Abstract
Several in vitro and animal studies have showed that inflammatory markers play a role in bone remodeling and pathogenesis of osteoporosis. Additionally, some human longitudinal studies showed suggestive associations between elevated inflammatory markers and increased risk of non-traumatic fractures. We examined several inflammatory markers and multiple fracture types in a single study of older individuals with extensive follow-up. We assessed the association of four inflammatory markers with the risk of incident hip fractures among 5,265 participants of the Cardiovascular Health Study (CHS) and a composite endpoint of incident fractures of the hip, pelvis, humerus, or proximal forearm in 4,477 participants. Among CHS participants followed between 1992 and 2009, we observed 480 incident hip fractures during a median follow-up of 11 years. In the composite fracture analysis cohort of 4,477 participants, we observed 711 fractures during a median follow-up of 7 years. Adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) for hip fracture associated with doubling of IL-6 were HR 1.15 (95%CI 1.02–1.30) overall and HR 1.17 (95%CI 1.01–1.35) in women. We also observed a positive association between each unit increase in white blood cell (WBC) count and risk of hip fracture: HR 1.04 (95%CI 1.01–1.06) overall and HR 1.06 (95%CI 0.95–1.20) in women. We observed no significant associations between any of the four inflammatory markers and a composite fracture endpoint. Our findings suggest that chronic inflammatory and immune processes may be related to higher rates of incident hip fractures.
Keywords: cytokines, general population studies, fracture risk assessment, osteoimmunology
Introduction
Osteoporotic fractures are a serious public health issue with devastating consequences.(1) In 2005, more than 2 million fractures were reported in the U.S. By 2025, annual fractures are projected to increase by 50% to more than 3 million fractures.(2) The effect of fractures on survival varies by fracture type, with hip fractures being the most serious.(1) Hip fractures have been associated with 10–20% excess mortality in the first year after fracture.(3)
The inflammation of aging hypothesis implies that aging is a result of tissue damage due to chronic activation of inflammatory processes.(4) Increased levels of pro-inflammatory markers have been associated with higher risk of morbidity and mortality,(5–8) decreases in bone mineral density (BMD), and increased risk of fractures.(9–12) Additionally, in several in vitro and animal studies, pro-inflammatory markers have been shown to play a role in bone remodeling and pathogenesis of osteoporosis. In human longitudinal studies, the association between inflammatory markers and hip fractures has been generally consistent, although associations often fail to reach statistical significance. Although the type of marker may vary across different studies, most studies showed that elevated inflammatory markers are associated with increased risk of non-traumatic fractures. For example, the Study of Osteoporotic Fractures (SOF) showed that women in the highest quartile (Q4) of interleukin-6 (IL-6) had a significantly higher risk of hip fractures compared to women in the lower three quartiles (Q123), hazard ratio (HR) 1.64 (95% confidence interval [CI] 1.09–2.48).(13) In the same study, the association between IL-6 soluble receptor (IL-6 SR) and incident hip fractures was HR 1.43 (95%CI 0.95–2.14) for women in Q4 compared to women in Q123. In the Women’s Health Initiative (WHI) study, the association between IL-6 SR and incident hip fractures was RR 1.43 (95%CI 0.98–2.07).(11) Similarly, in the Health Aging and Body Composition (ABC) study, the association between IL-6 SR and non-traumatic fractures was RR 1.33 (95%CI 0.9–1.96) for participants in Q4 compared to participants in Q123.(14) Also, the Health ABC study showed that the relative risk of non-traumatic fractures for participants in Q4 of C-reactive protein (CRP) was 1.34 (95%CI 0.99–1.82) when compared to participants in Q123 of CRP.(14) Moreover, in the Osteoporotic Fractures in Men (MrOS) study, the association between IL-6 and fractures was HR 1.03 (0.73–1.44) and for IL-6SR and fractures was HR 1.12 (0.81–1.56) for men in Q4 compared to men in Q1.(15) In the same study, the association between CPR and fractures HR 1.03 (0.73–1.45) for men in Q4 compared to men in Q1. These associations would indicate a meaningful association between these biomarkers and fracture events, if they could be replicated in a setting with sufficient events to achieve statistical significance.
Exploring the relationship between inflammation and fractures in the Cardiovascular Health Study (CHS) allows for the examination of this association in a larger cohort of older individuals with extensive follow-up. Additionally, our study offers the opportunity to assess multiple inflammatory markers and multiple fracture types in a single study. Moreover, inflammatory markers have high within-person variability over time, and a single measurement may be influenced by short-term changes in biomarker levels due to recent activity (e.g., exercise).(16) While studies of biomarker variability suggest that the exposure measurement error for some measures is within acceptable levels, examining a larger sample improves estimation of associations.(17) Most crucially, the current studies in the literature are limited in statistical power due to the number of endpoints, resulting in estimates of association that are suggestive but often fail to achieve statistical significance. The use of CHS allows us to make much stronger claims about these associations due to the large number of events during the CHS follow-up and therefore greatly improve the estimate of the longitudinal association between chronic inflammation and osteoporotic fractures.
Materials and Methods
Study Population
CHS is a population-based, multicenter prospective cohort study of cardiovascular risk factors in community-dwelling adults aged 65 years and older.(18) The CHS cohort enrolled 5,201 participants in 1989–1990 and an additional 687 African-American participants in 1992–1993. The participants comprised a random sample of Medicare-eligible individuals from four U.S. communities: Forsyth County, North Carolina; Sacramento County, California; Washington County, Maryland; and Allegheny County, Pennsylvania. Those eligible to participate met the following inclusion criteria: 1) ≥ 65 years old, 2) non-institutionalized, 3) expected to remain in the area for at least 3 years, and 4) able to give informed consent with no need for a proxy respondent. Participants follow-up was conducted every 6 months for the first 10 years (in-clinic examinations alternating with phone interviews) and every 6 months thereafter (phone interviews). The CHS design and recruitment details are described elsewhere (19).
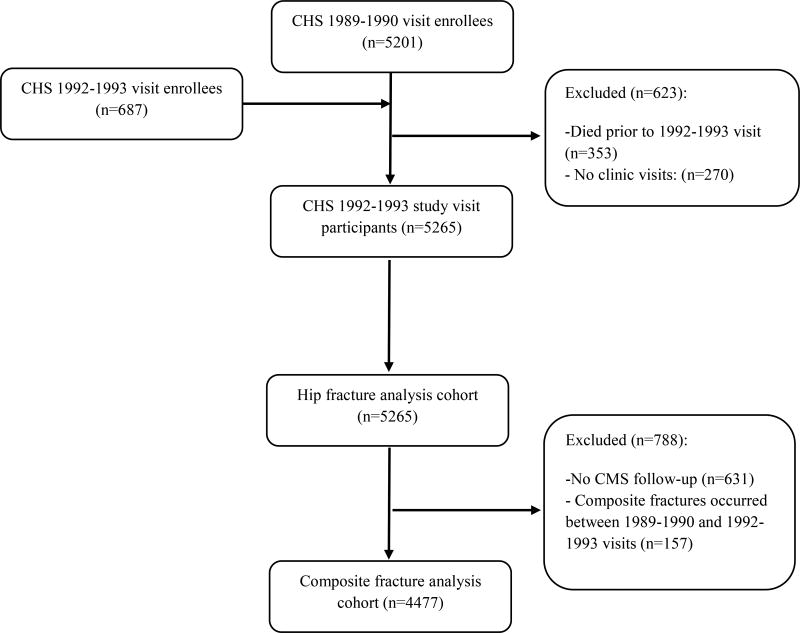
For the analyses assessing the association between inflammatory markers and incident hip fractures (i.e., hip fracture analysis cohort), we included all CHS participants from the 1992–1993 study visit (analytic baseline year). At that time, 353 of the original 5,888 participants had died, and 270 had no clinic visits (Figure 1), leaving 5,265 participants for analysis.
Figure 1.
Derivation of Study Analytic Cohort
For the analyses assessing the association between inflammatory markers and hip, pelvis, humerus, or distal forearm fractures (i.e., composite fracture analysis cohort), we limited analyses to participants who were enrolled in fee-for-service (FFS) Medicare (Centers for Medicare & Medicaid Services [CMS]) any time after the 1992–1993 study visit and did not have a CMS documented hip, pelvis, humerus, or distal forearm fracture between the 1989–1990 baseline study visit and the 1992–1993 study visit. The total number of participants in composite fracture analysis cohort was 4,477.
Measurements
Inflammatory Markers
Levels of IL-6, CRP, white blood cell (WBC) count, and fibrinogen were measured using blood specimens collected at the 1992–1993 study visit and stored at −70°C. Details of blood collection, laboratory procedures, and measurements of inflammatory markers in CHS have been previously described in detail.(20) Briefly, participants underwent phlebotomy after an 8–12-hour fast. Blood samples were collected in EDTA tubes and sent to clinical laboratories near the field centers for WBC measurements. For all other measures, frozen aliquots were shipped on dry ice to the Central Blood Analysis Laboratory (CBAL), following standardized procedures.
IL-6 levels were measured by ultrasensitive enzyme-linked immunosorbent assay (Quantikine HS Human IL-6 Immunoassay; R&D Systems, Minneapolis, Minn). The analytical coefficient of variation for this assay was 6.3%.(21, 22) CRP was measured with a high-sensitivity enzyme-linked immunosorbent assay using purified protein and polyclonal anticlonal anti-CRP antibodies (Calbiochem, La Jolla, California).(21, 22) The interassay coefficient of variation was 5.50%. Fibrinogen levels were measured using a semiautomated modified clot-rate method with a BBL Fibrometer (Becton Dickinson and Company, Bedford, Mass).(20, 22, 23) The mean monthly coefficient of variation for the fibrinogen assay was 3.09%. WBC counts were evaluated at each of the four local CHS laboratories using automated counters (Coulter Stack S cell counter; Beckman Coulter Inc, Fullerton, Calif; or the SysmexNE8000 counter; Toa Electronics Inc, South San Francisco, Calif).(18, 22)
Incident Fractures
The primary outcome was incident hip fracture, identified by the International Classification of Diseases, Ninth Revision (ICD-9) codes (820.xx, where x is any digit) from hospitalization records. Hip fracture data were obtained through participant reports and confirmed by the hospital discharge diagnosis codes. All hospitalization data, including discharge summaries, were collected prospectively every six months. To ensure completeness of hospitalization records, CHS data were checked against Medicare claims data to identify any hospitalizations unreported by the participants. We excluded admissions for pathologic fractures (ICD-9 code 773.1x) and motor vehicle accidents (E810.xx- E825.xx). We followed participants until incident hip fracture, death, loss to follow-up, or December 31, 2010.
The secondary outcome was a composite of incident fracture of the hip, pelvis, humerus, and distal forearm (time to the first of any of the four types of fractures). We ascertained composite fractures from a merged CHS-Medicare dataset, based on inpatient, outpatient, and physician services claims.(24–26) Composite fracture ICD-9 codes included hip (820.xx), pelvis (808.xx), humerus (812.xx), and distal forearm (813.4 or 813.5). Fracture inpatient claims required one ICD-9 code, while fractures identified by outpatient claims required an additional Current Procedural Terminology (CPT) procedure code for the corresponding fracture type (Appendix Table 1). We required concomitant CPT codes for outpatient claims for fractures to minimize misclassification from rule-out diagnoses, as defined by previously validated algorithms.(24, 26) We followed participants until incident composite fracture, death, loss to follow-up, or July 30, 2009. If participants left FFS Medicare, we censored them from analyses.
Other Measurements
Demographic characteristics (age, sex, race), physical activity (total kcal/week) derived from the Modified Minnesota Leisure Time Questionnaire, smoking status (current, former, never), alcohol consumption (nondrinker, 1–7 drinks/week, or ≥7 drinks/week), impairment in instrumental activities of daily living (IADLs), and health status (excellent/very good, good, or fair/poor) were obtained by standardized questionnaires at the time of the 1992–1993 study visit. Other variables included medical history (self-reported number of falls in the past year, physician-diagnosed osteoporosis, heart failure, and centrally adjudicated coronary heart disease). Clinic visits also included measurements of height and weight (used for calculating body mass index [BMI, kg/m2]), depression scores (modified version of the Centers for Epidemiologic Studies Depression [CES-D] scale score)(27), cognitive function scores (Modified Mini-Mental State Examination, 3MSE),(28) and physical performance (computed from the time to complete a 15 foot walk test). We defined diabetes as fasting plasma glucose level ≥ 126 mg/dl, random plasma glucose ≥ 200 mg/dl, or the use of any diabetes medications. Laboratory measures included serum cystatin-derived estimated glomerular filtration rate (eGFRcys), serum albumin, and thyroid stimulating hormone (TSH). Total hip, femoral neck and lumbar spine areal BMD were measured in 1,591 participants at two clinic sites (Pittsburgh, Pennsylvania and Sacramento, California). Complete information on prescription and non-prescription medications was obtained at the 1992–1993 study visit by a previously validated medication inventory approach. Assessment of current medications included the following categories (yes/no): thiazides, estrogen, thyroid medications, psychotropic medications (i.e., tri or tetra cyclic antidepressants, non-tricyclic antidepressants, antipsychotics, or benzodiazepines), oral corticosteroids, osteoporosis medications (i.e., calcium, vitamin D, bisphosphonates). For chronic medication ascertainment, the medication inventory approach has been demonstrated as more reliable than recall methods.21
Statistical Analysis
We compared the baseline characteristics of the CHS participants with and without data for inflammatory markers, and of the participants with and without Medicare data using the t-test for continuous variables and chi-square for categorical variables. Among participants with data for inflammatory markers, we compared baseline characteristics by Q123 and Q4 of IL-6 based on literature review a priori. We graphically examined the distribution of inflammatory markers. IL-6 and CRP values were skewed and were log base 2 transformed so that those biomarkers could be interpreted as risk per each doubling.
For each fracture outcome (i.e., hip and composite), we used Kaplan-Meier curves to describe the association between high versus low levels of inflammatory markers (Q4 vs. Q123) and time to incident fracture. Curves were compared with a log rank test. We used Cox proportional hazards models to estimate HR (95%CI) of incident fractures associated with doubling in IL-6 and CRP, and with a unit increase in WBC and fibrinogen. We used three nested adjustment models. In model 1, we adjusted for age, sex, race, and study clinic site. In model 2, we added anthropometric and behavioral characteristics as possible confounders (BMI, alcohol consumption, and smoking history). In the full model 3, our primary model, we added the following baseline covariates: medical history (diabetes, adjudicated coronary heart disease, and heart failure), medications (thiazides, estrogen, thyroid medications, psychotropic medications, oral corticosteroids, and osteoporosis medications), any fall in the past year, 15 foot walk time, self-reported general health, physical activity, IADLs, cognitive function score, depression score, and selected laboratory measures (eGFRcys, serum albumin, and TSH). We evaluated the validity of the proportional hazards assumption graphically and numerically using Schoenfeld residuals and found no meaningful departures. We incorporated sex in two ways, first by allowing sex-specific hazards and secondary by evaluating men and women separately.
We conducted analyses using R (R Development Core Team (2014)).
Results
Of the 5,265 CHS participants at year 1992–1993, 4,330 individuals (82%) had at least one inflammatory marker measured. At baseline, participants in Q123 of IL-6 were younger than those in Q4 of IL-6 (74.6 ±5.2 years vs. 76.5±5.6 years) (Table 1). Additionally, they were less likely to be males (39.6% vs. 48.3%) and black race (16.9% vs. 20.0%), had on average lower BMI and CESD, higher 3MSE scores, and were less likely to experience a fall in the past year than participants in Q4 of IL-6.
Table 1.
Participant characteristics at baseline in 1992–1993 by IL-6 values
| Participant Characteristic | IL-6 Q123 | IL-6 Q4 |
|---|---|---|
| Demographics | ||
| Age, years, mean (SD) | 74.6 ± 5.2 | 75.6 ± 5.6 † |
| Male sex, n (%) | 1285 (39.6) | 523 (48.3) † |
| Black race, n (%) | 549 (16.9) | 217 (20.0) ‡ |
| Alcohol use, drinks per week, n (%) | 1.95 ± 5.0 | 2.5 ± 9.0 ‡ |
| 0 | 1790 (55.2) | 610 (56.5) |
| 1–7 | 1113 (34.3) | 363 (33.6) |
| >7 | 340 (10.5) | 107 (9.9) |
| Smoking status | ||
| Current | 284 (8.9) | 141 (13.2) |
| Past | 1374 (43.2) | 515 (48.4) |
| Never | 1526 (47.9) | 409 (38.4) |
| BMI, kg/m2, mean (SD) | 26.5 ± 4.4 | 27.9 ± 5.6 † |
| Medical History, n (%) | ||
| Diabetes | 424 (13.1) | 243 (22.5) † |
| Coronary heart disease | 631 (19.4) | 312 (28.8) † |
| Heart failure | 132 (4.1) | 129 (11.9) † |
| Medications, current use, n (%) | ||
| Thiazides | 519 (16.0) | 211 (19.5) ‡ |
| Oral estrogens* | 283 (14.4) | 55 (9.8) ‡ |
| Thyroid medications | 334 (10.3) | 98 (9.0) |
| Psychotropics | 38 (1.2) | 52 (4.8) † |
| Oral corticosteroids | 319 (9.8) | 109 (10.1) |
| Osteoporosis medications | 651 (20.0) | 138 (12.7) † |
| Any fall in the past year, n (%) | 490 (15.1) | 199 (18.5) ‡ |
| Self-rated good health, n (%) | 2623 (80.8) | 790 (73.1) † |
| 15 foot walk time, seconds, mean (SD) | 5.4 ± 2.0 | 6.0 ± 4.0 † |
| IADL impairment, n (%) | 268 (8.3) | 168 (15.6) † |
| Depression score, CES-D, mean (SD) | 5.2 ± 4.8 | 6.1 ± 5.1 † |
| Cognitive score, 3MSE, mean (SD) | 90.5 ± 9.3 | 88.4 ±11.3 † |
| Laboratory Measures, mean (SD) | ||
| Glucose, mg/dL | 106.5 ± 34.7 | 113.8 ± 37.7 † |
| Serum eGFRcys, mL/min/1.73 m2 | 72.1 ± 18.0 | 69.3 ± 20.5 † |
| Serum albumin, g/dL | 3.9 ± 0.3 | 3.9 ± 0.3 † |
p < 0.001;
p < 0.05;
Reported for women only (n=2519);
BMI = body mass index; IADL = instrumental activities of daily living; 3MSE = modified mini-mental state exam; SBP=systolic blood pressure; DBP=diastolic blood pressure; eGFRcys = serum cystatin derived estimated glomerular filtration rate.
In the hip fracture analysis cohort, the median level for IL-6 was 2.79 pg/mL (interquartile range [IQR] 1.92–4.21), 2.66 mg/L for CRP (IQR 1.22–5.94), 321 mg/dL (IQR 284–365) for fibrinogen, and 6.1 × 103/mm3 (IQR 5.1–7.3) for WBC.
Association of Inflammatory Markers and Incident Fractures
The median follow-up times for the hip fracture and composite fracture analysis cohorts were 11.2 years (IQR 6.2–16.9) and 6.6 years (IQR 3.5–12.4), respectively (Table 2). The follow-up time was shorter in the composite fracture analysis cohort because composite fractures were censored once the participants left FFS Medicare. Approximately 11% of participants developed a hip fracture and 19% developed a composite fracture during follow-up.
Table 2.
Event summary during follow-up
| Hip fractures | Composite fractures | |||||
|---|---|---|---|---|---|---|
| Total | Q123 | Q4 | Total | Q123 | Q4 | |
| IL-6 | ||||||
| Sample size, n | 4330 | 3247 | 1083 | 3720 | 2793 | 927 |
| Fractures, n | 480 | 363 | 117 | 711 | 568 | 143 |
| Person-years follow-up | 47747 | 38061 | 9686 | 30652 | 24251 | 6402 |
| Incidence rate per 100 person-years | 1.01 | 0.95 | 1.21 | 2.32 | 2.34 | 2.23 |
| CRP | ||||||
| Sample size, n | 4701 | 3523 | 1178 | 4009 | 2997 | 1012 |
| Fractures, n | 514 | 399 | 115 | 758 | 594 | 164 |
| Person-years follow-up | 51888 | 39883 | 12006 | 32862 | 25247 | 7616 |
| Incidence rate per 100 person-years | 0.99 | 1.00 | 0.96 | 2.31 | 2.35 | 2.15 |
| Fibrinogen | ||||||
| Sample size, n | 4713 | 3514 | 1199 | 4022 | 3012 | 1010 |
| Fractures, n | 519 | 396 | 123 | 763 | 614 | 149 |
| Person-years follow-up | 52086 | 40050 | 12037 | 32972 | 25194 | 7779 |
| Incidence rate per 100 person-years | 1.00 | 0.99 | 1.02 | 2.31 | 2.44 | 1.92 |
| WBC | ||||||
| Sample size, n | 4670 | 3494 | 1176 | 3990 | 3001 | 989 |
| Fractures, n | 514 | 371 | 143 | 756 | 562 | 194 |
| Person-years follow-up | 51632 | 39922 | 11710 | 32735 | 25294 | 7442 |
| Incidence rate per 100 person-years | 1.00 | 0.93 | 1.22 | 2.31 | 2.22 | 2.61 |
IL-6-interleukin-6; CRP-C-reactive protein; WBC-white blood cells
In the hip fracture analysis cohort, doubling of IL-6 was associated with a significantly higher risk of incident hip fractures (unadjusted model HR 1.13, 95% CI 1.01–1.26; Table 3). Results were essentially unchanged in the fully adjusted model (HR 1.15, 95% CI 1.02–1.30). In analyses stratified by sex, the fully adjusted HR was 1.17 (95%CI 1.01–1.35) in women, whereas in men the HR was not significantly different from 1. In the composite fracture analysis cohort, there were no significant associations of IL-6 with incident composite fractures.
Table 3.
Incident hip and composite fracture risk associated with each doubling of IL-6 level, HR (95%CI) for 480 hip fractures and 711 composite fractures
| IL-6* | |||
|---|---|---|---|
| Women | Men | Overall | |
| Hip Fracture | |||
| Unadjusted | 1.12 (0.0.99–1.28) | 1.23 (1.00–1.51) | 1.13 (1.01–1.26) |
| Model 1† | 1.14 (1.00–1.30) | 1.16 (0.94–1.43) | 1.15 (1.03–1.28) |
| Model 2‡ | 1.21 (1.06–1.39) | 1.18 (0.95–1.46) | 1.20 (1.07–1.35) |
| Model 3** | 1.17 (1.01–1.35) | 1.11 (0.89–1.39) | 1.15 (1.02–1.30) |
| Composite Fracture | |||
| Unadjusted | 1.02 (0.92–1.13) | 1.12 (0.93–1.34) | 1.00 (0.91–1.10) |
| Model 1† | 1.03 (0.93–1.14) | 1.05 (0.87–1.27) | 1.03 (0.94–1.13) |
| Model 2‡ | 1.07 (0.96–1.20) | 1.08 (0.89–1.31) | 1.07 (0.97–1.18) |
| Model 3** | 1.06 (0.95–1.20) | 1.08 (0.88–1.32) | 1.06 (0.96–1.17) |
Bold values are statically significant results.
HR = hazard ratio; CI = confidence intervals; BMI = body mass index; CHD = coronary heart disease; CHF = congestive heart failure; IADL = instrumental activities of daily living; MMSE = mini-mental status exam; eGFRcys = serum cystatin derived estimated glomerular filtration rate; TSH = thyroid stimulating hormone
Per doubling of IL-6 levels
Model 1 adjusted for age, race, study clinic site, and sex (in non- sex stratified models only)
Model 2 additionally adjusts for alcohol consumption, smoking status, and BMI
Model 3 additionally adjusts for medical history (diabetes, CHD, HF), current medication use (thiazides, estrogen, thyroid medication, psychotropic medications, oral corticosteroids, osteoporosis medications), any fall in the past year, 15 foot walk time, self-reported general health, physical activity, IADL impairment, 3MS score, depression scale score, eGFRcys, serum albumin, and TSH.
Doubling of CRP was not associated with a significantly higher risk of incident hip or composite fractures in any of the models (Table 4). Results were unchanged in the analyses stratified by sex.
Table 4.
Incident hip and composite fracture risk associated with each doubling of CRP level, HR (95%CI) for 514 hip fractures and 758 composite fractures
| CRP* | |||
|---|---|---|---|
| Women | Men | Overall | |
| Hip Fracture | |||
| Model 1† | 1.01 (0.94–1.08) | 1.05 (0.94–1.16) | 1.02 (0.96–1.08) |
| Model 2‡ | 1.06 (0.98–1.13) | 1.08 (0.97–1.20) | 1.06 (1.00–1.13) |
| Model 3** | 1.06 (0.99–1.14) | 1.05 (0.94–1.18) | 1.06 (1.00–1.13) |
| Composite Fracture | |||
| Unadjusted | 0.94 (0.89–0.99) | 0.99 (0.91–1.09) | 0.97 (0.93–1.01) |
| Model 1† | 0.98 (0.93–1.03) | 1.02 (0.93–1.12) | 0.99 (0.95–1.04) |
| Model 2‡ | 1.01 (0.95–1.07) | 1.04 (0.95–1.15) | 1.02 (0.97–1.07) |
| Model 3** | 1.02 (0.96–1.08) | 1.06 (0.96–1.17) | 1.03 (0.98–1.08) |
CRP=C-reactive protein; HR = hazard ratio; CI = confidence intervals; BMI = body mass index; CHD = coronary heart disease; CHF = congestive heart failure; IADL = instrumental activities of daily living; MMSE = mini-mental status exam; eGFRcys = serum cystatin derived estimated glomerular filtration rate; TSH = thyroid stimulating hormone.
Per doubling of CRP levels
Model 1 adjusted for age, race, study clinic site, and sex (in non- sex stratified models only)
Model 2 additionally adjusts for alcohol consumption, smoking status, and BMI
Model 3 additionally adjusts for medical history (diabetes, CHD, HF), current medication use (thiazides, estrogen, thyroid medication, psychotropic medications, oral corticosteroids, osteoporosis medications), any fall in the past year, 15 foot walk time, self-reported general health, physical activity, IADL impairment, 3MS score, depression scale score, eGFRcys, serum albumin, and TSH.
In fully adjusted models, a unit increase in WBC was associated with a significantly higher risk of incident hip fracture overall (HR 1.04, 95%CI 1.01–1.06), and, in models stratified by sex, in women (HR 1.08, 95%CI 1.03–1.14) but not men. However, we found no significant associations between WBC and incident composite fractures.
In all models, the HR for hip and composite fracture per unit increase in fibrinogen was not significantly different from 1 (Supplementary Table 1).
Discussion
In this prospective population-based cohort of older adults, baseline levels of IL-6 and WBC were associated with higher incidence of hip fractures among women and the overall cohort during an 11.2-year follow-up period. These associations were evident after adjusting for a broad range of covariates. However, we found no association between any of the inflammatory markers and risk of composite fracture outcomes (hip, pelvis, humerus, and proximal forearm). In the composite fracture cohort, results were similar among men and women.
Although the role of the immune system in the pathophysiology of osteoporosis is increasingly recognized, assessing the relative significance of individual biomarkers is challenging.(11, 29) The role of IL-6 in bone metabolism is multifunctional.(14, 30) Most longitudinal studies have shown a positive association between IL-6/IL-6 SR and fractures, though the strength of these associations have varied considerably across studies. In the Health ABC study, the risk of fractures for participants in Q4 of IL-6 compared to participants in Q123 of IL-6 was HR 1.28 (95%CI 0.95–1.74).(14) In the SOF, women in Q4 of IL-6 had a significantly higher risk of hip fractures compared to women in Q123, HR 1.64 (1.09–2.48).(13) Also, participants in Q4 of IL-6 SR had a higher risk of fractures than those in Q123 of IL-6 SR in the Health ABC study [(HR 1.33 (95%CI 0.90–1.96)].(14) Similarly, women in Q4 of IL-6 SR had a higher risk of hip fractures compared to those in Q123 of IL-6 SR in the WHI study (HR 1.43 , 95% CI 0.98–1.07)(11) and in the SOF study [HR 1.43 (95%CI 0.95–2.14)].(13) Moreover, the association between IL-6 and fractures was HR 1.03 (0.73–1.44) and for IL-6SR and fractures was HR 1.12 (0.81–1.56) for men in Q4 compared to men in Q1 in the MrOS study.(15)
CRP has been most studied with respect to risk of cardiovascular disease but evidence from prospective longitudinal studies examining whether CRP predicts fractures is limited. Additionally, CRP levels have been reported to be elevated among patients with fractures.(31) The Health ABC study showed the relative risk of fracture for participants in Q4 of CRP was 1.34 (95%CI 0.99–1.82) when compared to Q123.(14) Also, the association between CPR and fractures HR 1.03 (0.73–1.45) for men in Q4 compared to men in Q1 in the MrOS study.(15)
This study had a number of strengths. It contained a large number of participants with extensive follow-up. Additionally, we assessed multiple inflammatory markers in association with incident fractures, the main clinical outcome of osteoporosis. We evaluated inflammatory markers as continuous variables, therefore decreasing the possibility of misclassification related to measurement error, as well as the bias related to the values close to the cut-off point. Also, we assessed multiple fracture types, ascertained from a merged CHS-Medicare dataset. Moreover, we adjusted for a comprehensive list of possible confounders. We used the validated medication inventory method, which is a more reliable method for obtaining medication information than self-report in epidemiologic studies.(32)
There were several limitations to this study. The composite fracture cohort follow-up was available only for the participants enrolled in FFS Medicare, so censoring could introduce bias. Although we were able to account for a large number of possible confounders, some of the variables were assessed by self-report, possibly introducing bias. Also, we measured cytokines in the circulation, while levels in the bone microenvironment may be more important. Similarly, the soluble receptors for inflammatory markers, whose levels appear to be more stable than the markers themselves, were not available in our study.(33) Previous studies suggest that increased levels of soluble receptors may represent a more prolonged inflammatory state.(34, 35) Therefore, it is possible that our findings are affected by transient changes and do not represent more severe or prolonged inflammation. Another limitation of our study is that BMD was only measured at two study sites. Thus, we were unable to account for its impact on observed associations between these cytokines and risk of incident fractures. A previous study found that BMD attenuated the association between inflammatory markers and incident fractures by 15%, suggesting a potential mediating role.(13)
In conclusion, we observed that higher baseline levels of IL-6 and WBC were associated with higher incidence of hip fractures among women and in the overall cohort of older adults. Our findings support the direction of the association between IL-6 and hip fractures in other longitudinal studies and have allowed us to generate a more precise and statistically significant estimate of the IL-6 and fracture association. Although significant progress has been made in unraveling the complex role of the immune system in the pathophysiology of osteoporosis, the clinical implications of this relationship remain to be determined.
Supplementary Material
Acknowledgments
DS is an employee of the U.S. Food and Drug Administration. This work represents the opinions of the authors and not necessarily that of the U.S. Food and Drug Administration; BMP reports service on the DSMB of a clinical trial funded by Zoll LifeCor and service on the Steering Committee of the Yale Open Data Access Project funded by Johnson & Johnson; LHC and SH received grants from NHLBI (NIH); MB received grants from General electric.
This research was supported by contracts HHSN268201200036C, HHSN268200800007C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and grants U01HL080295 and U01HL130114 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by R01AG023629 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Appendix
Appendix Table 1.
| Fracture Type | ICD-9 Codes | CPT Procedure Codes |
|---|---|---|
| Hip | 820.0–82.9 | 27125, 27130–27131, 27230–27248, 29010–29046, 29305–29365, 29505–29520, 29799 |
| Distal Forearm | 813.4–813.5 | 24580–24588, 25600–25620, 29065–29085, 29105, 29125–29126, 29799 |
| Humerus | 812.0–812.5 | 23600–23630, 23665–23680, 24500–24588, 29035–29065, 29105, 29799 |
| Pelvis | 808.0–808.9 | 27120, 27122, 27130, 27131–27132, 27190–27192, 27200, 27210–27214, 27220–27225 |
Inclusion
For inclusion, inpatient claims required a qualifying ICD-9 fracture diagnosis code and outpatient and physician claims required both a qualifying ICD-9 fracture diagnosis code and a corresponding CPT procedure code consistent with treatment of this fracture type.
Exclusion
Inpatient, outpatient and physician claims with codes consistent with care of old fractures or other bone diseases were excluded, including codes for late effects of fracture (ICD-9 905.4), implant complication (ICD-9 996.4, 996.6, 996.7, E878.1), aseptic necrosis (ICD-9 733.4), malunion of bone (ICD-9 733.8), other disorders of bone or cartilage (ICD-9 733.9), and fracture follow-up care (V540, V664, V674).
Footnotes
Disclosures
All other authors state that they have no conflicts of interest.
Author’s roles: Conception and study design : DS, PB, JAC. Analysis and interpretation of data: DS, PB, KM, HAF, JAC. Drafting the manuscript: DS, PB, HAF, JAC. Revising the manuscript: DS, PB, KM, SH, BP, JC, EW, LH, CH, MB, DL, RY, DJ, HAF, JAC. Approving the final version of the manuscript: DS, PB, KM, SH, BP, JC, EW, LH, CH, MB, DL, RY, DJ, HAF, JAC. DS and PB take responsibility for the integrity of the data analyses.
References
- 1.Sambrook P, Cooper C. Osteoporosis. Lancet. 2006;367(9527):2010–8. doi: 10.1016/S0140-6736(06)68891-0. [DOI] [PubMed] [Google Scholar]
- 2.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22(3):465–75. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 3.Abrahamsen B, van Staa T, Ariely R, Olson M, Cooper C. Excess mortality following hip fracture: a systematic epidemiological review. Osteoporos Int. 2009;20(10):1633–50. doi: 10.1007/s00198-009-0920-3. [DOI] [PubMed] [Google Scholar]
- 4.Tracy RP. Emerging relationships of inflammation, cardiovascular disease and chronic diseases of aging. Int J Obes Relat Metab Disord. 2003;27(Suppl 3):S29–34. doi: 10.1038/sj.ijo.0802497. [DOI] [PubMed] [Google Scholar]
- 5.Pai JK, Pischon T, Ma J, Manson JE, Hankinson SE, Joshipura K, et al. Inflammatory markers and the risk of coronary heart disease in men and women. N Engl J Med. 2004;351(25):2599–610. doi: 10.1056/NEJMoa040967. [DOI] [PubMed] [Google Scholar]
- 6.Harris TB, Ferrucci L, Tracy RP, Corti MC, Wacholder S, Ettinger WH, Jr, et al. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999;106(5):506–12. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- 7.Weaver JD, Huang MH, Albert M, Harris T, Rowe JW, Seeman TE. Interleukin-6 and risk of cognitive decline: MacArthur studies of successful aging. Neurology. 2002;59(3):371–8. doi: 10.1212/wnl.59.3.371. [DOI] [PubMed] [Google Scholar]
- 8.Ferrucci L, Harris TB, Guralnik JM, Tracy RP, Corti MC, Cohen HJ, et al. Serum IL-6 level and the development of disability in older persons. J Am Geriatr Soc. 1999;47(6):639–46. doi: 10.1111/j.1532-5415.1999.tb01583.x. [DOI] [PubMed] [Google Scholar]
- 9.Park JH, Park KH, Cho S, Choi YS, Seo SK, Lee BS, et al. Concomitant increase in muscle strength and bone mineral density with decreasing IL-6 levels after combination therapy with alendronate and calcitriol in postmenopausal women. Menopause. 2013;20(7):747–53. doi: 10.1097/GME.0b013e31827cabca. [DOI] [PubMed] [Google Scholar]
- 10.Wang Z, Yang Y, He M, Wang R, Ma J, Zhang Y, et al. Association between interleukin-6 gene polymorphisms and bone mineral density: a meta-analysis. Genet Test Mol Biomarkers. 2013;17(12):898–909. doi: 10.1089/gtmb.2013.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barbour KE, Boudreau R, Danielson ME, Youk AO, Wactawski-Wende J, Greep NC, et al. Inflammatory markers and the risk of hip fracture: the Women's Health Initiative. J Bone Miner Res. 2012;27(5):1167–76. doi: 10.1002/jbmr.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaneshiro S, Ebina K, Shi K, Higuchi C, Hirao M, Okamoto M, et al. IL-6 negatively regulates osteoblast differentiation through the SHP2/MEK2 and SHP2/Akt2 pathways in vitro. J Bone Miner Metab. 2014;32(4):378–92. doi: 10.1007/s00774-013-0514-1. [DOI] [PubMed] [Google Scholar]
- 13.Barbour KE, Lui LY, Ensrud KE, Hillier TA, LeBlanc ES, Ing SW, et al. Inflammatory markers and risk of hip fracture in older white women: the study of osteoporotic fractures. J Bone Miner Res. 2014;29(9):2057–64. doi: 10.1002/jbmr.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cauley JA, Danielson ME, Boudreau RM, Forrest KY, Zmuda JM, Pahor M, et al. Inflammatory markers and incident fracture risk in older men and women: the Health Aging and Body Composition Study. J Bone Miner Res. 2007;22(7):1088–95. doi: 10.1359/jbmr.070409. [DOI] [PubMed] [Google Scholar]
- 15.Cauley JA, Barbour KE, Harrison SL, Cloonan YK, Danielson ME, Ensrud KE, et al. Inflammatory markers and the risk of hip and vertebral fractures in men: the Osteoporotic Fractures in Men (MrOS) Journal of Bone and Mineral Research. 2016;31(12):2129–38. doi: 10.1002/jbmr.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gray SR, Clifford M, Lancaster R, Leggate M, Davies M, Nimmo MA. The response of circulating levels of the interleukin-6/interleukin-6 receptor complex to exercise in young men. Cytokine. 2009;47(2):98–102. doi: 10.1016/j.cyto.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 17.Sakkinen PA, Macy EM, Callas PW, Cornell ES, Hayes TE, Kuller LH, et al. Analytical and biologic variability in measures of hemostasis, fibrinolysis, and inflammation: assessment and implications for epidemiology. Am J Epidemiol. 1999;149(3):261–7. doi: 10.1093/oxfordjournals.aje.a009801. [DOI] [PubMed] [Google Scholar]
- 18.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, et al. The cardiovascular health study: design and rationale. Annals of epidemiology. 1991;1(3):263–76. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 19.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1(3):263–76. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 20.Cushman M, Cornell ES, Howard PR, Bovill EG, Tracy RP. Laboratory methods and quality assurance in the Cardiovascular Health Study. Clin Chem. 1995;41(2):264–70. [PubMed] [Google Scholar]
- 21.Macy EM, Hayes TE, Tracy RP. Variability in the measurement of C-reactive protein in healthy subjects: implications for reference intervals and epidemiological applications. Clinical chemistry. 1997;43(1):52–8. [PubMed] [Google Scholar]
- 22.Barzilay JI, Blaum C, Moore T, Xue QL, Hirsch CH, Walston JD, et al. Insulin resistance and inflammation as precursors of frailty: the Cardiovascular Health Study. Archives of internal medicine. 2007;167(7):635–41. doi: 10.1001/archinte.167.7.635. [DOI] [PubMed] [Google Scholar]
- 23.Cushman M, Meilahn EN, Psaty BM, Kuller LH, Dobs AS, Tracy RP. Hormone replacement therapy, inflammation, and hemostasis in elderly women. Arteriosclerosis, Thrombosis, and Vascular Biology. 1999;19(4):893–9. doi: 10.1161/01.atv.19.4.893. [DOI] [PubMed] [Google Scholar]
- 24.Ray WA, Griffin MR, Fought RL, Adams ML. Identification of fractures from computerized Medicare files. Journal of clinical epidemiology. 1992;45(7):703–14. doi: 10.1016/0895-4356(92)90047-q. [DOI] [PubMed] [Google Scholar]
- 25.Fisher ES, Whaley FS, Krushat WM, Malenka DJ, Fleming C, Baron JA, et al. The accuracy of Medicare's hospital claims data: progress has been made, but problems remain. American journal of public health. 1992;82(2):243–8. doi: 10.2105/ajph.82.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baron JA, Karagas M, Barrett J, Kniffin W, Malenka D, Mayor M, et al. Basic epidemiology of fractures of the upper and lower limb among Americans over 65 years of age. Epidemiology. 1996;7(6):612–8. doi: 10.1097/00001648-199611000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: Evaluation of a short form of the CES-D. American journal of preventive medicine. 1994 [PubMed] [Google Scholar]
- 28.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. Journal of Clinical Psychiatry. 1987 [PubMed] [Google Scholar]
- 29.Ing SW, Orchard TS, Lu B, LaMonte MJ, Barbour KE, Cauley JA, et al. TNF receptors predict hip fracture risk in the WHI study and fatty acid intake does not modify this association. The Journal of Clinical Endocrinology & Metabolism. 2015;100(9):3380–7. doi: 10.1210/JC.2015-1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clowes JA, Riggs BL, Khosla S. The role of the immune system in the pathophysiology of osteoporosis. Immunological reviews. 2005;208(1):207–27. doi: 10.1111/j.0105-2896.2005.00334.x. [DOI] [PubMed] [Google Scholar]
- 31.Mold C, Gewurz H, Du Clos TW. Regulation of complement activation by C-reactive protein. Immunopharmacology. 1999;42(1):23–30. doi: 10.1016/s0162-3109(99)00007-7. [DOI] [PubMed] [Google Scholar]
- 32.Psaty BM, Lee M, Savage PJ, Rutan GH, German PS, Lyles M. Assessing the use of medications in the elderly: methods and initial experience in the Cardiovascular Health Study. The Cardiovascular Health Study Collaborative Research Group. J Clin Epidemiol. 1992;45(6):683–92. doi: 10.1016/0895-4356(92)90143-b. [DOI] [PubMed] [Google Scholar]
- 33.Aderka D, Engelmann H, Shemer-Avni Y, Hornik V, Galil A, Sarov B, et al. Variation in serum levels of the soluble TNF receptors among healthy individuals. Lymphokine Cytokine Res. 1992;11(3):157–9. [PubMed] [Google Scholar]
- 34.Honda M, Yamamoto S, Cheng M, Yasukawa K, Suzuki H, Saito T, et al. Human soluble IL-6 receptor: its detection and enhanced release by HIV infection. J Immunol. 1992;148(7):2175–80. [PubMed] [Google Scholar]
- 35.Van Zee KJ, Kohno T, Fischer E, Rock CS, Moldawer LL, Lowry SF. Tumor necrosis factor soluble receptors circulate during experimental and clinical inflammation and can protect against excessive tumor necrosis factor alpha in vitro and in vivo. Proceedings of the National Academy of Sciences. 1992;89(11):4845–9. doi: 10.1073/pnas.89.11.4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.