and α6β4 integrins (receptors for collagen or laminin) on keratinocyte surface, activated expression of α5β1, αvβ6, α9β1, and αvβ5 integrin (receptors for fibronectin, tenascin or vitronectin), as well as increased metalloproteinase activity that facilitates keratinocyte migration by promoting ECM remodeling and hemidesmosome breakdown (Arnoux et al., 2005).
While reducing epithelial traits is an integral part of keratinocyte activation and migration during wound re-epithelialization and is reminiscent of partial EMT, in vivo evidence for gain of mesenchymal features is sparse. Elevated expression of vimentin and fibroblast-specific protein 1 (FSP1) has been noted in the migrating epithelial tongues of acute wounds of thermal burn patients and in hypertrophic scars (Yan et al., 2010). In a recent study, the spatiotemporal profile of keratinocyte migration and proliferation during wound healing in mouse tail was carefully dissected, again showing that these cellular events can be uncoupled (Aragona et al., 2017). Gene expression analysis of the migrating leading edge revealed an enrichment of genes involved in cell migration (e.g., metalloproteinases) and cell adhesion (e.g., protocadherins, α5-integrin, desmosome and gap junction proteins). Genes controlling cytoskeleton and actin remodeling (e.g., actin regulators, myosin, and tubulin) are also part of the leading edge molecular signature, consistent with epidermal migration being driven by actin-myosin filaments that generate traction forces and actin polymerization that generates protrusions (Mitchison and Cramer, 1993; Shaw and Martin, 2009). Interestingly, EMT genes were not noted as part of the leading edge signature (Aragona et al., 2017).
The downregulation of proliferation in migrating epidermal cells of the healing wounds (Arnoux et al., 2005; Aragona et al., 2017; Park et al., 2017) is worth noting, as an inverse correlation between EMT and cell proliferation has been noted in multiple contexts (Arnoux et al., 2005; Brabletz and Brabletz, 2010; Lim and Thiery, 2012). This said, EMT has also been suggested to promote cancer stem cell characteristics, which encompass the ability to self-renew and proliferate (Mani et al., 2008; Scheel et al., 2011). As such, complex and even unrelated mechanisms may underlie the observed parallel in proliferative activity between wound re-epithelialization and EMT.
Conceivably, the adhesive and cytoskeletal changes that occur in the leading edge must be kept in check so that migrating epidermal cells are able to eventually resume their full epithelial state (Figure 1C) in order to execute a terminal differentiation program to regenerate a stratified epithelium. Indeed, E-cadherin returns to normal levels soon after the two migrating fronts meet (Kuwahara et al., 2001). Moreover, the expression of genes within the leading edge signature decreases as wound re-epithelialization progresses, and disappears upon fusion of the two edges whereas proliferation is resumed at the wound center (Aragona et al., 2017). Furthermore, loss of a desmosomal component Perp leads to impaired re-epithelialization due to enhanced keratinocyte migration while proliferation is unaffected (Beaudry et al., 2010). These findings implicate the transient and reversible nature of the molecular/cellular events that occur during wound re-epithelialization. However, a systematic comparison between the reverse events in the neoepidermis and MET has not yet been performed.
EMT regulators in cutaneous wound healing
EMT-inducing signals in the wound bed
Signaling in the wound bed is a complicated and intertwining affair involving epidermal, dermal, and immune cells, as well as both paracrine and autocrine mechanisms. Platelets and neutrophils represent some of the key initial signaling sources that release factors to activate/recruit fibroblasts and keratinocytes (Shaw and Martin, 2009). Important among the complex signaling milieu are EGF, FGF, HGF, keratinocyte growth factor (KGF), and TGF-β (Arnoux et al., 2005; Eming et al., 2014) (Table 1). While these signaling molecules ultimately all influence the proliferation and/or migration of epidermal keratinocytes around the wound edge, their cellular origins and underlying mechanisms vary and do not necessarily indicate a direct involvement in regulating the EMT-like aspects of keratinocyte activation.
Particularly relevant to the regulation of partial EMT are EGF and TGF-β (Figure 1A–B). EGF signaling, mediated through EGF receptor (EGFR) and particularly extracellular-signal-regulated kinase 5, is thought to control Slug expression and keratinocyte activation during wound healing (Arnoux et al., 2008). EGFR signaling is enhanced in N-acetylglucosaminyltransferase V transgenic mice, and is associated with EMT-like phenotypes (elevated levels of Snai1, Twist and N-cadherin; lower level of E-cadherin) and enhanced re-epithelialization (Terao et al., 2011). TGF-β signaling is well-known for its EMT-inducing activity in a myriad of tissue, developmental, and cancer contexts (Nieto et al., 2016; Stone et al., 2016). Its role in cutaneous wound healing has been demonstrated by a number of studies (extensively reviewed in (Bielefeld et al., 2013)), dating back to as early as the 80s (Mustoe et al., 1987). However, complicating the interpretation of its net effect on wound re-epithelialization (Arnoux et al., 2005; Nieto et al., 2016; Stone et al., 2016) is the different and even opposite roles of the three TGF-β ligand isoforms (TGF-β1, TGF-β2, and TGF-β3), its plethora of actions on multiple cellular components in the wound bed, including its ability to promote a fibrotic response (TGF-β1) and induce epithelial cell growth arrest (Le et al., 2012; Bielefeld et al., 2013).
Other developmental signaling pathways such as Wnt, Hh, and Notch have also been implicated in wound healing (Bielefeld et al., 2013). A functional involvement of Wnt signaling has been shown for hair follicle regeneration in large wounds (Ito et al., 2007), whereas a specific effect on wound re-epithelialization in vivo remains elusive. Genetic or pharmacological perturbation of Notch signaling compromises wound closure, but the effects appear pleiotropic and are not limited to that on keratinocyte migration (Chigurupati et al., 2007).
EMT–inducing transcription factor in cutaneous wound healing: Slug
Existing evidence supports the in vivo functional involvement of Slug in cutaneous wound healing. Slug belongs to the Snail superfamily of well-conserved zinc finger transcriptional repressors first identified in Drosophila melanogaster and shown to induce EMT initiation (Nieto, 2002). In the chick, Slug was identified as an important regulator of mesoderm and neural crest formation – two classical developmental processes that require EMT (Nieto et al., 1994). In the mouse, Snail is critical for mesoderm formation and Snai1 null mice die at gastrulation, whereas Slug is not required for mesoderm or neural crest formation (Jiang et al., 1998). This said, early studies show that Slug overexpression in a rat urinary bladder carcinoma cell line is able to reduce desmosomal association between cells (Savagner et al., 1997), a notion that is later corroborated by other studies in other cell types including keratinocytes (Shirley et al., 2010). Whether Slug is normally expressed in skin epithelia is controversial, but its loss in mice results in a thinner epidermis and transient delay of hair growth (Shirley et al., 2010). Slug expression is elevated in keratinocytes at the wound margins (Figure 1B) both in vivo and in vitro, and Snai2 null mice display compromised epidermal migration as soon as 72 hours after wounding with cells at leading edge displaying blunted epithelial extensions (Arnoux et al., 2005; Hudson et al., 2009). Moreover, enhanced expression of E-cadherin and K8 is seen at the migrating front tips in these mice, whereas the rate of wound closure does not appear to be affected. Together, these studies portrait a modulatory, but non-essential function of Slug in wound re-epithelialization.
Other potential in vivo regulators of EMT in cutaneous wound healing
In vivo studies have identified other potential regulators of wound re-epithelialization. A recent study reported the co-expression of transcription factor Foxn1 with EMT markers Snai1, MMP9, and N-cadherin during wound re-epithelialization (Gawronska-Kozak et al., 2016). However, evidence for a functional involvement is lacking. Mice deficient in EMT marker vimentin show wound re-epithelialization defects that appear to be associated with defects in keratinocyte migration, decreased molecular features associated with EMT, as well as defects in maturation and stratification of the neoepidermis (Cheng et al., 2016). However, the predominant mode of action seems to involve fibroblasts via a paracrine mechanism. The expression of transcription factor Citp2 is activated in keratinocytes upon wounding, and epidermal-specific deletion of Citp2 results in delayed wound healing (Liang et al., 2012). Here defective re-epithelialization stems from delayed proliferation in the epidermis as well as inability of the keratinocytes to suppress E-cadherin expression in the migrating tongues. Lipocalin 2, which when overexpressed can downregulate E-cadherin expression and upregulate mesenchymal markers, has been shown to act downstream of transcription factor TCF3 to promote epidermal cell migration and wound healing (Miao et al., 2014). Despite tantalizing clues, the molecular mechanisms underlying the actions of these EMT-inducing factors are not fully understood.
Conclusions and perspectives
Conceptual and technological advances have been made in the study of cutaneous wound repair. Wound re-epithelialization – more specifically, keratinocyte activation and migration - share many of the cellular and molecular changes with EMT, particularly decreased epithelial traits (e.g., cell adhesion) and increased motility. However, a typical EMT core signature (e.g., activation of mesenchymal genes) does not appear to be a prominent feature of the migrating wound epidermal cells. Wound re-epithelialization and EMT are also regulated by common signaling pathways, yet the underlying mechanisms might be distinct. Moreover, Slug is the only well-known EMT-inducing transcription factor for which a function in wound re-epithelialization has been shown. It remains possible that Slug function in this context is in part independent of its classical EMT-inducing activity. Ideally, accepting the notion that wound re-epithelialization is a partial and reversible EMT process as a fact rather than a hypothesis entails experimental proof that activating/migrating epidermal cells in the wound are indeed capable of adopting a mesenchymal fate in vivo if appropriate conditions were met. Without such proof, the changes associated with wound keratinocyte activation and migration are best viewed as a mild form of epithelial plasticity, rather than a partial EMT. Alternatively, one may relax the definition of EMT as discussed above (Nieto et al., 2016) to include this form of plasticity that occurs predominantly within an epithelial range with no strong indication of mesenchymal state as the end point.
Semantics aside, important issues remain regarding epithelial plasticity in wound re-epithelialization. How heterogeneous is the epidermal cell population that undergoes activation and migration? Are cells in a continuum of transitional states, or in stable or metastable intermediate states? Single-cell RNA-seq analysis at different post-wounding time points, although technically challenging given the small number of cells at the early-stage migrating fronts, should provide insights into the issues of cellular heterogeneity and cell state transitions. The identification of leading edge-specific surface markers such as α5 integrin (CD51) (Aragona et al., 2017) will facilitate such effort.
Is partial EMT used during wound re-epithelialization to solely gain motility, or to also facilitate cell fate choices – such as when and how to divide, whether or not to commit to terminal differentiation or to adopt expanded lineage potential? Interesting leads are emerging from recent profiling and imaging studies (Aragona et al., 2017; Park et al., 2017), which when combined with genetic and chemical perturbations will help establish causal effects. The issue of expanding lineage potential might be particularly relevant to large wounds, where healing is geared towards regeneration of not only the interfollicular epidermis but also epidermal appendages and fat (Plikus et al., 2017). The existence of stable or metastable intermediate states within an EMT spectrum could potentially lower the energy barrier for such lineage reprogramming during regeneration.
Another area of interest is the signaling and transcriptional mechanisms that regulate epithelial plasticity during wound re-epithelialization. For growth factor signals that are known to affect wound healing, elucidating their direct effect (if any) on keratinocyte activation and migration as well as the major contributing source(s) of such signals entails cell type- and/or temporally controlled genetic manipulations. The role of typical EMT-inducing transcription factors other than Slug (e.g., Zeb1 and Zeb2) in promoting keratinocyte activation and migration during wound re-epithelialization can be systematically examined. Mechanistic dissections can be performed to understand whether they act by regulating EMT or non-EMT processes. As one of the major reasons for controversy in the EMT field is that a large number of studies have been performed using cultured cells that may present in vitro artifacts not relevant to physiological conditions, studying the involvement of potential EMT regulators needs to use animal models or at least organotypic culture systems. Moreover, the toolbox of EMT characterizations here needs to be expanded beyond simply examining the expression of a small number of EMT markers to include a more exhaustive list of genes (e.g., Table 1), and more sophisticated molecular and cellular techniques such as gene profiling, single cell RNA-seq, and intravital imaging. New insights from intravital imaging of cutaneous wounding healing showcase remarkable spatiotemporal coordination and organization of multiple cellular events during re-epithelialization (Park et al., 2017). It is expected that this technology will be incorporated into future studies to examine the precise mode of epidermal migration during wound healing in wild-type vs. EMT-misregulated animals, the behaviors of not only epidermal cells but also other cellular constituents (e.g., fibroblasts and immune cells) in the healing wounds, as well as how such behaviors are modified when signaling and gene expression programs are altered in the wound microenvironment.
Importantly, we know very little about the regulatory mechanisms that prevent migrating epidermal cells from completely losing epithelial traits (e.g., undergoing complete EMT). What mechanisms confer reversibility to the partial EMT process so that a full epithelial state is properly restored to allow terminal differentiation and neoepidermal stratification? Known negative regulators of EMT or positive regulators of MET, such as the Grhl2 and Ovol1/2 transcription factors, are obvious candidates that can be experimentally tested.
A better understanding of epithelial plasticity regulation during wound healing has important clinical implications. Managing chronic wounds represents major health care costs, and our ability to manipulate such plasticity holds promise in improving wound repair in human patients. Insights from wound studies are likely also applicable to cancer research, where EMT has been considered a major contributing factor to metastasis and/or chemoresistance, and to tissue fibrosis, which is shown to be associated with enhanced/prolonged EMT (Nieto et al., 2016).
Acknowledgments
Work in the Dai lab has been supported by NIH Grants R01-AR47320, R01-AR068074, and R56-AR064532 (to X.D.).
Abbreviations
- EMT
Epithelial-to-mesenchymal transition
- ECM
extracellular cellular matrix
- EGF
epidermal growth factor
- EGFR
EGF receptor
- FGF
fibroblast growth factor
- HGF
hepatocyte growth factor
- Hh
hedgehog
- KGF
keratinocyte growth factor
- TGF-β
transforming growth factor beta
References
- Aragona M, Dekoninck S, Rulands S, Lenglez S, Mascre G, Simons BD, Blanpain C. Defining stem cell dynamics and migration during wound healing in mouse skin epidermis. Nat Commun. 2017;8:14684. doi: 10.1038/ncomms14684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnoux V, Come C, Kusewitt DF, Hudson LG, Savagner P. Cutaneous wound reepithelialization: a partial and reversible EMT. Rise and fall of epithelial phenotype 2005 [Google Scholar]
- Arnoux V, Nassour M, L’Helgoualc’h A, Hipskind R, Savagner P. Erk5 controls slug expression and keratinocyte activation during wound healing. Mol Biol Cell. 2008;19:4738–4749. doi: 10.1091/mbc.E07-10-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arwert EN, Hoste E, Watt FM. Epithelial stem cells, wound healing and cancer. Nat Rev Cancer. 2012;12:170–180. doi: 10.1038/nrc3217. [DOI] [PubMed] [Google Scholar]
- Beaudry VG, Ihrie RA, Jacobs SB, Nguyen B, Pathak N, Park E, Attardi LD. Loss of the desmosomal component perp impairs wound healing in vivo. Dermatol Res Pract. 2010;2010:759731. doi: 10.1155/2010/759731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielefeld KA, Amini-Nik S, Alman BA. Cutaneous wound healing: recruiting developmental pathways for regeneration. Cell Mol Life Sci. 2013;70:2059–2081. doi: 10.1007/s00018-012-1152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabletz S, Brabletz T. The ZEB/miR-200 feedback loop–a motor of cellular plasticity in development and cancer? EMBO Rep. 2010;11:670–677. doi: 10.1038/embor.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng F, Shen Y, Mohanasundaram P, Lindstrom M, Ivaska J, Ny T, Eriksson JE. Vimentin coordinates fibroblast proliferation and keratinocyte differentiation in wound healing via TGF-beta-Slug signaling. Proc Natl Acad Sci U S A. 2016;113:E4320–4327. doi: 10.1073/pnas.1519197113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chigurupati S, Arumugam TV, Son TG, Lathia JD, Jameel S, Mughal MR, Tang SC, Jo DG, Camandola S, Giunta M, Rakova I, McDonnell N, Miele L, Mattson MP, Poosala S. Involvement of notch signaling in wound healing. PLoS One. 2007;2:e1167. doi: 10.1371/journal.pone.0001167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulombe P. Towards a moleclar definition of keratinocyte activation after acute injury to stratified epithelia. Biochemical and Biophysical Reseach Communications. 1997;236:231–238. doi: 10.1006/bbrc.1997.6945. [DOI] [PubMed] [Google Scholar]
- Coulombe PA. Wound epithelialization: accelerating the pace of discovery. J Invest Dermatol. 2003;121:219–230. doi: 10.1046/j.1523-1747.2003.12387.x. [DOI] [PubMed] [Google Scholar]
- Eming SA, Martin P, Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med. 2014;6:1–16. doi: 10.1126/scitranslmed.3009337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson MW, O’Kane S. Scar-free healing: from embryonic mechanisms to adult therapeutic intervention. Philos Trans R Soc Lond B Biol Sci. 2004;359:839–850. doi: 10.1098/rstb.2004.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrod DR, Berika MY, Bardsley WF, Holmes D, Tabernero L. Hyper-adhesion in desmosomes: its regulation in wound healing and possible relationship to cadherin crystal structure. J Cell Sci. 2005;118:5743–5754. doi: 10.1242/jcs.02700. [DOI] [PubMed] [Google Scholar]
- Gawronska-Kozak B, Grabowska A, Kur-Piotrowska A, Kopcewicz M. Foxn1 Transcription Factor Regulates Wound Healing of Skin through Promoting Epithelial-Mesenchymal Transition. PLoS One. 2016;11:e0150635. doi: 10.1371/journal.pone.0150635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell F. Wound repair, keratinocyte activation and integrin modulation. Journal of Cell Science. 1992;101:1–5. doi: 10.1242/jcs.101.1.1. [DOI] [PubMed] [Google Scholar]
- Hsu YC, Li L, Fuchs E. Emerging interactions between skin stem cells and their niches. Nat Med. 2014;20:847–856. doi: 10.1038/nm.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson LG, Newkirk KM, Chandler HL, Choi C, Fossey SL, Parent AE, Kusewitt DF. Cutaneous wound reepithelialization is compromised in mice lacking functional Slug (Snai2) J Dermatol Sci. 2009;56:19–26. doi: 10.1016/j.jdermsci.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Yang Z, Andl T, Cui C, Kim N, Millar SE, Cotsarelis G. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature. 2007;447:316–320. doi: 10.1038/nature05766. [DOI] [PubMed] [Google Scholar]
- Jiang R, Lan Y, Norton C, Sundberg J, Gridley T. The slug gene is not essential for mesoderm or neural crest development in mice. Developmental Biology. 1998;198:227–285. [PubMed] [Google Scholar]
- Kuwahara M, Hatoko M, Tada H, Tanaka A. E-cadherin expression in wound healing of mouse skin. J Cutan Pathol. 2001;28:191–199. doi: 10.1034/j.1600-0560.2001.028004191.x. [DOI] [PubMed] [Google Scholar]
- Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le M, Naridze R, Morrison J, Biggs LC, Rhea L, Schutte BC, Kaartinen V, Dunnwald M. Transforming growth factor Beta 3 is required for excisional wound repair in vivo. PLoS One. 2012;7:e48040. doi: 10.1371/journal.pone.0048040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, Villarreal-Ponce A, Fallahi M, Ovadia J, Sun P, Yu QC, Ito S, Sinha S, Nie Q, Dai X. Transcriptional mechanisms link epithelial plasticity to adhesion and differentiation of epidermal progenitor cells. Dev Cell. 2014;29:47–58. doi: 10.1016/j.devcel.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Bhattacharya S, Bajaj G, Guha G, Wang Z, Jang HS, Leid M, Indra AK, Ganguli-Indra G. Delayed cutaneous wound healing and aberrant expression of hair follicle stem cell markers in mice selectively lacking Ctip2 in epidermis. PLoS One. 2012;7:e29999. doi: 10.1371/journal.pone.0029999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J, Thiery JP. Epithelial-mesenchymal transitions: insights from development. Development. 2012;139:3471–3486. doi: 10.1242/dev.071209. [DOI] [PubMed] [Google Scholar]
- Losick VP, Fox DT, Spradling AC. Polyploidization and cell fusion contribute to wound healing in the adult Drosophila epithelium. Curr Biol. 2013;23:2224–2232. doi: 10.1016/j.cub.2013.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P, Lewis J. Actin cables and epidermal movement in embryonic wound healing. Nature. 1992;360:179–183. doi: 10.1038/360179a0. [DOI] [PubMed] [Google Scholar]
- McCluskey J, Martin P. Analysis of the tissue movements of embryonic wound healing–Dil studies in the limb bud stage mouse embryo. Dev Biol. 1995;170:102–114. doi: 10.1006/dbio.1995.1199. [DOI] [PubMed] [Google Scholar]
- Miao Q, Ku AT, Nishino Y, Howard JM, Rao AS, Shaver TM, Garcia GE, Le DN, Karlin KL, Westbrook TF, Poli V, Nguyen H. Tcf3 promotes cell migration and wound repair through regulation of lipocalin 2. Nat Commun. 2014;5:4088. doi: 10.1038/ncomms5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison T, Cramer L. Actin-based cell motility and cell locomotion. Cell. 1993;84:371–379. doi: 10.1016/s0092-8674(00)81281-7. [DOI] [PubMed] [Google Scholar]
- Mustoe T, Pierce G, Thomason A, Gramates P, Sporn M, Deuel T. Accelerated healing of incisional wounds in rats induced by transforming growth factor-beta. Science. 1987;237:1333–1336. doi: 10.1126/science.2442813. [DOI] [PubMed] [Google Scholar]
- Nieto MA. The snail superfamily of zinc-finger transcription factors. Nat Rev Mol Cell Biol. 2002;3:155–166. doi: 10.1038/nrm757. [DOI] [PubMed] [Google Scholar]
- Nieto MA, Huang RY, Jackson RA, Thiery JP. Emt: 2016. Cell. 2016;166:21–45. doi: 10.1016/j.cell.2016.06.028. [DOI] [PubMed] [Google Scholar]
- Nieto MA, Sargent M, Wilkinson D, Cooke J. Control of cell behavior during vertebrate development by Slug, a zinc finger gene. Science. 1994;264:835–839. doi: 10.1126/science.7513443. [DOI] [PubMed] [Google Scholar]
- Nunan R, Campbell J, Mori R, Pitulescu ME, Jiang WG, Harding KG, Adams RH, Nobes CD, Martin P. Ephrin-Bs Drive Junctional Downregulation and Actin Stress Fiber Disassembly to Enable Wound Re-epithelialization. Cell Rep. 2015;13:1380–1395. doi: 10.1016/j.celrep.2015.09.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odland G, Ross R. Human wound repair. J Cell Biol. 1968;39:135–151. doi: 10.1083/jcb.39.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Gonzalez DG, Gurirao B, Boucher JD, Cockburn K, Marsh ED, Mesa KR, Brown S, Rompolas P, Haberman AM, Bellaiche Y, Greco V. Tissue-scale coordination of cellular behavior promotes epidermal wound repair in live mice. Nat Cell Biol. 2017;19:155–164. doi: 10.1038/ncb3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plikus MV, Gay DL, Treffeisen E, Wang A, Supapannachart RJ, Cotsarelis G. Epithelial stem cells and implications for wound repair. Semin Cell Dev Biol. 2012;23:946–953. doi: 10.1016/j.semcdb.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plikus MV, Guerrero-Juarez CF, Ito M, Li YR, Dedhia PH, Zheng Y, Shao M, Gay DL, Ramos R, Hsi T-C, Oh JW, Wang X, Ramirez A, Konopelski SE, Elzein A, Wang A, Supapannachart RJ, Lee H-L, Lim CH, Nace A, Guo A, Treffeisen E, Andl T, Ramirez RN, Murad R, Offermanns S, Metzger D, Chambon P, Widgerow AD, Tuan T-L, Mortazavi A, Gupta RK, Hamilton BA, Millar SE, Seale P, Pear WS, Lazar MA, Cotsarelis G. Regeneration of fat cells from myofibroblasts during wound healing. Science. 2017;355:748–752. doi: 10.1126/science.aai8792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzell W, Wood W, Martin P. Swatting flies: modelling wound healing and inflammation in Drosophila. Dis Model Mech. 2011;4:569–574. doi: 10.1242/dmm.006825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redd MJ, Cooper L, Wood W, Stramer B, Martin P. Wound healing and inflammation: embryos reveal the way to perfect repair. Philos Trans R Soc Lond B Biol Sci. 2004;359:777–784. doi: 10.1098/rstb.2004.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson R, Slanchev K, Kraus C, Knyphausen P, Eming S, Hammerschmidt M. Adult zebrafish as a model system for cutaneous wound-healing research. J Invest Dermatol. 2013;133:1655–1665. doi: 10.1038/jid.2013.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savagner P, Yamada K, Thiery JP. The zinc-finger protein slug causes desmosome dissociation, an initial and necessary step for growth factor-induced epithelial-mesenchymal transition. J Cell Biol. 1997;137:1403–1419. doi: 10.1083/jcb.137.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheel C, Eaton EN, Li SH, Chaffer CL, Reinhardt F, Kah KJ, Bell G, Guo W, Rubin J, Richardson AL, Weinberg RA. Paracrine and autocrine signals induce and maintain mesenchymal and stem cell states in the breast. Cell. 2011;145:926–940. doi: 10.1016/j.cell.2011.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw TJ, Martin P. Wound repair at a glance. J Cell Sci. 2009;122:3209–3213. doi: 10.1242/jcs.031187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirley SH, Hudson LG, He J, Kusewitt DF. The skinny on Slug. Mol Carcinog. 2010;49:851–861. doi: 10.1002/mc.20674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone RC, Pastar I, Ojeh N, Chen V, Liu S, Garzon KI, Tomic-Canic M. Epithelial-mesenchymal transition in tissue repair and fibrosis. Cell Tissue Res. 2016;365:495–506. doi: 10.1007/s00441-016-2464-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terao M, Ishikawa A, Nakahara S, Kimura A, Kato A, Moriwaki K, Kamada Y, Murota H, Taniguchi N, Katayama I, Miyoshi E. Enhanced epithelial-mesenchymal transition-like phenotype in N-acetylglucosaminyltransferase V transgenic mouse skin promotes wound healing. J Biol Chem. 2011;286:28303–28311. doi: 10.1074/jbc.M111.220376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Thomason HA, Cooper NH, Ansell DM, Chiu M, Merrit AJ, Hardman MJ, Garrod DR. Direct evidence that PKCalpha positively regulates wound re-epithelialization: correlation with changes in desmosomal adhesiveness. J Pathol. 2012;227:346–356. doi: 10.1002/path.4016. [DOI] [PubMed] [Google Scholar]
- Wood W, Jacinto A, Grose R, Woolner S, Gale J, Wilson C, Martin P. Wound healing recapitulates morphogenesis in Drosophila embryos. Nat Cell Biol. 2002;4:907–912. doi: 10.1038/ncb875. [DOI] [PubMed] [Google Scholar]
- Yan C, Grimm WA, Garner WL, Qin L, Travis T, Tan N, Han YP. Epithelial to mesenchymal transition in human skin wound healing is induced by tumor necrosis factor-alpha through bone morphogenic protein-2. Am J Pathol. 2010;176:2247–2258. doi: 10.2353/ajpath.2010.090048. [DOI] [PMC free article] [PubMed] [Google Scholar]


