Abstract
Thymic dendritic cells (tDCs) play an important role in central tolerance by eliminating self-reactive thymocytes or differentiating them to regulatory T (Treg) cells. However, the molecular and cellular mechanisms underlying these functions are not completely understood. We found that mouse tDCs undergo maturation following cognate interaction with self-reactive CD4+ thymocytes and that this maturation is dependent on CD40 signaling. Ablation of CD40 expression in tDCs resulted in a significant reduction in the number of Treg cells in association with a significant reduction in the number of mature tDCs. In addition, CD40-deficient DCs failed to fully mature upon cognate interaction with CD4+ thymocytes in vitro and failed to differentiate them into Treg cells to a sufficient number. These findings suggest that tDC mature and potentiate Treg cell development in feedback response to self-reactive CD4+ thymocytes.
Keywords: Dendritic cell, DC maturation, CD40, regulatory T cell, thymus
Introduction
Thymus is a primary immune organ that supports the development of T cells. Hematopoietic precursors originate from bone marrow (BM), migrate to the thymus, and generate T cell receptors (TCRs) through random gene rearrangement. This gene rearrangement generates infinite diversity to the repertoire of TCRs and enables the organism to mount T cell immunity to a broad range of pathogens. Inevitably, however, this diversity also puts the organism in danger because some T cells will express TCRs reactive to self-antigens and potentially mount autoimmunity. Thus, it is critical to remove these self-reactive thymocytes before they emigrate to periphery. This selection process occurs through the interaction of the self-reactive thymocytes with antigen presenting cells (APCs) in the thymus (1). This interaction drives interacting thymocytes to either apoptotic cell death or differentiation to Treg cells (2, 3).
DCs represent one of the major APCs in the thymus. Two subsets are present, one being Sirpα+ and the other being CD8+. Sirpα+ thymic DCs (tDCs) originate from the periphery (4). They localize in the cortico-medullary junction and present circulating antigens (5, 6). CD8+ tDCs develop in situ in the thymus from the DC-thymocyte common precursors (7). They primarily localize in the medullary region and present antigens derived from medullary thymic epithelial cells including the tissue-restricted antigens (8–10). Antigen-presentation by both tDC subsets contributes substantially to the elimination of self-reactive thymocytes or differentiating them into Treg cells, although the specific molecular and cellular mechanisms underlying these functions are not completely understood (11).
DC function is often associated with its maturation (12). In steady-state, DCs are phenotypically “immature”. They express low level of MHC class II molecules at the surface and even lower levels of T cell co-stimulatory molecules such as CD80 and CD86. Despite MHC class II is expressed abundantly in immature DCs, it turns over rapidly (13, 14). Accordingly, immature DCs are inefficient at accumulating peptide-MHC complexes (pMHCII) in the surface and incompetent at stimulating T cells (15). In contrast, “mature” DCs express elevated levels of MHC and costimulatory molecules and are highly adept at presenting antigens to T cells for their stimulation (16, 17). Previous cell biological analysis has demonstrated that DC maturation is triggered by microbial products stimulating the innate immune signaling pathways (18). After the activation of innate immune signaling pathway, DCs downregulate their ability to capture antigens and upregulate the expressions of costimulatory molecules and chemokine receptors (e.g. CCR7) that facilitate DC migration to lymph nodes (19–21). Given these alterations and concomitant secretion of pro-inflammatory cytokines, the maturation effectively couples the detection of innate immune signals to the activation of antigen-specific T cell immune responses by DCs.
DC maturation also occurs in steady-state. A fraction of DCs in the periphery homeostatically upregulate MHCII, costimulatory molecules, and CCR7, migrate to the lymph nodes, and present antigens to T cells (22). However, this homeostatic maturation appears to take different signaling pathways and functions from the microbial product-induced maturation (23). They do not secrete proinflammatory cytokines but upregulate the expression of immune-modulatory molecules (10, 24). Accordingly, they convert the antigen-specific naive T cells into Treg cells (25).
tDCs appear to be comprised of immature and mature cells. DC-Lamp, a marker of human mature DCs, is expressed in a subpopulation of DCs in human thymus (26). In mouse thymi, CD8+ tDCs are segregated into two populations by differential expression of CCR7 and costimulatory molecules (10). However, it is not clearly understood how tDCs mature, whether this maturation is associated with any of their function, and if so, which functions are associated with the maturation. We addressed these questions as presented below.
Materials and Methods
Mice
C57BL/6 (B6), B6(Cg)-Zbtb46tm1(HBEGF)Mnz/J (Zbtb46-DTR), B6.SJL-Ptprca Pepcb/BoyJ (BoyJ), BALB/cJ, B6.129S2-Tcratm1Mom/J (TCRα−/−), B6.129S7-Rag1tm/1Mom/J (RAG1−/−), B6.Cg-Tg(TcraTcrb)425Cbn/J (OT-II), B6.129P2-B2mtm1Unc/J (β2m−/−), B6.129P2-Cd40tm1Kik/J (Cd40−/−), B6.Cg-Tg(Itgax-cre)1-1Reiz/J (CD11c-Cre), B6.129P2-Gt(ROSA)26Sortm1(DTA)Lky/J (ROSA26-floxDTA), and B6.PL-Thy1a/CyJ (Thy1.1) mice were purchased from Jackson laboratory. B6.129-H20Ab1tm1GruN12 (MHCII−/−) and B6.129S6-Rag2tm1Fwa Tg(TcraTcrb)1100Mjb (OT-I) mice were purchased from Taconic. Foxp3GFP (27), Myd88−/− (28), TRIF−/− (29), MAVS−/− (30), TSLPR−/− (31) mice were previously described. TSLPR−/− mice were in BAB/cJ background and compared with WT mice of the same background. Male and female mice were both used in the study, and the mice were at the age of 5 – 8 weeks unless stated otherwise. All mice were maintained in the UCSF mouse facility, and all animal studies were performed according to protocols approved by the UCSF Institutional Animal Ethics Committee.
Antibodies and reagents
The following antibodies were purchased from BioLegend, eBioscience, or BD Biosciences, and used for flow cytometry or enrichment of specific cell types: FITC-Sirpα, PE/Cy7-Sirpα PerCP/Cy5.5-B220, PE/Cy7-CD11c, Brilliant Violet 605-CD11c, Pacific Blue-IA/IE, FITC-YAe, PerCP/Cy5.5-CD45.1, APC-CD45.2, PE/Cy7-CD86, APC-CD86, PE-PD-L1, APC-CD200, PE/Cy7-CD40, PE-Foxp3, Pacific Blue-Foxp3, PerCP/Cy5.5-Thy1.1, APC-TCRβ, FITC-CD8α, Alexa700-CD8α, Alexa700-CD4, Brilliant Violet 605-CD4, Brilliant Violet 605-CD25, APC-CD25, PE/Cy7-CD3, PE/Cy7-CD24, Propidium iodide solution, Fixable Viability Dye eFluor780, Biotin-CD8β, Biotin-CD4, Biotin-CD25, and Biotin-B220. AlexaFluor647-conjugated OVA and crimson bead (0.02 μm) were purchased from ThermoFisher Scientific. Streptavidin-RapidSpheres isolation kit and CD11c-MicroBeads were purchased from STEMCELL technologies and Miltenyi Biotec, respectively.
Enrichment of thymic and splenic DCs
Thymi or spleens were isolated and digested with 125 mg/ml of collagenaseD (Roche) and 62.5 μg/ml of DNaseI (Roche) for 20 min with gentle rocking at 37°C. Digests were filtered through a 70 μm filter cap strainer (BD Falcon), re-suspended in 1 ml of high-density (1.115 g/ml) Percoll solution (GE healthcare), and layered with 1 ml of low-density (1.065 g/ml) Percoll solution followed by 1 ml of PBS. This Percoll gradient was centrifuged at 2,700 rpm for 30 min at 4°C to enrich DCs. Cells between the PBS and low-density Percoll layer were isolated and washed for further use.
Flow cytometry and cell sorting
For surface staining, cells were incubated with fluorophore-conjugated antibodies in FACS buffer (1% BSA in PBS) containing propidium iodide solution for 30 min on ice. When staining DCs, cells were pre-incubated with CD16/32 Fc block antibody for 10 min on ice. For intracellular staining of Foxp3, fixable viability dye and Foxp3/Transcription Factor Staining Buffer Set were used as instructed (eBioscience). Samples were analyzed and sorted by the FACS LSRII system (BD Biosciences) and BD FACS Aria3 Cell Sorter (BD Biosciences), respectively.
BM chimeric mice
BM cells were isolated from femurs and/or tibiae of the indicated donor mice. Recipient mice were lethally irradiated (1,200 rad) and i.v. injected with 3–5×106 BM cells from the donors. For the generation of mixed BM chimeric mice, donor BM cells were mixed in a 1:1 ratio, except for the WT:CD11c-DTA and Cd40−/−:CD11c-DTA mice, in which they were mixed in a 1:4 ratio. Mice were examined 6 weeks later.
DTx treatment of zbtb46-DTR mice
BM cells of Zbtb46-DTR mice were i.v. injected to lethally irradiated WT mice. The reconstituted mice were intraperitoneally injected with 20 ng of DTx (Sigma) per mouse weight (g) on 11, 7, 3, 1, and 0 day before analysis. tDCs of the mice were enriched and analyzed by flow cytometry.
DC transfer
Flt3L-expressing B16 tumor cells were cultured in DMEM supplemented with 10% FBS. Cells were harvested, and ~2×106 cells were subcutaneously injected to B6 mice. When the tumor became 10~20 mm in diameter, mice were sacrificed and DCs were isolated from the spleen by using CD11c-MicroBeads. The purity was confirmed to be over 95 % by flow cytometry. ~1×107 isolated DCs were injected i.v. to BoyJ mice. On the indicated days after the injection, the thymi and spleens of the recipient mice were enriched for DCs and analyzed by flow cytometry.
Antigen capture and presentation by tDCs
tDCs isolated by CD11c-MicroBeads were incubated with AlexaFluor647-conjugated OVA (5 μg/ml), 0.02 μm crimson bead (0.02 %), or I-Eα protein (20 μg/ml) for 1 hr with 10 % FBS-supplemented RPMI media in 37°C incubator. Cells were washed three times and stained with antibodies to be analyzed by flow cytometry.
OVA i.v. injection to mice
OT-II, OT-I, or B6 mice were i.v. injected with PBS or 4 mg of OVA (Worthington). After one or three days, tDCs were enriched and analyzed by flow cytometry.
BMDC culture and cDC (conventional DC) isolation
BM cells were cultured with RPMI media supplemented with 10 % FBS and 100 ng/ml of Flt3-ligand (PeproTech) for 12 days. To isolate cDCs, cells were harvested and pre-incubated with CD16/32 Fc block (UCSF CCF) for 10 min on ice in MACS buffer (PBS with 1 % BSA and 2 mM EDTA), and then with biotin-B220 antibody and Streptavidin-RapidSpheres isolation kit as instructed.
BMDC maturation by thymocytes
Conventional DCs isolated from BMDC culture (4×104) were co-cultured with OT-II or OT-I thymocytes (2×105) in the presence of increasing amounts of OVA323-339 or OVA257-264 peptides, respectively. DC maturation was determined after 24 hours by analyzing the surface levels of MHCII, CD86, PD-L1, CD200, and CD40 among B220−CD11c+MHCII+ cDC population by flow cytometry. CD4SP thymocytes were enriched from OT-II thymocytes by negative selection using biotin-CD8β and biotin-CD25 antibodies and the Streptavidin-RapidSpheres isolation kit. CD8SP thymocytes were enriched from OT-I thymocytes by negative selection using biotin-CD4 and biotin-CD25 antibodies and the Streptavidin-RapidSpheres isolation kit.
Treg cell differentiation by BMDCs
CD4+CD8−CD24+ EGFP− thymocytes (5×104) isolated from Thy1.1: Foxp3-EGFP: OT-II mice were co-cultured with BMcDCs (1×104) derived from WT and CD40−/− mice together with OVA323-339 peptides. After two days, the percentages of CD25+EGFP+ cells among Thy1.1+MHCII− were determined by flow cytometry.
Microarray of tDC subsets
We enriched thymic DCs to FACS-sort MHCIIlowCD86low and MHCIIhighCD86high populations of both Sirpα+ and CD8+ DCs as shown in Fig. 1A. RNA was isolated from duplicate sets of sorted cells using RNeasy mini kit (Qiagen). Sample preparation, labeling, and array hybridizations were performed according to standard protocols from the UCSF Shared Microarray Core Facilities and Agilent Technologies http://www.arrays.ucsf.edu and http://www.agilent.com). Total RNA quality was assessed using a Pico Chip on an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA). RNA was amplified and labeled with Cy3-CTP using the Agilent low RNA input fluorescent linear amplification kits following the manufacturer’s protocol. Labeled cRNA was assessed using the Nanodrop ND-100 (Nanodrop Technologies, Inc., Wilmington DE), and equal amounts of Cy3 labeled target were hybridized to Agilent whole mouse genome 4x44K Ink-jet arrays. Hybridizations were performed for 14 h, according to the manufacturer’s protocol. Arrays were scanned using the Agilent microarray scanner and raw signal intensities were extracted with Feature Extraction v10.5 software.
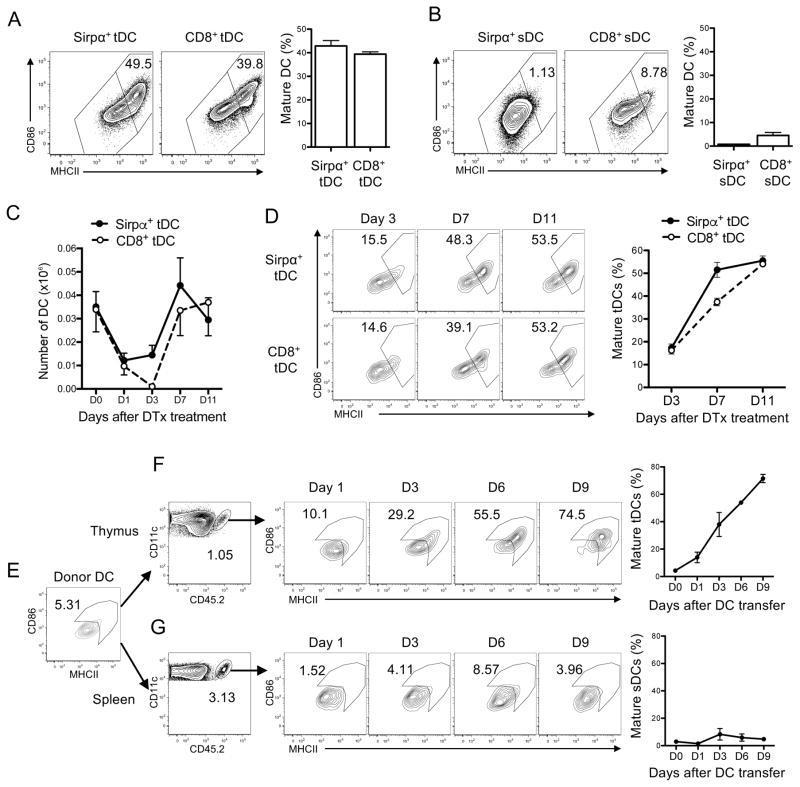
Figure 1. tDCs mature intrathymically.
(A–B) Maturation status of tDCs (A) and splenic DCs (sDCs) (B). MHCIIlowCD86low DCs and MHCIIhighCD86high DCs were defined as immature and mature DCs, respectively. The representative flow cytometry plots with the gating of immature and mature DCs. The percentage numbers of mature tDCs are shown in the left and the quantitated summaries are shown in the right. (C) Recovery of tDCs in Zbtb46-DTR BM chimeric mice injected with DTx. (D) Maturation status of tDCs in the Zbtb46-DTR BM chimeric mice on day 3, 7, and 11 post-DTx injection. The representative flow cytometry plots are shown in the left, and the quantitated summary is shown in the right. (E–G) Maturation of DCs in thymus but not spleen. DCs isolated from spleens of CD45.2+ mice pre-transplanted with the Flt3L-expressing B16 cells were i.v. injected to CD45.1+ mice and analyzed on day 1, 3, 6, and 9 post-DC transfer. The maturation status of donor DCs prior to transfer is shown in E. Maturation status of donor DCs that have homed to the thymus or spleen in the recipient mice are shown in F and G, respectively. The representative flow cytometry plots with the gating and the percentage numbers of mature tDCs (MHCIIhiCD86hi) are shown in the left and the quantitated summaries are shown in the right. Each data represents at least three (A–D) and two (E–G) independent experiments with two to six mice per group. Error bars represent SEM.
Software and statistical analyses
Flow cytometry data were analyzed by flowjo. Graphs were drawn by PRISM (GraphPad). Unpaired two-tailed student’s t-test was performed to assess the significance. For statistical analysis of microarray data, the dataset was normalized using quantile normalization (32), and a one-way ANOVA linear model was fit to the comparison to estimate the mean values and calculated moderated t-statistic (33). Adjusted p values were produced by the method proposed by Holm (34). All procedures were carried out using functions in the R package limma in Bioconductor (35).
Results
tDCs mature intrathymically
We examined the maturation status of tDCs using antibodies specific for MHCII or CD86, the representative DC maturation markers. We found that both Sirpα+ tDCs and CD8+ tDCs were segregated into MHCIIlowCD86low and MHCIIhighCD86high compartments at a near 1:1 ratio (Fig. 1A). In contrast, DCs in the spleen, which are mostly immature, consisted of largely MHCIIlowCD86low cells (Fig. 1B). This finding indicates tDCs are comprised of immature and mature cells at steady-state. Male and female mice had a similar fraction of mature tDCs (Supplemental Fig. 1A). New born, young, and old mice all had a similar fraction of mature tDCs (Supplemental Fig. 1B)
Next, we examined the relationship between the immature and mature tDCs. For this examination, Zbtb46-diphtheria toxin receptor (DTR) mice were utilized, in which DTR is specifically expressed in conventional DCs (36). A previous study has shown that diphtheria toxin (DTx) injection to the Zbtb46-DTR bone marrow chimeric mice in which hematopoietic cells were derived from Zbtb46-DTR mice results in efficient and specific depletion of cDCs (36). Indeed, injection of the Zbtb46-DTR BM chimeric mice with DTx resulted in a reduction in the number of of Sirpα+ tDCs and CD8+ tDCs near to 20 % of uninjected mice by day 1 post-injection (Fig. 1C). Then, we monitored restoration of tDCs by determining the number of tDCs on days 3, 7, and 11 post-DT injection and also examined maturation status of the newly appearing DCs by flow cytometry. We did not find any appreciable recovery of Sirpα+ tDCs or CD8+ tDCs on day 3 post-injection, but found full recovery of both tDC subsets by day 7 post-injection (Fig. 1C). The newly appearing tDCs of both subsets were mostly immature but gradually segregated into immature and mature populations (Fig. 1D), raising a possibility that the mature tDCs represent tDCs that matured intrathymically. To test this possibility, we took an approach of DC transfer. Immature DCs were enriched and isolated from spleens of mice pre-transplanted with Flt3L-expressing B16 cells and injected into congenic mice (Fig. 1E). Maturation of the donor DCs was monitored along the days after transfer. We found that the donor DCs reached the thymus within a day as immature (Fig. 1F). However, they gradually upregulated the expression of MHCII and CD86 resulting in 75 % of them being MHCIIhighCD86high cells by day 9 post-DC transfer (Fig. 1F). This finding strongly supports “intrathymic” maturation of DCs. To determine whether this intra-organ DC maturation is unique to the thymus, we traced the donor DCs in the spleen. We found that the donor DCs reached the spleen as largely immature and remained immature during the entire period of examination (Fig. 1G) suggesting that splenic environment does not support DC maturation. This finding implicates the presence of a thymus-specific factor inducing DC maturation.
A similar set of genes is regulated by tDCs and lymph node migratory DCs during maturation
We examined the gene expression profile of immature and mature Sirpα+ tDCs and CD8+ tDCs by a DNA microarray analysis. 149 and 306 of analyzed genes were differentially expressed in mature cells from immature cells in Sirpα+ DCs and CD8+ DCs, respectively, (Fig. 2A). The majority of these genes were upregulated in mature cells (Fig. 2A). Notably, the list of genes upregulated in Sirpα+ DCs and CD8+ DCs largely overlapped although there were some genes preferentially upregulated in one subset over the other (Fig. 2B, Supplemental Fig. 2). The genes upregulated by both subsets included molecules previously characterized as DC maturation markers including Ccr7, Il15rα, Cd200, and Pd-l1 (Fig. 2C). Flow cytometry confirmed that these molecules are indeed expressed at higher levels in MHCIIhigh mature tDCs than MHCIIlow immature tDCs (Fig. 2D).
Figure 2. A similar set of genes is regulated by tDCs and LN migratory DCs during maturation.
(A) Volcano plots depicting the fold changes of mRNA transcripts (x-axis) during the tDC maturation with the p value (y-axis) of each gene. Genes differentially expressed during maturation of Sirpα+ tDCs (green dots in the left panel) and CD8+ tDCs (blue dots in the right panel) are represented with colored dots and the numbers of genes significantly increased or decreased in each population are shown. Adjusted p value <0.05 (B) A scatter plot depicting the fold changes of mRNA transcripts during the maturation in Sirpα+ DCs (y-axis) and CD8+ tDCs (x-axis). Shown in red are the genes regulated equally in Sirpα+ DCs and CD8+ tDCs during maturation. Shown in blue are the genes regulated in CD8+ tDCs preferentially to Sirpα+ tDCs. Shown in green are the genes regulated in Sirpα+ tDCs preferentially to CD8+ tDCs. Adjusted p value <0.05. (C) Heat map showing the expression levels of representative genes upregulated during maturation of tDCs. (D) Flow cytometry analysis of surface molecules. Note that MHCIIhigh mature tDCs express CCR7, CD200, and PD-L1 at higher levels than MHCIIlow immature tDCs. (E) A scatter plot with its x-axis representing the fold difference between LN-migratory and tissue resident DCs and its y-axis representing the fold difference between mature and immature tDCs. The mRNA transcripts that are significantly (p<0.05) increased or decreased by more than two fold in LN-migratory DCs compared to tissue resident DCs were selected from the microarray data published by Miller et al., 2012. The changes in these transcripts in maturing Sirpα+ and CD8+ tDCs were averaged and plotted. Note the strongly positive correlation (Pearson’s r=0.8465, p<0.0001). (F–G) Heat maps showing the expression levels of representative immune-modulatory genes (F) and inflammatory genes (G).
Next, we compared maturation of tDCs to that of the lymph node (LN) migratory DCs by using a meta-analysis method (37, 38). Although comparing directly between one sample from one microarray experiment and one sample from another microarray experiment is not possible because there are always technical differences in two separate experiments, one can reasonably assess whether the genes found to be differentially expressed in one data set is also found differentially expressed in another data set and if so, whether trend of the difference is similar. Using the published microarray data (24), we identified the genes expressed in LN migratory mature DCs differentially from tissue resident immature DCs. We found that almost all of the genes upregulated in LN migratory DCs were also upregulated in mature tDCs whereas all of the genes downregulated in LN migratory DCs were also downregulated in mature tDCs (Fig. 2E). Additionally, we found that the genes upregulated during maturation of tDCs included many immune-modulatory molecules (Fig. 2F) while the expression of many inflammatory molecules remained low during maturation of tDCs (Fig. 2G), similarly to what was observed to the LN migratory DC maturation (24). Thus, a large set of genes is regulated in a similar fashion by tDCs and LN migratory DCs during maturation.
Mature tDCs capture and present antigens as efficiently as immature tDCs
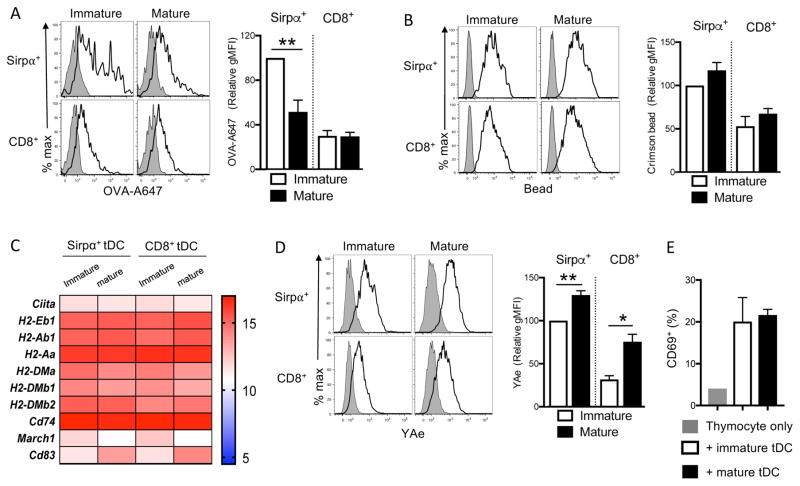
DCs maturing upon contact with microbial products rapidly cease their activities of endocytosing antigens, synthesizing MHCII, and degrading pre-existing MHCII (19, 39–42). This coordinated cellular change enables DCs to present microbial antigens at the maximal amounts and for a prolonged time so that they effectively induce antigen-specific immunity at the early stage of infection. We wondered whether this coordinated regulation of antigen processing and presentation similarly occurs to tDCs during maturation. Interestingly, mature tDCs endocytosed soluble as well as particulate antigens as efficiently as immature tDCs (Fig. 3A, 3B). Besides, mature tDCs transcribed MHCII and the molecules that promote MHCII synthesis and transport as highly as immature tDCs (Fig. 3C). On the other hand, mature tDCs downregulated MARCH1, the ubiquitin ligase that degrades MHCII, and upregulated CD83, an antagonist of MARCH1, thus augmenting the half-life of MHCII (43, 44) (Fig. 3C). Taken together, these findings suggest that mature tDCs continue to internalize surrounding antigens, load them onto newly synthesized MHCII, and present them at the surface for a prolonged time. To verify this possibility, we incubated immature and mature tDCs with the I-Eα recombinant protein and monitored the surface appearance of I-Eα52-68 peptide/MHCII complexes using the specific YAe antibody. Indeed, mature tDCs displayed I-Eα52-68 peptide/MHCII complexes at higher levels than immature tDCs (Fig. 3D). Lastly, we measured the ability of immature and mature DCs to activate antigen-specific CD4+ thymocytes. Mature tDCs loaded with ovalbumin activated ovalbumin-specific CD4+ thymocytes as efficiently as immature tDCs (Fig. 3E). These data suggest that tDCs can continually capture and present surrounding antigens to antigen-specific CD4 thymocytes after maturation.
Figure 3. Mature tDCs internalize and present antigens as efficiently as immature tDCs.
(A–B) Antigen uptake by immature and mature tDCs. Isolated tDCs were incubated with PBS, AlexaFluo647-conjugated OVA (OVA-A647) (A), or 0.02 μm crimson bead (Bead) (B) for 1 hr, and the fluorescence intensity of MHCIIlow tDC (Immature tDC) and MHCIIhigh tDCs (Mature tDC) was determined by flow cytometry. The representative flow cytometry histograms are shown in the left and the quantitated summaries are shown in the right. (C) Heat map showing the expression levels of genes related to MHCII synthesis, transport, and turnover. (D) Antigen uptake and presentation by immature and mature tDCs. Isolated tDCs were incubated with PBS or I-Eα recombinant protein for 1 hr, and the antigen presentation was measured using YAe antibody in MHCIIlow tDC (Immature tDC) and MHCIIhigh tDC (Mature tDC) by flow cytometry. The representative flow cytometry histograms are shown in the left and the quantitated summary is shown in the right. Each data were averaged from three independent experiments. Error bars represent SEM. *p<0.05 and **p<0.01 (E) Activation of antigen-specific thymocytes by immature and mature tDCs. tDCs were isolated and incubated with ovalbumin (2.5mg) for 1hr. Immature and mature cells were sorted and cocultured with CD4 SP thymocytes derived from OT-II mice at a 1:5 ratio for 24 hours. The expression of CD69 in the thymocytes was determined by flow cytometry. Error bars represent SEM.
tDC maturation in part depends on CD4+CD8− single positive (CD4SP) thymocytes and MHCII expressed in tDCs
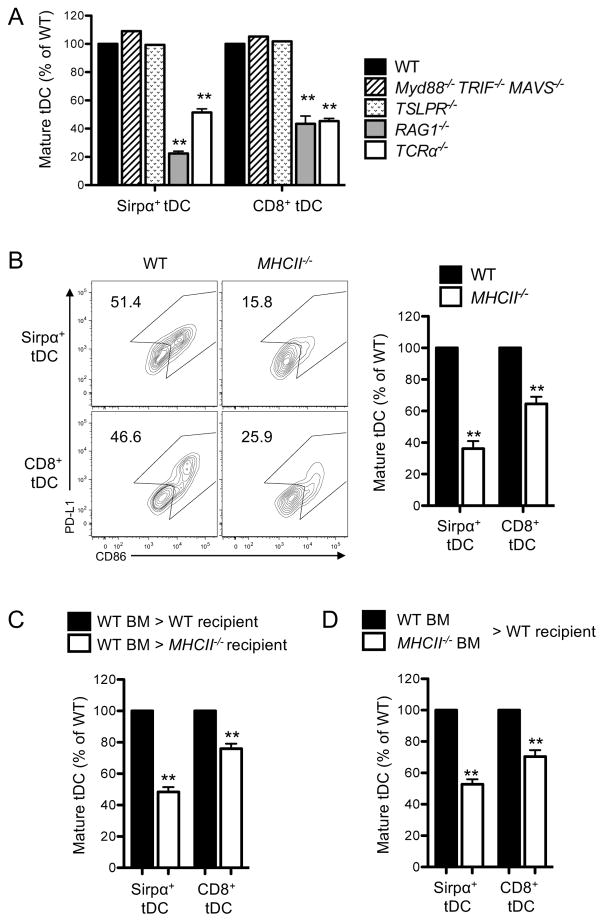
We sought to determine the specific mechanisms by which tDCs mature intrathymically. First, the role of innate immune signaling was examined by using the mice deficient in Myd88, TRIF, and MAVS, the adaptor molecules that mediate innate immune signaling. We found that these triple knock-out mice had a comparable fraction of mature tDCs to wild-type (WT) mice (Fig. 4A) suggesting that innate immune signaling is not crucial for tDC maturation. Next, the role of TSLPR signaling was examined using mice deficient in TSLPR. TSLP is produced by medullary thymic epithelial cells and was shown to induce maturation of human DCs in vitro (26). However, we found that the mice deficient in TSLPR had the same percentage of mature tDCs to WT mice (Fig. 4A). Then, the role of developing thymocytes was examined, considering the presence of these cells uniquely in the thymus and the intra-organ maturation occurring distinctively in the thymus. We found the RAG1−/− mice and TCRα−/− mice, both of which are deficient in mature thymocytes, had a markedly reduced fraction of mature tDCs (Fig. 4A), suggesting that mature thymocytes play a crucial role in tDC maturation.
Figure 4. tDC maturation in part depends on CD4SP thymocytes and MHCII expressed in tDCs.
(A) Maturation status of tDCs in the indicated mouse strains. Relative percentages of mature tDCs (MHCIIhighCD86high) in each mouse strain in comparison to those of WT mice were summarized as graphs. (B) Maturation status of tDCs in WT and MHCII−/− mice. Mature tDCs were defined as PD-L1highCD86high tDCs as shown in the flow cytometry plots. The quantitated summary is shown in the right. (C) Maturation status of tDCs in the indicated BM chimeric mice. (D) Maturation status of tDCs derived from indicated origin of BM in the indicated mixed BM chimeric mice. WT mice refer to BoyJ mice. Origins of tDCs were distinguished by congenic markers, CD45.1+ and CD45.2+. Mature tDCs were defined as PD-L1highCD86high tDCs and the quantitated summary are shown. Each data represents one (Myd88−/−TRIF−/−MAVS−/− and TSLPR−/−) and at least three independent experiments with three to five mice per group. Error bars represent SEM. **p<0.01
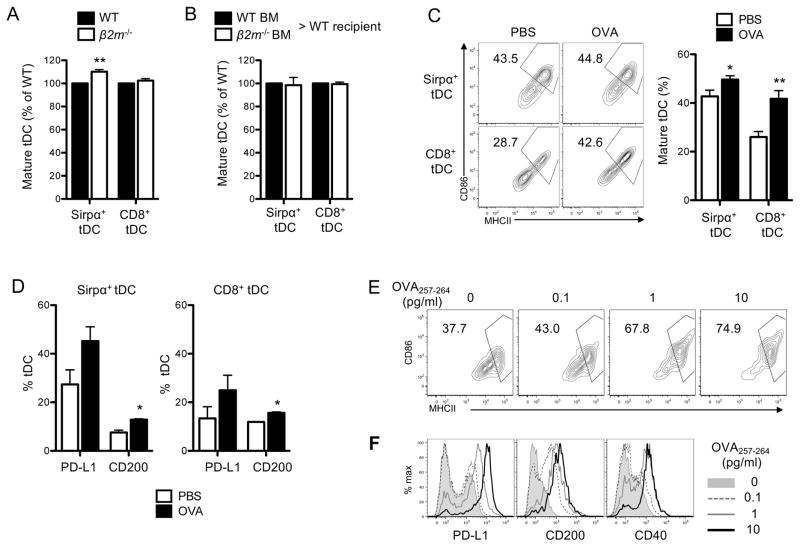
Next, we examined which subset(s) of mature thymocytes mediate tDC maturation. First, we tested the role of CD4SP thymocytes by using MHCII−/− mice, in which CD4SP thymocytes are specifically absent. Because these mice do not express MHCII in DCs, anti-PD-L1 antibody was used instead of anti-MHCII antibody to determine tDC maturation (Fig. 4B). We found that MHCII−/− mice had a significantly reduced fraction of mature tDCs compared to WT mice (Fig. 4B), implicating the involvement of CD4SP thymocytes. For further confirmation, we generated the BM chimeric mice by lethally irradiating MHCII−/− mice and reconstituting them with WT BM. Along with the absence of MHCII in the radio-resistant thymic epithelial cells, these chimeric mice had CD4SP thymocytes as few as MHCII−/− mice had (Supplemental Fig. 3). We found that these chimeric mice had a significantly lower fraction of mature tDCs than the control chimeric mice (Fig. 4C). This finding confirms that CD4SP thymocytes play a pivotal role in tDC maturation.
Since tDCs interact with CD4SP thymocytes through MHCII, we examined whether MHCII expressed in tDCs is required for maturation of the cells. For this examination, WT mice were lethally irradiated and injected with the mixture of MHCII-sufficient and -deficient BMs, and the maturation status of tDCs derived from each BM donor was examined. We found that tDCs derived from the MHCII-deficient BM cells matured poorly compared to those derived from MHCII-sufficient BM cells (Fig. 4D). This finding indicates that MHCII expressed in tDCs is involved in the tDC maturation. Thus, tDC maturation is mediated by both CD4SP thymocytes and MHCII expressed in DCs.
tDC maturation is triggered by cognate interaction with CD4SP thymocytes
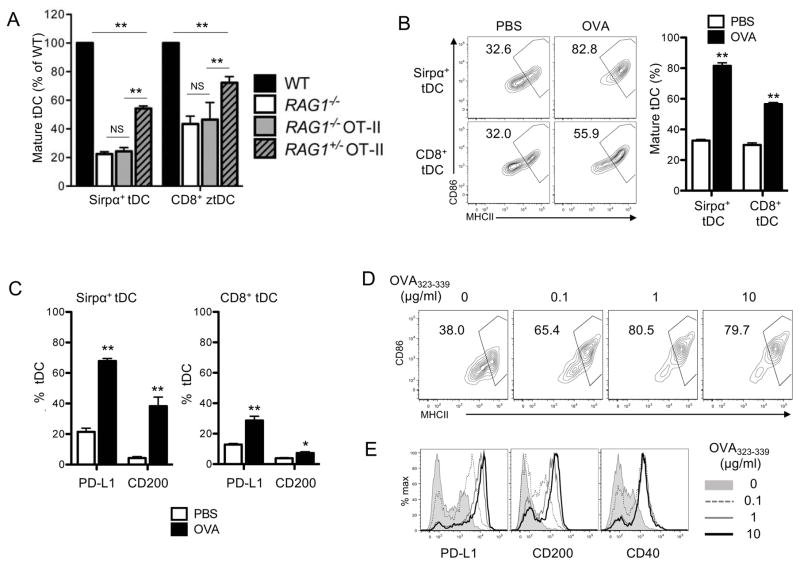
Although both CD4 T cells and MHCII-expressing DCs are present, spleens do not support DC maturation (Fig. 1F, 1G). Because CD4SP thymocytes developing in the thymus but not CD4 T cells present in the spleen are largely self-reactive, we wondered whether this self-reactivity is essential for the tDC maturation. To test this possibility, we examined tDC maturation in RAG1−/− OT-II mice. RAG1−/− OT-II mice have CD4SP thymocytes, but all of the thymocytes express TCRs specific for a non-self ovalbumin (OVA) antigen. We found that these mice had a reduced fraction of mature tDCs similarly to what was observed in RAG1−/− mice. We also examined RAG1+/− OT-II mice, in which some thymocytes express self-reactive TCRs although majority still express TCRs specific for OVA. These mice had an increased fraction of mature tDCs compared to RAG1−/− OT-II mice, although they still had a significantly reduced fraction of mature tDCs compared to WT mice (Fig. 5A). These findings strongly suggest that self-reactivity is critical for tDC maturation driven by CD4SP thymocytes.
Figure 5. tDCs mature by the cognate interactions with CD4SP thymocytes.
(A) Maturation status of tDCs in the indicated mouse strains. Relative percentages of mature tDCs (MHCIIhighCD86high) in each mouse strain in comparison to those of WT mice are summarized. (B) Maturation status of tDCs in OT-II mice following iv injection with PBS or OVA. The representative flow cytometry plots are shown in the left and the quantitated summary is shown in the right. (C) The increase in the expression of PD-L1 and CD200 in tDCs of OT-II mice following iv injection with OVA. The fraction of DCs that express PD-L1 CD200 were quantitated and summarized. (D–E) Maturation of BMDCs following 24 hr co-culture with OT-II CD4SP thymocytes and indicated doses of OVA323-339 peptides. The flow cytometry plots of MHCII and CD86 are shown in (D) and histograms of PD-L1, CD200, and CD40 are shown in (E). Each data represents at least three independent experiments with three to six mice per group (A–C) or triplicate per experiment (D and E). Error bars represent SEM. **p<0.01 and NS ‘not significant’
Then, we directly tested whether the cognate interaction between self-reactive CD4SP thymocytes and tDCs drives maturation of the interacting tDCs. Previous studies have shown that the circulating antigens are taken up and presented by tDCs (5, 45). Thus, we i.v. injected OVA or PBS into OT-II mice and examined the maturation status of tDCs in the mice 24 hr later. Mice that received OVA had an increased fraction of mature tDCs compared to those that received PBS (Fig. 5B) suggesting that OVA triggered the maturation of tDCs. The maturation was further confirmed by the finding that the expressions of PD-L1 and CD200 also increased in the tDCs (Fig. 5C). Notably, Sirpα+ DCs matured more efficiently than CD8+ DCs in accordance with their accessibility to the circulation (Fig. 5B, 5C). To make sure that the maturation occurred by the cognate interaction of the OVA-presenting DCs with the specific CD4SP thymocytes, but not by the endotoxins possibly contaminating the OVA, OVA was injected to WT mice, which do not have OVA-specific CD4SP thymocytes, and tDC maturation was examined. These WT mice did not show increased tDC maturation compared to the mice that received PBS (data not shown).
We also examined whether DC-thymocyte interaction triggers maturation of DCs in vitro. Bone marrow-derived dendritic cells (BMDCs) were loaded with OVA323-339 peptides and co-cultured with OT-II CD4SP thymocytes, and the maturation status was examined 24 hrs later. We found that BMDCs loaded with OVA323-339 peptides upregulated the expression of many maturation markers including MHCII, CD86, PD-L1, CD200, and CD40. Moreover, the degree of maturation strongly correlated with the amount of the peptide loaded (Fig. 5D, 5E). These findings demonstrate that an antigen-specific cognitive interaction with CD4SP thymocytes elicits maturation of the interacting DCs.
tDC maturation requires neither CD4−CD8+ single positive (CD8SP) thymocytes nor MHCI expression in tDCs but can be triggered by cognate interaction with CD8SP thymocytes
We also tested the involvement of CD8SP thymocytes in tDC maturation. β2m−/− mice, in which CD8SP thymocytes are absent due to the lack of MHCI expression, were not defective in tDC maturation compared to WT mice (Fig. 6A). Also, MHCI-deficient tDCs matured similarly to MHCI-sufficient tDCs in the mixed BM chimeric mice (Fig. 6B). However, we speculated that CD8SP thymocytes and MHCI expressed in DCs, although not required in steady-state, might still contribute to tDC maturation and that this contribution might be manifested when their interaction is forced. To test this possibility, OT-I mice, in which the CD8SP thymocytes express TCRs specific for OVA257-264 peptide, were i.v. injected with OVA or PBS, and the maturation status of tDCs was examined. No significant difference was observed in the maturation status of tDCs in mice that received OVA or PBS by day 1 post-OVA injection (data not shown). However, an appreciable increase in the fraction of mature tDCs was observed on day 3 post-OVA injection. Interestingly, the degree of maturation was higher in CD8+ DCs than in Sirpα+ DCs, which contrasted to the earlier finding that Sirpα+ DCs matured more readily than CD8+ DCs in OT-II mice that received OVA (Fig. 6C, 6D).
Figure 6. tDC maturation requires neither CD8SP thymocytes nor MHCI expression in tDCs but can be triggered by the cognate interactions with CD8SP thymocytes.
(A) Maturation status of tDCs in WT and β2m−/− mice. Relative percentages of mature tDCs (MHCIIhiCD86hi) were summarized. (B) Maturation status of tDCs derived from each BM. Relative percentages of mature tDCs (MHCIIhiCD86hi) were summarized. (C) Maturation status of tDCs in OT-I mice 3 days after injection with PBS or OVA. The representative flow cytometry plots are shown in the left and the quantitated summary is shown in the right. (D) The increased expressions of PD-L1 and CD200 in tDCs of OT-I mice injected with OVA. The fraction of DCs that express PD-L1 or CD200 were quantitated and summarized. (E–F) Maturation of BMDCs following 24 hr co-culture with OT-I CD8SP thymocytes and OVA257-264 peptides. The flow cytometry plots of MHCII and CD86 are shown in (E) and histograms of PD-L1, CD200, and CD40 are shown in (F). Each data represents at least three independent experiments with three to five mice per group (A–D) or triplicate per experiment (E and F). Error bars represent SEM. *p<0.05 and **p<0.01
We further tested the ability of antigen-specific CD8SP thymocytes to induce maturation of DCs in vitro. BMDCs were loaded with OVA257-264 peptide and co-cultured with OT-I CD8SP thymocytes, and the maturation status was examined 24 hours later. BMDCs loaded with the OVA257-264 peptide upregulated the expression of all the maturation markers examined, including MHCII, CD86, PD-L1, CD200, and CD40 (Fig. 6E, 6F). These findings indicate that tDC maturation requires neither CD8SP thymocytes nor MHCI expression in tDCs in steady-state but can be triggered by a cognitive interaction with CD8SP thymocytes.
CD4SP thymocyte-driven tDC maturation is mediated by CD40
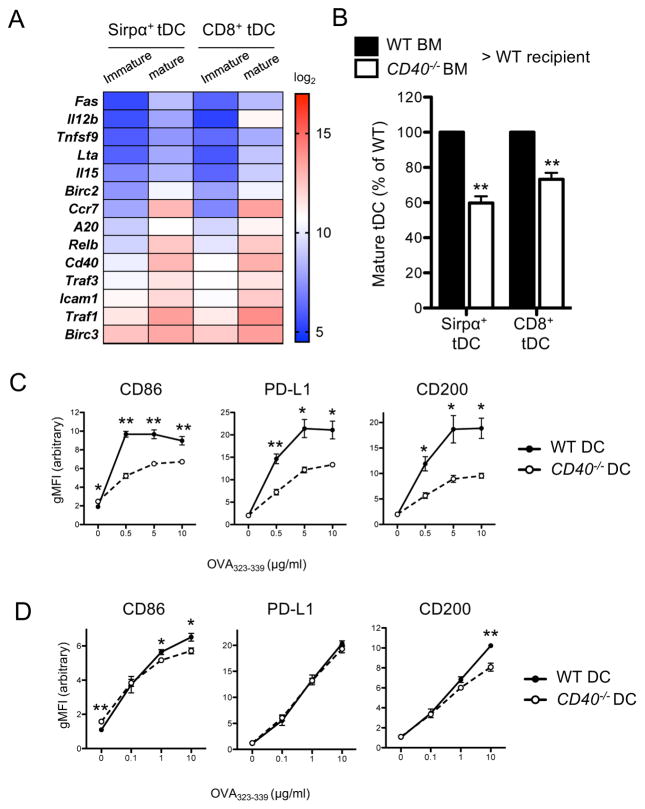
We sought to identify the molecular mechanism by which tDCs mature following the cognate interaction with CD4SP thymocytes. CD40 has been shown to mediate maturation of DCs in the periphery, and its ligand CD40L is constitutively expressed in CD4SP thymocytes (46–48). Therefore, we examined the possibility that CD40 may mediate the maturation of tDCs driven by CD4SP thymocytes. First, the gene expression microarray data were probed for indication of the activation of CD40 signaling pathway in association with tDC maturation. Interestingly, the genes known to be upregulated following CD40 signaling, such as Fas (Cd95), Tnfsf9 (4-1bbl), Lta (Tnfsf1), Il15, Birc2 (cIAP1), A20, Traf3, Traf1, and Birc3 (cIAP2) as well as Cd40 itself, were expressed highly in mature tDCs relative to immature tDCs (49–53) (Fig. 7A). We then directly examined the role of CD40 in tDC maturation. CD40-deficient mice are severely compromised in thymic organization, and this alteration may impair tDC maturation independent of CD40 signaling in DCs (54). Therefore, we made BM chimeric mice using the mixtures of CD40-sufficient and -deficient BMs and examined the maturation status of tDCs derived from each BM. We found that tDCs derived from CD40-deficient BM matured poorly compared to those from CD40-sufficient BM (Fig. 7B). This finding indicates that CD40 signaling is indeed involved in tDC maturation in a cell-intrinsic manner.
Figure 7. CD4SP thymocyte-driven maturation of tDCs is mediated by CD40.
(A) Heat map showing the expression levels of genes known to be upregulated following CD40 signaling. (B) Maturation status of tDCs derived from each BM. Relative percentages of mature tDCs (MHCIIhiCD86hi) were summarized. Data represent two independent experiments with three to six mice per group. Error bars represent SEM. (C) Maturation of WT or Cd40−/− BMDCs following 24 hr co-culture with OT-II CD4SP thymocytes and OVA323-339 peptides. (D) Maturation of WT or Cd40−/− BMDCs following 24 hr co-culture with OT-I CD8SP thymocytes and OVA257-264 peptides. The changes in the expression of CD86, PD-L1, and CD200 were determined by flow cytometry. Data represent three independent experiments with at least duplicate per experiment. Error bars represent SEM. *p<0.05 and **p<0.01
Next, we examined whether CD40 is involved in the CD4SP thymocyte-driven tDC maturation. BMDCs cultured from CD40-sufficient or -deficient mice were loaded with OVA323-339 peptide and co-cultured with OT-II CD4SP thymocytes as described previously. We found that BMDCs cultured from CD40-deficient mice matured poorly compared to those from CD40-sufficient mice (Fig. 7C). We also examined whether CD40 is involved in CD8SP thymocyte-driven DC maturation by employing the similar in vitro co-culture experiment. We found that CD40-deficient DCs and CD40-sufficient DCs matured comparably following engagement with OT-I CD8SP thymocytes (Fig. 7D). These findings indicate that the DC maturation triggered by antigen-specific CD4SP thymocytes, but not CD8SP thymocytes, largely depends on CD40 expression.
DC expression of CD40 supports regulatory T cell development in the thymus
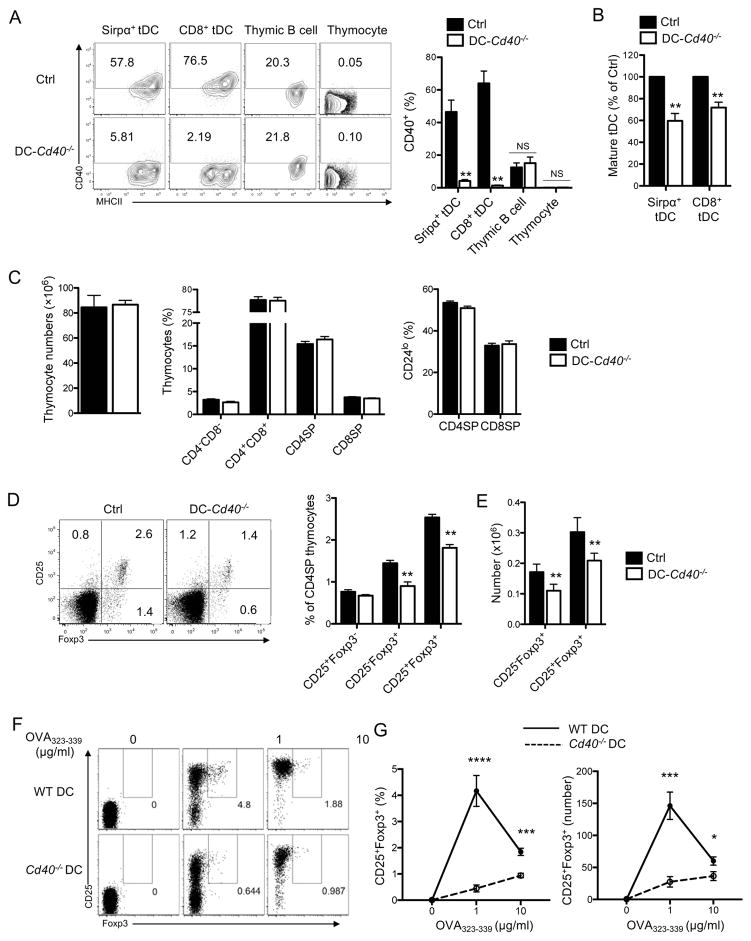
Having found that CD40 mediates maturation of tDCs, we explored functional role of tDC maturation by investigating whether CD40 deficiency in tDCs causes any functional defect in the thymus, particularly on thymocyte development. In order to make mice deficient in CD40 specifically in DCs, mixed BM chimeric mice were generated by reconstituting lethally irradiated WT mice with CD11c-diphtheria toxin A (DTA) mouse BM mixed with CD40−/− mouse BM at a 4:1 ratio; CD11c-DTA mice refers to CD11c-Cre mice crossed to ROSA26-floxDTA mice (55, 56). For comparison, control chimeric mice were generated by using WT mouse BM instead of CD40−/− mouse BM. We first confirmed that CD40−/−:CD11c-DTA (DC-CD40−/−) mice did not express CD40 in DCs while WT:CD11c-DTA (Ctrl) mice did (Fig. 8A). B cells, however, expressed CD40 in both chimeric mice (Fig. 8A).
Figure 8. DC expression of CD40 supports Treg cell development in the thymus.
(A) Surface expression of CD40 in WT:CD11c-DTA (Ctrl)- and Cd40−/−:CD11c-DTA (DC-Cd40−/−)-mixed BM chimeric mice. The representative flow cytometry plot showing the expression level of CD40 is shown in the left and the quantitated summary of the percentages of CD40+ cells is shown in the right. (B) Maturation status of tDCs in Ctrl- and DC-Cd40−/−-mixed BM chimeric mice. Relative percentages of mature tDCs (MHCIIhighCD86high) were summarized. (C) Cellularity, CD4 and CD8 thymocyte composition, and the percentages of CD24lo fully mature thymocytes in the Ctrl- and the DC-Cd40−/−-mixed BM chimeric mice. (D) The frequency of Treg cell precursors and Treg cells among CD4SP thymocytes in the Ctrl- and the DC-Cd40−/−-mixed BM chimeric mice. The representative flow cytometry plots are shown in the left and the quantitated summary is shown in the center and right. (E) Antigen-specific Treg cell differentiation by WT and Cd40−/− BMDCs. WT or Cd40−/− BMDCs were co-culture with immature OT-II CD4SP thymocytes and OVA323-339 peptides. The expression of Foxp3 and CD25 by the thymocytes was determined by flow cytometry after 2 days of the co-culture. The representative flow cytometry plots are shown in the left and the quantitated summary is shown in the right. Each data represents at least three independent experiments with four to seven mice per group (A–D) or at least duplicate per experiment (E). Error bars represent SEM. *p<0.05 **p<0.01 and NS ‘not significant’
We then determined tDC maturation and thymocyte development in the DC-CD40−/− chimeric mice in comparison to the Ctrl chimeric mice. The DC-CD40−/− chimeric mice had a significantly lower fraction of mature tDCs than the Ctrl mice in accordance with the role of CD40 in tDC maturation described in Fig. 7 (Fig. 8B and Supplemental Fig. 4A). However, thymic cellularity, CD4 and CD8 thymocyte profile, and the fraction of CD24lo mature cells among CD4SP and CD8SP thymocytes, and the fraction of CD69−CD62L+ mature CD4SP thymocytes were not significantly altered (Fig. 8C and Supplemental Fig. 4B). Remarkably however, the frequencies of Foxp3+ Treg cells, both CD25− and CD25+ subsets, were significantly reduced in DC-CD40−/− chimeric mice compared to the Ctrl mice (Fig. 8D) although no significant difference was observed in the fraction of proliferating or apoptotic Treg cells (Supplemental Fig. 4C). To determine whether this reduction in the frequency of Treg cells reflects the reduction in DC ability to support Treg cell differentiation, we examined the ability of CD40-sufficient or -deficient DCs to differentiate antigen-specific Treg cells in vitro. BMDCs were cultured from CD40-sufficient or -deficient mice, loaded with OVA323-339 peptide, and co-cultured with immature non-Treg CD4SP thymocytes isolated from Foxp3GFPOT-II mice. We found that the thymocytes co-cultured with CD40-deficient DCs became CD25+Foxp3+ Treg cells to a much lesser degree than those co-cultured with CD40-sufficient DCs (Fig. 8E) indicating that CD40 plays a significant role in DC ability to differentiate antigen-specific Treg cells in vitro. Taken together, CD40 expressed in DCs supports Treg cell development in the thymus in association with its role in mediating maturation of tDCs.
Discussion
DCs play an important role in the development of T cells in the thymus by negatively selecting self-reactive thymocytes or differentiating them into Treg cells. However, the molecular and cellular mechanisms underlying these functions have not been clearly identified. The study presented here shows that tDCs mature following engagement with self-reactive CD4SP thymocytes by involving CD40 signaling and that CD40 signaling conditions the tDC to differentiate self-reactive CD4SP thymocytes into Treg cells. Thus, DC function of generating Treg cells involves a feedback mechanism that includes cognate interaction between tDCs and self-reactive thymocytes.
The mode of maturation occurring to tDCs is quite distinct from those occurring to the peripheral DCs. Firstly, tDC maturation is not accompanied by the relocation of DCs from a tissue to lymph nodes. The DC maturation triggered by microbes involves upregulation of CCR7, and CCR7 mediates the relocation of maturing DCs to lymph nodes (21, 57). The homeostatic DC maturation occurring at steady-state in the periphery is also accompanied by CCR7 upregulation and associated with their migration to lymph nodes (21). While tDC maturation also involves upregulation of CCR7, mature tDCs stay in the thymus. Secondly, tDCs can mature independent of innate immune signaling but poorly mature in the absence of cognate interactions with self-reactive CD4SP thymocytes. The role of thymocytes in tDC maturation has been implicated in a previous study that showed the numbers of mature tDCs were markedly low in lymphopenic mice (58). Our study confirms this previous study but also establishes the requirement of a direct conjugation between pMHCII in tDCs and the antigen-specific TCR in CD4SP thymocytes. This requirement was demonstrated in vivo in the mixed BM chimeric mice in which MHCII-sufficient tDCs matured normally while MHCII-deficient tDCs did not. It was further confirmed both in vivo and in vitro experiments using OT-II mice and OVA antigens, in which the antigen-mediated cognate interaction elicited DC maturation. Notably, we do not rule out the possibility that other thymocyte compartments may also contribute to tDC maturation. Indeed, TCRα−/− mice, which have CD4+CD8+ DP thymocytes, showed a significant increase in the proportion of the Sirpα+ subset of mature tDCs as compared to RAG−/− mice that lack DP thymocytes implicating the potential contribution of DP thymocytes to tDC maturation. Lastly, tDCs uniquely retain antigen-presenting capacity after maturation. Mature tDCs endocytosed both soluble and particulate antigens as efficiently as immature tDCs. Mature tDCs transcribed MHCII, MHCII transactivator CIITA, and the MHCII chaperon CD74 at high levels similarly to immature tDCs, while limiting the expression of genes that mediate MHCII degradation thus extending its half-life. Accordingly, mature tDCs loaded newly endocytosed antigens to newly synthesized MHCII, transported them to the plasma membrane, and presented them in in a prolonged period. This constant activity of endocytosing and presenting antigens after maturation is likely to maximize the amount and species of self-antigens that tDCs present to developing thymocytes, enabling tDCs to fulfill their function of thymocyte selection.
The ability of CD40 to mediate DC maturation was established before. CD40L and CD40 antibody can induce maturation of DCs in vitro (48, 59). Injection of CD40 antibody into mice also triggers maturation of tissue DCs and enhances their function of inducing immunity (47, 58, 60, 61). However, it has not been shown whether CD40 mediates maturation of DCs under any physiological condition and if so whether CD40-mediated maturation plays any important homeostatic function. In this study, we demonstrate that CD40 signaling mediates DC maturation in the thymus. This was evidenced by the finding that Cd40-deficient tDCs mature lesser than CD40-sufficient tDCs in the mixed BM chimeric mice. Also, CD40-deficient DCs matured poorly than CD40-sufficient DCs upon cognate interaction with CD4SP thymocytes in vitro. We believe this finding provides the first evidence that CD40 mediates maturation of DCs under physiologic condition. Notably, a recent study showed that the maturation occurring to peripheral DCs that migrate to the lymph nodes at steady-state is dependent on IκB kinase β (IKKβ) signaling (62). IKKβ singling is activated following CD40 ligation. Thus, it is plausible that CD40 signaling also mediates this homeostatic peripheral DC maturation.
We showed that CD40-dependent maturation potentiates the ability of tDCs to generate Treg cells. Mice lacking CD40 expression in DCs had a reduced number of thymic Treg cells in addition to a reduced number of thymic mature DCs. This finding is corroborated by a recent study that showed CD40flox/floxCD11c-Cre mice had a reduced number of Treg cells in the thymus (63). Although the specific molecular mechanisms remain to be defined, CD40 signaling increases the expressions of many costimulatory molecules and adhesion molecules in tDCs, such as CD86, CD80, CD70, OX40-L, TNF, PD-L1, and ICAM1 that play a role in Treg cell development (64–70). These surface molecules are likely to deliver enhanced TCR signaling to the interacting thymocytes and thus promote Treg cell differentiation (71). In line with this, NF-κB-inducing kinase (NIK), a downstream mediator of CD40 signaling, has been shown to promote the expression of costimulatory molecules in tDCs and also promote the development of Treg cells in the thymus (72). It is notable that not only cDCs but also plasmacytoid DCs (pDCs) express CD11c in mice and that human pDCs have been shown to drive Treg cell differentiation involving CD40 signaling (73). Therefore, reduced Treg cell number observed in CD40flox/floxCD11c-Cre mice may be in part due to the deficiency in CD40 signaling in pDCs in addition to that in cDCs. CD40 also mediates maturation of medullary thymic epithelial cells and thymic B cells involving the upregulation of costimulatory molecules. The immature medullary thymic epithelial cells upregulate the expression of CD80 upon cognate interaction with CD4SP thymocytes and fully mature into the cells capable of negatively selecting self-reactive thymocytes, which in part is mediated by CD40 signaling (54, 74). Thymic B cells also upregulate MHCII and CD80 via the CD40 signaling following cognate interaction with CD4SP thymocytes and this signaling licenses the B cells to execute the negative selection of self-reactive thymocytes (75). Taken together, CD40 signaling appears to be an upstream core signaling pathway that mediate maturation of various thymic APCs and condition them to properly implement their function in central tolerance. The specific molecular mechanisms mediating negative selection vs. Treg cell differentiation by each APC remain to be further delineated.
Supplementary Material
Acknowledgments
This work was supported by the National Institute of Health (R01GM105800) and the UCSF Sandler Asthma Basic Research Center.
We thank Steven Ziegler, Richard Locksley, Lewis Lanier, Mehrdad Matloubian, Arthur Weiss, and Qizhi Tang for sharing mice and the previous and current members of the Shin lab for helpful discussions, comments, and technical assistance.
Abbreviations used in this article
- tDC
thymic dendritic cells
- APC
antigen presenting cell
- CD4SP thymocyte
CD4+CD8-single positive thymocyte
- BMDCs
bone marrow-derived dendritic cells
- OVA
ovalbumin
- Treg cell
regulatory T cell
Footnotes
Disclosures
The authors have no conflicting financial interests.
The microarray data presented in this article have been submitted to the Gene Expression Omnibus (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE107571) under accession number GSE107571.
References
- 1.Klein L, Kyewski B, Allen PM, Hogquist KA. Positive and negative selection of the T cell repertoire: what thymocytes see (and don’t see) Nat Rev Immunol. 2014;14:377–391. doi: 10.1038/nri3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hsieh CS, Lee HM, Lio CW. Selection of regulatory T cells in the thymus. Nat Rev Immunol. 2012;12:157–167. doi: 10.1038/nri3155. [DOI] [PubMed] [Google Scholar]
- 3.Palmer E. Negative selection--clearing out the bad apples from the T-cell repertoire. Nat Rev Immunol. 2003;3:383–391. doi: 10.1038/nri1085. [DOI] [PubMed] [Google Scholar]
- 4.Li J, Park J, Foss D, Goldschneider I. Thymus-homing peripheral dendritic cells constitute two of the three major subsets of dendritic cells in the steady-state thymus. J Exp Med. 2009;206:607–622. doi: 10.1084/jem.20082232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baba T, Nakamoto Y, Mukaida N. Crucial contribution of thymic Sirp alpha+ conventional dendritic cells to central tolerance against blood-borne antigens in a CCR2-dependent manner. J Immunol. 2009;183:3053–3063. doi: 10.4049/jimmunol.0900438. [DOI] [PubMed] [Google Scholar]
- 6.Bonasio R, Scimone ML, Schaerli P, Grabie N, Lichtman AH, von Andrian UH. Clonal deletion of thymocytes by circulating dendritic cells homing to the thymus. Nat Immunol. 2006;7:1092–1100. doi: 10.1038/ni1385. [DOI] [PubMed] [Google Scholar]
- 7.Ardavin C, Wu L, Li CL, Shortman K. Thymic dendritic cells and T cells develop simultaneously in the thymus from a common precursor population. Nature. 1993;362:761–763. doi: 10.1038/362761a0. [DOI] [PubMed] [Google Scholar]
- 8.Lei Y, Ripen AM, Ishimaru N, Ohigashi I, Nagasawa T, Jeker LT, Bosl MR, Hollander GA, Hayashi Y, de Malefyt WR, Nitta T, Takahama Y. Aire-dependent production of XCL1 mediates medullary accumulation of thymic dendritic cells and contributes to regulatory T cell development. J Exp Med. 2011;208:383–394. doi: 10.1084/jem.20102327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koble C, Kyewski B. The thymic medulla: a unique microenvironment for intercellular self-antigen transfer. J Exp Med. 2009;206:1505–1513. doi: 10.1084/jem.20082449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ardouin L, Luche H, Chelbi R, Carpentier S, Shawket A, Montanana Sanchis F, Santa Maria C, Grenot P, Alexandre Y, Gregoire C, Fries A, Vu Manh TP, Tamoutounour S, Crozat K, Tomasello E, Jorquera A, Fossum E, Bogen B, Azukizawa H, Bajenoff M, Henri S, Dalod M, Malissen B. Broad and Largely Concordant Molecular Changes Characterize Tolerogenic and Immunogenic Dendritic Cell Maturation in Thymus and Periphery. Immunity. 2016;45:305–318. doi: 10.1016/j.immuni.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 11.Oh J, Shin JS. The Role of Dendritic Cells in Central Tolerance. Immune Netw. 2015;15:111–120. doi: 10.4110/in.2015.15.3.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106:255–258. doi: 10.1016/s0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- 13.Villadangos JA, Schnorrer P, Wilson NS. Control of MHC class II antigen presentation in dendritic cells: a balance between creative and destructive forces. Immunol Rev. 2005;207:191–205. doi: 10.1111/j.0105-2896.2005.00317.x. [DOI] [PubMed] [Google Scholar]
- 14.Shin JS, Ebersold M, Pypaert M, Delamarre L, Hartley A, Mellman I. Surface expression of MHC class II in dendritic cells is controlled by regulated ubiquitination. Nature. 2006;444:115–118. doi: 10.1038/nature05261. [DOI] [PubMed] [Google Scholar]
- 15.Trombetta ES, Mellman I. Cell biology of antigen processing in vitro and in vivo. Annu Rev Immunol. 2005;23:975–1028. doi: 10.1146/annurev.immunol.22.012703.104538. [DOI] [PubMed] [Google Scholar]
- 16.Cella M, Engering A, Pinet V, Pieters J, Lanzavecchia A. Inflammatory stimuli induce accumulation of MHC class II complexes on dendritic cells. Nature. 1997;388:782–787. doi: 10.1038/42030. [DOI] [PubMed] [Google Scholar]
- 17.Pierre P, Turley SJ, Gatti E, Hull M, Meltzer J, Mirza A, Inaba K, Steinman RM, Mellman I. Developmental regulation of MHC class II transport in mouse dendritic cells. Nature. 1997;388:787–792. doi: 10.1038/42039. [DOI] [PubMed] [Google Scholar]
- 18.Kaisho T, Akira S. Dendritic-cell function in Toll-like receptor- and MyD88-knockout mice. Trends Immunol. 2001;22:78–83. doi: 10.1016/s1471-4906(00)01811-1. [DOI] [PubMed] [Google Scholar]
- 19.Garrett WS, Chen LM, Kroschewski R, Ebersold M, Turley S, Trombetta S, Galan JE, Mellman I. Developmental control of endocytosis in dendritic cells by Cdc42. Cell. 2000;102:325–334. doi: 10.1016/s0092-8674(00)00038-6. [DOI] [PubMed] [Google Scholar]
- 20.Yanagihara S, Komura E, Nagafune J, Watarai H, Yamaguchi Y. EBI1/CCR7 is a new member of dendritic cell chemokine receptor that is up-regulated upon maturation. J Immunol. 1998;161:3096–3102. [PubMed] [Google Scholar]
- 21.Ohl L, Mohaupt M, Czeloth N, Hintzen G, Kiafard Z, Zwirner J, Blankenstein T, Henning G, Forster R. CCR7 governs skin dendritic cell migration under inflammatory and steady-state conditions. Immunity. 2004;21:279–288. doi: 10.1016/j.immuni.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 22.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 23.Vander Lugt B, Riddell J, Khan AA, Hackney JA, Lesch J, DeVoss J, Weirauch MT, Singh H, Mellman I. Transcriptional determinants of tolerogenic and immunogenic states during dendritic cell maturation. J Cell Biol. 2017;216:779–792. doi: 10.1083/jcb.201512012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller JC, Brown BD, Shay T, Gautier EL, Jojic V, Cohain A, Pandey G, Leboeuf M, Elpek KG, Helft J, Hashimoto D, Chow A, Price J, Greter M, Bogunovic M, Bellemare-Pelletier A, Frenette PS, Randolph GJ, Turley SJ, Merad M C. Immunological Genome. Deciphering the transcriptional network of the dendritic cell lineage. Nat Immunol. 2012;13:888–899. doi: 10.1038/ni.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Idoyaga J, Fiorese C, Zbytnuik L, Lubkin A, Miller J, Malissen B, Mucida D, Merad M, Steinman RM. Specialized role of migratory dendritic cells in peripheral tolerance induction. J Clin Invest. 2013;123:844–854. doi: 10.1172/JCI65260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watanabe N, Wang YH, Lee HK, Ito T, Wang YH, Cao W, Liu YJ. Hassall’s corpuscles instruct dendritic cells to induce CD4+CD25+ regulatory T cells in human thymus. Nature. 2005;436:1181–1185. doi: 10.1038/nature03886. [DOI] [PubMed] [Google Scholar]
- 27.Haribhai D, Lin W, Relland LM, Truong N, Williams CB, Chatila TA. Regulatory T cells dynamically control the primary immune response to foreign antigen. J Immunol. 2007;178:2961–2972. doi: 10.4049/jimmunol.178.5.2961. [DOI] [PubMed] [Google Scholar]
- 28.Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, Akira S. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 30.Sun Q, Sun L, Liu HH, Chen X, Seth RB, Forman J, Chen ZJ. The specific and essential role of MAVS in antiviral innate immune responses. Immunity. 2006;24:633–642. doi: 10.1016/j.immuni.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 31.Zhou B, Comeau MR, De Smedt T, Liggitt HD, Dahl ME, Lewis DB, Gyarmati D, Aye T, Campbell DJ, Ziegler SF. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat Immunol. 2005;6:1047–1053. doi: 10.1038/ni1247. [DOI] [PubMed] [Google Scholar]
- 32.Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics. 2003;19:185–193. doi: 10.1093/bioinformatics/19.2.185. [DOI] [PubMed] [Google Scholar]
- 33.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate - a Practical and Powerful Approach to Multiple Testing. J Roy Stat Soc B Met. 1995;57:289–300. [Google Scholar]
- 34.Holm S. A Simple Sequentially Rejective Multiple Test Procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- 35.Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge YC, Gentry J, Hornik K, Hothorn T, Huber W, Iacus S, Irizarry R, Leisch F, Li C, Maechler M, Rossini AJ, Sawitzki G, Smith C, Smyth G, Tierney L, Yang JYH, Zhang JH. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 2004:5. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meredith MM, Liu K, Darrasse-Jeze G, Kamphorst AO, Schreiber HA, Guermonprez P, Idoyaga J, Cheong C, Yao KH, Niec RE, Nussenzweig MC. Expression of the zinc finger transcription factor zDC (Zbtb46, Btbd4) defines the classical dendritic cell lineage. J Exp Med. 2012;209:1153–1165. doi: 10.1084/jem.20112675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Normand SL. Meta-analysis: formulating, evaluating, combining, and reporting. Stat Med. 1999;18:321–359. doi: 10.1002/(sici)1097-0258(19990215)18:3<321::aid-sim28>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 38.Hong F, Breitling R. A comparison of meta-analysis methods for detecting differentially expressed genes in microarray experiments. Bioinformatics. 2008;24:374–382. doi: 10.1093/bioinformatics/btm620. [DOI] [PubMed] [Google Scholar]
- 39.Landmann S, Muhlethaler-Mottet A, Bernasconi L, Suter T, Waldburger JM, Masternak K, Arrighi JF, Hauser C, Fontana A, Reith W. Maturation of dendritic cells is accompanied by rapid transcriptional silencing of class II transactivator (CIITA) expression. J Exp Med. 2001;194:379–391. doi: 10.1084/jem.194.4.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Villadangos JA, Cardoso M, Steptoe RJ, van Berkel D, Pooley J, Carbone FR, Shortman K. MHC class II expression is regulated in dendritic cells independently of invariant chain degradation. Immunity. 2001;14:739–749. doi: 10.1016/s1074-7613(01)00148-0. [DOI] [PubMed] [Google Scholar]
- 41.Wilson NS, El-Sukkari D, Villadangos JA. Dendritic cells constitutively present self antigens in their immature state in vivo and regulate antigen presentation by controlling the rates of MHC class II synthesis and endocytosis. Blood. 2004;103:2187–2195. doi: 10.1182/blood-2003-08-2729. [DOI] [PubMed] [Google Scholar]
- 42.Platt CD, Ma JK, Chalouni C, Ebersold M, Bou-Reslan H, Carano RA, Mellman I, Delamarre L. Mature dendritic cells use endocytic receptors to capture and present antigens. Proc Natl Acad Sci U S A. 2010;107:4287–4292. doi: 10.1073/pnas.0910609107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tze LE, Horikawa K, Domaschenz H, Howard DR, Roots CM, Rigby RJ, Way DA, Ohmura-Hoshino M, Ishido S, Andoniou CE, Degli-Esposti MA, Goodnow CC. CD83 increases MHC II and CD86 on dendritic cells by opposing IL-10-driven MARCH1-mediated ubiquitination and degradation. J Exp Med. 2011;208:149–165. doi: 10.1084/jem.20092203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matsuki Y, Ohmura-Hoshino M, Goto E, Aoki M, Mito-Yoshida M, Uematsu M, Hasegawa T, Koseki H, Ohara O, Nakayama M, Toyooka K, Matsuoka K, Hotta H, Yamamoto A, Ishido S. Novel regulation of MHC class II function in B cells. EMBO J. 2007;26:846–854. doi: 10.1038/sj.emboj.7601556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oh J, Wu N, Baravalle G, Cohn B, Ma J, Lo B, Mellman I, Ishido S, Anderson M, Shin JS. MARCH1-mediated MHCII ubiquitination promotes dendritic cell selection of natural regulatory T cells. J Exp Med. 2013;210:1069–1077. doi: 10.1084/jem.20122695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hikosaka Y, Nitta T, Ohigashi I, Yano K, Ishimaru N, Hayashi Y, Matsumoto M, Matsuo K, Penninger JM, Takayanagi H, Yokota Y, Yamada H, Yoshikai Y, Inoue J, Akiyama T, Takahama Y. The cytokine RANKL produced by positively selected thymocytes fosters medullary thymic epithelial cells that express autoimmune regulator. Immunity. 2008;29:438–450. doi: 10.1016/j.immuni.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 47.Yang Y, Wilson JM. CD40 ligand-dependent T cell activation: requirement of B7-CD28 signaling through CD40. Science. 1996;273:1862–1864. doi: 10.1126/science.273.5283.1862. [DOI] [PubMed] [Google Scholar]
- 48.Schuurhuis DH, Laban S, Toes RE, Ricciardi-Castagnoli P, Kleijmeer MJ, van der Voort EI, Rea D, Offringa R, Geuze HJ, Melief CJ, Ossendorp F. Immature dendritic cells acquire CD8(+) cytotoxic T lymphocyte priming capacity upon activation by T helper cell-independent or -dependent stimuli. J Exp Med. 2000;192:145–150. doi: 10.1084/jem.192.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dieu MC, Vanbervliet B, Vicari A, Bridon JM, Oldham E, Ait-Yahia S, Briere F, Zlotnik A, Lebecque S, Caux C. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J Exp Med. 1998;188:373–386. doi: 10.1084/jem.188.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schulz O, Edwards AD, Schito M, Aliberti J, Manickasingham S, Sher A, Reis e Sousa C. CD40 triggering of heterodimeric IL-12 p70 production by dendritic cells in vivo requires a microbial priming signal. Immunity. 2000;13:453–462. doi: 10.1016/s1074-7613(00)00045-5. [DOI] [PubMed] [Google Scholar]
- 51.Dadgostar H, Zarnegar B, Hoffmann A, Qin XF, Truong U, Rao G, Baltimore D, Cheng G. Cooperation of multiple signaling pathways in CD40-regulated gene expression in B lymphocytes. Proc Natl Acad Sci U S A. 2002;99:1497–1502. doi: 10.1073/pnas.032665099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aicher A, Shu GL, Magaletti D, Mulvania T, Pezzutto A, Craxton A, Clark EA. Differential role for p38 mitogen-activated protein kinase in regulating CD40-induced gene expression in dendritic cells and B cells. J Immunol. 1999;163:5786–5795. [PubMed] [Google Scholar]
- 53.O’Sullivan BJ, Thomas R. CD40 ligation conditions dendritic cell antigen-presenting function through sustained activation of NF-kappaB. J Immunol. 2002;168:5491–5498. doi: 10.4049/jimmunol.168.11.5491. [DOI] [PubMed] [Google Scholar]
- 54.Akiyama T, Shimo Y, Yanai H, Qin J, Ohshima D, Maruyama Y, Asaumi Y, Kitazawa J, Takayanagi H, Penninger JM, Matsumoto M, Nitta T, Takahama Y, Inoue J. The tumor necrosis factor family receptors RANK and CD40 cooperatively establish the thymic medullary microenvironment and self-tolerance. Immunity. 2008;29:423–437. doi: 10.1016/j.immuni.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 55.Birnberg T, Bar-On L, Sapoznikov A, Caton ML, Cervantes-Barragan L, Makia D, Krauthgamer R, Brenner O, Ludewig B, Brockschnieder D, Riethmacher D, Reizis B, Jung S. Lack of conventional dendritic cells is compatible with normal development and T cell homeostasis, but causes myeloid proliferative syndrome. Immunity. 2008;29:986–997. doi: 10.1016/j.immuni.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 56.Ohnmacht C, Pullner A, King SB, Drexler I, Meier S, Brocker T, Voehringer D. Constitutive ablation of dendritic cells breaks self-tolerance of CD4 T cells and results in spontaneous fatal autoimmunity. J Exp Med. 2009;206:549–559. doi: 10.1084/jem.20082394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.MartIn-Fontecha A, Sebastiani S, Hopken UE, Uguccioni M, Lipp M, Lanzavecchia A, Sallusto F. Regulation of dendritic cell migration to the draining lymph node: impact on T lymphocyte traffic and priming. J Exp Med. 2003;198:615–621. doi: 10.1084/jem.20030448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Spidale NA, Wang B, Tisch R. Cutting edge: Antigen-specific thymocyte feedback regulates homeostatic thymic conventional dendritic cell maturation. J Immunol. 2014;193:21–25. doi: 10.4049/jimmunol.1400321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med. 1996;184:747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Diehl L, den Boer AT, Schoenberger SP, van der Voort EI, Schumacher TN, Melief CJ, Offringa R, Toes RE. CD40 activation in vivo overcomes peptide-induced peripheral cytotoxic T-lymphocyte tolerance and augments anti-tumor vaccine efficacy. Nat Med. 1999;5:774–779. doi: 10.1038/10495. [DOI] [PubMed] [Google Scholar]
- 61.Bennett SR, Carbone FR, Karamalis F, Flavell RA, Miller JF, Heath WR. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 62.Baratin M, Foray C, Demaria O, Habbeddine M, Pollet E, Maurizio J, Verthuy C, Davanture S, Azukizawa H, Flores-Langarica A, Dalod M, Lawrence T. Homeostatic NF-kappaB Signaling in Steady-State Migratory Dendritic Cells Regulates Immune Homeostasis and Tolerance. Immunity. 2015;42:627–639. doi: 10.1016/j.immuni.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 63.Garg G, Nikolouli E, Hardtke-Wolenski M, Toker A, Ohkura N, Beckstette M, Miyao T, Geffers R, Floess S, Gerdes N, Lutgens E, Osterloh A, Hori S, Sakaguchi S, Jaeckel E, Huehn J. Unique properties of thymic antigen-presenting cells promote epigenetic imprinting of alloantigen-specific regulatory T cells. Oncotarget. 2017;8:35542–35557. doi: 10.18632/oncotarget.16221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Coquet JM, Ribot JC, Babala N, Middendorp S, van der Horst G, Xiao Y, Neves JF, Fonseca-Pereira D, Jacobs H, Pennington DJ, Silva-Santos B, Borst J. Epithelial and dendritic cells in the thymic medulla promote CD4+Foxp3+ regulatory T cell development via the CD27-CD70 pathway. J Exp Med. 2013;210:715–728. doi: 10.1084/jem.20112061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mahmud SA, Manlove LS, Schmitz HM, Xing Y, Wang Y, Owen DL, Schenkel JM, Boomer JS, Green JM, Yagita H, Chi H, Hogquist KA, Farrar MA. Costimulation via the tumor-necrosis factor receptor superfamily couples TCR signal strength to the thymic differentiation of regulatory T cells. Nat Immunol. 2014;15:473–481. doi: 10.1038/ni.2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gottrand G, Courau T, Thomas-Vaslin V, Prevel N, Vazquez T, Ruocco MG, Lambrecht B, Bellier B, Colombo BM, Klatzmann D. Regulatory T-cell development and function are impaired in mice lacking membrane expression of full length intercellular adhesion molecule-1. Immunology. 2015;146:657–670. doi: 10.1111/imm.12533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tai X, Cowan M, Feigenbaum L, Singer A. CD28 costimulation of developing thymocytes induces Foxp3 expression and regulatory T cell differentiation independently of interleukin 2. Nat Immunol. 2005;6:152–162. doi: 10.1038/ni1160. [DOI] [PubMed] [Google Scholar]
- 68.Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, Naji A, Caton AJ. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol. 2001;2:301–306. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 69.Francisco LM, V, Salinas H, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, Sharpe AH. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Proietto AI, van Dommelen S, Zhou P, Rizzitelli A, D’Amico A, Steptoe RJ, Naik SH, Lahoud MH, Liu Y, Zheng P, Shortman K, Wu L. Dendritic cells in the thymus contribute to T-regulatory cell induction. Proc Natl Acad Sci U S A. 2008;105:19869–19874. doi: 10.1073/pnas.0810268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li MO, Rudensky AY. T cell receptor signalling in the control of regulatory T cell differentiation and function. Nat Rev Immunol. 2016;16:220–233. doi: 10.1038/nri.2016.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hofmann J, Mair F, Greter M, Schmidt-Supprian M, Becher B. NIK signaling in dendritic cells but not in T cells is required for the development of effector T cells and cell-mediated immune responses. J Exp Med. 2011;208:1917–1929. doi: 10.1084/jem.20110128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Martin-Gayo E, Sierra-Filardi E, Corbi AL, Toribio ML. Plasmacytoid dendritic cells resident in human thymus drive natural Treg cell development. Blood. 2010;115:5366–5375. doi: 10.1182/blood-2009-10-248260. [DOI] [PubMed] [Google Scholar]
- 74.Irla M, Hugues S, Gill J, Nitta T, Hikosaka Y, Williams IR, Hubert FX, Scott HS, Takahama Y, Hollander GA, Reith W. Autoantigen-specific interactions with CD4+ thymocytes control mature medullary thymic epithelial cell cellularity. Immunity. 2008;29:451–463. doi: 10.1016/j.immuni.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 75.Yamano T, Nedjic J, Hinterberger M, Steinert M, Koser S, Pinto S, Gerdes N, Lutgens E, Ishimaru N, Busslinger M, Brors B, Kyewski B, Klein L. Thymic B Cells Are Licensed to Present Self Antigens for Central T Cell Tolerance Induction. Immunity. 2015;42:1048–1061. doi: 10.1016/j.immuni.2015.05.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.