Abstract
Point mutations in the TERT gene promoter occur at high frequency in multiple cancers, including urothelial carcinoma (UC). However, the relationship between TERT promoter mutations and UC patient outcomes is unclear owing to conflicting reports in the literature. In this study we examined the association of TERT alterations, tumor mutational burden per megabase (Mb), and copy number alteration (CNA) burden with clinical parameters and their prognostic value in a cohort of 398 urothelial tumors. The majority of TERT mutations were located at two promoter region hotspots (chromosome 5, 1 295 228 C>T and 1 295 250 C>T). TERT alterations were more frequently present in bladder tumors than in upper tract tumors (73% vs 53%; p = 0.001). ARID1A, PIK3CA, RB1, ERCC2, ERBB2, TSC1, CDKN1A, CDKN2A, CDKN2B, and PTPRD alterations showed significant co-occurrence with TERT alterations (all p < 0.0025). TERT alterations and the mutational burden/Mb were independently associated with overall survival (hazard ratio[HR] 2.31, 95% confidence interval [CI] 1.46–3.65; p < 0.001; and HR 0.96, 95% CI 0.93–0.99; p = 0.002), disease-specific survival (HR 2.23, 95% CI 1.41–3.53; p < 0.001; and HR 0.96, 95% CI 0.93–0.99; p = 0.002), and metastasis-free survival (HR 1.63, 95% CI 1.05–2.53; p = 0.029; and HR 0.98, 95% CI 0.96–1.00; p = 0.063) in multivariate models.
Point mutations in the TERT gene promoter occur at high frequency in multiple cancers, including urothelial carcinoma (UC). These mutations create novel consensus binding sites for ETS family transcription factors, leading to upregulated telomerase expression. Borah et al [1] showed that TERT promoter mutations correlate with higher levels of TERT mRNA and protein expression, telomerase enzyme activity, and telomere length in a study of 23 human UC cell lines. However, the relationship between TERT promoter mutations and UC patient outcomes is unclear owing to conflicting reports in the literature [2,3]. Furthermore, the genomic characterization of bladder UC performed by The Cancer Genome Atlas (TCGA) using-whole exome sequencing did not include the TERT promoter region in their analysis [4]. We previously showed that a subset of bladder tumors have a high burden of copy number alterations (CNAs) using array comparative genomic hybridization [5]. However, the extent of the CNA burden did not predict survival in a cohort of 97 high-grade bladder tumors [5]. In addition, among tumor types subjected to TGCA analysis, bladder tumors have a relatively high mutational burden per megabase (Mb). Of note, it has been shown that a high mutational count is associated with better clinical outcome among UC patients treated with immunotherapy [6].
In this study, we examined the association between TERT alterations and tumor mutational and CNA burden with clinical parameters in a cohort of 398 urothelial tumors. Tumors were profiled using the Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT) next-generation sequencing assay [7] in a clinical laboratory with Clinical Laboratory Improvement Amendments certification according to an institutional review board–approved prospective sequencing protocol. MSK-IMPACT uses paired tumor and germline DNA to identify somatic point mutations, insertions/deletions, CNAs, and select translocations in all exons and select introns for 341 oncogenes and tumor suppressor genes. Notably, MSK-IMPACT covers the entire TERT promoter region. CNA burden was defined as fraction of the tumor genome affected by CNAs.
Among the 398 UC patients analyzed, 286 mutations and seven TERT gene amplifications were identified in 276 tumors (Supplementary Table 1). The majority of the TERT mutations were localized to two promoter region hotspots (chromosome 5, 1 295 228 C>T and 1 295 250 C>T; Fig. 1A). The median allele frequency for TERT mutations was 32% (interquartile range 19–44%). Patients with no TERT mutation (n = 122), TERT promoter mutations (n = 259), TERT gene amplification (n = 2), and concomitant TERT promoter mutations and gene amplification (n = 5) were included in this analysis. We excluded cases with TERT mutations of unknown significance (n = 10) for the analysis. Of note, inclusion of cases with TERT mutations of unknown significance (n = 10) did not alter the results.
Fig. 1.
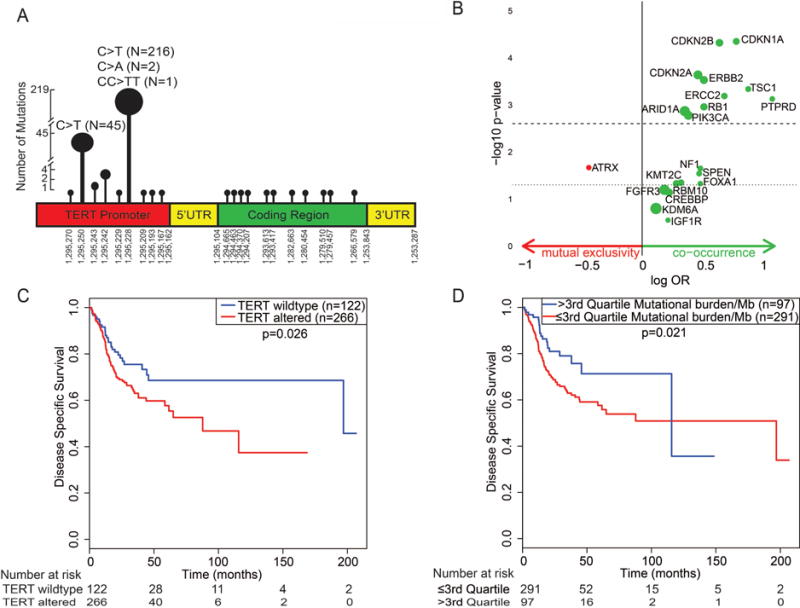
(A) Location of TERT mutations in the promoter region and coding regions. In our cohort of 398 urothelial tumors, 276 had a total of 286 TERT mutations. The most common location of TERT mutations in urothelial tumor was chromosome 5 1 295 228 (76.6%), followed by chromosome 5 1 295 250 (15.7%). Consistent with prior reports, C>T substitution was common at both of these positions. (B) Volcano plot showing co-occurrence and mutual exclusivity for TERT alterations and other gene alterations. The dotted line represents the unadjusted p value (<0.05) and the dashed line represents the adjusted p value (<0.0025) for multiple comparisons. The bubble size corresponds to the proportion of patients with a mutation in that gene. ARID1A, PIK3CA, RB1, ERCC2, ERBB2, TSC1, CDKN1A, CDKN2A, CDKN2B, and PTPRD alterations were significantly associated with TERT alteration after adjusting for multiple comparisons (all p < 0.0025). (C) Kaplan-Meier plots of disease-specific survival for patients with and without TERT alterations (promoter mutations and/or TERT amplification). Urothelial carcinoma with TERT alterations had worse disease-specific survival compared to tumors without TERT alteration. (D) Kaplan-Meier plots of disease-specific survival for tumor mutational burden/Mb. Urothelial carcinoma with a high mutational burden (>3rd quartile, ie, >14 mutations/Mb) had better disease-specific survival compared to tumors with a low mutational burden (≤3rd quartile, ≤14 mutations/Mb). OR = odds ratio.
The demographic, clinical, and pathological characteristics of the patients in the cohort are summarized in Supplementary Table 2. Patients with TERT alterations (promoter mutations and/or amplification) were significantly older than those without TERT alterations (median age 67 vs 64 yr; p = 0.03). UC with TERT alterations had a significantly higher mutational burden/Mb (median 8 vs 4; p < 0.001) and a significantly higher CNA burden (median 0.12 vs 0.05; p < 0.001). TERT alterations were more frequently present in bladder UC than in upper tract UC (73% vs 53%; p = 0.001). There was no association between TERT alterations and tumor stage or tumor grade (Supplementary Table 2). Supplementary Figure 1 shows the association of mutational burden/Mb and CNA burden with tumor stage and tumor grade. There was a significant association between CNA burden and tumor stage (p < 0.001) and tumor grade (p < 0.001). Supplementary Figure 2 shows the relationship between mutational burden/Mb and CNA burden (Spearman’s ρ = 0.138; p = 0.006).
We also examined whether TERT alterations co-occurred with or mutually exclusive from alterations in other known oncogenes and tumor suppressor genes. ARID1A, PIK3CA, RB1, ERCC2, ERBB2, TSC1, CDKN1A, CDKN2A, CDKN2B, and PTPRD alterations showed significant co-occurrence with TERT alterations after Bonferroni correction to adjust for multiple comparisons (all p < 0.0025; Fig. 1B and Supplementary Table 3). TERT and ATRX gene alterations were mutually exclusive, but this association was not significant after adjusting for multiple comparisons (p = 0.02). Notably, it has been shown that TERT alterations are mutually exclusive from ATRX alterations, as tumors with ATRX alterations utilize the telomerase-independent alternative lengthening of telomeres mechanism to maintain telomere length [8,9]. Heidenreich et al [10] reported that TERT alterations co-occurred with CDKN2A alterations. A few investigators [3] reported co-occurrence of TERT alterations and FGFR3 alterations; however, we did not find a significant association between FGFR3 and TERT alterations in our cohort.
Supplementary Table 4 shows the results of a univariate Cox regression analysis of the clinical and pathological parameters evaluated for overall survival (OS), disease-specific survival (DSS), and metastasis-free survival (MFS). TERT alterations and the mutational burden/Mb were independent predictors for OS (p < 0.001 and 0.002), DSS (p < 0.001 and 0.002), and MFS (p = 0.029 and 0.063) in multivariate Cox regression models (Table 1).
Table 1.
Multivariate Cox regression models for OS, DSS, and MFS
| Characteristic | OS (n = 388; 120 events) | DSS (n = 388; 118 events) | MFS (n = 289; 114 events) | |||
|---|---|---|---|---|---|---|
|
| ||||||
| HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | |
| TERT alteration | 2.31 (1.46–3.65) | <0.001 | 2.23 (1.41–3.53) | <0.001 | 1.63 (1.05–2.53) | 0.029 |
| Mutational burden/Mb | 0.96 (0.93–0.99) | 0.002 | 0.96 (0.93–0.99) | 0.002 | 0.98 (0.96–1.00) | 0.063 |
| Age | 1.02 (1.01–1.04) | 0.012 | 1.02 (1.01–1.04) | 0.013 | 1.02 (1.00–1.04) | 0.016 |
| Tumor stage | ||||||
| NMIBC | Reference | Reference | Reference | |||
| MIBC | 16.77 (7.11–39.55) | <0.001 | 23.95 (8.93–64.23) | <0.001 | 11.35 (6.21–20.74) | <0.001 |
| Localized UTUC | 11.85 (4.68–30.02) | <0.001 | 16.03 (5.64–45.54) | <0.001 | 8.93 (4.79–16.66) | <0.001 |
| Metastatic | 56.09 (23.57–133.47) | <0.001 | 74.26 (27.57–200.02) | <0.001 | – | – |
| Variant histology | 1.54 (1.02–2.33) | 0.040 | 1.52 (1.01–2.31) | 0.047 | 1.77 (1.40–2.75) | 0.011 |
OS = overall survival; DSS = disease-specific survival; MFS = metastasis-free survival; HR = hazard ratio; CI = confidence interval; NMIBC = non–muscle-invasive bladder cancer; MIBC = muscle-invasive bladder cancer;
Tumors with TERT alterations had worse prognosis compared to tumors without TERT alterations, whereas tumors with a higher mutational burden/Mb had a more favorable outcome compared to tumors with a low mutational burden/Mb. Figure 1C,D shows Kaplan Meir DSS plots for TERT alterations and mutational burden/Mb. Supplementary Figure 3A–D shows Kaplan-Meier plots for TERT alterations and mutational burden/Mb for OS and MFS. Of note, the prognostic ability of mutational burden/Mb depends on all mutations detected in tumor, not just on TERT alterations. Supplementary Table 5 shows details of the mutational burden/Mb in tumors with wild-type TERT and TERT alterations. Supplementary Figure 4A–C shows risk stratification based on TERT status and the mutational burden/Mb. Urothelial tumors with both risk factors (TERT alterations and low mutational burden/Mb) had the worst prognosis, while tumors with no risk factor (wild-type TERT and high mutational burden/Mb) had better prognosis. Tumors with one risk factor (TERT alterations and high mutational burden/Mb, or wild-type TERT and low mutational burden/Mb) had intermediate prognosis. Patient age, tumor stage, and variant histology were also predictors of OS (p = 0.012, <0.001, and 0.040), DSS (p = 0.013, <0.001, and 0.047) and MFS (p = 0.016, <0.001 and 0.011) in multivariate Cox regression models. CNA burden was also associated with OS (p < 0.001), DSS (p < 0.001) and MFS (p = 0.004) in univariate analysis, but this association was not significant after covariate adjustment in multivariate models.
In summary, the majority of TERT mutations are located at two promoter region hotspots. Tumors with TERT alterations had worse prognosis compared to tumors without TERT alterations, whereas tumors with a higher mutational burden/Mb had more favorable outcome compared to tumors with a low mutational burden/Mb. The results suggest that tumor genomic profiling may aid in the risk stratification of UC patients.
Supplementary Material
Patient summary.
The majority of TERT gene mutations we detected in urothelial carcinoma are located at two promoter hotspots. Urothelial tumors with TERT alterations had worse prognosis compared to tumors without TERT alterations, whereas tumors with a higher mutational burden had more favorable outcome compared to tumors with low mutational burden.
Take Home Message.
The majority of TERT mutations are located at two promoter region hotspots. Tumors with TERT alterations had worse prognosis compared to tumors without TERT alterations, whereas tumors with a higher mutational burden/Mb had a more favorable outcome compared to tumors with a low mutational burden/Mb.
Acknowledgments
Funding/Support and role of the sponsor: This study was supported by the Cycle for Survival and Ruth L. Kirschstein National Research Service Award T32CA082088. The study was also funded in part by the Sloan Kettering Institute for Cancer Research Cancer Center Support Grant P30CA008748 and the Marie-Josée and Henry R. Kravis Center for Molecular Oncology. The sponsors played no direct role in the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions: Hikmat A. Al-Ahmadie had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Isharwal, Solit, Al-Ahmadie.
Acquisition of data: Isharwal, Audenet, Pietzak, Cha, Arcila, Jayakumaran, Berger, Donahue, Rosenberg, Bajorin, Coleman, Dalbagni, Reuter.
Analysis and interpretation of data: Drill, Ostrovnaya, Isharwal, Solit, Al-Ahmadie.
Drafting of the manuscript: Isharwal, Iyer, Solit, Al-Ahmadie, Pietzak, Donahue, Rosenberg, Bajorin, Coleman, Dalbagni, Reuter.
Critical revision of the manuscript for important intellectual content: Isharwal, Iyer, Solit, Bochner, Al-Ahmadie.
Statistical analysis: Drill, Ostrovnaya, Isharwal.
Obtaining funding: Solit, Bochner, Al-Ahmadie.
Administrative, technical, or material support: None.
Supervision: Solit, Bochner, Al-Ahmadie.
Other: None.
Financial disclosures: Hikmat A. Al-Ahmadie certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
References
- 1.Borah S, Xi L, Zaug AJ, et al. Cancer. TERT promoter mutations and telomerase reactivation in urothelial cancer. Science. 2015;347:1006–10. doi: 10.1126/science.1260200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu S, Huang P, Li C, et al. Telomerase reverse transcriptase gene promoter mutations help discern the origin of urogenital tumors: a genomic and molecular study. Eur Urol. 2014;65:274–7. doi: 10.1016/j.eururo.2013.10.038. [DOI] [PubMed] [Google Scholar]
- 3.Allory Y, Beukers W, Sagrera A, et al. Telomerase reverse transcriptase promoter mutations in bladder cancer: high frequency across stages, detection in urine, and lack of association with outcome. Eur Urol. 2014;65:360–6. doi: 10.1016/j.eururo.2013.08.052. [DOI] [PubMed] [Google Scholar]
- 4.Cancer Genome Atlas Research Network. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 2014;507:315–22. doi: 10.1038/nature12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iyer G, Al-Ahmadie H, Schultz N, et al. Prevalence and co-occurrence of actionable genomic alterations in high-grade bladder cancer. J Clin Oncol. 2013;31:3133–40. doi: 10.1200/JCO.2012.46.5740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387:1909–20. doi: 10.1016/S0140-6736(16)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng DT, Mitchell TN, Zehir A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): a hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn. 2015;17:251–64. doi: 10.1016/j.jmoldx.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Killela PJ, Reitman ZJ, Jiao Y, et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci U S A. 2013;110:6021–6. doi: 10.1073/pnas.1303607110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heaphy CM, de Wilde RF, Jiao Y, et al. Altered telomeres in tumors with ATRX and DAXX mutations. Science. 2011;333:425. doi: 10.1126/science.1207313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heidenreich B, Nagore E, Rachakonda PS, et al. Telomerase reverse transcriptase promoter mutations in primary cutaneous melanoma. Nat Commun. 2014;5:3401. doi: 10.1038/ncomms4401. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


