Abstract
Chronic stress is a risk factor for a number of physiological disorders including cardiovascular disease, obesity and gastrointestinal disorders, as well as psychiatric and neurodegenerative disorders. There are a number of underlying molecular and cellular mechanisms altered in the course of chronic stress, which may increase the vulnerability of individuals to develop psychiatric disorders such as depression, and neurodegenerative disorders such as Alzheimer’s Disease (AD). This is evident in the influence of stress on large-scale brain networks, including the resting state Default Mode Network (DMN), the effects of stress on neuronal circuitry and architecture, and the cellular and molecular adaptations to stress, which may render individuals with stress related psychiatric disorders more vulnerable to neurodegenerative disease later in life. These alterations include decreased negative feedback inhibition of the hypothalamic pituitary axis (HPA) axis, decreased dendritic arborization and spine density in the prefrontal cortex (PFC) and hippocampus, and the release of proinflammatory cytokines, which may suppress neurogenesis and promote neuronal cell death. Each of these factors are thought to play a role in stress-related psychiatric disease as well as AD, and have been observed in clinical and post-mortem studies of individuals with depression and AD. The goal of the current review is to summarize clinical and preclinical evidence supporting a role for chronic stress as a putative link between neuropsychiatric and neurodegenerative disease. Moreover, we provide a rationale for the importance of taking a medical history of stress-related psychiatric diseases into consideration during clinical trial design, as they may play an important role in the etiology of AD in stratified patient populations.
Keywords: stress, amyloid, norepinephrine, dopamine-β-hydroxylase, depression, adrenergic receptors
I. Introduction
In 1907, Alois Alzheimer published his observations of a 51-year-old woman in an insane asylum of Frankford am Main, Germany. The first clinical symptoms reported were disorientation, paranoia, hallucinations, delirium and mood changes that were accompanied by memory loss. While her state of awareness gradually eroded over time, Alzheimer noted that these complex symptoms were at times stronger or weaker in appearance. Upon post mortem analysis, Alzheimer noted an evenly atrophic brain without macroscopic focal degeneration, but with neurofibrils and unusual morphology of glial cells. He concluded that this was a distinctive illness, one that should not be classified amongst the psychiatric illnesses that had previously been described, and that histological analyses of individual cases may facilitate clinical distinction of specific illnesses from general categories of disease (Alzheimer 1907, Alzheimer, Stelzmann et al. 1995).
Over one-hundred years later, scientists are still mystified by Alzheimer’s Disease (AD). While much progress has been made, there are still no disease modifying therapeutics for AD patients, affecting over 5 million Americans (Alzheimer’s 2015). Traditionally, AD is characterized by histological analysis of post mortem brain tissue revealing aggregates of synaptic proteins including amyloid beta (Aβ) peptides that are forty (Aβ40) or forty-two (Aβ42) amino acids in length, and abnormally phosphorylated tau (tau-p) proteins in a spatially distinct pattern outlined by Thal (Thal, Rub et al. 2002) and Braak staging (Braak, Alafuzoff et al. 2006), respectively. In their pathogenic form, Aβ aggregates are known as senile plaques (SP), and aggregated tau as neurofibrillary tangles (NFT). Aβ plaques may take several forms, ranging from diffuse to neuritic, with the latter including dystrophic neurites and tau-p material (Thal, Rub et al. 2002). Synapse loss is the major correlate of disease progression (Selkoe 2002, Jack, Knopman et al. 2010), and while the exact mechanisms by which SP and NFT disrupt synaptic function are unclear, there appears to be a synergistic relationship in promoting synapse loss and ultimately neuronal death (Jack 2013). The most current hypothesis of biomarker staging suggests that tau-p, a product of normal aging, starts gradually accumulating early in life but becomes severely accelerated when the global Aβ burden exceeds a certain threshold, initiating neuronal injury and neurodegeneration (Jack et al 2013, 2016). Generally, it is believed that NFT accumulation begins in the transentorhinal cortex, progressing to entorhinal, hippocampal, temporal, parietal and occipital cortices (Braak 2006). A fascinating retrospective study identified neuropathologically defined subtypes of AD based on atypical distributions of NFT when compared to typical AD. Of 889 patients, 665 cases (75%) were classified as typical AD, 97 cases (11%) were classified as hippocampal sparing based on the observation that NFT in the hippocampus were sparse compared with the three association cortices, and 127 cases (14%) were classified as limbic predominant, where NFT count was higher in the hippocampus compared to that in three association cortices (Murray, Graff-Radford et al. 2011). Of the hippocampal sparing group, a higher proportion of cases were men (63%), younger at death, and had a higher NFT count in cortical regions. The limbic predominant group, was primarily composed of women (69%), that were older at death, and had a gradual slope of decline when compared to hippocampal sparing cases (Murray, Graff-Radford et al. 2011). The age of onset, education level, and results of a questionnaire used to assess cognitive impairment, Mini Mental State Exam (MMSE) scores, were recorded from the clinical history of these participants, but previous medical history of depression, or other stress-related psychiatric disorders were not taken into account. In the upcoming sections, we will provide a rationale for the importance of taking such factors into consideration, as they may play an important role in the etiology of AD in stratified patient populations.
This review will discuss a number of striking similarities in the pathogenesis of AD and neuropsychiatric disorders that have been observed in clinical studies, as well as in preclinical models. While there are significant distinctions between these disparate disease states, we will discuss homologous elements such as adaptation to stress that may become maladaptive following chronic exposure, resulting in large-scale network reorganization and alterations in neuronal connectivity. Further, the heterogeneity of disease presentation, onset, environmental and genomic factors that are prominent in both psychiatric disorders, e.g. depression, and AD support the notion that the stratification of patients, and potential opportunities for individualized therapies is crucial for the advent of effective disease modifying therapeutics. Elucidating the link between neuropsychiatric and neurodegenerative disorders is paramount to advancing knowledge for both conditions. First, understanding that a subpopulation of individuals that are vulnerable to stress-related psychiatric diseases may be predisposed to AD presents opportunities for the development of therapeutic interventions geared towards stress related targets that may be advantageous in preventing AD. Second, defining the abnormalities in Aβ and tau-p that are thought to drive the progression of AD, may be an important area of investigation for treatment of broader neuropsychiatric disease states, including depression.
In the following sections we will propose a hypothesized framework by which chronic stress may contribute to underlying neuropsychiatric pathology evident in depressed patients, which may render some individuals vulnerable to neurodegenerative disease later in life (Figure 1). We begin by reviewing clinical studies supporting the notion that depression is an independent risk factor for AD, and additional clinical and preclinical findings linking chronic stress with depression and AD. Next we provide a foundation for the the importance of aminergic circuitry in depression and AD, and discuss the large-scale network adaptations, cellular and molecular alterations to neurons within these networks, that are induced by stress and may contribute to the pathophysiology of disease. Undoubtedly, extensive research has demonstrated the importance of a balance in excitatory and inhibitory neurotransmission in mood related psychiatric disorders (Kendell, Krystal et al. 2005, Kugaya and Sanacora 2005, McEwen 2005) and AD (Palop and Mucke 2010, Palop and Mucke 2010), and the influence of glucocorticoids (GC) (McEwen 2005, Oakley and Cidlowski 2013). There are excellent previously published reviews on these topics, thus the focus of this review will be on stress-related aminergic circuit dysfunction with emphasis on the locus coeruleus (LC)- norepinephrine (NE) system, an underappreciated circuit in the context of AD (Ross, McGonigle et al. 2015).
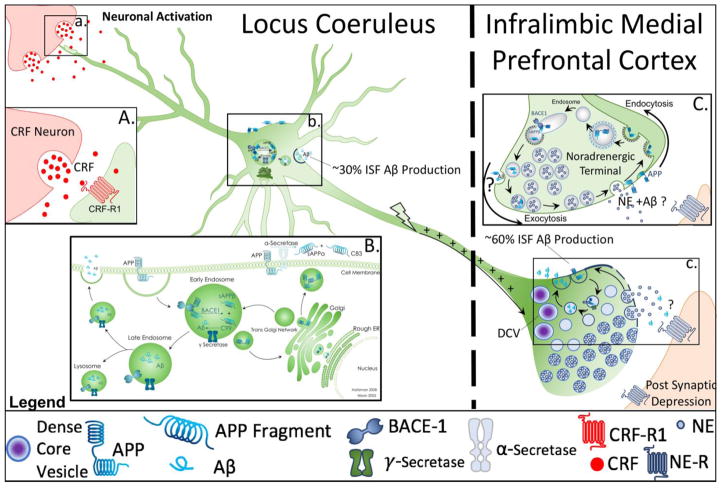
Figure 1.
Cellular mechanisms of amyloid β (Aβ) production and secretion from somatodendritic and synaptic sites. This schematic reflects hypothetical and known mechanisms of Aβ production and secretion described in the literature with respect to locus coeruleus (LC)- norepinephrine (NE) circuitry. Figure 1a. points to the synaptic cleft of a corticotropin releasing factor (CRF) terminal releasing CRF onto and LC dendrite. Box 1A shows a higher magnification image of CRF release onto LC neurons under conditions of stress, engaging CRF Receptor 1, which has been implicated in Aβ production and abnormal phosphorylated tau (taup). Figure 1b. illustrates amyloid precursor protein (APP) processing within the soma of LC neurons. Box 1B. expands on the mechanistic details of APP processing within the cell body, illustrating that the subcellular localization of APP determines its participation in two divergent processing pathways: the α-secretase mediated non-amyloidogenic pathway, or the beta amyloid cleaving enzyme 1 (BACE-1) and γ-secretase mediated amyloidogenic pathway. Once Aβ is formed, it may continue along the secretory pathway, resulting in release into the extracellular space, or degradation in the lysosome (Haass, Kaether et al. 2012). Studies utilizing microdialysis have estimated that approximately 30% of endogenous interstitial fluid (ISF) Aβ is produced in the secretory pathway and released from somatodendritic processes (Box 1B), while approximately 60% is produced at the synapse (Cirrito, May et al. 2003) (Figure 1c). Box 1C illustrates that the production and secretion of Aβ at the synapse is a tightly regulated process that occurs following changes in synaptic activity, in an endocytosis dependent manner (Cirrito, Yamada et al. 2005, Cirrito, Kang et al. 2008). It has been hypothesized that increases in synaptic activity correlate with increased vesicle recycling, which leads to greater internalization of APP, and subsequent increased amyloidogenic processing (Cirrito, Kang et al. 2008). Microdialysis studies utilizing acute behavior paradigms of stress or exogenous CRF have demonstrated increased levels of ISF levels of Aβ (Kang, Cirrito et al. 2007), however, the anatomical substrates of this interaction with respect to LC neurons has not yet been investigated.
II. Clinical Evidence
a. Behavioral and Psychological Symptoms of Dementia
Today, many of Alzheimer’s keen insights are still driving clinical investigations regarding not only the stratification of patients with complex neurodegenerative diseases based on clinical and histological differences between patients, but also regarding the behavioral and psychological symptoms of dementia (BPSD). McKeith and Cummings note that while cognitive decline has traditionally been seen as the defining feature of dementia, neuropsychiatric assessment is crucial for differential diagnosis and the management of symptoms (McKeith and Cummings 2005). McKeith and Cummings have extensively studied BPSD in the AD clinical population and have identified three basic behavioral syndromes that predominate; one group is characterized by few behavioral abnormalities, another by prominent symptoms of psychosis, and the third by a prominent mood disorder syndrome (McKeith and Cummings 2005). BPSD may be assessed using the neuropsychiatric Inventory (NPI), a questionnaire that detects 12 symptoms that may occur during the process of dementia, including delusions, hallucinations, agitation, depression, anxiety, euphoria, apathy, irritability, disinhibition, aberrant motor activity, sleep disturbances and appetite changes. Retrospective studies that have utilized this assessment approximate that onset of BPSD occurs between 45–50 months of diagnosis (Hart, Craig et al. 2003). The same study reported that behavioral changes were present in up to 88% of patients, and that depression was the most common psychological symptom in 56% of the cohort (Hart, Craig et al. 2003).
A clinical study of anxiety in AD patients utilizing the NPI anxiety subscale, revealed that anxiety is most common in those with more severe cognitive deterioration and an earlier age of onset after adjusting for age, age at onset, educational level, and MMSE score. Caregivers reported anxiety in AD patients in 30 of the 115 (26.1%) subjects examined. Interestingly, anxiety was also correlated with delusions, hallucinations, euphoria, and disinhibition, and 19 of 30 (63.3%) patients with reported anxiety also had depression (Porter, Buxton et al. 2003). Neuroimaging studies suggest that there are neurobiological changes that differentiate patients with BPSD from those without BPSD and further, the presence of BPSD in AD may be conferred with the presence of genetic polymorphisms in serotonergic, noradrenergic, and dopaminergic related synaptic machinery, and markers of inflammatory cascades, which are also found in depressed individuals (Klimek, Stockmeier et al. 1997, Ordway, Stockmeier et al. 1997, Ordway, Schenk et al. 2003, McCulley, Day et al. 2004, Szot, White et al. 2006, Borroni, Grassi et al. 2009, Grunblatt, Zehetmayer et al. 2009, Combarros, Warden et al. 2010).
b. Depression as a Risk Factor for AD
It is well known that depression can cause reversible cognitive impairment, even without any co-existent neurodegenerative disorder, and some investigators have reported hippocampal atrophy in clinical studies of recurrent major depression (Sheline, Wang et al. 1996). On the other hand, depressive symptoms in older persons are associated with clinical AD in cross sectional studies, and with cognitive decline and risk of dementia or AD in longitudinal studies (Green, Cupples et al. 2003, Wilson, Evans et al. 2003, Ownby, Crocco et al. 2006). These observations have lead to clinical studies investigating whether depressive symptomology is a risk factor for AD, or if it is an early sign of the pathology of the disease. To this end, it has been shown that depressive symptoms are associated with an increased risk in incident AD and rate of cognitive decline in a cohort of older Catholic nuns, priests and brothers that were annually evaluated for physical and mental health until post mortem analysis as part of the Religious Orders Study. Further studies on this cohort have demonstrated that depressive symptoms were not significantly related to plaques and tangles, and did not significantly alter the relation of SP and NFT to clinical manifestations of the disease. Thus, it would appear that depressive symptoms are associated with risk of clinical AD through a mechanism independent of SP and NFT (Wilson, Schneider et al. 2003). In agreement with these findings, a systematic meta-analysis and meta-regression analysis of clinical studies examining the relationship between depressive symptoms and AD concluded that a history of depression may possibly confer an increased risk for later developing AD, thus reflecting an independent risk factor for the disease rather than a prodrome of the disease. The authors do caution, however, that their findings do not rule out the possibility that depression is both a remote risk factor for AD and a proximal prodromal feature of it (Ownby, Crocco et al. 2006). In line with these results, the Multi-institutional Research in Alzheimer’s Genetic Epidemiology (MIRAGE) study, which included 4,046 subjects found that depression symptoms before the onset of AD are associated with the development of AD. Strikingly, depression symptoms occurring more than 25 years before memory impairment were associated with the development of AD (Green, Cupples et al. 2003). These data and others suggest that depression symptoms are a risk factor for later development of AD. Thus, a culmination of compelling evidence from clinical and epidemiological studies exist to suggest common underlying mechanisms that may play important role in the neuropathology of depression and AD.
i. Chronic Stress as a Putative Link between Depression and AD
1. Chronic Stress, Depression, and AD: clinical studies
Clinical and post mortem studies of depressed patients, untreated suicide victims and AD have revealed striking parallels in the nature of these neurological disorders. The evidence suggests an intersection of neuropsychiatric and neurodegenerative disorders, whereby chronic stress may link these seeming disparate classes of disorders in certain patient subpopulations. Clinical studies have revealed that there exists an underlying relationship between depression and AD, although the precise mechanisms are not fully understood. Based on accumulating evidence in the literature, hypotheses have been put forth that pathologic mechanisms induced by chronic stress, often a significant factor in the etiology of depression, may be a key factor predisposing individuals with chronic stress or stress-related psychiatric diseases such as depression, to long term neurodegenerative diseases such as AD (Pomara and Sidtis 2009, Aznar and Knudsen 2011).
a. Hyperarousal
The first line of evidence that chronic stress may bridge neuropsychiatric and neurodegenerative pathology stems from clinical studies demonstrating hyper-activation of the hypothalamo-pituitary-adrenal (HPA) axis and indicators of decreased negative feedback inhibition in both depressed and suicidal individuals, and AD patients. Exposure to stress is associated with the onset and severity of depression and several anxiety disorders (Kendler, Kessler et al. 1995). In addition to an association with stress, anxiety disorders and melancholic features of depression share a core symptom of hyperarousal, which is a defining feature of the disease and is characterized by sleep disturbances, inability to concentrate, restlessness, and increased vigilance (Southwick, Paige et al. 1999, Gold and Chrousos 2002). In both animal models and humans, corticotropin releasing factor (CRF) is the neurohormone that initiates both the peripheral and central responses to stress, and has been shown to be hypersecreted in patients with depression, evident in elevated levels of CRF immunoreactivity in the cerebrospinal fluid (CSF) of depressed patients (Nemeroff, Widerlov et al. 1984). CRF engages the central response to stress by exciting the LC, a cluster of NE neurons located at the base of the fourth ventricle that are recognized as the sole providers of NE to the frontal cortex and hippocampus, and whose broad reaching afferents provide NE to the entire neuraxis. The LC-NE system is highly responsive to CRF during both acute and chronic stressors, both cognitive and physical, and individuals experiencing melancholic depression exhibit a state of high CRF/NE tone (Gold and Chrousos 2002). Clinical studies have also demonstrated that inpatients with chronic, treatment-resistant depression have cortisol outputs throughout the day which are double those of healthy controls (Juruena, Cleare et al. 2006). Finally, many of the symptoms in a subset of individuals with severe melancholic depression, resemble over-activity of the stress axis, including increased basal cortisol secretion, blunted rhythm, and resistance to dexamethasone suppression (Gold 1988, Gold, Goodwin et al. 1988, Gold and Chrousos 2002, Gold, Gabry et al. 2002). Thus, hyperarousal of the stress system is an important factor that dictates both the onset and severity of psychiatric disorders. However, it is imperative to recognize the inherent heterogeneity of depression, reflected in the characterization of depressive subtypes that have opposing features such as enhanced or suppressed HPA axis activity exemplified by high or low CRF/NE states in melancholic and atypical depression, respectively. Such features have tremendous therapeutic implications, and may guide efforts to appropriately stratify patient populations, and neglecting to do so may confound the interpretation of results, especially those attempting to discern the role of CRF/NE stress responsivity in depression and neurodegenerative disease (Gold and Chrousos 2002, Gold, Gabry et al. 2002).
b. Cortisol
Based on a number of clinical studies on plasma cortisol and its metabolites in AD patients, there is also support for the notion of hypercortisolism as a contributing factor in the pathogenesis and progression of AD (Weiner, Vobach et al. 1997, Csernansky, Dong et al. 2006), and that chronic stress is a risk factor for AD (Wilson, Evans et al. 2003, Wilson, Schneider et al. 2003). Hypercortisolemia (Weiner, Vobach et al. 1997) and reduced negative feedback inhibition (O’Brien, Schweitzer et al. 1994) have been documented in several clinical studies of AD utilizing CSF isolated via lumbar puncture, evidenced by abnormalities in the dexamethasone suppression test (Balldin, Gottfries et al. 1983, Davis, Davis et al. 1986), supporting the notion that the peripheral and central stress systems are in a state of hyperarousal. While hypercortisolemia has been widely documented in AD patient populations (Davis, Davis et al. 1986, Masugi, Ogihara et al. 1989, Oxenkrug, Gurevich et al. 1989, Martignoni, Petraglia et al. 1990, Franceschi, Airaghi et al. 1991, Maeda, Tanimoto et al. 1991, Weiner, Vobach et al. 1997, Lupien, de Leon et al. 1998, Carlson, Sherwin et al. 1999, Umegaki, Ikari et al. 2000, Peskind, Wilkinson et al. 2001, Rasmuson, Andrew et al. 2001, Csernansky, Dong et al. 2006, Huang, Lui et al. 2009, Arsenault-Lapierre, Chertkow et al. 2010, Lara, Caramelli et al. 2013, Zverova, Fisar et al. 2013, Popp, Wolfsgruber et al. 2015), it is also important to consider that cortisol and HPA axis activity may increase with age (Friedman, Green et al. 1969, Dodt, Dittmann et al. 1991, Raadsheer, Sluiter et al. 1993, Raadsheer, Oorschot et al. 1994), though this remains controversial as some studies have reported no change or decline in HPA activity with age (Sharma, Palacios-Bois et al. 1989). Perhaps, some of these differences may be reconciled by a longitudinal study of healthy elderly adults providing evidence for subgroups of individuals characterized by cortisol levels that increased with years in one subgroup, to decrease in another, and to remain stable in a third, and in which the age of the subjects was not related to either cortisol levels or to the pattern of change in cortisol secretion over years (Lupien, Lecours et al. 1996). In a longitudinal study designed to assess the relationship of cortisol levels to progression of AD, it was determined that cortisol levels were significantly related to the clinical progression of the disease, but not to aging or survival (Weiner, Vobach et al. 1997). However, another study found that hypercortisolemia and associated HPA axis dysfunction which correlated with severity of dementia in cross-sectional sampling did not correlate with a longitudinal follow-up sampling in AD patients (Swanwick, Kirby et al. 1998). It should also be noted, that hypercortisolemia alone has deleterious effects on cognitive function and hippocampal volume, such as that observed in Cushing’s Syndrome patients, (Starkman, Gebarski et al. 1992, Forget, Lacroix et al. 2000, Starkman, Giordani et al. 2001), or healthy adults with stress level cortisol treatment (Newcomer, Selke et al. 1999). More recent studies have further elucidated the relationship between cortisol and AD, demonstrating that the relationship between cortisol and memory may depend on the presence or absence of cognitive decline of the individual, such that AD patients may be disproportionately affected by cortisol induced hippocampal atrophy, or cognitive function evidenced by MMSE scores (Huang, Lui et al. 2009) or by Brief Cognitive Screening Battery, a screening tool used to diagnose AD (Souza-Talarico, Chaves et al. 2010). A study conducted in 2015 by Popp and colleagues demonstrated that after controlling for confounding variables including CSF measures of Aβ42 and total tau, higher levels of CSF cortisol were associated with accelerated disease progression, evidenced by faster clinical and cognitive decline (Popp, Wolfsgruber et al. 2015). Further, by stratifying patients assessed by baseline cognitive status, the study was able to establish that HPA axis dysregulation occurs at the mild cognitive impairment (MCI) stage, early in disease onset and development (Popp, Wolfsgruber et al. 2015). In support of this, stress is considered a risk factor highly correlated with AD pathology in rodents and humans (Pardon 2011), as well is in humans with depression (Kendler, Kessler et al. 1995). Interestingly, studies examining the effects of stress on apolipoprotein E (ApoE) allele status have demonstrated that prolonged stress in older, non-demented individuals in the presence of an ApoE ε4 allele leads to memory decline (Peavy, Lange et al. 2007).
c. Involvement of biogenic amine circuits
Among persons with AD, the presence of major depression has been related to neuronal loss in midbrain aminergic nuclei, and decreased cortical levels of NE and serotonin (Zubenko and Moossy 1988, Zubenko, Moossy et al. 1990, Forstl, Burns et al. 1992). The role of NE in the behavioral and psychological symptoms of AD has been reviewed, summarizing compelling evidence that degeneration of noradrenergic neurons and subsequent alterations in noradrenergic transmission result in behavioral changes related to depression, aggression, agitation and psychosis (Herrmann, Lanctot et al. 2004). In line with these findings, are genetic studies linking polymorphisms in NE synthesizing enzymes (Combarros, Warden et al. 2010). Even more fascinating, is the finding that LC neuronal loss exhibited in AD patients can be more profound in a subpopulation of individuals, originally identified by Blessed and colleagues in 1981 (Cross, Crow et al. 1981), and later affirmed by Bondareff et al, in 1987, who identified a group of AD patients with greater neuronal loss in the LC, which was associated with less choline acetyltransferase (ChAT) activity, smaller concentrations of somatostatin and NE, more plaques and tangles in cerebral cortex (Bondareff, Mountjoy et al. 1987). Further, it was noted that the duration of dementia was greater in these patients, but that the mean age of death was greater, however these differences were not found to be statistically significant (Bondareff, Mountjoy et al. 1987). Additional studies by Bondareff et al. demonstrated that loss of LC neurons correlated significantly with NE concentration, ChAT activity, and numbers of plaques and tangles on Brodmann area 24 (cingulate); ChAT and plaque counts in area 21 (temporal); and with ChAT activity in area 10 (frontal). In addition, LC neuronal counts, and the number of NFT in the LC were correlated significantly with the severity and estimated duration of dementia, suggesting that changes in central NE pathways are related to the pathophysiology of AD (Bondareff, Mountjoy et al. 1987). Interestingly, the Vienna-Transdanube Aging (VITA) study investigated the genetic link between depression and AD found significant associations with ChAT polymorphisms in depression and AD (Grunblatt, Zehetmayer et al. 2009). Thus, LC neuronal loss may influence not only NE transmission, but also acetylcholine transmission in the forebrain, which plays a significant role in the etiology of depression and AD.
Additional support for involvement of aminergic brain dysfunction in both neuropsychiatric and neurodegenerative disorders stems from studies of untreated suicide victims with neuronal loss of the LC. Tyrosine hydroxylase (TH) levels in the LC of untreated individuals who committed suicide were 108–171% higher than levels in control subjects (Ordway, Smith et al. 1994). Further, the finding that there are 23% fewer neurons in the LC of those who have committed suicide compared to controls has led some researchers to hypothesize that altered NE transmission in severe depression may be due to the presence of fewer NE neurons (Chan-Palay and Asan 1989). Additional findings demonstrate that in post mortem brain tissue of suicide victims exhibit signs of altered function of the α-2 adrenergic receptor (AR), further supporting the involvement of NE and AR in the pathogenesis of depression (Valdizan, Diez-Alarcia et al. 2010). While suicide as a cause of death does not necessarily indicate depression as a causal factor, the American Society for Suicide Prevention reports that ninety percent of individuals that commit suicide have a diagnosable psychiatric disorder at the time of their death, over fifty percent of which are diagnosed with depression (Prevention 2015). As previously discussed, a prominent loss of LC neurons is a well characterized feature of AD, which occurs more severely in a subpopulation of Alzheimer’s patients (Bondareff, Mountjoy et al. 1987, Bondareff, Mountjoy et al. 1987, Zarow, Lyness et al. 2003), and a prevailing hypothesis states that partial loss of noradrenergic neurons of the LC is accompanied by compensatory activation of surviving neurons through an increase in TH mRNA expression in remaining neurons, sprouting of dendrites into the peri-LC dendritic zone and sprouting of axonal projections to the hippocampus (Szot, White et al. 2006, Stefani, Olivola et al. 2015). These findings are further supported by studies that demonstrate significant positive correlations between indexes of noradrenergic activity in CSF, plasma and urine in depressed patients (Wyatt, Portnoy et al. 1971, Roy, Pickar et al. 1987, Roy, Pickar et al. 1988). However, other studies did not show significant differences in CSF NE between depressed individuals and control neurological patients, except where depressed patients also had high levels of anxiety (Post, Lake et al. 1978). Currently there are no clinical studies directly evaluating LC neuronal loss in patients with depression, however this may be an important area of future research considering that a common therapeutic effect of antidepressants with diverse pharmacological mechanisms is a reduction of LC neuronal firing that may be neuroprotective (Szabo, de Montigny et al. 2000, West, Ritchie et al. 2009). Importantly, several studies have found greater LC neuron loss in depressed subjects with AD (Zubenko and Moossy 1988, Zweig, Ross et al. 1988, Zubenko, Moossy et al. 1990, Forstl, Burns et al. 1992). In contrast, one study was able to confirm LC neuronal loss in AD patients, but did not find a significant difference in LC neuronal loss between AD patients with depression, transient depression or without depression (Hoogendijk, Sommer et al. 1999). It should be noted, however, that such studies are limited by heterogeneity of depression, as the presence or absence of melancholic features in depression were left unaccounted (see section on hyperarousal). This may have a significant influence on the interpretation of results and perhaps should be taken into account in future study design, as individuals with melancholic depression or displaying more prominent features of melancholia may have LC neurons that are disproportionately affected. Thus, there is a possibility that the pathophysiological mechanisms of depression may influence the integrity of the LC-NE system, resulting in the selective degeneration of LC neurons and predisposing a select population of individuals to AD neuropathology. While these separate studies cannot exclude this possibility, studies to date have not directly studied this issue, and it may be an important area of future investigation.
ii. Sex differences in Vulnerability to Stress Related Psychiatric Disorders and AD
Stress is a common underlying feature of psychiatric disorders, particularly those that are more prevalent in females such as depression. Strikingly, approximately two-thirds of AD patients are women (Alzheimer’s 2015), however, the most recent data on sex differences in incidence of AD suggests equivocal incidence of AD in men and women. Despite this equivalence, sex differences in risk factors and molecular drivers of pathology differ between men and women significantly (Rasmuson, Andrew et al. 2001, Beeri, Rapp et al. 2009, Corbo, Gambina et al. 2009, Craig and Murphy 2009), and women have increased severity of neuropathology compared to men (Koran, Wagener et al. 2016). There are neuroanatomical and physiological sex differences in brain regions that integrate the stress response, and in the signaling mechanisms that dictate responsivity to stress. The electrophysiological characteristics of LC neurons under basal conditions are mostly comparable between sexes, however, under conditions of stress, female rats exposed to stressors that activate the LC through CRF release in the LC, showed a greater magnitude of response than male rats independently of adult hormonal status of either males or females. Further, LC neurons exhibit morphological sex differences, such that LC dendrites of female rats are longer and more complex, having more branch points and extending further into the peri-LC than LC dendrites of male rats (Bangasser, Curtis et al. 2010, Bangasser, Zhang et al. 2011) thus allowing for a greater magnitude of arousal in response to emotion-related stimuli by favoring more contacts with limbic terminals that convey emotion- related information (Valentino, Van Bockstaele et al. 2013). Finally, there are molecular differences in CRF receptor 1 (CRFR1) signaling in the LC that alter its trafficking, males exhibit an adaptive response to stress by internalization of the CRFR1, while females do not internalize CRFR1 potentiating the response to stress (Reyes, Valentino et al. 2008, Reyes, Bangasser et al. 2014). Although coordinated CRF release in these circuits would be well designed for coping in a dynamic environment with potential life-threatening challenges, the initiation of these responses would be maladaptive in the absence of stressors or if the responses persisted after stress termination, especially in females (Valentino, Van Bockstaele et al. 2013, Bangasser, Wiersielis et al. 2016). Additionally, cortisol production and metabolism appear to be altered in women in early stages of AD (Rasmuson, Andrew et al. 2001). Interestingly, a retrospective study evaluating the effect of ApoE genotype and depressive symptoms in AD revealed that women who possessed the ApoE ε4 allele (conferring increased risk of AD) were almost four times more likely to be depressed in comparison to those who did not carry the allele, and ApoE ε4 status, but did not predict depression among men in the cohort. These results are consistent with recent suggestions that the ApoE ε4 genotype may be over-represented among women with AD and depression and highlight a need for additional research investigating the links between ApoE genotype, mood, and gender (Bondi, Rodriguez et al. 2008, Bondi, Jak et al. 2008).
c. Consequences of Chronic Stress on Development and Progression of Depression and AD
i. Allostasis, Allostatic Load, and Overload
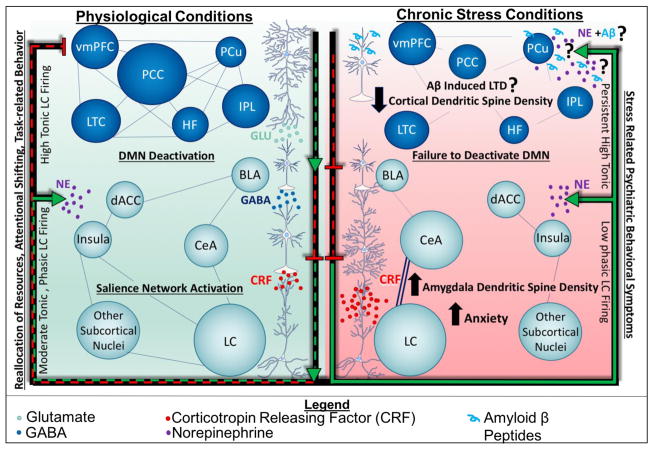
The adaptation to stressful physical or social stimuli requires the coordination of peripheral and central nervous system components through hormonal and neuroendocrine mediators such as cortisol, GC, and CRF. The active process of adapting to stressors, the engagement of the HPA axis, and the negative feedback mechanisms that are built into systems physiology that terminate the stress response and maintain homeostasis, have been termed allostasis (McEwen 2002, McEwen and Gianaros 2011). While these responses are necessary, their net effect are not without consequence; they may erode the integrity of these systems over time, leading to wear and tear on the body and the brain. The cumulative effect of this adaptation process is termed allostatic load (McEwen 2002, McEwen and Gianaros 2011). The state of allostatic overload is one defined by the inability to turn off the stress response efficiently, and may result from exposure to chronic stress, which leads to maladaptive responses and pathophysiology (McEwen and Gianaros 2011, McEwen and Morrison 2013, McEwen, Bowles et al. 2015) (Figure 2).
Figure 2.
Putative consequences of Aβ accumulation in stress-related psychiatric disorders. We propose that chronic stress results in the production and accumulation of Aβ in the PFC, initiating mechanisms of post synaptic depression. With decreased activity of PFC neurons, other limbic regions such as the amygdala may become hyperactive, exhibiting increased dendritic spine density. As a result, a potential feed-forward system may be engaged, creating a vicious cycle in which imbalances in neurotransmitters and neuronal activity are perpetuated, and may manifest behaviorally as a stress-related psychiatric disorder such as anxiety or depression. LTD, long term depression.
The maladaptive responses observed in a state of allostatic overload are profound: they may result in metabolic and inflammatory alterations both peripherally and centrally, and promote alterations in neuronal gene expression, neuronal morphology, and the architecture of greater neuronal networks. These alterations have significant effects on behavior, thus are crucial for the understanding and treatment of stress-related psychiatric disorders. Recent evidence in the literature supports the notion that the mechanisms by which chronic stress profoundly alters human behavior, large scale brain-networks, the smaller neuronal networks that compose them, and the individual synapses that control their activity, may provide a link between neuropsychiatric and neurodegenerative disease. In the following sections, we will review evidence that supports the hypothesis that allostatic overload, or chronic stress, results in a reorganization of neuronal circuitry such that limbic circuits are enhanced and cortical circuits are diminished, resulting in a disconnection syndrome evidenced by psychiatric symptoms, including those observed in dementia.
ii. Stress and Large-Scale Brain Network Reorganization: A Balancing Act
The activation or connectivity of large-scale brain networks, evident in reliable patterns of activity during specific tasks, may be referred to as brain states. The brain state when an individual is not focused on their external environment, but rather is concentrating on internal events and thoughts, engages a distinct anatomically defined brain system called the default mode network (DMN) (Buckner, Andrews-Hanna et al. 2008, Buckner 2013, Raichle 2015). The DMN is active during internally focused tasks, such as autobiographical memory retrieval, planning for the future, and conceiving the thoughts or perceptions of others (Buckner, Andrews-Hanna et al. 2008). Neuroimaging functional magnetic resonance imaging (fMRI) studies in non human primates and in human have converged to identify core regions associated with DMN; the ventral medial prefrontal cortex (vMPFC) (also known as the infralimbic region), posterior cingulate cortex (PCC), the adjacent precuneous (pCu), inferior parietal lobe (IPL), lateral temporal cortex (LTC), the dorsal medial prefrontal cortex (dMPFC) and the hippocampal formation (HF) (Buckner, Andrews-Hanna et al. 2008, Buckner 2013).
The discovery of DMN has enticed investigators to hypothesize about the maintenance of brain states, and the neural correlates of switching between states of internal mentation and interacting with the external environment (Tang, Rothbart et al. 2012). The suppression of the DMN is functionally important for successful operation of certain cognitive processes, such as focused attention, working memory and other executive functions (Anticevic, Cole et al. 2012). This effective switching between the states is at least partially dependent on the function of salience network, consisting of anterior insular and dorsal anterior cingulate regions (Sridharan, Levitin et al. 2008, Bonnelle, Ham et al. 2012).
The LC is thought to play an important role in this mechanism (Corbetta, Patel et al. 2008, Hermans, van Marle et al. 2011), as it is poised to switch between two modes of discharge activity that dictate behavioral outcomes in response to task-related stimuli [for extensive review see (Aston-Jones and Cohen 2005, Valentino and Van Bockstaele 2008)]. The LC-NE system is critically involved in promoting attention, wakefulness and cognition, and responding to stressful stimuli. Thus, it has been proposed that the activation of the LC and release of NE, terminates the resting state and begins an adjustment that involves cortical, subcortical, and autonomic activity, potentiating attention (Sestieri, Corbetta et al. 2011, Tang, Rothbart et al. 2012, Buckner 2013) and allowing for behavioral output of a task-related stimuli (Aston-Jones and Cohen 2005) (Figure 2). During passive task states, the LC-NE system is characterized by a high tonic baseline, putatively linked to spontaneous thoughts (Smallwood, Brown et al. 2012). During task-focused states, a decrease in tonic activity of the LC to moderate levels, may promote optimal engagement in the immediate task (Aston-Jones and Cohen 2005). Thus, it has been suggested that the deactivation of certain regions overlapping with DMN may be caused, at least in part, by high tonic activity associated with the LC-NE system, reflected by theta oscillations (Scheeringa, Bastiaansen et al. 2008). In support of this, it has been shown that Modafinil, a wakefulness promoting agent thought to act on monoaminergic transmitter systems, can augment task induced deactivation in the DMN to facilitate sensorimotor processing speed, an effect dependent on changes in vMPFC activity and is consistent with gain control function of catecholamine systems (Minzenberg, Yoon et al. 2011).
iii. Allostasis in the context of LC-NE Induced De-activation of DMN
In addition to its role in initiating the deactivation of the DMN for task-directed behavior, the LC-NE system is a key component of the stress response brain state. This is especially important in light of the recent evidence in human fMRI studies that stressed individuals displayed greater activation of the DMN, as well as impairments in the deactivation of resting state networks when compared to controls (Soares, Sampaio et al. 2013). Specifically, stress increased the functional connectivity of the medial prefrontal cortex (mPFC), medial orbitofrontal cortex, PCC and the pCu. Sousa and colleagues conclude that the increases in the posterior regions of the DMN observed in stressed participants, particularly the PCC and the inferolateral parietal lobes, are likely associated with longer processing of emotionally salient stimuli and episodic memory retrieval (Maddock, Garrett et al. 2003, Wagner, Shannon et al. 2005, Soares, Sampaio et al. 2013). Additionally, this increase in functional connectivity was associated with a contraction of the connectivity map of the DMN, with specific reductions in the left PCC and left and right parietal inferior regions. While whole brain analysis for relative intracranial volumes between stress and control groups did not differ, a significant reduction in the total DMN volume was observed in stressed patients, and more specifically in the left PCC and the left and right parietal inferior cortices. Sousa further speculates that these reductions are likely to reflect the stress-induced atrophic effects in cortical regions, observed in several previous reports (Liston, McEwen et al. 2009, Soares, Sampaio et al. 2012, Soares, Sampaio et al. 2013). In addition to fMRI, participants in this study were also examined on the perceived stress scale (PSS), Hamilton anxiety scale, and Hamilton Depression Scale. It was confirmed that stress individuals scored higher on the Hamilton anxiety scale, a finding that supports previous fMRI studies revealing lower deactivation in the pCu on anxious patients (Porter, Buxton et al. 2003, Carey, Warwick et al. 2004, Zhao, Wang et al. 2007, Gentili, Ricciardi et al. 2009, Soares, Sampaio et al. 2013) and in anxious patients with AD (Zhao, Wang et al. 2007). Thus, stress may act to precipitate behavioral alterations that reflect profound changes in DMN circuitry, particularly in the prefrontal cortex (PFC) areas, which have significant implications for psychiatric and neurodegenerative disease (discussed below) (Figure 2).
iv. Failure to deactivate DMN: Implications for Depression and AD
Buckner and colleagues note that perhaps the most compelling link between clinical disease and disruption of DMN occurs in AD (Buckner, Andrews-Hanna et al. 2008). From metabolism, Aβ Pittsburgh compound B (PIB)-positron emission tomography (PET), structure and activity based imaging studies in humans with AD, all approaches converge to suggest that the DMN is disrupted (Buckner, Andrews-Hanna et al. 2008). The neuroanatomical pattern of reduced metabolism in patients with AD and age-matched healthy controls reflects DMN anatomical substrates, and includes PCC, IPL and LTC (Wagner, Shannon et al. 2005). Further, patients at genetic risk for the disease show similar metabolic differences in these areas (Reiman, Caselli et al. 1996). Additional studies that have examined regional brain atrophy in AD patients have also shown prominent decreases in volume of the medial temporal lobe (Scahill, Schott et al. 2002, Thompson, Patterson et al. 2003, Buckner, Snyder et al. 2005), with accelerated atrophy in the PCC and medial temporal lobe in preclinical stages of the disease (Buckner, Snyder et al. 2005). Studies utilizing [18F] fluorodeoxyglucose (FDG)-PET, measuring neuronal activity through glucose metabolism have shown that DMN regions along the midline, especially the PCC, show disproportionately high resting glucose metabolism relative to other brain regions by about 20% (Gusnard, Raichle et al. 2001, Raichle, MacLeod et al. 2001). Compelling reports of AD pathology cite DMN as a network where Aβ accumulates (Sheline, Raichle et al. 2010, Myers, Pasquini et al. 2014, Koch, Myers et al. 2015), even before symptoms emerge (Klunk, Engler et al. 2004), suggesting early dysregulation of this system in the course of the disease. In line with this, studies in cognitively normal individuals show that within and between the DMN and other networks, Aβ accumulation induced alterations in functional connectivity, likely reflecting local network disruption and compensatory reorganization. Further, these changes occurred prior to biomarkers of hypometabolism, indicating that these profound changes occurred before the onset of cognitive decline (Elman, Madison et al. 2016). In older adults with memory impairment and sleep disturbances, Aβ also disrupted functional connectivity in the DMN (Mander, Marks et al. 2015). Consistent with these findings, Braak staging of Aβ deposition begins in the cortical regions of the brain (Braak and Braak 1991). Buckner and colleagues have proposed that the DMN’s continuous activity augments an activity-dependent or metabolism dependent cascade that is conducive to the formation of AD pathology (2005), and this is consistent with the known mechanisms of Amyloid Precursor Protein (APP) processing and Aβ formation, which is synaptic-activity dependent (Cirrito, Yamada et al. 2005).
A growing body of work using resting state-functional connectivity MRI (rs-fcMRI) implicates a failure to suppress DMN activity in cognitive impairments and symptoms associated with neuropsychiatric disorders (Benson, Kuhl et al. 1983, Buckner, Andrews-Hanna et al. 2008, Anticevic, Cole et al. 2012). A lack of DMN suppression has been reported in depression; Sheline and colleagues found that depressed individuals fail to reduce DMN activity while examining negative images (Sheline, Barch et al. 2009). In depression, DMN suppression deficits are linked to rumination (Hamilton, Furman et al. 2011) or negative internal thought, an effect thought to reflect activity in the mPFC (Hamilton, Furman et al. 2011, Anticevic, Cole et al. 2012, Lemogne, Delaveau et al. 2012). In support of this, neuroimaging and post-mortem analysis of patients with major depressive disorder have revealed decreased grey matter volume and cell counts with corresponding decreased glucose metabolism and cerebral blood flow in the dMPFC/anterolateral PFC, subgenual anterior cingulate, progenual anterior cingulate, orbital and ventrolateral and PCC (see (Drevets, Price et al. 2008) for an excellent review of the structural and functional alterations in mood disorders). Interestingly, in depressed participants, an increased fMRI connectivity pattern between dMPFC regions and the DMN has been reported (Kessler, Chiu et al. 2005), and this increased connectivity has recently been shown to be reduced by antidepressants (Drevets 2001). Strikingly, the study by Sousa and colleagues reveals an increased functional connectivity between mPFC and PCC in stressed participants, and the finding that stress increased activation in resting states of the anterior cingulate cortex is also of relevance for the affective processing of negative information, known to be altered in depressed patients (Surguladze, Brammer et al. 2005, Mogg, Bradbury et al. 2006).
Based on the cumulative evidence presented above, stress may be an important etiological factor in diminishing the brain’s ability to suppress DMN activity. Further, the role of LC-NE system in the stress response is well established, and with further examination of DMN we may come to better understand how LC-NE activity may participate in DMN suppression. The currently available literature suggests that the LC-NE system acts as a switch, allowing for the suppression of DMN while also enabling task-focused attention. What is unknown, and perhaps critical for future study, are the direct effects of chronic stress and downstream dysregulation of LC-NE activity on DMN activity. What may be inferred from the evidence presented, is that a dysregulation in the LC-NE system, possibly resulting from chronic stress, at least in part results in diminished ability to suppress DMN, and that this has significant consequences for stress-related disease states. Further, the resultant dysfunction of cortical regions involved in the DMN, may influence the neural architecture of downstream circuits involved in the neuroendocrine, neurotransmitter, and behavioral responses to emotional stimuli and stressors, creating a vicious cycle (discussed in detail in the following section). Thus, understanding the cellular and molecular processes by which NE may exert these effects may not only enhance our understanding of the maintenance and switching between large-scale networks and corresponding brain states, but also may facilitate and understanding the neural substrates of stress related-psychiatric disease and their behavioral correlates. In the following sections, we will review preclinical evidence that may provide mechanistic support for the discussed clinical findings.
III. Preclinical Mechanisms
a. Allostatic Reorganization of Stress Integrative Circuitry
The coordination between endocrine, cognitive, and emotional limbs of the stress response is required to facilitate an appropriate behavioral response to a perceived stressor. A crucial player in this orchestrated response is the neurohormone CRF, which engages the peripheral and central stress response systems in parallel when released from the paraventricular nucleus of the hypothalamus (PVN). CRF action on the anterior pituitary via the hypophyseal portal system results in the release of adrenocorticotropic hormone (ACTH) which stimulates the adrenal gland to produce cortisol and GC. Of particular importance are GC, as they participate in a negative feedback loop to terminate the stress response when they bind to and activate their receptors, that are abundant in the PVN, hippocampus, and the PFC (McEwen 1988, Ahima and Harlan 1990, Ahima and Harlan 1991). Thus, circulating blood cortisol levels and interstitial fluid (ISF) levels of GC may be used as indicators of the stress response state of arousal. Importantly, in addition to the peripheral stress response, CRF also engages the intricately connected cognitive and emotional limbs of the stress response when it stimulates the midbrain nucleus LC and amygdala, respectively (Valentino and Van Bockstaele 2008). As a crucial component of the cortico-limbic neurocircuitry, the mPFC has extensive downstream projections to regions as diverse as the amygdala and the brainstem (Sesack, Deutch et al. 1989), providing a mechanism of executive top-down regulation of autonomic and neuroendocrine balance (Thayer and Brosschot 2005), with influences on parasympathetic (Thayer and Sternberg 2006) and HPA activity (Diorio, Viau et al. 1993). Of particular importance, are the excitatory projections from the mPFC that synapse onto gamma-aminobutyric acid (GABA)ergic interneurons of the basolateral amygdala (BLA), which suppress the output of central nucleus of the amygdala (CeA) neurons. This has significant implications, because in the absence of top down modulation from the mPFC, hyperactivation of the CeA may result in increased LC neuronal activity and NE secretion, creating a vicious cycle of enhanced emotional and cognitive limbic excitability and reduced cortical control of those circuits (Figure 2).
i. Preclinical Models of Chronic Stress Reflect Neuropsychiatric Deficits in Humans with Depression and AD
Stress response areas in the brain are organized into functionally integrated networks that, when perturbed, disrupt cognitive function and emotional processing on a global level. Preclinical models of chronic and repeated stress have been developed to facilitate investigations of neurobiological correlates of mood disorders (Drevets, Price et al. 2008). In regions that appear homologous to the areas where grey matter reductions are evident in depressed humans (mPFC, hippocampus), repeated stress results in dendritic atrophy and reductions in glial counts and proliferation in rodents (McEwen and Magarinos 2001, Radley, Sisti et al. 2004, Banasr and Duman 2007, Banasr, Valentine et al. 2007, Radley, Rocher et al. 2008). Many of the regions reported to have dendritic atrophy under conditions of stress, particularly layer II/III pyramidal neurons in the right prelimbic and infralimbic cortex and left anterior cingulate cortex in preclinical models (Wellman 2001, Cook and Wellman 2004, Radley, Rocher et al. 2006, Cerqueira, Taipa et al. 2007, Shansky, Hamo et al. 2009, Sousa and Almeida 2012) are aligned with reports of reduced volume of grey matter volume in DMN structures (Sheline, Raichle et al. 2010, Soares, Sampaio et al. 2013, Elman, Madison et al. 2016), and other regions implicated in depression and AD in humans (Chan-Palay and Asan 1989, Chan-Palay and Asan 1989, Soares, Sampaio et al. 2013, McEwen, Nasca et al. 2016) (Figure 2). These findings have lead some investigators to hypothesize that homologous processes underlie reduction in grey matter volume in PFC and hippocampal structures in Major Depressive Disorder (MDD) (McEwen and Magarinos 2001), and can potentially provide insight on the long term changes that may increase vulnerability of depressed or chronically stressed individuals to AD (Pomara and Sidtis 2009).
Rodent studies utilizing chronic restraint stress for a duration of 21 days showed functional and structural changes in the PFC, amygdala, and hippocampus in a regionally specific manner (McEwen and Gianaros 2011). These alterations were such that dendritic spines in the mPFC were shortened (Wellman 2001, Cook and Wellman 2004, Radley, Sisti et al. 2004, Cerqueira, Catania et al. 2005, Radley, Rocher et al. 2006, Cerqueira, Taipa et al. 2007), while dendritic growth was evident in the amygdala (Vyas, Mitra et al. 2002). In line with these results, other studies of repeated stress in rodents have shown resultant hyper-excitability of BLA neurons (Shekhar, Truitt et al. 2005, Vyas, Jadhav et al. 2006). Other studies examining pyramidal neurons in layer III of the infralimbic, prelimbic, and cingulate cortices have shown 20% reduction in the length of distal portions of apical dendrites in male rats with exposure to chronic stress, an effect downstream of protein kinase C (PKC) signaling (Hains, Vu et al. 2009). This dendritic shrinkage was accompanied by spine loss, resulting in over 30% spine loss, which were primarily thin spines that are known to be involved in plasticity and cognitive performance, following chronic stress (Bloss, Janssen et al. 2011). These findings are consistent with human studies that demonstrate that the severity of MDD correlates positively with amygdala activity and negatively with activity in the PFC and lateral orbital cortex regions (Drevets, Price et al. 2008). Thus, a collective inhibition or down-regulation of activity in the mPFC and concurrent increase in activity of the amygdala may serve as a mechanism by which the modulation and organization of stress integrative circuitry is altered under conditions of chronic stress. In line with this reasoning, some investigators have speculated that the neuropathological changes evident in the mPFC in primary and some secondary mood disorders thus may impair the modulatory role of the mPFC over emotional expression, disinhibiting or enhancing limbic responses to stressors and emotional stimuli (Drevets, Price et al. 2008).
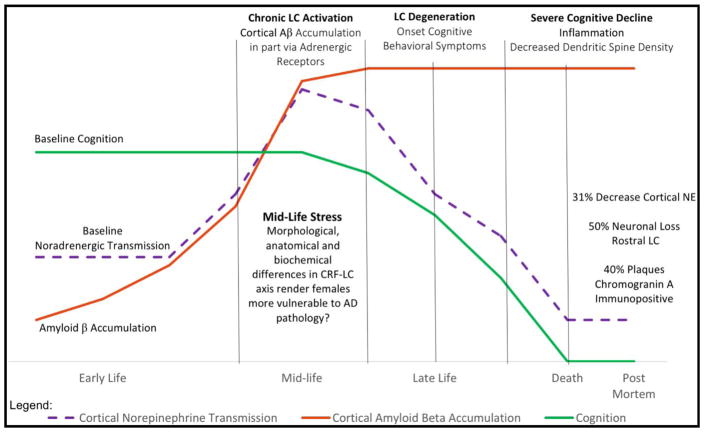
The evidence described thus far supports a model by which stress-induced remodeling of the PFC and amygdala is such that top-down executive control of the PFC is diminished by a reduction in dendritic spine density. Further, the retraction of dendritic spines in the PFC allows for enhanced emotional arousal, mediated by dendritic growth and hyper excitability of amygdalar neurons. Thus, the next important questions to consider are: 1. What influence do these alterations have on behavioral phenotypes? 2. Is there a concurrent effect on LC neurons? Few studies to date have examined LC neurons in models of stress-induced depression. Those that have, however, have indicated that LC axons degenerate under conditions of stress induced depression and may be regenerated with the administration of the antidepressants desipramine and imipramine. Thus, concluding that LC neurons degenerate in depression and regenerate in remission (Kitayama, Nakamura et al. 1994, Kitayama, Otani et al. 2008). More recent studies provide further support for this notion, as the injection of neurotoxin 6-hydroxydopamine (6-OHDA) directly into the LC results in a depressive phenotype indicated by increased immobility time in the forced swim test (FST), which could be reversed with a single dose of either L-1-3-4-dihydroxyphenylalanine (DOPA) or L-threo-3,4-dihydroxyphenylserine (DOPS) prior to behavioral assessment (Szot, Franklin et al. 2016). Investigators also report a significant positive correlation between the number of surviving LC neurons after 6-OHDA and FST immobility. Thus, concluding that even minimal loss of LC neurons is sufficient to induce depressive symptoms, and that early degeneration of LC neurons as observed in MCI patients may contribute to the observed BPSD, especially depression (Szot, Franklin et al. 2016). The investigation of abnormalities in tau-p and endogenous Aβ in such models will be critical to move forward our understanding of the intersection of neurodegeneration and neuropsychiatric disorders.
ii. Stress-induced changes in dendritic spine density: the role of GC and aging
In an excellent review on the role of stress in structural plasticity over the course of a lifetime, McEwen and Morrison discuss the important role of GC in dendritic spine turnover during chronic stress, noting that aging impinges significantly on neuronal resilience to stress, and mechanisms of plasticity in the cortex, hippocampus, and amygdala. GC play an important role in the maintenance and regulation of dendritic spine density under conditions of stress, acute or chronic. Further, areas such as the prefrontal cortex and hippocampus are abundant in GC receptors (Ahima and Harlan 1990, Ahima and Harlan 1991), and therefore may be more vulnerable to stress-related changes in dendritic morphology or atrophy.
McEwen and Morrison further discuss the vulnerability of PFC-dependent tasks to decline with age in humans, non-human primates, and rodents (reviewed in (Gallagher and Rapp 1997)), age-related decline in cognitive performance reliant on PFC may result from loss of thin, highly plastic spines that comprise axospinous synapses in pyramidal layer III of mPFC class of axospinous synapses on PFC pyramidal neurons (Dumitriu, Hao et al. 2010). Interestingly, in young animals, despite profound changes in dendritic morphology and cognitive performance following chronic stress, a rest period of three weeks is sufficient for full functional recovery, with full dendritic arbor recovery and partial spine density recovery (Radley, Rocher et al. 2008, Bloss, Janssen et al. 2011). In contrast, middle-aged (12-month old) and aged rats, neurons failed to recover with rest following chronic stress demonstrating a loss of neuronal resilience that is apparent by middle age (Bloss, Janssen et al. 2010). Upon further analysis, it was concluded that middle-aged and aged rats lose 30% of their spines in the absence of stress, and this loss is driven primarily by the loss of thin spines in the aged rats. Taken together, these studies provide evidence that mPFC pyramidal neurons from aged rats suffer losses of plasticity at multiple levels: first, neurons from aging animals lose a certain population of thin spines that may be critical for proper functioning within PFC circuitry; second, the remaining dendritic spines are less capable of rewiring in response to experience; and lastly, neuronal dendrites from aging animals lack recovery-related plasticity mechanisms. Importantly, all three of these age-related changes in plasticity were observed in both middle-aged and aged animals, suggesting that preventative measures against such plasticity deficits may be optimally effective when implemented during middle-age. These observations are consistent with reports that middle-aged humans subjected to chronic stress are more vulnerable to develop AD than non-stressed controls (Johansson, Guo et al. 2010), and that prolonged exposure of older, non-demented individuals to stress in the presence of an ApoE ε4 allele leads to memory decline (Peavy, Lange et al. 2007).
iii. Allostasis and Synapse Remodeling
AD is considered one of synaptic failure. Synapses are the primary mode of communication for neuronal and glial cells, dictate the activity of local neuronal circuits and wider circuits that connect disparate brain regions. Synapses are dynamic structures that undergo frequent remodeling and turnover in response to diverse stimuli, and are shaped by experience (Bloss, Janssen et al. 2011). Thus, while synaptic integrity is vital for brain function, it also requires plasticity for learning and memory processes, and also for adaptation to ever-changing environments. Synaptic remodeling may include alterations in the number of dendrites, via spine pruning, or the removal or regrowth of diverse types of dendritic spines (Sousa and Almeida 2012). Stress concurrently influences the tone of neuromodulators including GC, CRF, and NE, all of which may have profound molecular effects upon receptor binding, in a regionally specific manner. The mechanisms of stress-induced retraction and expansion of dendrites and synapse turnover, and the behavioral impact of stress induced synaptic plasticity have been reviewed (McEwen and Morrison 2013). While synaptic remodeling is pivotal for overall neuronal health in all brain regions, it is particularly important in the PFC and hippocampal regions, where complex cognitive processes occur (Wellman 2001). We begin this section by describing key components of the synapse, some of which are known to become drastically altered during the course of chronic stress, depression, and AD. As the cellular and molecular substrates reviewed in this section are likely to be key mediators of underlying mechanisms influencing neuronal structure, function, and viability in stress related psychiatric disorders, we postulate as to how chronic stress and a history of depression may render individuals more vulnerable to AD.
a. Cytoskeletal architecture of the synapse: the role of Tau
Structural plasticity, in the form of synaptic remodeling of neurons and glia, leads to functional plasticity (Sousa and Almeida 2012). The synapse is composed of a presynaptic neuron, defined synaptic cleft, and post synaptic neuron with a prominent post synaptic density (PSD). Tau, a member of the microtubule associated protein (MAP) family, is a soluble phospho-protein that stabilizes primarily axonal but also dendritic microtubules into bundles, to maintain cellular morphology. Tau binding and stabilization of axonal microtubules is crucial for trafficking intracellular organelles and vesicles. One of the primary events that regulate tau-microtubule interactions is phosphorylation, which alters tau binding and function. In contrast, post synaptic dendritic structures have protrusions that are known as dendritic spines, which make the connections with presynaptic terminal structures and are composed of diverse PSD proteins that are responsible for linking membrane components with cytoplasmic cytoskeletal proteins (Kondo and Okabe 2011). Actin is one of the primary cytoskeletal components of the synapse that regulate the activity dependent redistribution of scaffolding proteins, and may be altered by the strength of synaptic activity and the morphological enlargement of spine structure (Kondo and Okabe 2011).
b. Modulation of Neuronal Excitability at the Synapse: the role of Aβ
As alluded to earlier, Aβ peptides are produced at the synapse, in a synaptic activity- and endocytosis-dependent manner, approximately 60% of the time (Cirrito, Yamada et al. 2005, Cirrito, Kang et al. 2008). The production of Aβ is also largely dictated by the subcellular localization of its precursor, APP (Haass, Kaether et al. 2012). Typically, the internalization of APP favors amyloidogenic processing and the formation of Aβ, whereas its presence on the surface confers increased likelihood that it will encounter the α-secretase known to produce nontoxic, soluble APPα and C83 fragments (Hong, Huang et al. 2014). Based on the cumulative evidence that synaptic Aβ production is neuronal activity- and endocytosis- dependent, it has been proposed that following neuronal activation, the recycling of synaptic vesicles results in increased internalization of APP, thus increasing substrate for β- and γ-secretases that reside in intracellular compartments. Resultant Aβ fragments may then be released into the extracellular space (Cirrito, Kang et al. 2008). It has been shown that Aβ has the ability to enhance calcium permeability pre-synaptically resulting in increased probability of vesicle release (Abramov, Dolev et al. 2009), or may act post-synaptically to suppress neuronal activity by promoting receptor internalization (Snyder, Nong et al. 2005, Wang, Yuen et al. 2011, Ulrich 2015), and long term depression (LTD)(Li, Hong et al. 2009, Palop and Mucke 2010). In line with these findings, it has been demonstrated that optimal concentrations of Aβ will facilitate vesicle release and neurotransmission, however, too low or too high levels of Aβ will inhibit synaptic activity (Abramov, Dolev et al. 2009). Based on this evidence, it has been postulated that Aβ has a physiological role in the central nervous system as a modulator of neuronal activity (Palop and Mucke 2010, Palop and Mucke 2010), that participates in a negative feedback loop to modulate neuronal excitability. Low levels of neuronal activity promote the processing of APP into Aβ by promoting the activity of the β-secretase at the synapse, however, once an abundance of Aβ is formed, it acts post synaptically to suppress neuronal activity. This has significant implications because, as previously discussed, the synaptic connections between neurons can dictate the activity of groups of neurons such as the LC in downstream microcircuits circuits such as those in the PFC and their global networks, which exert top-down executive control over regions such as the amygdala and hippocampus under normal physiological conditions. Thus an imbalance in Aβ may have global implications for neuronal functioning, behavior, and mood.
c. Stress is a Precipitating Factor of Abnormalities in AD Related Synaptic Proteins
Stress may disturb the activity of multiple brain regions. Because Aβ production at the synapse is dictated by synaptic activity, and it has been demonstrated that chronic neuronal hyperactivity is a driving force in Aβ accumulation (Yamamoto, Arima et al. 2015), it is logical to put forth the notion that stress may increase synaptic levels of Aβ, perhaps to the level of dysfunction. Biochemical analyses of Aβ peptides have revealed they are highly hydrophobic approximately 4kDa proteins with a strong tendency to self aggregate into stable dimers, trimers, higher oligomers and ultimately, 8nm amyloid fibrils (Masters and Selkoe 2012). Thus the neuronal microenvironment, and the level of activity exhibited by certain conditions. In particular, stress, via chronic LC hyperactivation, may render the brain via its widespread projections, more vulnerable to an excessive accumulation of Aβ peptides, and subsequent deposition, forming plaques. Of great importance, would be excessive Aβ accumulation in the PFC, which would promote post-synaptic depression of GABA-ergic interneurons that control the amygdala, providing an anatomical substrate for enhanced limbic excitability.
Under normal physiological conditions clusters of neurons will fire together in synchrony, a process that is important for memory and learning processes (Jutras and Buffalo 2010). However, what has been observed in humans with AD, and in mouse models of AD that overexpress APP and consequently have increased production of Aβ, is that there are abnormal patterns of neuronal activity in circuits and wider networks (Palop and Mucke 2010, Palop and Mucke 2010). This suggests that the negative feedback loop in which Aβ normally participates, becomes altered in AD. For example at the circuit level, high levels of Aβ increase network synchrony and elicit epileptiform activity as illustrated in EEG recordings of the left and right parietal cortex of human APP transgenic mice and non-transgenic controls (Palop, Chin et al. 2007). However, when individual neurons are examined in vivo by calcium imaging, neurons recorded from non-transgenic controls exhibit an intermediate level of activity, whereas neurons from mice genetically modified to express presenilin 1 and human APP (PSAPP) mutations, yielding high Aβ levels, are either hypoactive or hyperactive (Busche, Eichhoff et al. 2008). Ultimately, this has lead to the perspective that AD is a disconnection syndrome, or global network desynchronization. The impairments of neural synchrony observed in schizophrenia, autism, and AD are consistent with current theories that emphasize a disconnection syndrome as the underlying pathophysiological mechanism (Palop, Chin et al. 2007, Palop and Mucke 2010, Palop and Mucke 2010). According to these theories, cognitive dysfunctions as well as symptoms of these disorders arise from a dysfunction in the coordination of distributed neural activity between and within functionally specialized regions of the cerebral cortex (Elman, Madison et al. 2016). Reduced neural synchronization can be a consequence of disconnection, but it can also be the cause of impaired coupling between brain areas because synchronization of neural responses is essential for their propagation across sparsely connected networks (Abeles 1991).
i. CRF and CRF Receptor 1
In the last decade, a number of studies have examined the contribution of stress in AD by utilizing behavioral models of acute and chronic stress as well as a number of genetic models that perturb CRFR1 signaling, either by overexpression of CRF (CRF OE) or ablation of CRF receptors. Seminal studies examining the effect of neuronal activation on Aβ and hyper-phosphorylated tau accumulation demonstrated that stress, or increased CRF neurotransmission, accelerates AD neuropathology (Kang, Cirrito et al. 2007, Dong, Murphy et al. 2012). Studies from the Rissman group have utilized a mouse line with genetic ablation of CRFR1 crossed with the PSAPP mouse model of AD, to examine the effects of CRFR1 ablation on Aβ accumulation. CRFR1 ablation resulted in significantly reduced levels of Aβ40, Aβ42 compared to PSAPP counterparts, with a concurrent dramatic reduction in AβPP C-terminal fragments (CTFs) in PSAPP-R1 mutant mice. Further, the data demonstrate that PSAPP-R1 mutant mice have reduced insulin degrading enzyme (IDE) and AβPP CTFs, suggesting that the mechanism underlying the observed change may involve Aβ production rather than clearance. One putative mechanism suggested is that CRFR1 is a modulator of β- or γ-secretase trafficking. Thus, CRFR1, the primary receptor responsible for stress-responsivity and abundantly expressed in the LC, amongst other regions, is a significant contributor of Aβ production in AD mouse models.
Equally compelling, are studies that explore the effects of CRFR1 activation or ablation on the phosphorylation of tau. In one study it was demonstrated that thirty-minute acute restraint stress resulted in rapid and reversible increased in tau-p at multiple AD relevant sites (S181, S199, S202/T205 (AT8), T212, T231, S396/404 (PHF-1), and S422) in soluble fractions of hippocampal tissue, independently of GC signaling. Additional studies determined that CRFRs differentially regulated tau-p, and that CRFR1 was predominantly responsible for stress-induced increases in tau-p. These findings were further supported by pharmacological studies using antalarmin, a selective CRFR1 antagonist, that demonstrated that CRFR1 antagonism was sufficient to prevent stress-induced increased in tau-p. These results support a specific involvement of CRFR1 signaling in stress induced tau-p of AT8 and PHF-1 sites (Rissman, Lee et al. 2007).
Subsequent studies focusing on the effects of chronic, repeated stress on tau-p revealed that CRFRs have differential effects on not only tau-p, but also its solubility and state of aggregation. To determine whether this effect generalized to chronic/repeated stress these investigators examined levels of AT8 and PHF-1 sites in mice lacking CRFR1, CRFR2, or both receptors and in both soluble and insoluble hippocampal fractions after a single exposure (acute stress) or fourteen consecutive daily exposures (repeated stress) to a restraint stress. While tau-p resulting from acute stress was transient and potentially involved in stress related neuroplasticity, tau-p induced by repeated stress or overexpression of CRF was present for extended periods after stress exposure, had a definable structure and was localized to detergent soluble cellular fractions (Rissman, Staup et al. 2012). Thus, tau-p induced by chronic stress via CRFR1 forms pre-pathological structures that may result in NFT formation over long periods of time.
Constitutive overexpression of CRF in mice is considered a valid animal model of chronic stress; CRF OE mice display significant brain atrophy at three to six months of age, and show hippocampal dependent learning deficits when tested at 9 months of age. Furthermore, studies using CRF OE mice have found increased levels of tau-p compared with wild type littermates. CRF OE mice have increased hippocampal tau-p; in particular, AT8, PHF-1 and S422 were 350%, 350% and 170%, respectively greater in CRF OE mice than their WT counterparts. Treatment for thirty days with R121919, a selective CRFR1 antagonist, completely prevented the rise in tau-P at AT8 and PHF-1 phosphorylation sites in CRF OE mice. By contrast, levels at the S262 and S422 sites were unchanged by R121919 pretreatment. These results demonstrate that overexpression of CRF can induce tau-p in the hippocampus, a process that requires CRFR1 for specific sites but not others. CRF OE cohorts show significantly increased phosphorylation levels of glycogen synthase kinase 3 beta (GSK-3B), mitogen-activated protein kinases (MAPK) p38, and extracellular signal-regulated kinase (ERK)1/2. Levels of cyclin-dependent kinase 5 (cdk5) were unchanged in CRF OE mice regardless of treatment with R12919. Only levels of activated c-Jun N-terminal kinase (JNK) were sensitive to R121919 pretreatment, indicating that the induction of tau-p via JNK activation is central to tau-p at the AT8 and PHF-1 sites in CRF OE. CRF OE also increased formation of globular tau aggregates. Requires more confirmation, but findings may represent evidence of pre-pathologic hippocampal tau aggregates in vivo (Campbell, Zhang et al. 2015). Importantly, the LC has not been investigated in these models, and given the abundance of CRFR1 and the literature supporting LC degeneration in stress-induced models of depression, studying tau-p and Aβ aggregates are of paramount importance.
Rissman and colleagues hypothesize that stress-induced tau-p is an essential process required for stress adaptation that becomes dysfunctional with advancing age and/or with chronic overstimulation (Rissman 2009). In support of this, studies examining the functional implications of CRF exposure on tau-p have revealed that CREB activation and BDNF transport was significantly reduced, while mitochondrial velocity and distance traveled was increased (Le, Weissmiller et al. 2016), suggesting a shift in priority away from growth factors and towards cellular energy sources under conditions of stress. Although phenomenology may be distinct, a similar case can be made for toxicity associated with Aβ. Aβ is produced in the brain throughout life, but with advancing age perhaps the ability to clear or process Aβ may become altered or lost, which causes accumulation (Campbell, Zhang et al. 2015).
ii. Noradrenergic Influence
The involvement of the stress-integrative LC-NE system and its putative role in potentiating AD-related pathology has recently been reviewed (Ross, McGonigle et al. 2015). Here, we will briefly reiterate the role of ARs in Aβ accumulation. Of particular importance, is the localization of β2 and α2AR to terminal regions of the LC, which have a direct influence on synaptic transmission and the APP processing machinery residing at the synapse, and that have been implicated in the production and secretion of Aβ. Electron microscopy studies have localized the β2AR on dendritic spines of pyramidal neurons of the PFC and on GABAergic interneurons (Aoki, Venkatesan et al. 1998), where it is proposed that via the joint endocytosis of β2 and APP from the plasma membrane following stimulation, will increase the co-localization and availability of APP to act as a substrate for γ-secretase in the endosomal compartment (Ni, Zhao et al. 2006). It has also been postulated that β2AR is able to modulate Aβ production via its association with β-arrestin2, which physically interacts with the α1A subunit of the γ-secretase complex, resulting in increased catalytic activity of the complex (Thathiah, Horre et al. 2013). In support of these findings, studies in a transgenic animal model of AD have shown that β2 antagonists diminish levels of Aβ40 and Aβ42 (Scullion, Kendall et al. 2011), and the therapeutic potential of targeting β-ARs in AD has been reviewed (Yu, Wang et al. 2011). The α2AR are coupled to Gi/cAMP systems and are predominantly localized presynaptically, acting as autoreceptors that regulate NE synthesis and release (Berridge and Waterhouse 2003). Early studies exploring catecholamine neurotransmitters and receptors in age-related cognitive disorders identified α2-AR as contributors to cognitive decline in the PFC (Arnsten and Goldman-Rakic 1985). In line with this, a recent study demonstrated that chronic treatment with the α-2AR antagonist fluparoxan, prevented age-related deficits in spatial working memory in APP/PS1 transgenic mice, independent of amyloid plaque burden (Scullion, Kendall et al. 2011). The α2aAR has also been implicated in the regulation of APP processing and subsequent Aβ production and secretion via the endocytic and secretory pathways (Chen, Peng et al. 2014). This is also interesting in light of the findings described above regarding altered function of α2ARs in the frontal cortex of suicide victims (Valdizan, Diez-Alarcia et al. 2010). Based on this evidence it is clear that the β2 and α2aARs play a role in the modulation of Aβ production and secretion. Further, the activation of β2AR and/or α2aAR following noradrenergic transmission and resultant Aβ production, suggests that aberrant activity of the LC induced by stress may contribute to increased Aβ production in the cortex during prodromal or early stages of the disease (Figures 1,3).
Figure 3.
Hypothesized influence of coeruleo-cortical network dysregulation on AD progression. Based on the cumulative evidence in the literature, we hypothesize that In the fifteen to twenty years prior to the onset of cognitive symptoms of AD, stress induced chronic activation of the LC and corresponding increases in noradrenergic transmission increases Aβ production, secretion and accumulation in cortical regions such as the infralimbic medial PFC. Further, we hypothesize that the aberrant accumulation of Aβ peptides results in a disconnection in communication, or desynchronization, between the LC and its cortical efferent projections.
The findings reviewed in this section are particularly important, as Aβ deposits are thought to first accumulate in layers II, III, IV and V of the neocortex, progressing down to limbic structures including hippocampus and amygdala, and finally accumulating in midbrain and brainstem structures according to Braak staging (1991), and many of these regions, particularly regions affected in early or prodromal periods of AD are abundant with ARs. Further, the classic pattern of NFT accumulation, starting in entorhinal and hippocampal formation, then progressing to association and primary cortices is relevant to the density of CRFR1, that play a role in tau-p. More recently, Braak proposed that the LC was the first region to exhibit abnormalities in tau-p, reflected in pre-tangle material in post-mortem brain tissue from individuals that died under thirty years old (Braak and Del Tredici 2011, Braak and Del Tredici 2011, Braak, Thal et al. 2011, Braak and Del Tredici 2012, Braak, Thal et al. 2013). As discussed earlier, the LC is also a region of CRFR1 abundance and the mechanisms of CRFR1 regulation of tau-p under conditions of stress described here may be relevant to these findings (Figure 1). The studies reviewed here suggest a direct role of CRF and AR in the accumulation of Aβ and in the hyperphosphorylation of tau.
V. Conclusions
The studies reviewed here suggest that stress induces widespread alterations to brain functioning, ranging from large-scale network activity and reorganization to the altered expression and function of synaptic proteins, including Aβ and tau-p, primary components of AD neuropathology. What is less understood, is the relationship between chronic stress and depression in the context of neuronal resilience over time, or with aging. A significant gap in our knowledge is whether neuronal resilience to chronic stress, or allostatic overload, is reduced in depressed individuals, and if so, the direct consequences on Aβ and tau-p accumulation. It is tempting to speculate that, given the role of Aβ in modulating neuronal activity, and the volumetric reductions in critical PFC networks involved in both the integration of the stress response, and resting state brain activity in the DMN, that perhaps Aβ is an overlooked neuropeptide with relevance in broader stress related psychiatric disorders such as depression. To illustrate this point, we propose that chronic hyperactivity of the LC results in the production and accumulation of Aβ in terminal regions such as the PFC, initiating mechanisms of post synaptic depression. It is also possible that during this time, with chronic hyperactivation, aberrant tau-p begins accumulating in the LC, activating reversible mechanisms of degeneration. With decreased activity of PFC neurons, other limbic regions such as the amygdala become hyperactive, and may exhibit increased dendritic spine density. As a result, a variety of imbalances in neurotransmitters and neuronal activity are perpetuated, and may manifest behaviorally as a stress-related psychiatric disorder such as anxiety or depression (Figure 2). With time, in mid-life, it would be expected that the severity of these alterations would increase given that mechanisms of neuronal resilience decline naturally with age. This may be a putative explanation for increased incidence of anxiety and depression in later life stages, and the co-morbidity with AD in specific subpopulations (Figure 3). While the current literature does not address this question, it may be an interesting and important area of future research. This hypothesis may be supported to a limited extent, by human clinical studies demonstrating volumetric changes in regions not only known to be influenced by mood disorders and stress-related psychiatric disorders such as depression, but also by the fact that AD consists of a 20+ year prodromal period in which Aβ and abnormal tau-p are thought to accumulate, and the first region of Aβ accumulation is the neocortex, while abnormal tau-p has been sited at earliest stages in the LC (Braak and Del Tredici 2011, Braak and Del Tredici 2011, Braak, Thal et al. 2011, Braak and Del Tredici 2012, Braak, Thal et al. 2013). This hypothesis has significant implications for understudied regions such as the LC, which not only may perpetuate dysfunction of cortical networks under conditions of chronic stress, but also may contribute to failure to suppress DMN activity, thus having broader implications for stress related psychiatric and neurodegenerative disorders (Figures 2, 3).
Highlights.
A hypothesized framework by which chronic stress may contribute to underlying neuropsychiatric pathology evident in depressed patients, which may render some individuals vulnerable to neurodegenerative disease later in life is discussed.
Stress induces widespread alterations to brain functioning, ranging from large-scale network activity and reorganization to the altered expression and function of synaptic proteins, including Aβ and phosphorylated tau, primary components of AD neuropathology.
Evidence is discus sed that supports the hypothesis that allostatic overload, or chronic stress, results in a reorganization of neuronal circuitry such that limbic circuits are enhanced and cortical circuits are diminished, resulting in a disconnection syndrome evidenced by psychiatric symptoms, including those observed in dementia.
What may be inferred from the evidence presented, is that a dysregulation in the Locus Coeruleus (LC)-Norepinephrine (NE) system, possibly resulting from chronic stress, at least in part results in diminished ability to suppress the Default Mode Network (DMN), and that this has significant consequences for stress-related disease states.
Further, the resultant dysfunction of cortical regions involved in the DMN, may influence the neural architecture of downstream circuits involved in the neuroendocrine, neurotransmitter, and behavioral responses to emotional stimuli and stressors, creating a vicious cycle.
Acknowledgments
This work was funded by NIH Grant DA 09082 and NIH Grant DA020129.
ABBREVIATIONS
- AD
Alzheimer’s Disease
- HPA
hypothalamo-pituitary adrenal
- PFC
prefrontal cortex
- DMN
default mode network
- SP
senile plaque
- NFT
neurofibrillary tangle
- MMSE
mini mental state exam
- LC
locus coeruleus
- NE
norepinephrine
- BPSD
behavioral and psychological symptoms of dementia
- NPI
neuropsychiatric inventory
- MIRAGE
multi-institutional research in Alzheimer’s genetic epidemiology
- CRF
corticotropin-releasing factor
- CSF
cerebrospinal fluid
- MCI
mild cognitive impairment
- Aβ
β-amyloid peptides
- ChAT
choline acetyltransferase
- VITA
Vienna-Transdanube Aging
- TH
tyrosine hydroxylase
- CRFR1
corticotropin-releasing factor receptor 1
- mPFC
medial prefronal cortex
- vMPFC
ventral medial prefrontal cortex
- PCC
posterior cingulate cortex
- pCu
precuneous
- IPL
inferior parietal lobe
- LTC
lateral temporal cortex
- dMPFC
dorsal medial prefrontal cortex
- HF
hippocampal formation
- fMRI
functional magnetic resonance imaging
- FDG-PET
[18F] Fludeoxyglucose positron emission tomography
- rs-fcMRI
resting state-functional connectivity magnetic resonance imaging
- CRF OE
corticotropin-releasing factor overexpressing
- PVN
paraventricular nucleus of the hypothalamus
- ACTH
adrenocorticotropic hormone
- GC
glucocorticoids
- BLA
basolateral nucleus of the amygdala
- CeA
central nucleus of the amygdala
- DOPA
L-1-3-4-dihydroxyphenylalanine
- DOPS
L-threo-3,4-dihydroxyphenylserine
- FST
forced swim test
- tau-p
phosphorylated tau
- 6-OHDA
6-hydroxydopamine
- PSD
post-synaptic density
- MAP
microtubule associated protein
- APP
amyloid precursor protein
- GSK-3B
glycogen synthase 3 beta kinase
- MAPK
mitogen activated protein kinase
- ERK
extracellular signal-regulated kinase
- cdk5
cyclin-dependent kinase
- JNK
c-Jun N-terminal kinase
- CREB
cyclic AMP response element binding protein
- BDNF
brain-derived neurotrophic factor
- AR
adrenergic receptors
- GABA
gamma-aminobutyric acid
- CTF
C-terminal fragments
- EEG
electroencephalogram
- LTD
long-term depression
- PKC
protein kinase C
- PSS
perceived stress scale
- PIB
Pittsburgh compound B
- PET
positron emission tomography
- PSAPP
presenilin 1 amyloid precursor protein
- IDE
insulin degrading enzyme
- ApoE
apolipoprotein E
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abeles M. Corticonics: Neural Circuits of the Cerebral Cortex. New York, NY: 1991. [Google Scholar]
- Abramov E, Dolev I, Fogel H, Ciccotosto GD, Ruff E, Slutsky I. Amyloid-beta as a positive endogenous regulator of release probability at hippocampal synapses. Nat Neurosci. 2009;12(12):1567–1576. doi: 10.1038/nn.2433. [DOI] [PubMed] [Google Scholar]
- Ahima RS, Harlan RE. Charting of type II glucocorticoid receptor-like immunoreactivity in the rat central nervous system. Neuroscience. 1990;39(3):579–604. doi: 10.1016/0306-4522(90)90244-x. [DOI] [PubMed] [Google Scholar]
- Ahima RS, Harlan RE. Differential corticosteroid regulation of type II glucocorticoid receptor-like immunoreactivity in the rat central nervous system: topography and implications. Endocrinology. 1991;129(1):226–236. doi: 10.1210/endo-129-1-226. [DOI] [PubMed] [Google Scholar]
- Alzheimer A. Über eine eigenartige Erkrankung der Hirnrinde. Allg Zschr Psychiatr Psych Gerichtl Med. 1907;64:146–148. [Google Scholar]
- Alzheimer A, Stelzmann RA, Schnitzlein HN, Murtagh FR. An English translation of Alzheimer’s 1907 paper, “Uber eine eigenartige Erkankung der Hirnrinde”. Clin Anat. 1995;8(6):429–431. doi: 10.1002/ca.980080612. [DOI] [PubMed] [Google Scholar]
- Alzheimer’s A. 2015 Alzheimer’s disease facts and figures. Alzheimers Dement. 2015;11(3):332–384. doi: 10.1016/j.jalz.2015.02.003. [DOI] [PubMed] [Google Scholar]
- Anticevic A, Cole MW, Murray JD, Corlett PR, Wang XJ, Krystal JH. The role of default network deactivation in cognition and disease. Trends Cogn Sci. 2012;16(12):584–592. doi: 10.1016/j.tics.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki C, Venkatesan C, Kurose H. Noradrenergic modulation of the prefrontal cortex as revealed by electron microscopic immunocytochemistry. Adv Pharmacol. 1998;42:777–780. doi: 10.1016/s1054-3589(08)60862-5. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Goldman-Rakic PS. Alpha 2-adrenergic mechanisms in prefrontal cortex associated with cognitive decline in aged nonhuman primates. Science. 1985;230(4731):1273–1276. doi: 10.1126/science.2999977. [DOI] [PubMed] [Google Scholar]
- Arsenault-Lapierre G, Chertkow H, Lupien S. Seasonal effects on cortisol secretion in normal aging, mild cognitive impairment and Alzheimer’s disease. Neurobiol Aging. 2010;31(6):1051–1054. doi: 10.1016/j.neurobiolaging.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. Adaptive gain and the role of the locus coeruleus-norepinephrine system in optimal performance. J Comp Neurol. 2005;493(1):99–110. doi: 10.1002/cne.20723. [DOI] [PubMed] [Google Scholar]
- Aznar S, Knudsen GM. Depression and Alzheimer’s disease: is stress the initiating factor in a common neuropathological cascade? J Alzheimers Dis. 2011;23(2):177–193. doi: 10.3233/JAD-2010-100390. [DOI] [PubMed] [Google Scholar]
- Balldin J, Gottfries CG, Karlsson I, Lindstedt G, Langstrom G, Walinder J. Dexamethasone suppression test and serum prolactin in dementia disorders. Br J Psychiatry. 1983;143:277–281. doi: 10.1192/bjp.143.3.277. [DOI] [PubMed] [Google Scholar]
- Banasr M, Duman RS. Regulation of neurogenesis and gliogenesis by stress and antidepressant treatment. CNS Neurol Disord Drug Targets. 2007;6(5):311–320. doi: 10.2174/187152707783220929. [DOI] [PubMed] [Google Scholar]
- Banasr M, Valentine GW, Li XY, Gourley SL, Taylor JR, Duman RS. Chronic unpredictable stress decreases cell proliferation in the cerebral cortex of the adult rat. Biol Psychiatry. 2007;62(5):496–504. doi: 10.1016/j.biopsych.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Bangasser DA, Curtis A, Reyes BA, Bethea TT, Parastatidis I, Ischiropoulos H, Van Bockstaele EJ, Valentino RJ. Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol Psychiatry. 2010;15(9):877, 896–904. doi: 10.1038/mp.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Wiersielis KR, Khantsis S. Sex differences in the locus coeruleus-norepinephrine system and its regulation by stress. Brain Res. 2016;1641(Pt B):177–188. doi: 10.1016/j.brainres.2015.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Zhang X, Garachh V, Hanhauser E, Valentino RJ. Sexual dimorphism in locus coeruleus dendritic morphology: a structural basis for sex differences in emotional arousal. Physiol Behav. 2011;103(3–4):342–351. doi: 10.1016/j.physbeh.2011.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeri MS, Rapp M, Schmeidler J, Reichenberg A, Purohit DP, Perl DP, Grossman HT, Prohovnik I, Haroutunian V, Silverman JM. Number of children is associated with neuropathology of Alzheimer’s disease in women. Neurobiol Aging. 2009;30(8):1184–1191. doi: 10.1016/j.neurobiolaging.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson DF, Kuhl DE, Hawkins RA, Phelps ME, Cummings JL, Tsai SY. The fluorodeoxyglucose 18F scan in Alzheimer’s disease and multi-infarct dementia. Arch Neurol. 1983;40(12):711–714. doi: 10.1001/archneur.1983.04050110029003. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev. 2003;42(1):33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- Bloss EB, Janssen WG, McEwen BS, Morrison JH. Interactive effects of stress and aging on structural plasticity in the prefrontal cortex. J Neurosci. 2010;30(19):6726–6731. doi: 10.1523/JNEUROSCI.0759-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloss EB, Janssen WG, Ohm DT, Yuk FJ, Wadsworth S, Saardi KM, McEwen BS, Morrison JH. Evidence for reduced experience-dependent dendritic spine plasticity in the aging prefrontal cortex. J Neurosci. 2011;31(21):7831–7839. doi: 10.1523/JNEUROSCI.0839-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondareff W, Mountjoy CQ, Roth M, Rossor MN, Iversen LL, Reynolds GP. Age and histopathologic heterogeneity in Alzheimer’s disease. Evidence for subtypes. Arch Gen Psychiatry. 1987;44(5):412–417. doi: 10.1001/archpsyc.1987.01800170026005. [DOI] [PubMed] [Google Scholar]
- Bondareff W, Mountjoy CQ, Roth M, Rossor MN, Iversen LL, Reynolds GP, Hauser DL. Neuronal degeneration in locus ceruleus and cortical correlates of Alzheimer disease. Alzheimer Dis Assoc Disord. 1987;1(4):256–262. doi: 10.1097/00002093-198701040-00005. [DOI] [PubMed] [Google Scholar]
- Bondi CO, Rodriguez G, Gould GG, Frazer A, Morilak DA. Chronic unpredictable stress induces a cognitive deficit and anxiety-like behavior in rats that is prevented by chronic antidepressant drug treatment. Neuropsychopharmacology. 2008;33(2):320–331. doi: 10.1038/sj.npp.1301410. [DOI] [PubMed] [Google Scholar]
- Bondi MW, Jak AJ, Delano-Wood L, Jacobson MW, Delis DC, Salmon DP. Neuropsychological contributions to the early identification of Alzheimer’s disease. Neuropsychol Rev. 2008;18(1):73–90. doi: 10.1007/s11065-008-9054-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnelle V, Ham TE, Leech R, Kinnunen KM, Mehta MA, Greenwood RJ, Sharp DJ. Salience network integrity predicts default mode network function after traumatic brain injury. Proc Natl Acad Sci U S A. 2012;109(12):4690–4695. doi: 10.1073/pnas.1113455109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borroni B, Grassi M, Archetti S, Costanzi C, Bianchi M, Caimi L, Caltagirone C, Di Luca M, Padovani A. BDNF genetic variations increase the risk of Alzheimer’s disease-related depression. J Alzheimers Dis. 2009;18(4):867–875. doi: 10.3233/JAD-2009-1191. [DOI] [PubMed] [Google Scholar]
- Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006;112(4):389–404. doi: 10.1007/s00401-006-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Demonstration of amyloid deposits and neurofibrillary changes in whole brain sections. Brain Pathol. 1991;1(3):213–216. doi: 10.1111/j.1750-3639.1991.tb00661.x. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K. Alzheimer’s pathogenesis: is there neuron-to-neuron propagation? Acta Neuropathol. 2011;121(5):589–595. doi: 10.1007/s00401-011-0825-z. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K. The pathological process underlying Alzheimer’s disease in individuals under thirty. Acta Neuropathol. 2011;121(2):171–181. doi: 10.1007/s00401-010-0789-4. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K. Where, when, and in what form does sporadic Alzheimer’s disease begin? Curr Opin Neurol. 2012;25(6):708–714. doi: 10.1097/WCO.0b013e32835a3432. [DOI] [PubMed] [Google Scholar]
- Braak H, Thal DR, Ghebremedhin E, Del Tredici K. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol. 2011;70(11):960–969. doi: 10.1097/NEN.0b013e318232a379. [DOI] [PubMed] [Google Scholar]
- Braak H, Thal DR, Matschke J, Ghebremedhin E, Del Tredici K. Age-related appearance of dendritic inclusions in catecholaminergic brainstem neurons. Neurobiol Aging. 2013;34(1):286–297. doi: 10.1016/j.neurobiolaging.2012.02.031. [DOI] [PubMed] [Google Scholar]
- Buckner RL. The brain’s default network: origins and implications for the study of psychosis. Dialogues Clin Neurosci. 2013;15(3):351–358. doi: 10.31887/DCNS.2013.15.3/rbuckner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, Sheline YI, Klunk WE, Mathis CA, Morris JC, Mintun MA. Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25(34):7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busche MA, Eichhoff G, Adelsberger H, Abramowski D, Wiederhold KH, Haass C, Staufenbiel M, Konnerth A, Garaschuk O. Clusters of hyperactive neurons near amyloid plaques in a mouse model of Alzheimer’s disease. Science. 2008;321(5896):1686–1689. doi: 10.1126/science.1162844. [DOI] [PubMed] [Google Scholar]
- Campbell SN, Zhang C, Monte L, Roe AD, Rice KC, Tache Y, Masliah E, Rissman RA. Increased tau phosphorylation and aggregation in the hippocampus of mice overexpressing corticotropin-releasing factor. J Alzheimers Dis. 2015;43(3):967–976. doi: 10.3233/JAD-141281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell SN, Zhang C, Roe AD, Lee N, Lao KU, Monte L, Donohue MC, Rissman RA. Impact of CRFR1 Ablation on Amyloid-beta Production and Accumulation in a Mouse Model of Alzheimer’s Disease. J Alzheimers Dis. 2015;45(4):1175–1184. doi: 10.3233/JAD-142844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey PD, Warwick J, Niehaus DJ, van der Linden G, van Heerden BB, Harvey BH, Seedat S, Stein DJ. Single photon emission computed tomography (SPECT) of anxiety disorders before and after treatment with citalopram. BMC Psychiatry. 2004;4:30. doi: 10.1186/1471-244X-4-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson LE, Sherwin BB, Chertkow HM. Relationships between dehydroepiandrosterone sulfate (DHEAS) and cortisol (CRT) plasma levels and everyday memory in Alzheimer’s disease patients compared to healthy controls. Horm Behav. 1999;35(3):254–263. doi: 10.1006/hbeh.1999.1518. [DOI] [PubMed] [Google Scholar]
- Cerqueira JJ, Catania C, Sotiropoulos I, Schubert M, Kalisch R, Almeida OF, Auer DP, Sousa N. Corticosteroid status influences the volume of the rat cingulate cortex - a magnetic resonance imaging study. J Psychiatr Res. 2005;39(5):451–460. doi: 10.1016/j.jpsychires.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Cerqueira JJ, Taipa R, Uylings HB, Almeida OF, Sousa N. Specific configuration of dendritic degeneration in pyramidal neurons of the medial prefrontal cortex induced by differing corticosteroid regimens. Cereb Cortex. 2007;17(9):1998–2006. doi: 10.1093/cercor/bhl108. [DOI] [PubMed] [Google Scholar]
- Chan-Palay V, Asan E. Alterations in catecholamine neurons of the locus coeruleus in senile dementia of the Alzheimer type and in Parkinson’s disease with and without dementia and depression. J Comp Neurol. 1989;287(3):373–392. doi: 10.1002/cne.902870308. [DOI] [PubMed] [Google Scholar]
- Chan-Palay V, Asan E. Quantitation of catecholamine neurons in the locus coeruleus in human brains of normal young and older adults and in depression. J Comp Neurol. 1989;287(3):357–372. doi: 10.1002/cne.902870307. [DOI] [PubMed] [Google Scholar]
- Chen Y, Peng Y, Che P, Gannon M, Liu Y, Li L, Bu G, van Groen T, Jiao K, Wang Q. alpha(2A) adrenergic receptor promotes amyloidogenesis through disrupting APP-SorLA interaction. Proc Natl Acad Sci U S A. 2014;111(48):17296–17301. doi: 10.1073/pnas.1409513111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirrito JR, Kang JE, Lee J, Stewart FR, Verges DK, Silverio LM, Bu G, Mennerick S, Holtzman DM. Endocytosis is required for synaptic activity-dependent release of amyloid-beta in vivo. Neuron. 2008;58(1):42–51. doi: 10.1016/j.neuron.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirrito JR, May PC, O’Dell MA, Taylor JW, Parsadanian M, Cramer JW, Audia JE, Nissen JS, Bales KR, Paul SM, DeMattos RB, Holtzman DM. In vivo assessment of brain interstitial fluid with microdialysis reveals plaque-associated changes in amyloid-beta metabolism and half-life. J Neurosci. 2003;23(26):8844–8853. doi: 10.1523/JNEUROSCI.23-26-08844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirrito JR, Yamada KA, Finn MB, Sloviter RS, Bales KR, May PC, Schoepp DD, Paul SM, Mennerick S, Holtzman DM. Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo. Neuron. 2005;48(6):913–922. doi: 10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- Combarros O, Warden DR, Hammond N, Cortina-Borja M, Belbin O, Lehmann MG, Wilcock GK, Brown K, Kehoe PG, Barber R, Coto E, Alvarez V, Deloukas P, Gwilliam R, Heun R, Kolsch H, Mateo I, Oulhaj A, Arias-Vasquez A, Schuur M, Aulchenko YS, Ikram MA, Breteler MM, van Duijn CM, Morgan K, Smith AD, Lehmann DJ. The dopamine beta-hydroxylase -1021C/T polymorphism is associated with the risk of Alzheimer’s disease in the Epistasis Project. BMC Med Genet. 2010;11:162. doi: 10.1186/1471-2350-11-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook SC, Wellman CL. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J Neurobiol. 2004;60(2):236–248. doi: 10.1002/neu.20025. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58(3):306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbo RM, Gambina G, Ulizzi L, Moretto G, Scacchi R. Genetic variation of CYP19 (aromatase) gene influences age at onset of Alzheimer’s disease in women. Dement Geriatr Cogn Disord. 2009;27(6):513–518. doi: 10.1159/000221832. [DOI] [PubMed] [Google Scholar]
- Craig MC, Murphy DG. Alzheimer’s disease in women. Best Pract Res Clin Obstet Gynaecol. 2009;23(1):53–61. doi: 10.1016/j.bpobgyn.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Cross AJ, Crow TJ, Perry EK, Perry RH, Blessed G, Tomlinson BE. Reduced dopamine-beta-hydroxylase activity in Alzheimer’s disease. Br Med J (Clin Res Ed) 1981;282(6258):93–94. doi: 10.1136/bmj.282.6258.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csernansky JG, Dong H, Fagan AM, Wang L, Xiong C, Holtzman DM, Morris JC. Plasma cortisol and progression of dementia in subjects with Alzheimer-type dementia. Am J Psychiatry. 2006;163(12):2164–2169. doi: 10.1176/appi.ajp.163.12.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KL, Davis BM, Greenwald BS, Mohs RC, Mathe AA, Johns CA, Horvath TB. Cortisol and Alzheimer’s disease, I: Basal studies. Am J Psychiatry. 1986;143(3):300–305. doi: 10.1176/ajp.143.3.300. [DOI] [PubMed] [Google Scholar]
- Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J Neurosci. 1993;13(9):3839–3847. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodt C, Dittmann J, Hruby J, Spath-Schwalbe E, Born J, Schuttler R, Fehm HL. Different regulation of adrenocorticotropin and cortisol secretion in young, mentally healthy elderly and patients with senile dementia of Alzheimer’s type. J Clin Endocrinol Metab. 1991;72(2):272–276. doi: 10.1210/jcem-72-2-272. [DOI] [PubMed] [Google Scholar]
- Dong H, Murphy KM, Meng L, Montalvo-Ortiz J, Zeng Z, Kolber BJ, Zhang S, Muglia LJ, Csernansky JG. Corticotrophin releasing factor accelerates neuropathology and cognitive decline in a mouse model of Alzheimer’s disease. J Alzheimers Dis. 2012;28(3):579–592. doi: 10.3233/JAD-2011-111328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging and neuropathological studies of depression: implications for the cognitive-emotional features of mood disorders. Curr Opin Neurobiol. 2001;11(2):240–249. doi: 10.1016/s0959-4388(00)00203-8. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213(1–2):93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitriu D, Hao J, Hara Y, Kaufmann J, Janssen WG, Lou W, Rapp PR, Morrison JH. Selective changes in thin spine density and morphology in monkey prefrontal cortex correlate with aging-related cognitive impairment. J Neurosci. 2010;30(22):7507–7515. doi: 10.1523/JNEUROSCI.6410-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elman JA, Madison CM, Baker SL, Vogel JW, Marks SM, Crowley S, O’Neil JP, Jagust WJ. Effects of Beta-Amyloid on Resting State Functional Connectivity Within and Between Networks Reflect Known Patterns of Regional Vulnerability. Cereb Cortex. 2016;26(2):695–707. doi: 10.1093/cercor/bhu259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forget H, Lacroix A, Somma M, Cohen H. Cognitive decline in patients with Cushing’s syndrome. J Int Neuropsychol Soc. 2000;6(1):20–29. doi: 10.1017/s1355617700611037. [DOI] [PubMed] [Google Scholar]
- Forstl H, Burns A, Luthert P, Cairns N, Lantos P, Levy R. Clinical and neuropathological correlates of depression in Alzheimer’s disease. Psychol Med. 1992;22(4):877–884. doi: 10.1017/s0033291700038459. [DOI] [PubMed] [Google Scholar]
- Franceschi M, Airaghi L, Gramigna C, Truci G, Manfredi MG, Canal N, Catania A. ACTH and cortisol secretion in patients with Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 1991;54(9):836–837. doi: 10.1136/jnnp.54.9.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman M, Green MF, Sharland DE. Assessment of hypothalamic-pituitary-adrenal function in the geriatric age group. J Gerontol. 1969;24(3):292–297. doi: 10.1093/geronj/24.3.292. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Rapp PR. The use of animal models to study the effects of aging on cognition. Annu Rev Psychol. 1997;48:339–370. doi: 10.1146/annurev.psych.48.1.339. [DOI] [PubMed] [Google Scholar]
- Gentili C, Ricciardi E, Gobbini MI, Santarelli MF, Haxby JV, Pietrini P, Guazzelli M. Beyond amygdala: Default Mode Network activity differs between patients with social phobia and healthy controls. Brain Res Bull. 2009;79(6):409–413. doi: 10.1016/j.brainresbull.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Gold PW. Stress-responsive neuromodulators. Biol Psychiatry. 1988;24(4):371–374. doi: 10.1016/0006-3223(88)90172-2. [DOI] [PubMed] [Google Scholar]
- Gold PW, Chrousos GP. Organization of the stress system and its dysregulation in melancholic and atypical depression: high vs low CRH/NE states. Mol Psychiatry. 2002;7(3):254–275. doi: 10.1038/sj.mp.4001032. [DOI] [PubMed] [Google Scholar]
- Gold PW, Gabry KE, Yasuda MR, Chrousos GP. Divergent endocrine abnormalities in melancholic and atypical depression: clinical and pathophysiologic implications. Endocrinol Metab Clin North Am. 2002;31(1):37–62. vi. doi: 10.1016/s0889-8529(01)00022-6. [DOI] [PubMed] [Google Scholar]
- Gold PW, Goodwin FK, Chrousos GP. Clinical and biochemical manifestations of depression. Relation to the neurobiology of stress (2) N Engl J Med. 1988;319(7):413–420. doi: 10.1056/NEJM198808183190706. [DOI] [PubMed] [Google Scholar]
- Green RC, Cupples LA, Kurz A, Auerbach S, Go R, Sadovnick D, Duara R, Kukull WA, Chui H, Edeki T, Griffith PA, Friedland RP, Bachman D, Farrer L. Depression as a risk factor for Alzheimer disease: the MIRAGE Study. Arch Neurol. 2003;60(5):753–759. doi: 10.1001/archneur.60.5.753. [DOI] [PubMed] [Google Scholar]
- Grunblatt E, Zehetmayer S, Bartl J, Loffler C, Wichart I, Rainer MK, Jungwirth S, Bauer P, Danielczyk W, Tragl KH, Riederer P, Fischer P. Genetic risk factors and markers for Alzheimer’s disease and/or depression in the VITA study. J Psychiatr Res. 2009;43(3):298–308. doi: 10.1016/j.jpsychires.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2(10):685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Haass C, Kaether C, Thinakaran G, Sisodia S. Trafficking and proteolytic processing of APP. Cold Spring Harb Perspect Med. 2012;2(5):a006270. doi: 10.1101/cshperspect.a006270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hains AB, Vu MA, Maciejewski PK, van Dyck CH, Gottron M, Arnsten AF. Inhibition of protein kinase C signaling protects prefrontal cortex dendritic spines and cognition from the effects of chronic stress. Proc Natl Acad Sci U S A. 2009;106(42):17957–17962. doi: 10.1073/pnas.0908563106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JP, Furman DJ, Chang C, Thomason ME, Dennis E, Gotlib IH. Default-mode and task-positive network activity in major depressive disorder: implications for adaptive and maladaptive rumination. Biol Psychiatry. 2011;70(4):327–333. doi: 10.1016/j.biopsych.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart DJ, Craig D, Compton SA, Critchlow S, Kerrigan BM, McIlroy SP, Passmore AP. A retrospective study of the behavioural and psychological symptoms of mid and late phase Alzheimer’s disease. Int J Geriatr Psychiatry. 2003;18(11):1037–1042. doi: 10.1002/gps.1013. [DOI] [PubMed] [Google Scholar]
- Hermans EJ, van Marle HJ, Ossewaarde L, Henckens MJ, Qin S, van Kesteren MT, Schoots VC, Cousijn H, Rijpkema M, Oostenveld R, Fernandez G. Stress-related noradrenergic activity prompts large-scale neural network reconfiguration. Science. 2011;334(6059):1151–1153. doi: 10.1126/science.1209603. [DOI] [PubMed] [Google Scholar]
- Herrmann N, Lanctot KL, Khan LR. The role of norepinephrine in the behavioral and psychological symptoms of dementia. J Neuropsychiatry Clin Neurosci. 2004;16(3):261–276. doi: 10.1176/jnp.16.3.261. [DOI] [PubMed] [Google Scholar]
- Hong L, Huang HC, Jiang ZF. Relationship between amyloid-beta and the ubiquitin-proteasome system in Alzheimer’s disease. Neurol Res. 2014;36(3):276–282. doi: 10.1179/1743132813Y.0000000288. [DOI] [PubMed] [Google Scholar]
- Hoogendijk WJ, I, Sommer E, Pool CW, Kamphorst W, Hofman MA, Eikelenboom P, Swaab DF. Lack of association between depression and loss of neurons in the locus coeruleus in Alzheimer disease. Arch Gen Psychiatry. 1999;56(1):45–51. doi: 10.1001/archpsyc.56.1.45. [DOI] [PubMed] [Google Scholar]
- Huang CW, Lui CC, Chang WN, Lu CH, Wang YL, Chang CC. Elevated basal cortisol level predicts lower hippocampal volume and cognitive decline in Alzheimer’s disease. J Clin Neurosci. 2009;16(10):1283–1286. doi: 10.1016/j.jocn.2008.12.026. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, Petersen RC, Trojanowski JQ. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9(1):119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson L, Guo X, Waern M, Ostling S, Gustafson D, Bengtsson C, Skoog I. Midlife psychological stress and risk of dementia: a 35-year longitudinal population study. Brain. 2010;133(Pt 8):2217–2224. doi: 10.1093/brain/awq116. [DOI] [PubMed] [Google Scholar]
- Juruena MF, Cleare AJ, Papadopoulos AS, Poon L, Lightman S, Pariante CM. Different responses to dexamethasone and prednisolone in the same depressed patients. Psychopharmacology (Berl) 2006;189(2):225–235. doi: 10.1007/s00213-006-0555-4. [DOI] [PubMed] [Google Scholar]
- Jutras MJ, Buffalo EA. Synchronous neural activity and memory formation. Curr Opin Neurobiol. 2010;20(2):150–155. doi: 10.1016/j.conb.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JE, Cirrito JR, Dong H, Csernansky JG, Holtzman DM. Acute stress increases interstitial fluid amyloid-beta via corticotropin-releasing factor and neuronal activity. Proc Natl Acad Sci U S A. 2007;104(25):10673–10678. doi: 10.1073/pnas.0700148104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendell SF, Krystal JH, Sanacora G. GABA and glutamate systems as therapeutic targets in depression and mood disorders. Expert Opin Ther Targets. 2005;9(1):153–168. doi: 10.1517/14728222.9.1.153. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Kessler RC, Walters EE, MacLean C, Neale MC, Heath AC, Eaves LJ. Stressful life events, genetic liability, and onset of an episode of major depression in women. Am J Psychiatry. 1995;152(6):833–842. doi: 10.1176/ajp.152.6.833. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitayama I, Nakamura S, Yaga T, Murase S, Nomura J, Kayahara T, Nakano K. Degeneration of locus coeruleus axons in stress-induced depression model. Brain Res Bull. 1994;35(5–6):573–580. doi: 10.1016/0361-9230(94)90171-6. [DOI] [PubMed] [Google Scholar]
- Kitayama IT, Otani M, Murase S. Degeneration of the locus ceruleus noradrenergic neurons in the stress-induced depression of rats. Ann N Y Acad Sci. 2008;1148:95–98. doi: 10.1196/annals.1410.059. [DOI] [PubMed] [Google Scholar]
- Klimek V, Stockmeier C, Overholser J, Meltzer HY, Kalka S, Dilley G, Ordway GA. Reduced levels of norepinephrine transporters in the locus coeruleus in major depression. J Neurosci. 1997;17(21):8451–8458. doi: 10.1523/JNEUROSCI.17-21-08451.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Bergstrom M, Savitcheva I, Huang GF, Estrada S, Ausen B, Debnath ML, Barletta J, Price JC, Sandell J, Lopresti BJ, Wall A, Koivisto P, Antoni G, Mathis CA, Langstrom B. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55(3):306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- Koch K, Myers NE, Gottler J, Pasquini L, Grimmer T, Forster S, Manoliu A, Neitzel J, Kurz A, Forstl H, Riedl V, Wohlschlager AM, Drzezga A, Sorg C. Disrupted Intrinsic Networks Link Amyloid-beta Pathology and Impaired Cognition in Prodromal Alzheimer’s Disease. Cereb Cortex. 2015;25(12):4678–4688. doi: 10.1093/cercor/bhu151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo S, Okabe S. Turnover of synapse and dynamic nature of synaptic molecules in vitro and in vivo. Acta Histochem Cytochem. 2011;44(1):9–15. doi: 10.1267/ahc.10035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koran ME, Wagener M, Hohman TJ I. Alzheimer’s Neuroimaging. Sex differences in the association between AD biomarkers and cognitive decline. Brain Imaging Behav. 2016 doi: 10.1007/s11682-016-9523-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugaya A, Sanacora G. Beyond monoamines: glutamatergic function in mood disorders. CNS Spectr. 2005;10(10):808–819. doi: 10.1017/s1092852900010403. [DOI] [PubMed] [Google Scholar]
- Lara VP, Caramelli P, Teixeira AL, Barbosa MT, Carmona KC, Carvalho MG, Fernandes AP, Gomes KB. High cortisol levels are associated with cognitive impairment no-dementia (CIND) and dementia. Clin Chim Acta. 2013;423:18–22. doi: 10.1016/j.cca.2013.04.013. [DOI] [PubMed] [Google Scholar]
- Le MH, Weissmiller AM, Monte L, Lin PH, Hexom TC, Natera O, Wu C, Rissman RA. Functional Impact of Corticotropin-Releasing Factor Exposure on Tau Phosphorylation and Axon Transport. PLoS One. 2016;11(1):e0147250. doi: 10.1371/journal.pone.0147250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemogne C, Delaveau P, Freton M, Guionnet S, Fossati P. Medial prefrontal cortex and the self in major depression. J Affect Disord. 2012;136(1–2):e1–e11. doi: 10.1016/j.jad.2010.11.034. [DOI] [PubMed] [Google Scholar]
- Li S, Hong S, Shepardson NE, Walsh DM, Shankar GM, Selkoe D. Soluble oligomers of amyloid Beta protein facilitate hippocampal long-term depression by disrupting neuronal glutamate uptake. Neuron. 2009;62(6):788–801. doi: 10.1016/j.neuron.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, McEwen BS, Casey BJ. Psychosocial stress reversibly disrupts prefrontal processing and attentional control. Proc Natl Acad Sci U S A. 2009;106(3):912–917. doi: 10.1073/pnas.0807041106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien S, Lecours AR, Schwartz G, Sharma S, Hauger RL, Meaney MJ, Nair NP. Longitudinal study of basal cortisol levels in healthy elderly subjects: evidence for subgroups. Neurobiol Aging. 1996;17(1):95–105. doi: 10.1016/0197-4580(95)02005-5. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, de Leon M, de Santi S, Convit A, Tarshish C, Nair NP, Thakur M, McEwen BS, Hauger RL, Meaney MJ. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat Neurosci. 1998;1(1):69–73. doi: 10.1038/271. [DOI] [PubMed] [Google Scholar]
- Maddock RJ, Garrett AS, Buonocore MH. Posterior cingulate cortex activation by emotional words: fMRI evidence from a valence decision task. Hum Brain Mapp. 2003;18(1):30–41. doi: 10.1002/hbm.10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda K, Tanimoto K, Terada T, Shintani T, Kakigi T. Elevated urinary free cortisol in patients with dementia. Neurobiol Aging. 1991;12(2):161–163. doi: 10.1016/0197-4580(91)90055-o. [DOI] [PubMed] [Google Scholar]
- Mander BA, Marks SM, Vogel JW, Rao V, Lu B, Saletin JM, Ancoli-Israel S, Jagust WJ, Walker MP. beta-amyloid disrupts human NREM slow waves and related hippocampus-dependent memory consolidation. Nat Neurosci. 2015;18(7):1051–1057. doi: 10.1038/nn.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martignoni E, Petraglia F, Costa A, Monzani A, Genazzani AR, Nappi G. Cerebrospinal fluid corticotropin-releasing factor levels and stimulation test in dementia of the Alzheimer type. J Clin Lab Anal. 1990;4(1):5–8. doi: 10.1002/jcla.1860040104. [DOI] [PubMed] [Google Scholar]
- Masters CL, Selkoe DJ. Biochemistry of amyloid beta-protein and amyloid deposits in Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2(6):a006262. doi: 10.1101/cshperspect.a006262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masugi F, Ogihara T, Sakaguchi K, Otsuka A, Tsuchiya Y, Morimoto S, Kumahara Y, Saeki S, Nishide M. High plasma levels of cortisol in patients with senile dementia of the Alzheimer’s type. Methods Find Exp Clin Pharmacol. 1989;11(11):707–710. [PubMed] [Google Scholar]
- McCulley MC, I, Day N, Holmes C. Association between interleukin 1-beta promoter (-511) polymorphism and depressive symptoms in Alzheimer’s disease. Am J Med Genet B Neuropsychiatr Genet. 2004;124B(1):50–53. doi: 10.1002/ajmg.b.20086. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Glucocorticoid receptors in the brain. Hosp Pract (Off Ed) 1988;23(8):107–111. 114, 119–121. doi: 10.1080/21548331.1988.11703523. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Sex, stress and the hippocampus: allostasis, allostatic load and the aging process. Neurobiol Aging. 2002;23(5):921–939. doi: 10.1016/s0197-4580(02)00027-1. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Glucocorticoids, depression, and mood disorders: structural remodeling in the brain. Metabolism. 2005;54(5 Suppl 1):20–23. doi: 10.1016/j.metabol.2005.01.008. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Bowles NP, Gray JD, Hill MN, Hunter RG, Karatsoreos IN, Nasca C. Mechanisms of stress in the brain. Nat Neurosci. 2015;18(10):1353–1363. doi: 10.1038/nn.4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Gianaros PJ. Stress- and allostasis-induced brain plasticity. Annu Rev Med. 2011;62:431–445. doi: 10.1146/annurev-med-052209-100430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Magarinos AM. Stress and hippocampal plasticity: implications for the pathophysiology of affective disorders. Hum Psychopharmacol. 2001;16(S1):S7–S19. doi: 10.1002/hup.266. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Morrison JH. The brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course. Neuron. 2013;79(1):16–29. doi: 10.1016/j.neuron.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Nasca C, Gray JD. Stress Effects on Neuronal Structure: Hippocampus, Amygdala, and Prefrontal Cortex. Neuropsychopharmacology. 2016;41(1):3–23. doi: 10.1038/npp.2015.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeith I, Cummings J. Behavioural changes and psychological symptoms in dementia disorders. Lancet Neurol. 2005;4(11):735–742. doi: 10.1016/S1474-4422(05)70219-2. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Yoon JH, Carter CS. Modafinil modulation of the default mode network. Psychopharmacology (Berl) 2011;215(1):23–31. doi: 10.1007/s00213-010-2111-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogg K, Bradbury KE, Bradley BP. Interpretation of ambiguous information in clinical depression. Behav Res Ther. 2006;44(10):1411–1419. doi: 10.1016/j.brat.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Murray ME, Graff-Radford NR, Ross OA, Petersen RC, Duara R, Dickson DW. Neuropathologically defined subtypes of Alzheimer’s disease with distinct clinical characteristics: a retrospective study. Lancet Neurol. 2011;10(9):785–796. doi: 10.1016/S1474-4422(11)70156-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers N, Pasquini L, Gottler J, Grimmer T, Koch K, Ortner M, Neitzel J, Muhlau M, Forster S, Kurz A, Forstl H, Zimmer C, Wohlschlager AM, Riedl V, Drzezga A, Sorg C. Within-patient correspondence of amyloid-beta and intrinsic network connectivity in Alzheimer’s disease. Brain. 2014;137(Pt 7):2052–2064. doi: 10.1093/brain/awu103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeroff CB, Widerlov E, Bissette G, Walleus H, Karlsson I, Eklund K, Kilts CD, Loosen PT, Vale W. Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science. 1984;226(4680):1342–1344. doi: 10.1126/science.6334362. [DOI] [PubMed] [Google Scholar]
- Newcomer JW, Selke G, Melson AK, Hershey T, Craft S, Richards K, Alderson AL. Decreased memory performance in healthy humans induced by stress-level cortisol treatment. Arch Gen Psychiatry. 1999;56(6):527–533. doi: 10.1001/archpsyc.56.6.527. [DOI] [PubMed] [Google Scholar]
- Ni Y, Zhao X, Bao G, Zou L, Teng L, Wang Z, Song M, Xiong J, Bai Y, Pei G. Activation of beta2-adrenergic receptor stimulates gamma-secretase activity and accelerates amyloid plaque formation. Nat Med. 2006;12(12):1390–1396. doi: 10.1038/nm1485. [DOI] [PubMed] [Google Scholar]
- O’Brien JT, Schweitzer I, Ames D, Tuckwell V, Mastwyk M. Cortisol suppression by dexamethasone in the healthy elderly: effects of age, dexamethasone levels, and cognitive function. Biol Psychiatry. 1994;36(6):389–394. doi: 10.1016/0006-3223(94)91214-9. [DOI] [PubMed] [Google Scholar]
- Oakley RH, Cidlowski JA. The biology of the glucocorticoid receptor: new signaling mechanisms in health and disease. J Allergy Clin Immunol. 2013;132(5):1033–1044. doi: 10.1016/j.jaci.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordway GA, Schenk J, Stockmeier CA, May W, Klimek V. Elevated agonist binding to alpha2-adrenoceptors in the locus coeruleus in major depression. Biol Psychiatry. 2003;53(4):315–323. doi: 10.1016/s0006-3223(02)01728-6. [DOI] [PubMed] [Google Scholar]
- Ordway GA, Smith KS, Haycock JW. Elevated tyrosine hydroxylase in the locus coeruleus of suicide victims. J Neurochem. 1994;62(2):680–685. doi: 10.1046/j.1471-4159.1994.62020680.x. [DOI] [PubMed] [Google Scholar]
- Ordway GA, Stockmeier CA, Cason GW, Klimek V. Pharmacology and distribution of norepinephrine transporters in the human locus coeruleus and raphe nuclei. J Neurosci. 1997;17(5):1710–1719. doi: 10.1523/JNEUROSCI.17-05-01710.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ownby RL, Crocco E, Acevedo A, John V, Loewenstein D. Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Arch Gen Psychiatry. 2006;63(5):530–538. doi: 10.1001/archpsyc.63.5.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxenkrug GF, Gurevich D, Siegel B, Dumlao MS, Gershon S. Correlation between brain-adrenal axis activation and cognitive impairment in Alzheimer’s disease: is there a gender effect? Psychiatry Res. 1989;29(2):169–175. doi: 10.1016/0165-1781(89)90031-0. [DOI] [PubMed] [Google Scholar]
- Palop JJ, Chin J, Roberson ED, Wang J, Thwin MT, Bien-Ly N, Yoo J, Ho KO, Yu GQ, Kreitzer A, Finkbeiner S, Noebels JL, Mucke L. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer’s disease. Neuron. 2007;55(5):697–711. doi: 10.1016/j.neuron.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palop JJ, Mucke L. Amyloid-beta-induced neuronal dysfunction in Alzheimer’s disease: from synapses toward neural networks. Nat Neurosci. 2010;13(7):812–818. doi: 10.1038/nn.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palop JJ, Mucke L. Synaptic depression and aberrant excitatory network activity in Alzheimer’s disease: two faces of the same coin? Neuromolecular Med. 2010;12(1):48–55. doi: 10.1007/s12017-009-8097-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardon MC. Therapeutic potential of some stress mediators in early Alzheimer’s disease. Exp Gerontol. 2011;46(2–3):170–173. doi: 10.1016/j.exger.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Peavy GM, Lange KL, Salmon DP, Patterson TL, Goldman S, Gamst AC, Mills PJ, Khandrika S, Galasko D. The effects of prolonged stress and APOE genotype on memory and cortisol in older adults. Biol Psychiatry. 2007;62(5):472–478. doi: 10.1016/j.biopsych.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peskind ER, Wilkinson CW, Petrie EC, Schellenberg GD, Raskind MA. Increased CSF cortisol in AD is a function of APOE genotype. Neurology. 2001;56(8):1094–1098. doi: 10.1212/wnl.56.8.1094. [DOI] [PubMed] [Google Scholar]
- Pomara N, Sidtis J. Does cortical thinning in persons at increased risk for major depression also increase their risk for Alzheimer’s disease? Proc Natl Acad Sci U S A. 2009;106(29):E82. doi: 10.1073/pnas.0903660106. author reply E83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popp J, Wolfsgruber S, Heuser I, Peters O, Hull M, Schroder J, Moller HJ, Lewczuk P, Schneider A, Jahn H, Luckhaus C, Perneczky R, Frolich L, Wagner M, Maier W, Wiltfang J, Kornhuber J, Jessen F. Cerebrospinal fluid cortisol and clinical disease progression in MCI and dementia of Alzheimer’s type. Neurobiol Aging. 2015;36(2):601–607. doi: 10.1016/j.neurobiolaging.2014.10.031. [DOI] [PubMed] [Google Scholar]
- Porter VR, Buxton WG, Fairbanks LA, Strickland T, O’Connor SM, Rosenberg-Thompson S, Cummings JL. Frequency and characteristics of anxiety among patients with Alzheimer’s disease and related dementias. J Neuropsychiatry Clin Neurosci. 2003;15(2):180–186. doi: 10.1176/jnp.15.2.180. [DOI] [PubMed] [Google Scholar]
- Post RM, Lake CR, Jimerson DC, Bunney WE, Wood JH, Ziegler MG, Goodwin FK. Cerebrospinal fluid norepinephrine in affective illness. Am J Psychiatry. 1978;135(8):907–912. doi: 10.1176/ajp.135.8.907. [DOI] [PubMed] [Google Scholar]
- Prevention, A. F. f. S. Facts and Figures. 2015 Retrieved May 10, 2017, from https://www.theovernight.org/?fuseaction=cms.page&id=1034.
- Raadsheer FC, Oorschot DE, Verwer RW, Tilders FJ, Swaab DF. Age-related increase in the total number of corticotropin-releasing hormone neurons in the human paraventricular nucleus in controls and Alzheimer’s disease: comparison of the disector with an unfolding method. J Comp Neurol. 1994;339(3):447–457. doi: 10.1002/cne.903390311. [DOI] [PubMed] [Google Scholar]
- Raadsheer FC, Sluiter AA, Ravid R, Tilders FJ, Swaab DF. Localization of corticotropin-releasing hormone (CRH) neurons in the paraventricular nucleus of the human hypothalamus; age-dependent colocalization with vasopressin. Brain Res. 1993;615(1):50–62. doi: 10.1016/0006-8993(93)91113-7. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Miller M, Janssen WG, Liston C, Hof PR, McEwen BS, Morrison JH. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex. 2006;16(3):313–320. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Rodriguez A, Ehlenberger DB, Dammann M, McEwen BS, Morrison JH, Wearne SL, Hof PR. Repeated stress alters dendritic spine morphology in the rat medial prefrontal cortex. J Comp Neurol. 2008;507(1):1141–1150. doi: 10.1002/cne.21588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Sisti HM, Hao J, Rocher AB, McCall T, Hof PR, McEwen BS, Morrison JH. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125(1):1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Raichle ME. The brain’s default mode network. Annu Rev Neurosci. 2015;38:433–447. doi: 10.1146/annurev-neuro-071013-014030. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmuson S, Andrew R, Nasman B, Seckl JR, Walker BR, Olsson T. Increased glucocorticoid production and altered cortisol metabolism in women with mild to moderate Alzheimer’s disease. Biol Psychiatry. 2001;49(6):547–552. doi: 10.1016/s0006-3223(00)01015-5. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Caselli RJ, Yun LS, Chen K, Bandy D, Minoshima S, Thibodeau SN, Osborne D. Preclinical evidence of Alzheimer’s disease in persons homozygous for the epsilon 4 allele for apolipoprotein E. N Engl J Med. 1996;334(12):752–758. doi: 10.1056/NEJM199603213341202. [DOI] [PubMed] [Google Scholar]
- Reyes BA, Bangasser DA, Valentino RJ, Van Bockstaele EJ. Using high resolution imaging to determine trafficking of corticotropin-releasing factor receptors in noradrenergic neurons of the rat locus coeruleus. Life Sci. 2014;112(1–2):2–9. doi: 10.1016/j.lfs.2014.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes BA, Valentino RJ, Van Bockstaele EJ. Stress-induced intracellular trafficking of corticotropin-releasing factor receptors in rat locus coeruleus neurons. Endocrinology. 2008;149(1):122–130. doi: 10.1210/en.2007-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissman RA. Stress-induced tau phosphorylation: functional neuroplasticity or neuronal vulnerability? J Alzheimers Dis. 2009;18(2):453–457. doi: 10.3233/JAD-2009-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissman RA, Lee KF, Vale W, Sawchenko PE. Corticotropin-releasing factor receptors differentially regulate stress-induced tau phosphorylation. J Neurosci. 2007;27(24):6552–6562. doi: 10.1523/JNEUROSCI.5173-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissman RA, Staup MA, Lee AR, Justice NJ, Rice KC, Vale W, Sawchenko PE. Corticotropin-releasing factor receptor-dependent effects of repeated stress on tau phosphorylation, solubility, and aggregation. Proc Natl Acad Sci U S A. 2012;109(16):6277–6282. doi: 10.1073/pnas.1203140109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross JA, McGonigle P, Van Bockstaele EJ. Locus Coeruleus, norepinephrine and Abeta peptides in Alzheimer’s disease. Neurobiol Stress. 2015;2:73–84. doi: 10.1016/j.ynstr.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A, Pickar D, De Jong J, Karoum F, Linnoila M. Norepinephrine and its metabolites in cerebrospinal fluid, plasma, and urine. Relationship to hypothalamic-pituitary-adrenal axis function in depression. Arch Gen Psychiatry. 1988;45(9):849–857. doi: 10.1001/archpsyc.1988.01800330081010. [DOI] [PubMed] [Google Scholar]
- Roy A, Pickar D, Linnoila M, Chrousos GP, Gold PW. Cerebrospinal fluid corticotropin-releasing hormone in depression: relationship to noradrenergic function. Psychiatry Res. 1987;20(3):229–237. doi: 10.1016/0165-1781(87)90083-7. [DOI] [PubMed] [Google Scholar]
- Scahill RI, Schott JM, Stevens JM, Rossor MN, Fox NC. Mapping the evolution of regional atrophy in Alzheimer’s disease: unbiased analysis of fluid-registered serial MRI. Proc Natl Acad Sci U S A. 2002;99(7):4703–4707. doi: 10.1073/pnas.052587399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheeringa R, Bastiaansen MC, Petersson KM, Oostenveld R, Norris DG, Hagoort P. Frontal theta EEG activity correlates negatively with the default mode network in resting state. Int J Psychophysiol. 2008;67(3):242–251. doi: 10.1016/j.ijpsycho.2007.05.017. [DOI] [PubMed] [Google Scholar]
- Scullion GA, Kendall DA, Marsden CA, Sunter D, Pardon MC. Chronic treatment with the alpha2-adrenoceptor antagonist fluparoxan prevents age-related deficits in spatial working memory in APPxPS1 transgenic mice without altering beta-amyloid plaque load or astrocytosis. Neuropharmacology. 2011;60(2–3):223–234. doi: 10.1016/j.neuropharm.2010.09.002. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science. 2002;298(5594):789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol. 1989;290(2):213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- Sestieri C, Corbetta M, Romani GL, Shulman GL. Episodic memory retrieval, parietal cortex, and the default mode network: functional and topographic analyses. J Neurosci. 2011;31(12):4407–4420. doi: 10.1523/JNEUROSCI.3335-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shansky RM, Hamo C, Hof PR, McEwen BS, Morrison JH. Stress-induced dendritic remodeling in the prefrontal cortex is circuit specific. Cereb Cortex. 2009;19(10):2479–2484. doi: 10.1093/cercor/bhp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M, Palacios-Bois J, Schwartz G, Iskandar H, Thakur M, Quirion R, Nair NP. Circadian rhythms of melatonin and cortisol in aging. Biol Psychiatry. 1989;25(3):305–319. doi: 10.1016/0006-3223(89)90178-9. [DOI] [PubMed] [Google Scholar]
- Shekhar A, Truitt W, Rainnie D, Sajdyk T. Role of stress, corticotrophin releasing factor (CRF) and amygdala plasticity in chronic anxiety. Stress. 2005;8(4):209–219. doi: 10.1080/10253890500504557. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Price JL, Rundle MM, Vaishnavi SN, Snyder AZ, Mintun MA, Wang S, Coalson RS, Raichle ME. The default mode network and self-referential processes in depression. Proc Natl Acad Sci U S A. 2009;106(6):1942–1947. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Raichle ME, Snyder AZ, Morris JC, Head D, Wang S, Mintun MA. Amyloid plaques disrupt resting state default mode network connectivity in cognitively normal elderly. Biol Psychiatry. 2010;67(6):584–587. doi: 10.1016/j.biopsych.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW. Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci U S A. 1996;93(9):3908–3913. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallwood J, Brown KS, Baird B, Mrazek MD, Franklin MS, Schooler JW. Insulation for daydreams: a role for tonic norepinephrine in the facilitation of internally guided thought. PLoS One. 2012;7(4):e33706. doi: 10.1371/journal.pone.0033706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY, Nairn AC, Salter MW, Lombroso PJ, Gouras GK, Greengard P. Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci. 2005;8(8):1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- Soares JM, Sampaio A, Ferreira LM, Santos NC, Marques F, Palha JA, Cerqueira JJ, Sousa N. Stress-induced changes in human decision-making are reversible. Transl Psychiatry. 2012;2:e131. doi: 10.1038/tp.2012.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares JM, Sampaio A, Ferreira LM, Santos NC, Marques P, Marques F, Palha JA, Cerqueira JJ, Sousa N. Stress Impact on Resting State Brain Networks. PLoS One. 2013;8(6):e66500. doi: 10.1371/journal.pone.0066500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa N, Almeida OF. Disconnection and reconnection: the morphological basis of (mal)adaptation to stress. Trends Neurosci. 2012;35(12):742–751. doi: 10.1016/j.tins.2012.08.006. [DOI] [PubMed] [Google Scholar]
- Southwick SM, Paige S, Morgan CA, 3rd, Bremner JD, Krystal JH, Charney DS. Neurotransmitter alterations in PTSD: catecholamines and serotonin. Semin Clin Neuropsychiatry. 1999;4(4):242–248. doi: 10.153/SCNP00400242. [DOI] [PubMed] [Google Scholar]
- Souza-Talarico JN, Chaves EC, Lupien SJ, Nitrini R, Caramelli P. Relationship between cortisol levels and memory performance may be modulated by the presence or absence of cognitive impairment: evidence from healthy elderly, mild cognitive impairment and Alzheimer’s disease subjects. J Alzheimers Dis. 2010;19(3):839–848. doi: 10.3233/JAD-2010-1282. [DOI] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A. 2008;105(34):12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkman MN, Gebarski SS, Berent S, Schteingart DE. Hippocampal formation volume, memory dysfunction, and cortisol levels in patients with Cushing’s syndrome. Biol Psychiatry. 1992;32(9):756–765. doi: 10.1016/0006-3223(92)90079-f. [DOI] [PubMed] [Google Scholar]
- Starkman MN, Giordani B, Berent S, Schork MA, Schteingart DE. Elevated cortisol levels in Cushing’s disease are associated with cognitive decrements. Psychosom Med. 2001;63(6):985–993. doi: 10.1097/00006842-200111000-00018. [DOI] [PubMed] [Google Scholar]
- Stefani A, Olivola E, Liguori C, Hainsworth AH, Saviozzi V, Angileri G, D’Angelo V, Galati S, Pierantozzi M. Catecholamine-Based Treatment in AD Patients: Expectations and Delusions. Front Aging Neurosci. 2015;7:67. doi: 10.3389/fnagi.2015.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surguladze S, Brammer MJ, Keedwell P, Giampietro V, Young AW, Travis MJ, Williams SC, Phillips ML. A differential pattern of neural response toward sad versus happy facial expressions in major depressive disorder. Biol Psychiatry. 2005;57(3):201–209. doi: 10.1016/j.biopsych.2004.10.028. [DOI] [PubMed] [Google Scholar]
- Swanwick GR, Kirby M, Bruce I, Buggy F, Coen RF, Coakley D, Lawlor BA. Hypothalamic-pituitary-adrenal axis dysfunction in Alzheimer’s disease: lack of association between longitudinal and cross-sectional findings. Am J Psychiatry. 1998;155(2):286–289. doi: 10.1176/ajp.155.2.286. [DOI] [PubMed] [Google Scholar]
- Szabo ST, de Montigny C, Blier P. Progressive attenuation of the firing activity of locus coeruleus noradrenergic neurons by sustained administration of selective serotonin reuptake inhibitors. Int J Neuropsychopharmacol. 2000;3(1):1–11. doi: 10.1017/S1461145700001772. [DOI] [PubMed] [Google Scholar]
- Szot P, Franklin A, Miguelez C, Wang Y, Vidaurrazaga I, Ugedo L, Sikkema C, Wilkinson CW, Raskind MA. Depressive-like behavior observed with a minimal loss of locus coeruleus (LC) neurons following administration of 6-hydroxydopamine is associated with electrophysiological changes and reversed with precursors of norepinephrine. Neuropharmacology. 2016;101:76–86. doi: 10.1016/j.neuropharm.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szot P, White SS, Greenup JL, Leverenz JB, Peskind ER, Raskind MA. Compensatory changes in the noradrenergic nervous system in the locus ceruleus and hippocampus of postmortem subjects with Alzheimer’s disease and dementia with Lewy bodies. J Neurosci. 2006;26(2):467–478. doi: 10.1523/JNEUROSCI.4265-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YY, Rothbart MK, Posner MI. Neural correlates of establishing, maintaining, and switching brain states. Trends Cogn Sci. 2012;16(6):330–337. doi: 10.1016/j.tics.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thal DR, Rub U, Orantes M, Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58(12):1791–1800. doi: 10.1212/wnl.58.12.1791. [DOI] [PubMed] [Google Scholar]
- Thathiah A, Horre K, Snellinx A, Vandewyer E, Huang Y, Ciesielska M, De Kloe G, Munck S, De Strooper B. beta-arrestin 2 regulates Abeta generation and gamma-secretase activity in Alzheimer’s disease. Nat Med. 2013;19(1):43–49. doi: 10.1038/nm.3023. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Brosschot JF. Psychosomatics and psychopathology: looking up and down from the brain. Psychoneuroendocrinology. 2005;30(10):1050–1058. doi: 10.1016/j.psyneuen.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Sternberg E. Beyond heart rate variability: vagal regulation of allostatic systems. Ann N Y Acad Sci. 2006;1088:361–372. doi: 10.1196/annals.1366.014. [DOI] [PubMed] [Google Scholar]
- Thompson SA, Patterson K, Hodges JR. Left/right asymmetry of atrophy in semantic dementia: behavioral-cognitive implications. Neurology. 2003;61(9):1196–1203. doi: 10.1212/01.wnl.0000091868.28557.b8. [DOI] [PubMed] [Google Scholar]
- Ulrich D. Amyloid-beta Impairs Synaptic Inhibition via GABA(A) Receptor Endocytosis. J Neurosci. 2015;35(24):9205–9210. doi: 10.1523/JNEUROSCI.0950-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umegaki H, Ikari H, Nakahata H, Endo H, Suzuki Y, Ogawa O, Nakamura A, Yamamoto T, Iguchi A. Plasma cortisol levels in elderly female subjects with Alzheimer’s disease: a cross-sectional and longitudinal study. Brain Res. 2000;881(2):241–243. doi: 10.1016/s0006-8993(00)02847-x. [DOI] [PubMed] [Google Scholar]
- Valdizan EM, Diez-Alarcia R, Gonzalez-Maeso J, Pilar-Cuellar F, Garcia-Sevilla JA, Meana JJ, Pazos A. alpha(2)-Adrenoceptor functionality in postmortem frontal cortex of depressed suicide victims. Biol Psychiatry. 2010;68(9):869–872. doi: 10.1016/j.biopsych.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino RJ, Van Bockstaele E. Convergent regulation of locus coeruleus activity as an adaptive response to stress. Eur J Pharmacol. 2008;583(2–3):194–203. doi: 10.1016/j.ejphar.2007.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino RJ, Van Bockstaele E, Bangasser D. Sex-specific cell signaling: the corticotropin-releasing factor receptor model. Trends Pharmacol Sci. 2013;34(8):437–444. doi: 10.1016/j.tips.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Jadhav S, Chattarji S. Prolonged behavioral stress enhances synaptic connectivity in the basolateral amygdala. Neuroscience. 2006;143(2):387–393. doi: 10.1016/j.neuroscience.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22(15):6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci. 2005;9(9):445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Wang D, Yuen EY, Zhou Y, Yan Z, Xiang YK. Amyloid beta peptide-(1–42) induces internalization and degradation of beta2 adrenergic receptors in prefrontal cortical neurons. J Biol Chem. 2011;286(36):31852–31863. doi: 10.1074/jbc.M111.244335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner MF, Vobach S, Olsson K, Svetlik D, Risser RC. Cortisol secretion and Alzheimer’s disease progression. Biol Psychiatry. 1997;42(11):1030–1038. doi: 10.1016/s0006-3223(97)00165-0. [DOI] [PubMed] [Google Scholar]
- Wellman CL. Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration. J Neurobiol. 2001;49(3):245–253. doi: 10.1002/neu.1079. [DOI] [PubMed] [Google Scholar]
- West CH, Ritchie JC, Boss-Williams KA, Weiss JM. Antidepressant drugs with differing pharmacological actions decrease activity of locus coeruleus neurons. Int J Neuropsychopharmacol. 2009;12(5):627–641. doi: 10.1017/S1461145708009474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Evans DA, Bienias JL, Mendes de Leon CF, Schneider JA, Bennett DA. Proneness to psychological distress is associated with risk of Alzheimer’s disease. Neurology. 2003;61(11):1479–1485. doi: 10.1212/01.wnl.0000096167.56734.59. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Schneider JA, Bienias JL, Arnold SE, Evans DA, Bennett DA. Depressive symptoms, clinical AD, and cortical plaques and tangles in older persons. Neurology. 2003;61(8):1102–1107. doi: 10.1212/01.wnl.0000092914.04345.97. [DOI] [PubMed] [Google Scholar]
- Wyatt RJ, Portnoy B, Kupfer DJ, Snyder F, Engelman K. Resting plasma catecholamine concentrations in patients with depression and anxiety. Arch Gen Psychiatry. 1971;24(1):65–70. doi: 10.1001/archpsyc.1971.01750070067009. [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Arima H, Sugiura T, Hirate H, Kusama N, Suzuki K, Sobue K. Midazolam inhibits the formation of amyloid fibrils and GM1 ganglioside-rich microdomains in presynaptic membranes through the gamma-aminobutyric acid A receptor. Biochem Biophys Res Commun. 2015;457(4):547–553. doi: 10.1016/j.bbrc.2015.01.022. [DOI] [PubMed] [Google Scholar]
- Yu JT, Wang ND, Ma T, Jiang H, Guan J, Tan L. Roles of beta-adrenergic receptors in Alzheimer’s disease: implications for novel therapeutics. Brain Res Bull. 2011;84(2):111–117. doi: 10.1016/j.brainresbull.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Zarow C, Lyness SA, Mortimer JA, Chui HC. Neuronal loss is greater in the locus coeruleus than nucleus basalis and substantia nigra in Alzheimer and Parkinson diseases. Arch Neurol. 2003;60(3):337–341. doi: 10.1001/archneur.60.3.337. [DOI] [PubMed] [Google Scholar]
- Zhao XH, Wang PJ, Li CB, Hu ZH, Xi Q, Wu WY, Tang XW. Altered default mode network activity in patient with anxiety disorders: an fMRI study. Eur J Radiol. 2007;63(3):373–378. doi: 10.1016/j.ejrad.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Zubenko GS, Moossy J. Major depression in primary dementia. Clinical and neuropathologic correlates. Arch Neurol. 1988;45(11):1182–1186. doi: 10.1001/archneur.1988.00520350020008. [DOI] [PubMed] [Google Scholar]
- Zubenko GS, Moossy J, Kopp U. Neurochemical correlates of major depression in primary dementia. Arch Neurol. 1990;47(2):209–214. doi: 10.1001/archneur.1990.00530020117023. [DOI] [PubMed] [Google Scholar]
- Zverova M, Fisar Z, Jirak R, Kitzlerova E, Hroudova J, Raboch J. Plasma cortisol in Alzheimer’s disease with or without depressive symptoms. Med Sci Monit. 2013;19:681–689. doi: 10.12659/MSM.889110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweig RM, Ross CA, Hedreen JC, Steele C, Cardillo JE, Whitehouse PJ, Folstein MF, Price DL. The neuropathology of aminergic nuclei in Alzheimer’s disease. Ann Neurol. 1988;24(2):233–242. doi: 10.1002/ana.410240210. [DOI] [PubMed] [Google Scholar]