Abstract
Objectives
This study compared subjective (questionnaire) and objective (actigraphy) sleep assessments, and examined agreement between these methods, in vulnerable older adults participating in a Veterans Administration Adult Day Health Care (ADHC) program.
Methods
59 ADHC participants (95% male, mean age = 78 years) completed sleep questionnaires and 72 continuous hours of wrist actigraphy. Linear regression was used to examine agreement between methods and explore discrepancies in subjective/objective measures.
Results
Disturbed sleep was common, yet there was no agreement between subjective and objective sleep assessment methods. Compared with objective measures, one-half of participants reported worse sleep efficiency (SE) on questionnaires while one-quarter over-estimated SE. Participants reporting worse pain had a greater discrepancy between subjective and objective SE.
Conclusions
Vulnerable older adults demonstrated unique patterns of reporting sleep quality when comparing subjective and objective methods. Additional research is needed to better understand how vulnerable older adults evaluate sleep problems.
Clinical Implications
Objective and subjective sleep measures may represent unique and equally important constructs in this population. Clinicians should consider utilizing both objective and subjective sleep measures to identify individuals who may benefit from behavioral sleep treatments, and future research is needed to develop and validate appropriate sleep assessments for vulnerable older adults.
Keywords: Adult day health care, discrepancy, insomnia, measurement, objective, sleep, subjective
Introduction
Many older adults participating in Adult Day Health Care (ADHC) programs are challenged by multiple chronic conditions, depression, pain, functional limitations, and cognitive impairment (Rivero, Hughes, Kramer, & Martin, 2012). These same factors also increase risk for sleep problems, including insomnia (Maggi et al., 1998; Maglione et al., 2012; McCrae, 2009; Smagula, Stone, Fabio, & Cauley, 2016). ADHC settings, with their focus on optimizing health and preserving functional independence of vulnerable older adults (Office of Public Affairs & Media Relations, 2005), may provide a unique opportunity for screening and treatment of sleep problems.
Insomnia is marked by difficulty falling or staying asleep, or sleep that is non-restorative and the cause of significant daytime impairment (American Academy of Sleep Medicine, 2014; American Psychiatric Association, 2013). Self-reported insomnia symptoms are associated with increased healthcare utilization (Kaufmann et al., 2013), and greater functional dependence in older adults (Spira et al., 2014). Objectively-recorded poor sleep, including short sleep duration, extended time awake during the night, and reduced sleep efficiency (time asleep/total time in bed), is associated with poor functional performance (Dam et al., 2008; Goldman et al., 2007; Spira et al., 2012) and nursing home placement (Spira et al., 2012).
Research among older adults with insomnia has found discrepancies between subjective reports and objective measures of sleep (e.g., polysomnography or actigraphy) wherein older adults perceive more severe sleep disturbances than is measured via objective means (Kay, Buysse, Germain, Hall, & Monk, 2015; Williams, Kay, Rowe, & McCrae, 2013). Most research on the discrepancy between objective sleep measures and patient-reported sleep quality has used sleep diaries as the subjective measure. Sleep diaries may be challenging for older adults with functional or cognitive limitations, and our experience with older adults in ADHC is that many are not able to complete standard sleep diary assessments (Martin et al., 2017).To our knowledge, there has been no examination of the agreement between single timepoint subjective sleep assessments, including those that could be used in clinical practice (e.g., Pittsburgh Sleep Quality Index, PSQI, or Insomnia Severity Index, ISI), and objective sleep measures (e.g., actigraphy) in vulnerable older adults.
In prior research, we found that older adults attending ADHC may have different patterns of reporting sleep-related symptoms compared with studies of older adults living independently in the community. Utilizing a brief insomnia screening questionnaire, we found that more than two-thirds of ADHC participants endorsed characteristics of insomnia (i.e., extended time to fall asleep or time awake at night); however, only 38% met all criteria for insomnia disorder (Hughes & Martin, 2015). Additionally, of those participants meeting diagnostic criteria for insomnia disorder, 60% rated their overall sleep quality as “good” (Hughes & Martin, 2015). In addition, in a clinical trial evaluating a brief sleep intervention program designed for implementation in ADHCs, we found treatment benefits in terms of objective, but not subjective, sleep outcomes (Martin et al., 2017). This differs from studies of older community-dwelling adults, which typically show larger improvements in subjective rather than objective outcomes (Alessi et al., 2016; Buysse et al., 2011; Rybarczyk, Lopez, Benson, Alsten, & Stepanski, 2002). Together, these findings raise questions about how best to identify older adults who may benefit from sleep-related interventions in ADHC settings.
This article aims to examine the correspondence between subjective and objective evaluations of sleep in vulnerable older adults participating in a Veterans Administration (VA) ADHC program, with the goal of informing providers on how to identify individuals with poor sleep who may be appropriate for sleep-related interventions. We also examined potential predictors of discrepancies, or differences, between subjectively and objectively measured sleep characteristics. More specifically, we set out to answer two research questions: (1) Are subjective questionnaires commonly used to assess sleep disturbance and insomnia predictive of objective sleep measures in this population?; and (2) Are there patient-level predictors of the differences between subjective and objective sleep characteristics, specifically (a) total sleep time (TST), and (b) sleep efficiency (SE). In accordance with our research questions, we hypothesized that subjective questionnaires would not be significant predictors of objective sleep measures, and worse physical health (more comorbid conditions), low cognitive status, and more severe pain would be significant predictors of subjective-objective discrepancies in total sleep time and sleep efficiency.
Methods
Participants
Data presented here were collected during the baseline phase of a randomized controlled trial of a behavioral sleep intervention for older adults participating in VA ADHC (Martin et al., 2017). All ADHC participants aged 60 years and older who were able to communicate verbally and had been enrolled in ADHC for at least one month were invited to participate in the research study. Of 123 individuals who attended the ADHC program during the study period (November 2010 to June 2012) and who were screened for sleep disturbance, 72 were eligible to participate, able to provide self-consent, and agreed to complete the in-depth sleep and health questionnaire, as described below. Reasons for ineligibility (n = 51, 42.5%) included discharge from ADHC prior to enrollment (n = 16), cognitive disorders (identified by ADHC staff; n = 15), refusal (n = 14), death (n = 2) or other reasons (n = 4; socially inappropriate, communication problem, enrolled in another research study, or study ended).
This article utilizes data from the 59 participants (out of 72 enrolled) who had complete data on all baseline sleep questionnaires and wrist actigraphy. Participants who did not complete wrist actigraphy (n = 13) had lower cognitive status (MMSE total score: 20.8 vs 25.5, p < .001), but otherwise there were no differences in demographic, health, or functional characteristics than those with complete actigraphy data. The Institutional Review Board of the VA Greater Los Angeles Healthcare System approved the study. Written informed consent was obtained from all participants. No proxy consent was attempted.
Data Collection
An in-person sleep and health assessment was conducted by a trained research assistant on-site at the ADHC program and included the components listed below. At the first visit, participants completed all demographic and health questionnaires. Participants also received a wrist actigraph and 4-item sleep diary at this visit. The actigraph and sleep diary were returned, and sleep questionnaires were completed at a second visit. This visit was scheduled 4 to 5 days after the first visit depending on the participant’s attendance at ADHC.
Demographics
Basic demographic information was collected including age, gender, race and ethnicity, marital status, years of education, and current living arrangement. Length of ADHC participation was verified with program staff based on medical record and enrollment dates. Electronic health records were reviewed for both health history and prescribed medications.
Physical Health
Functional status, including activities of daily living (ADLs) and instrumental activities of daily living (IADLs), was assessed using the Older Americans Resources and Services (OARS) Multidimensional Functional Assessment Questionnaire (Fillenbaum, 1988). Scores were computed for both ADLs and IADLs; total scores ranged from 0 to 28 with higher scores indicating more functional independence.
Self-rated health was captured with the Medical Outcomes Study Short Form Survey (SF-12) (Ware, Kosinski, & Keller, 1996). Overall health was rated with the single item (rated as poor, fair, good, or very good), and component scores were calculated for each physical and mental health-related quality-of-life component scores (PCS and MCS, respectively). Pain was assessed using the 24-item Geriatric Pain Measure (GPM) (Ferrell, Stein, & Beck, 2000) where scores range from 0 to 42 with higher scores representing more pain. The total number of comorbidities was calculated based on chronic physical and mental health conditions documented in the patient’s electronic health record.
Cognitive and Psychological Health
Cognitive status was assessed with the 19-item Folstein Mini-Mental Status Examination (MMSE) (Folstein, Folsten, & McHugh, 1975). The MMSE assesses orientation, registration, attention/calculation, recall, and language. Scores range from 0 to 30 where higher scores indicate better functioning; scores below 24 are associated with cognitive impairment or dementia. The 9-item Patient Health Questionnaire (PHQ-9) (Kroenke, Spitzer, & Williams, 2001) was used to assess depression. The sleep item in the PHQ-9 was removed in order to avoid inflation of the total score. Scores range from 0 to 27 where higher scores indicate greater depressive symptoms.
Objective Sleep Assessment
At the conclusion of the first set of assessment questionnaires, participants were provided with a wrist actigraph (Actiwatch Spectrum, Philips Respironics), which they were asked to wear on their dominant wrist for three consecutive days and nights (i.e., >72 hours). An actigraph is a small device resembling a sportswatch that utilizes accelerometry to measure activity, including sleep-wake patterns. Data were collected in one-minute epochs with medium threshold scoring applied. Pre-identified thresholds were used to identify periods of low wrist activity as “sleep” and periods of high wrist activity as “wake.” Four variables of interest were collected from wrist actigraphy: total sleep time (minutes), sleep efficiency (defined as total time asleep out of total time in bed), total number of nighttime awakenings, and total minutes awake during the night. These variables are typically evaluated during the course of insomnia treatment (Schutte-Rodin, Broch, Buysse, Dorsey, & Sateia, 2008), thereby representing key variables of interest. The discrepancy analyses focused on total sleep time (TST) and sleep efficiency (SE) since these metrics parallel, and therefore could be compared with, items included in the self-report questionnaires (PSQI). Defined as total amount of time asleep out of total amount of time in bed, SE is used as a key clinical outcome and thought to represent overall sleep quality.
To facilitate accurate actigraphy scoring, participants are often asked to complete a sleep diary while wearing the wrist actigraph (Ancoli-Israel et al., 2016). In this study, participants completed a simplified, written sleep diary while wearing the wrist actigraphy. The diary recorded bedtime, rise time, nighttime sleep quality, and daytime fatigue.
Patient-reported Sleep Assessment
At the conclusion of the actigraphy recordings, participants were asked a series of questionnaires about their sleep. The sleep assessments included questionnaires to assess the participant’s perception of overall sleep quality and insomnia symptoms. The Pittsburgh Sleep Quality Index (PSQI) (Buysse, Reynolds, Monk, Berman, & Kupfer, 1989) is an 18-item questionnaire that captures overall sleep quality based on items related to sleep schedule, frequency of sleep disturbances, daytime consequences of poor sleep, and overall sleep quality rating. Participants’ sleep schedule responses are then used to estimate total sleep time and sleep efficiency (total time asleep/total time in bed). Total scores range from 0 to 21, with clinically defined poor sleep quality indicated by scores greater than 5. In addition to the total score, we used the PSQI 3-factor scoring system that included sleep efficiency (Factor 1), perceived sleep quality (Factor 2), and daily disturbances (Factor 3) (Cole et al., 2006).
The 7-item Insomnia Severity Index (ISI) (Bastien, Vallieres, & Morin, 2001) assesses the frequency and perceived severity of insomnia symptoms. Scores range from 0 to 28 and are broken down into the following categories: no clinically significant insomnia (0 to 7), subthres-hold insomnia (8 to 14), clinically significant moderate insomnia (15 to 21), and clinically significant severe insomnia (22 to 28).
Given both the 1-month enrollment eligibility criteria and brief 1-month intervention that was being tested in the larger study, both the PSQI and ISI questionnaires were modified to ask about sleep over the prior 7 days rather than the prior month or past 2 weeks, respectively.
The 10-item version of the Dysfunctional Beliefs and Attitudes Scale (DBAS), plus three additional items from the original scale relating to age-related misperceptions about sleep (Edinger & Wohlgemuth, 2001), was used to assess the patients’ sleep-related cognitions. Each item was scored on a scale of 0 to 10, with higher scores indicating more disruptive sleep-related cognitions.
Analyses
All analyses were conducted using Stata 13.1 (StataCorp, 2013). Descriptive statistics were calculated for all demographic and sleep variables. The following analyses were used to examine each research question.
Research Question 1
Four individual linear regression models were constructed to examine the relationship between subjective (questionnaire) and objective (actigraphy) sleep characteristics. Subjective evaluations included PSQI sleep efficiency (Factor 1), PSQI sleep disturbance (Factor 2), PSQI daily disturbances (Factor 3), and ISI total score. Outcome variables included each of the following objective sleep characteristics: total sleep time, sleep efficiency, number of nighttime awakenings, and total time awake during the night.
Research Question 2
Based on measures available in both the PSQI and actigraphy, we calculated two additional variables to capture the subjective-objective discordance in total sleep time (TST) and sleep efficiency (SE). Each difference was calculated as [PSQI value – actigraphy value]. Positive values indicated participants reported sleeping better (i.e., higher sleep efficiency or greater sleep time) than the actigraphy estimate. Negative values indicated participants reported sleeping worse (i.e., lower sleep efficiency or total sleep time) than the actigraphy estimate. Multiple linear regressions were used to test for significant predictors of the discordance between subjective and objective sleep measures. Predictors were selected based on the literature and included age, number of chronic medical conditions, functional status, cognitive status, depressive symptoms, pain, and beliefs and attitudes about sleep.
Results
A total of 59 participants completed the baseline assessment and 72 hours of wrist actigraphy (95% male, mean age = 78.3 years). On average, participants had been enrolled in the ADHC program for 27 months and attended the ADHC program an average of 2.3 days per week. Participants had multiple chronic health conditions, impaired functional independence, and reduced quality-of-life. Full demographic and health characteristics are displayed in Table 1.
Table 1.
Demographics and health characteristics (N = 59).
| Characteristics/Demographics | M (SD) | Range |
|---|---|---|
| Demographics | ||
| Age at the time of enrollment, mean (SD) | 78.24 (9.99) | 60 – 97 |
| Male, N (%) | 56 (95%) | – |
| Race, non-Hispanic White, N (%) | 41 (69%) | – |
| Years of Education | 14.41 (2.52) | 8 – 20 |
| Married or living with someone as married, N (%) | 29 (49%) | – |
| Living arrangement, N (%) | – | |
| Your own home/apartment | 37 (63%) | |
| Home of relative or a friend | 8 (13%) | |
| Facility | 13 (22%) | |
| Years since ADHC enrollment | 2.25 (3.19) | .5 – 11 |
| Health characteristics | ||
| Cognitive status, MMSE total score | 25.50 (3.15) | 16 – 30 |
| Number of chronic conditions | 24.15 (14.72) | 1 – 68 |
| Pain Severity, GPM | 16.07 (11.81) | 0 – 39 |
| Depression, PHQ9 | 5.53 (5.22) | 0 – 20 |
| Functional Status, OARS total | 20.81 (4.92) | 7 – 28 |
| SF-12 Physical Subscale | 36.52 (9.21) | 13.92 – 58.49 |
| SF-12 Mental Subscale | 50.15 (11.90) | 22.69 – 69.21 |
Sleep problems were common among participants based on both subjective questionnaires and objective wrist actigraphy. Based on subjective questionnaires, almost two-thirds of participants (65%) fell above the cutoff for clinically significant sleep disturbance (PSQI total score > 5). Based on objective actigraphy, the average sleep efficiency in our sample was 83.8% (SD = 7.3%) while total time awake during the night was 89 minutes (SD = 41.4). Both values exceed normal clinical thresholds (i.e., suggesting insomnia), including 85% or lower sleep efficiency, and 30 minutes or more time awake during the night (Schutte-Rodin et al., 2008). Despite the high prevalence of sleep problems, only 24 percent of participants rated their overall sleep quality as “fairly bad” or “very bad.” Sleep characteristics are displayed in Table 2.
Table 2.
Sleep characteristics (N = 59).
| Sleep Characteristic | M (SD) | Range |
|---|---|---|
| Subjective Sleep Characteristics | ||
| Time to fall sleep (minutes) | 30.8 (38.04) | 0 – 240 |
| Total sleep time (hours) | 6.69 (1.98) | 2 – 11.5 |
| Sleep Efficiency Percent | 74.7% (17.9%) | 20.0 – 100.0% |
| Pittsburgh Sleep Quality Index, total score | 7.35 (4.40) | 1 – 17 |
| Factor 1: Sleep Efficiency | 2.36 (2.26) | 0 – 6 |
| Factor 2: Perceived Sleep Quality | 3.10 (2.25) | 0 – 8 |
| Factor 3: Daily Disturbances | 1.98 (1.05) | 0 – 5 |
| Sleep Quality Rating, N (%) | ||
| Fairly Good or Very Good | 45 (76%) | – |
| Fairly Bad or Very Bad | 14 (24%) | – |
| Insomnia Severity Index, mean (SD) | 7.71 (6.20) | 0 – 22 |
| No Insomnia, N (%) | 31 (53%) | – |
| Subthreshold, N (%) | 18 (31%) | – |
| Moderate Insomnia, N (%) | 8 (14%) | – |
| Severe Insomnia, N (%) | 2 (3%) | – |
| Objective Sleep Characteristics (wrist actigraphy) | ||
| Total number of nighttime awakenings | 24.89 (8.65) | 7 – 46 |
| Middle of night time awake (minutes) | 89.45 (41.36) | 26 – 229 |
| Total sleep time (hours) | 7.76 (1.38) | 4.90 – 11.99 |
| Sleep Efficiency Percent (Total sleep time/Total time in bed) | 83.8% (7.4%) | 56.3 – 96.4% |
Research Question 1
A series of linear regression models revealed that none of the self-reported sleep questionnaires, including PSQI factors (sleep efficiency, sleep quality, daily disturbances) and ISI total score, were significant predictors of actigraph-assessed sleep characteristics, including total sleep time, sleep efficiency, number of nighttime awakenings, or total time awake during the night (all p’s > .05) (Table 3). The R-squared values for the four models ranged from .05 to .08, suggesting that a notable amount of variability in objective sleep characteristics remained unexplained. These relationships were further examined in a series of bivariate scatter plots (not shown) in which no patterns of linear, or non-liner, association were identified.
Table 3.
Association between subjective sleep questionnaires and objective sleep recordings.
| Factor | Total Sleep Time (mins) | Sleep Efficiency (percent) | Number of Nighttime Awakenings (count) | Total Nighttime Time Awake (mins) |
|---|---|---|---|---|
| PQSI Factor 1: Sleep Efficiency | −2.371 (6.845) |
.054 (.602) |
−.222 (.700) |
−.708 (3.399) |
| PQSI Factor 2: Perceived Sleep Quality | 7.891 (6.766) |
.896 (.595) |
−1.103 (.692) |
−3.648 (3.360) |
| PQSI Factor 3: Daily Disturbances | −10.05 (12.72) |
−.118 (1.120) |
−1.385 (1.301) |
.676 (6.318) |
| Insomnia Severity, ISI Total | −1.537 (3.132) |
−.0481 (.276) |
.245 (.320) |
−.149 (1.555) |
| Observations | 57 | 57 | 57 | 57 |
| R2 | .049 | .062 | .082 | .052 |
| Adjusted R2 | −.024 | −.011 | .011 | −.021 |
Notes: Standard errors appear in parentheses.
p < .05
p < .01
p < .001.
Research Question 2a
The agreement between subjective (PSQI) and objective (actigraphy) total sleep time was r = .55. On average, participants underestimated their total sleep time by sixty-four minutes (range for TST difference = −367 to 142 minutes). Ninety-two percent of participants (N = 54) underestimated TST by 30 minutes or more while seven percent (N = 4) overestimated TST by 30 minutes or more. Only one participant reported TST within a 30-minute window of the actigraphy TST estimate. In a multiple regression model predicting differences in subjective and objective TST, no significant predictors were identified (Table 4). A series of scatterplots further depict a lack of linear, or non-linear, associations between the subjective and objective measures (Figure 1).
Table 4.
Predictors of discrepancies (differences) between subjective and objective sleep efficiency and total sleep time.
| Predictive Factor | Total Sleep Time | Sleep Efficiency |
|---|---|---|
|
| ||
| PSQI (TST) – Acti (TST) | PSQI (SE) – Acti (SE) | |
| Age | 1.648 (1.610) |
.292 (.267) |
| Depression, PHQ9 | .106 (4.517) |
.344 (.748) |
| Total chronic conditions | −1.181 (1.117) |
−.0723 (.185) |
| Cognitive status, MMSE | 4.293 (5.303) |
.677 (.878) |
| Functional status, OARS | .401 (3.287) |
.324 (.544) |
| Pain severity, GPM | −2.707 (1.579) |
−.587* (.261) |
| Beliefs and attitudes about sleep, DBAS | −1.324 (6.218) |
−.987 (1.030) |
| Observations | 57 | 57 |
| R2 | .138 | .171 |
| Adjusted R2 | .015 | .052 |
Notes: Standard errors appear in parentheses.
p < .05
p < .01
p < .001.
Figure 1.
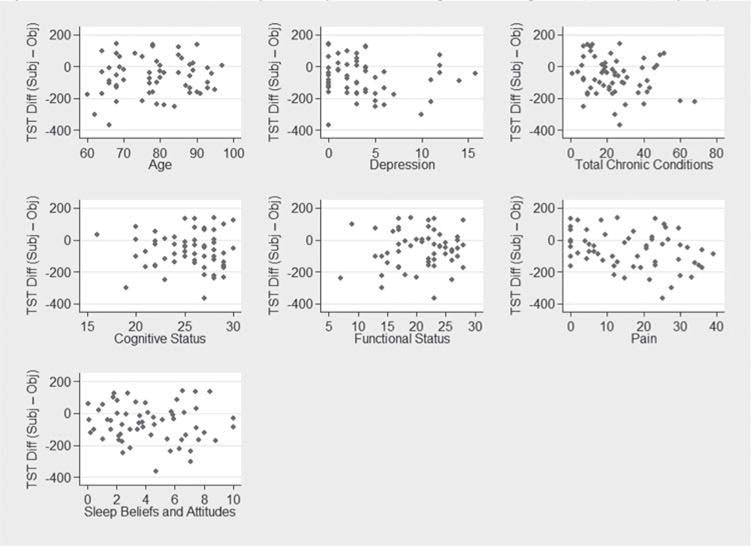
Patient-level predictors of subjective-objective total sleep time discrepancies (TST Diff (Subj-Obj)).
Differences calculated as PSQI (total sleep time) – actigraphy (total sleep time). Positive values suggest participant reports better sleep compared with wrist actigraphy recordings. Negative values suggest participant reports worse sleep compared with wrist actigraphy recordings.
Research Question 2b
The agreement between subjective and objective sleep efficiency (SE) was r = .16. On average, subjective estimates of sleep efficiency were 9% lower (range for SE difference = −56% to 28%) compared with actigraphy estimates. Fifty-four percent of participants (N = 32) underestimated SE by 5 percentage points or more, while 27% (N = 16) overestimated SE by 5 percentage points or more. Only 11 participants (19%) reported SE within five percentage points of the actigraphy SE estimate. In a multiple regression model predicting differences in subjective and objective sleep efficiency, more severe pain was a significant predictor while age, cognitive status, depression, functional status, number of comorbidities, and beliefs and attitudes about sleep were not significant predictors (Table 4). A series of scatterplots further depict no significant associations between all other predictors (Figure 2). The discrepancy between subjective and objective sleep efficiency percent increased as pain severity increased (higher GPM score). Figure 3 depicts this association showing the predicted mean of the discrepancy (with a 95% CI) as a function of GPM.
Figure 2.
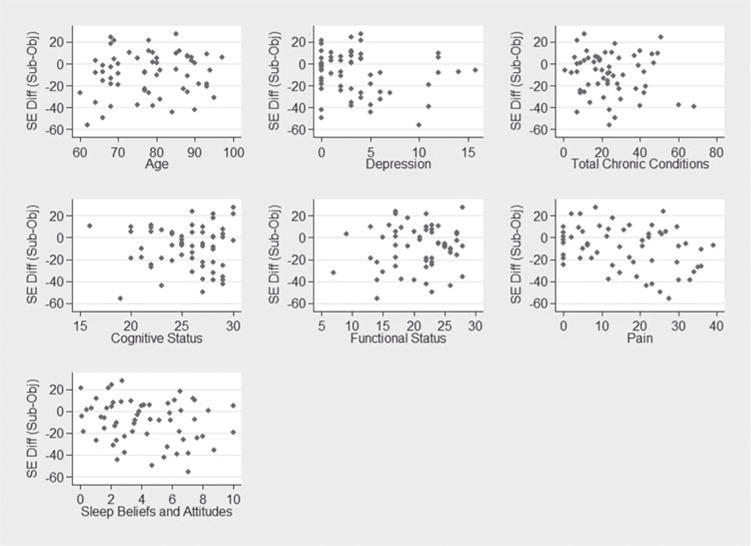
Patient-level predictors of subjective-objective sleep efficiency discrepancies (SE Diff (Subj-Obj)).
Differences calculated as PSQI (total sleep time) – actigraphy (total sleep time). Positive values suggest participant reports better sleep compared with wrist actigraphy recordings. Negative values suggest participant reports worse sleep compared with wrist actigraphy recordings.
Figure 3.
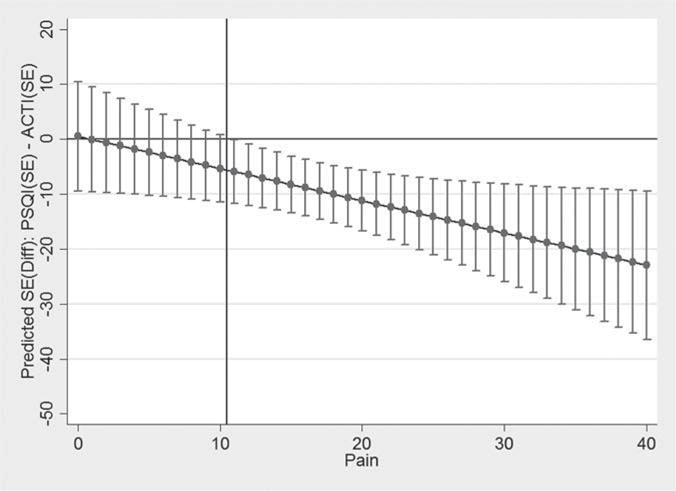
Predicted means (with 95% CIs) of subjective-objective sleep efficiency discrepancy by pain score.
Confidence intervals to the left of the X-line (≤10) include 0 while confidence intervals to the right of the X line (≥11) exclude 0.
Discussion
To our knowledge, this is the first study to conduct an in-depth assessment of sleep among older adults participating in ADHC services and the first to explore the discrepancies between commonly used, validated sleep questionnaires and wrist actigraphy in this vulnerable population. Sleep problems were common according to both subjective and objective sleep assessments; however, there was virtually no agreement between subjective sleep questionnaires and objective recordings captured by wrist actigraphy. Although more global sleep disturbance was common, as indicated by high scores on the PSQI, few participants met criteria for moderate or severe insomnia on the ISI.
Similar to other studies that found older adults with sleep complaints tend to be negative reporters of sleep (McCrae et al., 2005), we found that one-half of ADHC participants underestimated their sleep efficiency, meaning self-reported questionnaires indicated less, or worse, sleep compared with objective wrist actigraphy. However, we also found that twenty-seven percent of participants overestimated their sleep efficiency, perceiving their sleep quality to be better than that recorded by objective wrist actigraphy. This discrepancy mirrors our earlier work (Hughes & Martin, 2015) suggesting that ADHC participants may not negatively evaluate sleep problems. Our findings suggest that vulnerable older adults may evaluate poor sleep in unique ways not fully captured by existing questionnaires and/or in different ways compared with younger, healthier older adults.
We elected to explore predictors of discrepancies in subjective and objective sleep characteristics (TST and SE) with the goal of isolating clinical variables that could aid providers in identifying older patients who may benefit from additional sleep assessment or treatment. Our hypothesis was only partially supported. Dysfunctional attitudes about sleep and greater depressive symptoms were not significant predictors of discrepancies. However, more severe pain was associated with more negative reports of sleep efficiency. As shown in Figure 3, the difference between self-reported and actigraphy-measured sleep efficiency increased as pain severity also increased. Importantly, those without pain symptoms were most accurate in reporting their sleep patterns. These findings suggest older adults with chronic pain may be candidates for objective sleep assessments due to their tendency to more negatively evaluate their sleep.
Prior research comparing sleep assessment methods found that older adults who complained of a sleep problem exhibited a larger discrepancy between subjective questionnaires and objective wrist actigraphy (Williams et al., 2013). These findings suggest that presence of insomnia, including more severe symptoms, may be associated with a larger discrepancy. In a follow-up analysis, we added insomnia severity to regression models predicting TST and SE subjective-objective discrepancies (Table 5). These models revealed that more severe insomnia was associated with more negative sleep reports (i.e., subjective questionnaires indicated worse sleep compared with wrist actigraphy). Pain was no longer a significant predictor of SE discrepancies, suggesting that further researcher is needed to better understand how pain and insomnia interact in this population to impact subjective sleep evaluations.
Table 5.
Insomnia severity as predictor of discrepancies (differences) between subjective and objective sleep efficiency and total sleep time.
| Predictive Factor | Total Sleep Time
|
Sleep Efficiency
|
|---|---|---|
| PSQI (TST) – Acti (TST) | PSQI (SE) – Acti (SE) | |
| Age | 1.612 1.412) |
.285 .224) |
| Depression, PHQ9 | 2.921 4.026) |
.864 .640) |
| Total chronic conditions | −1.034 .981) |
−.0452 .156) |
| Cognitive status, MMSE | 3.461 4.657) |
.523 .740) |
| Functional status, OARS | −.156 2.887) |
.221 .459) |
| Pain severity, GPM | −.784 1.468) |
−.232 .233) |
| Beliefs and attitudes about sleep, DBAS | 1.968 5.518) |
−.379 .876) |
| Insomnia severity, ISI | −10.25*** 2.591) |
−1.895*** .412) |
| Observations | 57 | 57 |
| R2 | .350 | .425 |
| Adjusted R2 | .242 | .329 |
Notes: Standard errors appear in parentheses.
p < .05
p < .01
p < .001.
As reported in Research Question 1, we did not find a significant association between our self-reported insomnia measures, ISI, and any of our four objective actigraphy measures. Furthermore, only one-quarter of our participants (n = 15) met criteria for insomnia (ISI > 10). As described in the Introduction, an earlier screening phase of this study found several discrepancies in which ADHC participants did not complain about poor sleep quality on qualitative items despite reporting sleep disruption on quantitative items. For example, participants who endorsed taking more than 30 minutes to fall asleep reported “I don’t have sleep problems,” when asked about the duration of sleep related symptoms. We also found that a brief behavioral intervention for insomnia improved actigraphically measured sleep (compared with a control condition), but was not associated with improvements in self-reported sleep measures (Martin et al., 2017). As a result, we believe that few ADHC participants report being dissatisfied or distressed about their sleep problems, thereby causing ISI total scores to be lower than we would expect.
There are several potential explanations for discrepancies described above. First, the lack of agreement between subjective sleep evaluations and objective sleep characteristics may simply reflect the difficulty in accurately gauging the time to fall asleep and/or total hours of sleep, each of which form the basis for the calculation of subjective sleep efficiency using the PSQI. Second, discrepancies may be due to the notable chronicity of sleep problems in this population; sleep problems may be dismissed and simply accepted as “part of older age.” As a result, participants do not report that sleep problems cause significant distress or interference, as assessed by either the PSQI or ISI. Third, given ADHC’s strong emphasis on functional performance and physical rehabilitation, participants may not perceive poor sleep to be as bothersome as functional impairments and may not evaluate sleep problems as severe. Our earlier work found that compared with younger, healthier older adults, ADHC participants more frequently endorsed daytime consequences related to poor sleep (Hughes & Martin, 2015). Although daytime dysfunction is captured by both the PSQI and ISI, it may be that these questionnaires do not accurately or fully reflect how poor sleep impacts participants’ function.
Limitations of both the subjective and objective measures may have also contributed to observed discrepancies. Although both the PSQI and ISI are commonly used in adult outpatient settings (Luyster et al., 2015; Smith & Wegener, 2003), their length and item complexity may be prohibitive for both ADHC providers and participants. Our findings suggest additional research is needed to determine appropriate self-report and objective sleep assessments for frail and/or vulnerable older adults, including the extent to which each method provides unique information. While the Assessing Care of Vulnerable Elders (ACOVE) project advocated for regular screening for sleep disturbance in vulnerable older adults (Martin & Fung, 2007), there are no recommendations for how to best adapt screening procedures for this population. It is unknown how vulnerable older adults, including those challenged by competing cognitive and functional limitations, evaluate sleep problems and how such problems are prioritized in relationship to pain symptoms, and/or other health concerns. Furthermore, there has been little validation and/or revision of existing self-reported questionnaires with vulnerable older adults utilizing home and community-based long-term services. It is unknown whether traditional screening thresholds (i.e., poor sleep and insomnia defined as PSQI > 5 and ISI > 10, respectively) are appropriate for this population. Additional validation studies are needed to identify appropriate cutpoints associated with both clinically significant objective sleep complaints and other major outcomes of interest, including functional status, cognitive performance, quality-of-life, and healthcare utilization (Fung, Vitiello, Alessi, & Kuchel, 2016).
Wrist actigraphy should be viewed as an estimation of sleep parameters, rather than a direct measure. Although actigraphy scoring algorithms are optimized to correspond to EEG assessed sleep, it is possible that periods of motionless inactivity while awake are incorrectly scored as sleep, thereby overestimating total sleep time and sleep efficiency (Siverstein et al., 2006). Given this overestimation of sleep time, we believe true objective sleep characteristics, as captured by wrist actigraphy, to be worse than what was recorded in this study. An overestimation of sleep would result in a greater discrepancy than what our results show and further highlight the unique under-reporting patterns observed in ADHC participants and potential missed opportunities to identify and treat sleep problems if relying solely on subjective questionnaires.
Despite this study’s strengths, several limitations should be noted. First, retrospective self-report of sleep problems may bias results; however, similar questions are used routinely in clinical care, increasing the relevance of our findings. Second, it is unknown whether participants’ reports reflected sleep patterns over the past week timeframe, as indicated in the questionnaires, or more historical patterns. Additionally, although the wrist actigraphy and sleep questionnaires were collected over the same 7-day period, there was not direct overlap as participants were instructed to wear the actigraph for a minimum of three nights. Third, cognitive challenges and general poor adherence prevented collection of a full sleep diary data, which limited our ability to examine additional sleep characteristics, including sleep onset latency and number and duration of nighttime awakenings. Third, veterans tend to have more medical comorbidities and complexity compared with their non-veteran counterparts (Agha, Lofgren, VanRuiswyk, & Layde, 2000), thereby limiting the generalizability of our results beyond VA settings.
In closing, we believe the refinement of self-reported screening questionnaires and their integration into regular clinical care could be of tremendous benefit to ADHC programs and similar community-based long-term support services. However, additional research in sleep measurement and validation is warranted as subjective questionnaires and wrist actigraphy may capture unique aspects of sleep quality (Landry, Best, & Liu-Ambrose, 2015). Utilizing both assessment methods may provide a more complete picture of sleep patterns, particularly in patients with chronic pain. Sleep patterns and sleep quality are not routinely evaluated or addressed in ADHC clinical practices (Rivero et al., 2012), and developing validated, brief screening methods are a critical step toward improving clinical care in these patients. Given behavioral treatments have been shown to be safe and effective in treating sleep problems in older adults (Alessi et al., 2016; Bloom et al., 2009; Buysse et al., 2011; Morin, Colecchi, Stone, Sood, & Brink, 1999) and that sleep problems are associated with poor health outcomes, regular attention to sleep patterns and quality in vulnerable older adults is warranted.
Clinical Implications.
Among vulnerable older adults, subjective sleep questionnaires do not predict poor objective sleep characteristics, as measured by wrist actigraphy.
Severe pain and more severe insomnia symptoms may interfere with both subjective and objective assessments of sleep quality in older adults.
Additional research is needed to develop and validate appropriate sleep assessments for vulnerable older adults.
Clinicians should consider utilizing both objective and subjective methods to assess sleep problems in this population.
Acknowledgments
This work was completed at the Greater Los Angeles VA Health Care System Geriatric Research, Education and Clinical Center.
Funding
VA Rehabilitation Research and Development Service Merit Review Project (1RX000135-01, PI: Martin). Additional support from VA Greater Los Angeles Healthcare System, Geriatric Research, Education, and Clinical Center (GRECC); Center for Health Services Research in Primary Care, Durham VA Medical Center (Hughes, CIN 13-410); Office of Academic Affiliations, VA Health Services Research & Development (Hughes; TPH 21-000); National Institute on Aging of the National Institutes of Health (Dzierzewski; K23AG049955, Fung K23AG045937, Song K23AG055668), and the Beeson Career Development in Aging Research Award Program (Fung, supported by NIA, AFAR, The John A. Hartford Foundation, and the Atlantic Philanthropies).
Footnotes
Disclosure Statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article. The views of this manuscript are those of the authors and do not reflect the views of the funding agencies or the federal government.
References
- Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at VA medical centers sicker? A comparative analysis of health status and medical resource use. Archives of Internal Medicine. 2000;160:3252–3257. doi: 10.1001/archinte.160.21.3252. [DOI] [PubMed] [Google Scholar]
- Alessi CA, Martin JL, Fiorentino L, Fung CH, Dzierzewski JM, Rodriguez Tapia JC, Mitchell MN. Cognitive Behavioral Therapy for Insomnia in older veterans using nonclinician sleep coaches: Randomized controlled trial. Journal of American Geriatrics Society. 2016;64(9):1830–1838. doi: 10.1111/jgs.14304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Academy of Sleep Medicine. Insomnia, International Classification of Sleep Disorders. 3rd. Chicago, IL: Author; 2014. [Google Scholar]
- American Psychiatric Association. Desk Reference to the Diagnostic Criteria from DSM-5. Arlington, VA: 2013. [Google Scholar]
- Ancoli-Israel S, Martin JL, Blackwell TL, Buenaver L, Liu L, Meltzer LJ, Taylor DJ. The SBSM guide to actigraphy monitoring: Clinical and research applications. Behavioral Sleep Medicine. 2016;13:S4–S38. doi: 10.1080/15402002.2015.1046356. [DOI] [PubMed] [Google Scholar]
- Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Medicine. 2001;2:297–307. doi: 10.1016/S1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- Bloom HG, Ahmed I, Alessi CA, Ancoli-Israel S, Buysse DJ, Kryger MH, Zee PC. Evidence-based recommendations for the assessment and management of sleep disorders in older persons. Journal of the American Geriatrics Society. 2009;57(5):761–789. doi: 10.1111/j.1532-5415.2009.02220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Germain A, Moul DE, Franzen PL, Brar LK, Fletcher ME, Monk TH. Efficacy of brief behavioral treatment for chronic insomnia in older adults. Archives of Internal Medicine. 2011;171(10):887–895. doi: 10.1001/archinternmed.2010.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research. 1989;23:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Cole JC, Motivala SJ, Buysse DJ, Oxman MN, Levin MJ, Irwin MR. Validation of a 3-factor scoring mdoel for the Pittsburgh Sleep Quality Index in older adults. Journal of Sleep and Sleep Disorders Research. 2006;29(1):112–116. doi: 10.1093/sleep/29.1.112. [DOI] [PubMed] [Google Scholar]
- Dam TT, Ewing SK, Ancoli-Israel S, Ensrud KE, Redline S, Stone KL, Osteoporotic Fractures in Men Research Group Association between sleep and physical function in older men: The osteoporotic fractures in men sleep study. Journal of the American Geriatrics Society. 2008;56(9):1665–1673. doi: 10.1111/j.1532-5415.2008.01846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillenbaum GG. Multidimensional Functional Assessment of Older Adults: The Duke Older Americans Resources and Services Procedures. Lawrence Erlbaum Associates; Hillsdale, NJ: 1988. [Google Scholar]
- Edinger JD, Wohlgemuth WK. Psychometric comparisons of the standard and abbreviated DBAS-10 versions of the dysfunctional beliefs and attitudes about sleep questionnaire. Sleep Medicine. 2001;2:493–500. doi: 10.1016/S1389-9457(01)00078-8. [DOI] [PubMed] [Google Scholar]
- Ferrell BA, Stein WM, Beck JC. The geriatric pain measure: Validity, reliability, and factor analysis. Journal of the American Geriatrics Society. 2000;48:1669–1673. doi: 10.1111/jgs.2000.48.issue-12. [DOI] [PubMed] [Google Scholar]
- Folstein M, Folsten M, McHugh P. Mini-Mental State: A practical method for grading the cognitive state of patients for the clinican. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fung CH, Vitiello MV, Alessi CA, Kuchel GA, AGS/NIA Sleep Conference Planning Committee and Faculty Report and research agenda of the american geriatrics society and national institute on aging bedside-to-bench conference on sleep, circadian rhythms, and aging: New avenues for improving brain health, physical health, and functioning. Journal of the American Geriatrics Society. 2016;64(12):e238–e247. doi: 10.1111/jgs.2016.64.issue-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman SE, Stone KL, Ancoli-Israel S, Blackwell TL, Ewing SK, Boudreau R, Newman AB. Poor sleep is associated with poorer physical performance and greater functional limitations in older women. Sleep. 2007;30(10):1317–1324. doi: 10.1093/sleep/30.10.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JM, Martin JL. Sleep characteristics of Veterans Affairs Adult Day Health Care participants. Behavioral Sleep Medicine. 2015;13(3):197–207. doi: 10.1080/15402002.2013.855212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann CN, Canham SL, Mojtabai R, Gum AM, Dautovich ND, Kohn R, Spira AP. Insomnia and health services utilization in middle-aged and older adults: Results from the Health and Retirement Study. The Journals of Gerontology: Series A. 2013;68(12):1512–1517. doi: 10.1093/gerona/glt050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay DB, Buysse DJ, Germain A, Hall M, Monk TH. Subjective-objective sleep discrepancy among older adults: Associations with insomnia diagnosis and insomnia treatment. Journal of Sleep Research. 2015;24(1):32–39. doi: 10.1111/jsr.2015.24.issue-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JBW. Validity of a brief depression severity measure. Journal General Internal Medicine. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry GJ, Best JR, Liu-Ambrose T. Measuring sleep quality in older adults: A comparison using subjective and objective methods. Frontiers in Aging Neuroscience. 2015;7 doi: 10.3389/fnagi.2015.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyster FS, Choi J, Yeh CH, Imes CC, Johansoon AEE, Chassens ER. Sceening and evaluation tools for sleep disorders in older adults. Applied Nursing Research. 2015;28(4):334–340. doi: 10.1016/j.apnr.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi S, Langlois JA, Minicuci N, Grigoletto F, Pavan M, Foley DJ, Enzi G. Sleep complaints in community-dwelling older persons: Prevalence, associated factors, and reported causes. Journal of the American Geriatrics Society. 1998;46:161–168. doi: 10.1111/jgs.1998.46.issue-2. [DOI] [PubMed] [Google Scholar]
- Maglione JE, Ancoli-Israel S, Peters KW, Paudel ML, Yaffe K, Ensrud KE, Stone KL. Depressive symptoms and subjective and objective sleep in community-dwelling older women. Journal of the American Geriatrics Society. 2012;60:635–643. doi: 10.1111/jgs.2012.60.issue-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JL, Fung CH. Quality Indicators for the Care of Sleep Disorders in Vulnerable Elders. Journal of the American Geriatrics Society. 2007;55:S424–S430. doi: 10.1111/jgs.2007.55.issue-s2. [DOI] [PubMed] [Google Scholar]
- Martin JL, Song Y, Hughes JM, Jouldjian S, Dzierzewski JM, Fung CH, Alessi CA. A four-session sleep intervention program improves sleep for older adult day health care participants: Results of a randomized controlled trial. Sleep. 2017;40(8):097. doi: 10.1093/sleep/zsx079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrae CS. Late-life comorbid insomnia: Diagnosis and treatment. American Journal of Managed Care. 2009;15:S14–S23. [PubMed] [Google Scholar]
- McCrae CS, Rowe MA, Tierney CG, Dautovich ND, DeFinis AL, McNamara JPH. Sleep complaints, subjective and objective sleep pattersn, health, psychological adjustment, and daytime functioning in community-dwelling older adults. Journal of Gerontology: Psychological Sciences. 2005;60B(4):182–189. doi: 10.1093/geronb/60.4.P182. [DOI] [PubMed] [Google Scholar]
- Morin CM, Colecchi C, Stone J, Sood R, Brink D. Behavioral and pharmacological therapies for late-life insomnia: A randomized controlled trail. Journal of the American Medical Association. 1999;281(11):991–999. doi: 10.1001/jama.281.11.991. [DOI] [PubMed] [Google Scholar]
- Office of Public Affairs & Media Relations. VA Long-term Care. Washington, DC: Department of Veterans Affairs; 2005. [Google Scholar]
- Rivero T, Hughes JM, Kramer BJ, Martin JL. Veterans Health Administration Adult Day Health Care programs: Variations and common features. Paper presented at the Gerontological Society of America; San Diego, CA. Nov, 2012. [Google Scholar]
- Rybarczyk B, Lopez M, Benson R, Alsten C, Stepanski EJ. Efficacy of two behavioral treatment programs for comorbid geriatric insomnia. Psychology and Aging. 2002;17:288–298. doi: 10.1037/0882-7974.17.2.288. [DOI] [PubMed] [Google Scholar]
- Schutte-Rodin S, Broch L, Buysse DJ, Dorsey CM, Sateia M. Clinical guidelines for the evaluation and management of chronic insomnia in adults. Journal of Clinical Sleep Medicine. 2008;4(5):487–504. [PMC free article] [PubMed] [Google Scholar]
- Siverstein B, Omvik S, Havik OE, Pallesen S, Bjorvatn B, Hostmark Nielsen G, Hilde Nordhus I. A comparison of actigraphy and polysomnography in older adults treated for chronic primary insomnia. Sleep. 2006;29(10):1353–1358. doi: 10.1093/sleep/29.10.1353. [DOI] [PubMed] [Google Scholar]
- Smagula SF, Stone KL, Fabio A, Cauley JA. Risk factors for sleep disturbances in older adults: Evidence from prospective studies. Sleep Medicine Reviews. 2016;25:21–30. doi: 10.1016/j.smrv.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MT, Wegener ST. Measures of sleep: The Insomnia Severity Index, Medical Outcomes Study (MOS) Sleep Scale, Pittsburgh Sleep Diary (PSD), and Pittsburgh Sleep Quality Index (PSQI) Arthritis & Rheumatism. 2003;49(S5):S184–S196. doi: 10.1002/(ISSN)1529-0131. [DOI] [Google Scholar]
- Spira AP, Covinsky K, Rebok GW, Punjabi NM, Stone KL, Hillier TA, Yaffe K. Poor sleep quality and functional decline in older women. Journal of American Geriatrics Society. 2012;60:1092–1098. doi: 10.1111/j.1532-5415.2012.03968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spira AP, Covinsky K, Rebok GW, Stone KL, Redline S, Yaffe K. Objectively measured sleep quality and nursing home placement in older women. Journal of the American Geriatrics Society. 2012;60(7):1237–1243. doi: 10.1111/jgs.2012.60.issue-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spira AP, Kaufmann CN, Kasper JD, Ohayon MM, Rebok GW, Skidmore E, Reynolds CF., III Association between insomnia symptoms and functional status in US older adults. Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 2014;69(7):S35–S41. doi: 10.1093/geronb/gbu116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- StataCorp. Stata Statistical Software: Release 13.1. College Station, TX: StataCrop LP; 2013. [Google Scholar]
- Ware JE, Kosinski M, Keller SD. A 12-item short-form health survey: Construction of scales and preliminary tests of reliability and validity. Medical Csare. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- Williams JM, Kay DB, Rowe M, McCrae CS. Sleep discrepancy, sleep complaint, and poor sleep among older adults. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2013;68(5):712–720. doi: 10.1093/geronb/gbt030. [DOI] [PMC free article] [PubMed] [Google Scholar]


