Abstract
Background
Patients with sarcomatoid renal cell carcinomas (sRCC) have poor outcomes and limited treatment options. Pre-clinical and clinical data suggest susceptibility to cytotoxic agents and vascular endothelial growth factor-targeted therapies. We designed a phase II trial to evaluate the efficacy and safety of capecitabine, gemcitabine and bevacizumab in sRCC.
Methodology
Patients with metastatic or unresectable sRCC were eligible for inclusion. Patients received oral capecitabine 800 mg/m2 twice daily on days 1–21 of a 28-day cycle, intravenous gemcitabine 900 mg/m2 on days 1 and 15, and intravenous bevacizumab 10 mg/kg on days 1 and 15. Primary end-points were progression-free survival (PFS) and time to treatment failure (TTF). Secondary end points were safety, objective response rate (ORR), and overall survival (OS).
Results
Thirty-four patients were enrolled on trial. One patient was excluded from survival analysis and four from response analysis due to missing data. Median PFS was 5.5 months (95% confidence interval [CI], 3.4–7.7), median TTF was 4.2 months (95% CI, 2.4–6.0) and median OS was 12 months (95% CI, 10.6–13.4). ORR was 20% (5 partial responses, 1 complete response) and disease control rate was 73%. Thirty-one of the 34 patients (91%) discontinued treatment. Most common reason for discontinuation was progressive disease in 24 patients (71%). The most common grade 3 toxicity was rash (including hand-foot syndrome) in 24% patients.
Conclusion
The combination of capecitabine, gemcitabine and bevacizumab is an option for patients with sRCC, however, response rates are low. Novel therapies are needed to improve outcomes in patients with sRCC.
Keywords: sarcomatoid renal cell carcinoma, metastatic renal cell carcinoma, capecitabine, gemcitabine, bevacizumab
Introduction
Renal-cell carcinoma (RCC) is the most common primary neoplasm of the kidney with worldwide incidence of over 330,000 new cases annually.1 Sarcomatoid differentiation of RCC is characterized by spindle cell morphology and has been reported to occur in 1–29% of cases.2–4 It has been suggested to represent clonal evolution and can involve any histologic subtype of RCC.5–8 Sarcomatoid features classify RCC as Fuhrman grade 4 tumors and these typically have higher Ki-67, and frequent p53 mutations.8–11 Sarcomatoid RCC (sRCC) has an aggressive biology and confers 3-fold higher risk of mortality compared to clear cell RCC (ccRCC).2,12,13 Nearly half of the patients with sRCC present with metastatic disease and consequently have poor prognosis with a median overall survival (OS) of 3 to 10 months.12,14,15
Patients with sRCC have historically shown limited response to treatment, and prospective trials with limited numbers of patients have shown poor response rates.14,16–21 A summary of studies reporting on systemic therapy options for sRCC is summarized in Supplementary Table 2. Despite recent advances in targeted therapies, experience with these agents in sRCC has been limited. Recent phase III trials have either excluded or have not specified details of inclusion of sRCC.22–27 Rapid growth and pathologic resemblance with sarcomas have generated interest in cytotoxic chemotherapy similar to the ones used in soft-tissue sarcomas with anecdotal reports of durable responses with doxorubicin-based regimens.28–32 The chemotherapy combination of infusional fluorouracil and weekly gemcitabine in metastatic RCC demonstrated an overall response rate of 17% in the phase II setting and was regarded as a therapeutic option in the early days of targeted therapy development.33 Similar results have been reported for capecitabine and gemcitabine, with response rates of 8–16% in metastatic RCC.34–36
Pre-clinical data has shown overexpression of hypoxia-inducible factor (HIF) pathway markers in sRCC.37 Vascular endothelial growth factor (VEGF)-targeted therapy has shown variable results with partial response (PR) rates of 10–20%, stable disease (SD) in nearly 50%, and OS of 10–12 months, with concerns for worse outcomes with higher component of sarcomatoid element.14,38,39 At the time of study development, bevacizumab was regarded as a promising anti-angiogenic agent and was being used in several combination regiments in the treatment of metastatic RCC.40 The aim of this phase II trial was to evaluate outcomes in patients with sRCC treated with the combination of cytotoxic chemotherapy and antiangiogenic therapy can improve outcomes in patients with sRCC.
Patients and methods
This was a single-arm, open-label, single-center, phase II trial of capecitabine, gemcitabine, and bevacizumab for the treatment of metastatic or unresectable sRCC (ClinicalTrials.gov identifier: NCT00496587). The trial was approved by the institutional review board at The University of Texas MD Anderson Cancer Center, and complied with the Declaration of Helsinki, Good Clinical Practice guidelines, and local laws. Written informed consent was obtained from patients at the time of enrollment.
Eligibility
Adult patients with metastatic or unresectable sRCC were enrolled on this trial. Eligible patients had at least 10% sarcomatoid histology on the tumor specimens (nephrectomy or metastasectomy), and could have any WHO-recognized RCC epithelial component. Patients diagnosed with sRCC via needle biopsy of the primary or a metastatic site were eligible regardless of the percentage of sarcomatoid dedifferentiation found in biopsy sample. Patients were required to have Eastern Cooperative Oncology Group performance status of 0–2, adequate renal, hepatic, hematologic, and cardiac function, and at least one site of measurable disease. Patients should not have received any prior cytotoxic chemotherapy, or bevacizumab. Prior sorafenib, sunitinib, or immunotherapy treatment was permitted.
Treatment plan
Patients received oral capecitabine 800 mg/m2 twice daily on days 1–21, intravenous gemcitabine 900 mg/m2 over 30 minutes on days 1 and 15, and intravenous bevacizumab 10 mg/kg on days 1 and 15 of a 28-day cycle for a planned total of 12 cycles. The dose of individual agents was determined on the basis of our prior retrospective and phase II experience.35,41 Growth factors were allowed as per discretion of the treating physician. Surgical consolidation, when appropriate, was allowed for responding patients. Dose reductions for capecitabine and gemcitabine, and interruptions were allowed based on pre-specified adverse events (AE) (described in supplemental methods).
Diagnostic evaluation and assessment of efficacy and safety
Patients were evaluated at baseline with a history and physical examination; laboratory studies; urinalysis; 12-lead electrocardiogram; and computed tomography (CT) scans of chest, abdomen, and pelvis. Imaging studies were performed every 8 weeks. Tumor response was assessed using the Response Evaluation Criteria in Solid Tumors (RECIST), v.1.0.42 Progression was defined per RECIST as an increase in disease burden by 20% or more in the sum of the largest tumor diameters compared to baseline or smallest measurement. AE were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events v3.0.43
Study end points and statistical analysis
A maximum accrual of 40 patients was planned. The primary end-point was progression-free survival (PFS) and time to failure (TTF), with failure defined as death, disease progression, or unacceptable toxicity. Objective response rate (ORR), and OS were secondary end points. PFS, TTF, and OS were calculated from the first day of treatment. Early stopping rules were established for both TTF and AEs (described in the supplemental methods). Descriptive statistics were used to summarize continuous and categorical variables. Kaplan-Meier curves were constructed using SPSS 23.0 (IBM Corp, Armonk, NY). Chi-square test was used to compare proportion of categorical variables between groups. P values <0.05 were considered as significant.
Results
Patient characteristics
The study opened for enrollment in July 2007 and was terminated in January 2012 due to low accrual after enrollment of 34 patients. Thirty-three patients were included in the final survival analysis. One patient went off protocol after receiving one cycle due to delay in starting his second cycle and was never restaged, and hence was not included in the survival analysis due to missing data. Patient characteristics and prior therapies are summarized in Table 1. Two patients were treated with sorafenib prior to study start and both had outright progressive disease. Three patients received sunitinib prior to study start; two of these patients received sunitinib in the frontline setting and one in second-line setting. In the front-line sunitinib patients, one had stable disease as best response and the other had progressive disease. The second-line patient was treated temsirolimus prior to sunitinib and had stable disease on sunitinib for some time prior to progression.
Table 1.
Patient characteristics (n=34)
| Characteristic | Patients, n (%) |
|---|---|
|
| |
| Gender | |
| Male | 26 (76) |
| Female | 8 (24) |
|
| |
| Age, y | |
| Median | 54 |
| Range | 38–77 |
|
| |
| Race | |
| Caucasian | 30 (88) |
| Latin-American | 2 (6) |
| African-American | 2 (6) |
|
| |
| ECOGa performance status | |
| 0 | 12 (35) |
| 1 | 16 (47) |
| 2 | 6 (18) |
|
| |
| Disease status at enrollment | |
| Newly diagnosed metastatic disease | 21 (62) |
| Recurrent metastatic disease | 10 (29) |
| Progressive metastatic disease | 3 (9) |
|
| |
| Number of prior lines of therapy received | |
| 0 | 27 (79) |
| 1 | 5 (15) |
| 2 | 2 (6) |
|
| |
| Prior therapies | |
| Sunitinib | 3 (9) |
| Sorafenib | 2 (6) |
| Radiation | 1 (3) |
| Immunotherapy | 0 (0) |
|
| |
| Nephrectomy | |
| Prior to enrollment | 26 (77) |
| After enrollment | 0 (0) |
|
| |
| Epithelial component | |
| Clear cell | 16 (47) |
| Chromophobe | 1 (3) |
| Papillary type 2 | 1 (3) |
| Undeterminedb | 16 (47) |
|
| |
| Sarcomatoid component | |
| > 40% | 12 (35) |
| ≤ 20% | 7 (21) |
| 21–40% | 2 (6) |
| Unquantified | 13 (38) |
ECOG = Eastern Cooperative Oncology Group;
Diagnosis of renal cell carcinoma was based on typical imaging findings
Treatment administration and safety
Thirty-four patients were evaluable for safety. The median number of cycles administered were 5 (range 1 to 12). During the study period, there was a total 594 AEs regardless of causality. Out of these, 377 were thought to be related (possibly, probably or definitely) to the study regimen (Table 2). There was no grade 4 toxicity reported. The most common non-hematological AEs were pain (body, limb, head) in 82% of patients, rash including hand-foot syndrome in 76% of patients, and fatigue in 65% of patients. The most common hematological AEs were anemia in 38% of patients and lymphopenia in 15% patients. The most common grade 3 toxicities were rash in 24% patients, fatigue in 9% of patients and catheter related thrombosis in 6% of patients. Twelve patients required dose reductions of one or more. One patient had sudden death from unknown etiology while on study. At the time of last follow-up, only one patient was alive.
Table 2.
Treatment-related adverse eventsa
| Adverse event | Any grade (n) | % | Grade 3/4 (n) | % |
|---|---|---|---|---|
| Non-hematological adverse events | ||||
| Pain (body, limb, head) | 28 | 82 | 1 | 3 |
| Rash | 26 | 76 | 8 | 24 |
| Fatigue | 22 | 65 | 3 | 9 |
| Anorexia | 16 | 47 | 0 | 0 |
| Nausea | 14 | 41 | 0 | 0 |
| Dry skin | 13 | 38 | 0 | 0 |
| Proteinuria | 13 | 38 | 0 | 0 |
| Dyspnea | 12 | 35 | 0 | 0 |
| Sensory neuropathy | 11 | 32 | 1 | 3 |
| Diarrhea | 10 | 29 | 1 | 3 |
| Hyperglycemia | 10 | 29 | 0 | 0 |
| Oral mucositis | 10 | 29 | 0 | 0 |
| Hemorrhage (epistaxis, hemoptysis, genitourinary) | 8 | 24 | 0 | 0 |
| Elevated AST | 7 | 21 | 0 | 0 |
| Taste alteration | 7 | 21 | 0 | 0 |
| Elevated ALT | 6 | 18 | 0 | 0 |
| Constipation | 6 | 18 | 0 | 0 |
| Elevated creatinine | 6 | 18 | 0 | 0 |
| Hyperpigmentation | 6 | 18 | 0 | 0 |
| Vomiting | 6 | 18 | 0 | 0 |
| Hypoalbuminemia | 5 | 15 | 0 | 0 |
| Edema | 5 | 15 | 0 | 0 |
| Oral sensitivity | 5 | 15 | 0 | 0 |
| Abdominal symptoms (cramps, diarrhea) | 5 | 15 | 0 | 0 |
| Hyponatremia | 5 | 15 | 1 | 3 |
| Elevate alkaline phosphatase | 4 | 12 | 0 | 0 |
| Hyperbilirubinemia | 4 | 12 | 1 | 3 |
| Cough | 4 | 12 | 0 | 0 |
| Dry mouth | 4 | 12 | 0 | 0 |
| Cold intolerance | 4 | 12 | 0 | 0 |
| Motor neuropathy | 4 | 12 | 1 | 3 |
| Hypocalcemia | 3 | 9 | 0 | 0 |
| Alopecia | 3 | 9 | 0 | 0 |
| Hypertension | 3 | 9 | 1 | 3 |
| Nail changes | 3 | 9 | 0 | 0 |
| Hyperkalemia | 3 | 9 | 0 | 0 |
| Sweating | 3 | 9 | 0 | 0 |
| Catheter related thromboembolism | 2 | 6 | 2 | 6 |
| Prolonged PTT | 2 | 6 | 0 | 0 |
| Night sweats | 2 | 6 | 0 | 0 |
| Dyspepsia | 2 | 6 | 0 | 0 |
| Hiccups | 2 | 6 | 0 | 0 |
| Hyperkalemia | 2 | 6 | 0 | 0 |
| Insomnia | 2 | 6 | 0 | 0 |
| Pruritus | 2 | 6 | 0 | 0 |
| Thromboembolism | 1 | 3 | 1 | 3 |
| Hypernatremia | 1 | 3 | 1 | 3 |
| Hematological adverse events | ||||
| Anemia | 13 | 38 | 0 | 0 |
| Lymphopenia | 5 | 15 | 1 | 3 |
| Thrombocytopenia | 5 | 15 | 0 | 0 |
| Leukopenia | 3 | 9 | 1 | 3 |
| Neutropenia | 3 | 9 | 1 | 3 |
Treatment-related adverse events were defined as events that the investigators deemed to have a possible, probable, or definite relationship to the study regimen. Listed are the treatment-related adverse events that were reported in at least 5% of the patients, along with any incidence of grade 3 or 4 events in more than 1% of the study population.
Efficacy
Thirty-four patients were treated based on the protocol. Thirty-three patients were evaluable for survival analyses (Figure 1). Four patients were not evaluable for response by diagnostic imaging due to discontinuation of therapyafter one cycle. Best responses in 30 evaluable patients include complete response (CR) in 1 patient, PR in 5 patients, SD in 16 patients, and progressive disease (PD) in 8 patients. In terms of the primary end-points of the study, the median PFS was 5.5 months (95% confidence interval [CI], 3.4–7.7), and the median TTF was 4.2 months (95% CI, 2.4–6.0). In terms of the secondary endpoints of the study, the ORR was 20% (6/30) and the disease control rate (CR+PR+SD) was 73% (22/30). Median OS was 12 months (95% CI, 10.6–13.4). Three patients completed study per protocol after receiving 12 cycles of chemotherapy. Thirty-one of the 34 patients (91%) discontinued treatment. Reasons for discontinuation were PD in 24 patients (71%), toxicity in 2 patients (6%, acute congestive heart failure in 1 patient, grade 3 hand-foot syndrome in the other patient), patient’s choice in 2 cases (6%), other reasons in 2 cases (6%, 1 patient had acute kidney injury unrelated to investigational regimen, and the other patient had delay in treatment per protocol), and sudden death of unknown etiology in 1 patient (3%). Among patients evaluable for response, disease control rate between groups with ≤20% versus >20 sarcomatoid element was 71% (5/7) and 73% (8/11) respectively, p=0.95. No patients went on to receive consolidative surgery in this cohort.
Figure 1.
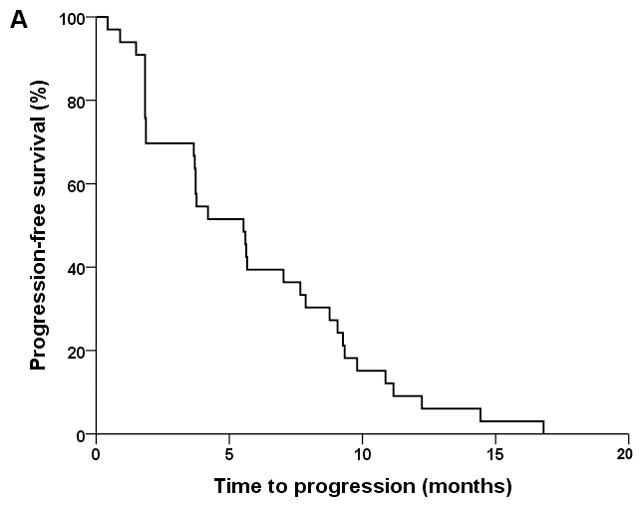
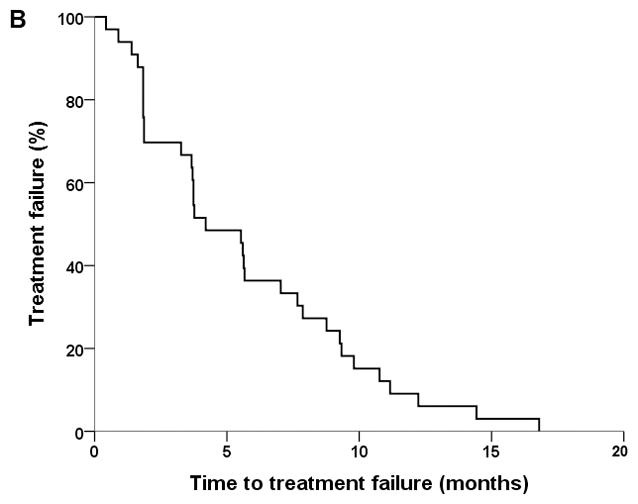
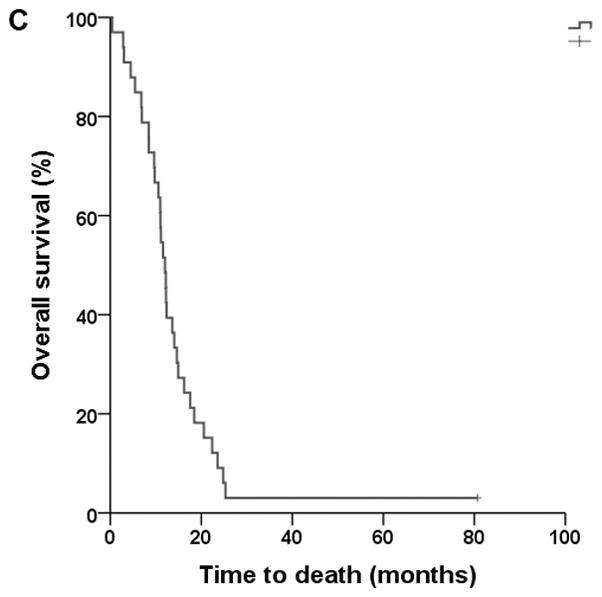
Kaplan-Meier plots of progression-free survival (A), time to treatment failure (B), and overall survival (C).
Discussion
We demonstrated feasibility and objective response with combined cytotoxic and anti-angiogenic therapy in patients with sRCC. The ORR was 20%, median TTF was 4.2 months, and median OS was 12 months. These results are comparable with the phase II trial by Michaelson et al. using sunitinib and gemcitabine in 39 patients with sRCC, which showed ORR of 26%, a median time to progression (TTP) of 5 months, and a median OS of 10 months.16 The most common grade 3/4 toxicities encountered were neutropenia (51%), anemia (28%), and fatigue (21%). Conversely, the most common grade 3 toxicities in our cohort were rash in 24% patients, fatigue in 9% of patients and catheter related thrombosis in 6% of patients. There were no grade 4 toxicities in our cohort.
A summary of studies reporting on systemic treatments in sRCC is provided in Supplementary Table 2. The French federation genitourinary group phase II trial of doxorubicin and ifosfamide in 25 patients with sRCC did not show any objective responses and had a median TTP of 2.2 months and median OS of 3.9.19 Nanus et al reported some activity with gemcitabine and doxorubicin (GD).32 Out of 10 sRCC patients evaluated 2 had complete response (CR), 1 had PR, 1 had SD, 1 had mixed response, while 4 patients had no response. However, the ECOG 8802 phase II trial of GD in 39 patients concluded that the regimen was inactive in sRCC showing a median PFS of 3.5 months and a median OS of 8.8 months.17 They noted more responses in patients with higher proportion of sarcomatoid elements. The study by Staehler et al. with GD followed by sorafenib in 15 patients showed median TTP of 6.6 months with GD and 10.9 months for those who received sorafenib after GD.18 No responses were seen with GD, but out of the patients who received sorafenib one had PR and four had SD. The OS for patients with metastatic disease who received both GD and sorafenib was 8 months.18
One retrospective study by Golshayan et al. on VEGF-targeted therapy in 48 patients with sRCC reported a PR rate of 19%, SD in 49%, TTP of 5.3 months, and OS of 11.8 months.14 In another report by Molina et al, out of 34 sRCC patients who received sunitinib as frontline or second-line therapy, CR was observed in 1 (3%), PR in 4 (12%), and SD in 17 (50%) of patients with a median PFS of 4.4 months and OS of 10 months. These responses to VEGF-targeted therapy are congruent with pre-clinical observations of HIF pathway proteins, e.g. VEGF expression in sRCC.37 However, one study by Beuseliunk et al reported that patients with >25% sarcomatoid component have very poor outcomes with anti-VEGF tyrosine kinase inhibitors.39 It is interesting to note that in the series from Golshayan et al, patients who had <20% sarcomatoid elements in their tumor fared better with VEGF-targeted therapy; however the differences were not statistically significant.14 The PRs in their patients were limited to this population, and they had better PFS (6.8 vs. 4.3 months) and OS (14.9 vs. 8.6 months) compared to those who had >20% sarcomatoid elements.14 In our cohort, there was no difference in the disease control rate between patients with ≤20% versus >20% sarcomatoid elements. However, the number of patients was very small. These results suggest that patients with higher degree of sarcomatoid component (more than 20–25%) may not benefit from single agent VEGF-targeted therapy. At the time of this trial design, data supporting the 20–25% prognostic cutoff was not availabe. Thus, while patients with >10% sarcomatoid component were included on trial, analysis of outcomes incorporated 20% cutoff. Epithelial to mesenchymal transition has been reported as one of the mechanisms involved in such resistance to anti-VEGF therapies in sarcomatoid tumors.44,45
One retrospective study from our institution on 108 patients by Mian et al. showed response rates of 32%, 33% and 14% to interferon-α, interleukin-2 and interferon-γ based regimens, respectively. However, there was considerable heterogeneity in the treatment regimens, many of which included cytotoxic agents such as 5-flurouracil, mitomycin C, cyclophosphamide, vincristine, doxorubicin, and dacarbazine. The higher grade, proliferation index, and rapid growth of the sarcomatoid component in sRCC can partly explain the susceptibility to such cytotoxic agents noted in the present report and in prior studies.
A difficulty in this study was patient accrual, in large part due to the rarity of this tumor. It is an unfortunate reality that rare and orphan tumors are often treated with data gleaned from case series and retrospective analysis. Multicenter collaboration is essential to gather larger cohorts of patients with rare tumors and perform prospective studies to better understand disease biology and advance the field.
Ongoing trials in patients with sRCC or advanced RCC with sarcomatoid features are currently evaluating sunitinib with or without gemcitabine (NCT01164228), pazopanib (NCT01767636), sorafenib with bevacizumab (NCT00126503), and sunitinib versus bevacizumab with atezolizumab (NCT02420821). sRCC has among the highest expression of c-MET among the different RCC subtypes making it an excellent candidate for clinical trials involving cabozantinib.46 There is also higher co-expression of programmed death ligand-1 (PD-L1) on tumor cells, as well as programmed death 1 (PD-1) on tumor-infiltrating lymphocytes in sRCC compared with ccRCC (50% vs. 3%)47 making anti-PD-1 / PD-L1 checkpoint inhibitors an attractive option in sRCC. Phase 1 data with atezolizumab, a PD-L1 antibody, showed early evidence of anti-tumor activity in sRR with an ORR of 22%; safety data indicated a favorable profile, with no grade 4 or 5 events. Further studies of immune checkpoint inhibition are needed in sRCC, both as single agent and combination therapy options.48
Conclusions
The combination of capecitabine, gemcitabine, and bevacizumab was well tolerated in patients with sRCC and had PFS, ORR, and OS comparable to other regimens tested in this population. Further studies need to establish the optimal combination of cytotoxic chemotherapy, targeted agents, and immunotherapy to improve outcomes in these patients.
Supplementary Material
Clinical Practice Points.
Patients with sarcomatoid renal cell carcinomas (sRCC) have poor outcomes and limited treatment options. Pre-clinical and clinical data suggest susceptibility to cytotoxic agents and vascular endothelial growth factor-targeted therapies.
In this phase II trial of capecitabine, gemcitabine, and bevacizumab including 34 patients, the median progression free survival was 5.5 months, median overall survival was 12 months, objective response rate was 20%, and disease control rate was 73%. Most common grade 3 toxicity was rash. There were no grade 4 toxicities.
Capecitabine, gemcitabine, and bevacizumab is an option for patients with sRCC, however, response rates are low. Future studies need to evaluate combination of cytotoxic, anti-angiogenic, and immunotherapy in sRCC.
Acknowledgments
Funding:
This original research was done in collaboration with Eli Lilly and Company. This study was supported in part by the M.D. Anderson Cancer Center Support Grant (5 P30 CA016672, PI: Dr Ronald DePinho) from the National Cancer Institute. PM is supported by the National Institutes of Health T32 CA009666 grant.
Footnotes
Clinical trial registration information: https://clinicaltrials.gov/ct2/show/NCT00496587
Authorship contribution:
AM and MNS contributed equally to this study. AM, MNS, and PM collected and analyzed the data, and drafted the manuscript. LCP and NMT designed the clinical trial, treated patients, analyzed the data, and co-wrote the manuscript. AYS, NMT, EJ, and LCP treated patients, acquired data, and revised the manuscript for important intellectual content. All authors reviewed and approved the final version to be published.
Prior presentation:
Preliminary results were presented in part at the 2010 Genitourinary Cancers Symposium, March 5–7, San Francisco, CA, USA.
Financial Disclosures:
AM: None; PM: None; MNS: None; NMT: has received grants, personal fees and non-financial support from Novartis, Bristol-Myers Squibb, and Exelixis; personal fees and non-financial support from GSK, Pfizer, and Nektar; and grants from Epizyme.
References
- 1.GLOBOCAN 2012: Estimated Cancer Incidence, Moratility and Prevalence Worldwide in 2012. [Internet] [cited 2016 Oct 3];Available from: http://globocan.iarc.fr/Pages/fact_sheets_population.aspx.
- 2.Shuch B, Bratslavsky G, Linehan WM, Srinivasan R. Sarcomatoid Renal Cell Carcinoma: A Comprehensive Review of the Biology and Current Treatment Strategies. The Oncologist. 2012;17(1):46–54. doi: 10.1634/theoncologist.2011-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Peralta-Venturina M, Moch H, Amin M, et al. Sarcomatoid differentiation in renal cell carcinoma: a study of 101 cases. Am J Surg Pathol. 2001;25(3):275–84. doi: 10.1097/00000478-200103000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Sella A, Logothetis CJ, Ro JY, Swanson DA, Samuels ML. Sarcomatoid renal cell carcinoma. A treatable entity. Cancer. 1987;60(6):1313–8. doi: 10.1002/1097-0142(19870915)60:6<1313::aid-cncr2820600625>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 5.Delahunt B. Sarcomatoid renal carcinoma: the final common dedifferentiation pathway of renal epithelial malignancies. Pathology (Phila) 1999;31(3):185–90. doi: 10.1080/003130299104945. [DOI] [PubMed] [Google Scholar]
- 6.Jones TD, Eble JN, Wang M, MacLennan GT, Jain S, Cheng L. Clonal divergence and genetic heterogeneity in clear cell renal cell carcinomas with sarcomatoid transformation. Cancer. 2005;104(6):1195–203. doi: 10.1002/cncr.21288. [DOI] [PubMed] [Google Scholar]
- 7.Cheville JC, Lohse CM, Zincke H, et al. Sarcomatoid renal cell carcinoma: an examination of underlying histologic subtype and an analysis of associations with patient outcome. Am J Surg Pathol. 2004;28(4):435–41. doi: 10.1097/00000478-200404000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Dal Cin P, Sciot R, Van Poppel H, Balzarini P, Roskams T, Van den Berghe H. Chromosome changes in sarcomatoid renal carcinomas are different from those in renal cell carcinomas. Cancer Genet Cytogenet. 2002;134(1):38–40. doi: 10.1016/s0165-4608(01)00615-x. [DOI] [PubMed] [Google Scholar]
- 9.Visapää H, Seligson D, Huang Y, et al. Ki67, gelsolin and PTEN expression in sarcomatoid renal tumors. Urol Res. 2003;30(6):387–9. doi: 10.1007/s00240-002-0284-z. [DOI] [PubMed] [Google Scholar]
- 10.Oda H, Nakatsuru Y, Ishikawa T. Mutations of the p53 gene and p53 protein overexpression are associated with sarcomatoid transformation in renal cell carcinomas. Cancer Res. 1995;55(3):658–62. [PubMed] [Google Scholar]
- 11.Ro JY, Ayala AG, Sella A, Samuels ML, Swanson DA. Sarcomatoid renal cell carcinoma: Clinicopathologic. A study of 42 cases. Cancer. 1987;59(3):516–26. doi: 10.1002/1097-0142(19870201)59:3<516::aid-cncr2820590327>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 12.Trudeau V, Larcher A, Sun M, et al. Comparison of oncologic outcomes between sarcomatoid and clear cell renal cell carcinoma. World J Urol. 2016;34(10):1429–36. doi: 10.1007/s00345-016-1780-z. [DOI] [PubMed] [Google Scholar]
- 13.Park I, Cho YM, Lee J-L, et al. Prognostic factors of metastatic renal cell carcinoma with extensive sarcomatoid component. J Cancer Res Clin Oncol. 2013;139(5):817–27. doi: 10.1007/s00432-013-1386-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Golshayan AR, George S, Heng DY, et al. Metastatic Sarcomatoid Renal Cell Carcinoma Treated With Vascular Endothelial Growth Factor–Targeted Therapy. J Clin Oncol. 2009;27(2):235–41. doi: 10.1200/JCO.2008.18.0000. [DOI] [PubMed] [Google Scholar]
- 15.Mian BM, Bhadkamkar N, Slaton JW, et al. Prognostic factors and survival of patients with sarcomatoid renal cell carcinoma. J Urol. 2002;167(1):65–70. [PubMed] [Google Scholar]
- 16.Michaelson MD, McKay RR, Werner L, et al. Phase 2 trial of sunitinib and gemcitabine in patients with sarcomatoid and/or poor-risk metastatic renal cell carcinoma. Cancer. 2015;121(19):3435–43. doi: 10.1002/cncr.29503. [DOI] [PubMed] [Google Scholar]
- 17.Haas NB, Lin X, Manola J, et al. A phase II trial of doxorubicin and gemcitabine in renal cell carcinoma with sarcomatoid features: ECOG 8802. Med Oncol. 2011;29(2):761–7. doi: 10.1007/s12032-011-9829-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Staehler M, Haseke N, Roosen A, et al. Sorafenib after combination therapy with gemcitabine plus doxorubicine in patients with sarcomatoid renal cell Carcinoma: a prospective evaluation. Eur J Med Res. 2010;15(7):287. doi: 10.1186/2047-783X-15-7-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Escudier B, Droz JP, Rolland F, et al. Doxorubicin and ifosfamide in patients with metastatic sarcomatoid renal cell carcinoma: a phase II study of the Genitourinary Group of the French Federation of Cancer Centers. J Urol. 2002;168(3):959–61. doi: 10.1016/S0022-5347(05)64551-X. [DOI] [PubMed] [Google Scholar]
- 20.Culine S, Bekradda M, Terrier-Lacombe MJ, Droz JP. Treatment of sarcomatoid renal cell carcinoma: is there a role for chemotherapy? Eur Urol. 1995;27(2):138–41. doi: 10.1159/000475145. [DOI] [PubMed] [Google Scholar]
- 21.Cangiano T, Liao J, Naitoh J, Dorey F, Figlin R, Belldegrun A. Sarcomatoid Renal Cell Carcinoma: Biologic Behavior, Prognosis, and Response to Combined Surgical Resection and Immunotherapy. J Clin Oncol. 1999;17(2):523–523. doi: 10.1200/JCO.1999.17.2.523. [DOI] [PubMed] [Google Scholar]
- 22.Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. The Lancet. 2007;370(9605):2103–11. doi: 10.1016/S0140-6736(07)61904-7. [DOI] [PubMed] [Google Scholar]
- 23.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus Interferon Alfa in Metastatic Renal-Cell Carcinoma. N Engl J Med. 2007;356(2):115–24. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 24.Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in Locally Advanced or Metastatic Renal Cell Carcinoma: Results of a Randomized Phase III Trial. J Clin Oncol. 2010;28(6):1061–8. doi: 10.1200/JCO.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- 25.Motzer RJ, Hutson TE, Cella D, et al. Pazopanib versus Sunitinib in Metastatic Renal-Cell Carcinoma. N Engl J Med. 2013;369(8):722–31. doi: 10.1056/NEJMoa1303989. [DOI] [PubMed] [Google Scholar]
- 26.Choueiri TK, Escudier B, Powles T, et al. Cabozantinib versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med. 2015;373(19):1814–23. doi: 10.1056/NEJMoa1510016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med. 2015;373(19):1803–13. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoshi S, Satoh M, Ohyama C, et al. Active chemotherapy for bone metastasis in sarcomatoid renal cell carcinoma. Int J Clin Oncol. 2003;8(2):0113–7. doi: 10.1007/s101470300020. [DOI] [PubMed] [Google Scholar]
- 29.Bangalore N, Bhargava P, Hawkins MJ, Bhargava P. Sustained response of sarcomatoid renal-cell carcinoma to MAID chemotherapy: Case report and review of the literature. Ann Oncol. 2001;12(2):271–4. doi: 10.1023/a:1008352024762. [DOI] [PubMed] [Google Scholar]
- 30.Rashid MH, Welsh CT, Bissada NK, Chaudhary UB. Complete response to adriamycin and ifosfamide in a patient with sarcomatoid renal cell carcinoma. Am J Clin Oncol. 2005;28(1):107–8. doi: 10.1097/01.coc.0000139938.76332.6e. [DOI] [PubMed] [Google Scholar]
- 31.Roubaud G, Gross-Goupil M, Wallerand H, de Clermont H, Dilhuydy MS, Ravaud A. Combination of Gemcitabine and Doxorubicin in Rapidly Progressive Metastatic Renal Cell Carcinoma and/or Sarcomatoid Renal Cell Carcinoma. Oncology. 2011;80(3–4):214–8. doi: 10.1159/000329078. [DOI] [PubMed] [Google Scholar]
- 32.Nanus DM, Garino A, Milowsky MI, Larkin M, Dutcher JP. Active chemotherapy for sarcomatoid and rapidly progressing renal cell carcinoma. Cancer. 2004;101(7):1545–51. doi: 10.1002/cncr.20541. [DOI] [PubMed] [Google Scholar]
- 33.Rini BI, Vogelzang NJ, Dumas MC, Wade JL, Taber DA, Stadler WM. Phase II trial of weekly intravenous gemcitabine with continuous infusion fluorouracil in patients with metastatic renal cell cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2000;18(12):2419–26. doi: 10.1200/JCO.2000.18.12.2419. [DOI] [PubMed] [Google Scholar]
- 34.Stadler WM, Halabi S, Rini B, et al. A phase II study of gemcitabine and capecitabine in metastatic renal cancer: a report of Cancer and Leukemia Group B protocol 90008. Cancer. 2006;107(6):1273–9. doi: 10.1002/cncr.22117. [DOI] [PubMed] [Google Scholar]
- 35.Tannir NM, Thall PF, Ng CS, et al. A Phase II Trial of Gemcitabine Plus Capecitabine for Metastatic Renal Cell Cancer Previously Treated With Immunotherapy and Targeted Agents. J Urol. 2008;180(3):867–72. doi: 10.1016/j.juro.2008.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Waters JS, Moss C, Pyle L, et al. Phase II clinical trial of capecitabine and gemcitabine chemotherapy in patients with metastatic renal carcinoma. Br J Cancer. 2004;91(10):1763–8. doi: 10.1038/sj.bjc.6602209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tickoo SK, Alden D, Olgac S, et al. Immunohistochemical Expression of Hypoxia Inducible Factor-1α and its Downstream Molecules in Sarcomatoid Renal Cell Carcinoma. J Urol. 2007;177(4):1258–63. doi: 10.1016/j.juro.2006.11.100. [DOI] [PubMed] [Google Scholar]
- 38.Molina AM, Tickoo SK, Ishill N, et al. Sarcomatoid-variant renal cell carcinoma: treatment outcome and survival in advanced disease. Am J Clin Oncol. 2011;34(5):454–9. doi: 10.1097/COC.0b013e3181f47aa4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beuselinck B, Lerut E, Wolter P, et al. Sarcomatoid Dedifferentiation in Metastatic Clear Cell Renal Cell Carcinoma and Outcome on Treatment With Anti–Vascular Endothelial Growth Factor Receptor Tyrosine Kinase Inhibitors: A Retrospective Analysis. Clin Genitourin Cancer. 2014;12(5):e205–14. doi: 10.1016/j.clgc.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 40.Hainsworth JD, Sosman JA, Spigel DR, Edwards DL, Baughman C, Greco A. Treatment of metastatic renal cell carcinoma with a combination of bevacizumab and erlotinib. J Clin Oncol Off J Am Soc Clin Oncol. 2005;23(31):7889–96. doi: 10.1200/JCO.2005.01.8234. [DOI] [PubMed] [Google Scholar]
- 41.Jonasch E, Lal LS, Atkinson BJ, et al. Treatment of metastatic renal carcinoma patients with the combination of gemcitabine, capecitabine and bevacizumab at a tertiary cancer centre. BJU Int. 2011;107(5):741–7. doi: 10.1111/j.1464-410X.2010.09626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Therasse P, Arbuck SG, Eisenhauer EA, et al. New Guidelines to Evaluate the Response to Treatment in Solid Tumors. J Natl Cancer Inst. 2000;92(3):205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 43.Common Terminology Criteria for Adverse Events v3.0. [Internet] [cited 2016 Oct 2];Available from: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf.
- 44.Loges S, Mazzone M, Hohensinner P, Carmeliet P. Silencing or fueling metastasis with VEGF inhibitors: antiangiogenesis revisited. Cancer Cell. 2009;15(3):167–70. doi: 10.1016/j.ccr.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 45.Hammers HJ, Verheul HM, Salumbides B, et al. Reversible epithelial to mesenchymal transition and acquired resistance to sunitinib in patients with renal cell carcinoma: evidence from a xenograft study. Mol Cancer Ther. 2010;9(6):1525–35. doi: 10.1158/1535-7163.MCT-09-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gibney GT, Aziz SA, Camp RL, et al. c-Met is a prognostic marker and potential therapeutic target in clear cell renal cell carcinoma. Ann Oncol. 2013;24(2):343–9. doi: 10.1093/annonc/mds463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Joseph RW, Millis SZ, Carballido EM, et al. PD-1 and PD-L1 Expression in Renal Cell Carcinoma with Sarcomatoid Differentiation. Cancer Immunol Res. 2015;3(12):1303–7. doi: 10.1158/2326-6066.CIR-15-0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McDermott DF, Sosman JA, Sznol M, et al. Atezolizumab, an Anti–Programmed Death-Ligand 1 Antibody, in Metastatic Renal Cell Carcinoma: Long-Term Safety, Clinical Activity, and Immune Correlates From a Phase Ia Study. J Clin Oncol. 2016;34(8):833–42. doi: 10.1200/JCO.2015.63.7421. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


