Abstract
OBJECTIVES
To establish the prevalence of sarcopenia in a long-term care population, assess agreement among different consensus sarcopenia diagnostic criteria, and examine agreement of a self-reported questionnaire with consensus guidelines.
DESIGN
Cross-sectional secondary analysis.
SETTING
Long-term care communities in the greater Pittsburgh, PA area.
PARTICIPANTS
Women age ≥ 65 years and older undergoing eligibility screening for a fracture reduction trial.
MEASUREMENTS
We measured appendicular lean muscle mass by dual-energy x-ray absorptiometry. Hand grip strength and usual gait speed were also evaluated. Sarcopenia status was determined according to European Working Group on Sarcopenia in Older People (EWGSOP) and the Foundation for the National Institutes of Health (FNIH) Sarcopenia Project criteria, and the SARC-F questionnaire.
RESULTS
Mean age was 83.6 in the 141 participants. The prevalence of sarcopenia was 7.8% (n=11) using the EWGSOP criteria, 4.3% (n=6) by FNIH conservative cut-point guidelines, and 32.6% (n=46) with intermediate FNIH cut-points. Only 2 of 141 participants met criteria for sarcopenia by all three guidelines. Sarcopenia was identified in 21.3% (n=30) of participants with the SARC-F questionnaire. Sensitivity of the SARC-F with consensus panel definitions ranged 18.2–33.3%. Specificity ranged 78.7–81.1%.
CONCLUSION
Current consensus criteria from the EWGSOP and FNIH Sarcopenia Project do not agree and have little overlap in older women residing in long-term care. The SARC-F questionnaire is a simple tool that could be implemented in long-term care, but has low sensitivity compared to current consensus guidelines in the identification of sarcopenic individuals.
Keywords: sarcopenia, long-term care, SARC-F
INTRODUCTION
Sarcopenia initially referred to loss of skeletal muscle mass with aging.1 However, loss of muscle mass alone is weakly correlated with poor physical performance and disability.2 Therefore, the definition of sarcopenia has evolved to describe loss of muscle mass combined with loss of muscle function. Sarcopenia is associated with several negative health outcomes including disability, falls, fractures and hospitalization – all of which lead to increased health care costs for older adults.3 A study by Janssen and colleagues estimated that in the year 2000, $18 billion in healthcare costs were related to sarcopenia.4 Sarcopenia is an important health issue in the geriatric patient population. Recent efforts to understand the prevalence of sarcopenia and its relationship to functional loss in older adults led to the attribution of an International Classification of Disease, Tenth Revision, Clinical Modification (ICD-10-CM) code to the syndrome in 2016. In order to fully understand the prevalence of sarcopenia, its impact on both patients and the healthcare system, and the optimal treatment options available, we must first understand how to diagnosis it.
Unfortunately, there is currently no consensus “gold standard” diagnostic criteria for sarcopenia. Several working groups have convened to develop recommendations for the assessment and identification of sarcopenia in older adults. Two such groups are the European Working Group on Sarcopenia in Older People (EWGSOP) and more recently the Foundation for the National Institutes of Health (FNIH) Sarcopenia Project. The EWGSOP and FNIH Sarcopenia Project had differing approaches to developing their recommendations for diagnosing sarcopenia. The EWGSOP defined sarcopenia as low muscle mass combined with either low muscle strength and/or reduced physical performance.5 They developed guidelines for assessing each component of sarcopenia (muscle mass, strength, and function) based on accuracy and availability of techniques in both research and clinical settings. Cut-points were determined based on previous studies in normative populations. The FNIH Sarcopenia Project took a slightly different approach and considered low physical performance as an outcome of sarcopenia.6 They sought to determine what values of muscle mass and muscle weakness were correlated with poor physical performance (slow walking), rather than developing cut-points based on normative data.6–10 Therefore, the FNIH definition of sarcopenia includes only low muscle mass combined with low muscle strength. Application of these consensus panel recommendations to regular clinical practice can be difficult due to the resources needed for assessment. In light of these challenges, the SARC-F was developed as a screening questionnaire to provide a simple and efficient method for identifying patients with sarcopenia during a regular office visit.11
Even though there has been much progress in the field to define and diagnose sarcopenia, questions remain. Consensus panel criteria were developed using data from relatively healthy, community-dwelling older adults.5–10 The SARC-F has also predominantly been tested in independent older populations.12–14 Community-dwelling adults are likely to have better functional abilities compared to the typical long-term care resident with multiple medical conditions and limited independence. Few studies have looked at prevalence estimates and agreement between sarcopenia criteria in older adults residing in long-term care communities, despite arguably greater clinical relevance. The objective of our study was to apply several current sarcopenia consensus diagnostic criteria and examine their performance in a long-term care population. We aimed to 1) establish the prevalence of sarcopenia based on each of the criteria; 2) determine the overlap between the EWSOP and FNIH criteria for identifying sarcopenia in a long-term care cohort; and 3) calculate sensitivity and specificity of the SARC-F questionnaire as a convenient screen compared to each of the consensus panel diagnostic criteria for sarcopenia.
METHODS
Study Design and Population
This study was a secondary cross-sectional analysis. We included 141 women age 65 years and older residing in a long-term care facility within the greater Pittsburgh, PA area. Participants with life expectancy < 3 years, known neuromuscular disorders, and those who could not perform a 4-meter walk to assess gait speed were excluded. Presence of comorbid conditions15 and frailty status16 were assessed to describe overall health and functional status of participants. Data was collected during eligibility screening for an ongoing fracture reduction trial, which was approved by the University of Pittsburgh Institutional Review Board. Written informed consent was obtained from participants or their representatives.
Sarcopenia Assessment
Appendicular lean muscle mass (ALM) was measured in grams by whole body dual x-ray absorptiometry (DXA) on a Discovery A densitometer (Hologic, Bedford, MA). Grip strength was measured in kilograms using a Jamar Plus dynamometer (Sammons Preston, Bolingbrook, IL). Three measurements were taken with each hand, and the maximum grip strength over all trials was used for analysis. Average gait speed (meters/second) between two trials was measured over a 4-meter walk according to established methods.17 Body mass index (BMI; kg/m2) was calculated from height and weight measurements.
Sarcopenia Classifications
Participants were identified as sarcopenic or non-sarcopenic according to the EWGSOP criteria, FNIH criteria, and the SARC-F. Participants were classified as sarcopenic by EWGSOP criteria for women if they had low muscle mass (ALM/height2 ≤ 5.5 kg/m2) combined with muscle weakness (grip strength < 20 kg) and/or low physical performance (gait speed < 0.8 m/s).5 We utilized two sets of sex-specific sarcopenia criteria from the FNIH panel recommendations. The first set, termed FNIH 1, followed the initial conservative cut-point recommendations for women of appendicular lean muscle mass standardized to body mass index (ALM/BMI) < 0.512 m2 combined with muscle weakness (grip strength < 16 kg).6–10 The second set of FNIH criteria (FNIH 2) were the intermediate cut-point values for women of low muscle mass (ALM/BMI < 0.591 m2) and weakness (grip strength < 19.99 kg).7,18 All FNIH cut-points were derived from pooled data sets using classification and regression tree (CART) analysis. Grip strength cut-points for weakness (<16 kg) or intermediate weakness (16–20 kg) were both associated with mobility impairment as assessed by slow gait speed.7 The FNIH 1 muscle mass cut-point was determined as the value which predicted weak grip strength (<16 kg).8 The FNIH 2 muscle mass cut-point was derived from CART analyses for predicting intermediate weakness.18 The SARC-F questionnaire consisted of 5 questions about Strength; Ability to walk, Rise from a chair, and Climb stairs; and Fall history.11 Questions were scored and summed to provide an overall score ranging from 0–10. A SARC-F score ≥ 4 was considered indicative of sarcopenia.11 The SARC-F was administered verbally to all participants by trained research personnel.
Statistical Analyses
We used appropriate summary statistics (means, standard deviations, frequencies, and percentages) to describe the participant characteristics. We constructed a three-factor Venn diagram to graphically assess the extent of overlap among the three consensus criteria for sarcopenia. Using each of the consensus criteria as the gold standard, we also examined the sensitivity and specificity of SARC-F ≥ 4 as a screening test to identify sarcopenia. SAS® version 9.3 (SAS Institute, Inc., Cary, North Carolina) was used for all statistical analyses.
RESULTS
Participant Characteristics
Participant characteristics are presented in Table 1. Our population ranged in age from 65 to 97 years old (mean ± standard deviation = 83.6 ± 7.0). The mean comorbidity burden was 3.1 ± 1.1 and 55.3% were frail or pre-frail. Average muscle mass was above both the EWGSOP and FNIH definitions for low lean muscle mass. Mean grip strength and gait speed were below the EWGSOP cut-points for muscle weakness (< 20 kg) and poor physical performance (gait speed < 0.8 m/s), respectively.
Table 1.
Participant Characteristics
| Characteristic | Value |
|---|---|
| Age, years, mean ± SD | 83.6 ± 7.0 |
| Race/ethnicity, n (%) | |
| Caucasian | 136 (96.5) |
| African-American | 5 (3.5) |
| Height, inches, mean ± SD | 62.0 ± 3.1 |
| Weight, pounds, mean ± SD | 153.6 ± 34.2 |
| BMI, kg/m2, mean ± SD | 28.1 (5.8) |
| Co-morbidity Index, mean ± SD, range (0–9) | 3.1 ± 1.1 |
| Frailty status, n (%) | |
| Frail | 9 (6.4) |
| Prefrail | 69 (48.9) |
| Non-frail | 63 (44.7) |
| ALM/height2, kg/m2, mean ± SD | 6.7 ± 1.1 |
| ALM/BMI, m2, mean ± SD | 0.6 ± 0.1 |
| Grip strength, kg, mean ± SD | 19.9 ± 4.9 |
| Gait speed, m/s, mean ± SD | 0.78 ± 0.22 |
SD = standard deviation, BMI = body mass index, ALM = appendicular lean muscle mass
Prevalence by EWGSOP and FNIH Sarcopenia Criteria
The prevalence of sarcopenia was 7.8% (n=11) using the EWGSOP criteria, 4.3% (n=6) according to FNIH conservative cut-point guidelines, and 32.6% (n=46) using intermediate FNIH cut-points. We found that many participants met some but not all of the criteria needed to be classified as having sarcopenia (Table 2). Over half of the participants had low muscle mass (ALM/BMI < 0.591 m2) according to FNIH 2 cut-points, even though only 32.6% were deemed to have sarcopenia. Similar findings occurred for muscle weakness defined by EWGSOP and FNIH 2 definitions with 53.2% of participants having low grip strength. According to EWGSOP guidelines, 52.5% of participants exhibited poor physical performance as indicated by slow gait speed, but only 7.8% had sarcopenia.
Table 2.
Participants Meeting Sarcopenia Criteria According to Consensus Definitions
| Criteria | EWGSOP | FNIH 1 | FNIH 2 |
|---|---|---|---|
| Have sarcopenia, n (%) | 11 (7.8) | 6 (4.3) | 46 (32.6) |
| Low muscle massa, n (%) | 15 (10.6) | 21 (14.9) | 73 (51.2) |
| Low grip strengthb, n (%) | 75 (53.2) | 27 (19.2) | 75 (53.2) |
| Slow gait speedc, n (%) | 74 (52.5) | – | – |
Appendicular lean muscle mass (ALM)/height2 ≤ 5.5 kg/m2 for EWGSOP, standardized to body mass index (ALM/BMI) < 0.512 m2 for FNIH 1, ALM/BMI < 0.591 m2 for FNIH2
Grip strength < 20kg by EWGSOP, < 16kg by FNIH 1, or < 19.99kg by FNIH 2
Usual gait speed < 0.8 m/s
EWGSOP = European Working Group on Sarcopenia in Older People. FNIH = Foundation for the National Institutes of Health Sarcopenia Project
Agreement among Consensus Guidelines
There was little agreement on who had sarcopenia based on the different consensus panel definitions (Figure 1). Six participants were considered sarcopenic by both FNIH definitions. The EWGSOP overlapped in the identification of 2 women by FNIH 1 and 6 women by FNIH 2 standards. Of note, only 2 women met the criteria for sarcopenia according to all three consensus definitions. The EWGSOP had sensitivity of 33.3% and specificity of 93.3% when compared to the FNIH 1 criteria as the gold standard. Sensitivity of the EWGSOP criteria was lower (13.0%) with similar specificity (94.7%) when using FNIH 2 as the gold standard.
Figure 1.
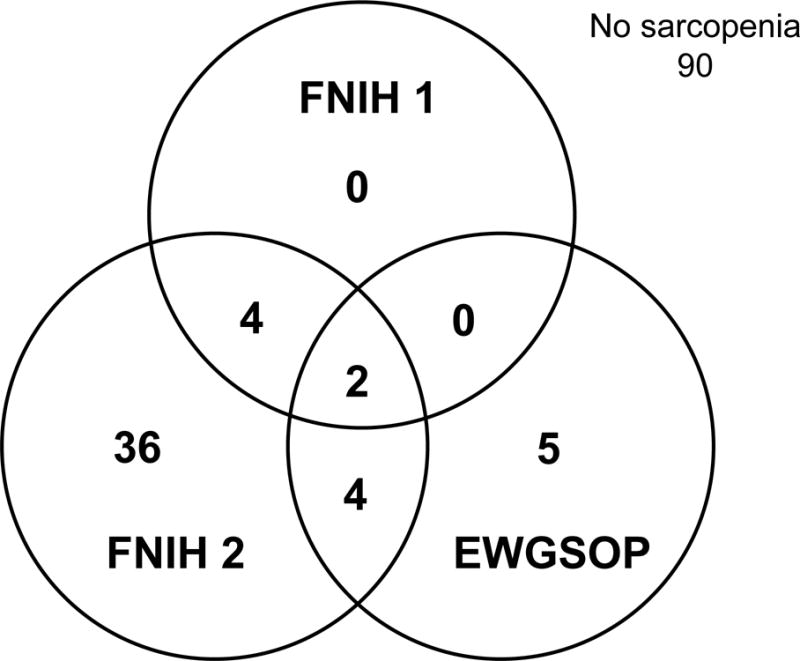
Agreement of Consensus Definitions in Identifying Sarcopenia. This Venn diagram illustrates the limited agreement between consensus definitions in identifying participants with sarcopenia. The FNIH 2 criteria classified the most participants with sarcopenia (total n=46) with 6 of these participants overlapping with EWGOSP or FNIH 1 criteria. Only 2 participants met criteria for sarcopenia across all three definitions. Ninety participants did not have sarcopenia by any of the three consensus criteria. EWGSOP = European Working Group on Sarcopenia in Older People. FNIH = Foundation for the National Institutes of Health Sarcopenia Project
SARC-F Questionnaire
The SARC-F questionnaire identified 30 participants (21.3%) with sarcopenia. The SARC-F did not consistently identify the same sarcopenic participants as the EWGSOP and FNIH consensus definitions (Table 3). SARC-F had low sensitivity with respect to each of the consensus panel guidelines (18.2% to 33.3%). Specificity was better, ranging 78.7–81.1%.
Table 3.
Agreement of SARC-F with Consensus Definitions in Classifying Sarcopenia Status
| SARC-F | Sensitivity (%) | Specificity (%) | |||
|---|---|---|---|---|---|
| Yes | No | ||||
| EWGSOP | Yes | 2 | 9 | 18.2 | 78.7 |
| No | 28 | 102 | |||
|
| |||||
| FNIH 1 | Yes | 2 | 4 | 33.3 | 79.3 |
| No | 28 | 107 | |||
|
| |||||
| FNIH 2 | Yes | 12 | 34 | 26.1 | 81.1 |
| No | 18 | 77 | |||
EWGSOP = European Working Group on Sarcopenia in Older People. FNIH = Foundation for the National Institutes of Health Sarcopenia Project
DISCUSSION
The prevalence of sarcopenia ranged 4.3–32.6% according to diagnostic criteria recommended by the EWGSOP and FNIH consensus panels. However, they were identifying different participants with little overlap. Prevalence of sarcopenia was 21.3% using a score of 4 or greater on the simple SARC-F questionnaire. The SARC-F had 78% specificity with the EWGSOP criterion, meaning SARC-F could reasonable exclude those who did not have sarcopenia. However, sensitivity was low at 18%. We found a similar pattern when comparing the SARC-F results to each of the two sets of FNIH panel cut-points, with specificity near 80% and sensitivity in the 26–34% range. Our results illustrate the discrepancies between current diagnostic paradigms for identifying sarcopenia in the long-term care population and are in agreement with other studies that have found varying prevalence of sarcopenia in community-dwelling older adults across definitions.9,19–21
Disagreement on how to diagnose sarcopenia complicates assessment of the geriatric patient. Patient diagnosis and intervention decisions will vary depending on the diagnostic criteria applied. The current interventions for sarcopenia include adequate nutrition with protein and vitamin D combined with resistance exercise.20 These interventions are advisable in general and would not necessarily be harmful to the non-sarcopenic patient. However, there are new pharmacological therapies in development.22,23 Researchers must be able to identify sarcopenia accurately in order to test the effectiveness of such therapies. Likewise, adoption of standard diagnostic criteria is essential for clinicians to target pharmacologic treatment to those who truly require it.
One of the major difficulties when assessing a patient for sarcopenia by the EWGSOP and FNIH criteria is simple availability and accessibility of testing resources, particularly for residents in long-term care communities. In their consensus statements, the EWGSOP and FNIH workgroups recommend DXA scan of the whole body to measure muscle mass.5–10 FNIH consensus cut-points for muscle mass were developed from DXA results obtained on Hologic and GE Healthcare Lunar densitometers that are not interchangeable and may contribute to discrepancies in the cut-point results. Furthermore, in some studies muscle mass data are measured with bioelectrical impedance analysis (BIA) that may also contribute to cut-point discrepancies. Conversion equations can be used to estimate comparable DXA results; however, they must be developed for specific populations and DXA technologies.24,25 Muscle mass cut-point recommendations may need to be adjusted for the type of DXA technology utilized. Additionally, the DXA scanners in a majority of facilities do not have the capability to perform the whole body scan required to assess lean muscle mass as this is not the standard method utilized for the clinical assessment of bone mineral density. Patients may be required to go to a specialized facility for testing, which raises concerns with cost, scheduling, and transportation. Currently, the whole body DXA scan is not a billable diagnostic test in the United States. Patients may be unwilling or unable to pay the increased out-of-pocket expenses associated with the whole body DXA scan for muscle mass assessment. In addition to issues related to accessibility and availability, the physical limitations of older adults can make the process of obtaining a whole body DXA scan challenging.
A simple questionnaire such as the SARC-F would be an ideal screening tool for geriatricians to implement into practice. Reliability and validity of the SARC-F has been established.14 In older Chinese people, SARC-F scores were associated with poor physical performance and impaired ability to perform instrumental activities of daily living.12 Higher SARC-F scores were also predictive of 4-year mortality in older Taiwanese adults.26 The SARC-F has potential as a clinical tool, but requires refinement to increase sensitivity and specificity. As with our current findings, several other studies have shown the SARC-F to demonstrate agreement with working group definitions on who does not have sarcopenia, but low consensus about those who do have sarcopenia.13,21,27 The SARC-F may require modification to increase concordance with current consensus definitions to better identify those that do have sarcopenia.
We acknowledge that our study does have limitations. We examined an all-female cohort from a single greater metropolitan area that was predominantly Caucasian; therefore, our results may have limited generalizability. Performance and agreement among these criteria may be different in other populations. The diagnosis of sarcopenia in older adults, particularly those in long-term care, is challenging given the presence of chronic diseases, mobility impairments, and other factors that can impact muscle mass and function. Consensus panel definitions based on information from relatively healthy cohorts may not be appropriate for the long-term care population.
Despite these limitations, our study also had several strengths. We were able to obtain lean muscle mass measures by DXA scan to comply with consensus panel recommendations. We used multiple criteria, including the most recent FNIH cut-points, to characterize the prevalence of sarcopenia in a long-term care population. Consensus panel definitions of sarcopenia were developed using data from previous studies that focused on community-dwelling older adults. The FNIH work group acknowledges this limitation and suggests more investigation into how their recommendations perform in populations that are less healthy and reside in long-term care communities.9 We also compared the latest expert consensus definitions with a simple questionnaire that could be more easily integrated into clinical practice with patients in long-term care.
In summary, current sarcopenia diagnostic guidelines are not in agreement with one another, leading to a wide range of sarcopenia prevalence estimates even in those residing in long-term care where sarcopenia is assumed to be more prevalent. The preferred methods of measurement proposed by the EWGSOP and FNIH consensus panels are not always feasible in primary care or long-term care settings. Currently, physicians can apply the ICD-10-CM code for sarcopenia without standard guidelines for how sarcopenia is determined. Given the challenges of obtaining the appropriate diagnostic measurements in clinical practice, there is a need for a screening tool to aide clinicians in identifying those who likely have sarcopenia and for whom further diagnostic testing is justified. Ultimately, the goal is to identify sarcopenia and intervene in order to prevent adverse health outcomes. Multiple consensus panel definitions and the SARC-F have similar modest predictive ability for adverse outcomes.28 However, our results demonstrate that patients with sarcopenia are not the same among the various definitions. It is possible that combining components from diagnostic measures and screening tools may improve the ability to predict adverse outcomes. Sarcopenia may turn out to be difficult to objectively define but geriatricians may “know it when we see it,” a characteristic shared by other geriatric syndromes such as frailty. If so, it would be valuable and clinically relevant to base identification of sarcopenia at least partially on a self-report questionnaire of functional ability. Further research is necessary to explore the optimal composite measures of sarcopenia that correlate with adverse health outcomes.
IMPACT STATEMENT.
We certify that this work is novel. The potential impact of this research on clinical care or health policy includes the following: diagnostic criteria for sarcopenia in long-term care communities are critical to appropriately target patients for future interventions. This study demonstrates that current consensus guidelines disagree and may identify different patients with sarcopenia.
Acknowledgments
We recognize the contributions of our research coordinators Christina Scenna, Jennifer Smith, Julie Wagner, and Kyle Walkiewicz for their assistance with data collection. We also are grateful to the participants and long-term care facilities for their involvement in this study. This research was funded by National Institutes of Health, National Institute on Aging grants R01AG050302 (SLG), K07AG052668 (SLG), T32AG021885 (SLG), and the Pittsburgh Claude D. Pepper Older Americans Independence Center (P30AG024827; SLG).
Funding: National Institutes of Health, National Institute on Aging grants R01AG050302 (SLG), K07AG052668 (SLG), T32AG021885 (SLG), and the Pittsburgh Claude D. Pepper Older Americans Independence Center (P30AG024827; SLG).
Sponsor’s Role: The funding agency had no role in design, conduct, data analysis, and manuscript preparation related to this study.
Footnotes
This paper was presented at the International Association of Gerontology and Geriatrics (IAGG) 21st World Congress, July 25, 2017, San Francisco, CA.
Conflict of Interest: The authors have no conflicts of interest.
Author Contributions: Study design and concept: MPK, SP, SLG. Acquisition of data: MPK. Analysis and interpretation of data: MPK, SP, DAN, NMR, SLG. Preparation and critical review of manuscript: MPK, SP, DAN, NMR, SLG.
References
- 1.Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147(8):755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 2.Manini TM, Clark BC. Dynapenia and aging: an update. J Gerontol A Biol Sci Med Sci. 2012;67(1):28–40. doi: 10.1093/gerona/glr010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beaudart C, Zaaria M, Pasleau F, et al. Health outcomes of sarcopenia: A systematic review and meta-analysis. PLoS One. 2017;12(1):e0169548. doi: 10.1371/journal.pone.0169548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Janssen I, Shepard DS, Katzmarzyk PT, et al. The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc. 2004;52(1):80–85. doi: 10.1111/j.1532-5415.2004.52014.x. [DOI] [PubMed] [Google Scholar]
- 5.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(4):412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Studenski SA, Peters KW, Alley DE, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014;69(5):547–558. doi: 10.1093/gerona/glu010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alley DE, Shardell MD, Peters KW, et al. Grip strength cutpoints for the identification of clinically relevant weakness. J Gerontol A Biol Sci Med Sci. 2014;69(5):559–566. doi: 10.1093/gerona/glu011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cawthon PM, Peters KW, Shardell MD, et al. Cutpoints for low appendicular lean mass that identify older adults with clinically significant weakness. J Gerontol A Biol Sci Med Sci. 2014;69(5):567–575. doi: 10.1093/gerona/glu023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dam TT, Peters KW, Fragala M, et al. An evidence-based comparison of operational criteria for the presence of sarcopenia. J Gerontol A Biol Sci Med Sci. 2014;69(5):584–590. doi: 10.1093/gerona/glu013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McLean RR, Shardell MD, Alley DE, et al. Criteria for clinically relevant weakness and low lean mass and their longitudinal association with incident mobility impairment and mortality: the Foundation for the National Institutes of Health (FNIH) sarcopenia project. J Gerontol A Biol Sci Med Sci. 2014;69(5):576–583. doi: 10.1093/gerona/glu012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malmstrom TK, Morley JE. SARC-F: a simple questionnaire to rapidly diagnose sarcopenia. J Am Med Dir Assoc. 2013;14(8):531–532. doi: 10.1016/j.jamda.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 12.Cao L, Chen S, Zou C, et al. A pilot study of the SARC-F scale on screening sarcopenia and physical disability in the Chinese older people. J Nutr Health Aging. 2014;18(3):277–283. doi: 10.1007/s12603-013-0410-3. [DOI] [PubMed] [Google Scholar]
- 13.Woo J, Leung J, Morley JE. Validating the SARC-F: a suitable community screening tool for sarcopenia? J Am Med Dir Assoc. 2014;15(9):630–634. doi: 10.1016/j.jamda.2014.04.021. [DOI] [PubMed] [Google Scholar]
- 14.Malmstrom TK, Miller DK, Simonsick EM, et al. SARC-F: a symptom score to predict persons with sarcopenia at risk for poor functional outcomes. J Cachexia Sarcopenia Muscle. 2016;7(1):28–36. doi: 10.1002/jcsm.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rigler SK, Studenski S, Wallace D, et al. Co-morbidity adjustment for functional outcomes in community-dwelling older adults. Clin Rehabil. 2002;16(4):420–428. doi: 10.1191/0269215502cr515oa. [DOI] [PubMed] [Google Scholar]
- 16.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 17.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55(4):M221–231. doi: 10.1093/gerona/55.4.m221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiles Shaffer N, Ferrucci L, Shardell M, et al. Agreement and predictive validity using less-conservative Foundation for the National Institutes of Health sarcopenia project weakness cutpoints. J Am Geriatr Soc. 2017;65(3):574–579. doi: 10.1111/jgs.14706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bischoff-Ferrari HA, Orav JE, Kanis JA, et al. Comparative performance of current definitions of sarcopenia against the prospective incidence of falls among community-dwelling seniors age 65 and older. Osteoporos Int. 2015;26(12):2793–2802. doi: 10.1007/s00198-015-3194-y. [DOI] [PubMed] [Google Scholar]
- 20.Cruz-Jentoft AJ, Landi F, Schneider SM, et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS) Age Ageing. 2014;43(6):748–759. doi: 10.1093/ageing/afu115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rolland Y, Dupuy C, Abellan Van Kan G, et al. Sarcopenia screened by the SARC-F questionnaire and physical performances of elderly women: A cross-sectional study. J Am Med Dir Assoc. 2017:000–000. doi: 10.1016/j.jamda.2017.05.010. 010.1016/j.jamda.2017.1005.1010. [DOI] [PubMed] [Google Scholar]
- 22.Brotto M, Abreu EL. Sarcopenia: pharmacology of today and tomorrow. J Pharmacol Exp Ther. 2012;343(3):540–546. doi: 10.1124/jpet.112.191759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morley JE. Pharmacologic options for the treatment of sarcopenia. Calcif Tissue Int. 2016;98(4):319–333. doi: 10.1007/s00223-015-0022-5. [DOI] [PubMed] [Google Scholar]
- 24.Buckinx F, Reginster JY, Dardenne N, et al. Concordance between muscle mass assessed by bioelectrical impedance analysis and by dual energy X-ray absorptiometry: a cross-sectional study. BMC Musculoskelet Disord. 2015;16:60. doi: 10.1186/s12891-015-0510-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sergi G, De Rui M, Stubbs B, et al. Measurement of lean body mass using bioelectrical impedance analysis: a consideration of the pros and cons. Aging Clin Exp Res. 2017;29(4):591–597. doi: 10.1007/s40520-016-0622-6. [DOI] [PubMed] [Google Scholar]
- 26.Wu TY, Liaw CK, Chen FC, et al. Sarcopenia screened with SARC-F questionnaire is associated with quality of life and 4-year mortality. J Am Med Dir Assoc. 2016;17(12):1129–1135. doi: 10.1016/j.jamda.2016.07.029. [DOI] [PubMed] [Google Scholar]
- 27.Barbosa-Silva TG, Menezes AM, Bielemann RM, et al. Enhancing SARC-F: Improving sarcopenia screening in the clinical practice. J Am Med Dir Assoc. 2016;17(12):1136–1141. doi: 10.1016/j.jamda.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 28.Woo J, Leung J, Morley JE. Defining sarcopenia in terms of incident adverse outcomes. J Am Med Dir Assoc. 2015;16(3):247–252. doi: 10.1016/j.jamda.2014.11.013. [DOI] [PubMed] [Google Scholar]


