Abstract
Background
The European Randomised Study of Screening for Prostate Cancer trial has shown a 21% reduction in prostate cancer (PC) mortality with prostate-specific antigen (PSA)-based screening. Sweden used a two-year screening interval and showed a larger mortality reduction than Finland with a four-year interval and higher PSA cut-off.
Objective
To evaluate the impact of screening interval and PSA cut-off on PC detection and mortality.
Design, Setting, and Participants
We analysed the core age groups (55–69 years at entry) of the Finnish (N=31,866) and Swedish (N=5,901) screening arms at 13 and 16 years of follow-up. Sweden used a screening interval of two years and a PSA cut-off of 3.0 ng/ml, while Finland the screening interval was four years and the PSA cut-off 4.0 ng/ml (or PSA 3.0–3.9 ng/ml with free PSA<16%).
Outcome Measurements and Statistical Analysis
We compared PC detection rate and PC mortality between the Finnish and Swedish centers and estimated the impact of different screening protocols.
Results and Limitations
If the Swedish screening protocol had been followed in Finland, 122 additional PC cases would have been diagnosed at screening, 84% of which would have been low-risk cancers, and four leading to PC death. In contrast, if a lower PSA threshold had been applied in Finland, at least 127 additional PC would have been found, with 19 PC deaths.
Conclusion
The small number of deaths among cases that would have been potentially detectable in Finland with the Swedish protocol (or those that would have been missed in Sweden with the Finnish approach) is unlikely to explain the differences in mortality in this long of a follow-up.
Patient summary
A PSA threshold of 3 ng/ml versus 4 ng/ml or a screening interval of two instead of four years is unlikely to explain the larger mortality reduction achieved in Sweden compared with Finland.
Keywords: Prostate cancer, Screening, Randomised trials, Prostate-specific antigen
Introduction
The European Randomized Study of Screening for Prostate Cancer (ERSPC) has shown that repeated prostate-specific antigen (PSA) screening can reduce prostate cancer (PC) mortality, though the balance of benefits and harms remains uncertain [1,2]. However, the optimal screening protocol in terms of PSA threshold, screening interval and target group [3] has not been established, and various organizations recommend different screening algorithms [4,5]. In addition, the length of the interval between screening rounds is controversial, as the Göteborg section of ERSPC with a two-year screening interval reported a 44% PC mortality reduction, but it was 32% in Rotterdam section of ERSPC with four-year screening interval. PLCO showed no mortality reduction with annual screening [6,7].
The ERSPC trial demonstrated a 21% overall reduction in PC mortality at 13 years with screening, but the effect sizes varied between centres[2]. A non-significant 15% reduction was achieved in the Finnish centre at 12 years. [8] There are some notable differences in the screening protocols between the two centres, in addition to the follow-up time, age at start of screening (50–64 in Sweden vs. 55–67 years in Finland), contamination in the control group (likely less opportunistic PSA testing in Sweden than Finland [9]) and different background risks in the populations (higher prostate cancer mortality in Sweden than Finland). Sweden used a lower PSA threshold value (3 ng/mL vs 4 ng/mL) and had a shorter screening interval than Finland (2 years vs. 4 years), but in Finland a free/total PSA ratio ≤16% was used as an ancillary test among men with PSA 3–3.99 ng/mL.
The purpose of this study is to evaluate the impact of the PSA threshold and screening interval on PC detection and mortality by comparing the Finnish and Swedish screening results. This information will be important in the future when designing screening studies or even national screening programs.
Materials and Methods
The study protocol of Finnish trial has been described in detail elsewhere [10]. Briefly, 32,000 men were randomly allocated into the screening arm (SA) in 1996–1999. Men with a PSA ≥4.0ng/ml or PSA 3.0–3.9ng/ml and free/total PSA < 16% were referred to further diagnostic examinations. If eligible (alive, free of PC and living in the study area), the men were re-invited after 4 years.
In Sweden, 5,901 men formed the screening arm [5]. The youngest men (born 1940–44) of Swedish part of ERSPC were excluded from these analyses, as they were outside the core age group in ERSPC and not comparable with the Finnish subjects. Swedish men with a PSA ≥3.0 ng/ml (WHO corrected value, further details[6]) were referred to similar diagnostic examinations as in Finland. Eligible men were re-invited after two years. Free/total PSA was not used in the Swedish study. We excluded screenings after the fifth round, as Finnish men were no longer invited after 8 years from the entry.
In our analyses, only the screening arms of the Finnish and Swedish centres were compared ad they are two of three largest centres of ERSPC. We compared Finland into Sweden as both screening interval and cut-off were different. An interval cancer was defined as any prostate cancer that was diagnosed outside the screening study protocol within a screening interval. PCs in non-participants were not regarded as interval cancers, neither were those detected more than 4 years after the previous screen. PC cases were categorized as low, moderate and high-risk or advanced cancers.
In our analyses, the second Finnish screening round (after a four-year interval) corresponds to the second and third screening rounds in Sweden, and the third Finnish screening round corresponds to the fourth and fifth Swedish rounds. However, if a Swedish subject was diagnosed with a PC in the second screening round and was therefore not included in the third round, we used the PSA value from the second round. If a Swedish man would participate on the second screening round, but not the third (and remain free of PC), we regarded him as a non-participant at the third “Finnish-protocol” screening round. A similar approach was applied with the fourth and fifth Swedish screening rounds. PCs diagnosed on Swedish sixth and seventh round, had no corresponding Finnish screening round.
The follow-up started on January 1st in the year of randomization (1995 for all men in Sweden and 1996–99 for Finland). The follow-up for incidence ended at PC diagnosis, death, emigration or the common closing date (December 31, 2010) and in survival analysis the start date was diagnosis and end death, emigration or closing date (December 31, 2015).
Statistical analysis
The Students t-test was used to compare differences in follow-up time, age and screening participation. As follow-up time varied between centers time-dependent cox regression was used to estimate age-adjusted hazard ratios for PC detection and PC mortality. In analyses, where no meaningful follow-up could be defined (positive PSA screening result, PC diagnosis, screen-detected cancer and interval cancer), we used binomial regression with a log link to calculate risk ratios with 95% confidence interval (CI). Statistical analyses were performed using IBM SPSS version 23.
Results
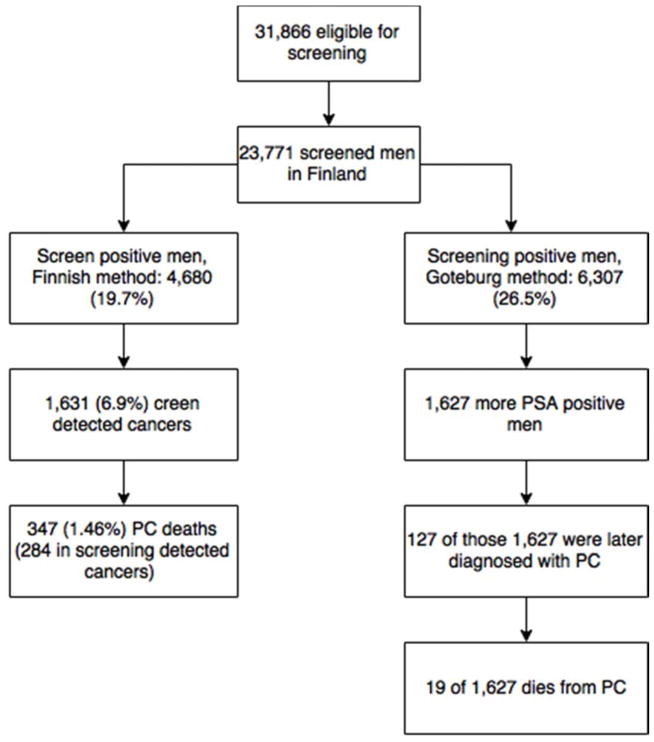
Overall 23,771 of the 31,866 (74.2%) invited men participated at least in one round of screening in Finland and similarly, 4,422 of the 5,901 (74.8%) men eligible participated in Sweden. The mean age at entry was 60.3 years in Finland and 60.7 years in Sweden (t-test p <0.01). The median follow-up time was 13 in Finland and 16 years in Sweden (with 362,433 and 76,241 person-years, respectively, p<0.01). Of the Finnish subjects, 4,680 had at least one positive screening test (19.7%) and 1,343 (29.9%) of Swedish men (HR 0.83, 95% CI 0.81–0.85). In Finland men with PSA 3–3.9 were referred to DRE 1996–1999, and 34 PCs were found for suspicious DRE, 29 of these cancers had f/t PSA< 16% (Table 1) (Figure 1 & 2).
Table 1.
Incidence of Positive PSA, Prostate Biopsy and Prostate Cancer by screening round. One person could have more than one positive PSA or prostate biopsy
| Finland (31,866 eligible) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Screening Round | 1 | 2 | 3 | Total | ||||
| n | % | n | % | n | % | n | % | |
| Participants | 20789 | 68.8 | 18613 | 70.4 | 12740 | 69.0 | 23771 | 74.0 |
| Positive PSA | 1977 | 9.5 | 2303 | 12.4 | 1644 | 12.9 | 5924 | - |
| Biopsies | 1877 | 9.0 | 2113 | 11.4 | 1469 | 11.5 | 5459 | - |
| Prostate Cancer | 676 | 4.1 | 1042 | 5.4 | 803 | 5.9 | 2521 | 10.6 |
| Sweden (5,901 eligible) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Screening Round | 1 | 2 | 3 | 4 | 5 | Total | ||||||
| n | % | n | % | n | % | n | % | n | % | n | % | |
| Participants | 3649 | 62.4 | 3227 | 58.0 | 2228 | 52.3 | 2492 | 60.7 | 1887 | 62.7 | 4422 | 74.8 |
| Positive PSA | 537 | 14.7 | 474 | 14.8 | 615 | 27.6 | 468 | 18.8 | 325 | 17.2 | 2419 | - |
| Biopsies | 466 | 12.8 | 385 | 11.9 | 551 | 24.7 | 394 | 15.8 | 286 | 15.2 | 2082 | - |
| Prostate Cancer | 115 | 3.7 | 111 | 3.7 | 154 | 7.8 | 111 | 5.0 | 169 | 9.6 | 660 | 14.0 |
Figure 1.
A Consort Style Flow Chart from Finnish Screening Arm
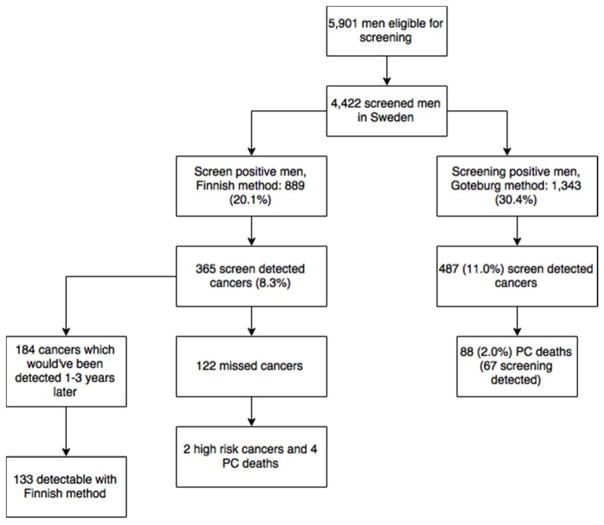
Figure 2.
A Consort Style Flow Chart from Finnish Screening Arm
Of the men who attended at least one screening round, 18.4% underwent a prostate biopsy in Finland compared to 28.4% in Sweden. Biopsy attendance among screen-positive men was 92.1% in Finland and 86.1% in Sweden. Of the biopsied men, 946 of 4,376 (21.6%) were biopsied more than once in Finland and 511 of 1,256 (40.1%) in Sweden (OR 0.74, 95% CI 0.71–0.77).
Of the 23,771 Finnish screening participants, 2,521 (7.0 per 1000-py) were diagnosed with PC during the follow-up, compared with 660 of 4,422 (8.8 per 1000-py) among the Swedish attendees hazard ratio (HR) of 1.01 (0.93–1.10). Out of these cancers, 1,631 (64.7%) in Finland and 570 (85.0%) in Sweden were screen-detected and similarly HR for screen-detected PC was 0.71 (95% CI 0.65–0.78). Of 570 Swedish screen detected cancers were diagnosed on Swedish screening round 6 or 7, which didn’t have a corresponding screening round in Finland. There were 356 cancers among non-attendees in Finland and 63 among Swedish ones.
In Finland, 1,627 additional men would have undergone at least one prostate biopsy with a PSA cut-off of 3 ng/ml. The number of men needed to undergo biopsy to detect one PC was 3.35 (95% CI 3.21–3.49) in Finland and 4.26 (95% CI 3.96–4.63) in Sweden.
The incidence of high-risk PC in the SA was 1.18 per 1000 person-years in Finland and 0.80 in Sweden (HR 1.93, CI 95% 1.47–2.52). Incidence of low and moderate-risk PCs is shown in (table 2).
Table 2.
Finnish screening arm had greater risk for high-risk prostate cancer. Risk groups were defined as follows: low risk was defined as stage T1–T2 with a Gleason score ≤6, M0 and N0. A moderate-risk PC was stage T1–T2 with a Gleason score 7, or T3 with a Gleason score ≤7, both with M0 and N0. High-risk PCs were either stage T1–T3 with a Gleason score 8–10, or stage T4 or M1 or N1 (with any Gleason score)
| Screen detected PCs | Finland | Sweden | ||||
|---|---|---|---|---|---|---|
| Total | Per 1000 person years | Total | Per 1000 person years | HR | CI 95% | |
| Low risk | 1139 | 3.14 | 452 | 5.93 | 0.62 | 0.55–0.69 |
| Moderate risk | 351 | 0.97 | 93 | 1.22 | 0.98 | 0.78–1.23 |
| High risk | 134 | 0.37 | 21 | 0.28 | 1.65 | 1.04–2.61 |
| Interval PCs | ||||||
|
| ||||||
| Low risk | 473 | 1.31 | 52 | 0.68 | 2.60 | 1.96–3.47 |
| Moderate risk | 259 | 0.71 | 27 | 0.35 | 3.01 | 2.01–4.47 |
| High risk | 137 | 0.39 | 16 | 0.21 | 2.56 | 1.52–4.32 |
| Total detected PCs (inc. non-attenders) | ||||||
|
| ||||||
| Low risk | 1795 | 4.95 | 525 | 6.89 | 0.88 | 0.80–9.80 |
| Moderate risk | 761 | 2.10 | 136 | 1.78 | 1.60 | 1.34–1.93 |
| High risk | 405 | 1.11 | 61 | 0.80 | 1.93 | 1.47–2.52 |
During the follow-up, 122 PCs (18.5% of 660) diagnosed in Sweden would have been missed had the Finnish protocol been applied. With the PSA>4ng/ml (or PSA 3.0–3.9 and f/t PSA<16) cut-off, 454 fewer Swedish men would have been screening-positive. In the second and fourth Swedish screening rounds, 187 PCs were diagnosed. With a four-year screening interval, these cancers would likely have been diagnosed at the subsequent screening round two years later (unless clinically detected earlier). Of these 187 cancers, 133 (71.1%) would have been positive with the Finnish cut-off as well, and thus, 54 cancers were found two years earlier in Sweden owing to the shorter screening interval and lower PSA threshold.
Of the 122 Swedish men, whose cancer would have been missed with the Finnish protocol, only two were high-risk cancers (one advanced) and four men died from prostate cancer during the follow-up. Of 769 Swedish men with first positive PSA result between 3–4 ng/ml, 9 died from PC during follow-up.
There were 308 interval PCs among the Finnish screening-positive men. Among those with PSA>3.0 ng/ml, 375 PC cases were detected outside screening. Hence, 67 cases were missed by the Finnish protocol due to higher PSA cut-off and these cancers could have been detected 1–3 years earlier with the Swedish protocol. Of the 1627 men who were potentially screen-positive with the Swedish screening-protocol in Finland, 127 (7.7%) were later diagnosed with a prostate cancer. Of these 1,672 men 19 (1.1%) died from PC.
In mortality analyses follow up time was 496,647 py in Finland and 122,019 py in Sweden. No statistically significant difference was observed in age-stratified prostate cancer mortality between the Finnish and Swedish screening arms (HR 0.88, 95% CI 0.67–1.15). In Finland, 284 (0.57 per 1000-py) and in Sweden 67 (0.55 per 1000-py) screening participants died from PC. Overall, 347 (0.70 per 1000-py) and 88 (0.72 per 1000-py) PC deaths were observed in Finland and Sweden during follow-up.
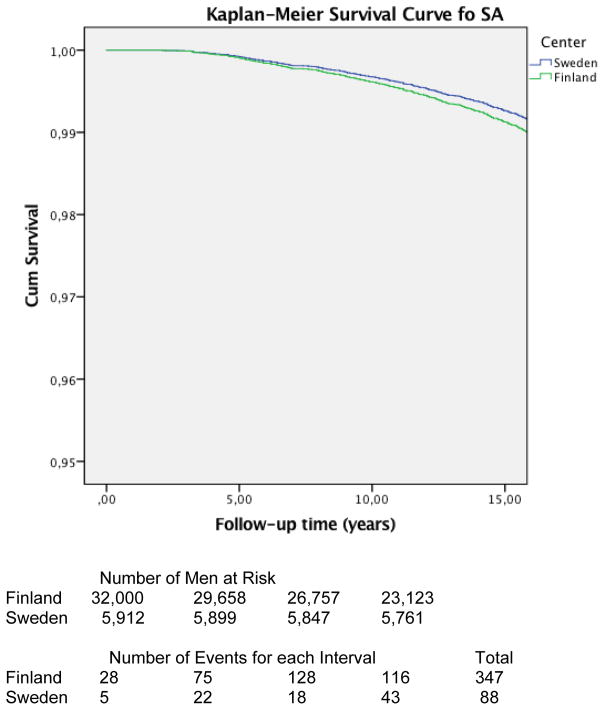
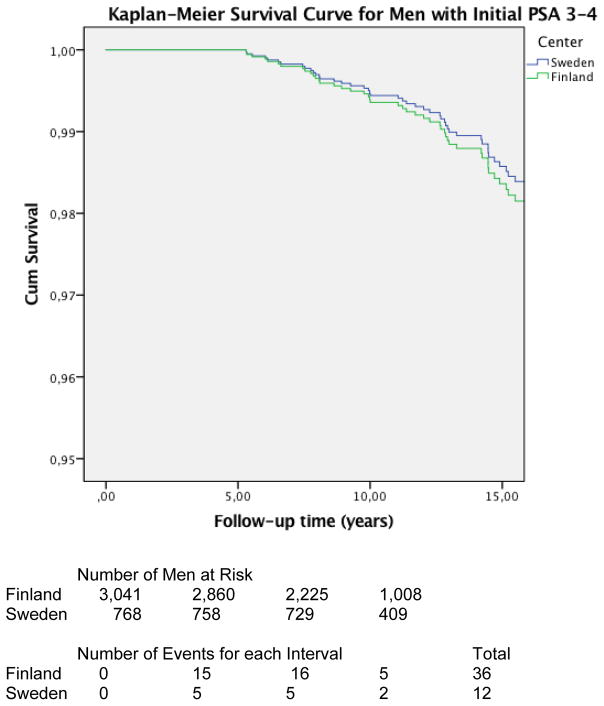
Also, no statistically significant difference in mortality was observed when comparing PC survival between Finnish and Swedish men with initial screening PSA 3–4 (Swedish threshold) HR 0.73 (CI 95% 0.33–1.63) (figure 3 & 4).
Figure 3.
Kaplan-Meier Survival Curve showing prostate cancer survival for all men in SA. No difference was shown in prostate cancer survival between Finnish and Swedish screening arms as hazard ratio was 0.88 (CI 95% 0.67–1.15)
Figure 4.
Kaplan-Meier Survival Curve showing the prostate cancer survival for men with PSA first time between 3–4 (free/total PSA not taken into account). No difference was observed as hazard ratio was 0.75 (95% CI 0.33–1.63). Although the number of events is low in both groups this suggest that if PSA is 3–4 ng/ml in PC screening, it does not matter whether to refer the men to further work-up or not
Discussion
Our results indicate that the lower PSA cut-off value and shorter screening interval in the Swedish ERSPC centre do not account for the larger mortality reduction compared to Finland. Only four of the 88 PC deaths in the Swedish screening arm was due to a PC detectable only with the Swedish screening protocol, but not with the Finnish one. Correspondingly, 19 of the 347 PC cases that turned out to be fatal in Finland would have been detectable with a lower PSA threshold. Of the 88 PC deaths in Sweden, 10 were PCs with initial PSA 3–4 and f/t PSA >16, indicating the lethal potential of even early cancers, but also failure screening to prevent all deaths from such cases. No difference of PC mortality was shown between screening arms.
Of the 122 Swedish PCs detected only with the lower PSA threshold, 84% had favourable prognostic features in terms Gleason score and stage. Only two cases that could potentially have been missed were poorly differentiated. Seven high-risk cancers would have been found two years later with the Finnish screening protocol.
The majority of the cancers diagnosed earlier in Sweden were detected by virtue of the shorter screening interval, rather than the lower PSA cut-off. Nevertheless, a third of the additional cancers were detectable only with the combined shorter screening interval and lower PSA threshold. Correspondingly, 67 Finnish interval cancers would have been detected 1–3 years earlier with the Swedish screening protocol due the lower PSA threshold. Use of DRE between 1996–1999 cannot explain differences, as vast majority of cancers found with DRE would have been found with F/T PSA as well.
Roobol et al. [11] conducted a research about PC screening intervals and found that more frequent screening doesn’t lower rates of aggressive cancers. Though they had a different approach as only interval PCs were compared. Also they did not compare PSA threshold (as they were same). Our study has 5 more years of follow-up in case of PC incidence and we also included the PC mortality.
The more intensive screening employed in Sweden resulted in higher frequency of positive screening results and screen-detected cases, particularly low-risk cancer, compared with Finland. On the other hand, the Swedish centre had lower interval cancer rates and there was no excess of moderate or high-risk PC in screening-detected cases or overall. An analysis comparing screening outcomes across ERSPC centres indicated that Sweden and Finland were in the opposite extremes, with a seven-fold higher absolute reduction in PC mortality (number needed to invite) and also more twice as large excess incidence compared with the control arm (number needed for overdiagnosis) in Sweden [8].
The frequency of biopsies in the Finnish trial was roughly 40% lower than in Sweden. Also multiple biopsies were more common in Sweden. Negative prostate biopsies are one of the adverse effects of PSA screening and they are commonly perceived as inconvenient or painful [12]. They also increase the screening costs. Frequent negative prostate biopsies may also decrease participation [13].
Due to the lack of PSA data, we cannot evaluate whether or when the 122 Swedish cases would have been detected that would have been missed with the Finnish protocol. There was, however, little mortality among the cases that would have been missed in Sweden (four deaths from PC), or might have been detected earlier in Finland (19 PC deaths). Overall, approximately 2000 more biopsies would have been needed to possibly avoid 23 deaths.
The incidence of high-risk prostate cancer in Finland was higher during the follow-up, despite the higher baseline risk for such cases in Sweden [6]. Previous reports indicate [6] that incidence of high-risk PC was 2.1% in the Swedish control arm and 1.4% in Finland (RR 1.51) [8]. In our study, incidence of high-risk PCs was 0.60 in Finland and 0.39 in Sweden per 1000-py (HR 1.93). Thus, the more intensive Swedish screening strategy decreases the incidence of high-risk prostate cancers by a third, which is likely a major factor in the mortality reduction.
Cumulative PC mortality was 0.64% in the SA and 1.04 in the CA in Sweden, while it was 0.53% in the Finnish SA and 0.59% in the CA, though the follow-up was longer in Sweden. This means an absolute mortality reduction of 0.40% in Sweden and 0.05% Finland. If Finland had used the Swedish screening protocol, the estimated potential mortality reduction would have been 0.07%, i.e. some increase in the screening effect, but far from the Swedish results. This means that other explanations need to be sought for the difference, such as number of screening rounds or duration of the screening period, contamination in the screening arm or management of PC. A recent modelling study suggested that contamination in the Finnish trial may have contributed to the smaller screening effect [9].
There are some potential weaknesses in this study. The follow-up might be too short, that the reduction of aggressive PCs is reflected to PC mortality. Also the follow-up times were different for centres, but this was compensated by using hazard ratios (Cox-regression). There are some other differences between centres of which effect is hard to establish, for example, Sweden used only sextant biopsies but Finland changed into 12 biopsy cores.
Conclusions
In conclusion, a more intensive screening regimen yields a higher sensitivity, for both low-risk and high-risk cancer. The lower threshold and shorter screening interval in Sweden is unlikely to explain the larger mortality reduction compared to Finland, as only 19 of the 284 lethal Finnish PCs could have been detected (and possibly cured) with the more intensive screening protocol. Therefore, other reasons for the differences need to be considered. The Swedish screening algorithm decreases the incidence of high-risk PC, which is likely to reflect as a mortality impact in longer follow-up as PC has long lead-time.
Acknowledgments
Funding: The study was financially supported by the Academy of Finland (Grant No 260931), Cancer Society of Finland and Pirkanmaa Hospital District Competitive Research Funding. Dr. Sigrid Carlsson’s work on this paper was supported in part by a Cancer Center Support Grant from the National Cancer Institute made to Memorial Sloan Kettering Cancer Center (P30 CA008748). Dr. Carlsson is also supported by a post-doctoral research grant from AFA Insurance.
Footnotes
Conflict of interest: None of the authors have conflicts of interest.
References
- 1.Schroder Fh, Hugosson J, Roobol Mj, Tammela Tl, Zappa M, Nelen V, et al. Screening And Prostate Cancer Mortality: Results Of The European Randomised Study Of Screening For Prostate Cancer (ERSPC) At 13 Years Of Follow-Up. Lancet. 2014;384:2027–35. doi: 10.1016/S0140-6736(14)60525-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Auvinen A, Moss Sm, Tammela Tl, Taari K, Roobol Mj, Schröder Fh, Bangma Ch, Carlsson S, Aus G, Zappa M, Puliti D, Denis Lj, Nelen V, Kwiatkowski M, Randazzo M, Paez A, Lujan M, Hugosson J. Absolute Effect Of Prostate Cancer Screening: Balance Of Benefits And Harms By Center Within The European Randomized Study Of Prostate Cancer Screening. Clinical Cancer Research. 2016;22:243–9. doi: 10.1158/1078-0432.CCR-15-0941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saarimaki L, Tammela Tl, Maattanen L, Taari K, Kujala Pm, Raitanen J, et al. Family History In The Finnish Prostate Cancer Screening Trial. International Journal Of Cancer. 2015;136:2172–7. doi: 10.1002/ijc.29243. [DOI] [PubMed] [Google Scholar]
- 4.Greene Kl, Albertsen Pc, Babaian Rj, Carter Bh, Gann Ph, Han M, et al. Prostate Specific Antigen Best Practice Statement: 2009 Update. J Urol. 2013;189:S2–S11. doi: 10.1016/j.juro.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 5.Heidenreich A, Bastian Pj, Bellmunt J, Bolla M, Joniau S, Van Der Kwast T, et al. EAU Guidelines On Prostate Cancer. Part 1: Screening, Diagnosis, and Local Treatment With Curative Intent-Update 2013. Eur Urol. 2014;65:124–37. doi: 10.1016/j.eururo.2013.09.046. [DOI] [PubMed] [Google Scholar]
- 6.Hugosson J, Carlsson S, Aus G, Bergdahl S, Khatami A, Lodding P, et al. Mortality Results From The Goteborg Randomised Population-Based Prostate-Cancer Screening Trial. Lancet Oncology. 2010;11:725–32. doi: 10.1016/S1470-2045(10)70146-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roobol Mj, Kranse R, Bangma C, Van Leenders A, Blijenberg B, Van Schaik R, et al. Screening For Prostate Cancer: Results Of The Rotterdam Section Of The European Randomized Study Of Screening For Prostate Cancer. Eur Urol. 2013;64:530–9. doi: 10.1016/j.eururo.2013.05.030. [DOI] [PubMed] [Google Scholar]
- 8.Kilpelainen Tp, Tammela Tl, Malila N, Hakama M, Santti H, Maattanen L, et al. Prostate Cancer Mortality In The Finnish Randomized Screening Trial. J Natl Cancer Inst. 2013;105:719–25. doi: 10.1093/jnci/djt038. [DOI] [PubMed] [Google Scholar]
- 9.Kilpelainen TP, Pogodin-Hannolainen D, Kemppainen K, Talala K, Raitanen J, Taari K, et al. Estimate Of Opportunistic Prostate-Specific Antigen Testing In The Finnish Randomized Study Of Screening For Prostate Cancer. J Urol. 2017;198:50–7. doi: 10.1016/j.juro.2017.01.048. [DOI] [PubMed] [Google Scholar]
- 10.Finne P, Stenman U, Maattanen L, Makinen T, Tammela Tlj, Martikainen P, et al. The Finnish Trial Of Prostate Cancer Screening: Where Are We Now? BJU Int. 2003;92(Suppl 2):22–6. doi: 10.1111/j.1465-5101.2003.04397.x. [DOI] [PubMed] [Google Scholar]
- 11.Roobol MJ, Grenabo A, Schröder F, Hugosson J. Interval Cancers In Prostate Cancer Screening: Comparing 2- And 4-Year Screening Intervals In The European Randomized Study Of Screening For Prostate Cancer, Gothenburg And Rotterdam. J Natl Cancer Inst. 2007;99:1296–303. doi: 10.1093/jnci/djm101. [DOI] [PubMed] [Google Scholar]
- 12.Zisman A, Leibovici D, Kleinmann J, Siegel Yi, Lindner A. The Impact Of Prostate Biopsy On Patient Well-Being: A Prospective Study Of Pain, Anxiety And Erectile Dysfunction. J Urol. 2001;165:445–54. doi: 10.1097/00005392-200102000-00023. [DOI] [PubMed] [Google Scholar]
- 13.Makinen T, Auvinen A, Hakama M, Stenman Uh, Tammela Tl. Acceptability And Complications Of Prostate Biopsy In Population-Based PSA Screening Versus Routine Clinical Practice: A Prospective, Controlled Study. Urology. 2002;60:846–50. doi: 10.1016/s0090-4295(02)01864-2. [DOI] [PubMed] [Google Scholar]