Abstract
Background
Accumulating evidence suggests the involvement of abnormal glutamateric neurotransmission and N-methyl-D-aspartate receptor (NMDAR) hypofunction in the pathophysiology of psychotic disorders. The purpose of this study was to quantify in vivo glutamate (Glu) and glycine (Gly) levels in patients with first-episode psychosis as well as age-matched healthy controls with MR spectroscopy.
Methods
The subjects were 46 patients with first-episode psychosis (20 schizophrenia-spectrum disorders (SZ), 26 bipolar disorder (BD)) and 50 age-matched healthy controls (HC). Glu and Gly levels were measured in vivo in the anterior cingulate cortex (ACC) and posterior cingulate cortex (PCC) of the subjects by using the echo-time (TE)-averaged proton MR spectroscopy technique (1H-MRS) at 4 T (i.e., modified PRESS sequence: 24 TE steps with 20 ms increments). Metabolite levels were quantified using LCModel with simulated basis sets.
Results
Significantly higher Glu and Gly levels were found in both the ACC and PCC of patients with first-episode psychosis as compared to healthy controls. Glu and Gly levels were positively correlated in patients. SZ and BD patients showed similar abnormalities.
Conclusions
Our findings demonstrate abnormally elevated brain Glu and Gly levels in patients with first-episode psychosis by means of TE-averaged 1H-MRS at 4 T. The findings implicate dysfunction of NMDAR and glutamatergic neurotransmission in the pathophysiology of the acute early phase of psychotic illnesses.
Keywords: N-methyl-D-aspartate receptor, (NMDAR), glutamate (Glu), glycine (Gly), first-episode psychosis, echo-time (TE)-averaged proton MR spectroscopy
Introduction
A large and consistent body of evidence suggests that glutamatergic dysregulation, and N-methyl-D-aspartate receptor (NMDAR) hypofunction in particular, are involved in the pathophysiology of psychotic disorders, including schizophrenia (SZ) and bipolar disorder (BD) (1). This pathophysiologic mechanism is supported by the observation that administration of NMDAR antagonist drugs, such as phencyclidine (PCP) and ketamine, in healthy subjects and/or animals mimics a broad range of psychotic symptoms that closely resemble those of SZ (2–7). While SZ has received the most attention as the disorder in which NMDAR hypofunction might exist, the fact that higher doses of NMDAR antagonists can induce mania, catatonic signs, and euphoria suggests that NMDAR hypofunction may also be responsible for some of the symptoms of BD (1, 8). Given that NMDAR plays a pivotal role in neurocognition and neurotoxicity, NMDAR function is of great interest in the field of psychiatric research.
A unique feature of the NMDAR is its glycine (Gly) binding site, where Gly or D-serine (D-Ser) acts as a co-agonist (9, 10). Both glutamate (Glu) and Gly/D-Ser binding are required to trigger opening of the ion channel (11). Since Gly can modulate glutamatergic neurotransmission, interventions enhancing Gly action may improve negative, cognitive and depressive symptoms in patients (12–15). Tsai and Lin conducted a systematic review and meta-analysis in 26 placebo-controlled clinical trials with over 800 subjects to evaluate the efficacy of NMDAR co-agonist drugs (i.e., Gly, D-Ser, D-cycloserine) and showed that agents that directly or indirectly activate the glycine modulatory site on the NMDAR significantly improved most symptom domains, in patients receiving concurrent antipsychotic treatment (16). Glu is the major excitatory neurotransmitter in the brain and interacts with metabotropic and ionotropic receptors (including NMDAR). Excessive stimulation of NMDAR by Glu released from presynaptic axon terminals can damage neurons by a process called “excitotoxicity” (17, 18). Given the converging roles of Glu and Gly in psychosis research and treatment, non-invasive in vivo measurements of these amino acids in the brains of patients with psychosis are of interest.
Although proton magnetic resonance spectroscopy (1H-MRS) can be used to non-invasively determine metabolite levels in vivo, limited spectral resolution, relatively narrow spectral range (i.e., 0 to 4 ppm), and overlapping resonances have restricted its utility. For example, the C4 proton signal of Glu at 2.35 ppm is contaminated by signal from the C3 protons of N-acetyl aspartate (NAA) as well as C3 and C4 protons of glutamine (Gln) even in short echo-time (TE) proton spectra. Further, Gly is present at very low concentration (below 1 mM) in the healthy adult brain and its resonances are largely overlapped with myo-inositol (mIns), which is normally present at higher concentrations than Gly. Hence, direct measurement of Gly remains challenging. It is therefore valuable to have a novel technique for simultaneous detections of Glu and Gly that overcomes the problem of overlapping spectral resonances seen in conventional 1H-MRS. TE-averaged 1H-MRS, which was initially developed for Glu detection distinct from Gln and NAA (19), has enabled reliable measure of the Gly singlet peak by attenuating the overlapping mIns resonance at 3.52 ppm (20). This approach was validated in an in vivo Gly supplementation study (21). Note that MRS measures reflect total pools of each metabolite in a volume of interest and metabolites such as Glu play multiple roles in the brain. Therefore we cannot assume our MRS measures reflect synaptic activity of Glu or Gly. To the best of our knowledge, there have been no reports on in vivo Gly levels in patients with psychotic disorders, despite multiple studies on the Gly metabolism in vitro in blood samples or cerebrospinal fluid (CSF) using biochemical analysis methods (12, 22, 23).
In this study, we aimed to simultaneously quantify Glu and Gly levels in vivo in patients with first-episode psychotic disorders using TE-averaged 1H-MRS at 4 Tesla and to assess possible interrelationships between Glu and/or Gly levels, diagnosis, and symptom severity. We hypothesized that we would see abnormalities consistent with NMDAR hypofunction in patients. Specifically, we expected elevated brain Glu levels, as observed in past studies of patients with established illness (24–27), and brain Gly levels would be lower in patients with first-episode psychosis based upon the studies of clinical trials of glycine supplementation (12, 13, 28).
Methods and Materials
Subjects
All protocols were approved and conducted in compliance with the Institutional Review Board at Partners Human Research Committee, and written informed consent was obtained from participants before the studies. Forty-six patients with a first-episode psychotic disorder (i.e., 20 diagnosed with a schizophrenia-spectrum disorder (SZ group) and 26 with BD) and 50 age-matched healthy controls (HC) were enrolled in our study. The clinical characteristics of participants are presented in Table 1 and additional details of subject assessments in Supplementary Materials.
Table 1.
Demographic and clinical characteristics of the subjects included in MRS analyses
| Characteristics | Healthy controls | Patients with first-episode psychosis | Statistics |
|---|---|---|---|
| Age (years, mean ± SD) | 25.14 ± 5.86 | 23.62 ± 4.73 | t=1.167, p=0.203 |
| Sex (M/F) | 19/30 | 34/6 | χ2=25.2, p<0.05 |
| Education (years, mean ± SD) | 16.08 ± 1.91 | 14.87 ± 2.04 | t=2.379, p=0.530 |
| Handedness (right/left) | 47/2 | 36/4 | χ2=1.227, p=0.268 |
| PANSS | |||
| Positive | - | 10.31 ± 5.12 | |
| Negative | - | 12.11 ± 5.45 | |
| General | - | 24.85 ± 7.75 | |
| YMRS | - | 5.00 ± 7.17 | |
| MADRS | - | 8.10 ± 8.37 | |
| MCAS | - | 45.20 ± 6.74 | |
| CPZ equivalent | - | 127.32 ± 178.08 | |
| Lithium users (%) | - | 16 (40.0 %) | |
| Antipsychotic users (%) | - | 26 (65.0 %) | |
All data are expressed as mean ± SD.
Abbreviations: BMI, Body Mass Index; PANSS, Positive and Negative Syndrome Scale; YMRS, Young Mania Rating Scale; MADRS, Montgomery-Asburg Depression Rating Scale; MCAS, Multnomah Community Ability Scale; CPZ, Chlorpromazine
Magnetic resonance imaging (MRI) and spectroscopy (1H-MRS) protocol
In vivo MRI and 1H-MRS measurements were conducted on a Varian Unity/Inova whole-body MR scanner operating at 4 Tesla (Varian Inc., Palo Alto, CA, USA) and 16 elements, single-tuned (1H), volumetric birdcage design RF coil (Robarts Research Institute, London, Canada) operating at 170.3 MHz was used for RF transmission and signal reception. Initially, the participants were scanned with a rapid 2D gradient-recall echo sequence to ensure their optimal positioning. High-contrast T1-weighted axial and sagittal images (TE/TR=6.2/11.4 ms, FOV=22×22×16 cm3, readout-duration= 4 ms, receive bandwidth= ±32 kHz, matrix size=256×256×64, in-plane resolution= 0.94×0.94, slice thickness=2.5 mm, flip angle=11°) were acquired to serve as an anatomical guide to positioning MRS voxels, which were placed on anterior cingulate cortex (ACC) with voxel size of 20×25×35 mm3 and posterior cingulate cortex (PCC) with voxel size of 20×20×20 mm3.
A modified PRESS sequence employing four-pulse WET (water suppression enhanced through T1-effects, WET flip angle: θ1: θ2: θ3: θ4 = 81.4°: 101.4°: 69.3°: 161.0°) (29) was used for the collection of 24 TE-stepped spectra with the echo-time ranging from 30 to 490 ms with 20 ms increments. Before acquiring the TE-stepped spectra, B0 homogeneity was manually adjusted for each subject. The localized unsuppressed water signal linewidth was less than 10 Hz for all in vivo measurements. The MRS acquisition parameters were: TR=2.0 sec, spectral width= 2kHz, number of excitation (NEX)=16 per echo, number of complex data points=1024, and total scan time= 13 min.
Test-retest data demonstrating the reliability of our TE-averaging quantification method for measuring Gly was previously published in work from our center (20). In this work, we further acquired the data from a single healthy control subject scanned in identical fashion 10 times over a 10-week period in order to provide test-retest reliability for Glu and Gly measure using TE-averaging approach.
Spectral processing and metabolites quantification
The TE-stepped 1H-MRS data were processed by averaging all 24 TE spectra. Before fast Fourier transformation, free induction decay (FID) was processed with Gaussian multiplication (i.e., f(t) = exp(−(t−tmax)2/2σ2, tmax = 0.05 sec, σ = 0.085 sec) and zero-filling to enhance spectral resolution (30). Frequency drift between each step of TE spectra acquisitions were corrected automatically using a cross-correlation algorithm (31). Eddy current correction was done using unsuppressed water signal. All spectral preprocessing was conducted using home-written software in MATLAB and fitting using LCModel. See Supplementary Materials for details of metabolite quantification and voxel segmentation.
Statistical analysis
We excluded TE-averaged 1H-MRS data with severe baseline distortion, or SNR lower than 15, and/or poor spectral resolution (i.e., full-width half-maximum higher than 0.06 ppm) (N=6 for psychosis group; N=1 for healthy group). The examples of bad spectral quality are shown in Supplementary Figure S1. As a result, data from 40 psychosis patients and 49 healthy controls were included in the statistical analysis. Our primary outcome measures were Glu and Gly levels, but the other metabolites with CRLB < 20 %, (NAA, Cr+PCr, PCh+GPC, mIns) were also examined. Three statistical approaches were used: (a) Analysis of Covariance (ANCOVA) with sex and GM/(GM+WM) as covariates to compare the differences in metabolite levels between two groups (i.e., patients with psychotic disorder vs. healthy controls); (b) one-way ANOVAs with sex as a covariant followed by post hoc (e.g., Bonferroni correction with factor of 0.05/3=0.017) comparison, and (c) independent t-tests for antipsychotic drug or lithium effects in the patient group. In the second approach, we divided the patient group into two groups (SZ and BD) in order to compare metabolite level differences among the three groups, SZ, BD and HC. In the third approach, metabolite levels in patients treated with antipsychotic drugs or lithium were compared with those who were not taking antipsychotic drugs or lithium. Since the ages were well matched among all groups, we only considered sex as a covariate to remove its influence. We considered corrected p values lower than 0.05 as statistically significant.
We next evaluated the interrelationships between Glu and/or Gly, and demographic/clinical parameters. A large number of correlation analyses were performed on an exploratory basis; therefore, we considered Pearson’s correlation coefficients (R values) greater than 0.30 and p values lower than 0.05 uncorrected for multiple comparisons as possibly significant. All statistical analysis was performed using PASW software (Window version 18.0, Chicago, IL, USA).
Results
Metabolite quantification
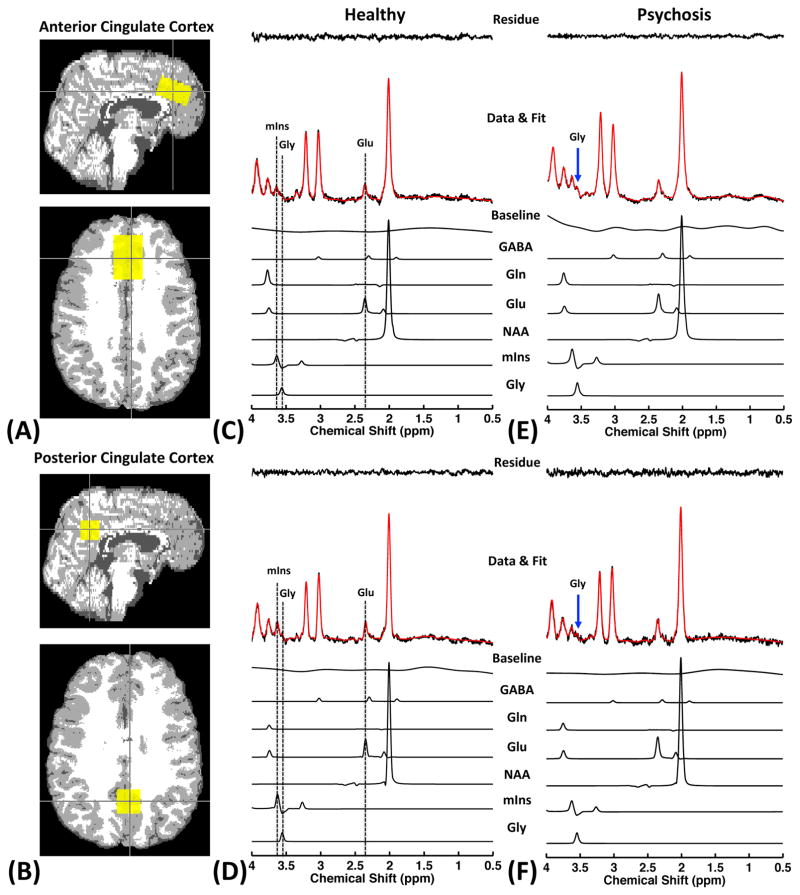
Figure 1 shows representative TE-averaged spectra obtained from a healthy control and a patient with psychosis; partial volume maps of tissue subtypes (GM, WM, and CSF) overlaid with the 1H-MRS voxel are also shown. There were no significant differences in voxel compositions of either brain region among HC and patients with psychosis, SZ or BD (all p > 0.05). As can be seen in Figure 1, TE-averaged 1H-MRS fully resolves the Glu signal at 2.35 ppm from the overlapping resonances of NAA and Gln, resulting in its unobstructed measurement. In addition, the resonance of Gly methylene protons can be clearly detected at 3.55 ppm in TE-averaged spectra, which is enabled by significantly attenuating the four groups of strongly J-coupled mIns resonances. This is confirmed in a density-matrix calculated 1H spectrum (See supplementary Figure S2). Compared to conventional PRESS measurement, TE-averaged 1H-MRS dramatically reduces the mIns resonance around 3.55 ppm, producing a clear separation between Gly and mIns signals, thus allowing greater sensitivity and specificity for detecting and quantifying Gly levels in vivo (Figure S2). However, because the peak detected at 3.55 ppm in TE-averaged spectrum is partially contaminated by the mIns signal, we labeled the glycine signal as Gly* to distinguish this peak from a “pure” Gly signal. Average CRLBs of Glu and Gly for all subjects were 6 % and 12 %, respectively. The CRLB values for two brain regions in both groups are listed in Table 2.
Figure 1.
Partial volume maps of tissue subtypes (GM: gray color; WM: white color; CSF: dark gray color) overlaid with reconstructed VOIs (yellow box) of 1H-MRS are shown (a: ACC, b: PCC). And representative TE-averaged proton spectra obtained from ACC and PCC of healthy control (c, d) and patient with psychosis (e, f) are also provided. Note that the Glu signal at 2.35 ppm is clearly resolved from the Gln and NAA resonances, and the Gly signal at 3.55 ppm is observed by suppressing the dominating mIns signal.
Table 2.
Relative Cramer-Rao Lower Bounds (CRLBs%) of Glu and Gly calculated by LCModel
| Brain region | Group | CRLBs (%) | |
|---|---|---|---|
| Glu | Gly | ||
| ACC | Healthy | 5.49 ± 2.01 | 12.77 ± 5.41 |
| Psychosis | 5.17 ± 1.37 | 11.31 ± 4.97 | |
|
| |||
| PCC | Healthy | 5.75 ± 1.77 | 10.92 ± 2.46 |
| Psychosis | 5.87 ± 1.64 | 12.25 ± 3.73 | |
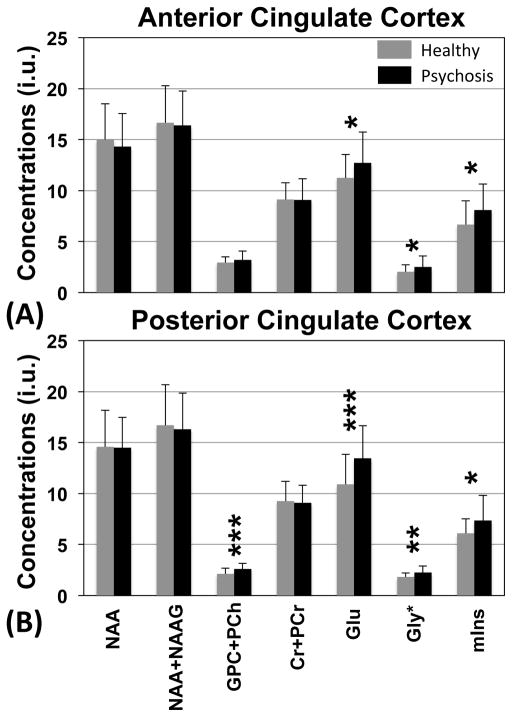
Figure 2 shows the differences in metabolite concentrations between patients with psychotic disorders and healthy controls. Statistical analysis revealed that there were significant elevations of Glu and Gly* concentrations in patients with psychotic disorder in both brain regions (ACC and PCC) as compared to healthy controls (ANCOVA: ACC, Glu: F = 5.935, p = 0.017, Gly*: F = 4.446, p = 0.038; PCC, Glu: F = 14.251, p < 0.001, Gly*: F = 9.599, p = 0.003). The Glu level in patient group was increased by 12.97 % and 23.5 % in ACC and PCC, respectively, while the Gly* level in patient group was increased by 23.83 % and 24.12 % in ACC and PCC, respectively. Furthermore, we found significantly higher mIns concentrations in ACC and PCC of patients with psychotic disorder compared to healthy controls (ANCOVA: ACC, mIns: F = 5.606, p = 0.02; PCC, mIns: F = 6.311, p = 0.014). GPC + PCh concentrations are significantly higher in the PCC of patients with psychosis as compared to healthy controls (F = 13.314, p < 0.001). Other metabolites quantified, including NAA and Cr+PCr, were not significantly different between the two groups, although the patient group showed a trend to numerically lower measures for each. In order to test possible differences of metabolite levels between SZ and BD patients, we next carried out one-way ANOVA followed by post hoc testing among the three groups. The post hoc tests revealed that there were no significant differences in Glu, Gly*, GPC+PCh, and mIns level in either brain region between SZ and BD patients (data not shown). We also compared metabolite level referenced to total Cr signal since water content might be different between healthy and patients with psychosis. Even if we use total Cr signal as internal reference, we still found significant differences in Glu/tCr and Gly*/tCr levels of both brain regions between groups. Detailed results are provided in Supplementary Table S1.
Figure 2.
Metabolite levels in ACC (a) and PCC (b) in patients with first-episode psychosis (N=40) and age-matched healthy controls (N=49) are shown. The quantifications were performed with LCModel and the concentrations are calculated using the unsuppressed water signal as an internal reference. The levels are expressed in institutional units (i.u.). The error bars indicate standard deviations of measurements of each metabolite. Statistical analyses were performed using Analysis of Covariance (ANCOVA) with sex as a covariate (significance level: *p < 0.05, **p < 0.005, ***p < 0.001).
Furthermore, we evaluated test-retest reliability of Glu and Gly measurement by calculating coefficient of variation (CV=standard deviation divided by mean) in a healthy control subject. We found that the CV of our measures was 7.4% for Glu and 12.4% for Gly, indicating excellent reliability. The data for this test-retest work are presented in Supplementary Figure S3.
Interrelationships between Glu and/or Gly, and clinical parameters
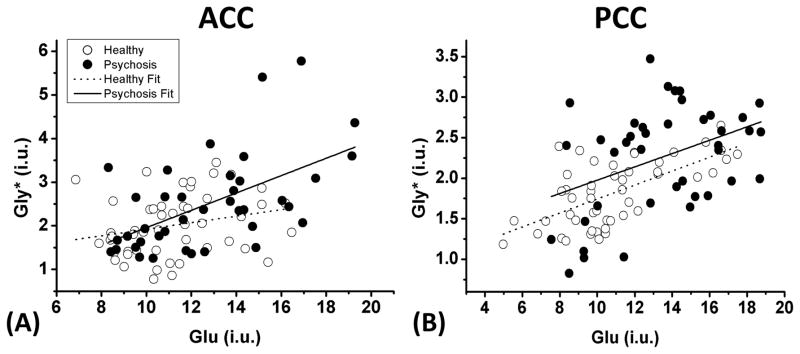
To investigate the interrelationships of Glu, Gly*, and clinical parameters, we performed Pearson’s correlation analyses. Interestingly, there were significant positive correlations between Glu and Gly* levels in both ACC (R = 0.558, p < 0.001) (Figure 3-a) and PCC (R = 0.401, p = 0.010) (Figure 3-b) of the psychosis group. A significant correlation between Glu and Gly levels was found only in the PCC of healthy controls (R = 0.636, p < 0.001) (Figure 3-b). In addition, we found relatively weak but significant positive correlations between brain Glu and the PANSS General score (R = 0.398, p = 0.012), and between brain GPC+PCh and the PANSS General score (R = 0.338, p = 0.035) in the region of ACC, but not in PCC. Those results are presented in supplementary Figure S4. We note that the latter correlations may be driven by skewed distribution in the PANSS General scores.
Figure 3.
The relationships between Glu and Gly levels in ACC (a) and PCC (b) in patients with first-episode psychosis (black-filled circles) and healthy controls (open circles) are shown. The Glu levels are positively correlated with Gly levels in both brain regions of patients with psychosis (ACC: R = 0.558, p < 0.001; PCC: R = 0.401, p = 0.010), while a significant correlation between these metabolites is observed only in PCC of healthy controls (R = 0.636, p < 0.001). Note that this figure presents data after correction for CSF contamination; although there was no between-group difference in voxel composition, a small number of participants with high CSF content in the MRS voxel show higher calculated Gly and Glu concentrations.
Subgroup analysis for antipsychotic drug effects
Table 3 summarizes the association between antipsychotic drug use and Glu and Gly* levels in two brain regions of the patients. In the ACC, there were no significant differences in Glu (p = 0.585) and Gly* levels (p = 0.205) between patients with and without antipsychotic drug use, while there was a trend association (p = 0.081) between antipsychotic drug use and Glu levels in PCC. In this instance, patients taking medication had lower Glu levels. Additionally, Gly* levels in the PCC were not significantly different between patients with and without taking antipsychotic drug use (p = 0.772).
Table 3.
The effects of antipsychotic drugs on measured Glu and Gly levels
| Region | Metabolites | Antipsychotic-free | Antipsychotic treatment | Statistics* | |
|---|---|---|---|---|---|
| t values | p values | ||||
| ACC | Glu | 12.31 ± 2.77 | 12.86 ± 3.14 | −0.551 | 0.585 |
| Gly | 2.17 ± 0.73 | 2.62 ± 1.18 | −1.288 | 0.205 | |
| PCC | Glu | 14.69 ± 3.29 | 12.92 ± 3.05 | 1.796 | 0.081 |
| Gly | 2.24 ± 0.74 | 2.32 ± 0.66 | 0.292 | 0.772 | |
Statistical analysis was performed with independent t-test.
The concentrations are expressed as mean ± standard deviation and the values are in institutional units (i.u.).
Moreover, we found no significant lithium effects on Glu and Gly* level in both brain regions. Detailed results for lithium effects are provided in Supplementary Table S2.
Discussion
In this study, we measured Glu and Gly levels using TE-averaged 1H-MRS in human brain at 4T. The measured Glu and Gly levels were significantly higher in both brain regions in patients with first-episode psychosis as compared to healthy controls. The findings were similar between the SZ and BD groups. We also found clear and significant positive correlations between Glu and Gly* levels in both brain regions among patients with psychosis but not as clearly among healthy controls. This pattern of findings suggests that early psychosis is characterized by abnormal and linked elevations in two endogenous NMDAR agonists. These findings are consistent with a multi-hit hypothesis of risk for psychosis, in which both glutamate and glycine levels are abnormal, or may possibly indicate a compensatory response to underlying NMDAR hypofunction.
The most striking finding in this study is that we were able to document abnormal in vivo brain Gly levels in patients with psychosis. Although the two uncoupled methylene protons of Gly exhibit a simple singlet resonance at 3.55 ppm in 1H MR spectra, reliable Gly detection in vivo with conventional 1H-MRS techniques is quite challenging. TE-averaged 1H-MRS as used in this study allows Gly detection by suppressing the dominant mIns signal that are greatly decreased due to J-coupling as well as T2 relaxation as TE is varied systematically. There are to date no published reports of 1H-MRS Gly measurement in patients with psychosis. Gly is both a co-agonist at the NMDAR as well as a precursor to D-Ser, the NMDAR co-agonist in forebrain. Occupancy of the glycine modulatory site on the NMDAR is essential for neural plasticity and memory (32). Synaptic Gly concentrations are tightly regulated by glycine transporters (33). Our finding of increased Gly levels in patients with first-episode psychosis is somewhat unexpected, but it is consistent with previous reports showing higher blood Gly, Glu, and serine levels in patients with SZ (34). Other studies also reported significantly higher levels of Gly in CSF (35) and in mesial aspects of temporal lobe of autopsied brain (36) in patients with SZ. Moreover, Heresco-Levy et al. observed a negative correlation between serum glycine levels and prepulse inhibition (PPI, a measure of sensorimotor gating) in SZ patients (23), indicating that patients with higher Gly levels have reduced PPI, as usually observed in SZ patients. However a recent meta-analysis found no observable differences in serum Gly levels between HC subjects and SZ patients (37). Given that Gly has been used as an adjunctive treatment to enhance glutamatergic neurotransmission and to ameliorate SZ symptoms (16), the higher brain Gly* levels in patients with psychosis observed in this study at first seem to be inconsistent with the NMDAR hypofunction hypothesis. However, MRS cannot determine whether it reflects a slower turnover, resulting in net accumulation, or increased synthesis, resulting in elevated levels. Nevertheless, the results of recent genome wide association study revealed a dozen risk genes that encode proteins that directly or indirectly affect NMDAR function or its down-stream mediators (32). A parsimonious interpretation is that the elevated Gly* levels represent a failed attempt at compensating for genetically determined NMDAR hypofunction. This interpretation is supported by the observation of the same pattern in patients on and off treatment in this study; i.e. we see no evidence of normalization with treatment indicating this may be a disease-related finding. Another possible interpretation of our findings is that there might be malfunction of the enzyme converting Gly to D-Ser, leading to a build-up of Gly but deficiency in D-Ser levels (38). Future research would be necessary to investigate how direct and indirect NMDAR agonist interventions affect brain Gly levels.
Our finding of increased Glu levels in patients with first-episode psychosis is broadly consistent with previous reports. For example, a study by Egerton et al. (2012) showed that the Glu/Cr ratio in ACC of 15 patients with first-episode psychosis were significantly higher compared to those in remission (25). The authors also found that higher Glu/Cr ratios in the ACC were associated with greater negative symptoms and lower global functioning. Another MRS study using a 3T MR scanner revealed that absolute Glu concentrations in the associative-striatum of ultra high-risk and antipsychotic naïve first-episode psychosis patients were elevated compared to healthy controls (24). Although Bustillo et al. (2010) observed no differences in Glu levels in the bilateral ACC, the left frontal white matter and the left thalamus of a minimally treated SZ group, they did see elevation of the Gln/Glu ratio in the bilateral ACC (39). Since Gln is a precursor and metabolite of Glu, Gln/Glu ratio may be a good measure of glutamatergic neurotransmission (40). 1H-MRS studies measuring Gln consistently showed elevation in the left ACC (41) and left thalamus (42) of antipsychotic naïve SZ patients. More recently, a meta-analysis of 1H-MRS studies in SZ showed significant elevations in Glu in the basal ganglia, Gln in the thalamus, and Glu+Gln (Glx) in the basal ganglia and medial temporal lobe of patients (27). A meta-analysis of 1H-MRS studies in BD revealed significant elevation of Glx in patients with BD as compared to HC when all brain areas were combined (26). The finding remained significant in medicated and non-medicated patients, and in frontal brain areas in adults (26). Our finding of elevated Glu level in patients with psychosis replicates previous reports and suggests some perturbation of the glutamatergic system that would need to be investigated by other means. Further study, for example 13C-MRS technique, would be required to investigate Glu synthesis and release in greater detail, because this technique permits direct quantitation of both Glu synthesis and catabolism rates using 13C-labled tracers. 1H-MRS cannot provide direct information regarding Glu neurotransmission but reflects the total amount of parenchymal Glu (i.e., metabolic and neurotransmitter pools of glutamate) within a specified VOI in the brain. Lastly, in exploratory analyses we found significantly higher mIns levels in ACC and PCC as well as higher GPC+PCh levels in the PCC of patients with psychosis as compared to healthy controls. The mIns and GPC+PCh signals have been proposed as markers of glial cell and membrane turnover, respectively, as those metabolites are mostly present within glial cells rather than in neurons (43). Thus elevated mIns and GPC+PCh levels in patients with psychosis might be tentatively interpreted as glial activation. Moreover, we found a significant positive correlation between Glu and mIns levels in patients with psychosis (data not shown). Since mIns and GPC+PCh levels are elevated in several neuroinflammatory disorders (44–46) our findings may motivate future studies on the relationship between neuroinflammation and mIns and GPC+PCh levels.
The present study has limitations. First, we were not able to reliably measure the Gln signal using TE-averaged 1H-MRS, since the signals for Gln C3 and C4 protons are cancelled out in TE-averaged spectra. Although one can estimate Gln levels by subtracting the integral of the Glu signal at 2.35 ppm from that of the Glx signal at 3.75 ppm, this method is not likely to be accurate. Simultaneous Glu and Gln detection methods such as J-modulated (47) or phase-adjusted echo time (PATE)-averaging MRS (48) would provide more detailed information about glutamatergic metabolites in psychosis. Second, we cannot exclude medication effects on Glu and Gly, levels since most of the patients participated in this study were taking medication. However patients who were taking antipsychotic or lithium medications showed no significant differences in Glu and Gly levels in either brain region compared to those who were not taking these medications, suggesting that medication effects do not account for our findings. Since the number of patients who were not taking any antipsychotic or lithium medication was small, replication in a larger population would be required to obtain a definitive finding. Third, differential T2 transverse relaxation effects between patients and controls may influence spectral pattern of each TE-step. The measurement of T2 for strongly coupled (e.g, Glu) and low concentration (e.g. Gly) metabolites in the brain is extremely difficult in vivo. Although apparent T2 values of Glu in healthy brain at 3 T (but not Gly) have been reported with 201 ± 18 ms (49), there are no reports about T2 values of Glu and Gly at 4 T. Further studies to investigate reliable measurement of T2 values for those metabolites at 4 T are required in order to observe possible differences between healthy controls and patients with psychosis. Lastly, the Gly levels, estimated using the TE-averaged approach of this study, might be partially contaminated by the mIns signal. However, we found 23.8 % and 21.3 % elevations of Gly* and mIns levels, respectively, in the ACC of patients of psychosis patients (corresponding to about a 0.5 and 1.2 i.u. elevation in concentration, respectively). Thus, a very substantial contamination from the mIns signal would be needed to account fully for our finding of Gly* elevation and that is inconsistent with previous reports showing that the mIns contribution was less than 2% of total Gly* signal in a 1:1 mIns/Gly mixture (20). Therefore we suggest that our findings reflect a true Gly elevation in patients with psychosis, even though we cannot completely rule out some contribution from mIns.
In conclusion, we report abnormal brain Glu and Gly levels in patients with first-episode psychosis by means of TE-averaged 1H-MRS at 4T, and that the pattern of our findings was consistent with the involvement of NMDAR hypofunction in the pathophysiology of early stage psychosis. Future research coupling these observations with intervention studies to address these dysfunctions would be of great interest.
Supplementary Material
Acknowledgments
This work was supported by grants from MH094594 (D.O.); MH104449 (D.O.); Program for Neuropsychiatric Research (B.M.C.).
Footnotes
Financial Disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Farber NB. The NMDA receptor hypofunction model of psychosis. Ann N Y Acad Sci. 2003;1003:119–130. doi: 10.1196/annals.1300.008. [DOI] [PubMed] [Google Scholar]
- 2.Chindo BA, Adzu B, Yahaya TA, Gamaniel KS. Ketamine-enhanced immobility in forced swim test: a possible animal model for the negative symptoms of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2012;38:310–316. doi: 10.1016/j.pnpbp.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 3.Itil T, Keskiner A, Kiremitci N, Holden JM. Effect of phencyclidine in chronic schizophrenics. Can Psychiatr Assoc J. 1967;12:209–212. doi: 10.1177/070674376701200217. [DOI] [PubMed] [Google Scholar]
- 4.Luby ED, Cohen BD, Rosenbaum G, Gottlieb JS, Kelley R. Study of a new schizophrenomimetic drug; sernyl. AMA Arch Neurol Psychiatry. 1959;81:363–369. doi: 10.1001/archneurpsyc.1959.02340150095011. [DOI] [PubMed] [Google Scholar]
- 5.Noda Y, Nabeshima T. Neuropsychopharmacological study on an animal model for negative symptom of schizophrenia induced by repeated phencyclidine treatment. Yakugaku Zasshi. 2000;120:677–682. [PubMed] [Google Scholar]
- 6.Lisman JE, Coyle JT, Green RW, Javitt DC, Benes FM, Heckers S, et al. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008;31:234–242. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim SY, Lee H, Kim HJ, Bang E, Lee SH, Lee DW, et al. In vivo and ex vivo evidence for ketamine-induced hyperglutamatergic activity in the cerebral cortex of the rat: Potential relevance to schizophrenia. NMR Biomed. 2011;24:1235–1242. doi: 10.1002/nbm.1681. [DOI] [PubMed] [Google Scholar]
- 8.Konopaske GT, Lange N, Coyle JT, Benes FM. Prefrontal cortical dendritic spine pathology in schizophrenia and bipolar disorder. JAMA Psychiatry. 2014;71:1323–1331. doi: 10.1001/jamapsychiatry.2014.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farber NB, Newcomer JW, Olney JW. Glycine agonists: what can they teach us about schizophrenia? Arch Gen Psychiatry. 1999;56:13–17. doi: 10.1001/archpsyc.56.1.13. [DOI] [PubMed] [Google Scholar]
- 10.Johnson JW, Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987;325:529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- 11.Mayer ML, Westbrook GL. The physiology of excitatory amino acids in the vertebrate central nervous system. Prog Neurobiol. 1987;28:197–276. doi: 10.1016/0301-0082(87)90011-6. [DOI] [PubMed] [Google Scholar]
- 12.Heresco-Levy U, Ermilov M, Lichtenberg P, Bar G, Javitt DC. High-dose glycine added to olanzapine and risperidone for the treatment of schizophrenia. Biol Psychiatry. 2004;55:165–171. doi: 10.1016/s0006-3223(03)00707-8. [DOI] [PubMed] [Google Scholar]
- 13.Heresco-Levy U, Javitt DC, Ermilov M, Mordel C, Silipo G, Lichtenstein M. Efficacy of high-dose glycine in the treatment of enduring negative symptoms of schizophrenia. Arch Gen Psychiatry. 1999;56:29–36. doi: 10.1001/archpsyc.56.1.29. [DOI] [PubMed] [Google Scholar]
- 14.Javitt DC, Silipo G, Cienfuegos A, Shelley AM, Bark N, Park M, et al. Adjunctive high-dose glycine in the treatment of schizophrenia. Int J Neuropsychopharmacol. 2001;4:385–391. doi: 10.1017/S1461145701002590. [DOI] [PubMed] [Google Scholar]
- 15.Javitt DC, Zylberman I, Zukin SR, Heresco-Levy U, Lindenmayer JP. Amelioration of negative symptoms in schizophrenia by glycine. Am J Psychiatry. 1994;151:1234–1236. doi: 10.1176/ajp.151.8.1234. [DOI] [PubMed] [Google Scholar]
- 16.Tsai GE, Lin PY. Strategies to enhance N-methyl-D-aspartate receptor-mediated neurotransmission in schizophrenia, a critical review and meta-analysis. Curr Pharm Des. 2010;16:522–537. doi: 10.2174/138161210790361452. [DOI] [PubMed] [Google Scholar]
- 17.Bruno V, Scapagnini U, Canonico PL. Excitatory amino acids and neurotoxicity. Funct Neurol. 1993;8:279–292. [PubMed] [Google Scholar]
- 18.Coyle JT, Bird SJ, Evans RH, Gulley RL, Nadler JV, Nicklas WJ, et al. Excitatory amino acid neurotoxins: selectivity, specificity, and mechanisms of action. Based on an NRP one-day conference held June 30, 1980. Neurosci Res Program Bull. 1981;19:1–427. [PubMed] [Google Scholar]
- 19.Hurd R, Sailasuta N, Srinivasan R, Vigneron DB, Pelletier D, Nelson SJ. Measurement of brain glutamate using TE-averaged PRESS at 3T. Magn Reson Med. 2004;51:435–440. doi: 10.1002/mrm.20007. [DOI] [PubMed] [Google Scholar]
- 20.Prescot AP, de BFB, Wang L, Brown J, Jensen JE, Kaufman MJ, et al. In vivo detection of brain glycine with echo-time-averaged (1)H magnetic resonance spectroscopy at 4.0 T. Magn Reson Med. 2006;55:681–686. doi: 10.1002/mrm.20807. [DOI] [PubMed] [Google Scholar]
- 21.Kaufman MJ, Prescot AP, Ongur D, Evins AE, Barros TL, Medeiros CL, et al. Oral glycine administration increases brain glycine/creatine ratios in men: a proton magnetic resonance spectroscopy study. Psychiatry Res. 2009;173:143–149. doi: 10.1016/j.pscychresns.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frye MA, Tsai GE, Huggins T, Coyle JT, Post RM. Low cerebrospinal fluid glutamate and glycine in refractory affective disorder. Biol Psychiatry. 2007;61:162–166. doi: 10.1016/j.biopsych.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 23.Heresco-Levy U, Bar G, Levin R, Ermilov M, Ebstein RP, Javitt DC. High glycine levels are associated with prepulse inhibition deficits in chronic schizophrenia patients. Schizophr Res. 2007;91:14–21. doi: 10.1016/j.schres.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 24.de la Fuente-Sandoval C, Leon-Ortiz P, Favila R, Stephano S, Mamo D, Ramirez-Bermudez J, et al. Higher levels of glutamate in the associative-striatum of subjects with prodromal symptoms of schizophrenia and patients with first-episode psychosis. Neuropsychopharmacology. 2011;36:1781–1791. doi: 10.1038/npp.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Egerton A, Brugger S, Raffin M, Barker GJ, Lythgoe DJ, McGuire PK, et al. Anterior cingulate glutamate levels related to clinical status following treatment in first-episode schizophrenia. Neuropsychopharmacology. 2012;37:2515–2521. doi: 10.1038/npp.2012.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gigante AD, Bond DJ, Lafer B, Lam RW, Young LT, Yatham LN. Brain glutamate levels measured by magnetic resonance spectroscopy in patients with bipolar disorder: a meta-analysis. Bipolar Disord. 2012;14:478–487. doi: 10.1111/j.1399-5618.2012.01033.x. [DOI] [PubMed] [Google Scholar]
- 27.Merritt K, Egerton A, Kempton MJ, Taylor MJ, McGuire PK. Nature of Glutamate Alterations in Schizophrenia: A Meta-analysis of Proton Magnetic Resonance Spectroscopy Studies. JAMA Psychiatry. 2016;73:665–674. doi: 10.1001/jamapsychiatry.2016.0442. [DOI] [PubMed] [Google Scholar]
- 28.Coyle JT, Tsai G. The NMDA receptor glycine modulatory site: a therapeutic target for improving cognition and reducing negative symptoms in schizophrenia. Psychopharmacology (Berl) 2004;174:32–38. doi: 10.1007/s00213-003-1709-2. [DOI] [PubMed] [Google Scholar]
- 29.Ogg RJ, Kingsley PB, Taylor JS. WET, a T1- and B1-insensitive water-suppression method for in vivo localized 1H NMR spectroscopy. J Magn Reson B. 1994;104:1–10. doi: 10.1006/jmrb.1994.1048. [DOI] [PubMed] [Google Scholar]
- 30.Tkac I, Andersen P, Adriany G, Merkle H, Ugurbil K, Gruetter R. In vivo 1H NMR spectroscopy of the human brain at 7 T. Magn Reson Med. 2001;46:451–456. doi: 10.1002/mrm.1213. [DOI] [PubMed] [Google Scholar]
- 31.Savorani F, Tomasi G, Engelsen SB. icoshift: A versatile tool for the rapid alignment of 1D NMR spectra. J Magn Reson. 2010;202:190–202. doi: 10.1016/j.jmr.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 32.Balu DT, Coyle JT. The NMDA receptor ‘glycine modulatory site’ in schizophrenia: D-serine, glycine, and beyond. Curr Opin Pharmacol. 2015;20:109–115. doi: 10.1016/j.coph.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bergeron R, Meyer TM, Coyle JT, Greene RW. Modulation of N-methyl-D-aspartate receptor function by glycine transport. Proc Natl Acad Sci U S A. 1998;95:15730–15734. doi: 10.1073/pnas.95.26.15730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Macciardi F, Lucca A, Catalano M, Marino C, Zanardi R, Smeraldi E. Amino acid patterns in schizophrenia: some new findings. Psychiatry Res. 1990;32:63–70. doi: 10.1016/0165-1781(90)90136-s. [DOI] [PubMed] [Google Scholar]
- 35.Reveley MA, De Belleroche J, Recordati A, Hirsch SR. Increased CSF amino acids and ventricular enlargement in schizophrenia: a preliminary study. Biol Psychiatry. 1987;22:413–420. doi: 10.1016/0006-3223(87)90163-6. [DOI] [PubMed] [Google Scholar]
- 36.Waziri R, Baruah S, Sherman AD. Abnormal serine-glycine metabolism in the brains of schizophrenics. Schizophr Res. 1993;8:233–243. doi: 10.1016/0920-9964(93)90021-a. [DOI] [PubMed] [Google Scholar]
- 37.Brouwer A, Luykx JJ, van Boxmeer L, Bakker SC, Kahn RS. NMDA-receptor coagonists in serum, plasma, and cerebrospinal fluid of schizophrenia patients: a meta-analysis of case-control studies. Neurosci Biobehav Rev. 2013;37:1587–1596. doi: 10.1016/j.neubiorev.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 38.Basu AC, Tsai GE, Ma CL, Ehmsen JT, Mustafa AK, Han L, et al. Targeted disruption of serine racemase affects glutamatergic neurotransmission and behavior. Mol Psychiatry. 2009;14:719–727. doi: 10.1038/mp.2008.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bustillo JR, Rowland LM, Mullins P, Jung R, Chen H, Qualls C, et al. 1H-MRS at 4 tesla in minimally treated early schizophrenia. Mol Psychiatry. 2010;15:629–636. doi: 10.1038/mp.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ongur D, Jensen JE, Prescot AP, Stork C, Lundy M, Cohen BM, et al. Abnormal glutamatergic neurotransmission and neuronal-glial interactions in acute mania. Biol Psychiatry. 2008;64:718–726. doi: 10.1016/j.biopsych.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Theberge J, Bartha R, Drost DJ, Menon RS, Malla A, Takhar J, et al. Glutamate and glutamine measured with 4.0 T proton MRS in never-treated patients with schizophrenia and healthy volunteers. Am J Psychiatry. 2002;159:1944–1946. doi: 10.1176/appi.ajp.159.11.1944. [DOI] [PubMed] [Google Scholar]
- 42.Theberge J, Williamson KE, Aoyama N, Drost DJ, Manchanda R, Malla AK, et al. Longitudinal grey-matter and glutamatergic losses in first-episode schizophrenia. Br J Psychiatry. 2007;191:325–334. doi: 10.1192/bjp.bp.106.033670. [DOI] [PubMed] [Google Scholar]
- 43.Griffin JL, Bollard M, Nicholson JK, Bhakoo K. Spectral profiles of cultured neuronal and glial cells derived from HRMAS (1)H NMR spectroscopy. NMR Biomed. 2002;15:375–384. doi: 10.1002/nbm.792. [DOI] [PubMed] [Google Scholar]
- 44.Chang L, Munsaka SM, Kraft-Terry S, Ernst T. Magnetic resonance spectroscopy to assess neuroinflammation and neuropathic pain. J Neuroimmune Pharmacol. 2013;8:576–593. doi: 10.1007/s11481-013-9460-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Inglese M, Li BS, Rusinek H, Babb JS, Grossman RI, Gonen O. Diffusely elevated cerebral choline and creatine in relapsing-remitting multiple sclerosis. Magn Reson Med. 2003;50:190–195. doi: 10.1002/mrm.10481. [DOI] [PubMed] [Google Scholar]
- 46.Miller BL, Moats RA, Shonk T, Ernst T, Woolley S, Ross BD. Alzheimer disease: depiction of increased cerebral myo-inositol with proton MR spectroscopy. Radiology. 1993;187:433–437. doi: 10.1148/radiology.187.2.8475286. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Y, Shen J. Simultaneous quantification of glutamate and glutamine by J-modulated spectroscopy at 3 Tesla. Magn Reson Med. 2016;76:725–732. doi: 10.1002/mrm.25922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prescot AP, Richards T, Dager SR, Choi C, Renshaw PF. Phase-adjusted echo time (PATE)-averaging 1 H MRS: application for improved glutamine quantification at 2.89 T. NMR Biomed. 2012;25:1245–1252. doi: 10.1002/nbm.2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Choi C, Coupland NJ, Bhardwaj PP, Kalra S, Casault CA, Reid K, et al. T2 measurement and quantification of glutamate in human brain in vivo. Magn Reson Med. 2006;56:971–977. doi: 10.1002/mrm.21055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.