Abstract
Background & Aims
Fluoroscopy during endoscopic retrograde cholangiopancreatography (ERCP) is increasingly performed by therapeutic endoscopists, many of whom have not received formal training in modulating fluoroscopy use to minimize radiation exposure. Exposure to ionizing radiation has significant health consequences for patients and endoscopists. We aimed to evaluate whether a 20-minute educational intervention for endoscopists would improve use of fluoroscopy and decrease ERCP-associated exposure to radiation for patients.
Methods
We collected data from 583 ERCPs, performed in California from June 2010 through November 2012; 331 were performed at baseline and 252 following endoscopist education. The educational intervention comprised a 20-min video explaining best practices for fluoroscopy, coupled with implementation of a formal fluoroscopy time out protocol before the ERCP was performed. Our primary outcome was the effect of the educational intervention on direct and surrogate markers of patient radiation exposure associated with ERCPs performed by high-volume endoscopists (HVEs, 200 or more ERCPs/year) vs low-volume endoscopists (LVEs, fewer than 200 ERCPs/year).
Results
At baseline, total radiation dose and dose area product were significantly higher for LVEs, but there was no significant difference between HVEs and LVEs following education. Education was associated with significant reductions in median fluoroscopy time (48% reduction for HVEs vs 30% reduction for LVEs), total radiation dose (28% reduction for HVEs vs 52% for LVEs) and dose area product (35% reduction for HVEs vs 48% reduction for LVEs). All endoscopists significantly increased their use of low magnification and collimation following education.
Conclusions
A 20-minute educational program with emphasis on ideal use of modifiable fluoroscopy machine settings results in an immediate and significant reduction in ERCP-associated patient radiation exposure for low-volume and high-volume endoscopists. Training programs should consider radiation education for advanced endoscopy fellows.
Keywords: safety, pancreatic and biliary disorder, treatment, side effect
Introduction
Endoscopic retrograde cholangio-pancreatography (ERCP) utilizes endoscopic and fluoroscopic guidance for the management of pancreatic and biliary disorders. In the early decades of ERCP, radiologists and subsequently radiology technicians performed fluoroscopy for the procedures. Over the last two decades, the responsibility for operating fluoroscopy during ERCP has increasingly been relegated to endoscopists performing ERCP. However, few U.S. endoscopists have received formal training in radiation protection and not all U.S. states require endoscopists to undergo formal credentialing in fluoroscopy.1
Fluoroscopy during ERCP exposes patients and endoscopy room personnel to ionizing radiation, with potentially harmful health effects. Controlled in vitro and in vivo experiments have demonstrated that exposure to high dose ionizing radiation is associated with genomic instability and an increased risk of developing some types of cancer.2 Whereas concentrated high-dose-rate radiation exposure was previously thought to confer a higher cancer risk than low-dose-rate exposure,3 a recent study of nuclear workers chronically exposed to low dose radiation indicated a linear increase in cancer mortality with increased exposure to radiation, regardless of the dose rate.4 A report from the National Council on Radiation Protection and Measurements indicated that in 2006, medical imaging accounted for 48% of the U.S. population radiation exposure.5 Computed tomography (CT) scans account for the majority of medical imaging related radiation exposure, and an estimated 2% of cancers diagnosed annually in the U.S. may be related to radiation exposure from CT scans.6
The radiology community has responded to this concern with an effective multi-pronged approach, including establishing appropriateness criteria for ordering CT scans, education of its membership with awareness campaigns, and creation of educational websites and decision support software.7, 8 The American College of Radiology has created a Dose Index Registry to collect radiation dose data from various institutions, allowing them to compare their radiation utilization to national averages.9, 10 In addition, several hospitals have established institutional CT dose reduction teams, conduct internal audits and utilize low dose CT protocols.11
In contrast, the response of the gastroenterology community in minimizing patient radiation exposure during ERCP and other endoscopic procedures has been relatively muted. The European ERCP society initially suggested a role for continuous education of endoscopists and supporting staff.12 The American Society for Gastrointestinal Endoscopy (ASGE) and the American College of Gastroenterology subsequently proposed quality indicators for ERCP, including the frequency with which fluoroscopy time and radiation dose were documented, and emphasizing that patient radiation exposure during ERCP should adhere to the ‘as low as reasonably achievable’ (ALARA) principal.13,14 However, there has not been a regulatory push in the U.S. to incorporate these proposed quality indicators, or to educate endoscopists in minimizing patient radiation exposure during ERCP.
Our group and others have reported significantly lower patient radiation exposure associated with ERCP performed by high volume endoscopists, compared to low volume endoscopists.15, 16 This may be related to high volume endoscopists having greater familiarity with the modifiable factors on the fluoroscopy equipment which modulate radiation exposure. As these are learnable elements, we postulated that education of endoscopists would result in a decrease in patient radiation exposure. We therefore implemented an educational program to decrease procedure related radiation exposure at our institution. We created a video highlighting best practices related to fluoroscopy use and incorporated a fluoroscopy ‘time out’ prior to performing ERCP, to reinforce the main points of the educational video. The aim of this study was to prospectively evaluate the impact of providing education to our endoscopists on minimizing ERCP-associated patient radiation exposure.
Methods
Participants
ERCPs were performed by 2 high volume endoscopists (HVE) and 7 low volume endoscopists (LVE). As with our previous study,15 a cutoff value of <200 ERCPs/year (self-reported) was chosen to define LVE because fluoroscopy time (FT) has been shown to be significantly higher in endoscopists who perform fewer than 200 ERCPs/year.15, 16 As required by our institution, all endoscopists held fluoroscopy x-ray supervisor and operator permits, issued by the California Department of Public Health. To obtain this permit one must pass an examination on biology and physics of radiation exposure, operation of fluoroscopy equipment, radiation exposure reduction, and image evaluation. Continuing education credits are required for renewal of this permit every three years.
Design
This study utilized a prospective, pre-post intervention design to evaluate the impact of endoscopist education on minimizing radiation exposure to patients during ERCP. The study was conducted with a pre-intervention phase (Phase I) and a post-intervention phase (Phase II) and with two arms, HVE and LVE, which were analyzed separately. The study was approved by the Stanford Institutional Review Board (Protocol #21236).
In the pre-intervention phase of the study, all ERCPs performed at our medical center over a 9-month period were analyzed. Baseline data on radiation parameters for HVE and LVE have been described in our previous study.15 Additional baseline data regarding utilization of modifiable fluoroscopy image parameters by HVE and LVE were extracted and analyzed for this study.
ERCPs performed by both groups of endoscopists over a period of five months in the post-intervention phase of the study were then analyzed for radiation exposure parameters as well as for procedural complexity).17 Advanced endoscopy fellows participated in post-intervention procedures performed by HVE only (baseline data were collected before our institution’s Advanced endoscopy fellowship program was initiated).
Intervention
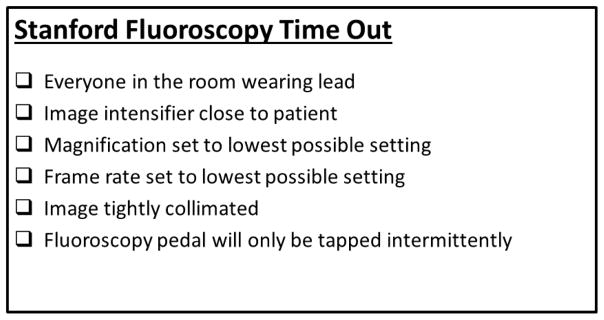
Prior to the post-intervention phase of the study, all endoscopists received a 20-minute audio-visual educational presentation. This detailed basic radiation physics, the risks of radiation exposure, best practices in fluoroscopy, detailed instruction on fluoroscopy machine use including ideal settings for modifiable factors which impact on radiation exposure, such as fluoroscopy frame rate, image magnification and collimation.15 A ‘Stanford Fluoroscopy Time-Out’ (Figure 1) was also instituted and verbalized prior to every ERCP performed during the post-intervention phase of the study. The fluoroscopy time-out included verification of the following elements: (1) everyone was wearing lead protective equipment, (2) image intensifier was set as close to the patient as possible, (3) image magnification was set to the lowest possible setting, (4) fluoroscopy frame rate was set at the lowest possible rate, (5) fluoroscopy image was collimated, and (6) reiteration that the fluoroscopy pedal was to be tapped only intermittently, rather than depressed for longer intervals.
Figure 1.
Stanford Fluoroscopy Time-Out placard posted on fluoroscopy machine. Each statement is verbalized prior to initiation of ERCP.
Procedure
All ERCPs were performed utilizing the same fluoroscopy equipment, a GE Precision 500D R&F System (GE Healthcare, Peawaukee, WI), installed in 2009. The fluoroscopy unit is equipped with an undercouch x-ray tube and collimator.
Data Collection
Data collected included patient demographics, procedure indication and interventions performed, fluoroscopy image and radiation parameters. Various measures of radiation dose quantify potential health consequences of exposure. We recorded Fluoroscopy Time (FT), as this remains the most widely utilized surrogate measure of radiation exposure. We have previously shown that FT is an imperfect surrogate for radiation exposure, given the evolution of newer fluoroscopy machines which allow modification of factors that modulate radiation dose over the same duration of FT.15 We therefore also included additional direct measures of radiation exposure in our analysis. We recorded Total Radiation Dose (TD) measured in units of milligray (mGy). Dose Area Product (DAP), measured in Gy-cm2 [defined as the product of the air kerma (Gy) and the exposed area (cm2)], which provides an easily measurable estimate of the total radiation delivered to patients, was recorded as well. We calculated Effective Dose (ED), in millisievert (mSv), by converting DAP readings with the conversion coefficient 0.26 mSv/(Gy-cm2). ED is a measure of dose distribution which allows comparison across different radiological procedures, is frequently used to quantify radiation exposure for correlation with health outcomes.18
Utilization of modifiable fluoroscopy image parameters, which are surrogates for awareness of and attention to minimizing radiation exposure, was evaluated in both study phases. It was assumed that the collimation and magnification settings noted in captured images reflected fluoroscopy use patterns. These image parameters include preferential use of collimation (defined as use of collimation for >50% of images captured in a given procedure), preferential collection of low magnification images (defined as use of low magnification for >50% of images captured in a given procedure) and the number of fluoroscopy images captured per procedure. Although frame rate is another modifiable parameter on our fluoroscopy machine (with the default frame rate set to 15/second during the study period), these data are not saved post-procedure by our fluoroscopy system software and are therefore not included in our study.
Outcome Measures
The primary outcomes of interest were patient radiation exposure during ERCP (assessed by FT, TD, DAP, and ED), number of fluoroscopy images captured and optimization of modifiable fluoroscopy image parameters (collimation and magnification). For all parameters, HVE and LVE were analyzed separately, prior to and following education. For post-intervention procedures, radiation exposure was evaluated for procedures with and without trainee (advanced endoscopy fellow) participation.
Statistical Analysis
Data analysis was performed using Stata statistical software version 13 (StataCorp LP, College Station, TX). Statistics for continuous variables are reported as mean ± standard deviation or median with interquartile range (IQR), dependent on whether the data distribution was parametric. All reported p-values are 2-sided and statistical significance was defined as p < 0.05 for all comparisons.
Results
Patient Characteristics
During the study period, 583 ERCPs were performed, 331 during Phase I and 252 during Phase II. During Phase I, 76.4% of ERCPs were performed by HVE and 23.5% by LVE. There was a small increase in the proportion of ERCPs performed by HVE (85.7%) during Phase II of the study. All ERCPs were performed with therapeutic intent, but therapeutic intervention was occasionally deemed unnecessary. Indications for ERCP, mean patient age and proportion of female patients were similar across both study groups and phases (Table 1).
Table 1.
Patient and procedural characteristics
| Phase I (n=331) | Phase II (n=252) | P values Phase I vs. II, TOTAL | P values Phase I vs II, LVE | P values Phase I vs II, HVE | |||||
|---|---|---|---|---|---|---|---|---|---|
| Total | LVE (n=78) | HVE (n=253) | Total | LVE (n=36) | HVE (n=216) | ||||
| Age (mean ± SD) | 60.1±17.1 | 62.8±19.7 | 59.2±16.2 | 60.0±17.1 | 64.5±14.5 | 59.2±17.4 | 0.9546 | 0.6460 | 0.9912 |
| Gender | 0.4437 | 0.0439 | 0.8553 | ||||||
| Male | 172 | 34 | 138 | 139 | 23 | 116 | |||
| Female | 159 | 44 | 115 | 113 | 13 | 100 | |||
| Indication | 0.0038 | 0.0119 | 0.0046 | ||||||
| Abnormal LFTs | 25 | 12 | 13 | 14 | 11 | 3 | |||
| Stone | 103 | 36 | 67 | 71 | 10 | 61 | |||
| Stricture | 158 | 23 | 135 | 103 | 6 | 97 | |||
| Others | 45 | 7 | 38 | 64 | 9 | 55 | |||
| Complexity Score (mean ± SD) | 2.28 ± 0.74 | 1.99 ± 0.61 | 2.37 ± 0.76 | 2.22 ± 0.84 | 1.53 ± 0.56 | 2.33 ± 0.82 | 0.371 | 0.0002 | 0.6384 |
ERCP Complexity
HVE performed ERCPs of Complexity Grade 3 or 4 more frequently than LVE in Phase I (42.7% vs 15.4%, p<0.001) and in Phase II (38.9% vs 2.8%, p<0.001)(Table 1).
Fluoroscopy Time
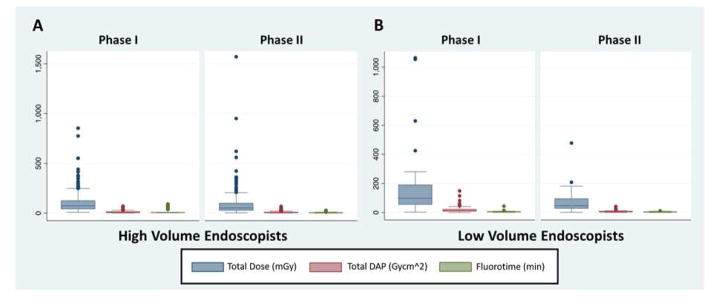
In Phase I, median FT for LVE was 5.4 minutes (IQR: 3.5–7.1 minutes) and for HVE was 6.4 minutes (IQR: 5.5–8.3 minutes) (p=0.0183, Figure 2). Post-education, FT for LVE decreased 30%, from 5.4 minutes to 3.8 minutes (IQR: 2.3–5.9 minutes) (p < 0.0001, Figure 2) and for HVE decreased 48%, from 6.4 minutes to 3.3 minutes (IQR: 1.7–5.5 minutes) (p< 0.0001, Table 3). There was a significantly higher procedural FT for HVE in comparison to LVE during Phase I of the study, but the difference in FT between HVE and LVE was not significant in the post-intervention phase (p=0.3648, Table 3).
Figure 2.
Boxplots comparing high-volume endoscopists (2A) and low-volume endoscopists (2B) prior to education (Phase I) and following education (Phase II). Radiation exposure parameters plotted are fluoroscopy time, total radiation dose and dose area product. Error bars span the 25th and 75th percentiles. Whiskers represent 5th and 95th percentiles, dots represent outliers.
Table 3.
Radiation exposure during ERCP
| Phase I | Phase II | Phase I vs. Phase II | ||||
|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | % reduction | P value | |
| FT (min) | ||||||
| HVE | 6.4 | 5.5 – 8.3 | 3.3 | 1.7 – 5.5 | 48 | <0.0001 |
| LVE | 5.4 | 3.5 – 7.1 | 3.8 | 2.3 – 5.9 | 30 | <0.001 |
| Total Dose (mGy) | ||||||
| HVE | 42.9 | 42.9 – 125.1 | 53.1 | 27.9 – 98.8 | −28 | <0.0001 |
| LVE | 98.3 | 56.4 – 189.9 | 46.9 | 28.6 – 95.5 | 52 | <0.001 |
| DAP (Gy-cm2) | ||||||
| HVE | 8.8 | 5.3 – 14.4 | 5.7 | 2.9 – 10.1 | 35 | <0.0001 |
| LVE | 13.9 | 8.8 – 23.1 | 7.2 | 4.2 – 9.9 | 48 | <0.0001 |
| ED (mSv) | ||||||
| HVE | 2.3 | 1.4 – 3.7 | 1.5 | 0.8 – 2.6 | 35 | <0.0001 |
| LVE | 3.6 | 2.3 – 6.0 | 1.9 | 1.1 – 2.6 | 48 | <0.0001 |
| HVE | LVE | HVE vs. LVE | ||||
| Median | IQR | Median | IQR | % reduction | P value | |
| FT (min) | ||||||
| Phase I | 6.4 | 5.5 – 8.3 | 5.4 | 3.5 – 7.1 | 15 | <0.0001 |
| Phase II | 3.3 | 1.7 – 5.5 | 3.8 | 2.3 – 5.9 | 13 | 0.363 |
| Total Dose (mGy) | ||||||
| Phase I | 74.1 | 43.0 – 125.1 | 98.3 | 56.4 – 189.9 | 33 | <0.0001 |
| Phase II | 53.1 | 27.9 – 98.8 | 46.9 | 28.6 – 95.5 | 11 | 0.545 |
| DAP (Gy-cm2) | ||||||
| Phase I | 8.8 | 5.3 – 14.4 | 13.9 | 8.8 – 23.1 | 59 | <0.0001 |
| Phase II | 5.7 | 2.9 – 10.1 | 7.2 | 4.2 – 9.9 | 26 | 0.465 |
| ED (mSv) | ||||||
| Phase I | 2.3 | 1.4 – 3.8 | 3.6 | 2.3 – 6.0 | 59 | <0.0001 |
| Phase II | 1.5 | 0.8 – 2.6 | 1.9 | 1.1 – 2.6 | 26 | 0.465 |
Total Dose
In Phase I, median TD for LVE was 98.3 mGy (IQR: 56.4 – 189.9 mGy) and for HVE was 74.1 mGy (IQR: 42.9–125.1 mGy) (p=0.0183, Table 3). Post-education, TD for LVE decreased 52.3% from 98.3 mGy to 46.9 mGy (IQR: 28.6–95.5 mGy) (p=0.0005) and for HVE decreased 28.3% from 74.1 mGy to 53.1 mGy (IQR: 27.9–98.8 mGy) (p<0.0001, Table 3). There was a significantly higher TD for LVE in comparison to HVE during Phase I of the study, but the difference in TD between HVE and LVE was not significant in the post-intervention phase (p=0.5464, Table 3).
Dose Area Product
In Phase I, median DAP for LVE was 13.9 Gy-cm2 (IQR: 8.8–23.1 Gy-cm2) and for HVE was 8.8 Gy-cm2 (IQR: 5.3–14.4 Gy-cm2) (p<0.0001, Table 3). Post-education, DAP for LVE decreased 48%, from 13.9 Gy-cm2 to 7.2 Gy-cm2 (IQR: 4.2–9.9 Gy-cm2) (p<0.0001, Table 3) and for HVE decreased by 35.1% from 8.8 Gy-cm2 to 5.7 Gy-cm2 (IQR: 2.9–10.1 Gy-cm2) (p< 0.0001, Table 3). There was a significantly higher DAP for LVE in comparison to HVE during Phase I of the study, but the difference in DAP between HVE and LVE was not significant in the post-intervention phase (p=0.4665, Figure 2).
Effective Dose
In Phase I, median ED for LVE was 3.6 mSv (IQR: 2.3–6.0 mSv) and median ED for HVE was 2.3 mSv (IQR: 1.4–3.7 mSv, Table 3). Post-education, ED among LVE decreased 48%, from 3.6 mSv to 1.9 mSv (IQR: 1.1–2.6 mSv) (p<0.0001, Table 3) and ED for HVE decreased by 35.1% from 2.3 mSv to 1.5 mSv (IQR: 0.8–2.6 mSv) (p< 0.0001, Table 3). There was a significantly higher ED for LVE in comparison to HVE during Phase I of the study, but the difference in ED between HVE and LVE was not significant in the post-intervention phase (p=0.4665, Table 3).
Collimation
In Phase I, preferential use of collimation was evident in 21.8% of ERCPs performed by LVE and 15.0% performed by HVE (p=0.1599, Table 2). Education had a positive impact in both groups, with preferential use of collimation increasing from 21.8% to 52.8% of ERCPs for LVE (p=0.0009) and from 15% to 71.8% for HVE (p<0.0001). HVE demonstrated preferential use of collimation in significantly more procedures relative to LVE in the post-intervention phase (p=0.0226).
Table 2.
Fluoroscopy parameters
| Phase I | Phase II | P value | |
|---|---|---|---|
| Collimation evident in >50% images | |||
| HVE | 38 (15.0%) | 155 (71.8%) | <0.0001 |
| LVE | 17 (21.8%) | 19 (52.8%) | 0.0009 |
| P value | 0.1599 | 0.0226 | |
| Magnification Setting at ‘Low’ in >50% images | |||
| HVE | 132 (52.2%) | 160 (74.1%) | <0.0001 |
| LVE | 16 (20.5%) | 29 (82.9%) | <0.0001 |
| P value | <0.0001 | 0.2637 | |
| Number of Images per procedure | |||
| HVE | 6.0 (4.0–8.0) | 5.0 (3.0–7.0) | <0.0001 |
| LVE | 6.0 (4.0–8.0) | 4.0 (2.5–7.0) | 0.0840 |
| P value | 0.4820 | 0.3675 | |
| Fluoroscopy Time | |||
| HVE | 6.4 (5.5–8.3) | 3.3 (1.7–5.5) | <0.0001 |
| LVE | 5.4 (3.5–7.1) | 3.8 (2.3–5.9) | <0.001 |
| P value | <0.0001 | 0.363 | |
Magnification
In Phase I, preferential use of low magnification was evident in 20.5% of ERCPs performed by LVE and 52.2% performed by HVE (p <0.0001, Table 2). Education had a positive impact in both groups, with preferential use of low magnification increasing from 20.5% to 82.9% of ERCPs for LVE (p<0.0001) and from 52.2% to 74.1% for HVE (p<0.0001). There was no significant difference in preferential use of low magnification between HVE and LVE in the post-intervention phase (p=0.2637).
Number of Images
In Phase I, LVE and HVE both captured a median of 6.0 (IQR: 4.0–8.0) digital fluoroscopy images per procedure (Table 2). Education had a positive impact in both groups, with LVE capturing a median of 4.0 (IQR: 2.5–7.0) images (p=0.0840), and HVE capturing a median of 5.0 (IQR: 3.0–7.0) images per ERCP (p<0.0001). There was no significant difference in number of images captured per procedure between HVE and LVE in the post-intervention phase (p=0.3675).
Trainee Involvement
Advanced endoscopy fellows participated in Phase II procedures under the supervision of HVE. There was no significant difference in FT, TD or DAP for procedures with trainee involvement compared to those without trainee involvement (Table 4).
Table 4.
Phase II radiation exposure for High Volume Endoscopists, stratified by Fellow Participation
| Without Trainee (Advanced Fellow) | With Trainee (Advanced Fellow) | With vs. Without Trainee (Advanced Fellow) | ||||
|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | % Change | ||
| FT (min) | ||||||
| Phase II | 2.8 | 1.5–4.9 | 3.4 | 1.7–5.5 | 21.4 | 0.4099 |
| Total Dose (mGy) | ||||||
| Phase II | 53.4 | 24.6–133.7 | 53.1 | 28.9–93.6 | −0.6 | 0.8379 |
| DAP (Gy-cm2) | ||||||
| Phase II | 5.9 | 2.7–14.1 | 5.7 | 2.9–9.6 | −3.4 | 0.5194 |
Discussion
Over the last three decades, there has been a 6-fold increase in patient radiation exposure in the U.S., attributed to increased medical utilization of radiologic investigation and therapy.19 The long-term risk of radiation exposure due to medical interventions is problematic and it is estimated that up to 2% of all cancer may be attributable to radiation from CT scans alone.18 The overall risk to patients from all sources of medical radiation exposure is therefore likely to be higher. There is also an unfortunate lack of awareness about radiation doses and associated cancer risk among physicians, including those in subspecialties that perform diagnostic studies and therapeutic procedures utilizing radiation.20
Radiation exposure during ERCP should be an issue of increasing importance for the endoscopy community, given the increasing number of therapeutic ERCPs performed in the last decade.21 However, few U.S. endoscopists have received formal education in radiation protection during fellowship training,1 and only a minority of endoscopists receive formal instruction in operating the fluoroscopy machine at their hospital of employment.22 HVE appear able to circumvent this lack of formal radiation training. We and others have previously shown that high endoscopist ERCP volume is associated with lower patient radiation exposure.15, 16 However, the majority of ERCPs in the U.S. are unfortunately performed by LVE in smaller hospitals.23
Although the radiology community has undertaken several concrete and effective steps to minimize patient radiation exposure, only nascent efforts are evolving within the endoscopy community. Both U.S. and European endoscopy societies now appear to be recognizing this as a problematic issue.24 However, there has been no regulatory push towards formal radiation training for endoscopists.23
This combination of regulatory permissiveness and lack of training during fellowship continues to result in patients receiving a higher radiation exposure during ERCP than can be achieved in the best circumstances. The unrelenting march of technology has mitigated this issue to some extent, resulting in an overall reduction in radiation exposure despite the lack of formal radiation training of endoscopists. In 2001, Larkin et al. reported an ED of 12.4 mSv for therapeutic ERCPs, estimated to confer a lifetime risk of cancer of 1 in 1700.25 In contrast, our group in 2015 reported approximately 3 fold lower overall ED associated with ERCPs performed by LVE and over 5 fold lower in those performed by HVE.15 Overall, ED associated with ERCPs performed by LVE was 59% higher than those performed by HVE. The lower radiation exposure associated with current ERCPs is related to technological advances in ERCP accessory devices, which facilitate procedural efficiency and to evolution of fluoroscopy machines, which allow minimization of radiation exposure.
A possible explanation contributing to the lower patient radiation exposure associated with ERCPs performed by HVE in our prior study,15 is greater utilization by HVE of modifiable fluoroscopy machine factors which can minimize radiation for any given fluoroscopy time. These modifiable factors include minimization of frame rate, avoidance of continuous fluoroscopy in favor of intermittent brief use of fluoroscopy,26 use of low magnification imaging and use of collimation.15 The radiation exposure gap between LVE and HVE suggests a potential role for endoscopist education, regarding the risks of radiation exposure and best practices for fluoroscopy utilization.
Until the present study, it was unclear whether providing education could affect utilization of these modifiable factors by endoscopists and result in lower ERCP associated radiation exposure. Our results indicate that education of endoscopists on the risks of radiation and on implementation of simple measures to minimize radiation exposure results in an immediate and significant reduction in patient radiation exposure. The most pronounced decrease in radiation exposure after education was noted for LVE (52% reduction in Total Dose). However, importantly, a decrease in radiation exposure was also evident for HVE (28% reduction in Total Dose), who at baseline already had significantly lower radiation exposure than LVE. Our study therefore highlights the value of radiation education for all endoscopists across a range of ERCP volumes.
Furthermore, after endoscopist education, no differences were noted between LVE and HVE for Total Dose, Dose Area Product, Effective Dose or Fluoroscopy Time (Figure 2). This may be partly due to the fact that HVE perform procedures of greater endoscopic and fluoroscopic complexity than LVE.
Although advanced endoscopy fellows participated in all post education procedures for HVE, and one might hypothesize that trainee involvement would typically result in increased radiation exposure, we found no significant difference in radiation exposure parameters for procedures with and without trainee involvement. The minimal impact of fellow participation on radiation exposure in this study may be explained by our institutional emphasis on minimizing radiation use, close attending supervision of fellows, and the environment of this study with a ‘Fluoroscopy Time Out’ called out prior to initiating procedures in Phase II.
In particular, our study highlights the fact that endoscopists can minimize radiation exposure by optimizing modifiable fluoroscopy parameters. Our review of captured images confirms that education was associated with a change in patterns of fluoroscopy use, with increased use of collimation and low magnification, both of which are associated with lower radiation exposure.27 Although fluoroscopic frame rate data were not captured in this study, one might infer that endoscopists were also increasingly attentive to minimizing the frame rate, as emphasized in our educational intervention and ‘Fluoroscopy Time Out.’ Using a lower frame rate has been shown to have no impact on image quality in gastrointestinal and cardiovascular interventions,28, 29 and we suggest that the default frame rate be set as low as possible for all endoscopy unit fluoroscopy machines.
Limitations include the fact that this is a single center study. However, our institution is unique, in that although it is an academic tertiary care center, it allows high and low volume academic and community physicians to use the same endoscopic and fluoroscopic equipment and staff for their ERCPs. This allows comparison between LVE and HVE in the same environment, thereby eliminating several confounding factors inherent in comparisons between these groups across large tertiary care hospitals and community hospitals. Baseline data for this study were collected retrospectively to determine radiation exposure associated with ERCPs performed by HVE and LVE while avoiding the Hawthorne effect, a phenomenon whereby participants modify their behavior due to their awareness of being studied. The post-intervention phase of this study is notably susceptible to the Hawthorne effect, because endoscopists were aware that their fluoroscopy use patterns would be monitored. However, any contribution from the Hawthorn effect to minimization of radiation exposure simply emphasizes the positive impact of continuous monitoring in improving endoscopist performance, as observed with quality measures such as the adenoma detection rate.
In conclusion, although previous studies have indicated that ERCPs performed by LVE are associated with higher patient radiation exposure compared to those performed by HVE, this is the first study to demonstrate that simple radiation education results in an immediate and significant reduction in ERCP associated patient radiation exposure to similar ranges for both LVE and HVE. Additionally, we have evaluated direct radiation parameters including the Total Dose, Dose Area Product and Effective Dose, which are highly accurate, unlike prior studies that have relied solely on Fluoroscopy Time as a surrogate measure of radiation exposure. This study indicates a need for all interventional endoscopy programs to strongly consider including radiation training for their advanced fellows and indeed for all interventional endoscopists. Gastroenterology societies may wish to consider minimization of patient radiation exposure as a potential future quality measure for ERCP.
Footnotes
Author Contributions: NCT, SK, RK and SB were involved in conception and design of the study; RJH, NCT, RK, SK, MTB, and SB were involved in collection, analysis and interpretation of the data; AC, MTB and SB were involved in drafting and critical revision of the article for important intellectual content; SB granted final approval of the article
Conflict of Interest: None of the authors have conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Saurabh Sethi SF, Banerjee Subhas. US survey assessing current ERCP-related radiation protection practices. Gastrointest Endosc. 2015;81:AB352. [Google Scholar]
- 2.Jaffe D, Bowden GT. Ionizing radiation as an initiator: effects of proliferation and promotion time on tumor incidence in mice. Cancer Res. 1987;47:6692–6. [PubMed] [Google Scholar]
- 3.Cardis E, Gilbert ES, Carpenter L, et al. Effects of low doses and low dose rates of external ionizing radiation: cancer mortality among nuclear industry workers in three countries. Radiat Res. 1995;142:117–32. [PubMed] [Google Scholar]
- 4.Richardson DB, Cardis E, Daniels RD, et al. Risk of cancer from occupational exposure to ionising radiation: retrospective cohort study of workers in France, the United Kingdom, and the United States (INWORKS) BMJ. 2015;351:h5359. doi: 10.1136/bmj.h5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schauer DA, Linton OW. National Council on Radiation Protection and Measurements report shows substantial medical exposure increase. Radiology. 2009;253:293–6. doi: 10.1148/radiol.2532090494. [DOI] [PubMed] [Google Scholar]
- 6.Berrington de Gonzalez A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169:2071–7. doi: 10.1001/archinternmed.2009.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nolan DJ. Minimizing radiation in diagnostic radiology. Br J Hosp Med. 1991;45:337. [PubMed] [Google Scholar]
- 8.Chintapalli KN, Montgomery RS, Hatab M, et al. Radiation dose management: part 1, minimizing radiation dose in CT-guided procedures. AJR Am J Roentgenol. 2012;198:W347–51. doi: 10.2214/AJR.11.7958. [DOI] [PubMed] [Google Scholar]
- 9.Little BP, Duong PA, Knighton J, et al. A Comprehensive CT Dose Reduction Program Using the ACR Dose Index Registry. J Am Coll Radiol. 2015;12:1257–65. doi: 10.1016/j.jacr.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 10.Bhargavan-Chatfield M, Morin RL. The ACR Computed Tomography Dose Index Registry: the 5 million examination update. J Am Coll Radiol. 2013;10:980–3. doi: 10.1016/j.jacr.2013.08.030. [DOI] [PubMed] [Google Scholar]
- 11.Robinson TJ, Robinson JD, Kanal KM. Implementation of the ACR dose index registry at a large academic institution: early experience. J Digit Imaging. 2013;26:309–15. doi: 10.1007/s10278-012-9546-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dumonceau JM, Garcia-Fernandez FJ, Verdun FR, et al. Radiation protection in digestive endoscopy: European Society of Digestive Endoscopy (ESGE) guideline. Endoscopy. 2012;44:408–21. doi: 10.1055/s-0031-1291791. [DOI] [PubMed] [Google Scholar]
- 13.Adler DG, Lieb JG, 2nd, Cohen J, et al. Quality indicators for ERCP. Gastrointest Endosc. 2015;81:54–66. doi: 10.1016/j.gie.2014.07.056. [DOI] [PubMed] [Google Scholar]
- 14.Garg MS, Patel P, Blackwood M, et al. Ocular Radiation Threshold Projection Based off of Fluoroscopy Time During ERCP. Am J Gastroenterol. 2016 doi: 10.1038/ajg.2016.540. [DOI] [PubMed] [Google Scholar]
- 15.Liao C, Thosani N, Kothari S, et al. Radiation exposure to patients during ERCP is significantly higher with low-volume endoscopists. Gastrointest Endosc. 2015;81:391–8. e1. doi: 10.1016/j.gie.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Jorgensen JE, Rubenstein JH, Goodsitt MM, et al. Radiation doses to ERCP patients are significantly lower with experienced endoscopists. Gastrointest Endosc. 2010;72:58–65. doi: 10.1016/j.gie.2009.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cotton PB, Eisen G, Romagnuolo J, et al. Grading the complexity of endoscopic procedures: results of an ASGE working party. Gastrointest Endosc. 2011;73:868–74. doi: 10.1016/j.gie.2010.12.036. [DOI] [PubMed] [Google Scholar]
- 18.Brenner DJ, Hall EJ. Computed tomography--an increasing source of radiation exposure. N Engl J Med. 2007;357:2277–84. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 19.Mettler FA, Jr, Bhargavan M, Faulkner K, et al. Radiologic and nuclear medicine studies in the United States and worldwide: frequency, radiation dose, and comparison with other radiation sources--1950–2007. Radiology. 2009;253:520–31. doi: 10.1148/radiol.2532082010. [DOI] [PubMed] [Google Scholar]
- 20.Wong CS, Huang B, Sin HK, et al. A questionnaire study assessing local physicians, radiologists and interns' knowledge and practice pertaining to radiation exposure related to radiological imaging. Eur J Radiol. 2012;81:e264–8. doi: 10.1016/j.ejrad.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 21.Huang RJ, Thosani NC, Barakat MT, et al. Evolution in the Utilization of Biliary Interventions in the United States: Results of a Nationwide Longitudinal Study from 1998 to 2013. Gastrointest Endosc. 2017 doi: 10.1016/j.gie.2016.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sethi S, Friedland S, Banerjee S. U.S. Survey Assessing Current ERCP-Related Radiation Protection Practices. Gastrointest Endosc. 2015;81:AB352. [Google Scholar]
- 23.Cote GA, Imler TD, Xu H, et al. Lower provider volume is associated with higher failure rates for endoscopic retrograde cholangiopancreatography. Med Care. 2013;51:1040–7. doi: 10.1097/MLR.0b013e3182a502dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ho IK, Cash BD, Cohen H, et al. Radiation exposure in gastroenterology: improving patient and staff protection. Am J Gastroenterol. 2014;109:1180–94. doi: 10.1038/ajg.2014.122. [DOI] [PubMed] [Google Scholar]
- 25.Larkin CJ, Workman A, Wright RE, et al. Radiation doses to patients during ERCP. Gastrointest Endosc. 2001;53:161–4. doi: 10.1067/mge.2001.111389. [DOI] [PubMed] [Google Scholar]
- 26.Churrango G, Deutsch JK, Dinneen HS, et al. Minimizing Radiation Exposure During ERCP by Avoiding Live or Continuous Fluoroscopy. J Clin Gastroenterol. 2015;49:e96–100. doi: 10.1097/MCG.0000000000000385. [DOI] [PubMed] [Google Scholar]
- 27.Thosani N, Chen AM, Friedland S, Banerjee S. Prospective Evaluation of Predictors of Increased Patient Radiation During ERCP: the Stanford ERCP Radiation Safety (Sers) Study. Gastrointestinal Endoscopy. 2014;79(5):AB340–AB341. [Google Scholar]
- 28.Schultz C, Dixon S. SU-F-I-77: Radiation Dose in Cardiac Catheterization Procedures: Impact of a Systematic Reduction in Pulsed Fluoroscopy Frame Rate. Med Phys. 2016;43:3404. [Google Scholar]
- 29.Boland GW, Murphy B, Arellano R, et al. Dose reduction in gastrointestinal and genitourinary fluoroscopy: use of grid-controlled pulsed fluoroscopy. AJR Am J Roentgenol. 2000;175:1453–7. doi: 10.2214/ajr.175.5.1751453. [DOI] [PubMed] [Google Scholar]