Summary
Platelets are small anucleated cells that constantly patrol the cardiovascular system to preserve its integrity and prevent excessive blood loss where the vessel lining is breached. Their key challenge is to form a hemostatic plug under conditions of high shear forces. To do so, platelets have evolved a molecular machinery that enables them to sense trace amounts of signals at the site of damage and to rapidly shift from a non-adhesive to a pro-adhesive state. However, this highly efficient molecular machinery can also lead to unintended platelet activation and cause clinical complications such as thrombocytopenia and thrombosis. Thus, several checkpoints are in place to tightly control platelet activation and adhesiveness in space and time. In this review, we will discuss select negative regulators of platelet activation, which are critical to maintain patrolling platelets in a quiescent, non-adhesive state and/or to limit platelet adhesion to sites of injury.
Keywords: negative regulators, platelet reactivity, platelet adhesion, thrombosis, hemostasis
Key features of the molecular machinery that positively regulates platelet adhesiveness
Platelets are highly specialized cells able to trigger an extremely sensitive and fast activation response. Their small size and disc shape morphology ensure that they concentrate in a fluid layer adjacent to the vessel wall, so that they can immediately sense a biochemical or physical alteration of the endothelial lining[1]. They are equipped with a repertoire of unique surface receptors required for adhesion and activation at sites of vascular injury under high shear stress conditions. Transient interactions between platelet-specific glycoprotein (GP)Ibα and Von Willebrand factor (VWF) decelerate platelets to facilitate full activation in response to components of the exposed extracellular matrix (ECM), e.g. collagen, and locally-generated soluble agonists, e.g. thrombin[2]. Collagen triggers platelet activation via GPVI, a member of the immunoglobulin family that associates with the Fc receptor γ (FcRγ)-chain, a homodimer containing an immune receptor tyrosine-based activation motif (ITAM) in its cytoplasmic tail. Like other ITAM-coupled receptors, the GPVI/FcRγ complex has a consensus sequence with two tyrosine residues (YxxI/Lx(6–12)YxxI/L, where x is any amino acid), that once phosphorylated by Src kinases, such as FYN and LYN, provide docking for the SH2 domain-containing kinase SYK, which in turn phosphorylates and activates many downstream signaling proteins including phospholipase C(PLC)γ2. However, compared to its immune receptor homologues, GPVI is primed to constitutively bind the Src-family kinase LYN so that it can meet the accelerated temporal requirements of hemostasis[3]. Soluble agonists ensure an even faster response through the engagement of G protein-coupled receptors (GPCRs) and, being soluble, are able to recruit free flowing platelets at the site of damage. In platelets, the activatory GPCRs are coupled to Gq that activates PLCβ, G12/13 that induces RHOA-dependent shape change, and Gi/z that inhibits adenylyl cyclase and activates phosphoinositide 3-kinase (PI3K). Although with different kinetics Gq-coupled and ITAM-coupled receptors converge at the level of PLC. Both PLCβ and PLCγ2 cleave phosphatidylinositol 4,5-biphosphate (PIP2) to inositol 1,4,5-triphosphate (IP3) and the second messenger diacylglycerol (DAG). IP3 binds to IP3 receptors (IP3R) on the dense tubular system and induces the release of the second messenger calcium ions (Ca2+) into the cytosol. DAG triggers the activation of the various protein kinase C (PKC) isoforms important for the exocytosis of storage granules[4], including PKCα, PKCβ, PKCθ, PKCδ and PKCε. The rise in cytosolic Ca2+ concentrations upon agonist receptor engagement is extremely fast and thus drives the initial burst of multiple platelet responses. The guanine nucleotide exchange factor, CALDAG-GEFI, is one example for how platelets utilize Ca2+ to initiate platelet adhesion. CALDAG-GEFI senses minimal changes in intracellular Ca2+ levels[5], leading to a near-immediate activation of the small GTPase RAP1[6], a key player in platelet adhesion (see below). Consistent with the transient nature of the Ca2+ signal, CALDAG-GEFI-mediated RAP1 activation is also a transient process[7–9]. A more sustained signal for RAP1 activation is required to allow for firm platelet adhesion. This signal depends on PKC activation, granule secretion and feedback signaling through the Gi-coupled receptor for ADP, P2Y12[7–9]. Once active, RAP1 recruits the cytoskeletal protein TALIN to the plasma membrane and thereby promotes the activation of β1 and β3 integrin receptors (inside-out signaling)[10], which enable firm platelet adhesion to the site of injury. The platelet surface is covered with ~80,000 copies of the platelet-specific integrin αIIbβ3[11]. Active αIIbβ3 receptors bind multivalent ligands, such as plasma fibrinogen, and support platelet-ECM and platelet-platelet adhesion required for the formation of three-dimensional aggregates. Both rapid and sustained RAP1/integrin activation are critical to ensure the formation of stable shear-resistant hemostatic plugs[7]. Once engaged, the integrin initiates outside-in signaling pathways that orchestrate cytoskeletal rearrangements necessary for the consolidation of the hemostatic plug through platelet spreading and clot retraction[12].
Checkpoints of platelet activation
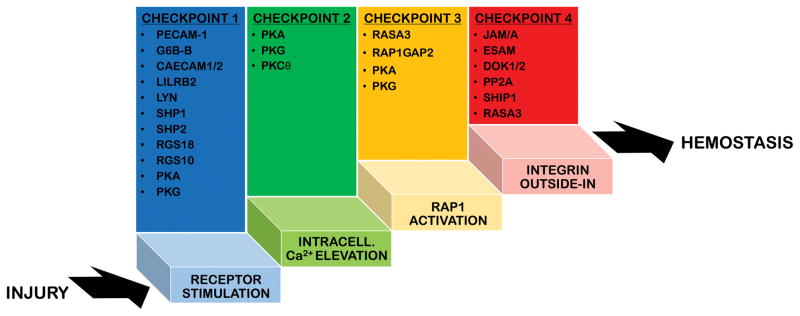
In physiological conditions the molecular mechanisms underlying the conversion to a pro-adhesive state are self-limiting. Various regulatory elements provide important negative feedback at critical steps during the activation process, checkpoints that need to be passed in order for platelet integrins to shift to and maintain a high-affinity state (Figure 1). Platelets that do not make it past these checkpoints return into circulation. These thresholds are set by a number of negative regulators that have two main functions: 1) when the endothelial lining is intact, they maintain patrolling platelets in a resting non-adhesive state; 2) at sites of injury, they limit the duration and intensity of the activation response to avoid the accumulation of too many platelets and the growth of occlusive thrombi (thrombosis).
Figure 1. Checkpoints of platelet activation.
In physiological conditions the molecular mechanisms regulating platelet adhesiveness and activation are self-limiting. Several checkpoints are in place 1) to prevent unwanted activation of patrolling platelets, when the endothelial lining is biochemically and physically healthy, and 2) to limit the duration and intensity of the activation response at sites of vascular injury. This schematic figure represents platelet activation as a ladder and shows the four main checkpoints that need to be passed/climbed in order for platelets to shift from a non-adhesive patrolling state to a pro-adhesive state that ensures hemostasis. Platelets that do not make it past these checkpoints return into circulation. The regulatory elements that provide important negative feedback at these critical checkpoints are listed above each step of the activation process. The list is not exhaustive but only includes the best characterized negative regulators, which we have described in the text.
The main signaling nodes where the positive and negative signals are integrated to control platelet activation/adhesiveness are at the level of 1) receptor stimulation, 2) intracellular Ca2+ elevation, 3) RAP1 activation, and 4) outside-in signaling.
Checkpoints at the level of Receptor stimulation
The first signaling node where negative regulators control initiation, intensity and duration of platelet activation is at the level of receptor stimulation (Figure 2). When the endothelial lining is intact and healthy, circulating platelets are maintained in a non-adhesive state. The intact endothelium contributes to the negative regulation of platelets by releasing prostacyclin (prostaglandin I2, PGI2) and nitric oxide (NO), which are potent platelet antagonist. PGI2 exerts its function by stimulating the prostacyclin receptor (IP-R) on the platelet surface. IP-R is a GPCR coupled to heterotrimeric G proteins that stimulates adenylyl cyclase to synthetize cyclic AMP (cAMP), which in turn activates protein kinase A (PKA). NO is a neutral oxide that can freely permeate the plasma membrane and bind directly to soluble guanylyl cyclase (sGC) in the cytosol. sGC responds to NO binding by generating cyclic GMP (cGMP), which in turn activates protein kinase G (PKG). PKA and PKG translate the effect of the endothelial-derived platelet antagonist by phosphorylating and inhibiting numerous proteins important for platelet activation, examples of which are provided later in the review. However, both PGI2 and NO have a very short half-life and their inhibitory effect is reversible and can be bypassed by high enough agonist concentrations, as those present at sites of injury. Defects in prostacyclin[13] or NO[14] signaling result in hyper-reactive platelets, a pro-thrombotic state and shortened bleeding times. Among the cell surface receptors, PKA phosphorylates the TxA2 receptor (TPα) on a site which could lead to receptor desensitization[15,16]. We refer the reader to the extensive review by Smolenski[17] for further details on mechanisms regulating this complex signaling pathway.
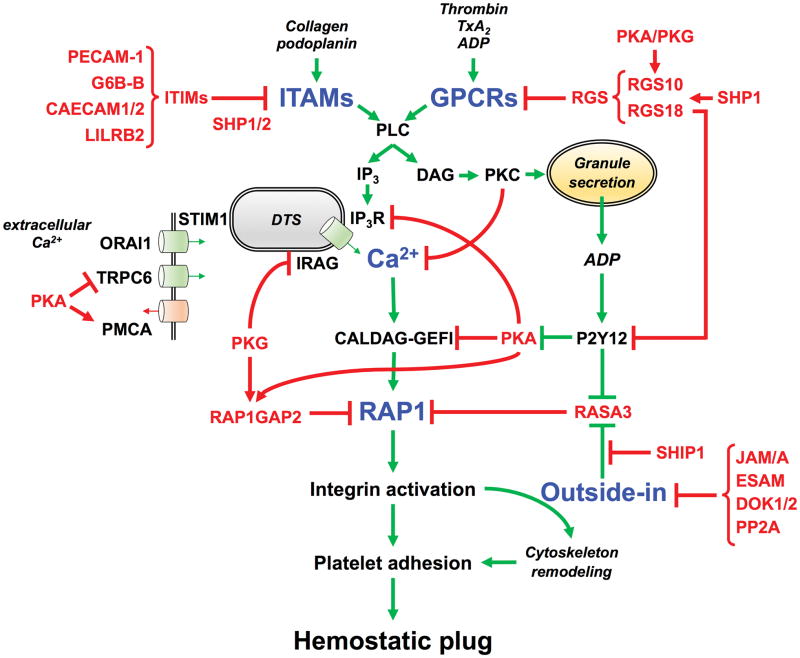
Figure 2. Negative regulators that control platelet activation and adhesiveness.
The tight balance between inhibitory (red arrows) and activatory (green arrows) signaling pathways is critical to maintain patrolling platelets in a quiescent, non-adhesive state and/or to limit platelet adhesion to sites of injury. This schematic figure shows the critical signaling nodes (blue) where the positive and negative signals are integrated to finely control platelet activation and adhesiveness in space and time, most importantly at the level of 1) receptor stimulation, 2) intracellular Ca2+ elevation, 3) Rap1 activation, and 4) outside-in signaling. The regulatory elements that provide important negative feedback at these critical checkpoints of the activation process are labelled in red. Abbreviations: ITAMs (immune receptor tyrosine-based activation motif), ITIMs (Immunoreceptor Tyrosine-based Inhibition Motif), PECAM-1 (Platelet Endothelial Cell Adhesion Molecule-1, CD31), G6B-B (Megakaryocyte and Platelet Inhibitory Receptor), CAECAM1/2 (Carcinoembryonic antigen-related cell adhesion molecule 1/2), LILRB2 (Leukocyte immunoglobulin-like receptor subfamily B member 2), GPCRs (G protein-coupled receptors), RGS (Regulators of G-protein Signaling), TxA2 (thromboxane A2), PLC (phospholipase C), IP3 (inositol 1,4,5-triphosphate), DTS (dense tubular system), Ca2+ (calcium ions), IP3R (IP3 receptor), IRAG (IP3R-associated cGMP kinase substrate), STIM1 (Stromal interaction molecule 1), ORAI1 (Calcium Release-Activated Calcium Modulator 1), TRPC6 (Transient receptor potential cation channel, subfamily C, member 6), PMCA (plasma membrane Ca2+-ATPase), DAG (diacylglycerol), RAP1 (Ras-proximate-1), CALDAG-GEFI (Ca2+-regulated guanine nucleotide exchange factor), PKC (protein kinase C), ADP (adenosine diphosphate), PKA (protein kinase A), PKG (protein kinase G), P2Y12 (G-protein coupled purinergic receptor), RASA3 (RAS p21 protein Activator 3), RAP1GAP2 (RAP1 GTPase activating protein 2), SHIP1 (phosphatidylinositol-3,4,5-trisphosphate 5-phosphatase 1), SHP1/2 (Src-homology 2 domain (SH2)-protein tyrosine phosphatase), JAM/A (Junctional adhesion molecule-A), ESAM (Endothelial Cell Adhesion Molecule), DOK1/2 (Docking protein 1/2), PP2A (Protein phosphatase 2A).
When the endothelium is damaged, PGI2 and NO concentrations decrease in proximity of the injury and platelets respond to exposed ECM components and locally-generated soluble agonists through ITAM-coupled receptors and GPCRs, respectively. ITAM-coupled receptors are typically antagonized by Immunoreceptor Tyrosine-based Inhibition Motif (ITIM)-coupled receptors. The cytoplasmic tail of these receptors contains at least one ITIM consensus sequence defined as I/V/LxYxxL/V, and may also include an immunoreceptor tyrosine–based switch motif (ITSM, consensus sequence TxYxxV/I) and a proline-rich region (PRR, SH3 binding domain) in close proximity. These structural features enable these receptors to attenuate ITAM signaling with two modes of action: 1) by recruiting lipid or protein tyrosine phosphatases (PTPs) in proximity of ITAM-coupled receptors to counteract the action of activating kinases or 2) by sequestering key components of the ITAM signaling pathway.
The best characterized ITIM-containing receptor present in platelets is the Platelet Endothelial Cell Adhesion Molecule-1 (PECAM-1, CD31)[18], which is highly expressed in non-erythroid hematopoietic cells and in endothelial cells. PECAM-1 signaling is initiated through the formation of trans homophilic interactions[19,20] between their immunoglobulin-like extracellular domains at the interface between platelets or between platelets and endothelial cells. Since these interactions are weak they are most likely to happen not among circulating cells in a healthy vessel but within a growing thrombus with the effect of limiting thrombus size. Indeed, genetic ablation of Pecam1 results in enhanced thrombus size and stability[21]. The paradoxical observation that Pecam1−/− mice exhibit a prolonged bleeding time is likely due to the loss of PECAM-1 in endothelial cells, as transplantation of wild type bone marrow-derived hematopoietic precursors[22] did not correct the hemostasis defect.
In its cytoplasmic tail PECAM-1 contains both an ITIM and an ITSM sequence and both have been shown to be phosphorylated to a low stoichiometry in resting platelets, supporting the fact that PECAM-1 does not contribute to the quiescent state of circulating platelets. Direct stimulation of PECAM-1 via its crosslinking or platelet stimulation by thrombin or collagen induces the phosphorylation of the ITIM/ITSM domains and inhibition of platelet activation. This inhibition can, however, be overcome with high concentrations of agonists[18]. In order to provide a docking site for SH2-containing phosphatases, both tyrosine residues need to be phosphorylated. In GPVI stimulated platelets the first phosphorylation is mediated by the Src-family kinase LYN. This kinase is implicated in the near-immediate activation of GPVI[3], but it is also known to have a late inhibitory role in GPVI-dependent platelet activation[23]. Consistently LYN and PECAM-1 deficient platelets are equally hyper-responsive to GPVI stimulation[24]. The second ITIM tyrosine residue is phosphorylated by a distinct SH2-containing kinase, such as BTK or CSK, which is activated later in the ITAM-signaling pathway and is recruited to the LYN-phosphorylated ITIM[25,26]. This sequential two-enzyme mechanism ensures a delayed onset of PECAM-1 activity in platelets, which is important to attenuate the intensity and the duration of the ITAM signal. Once the tyrosine residues are phosphorylated, the ITIM/ITSM provide a docking site for the protein tyrosine phosphatases (PTPs), SHP1 and SHP2, which counteract the stimulatory functions of tyrosine kinases. In addition, ITIM-bound SHP2 has been shown to dampen the ITAM signaling pathway by sequestering the p85 subunit of PI3K and interfering with the formation of functional signaling complexes bound to the ITAM cytoplasmic tail[27] (for a more complete review of the function of platelet phosphatases see [28]). Importantly, PECAM-1 negatively regulates the platelet response to multiple agonists, not just those stimulating ITAM-coupled receptors[29–31]. In particular crosslinking of PECAM-1 was shown to induce the internalization of the GPIb-IX-V complex and therefore inhibit platelet activation induced by thrombin (not thrombin receptors agonist peptide) and VWF[32].
Another relevant platelet ITIM receptor is G6B-B[33,34], which is highly restricted to the platelet/megakaryocyte lineage. Like PECAM-1, its cytoplasmic tail includes an ITIM and an ITSM consensus sequence. Interestingly though, while PECAM-1 is dephosphorylated in resting/patrolling platelets, G6B-B is highly phosphorylated in resting conditions[34], suggesting that this receptor may be necessary to transmit tonic inhibitory signals to prevent unwanted activation of circulating platelets. Consistently, G6b−/−[35] mice are characterized by a dramatic reduction in platelet count (~77%), which is in part due to platelet pre-activation and premature clearance, and their thrombocytopenia is partially rescued by ablation of the ITAM-coupled receptors GPVI and CLEC-2. Moreover, G6b−/− platelets also show a marked increase in GPVI shedding, which could be a compensatory mechanism to quench the hyper-reactivity of these platelets. The thrombocytopenia combined with the low levels of GPVI lead to a prolonged bleeding diathesis[35]. However, G6b−/− platelets have an enhanced response to CLEC-2 stimulation and platelets heterozygous for both G6B-B and GPVI (G6b+/−Gp6+/−), which do not show increased GPVI shedding, hyper-respond to GPVI agonists compared to Gp6+/−[35]. Thus, G6B-B is a negative regulator of ITAM-coupled receptors signaling[36]. Its inhibitory function is largely mediated through the recruitment of SHP1 and SHP2, in fact Shp1/Shp2 conditional knockout mice partially phenocopy the G6b knockout mice[37]. Although G6B-B primarily targets ITAM signaling it has been shown to inhibit also GPCR-induced platelet aggregation and Ca2+ flux[38], but the underlying mechanism is yet undefined.
In addition to PECAM-1 and G6b-B, platelets express very low levels[11] of the ITIM-bearing receptors CEACAM1[39], CEACAM2[40] and LILRB2 (PIR-B)[41] that contribute to the inhibition of ITAM signaling and limit thrombus growth. It should be noted that ITIM-bearing receptors are not exclusively negative regulators of platelet activation and this aspect was recently reviewed by Coxon and coauthors[42]. TLT-1, which is actually the most highly expressed ITIM-bearing receptor in platelets, has a positive regulatory role on platelet activation[43]. PECAM-1[44], G6b-B[37] and CEACAM-1/2[45,46] positively regulate integrin outside-in signaling, spreading and clot retraction with a yet unknown mechanism.
GPVI, but not the hemITAM receptor CLEC-2, is also regulated by proteolysis of its ectodomain that reduces the density of receptors that can undergo stimulation. Shedding occurs upon GPVI activation or after exposure of platelets to high shear stress and is a marker of platelet activation in the clinic (for review see [47]).
Soluble agonists stimulate platelet activation through GPCRs, which act by activating heterotrimeric (αβγ) GTP-binding proteins. G proteins are very efficient on/off switches that in their resting (off) state bind GDP with the α subunits (Gα). Upon ligand binding, the receptor promotes the exchange of GTP for GDP, partially dissociating the heterotrimer and allowing signaling downstream of both Gα-GTP and Gβγ (on). Signaling is then terminated by hydrolysis of the GTP bound to Gα. Duration and intensity of the GPCR signals is limited by the Regulators of G-protein Signaling (RGS), a class of proteins with GTPase-activating function that accelerate the rate of GTP hydrolysis and promote GPCR signal termination.
First evidence that RGS proteins are important negative regulators of platelet activation was provided analyzing mice with a single amino acid substitution (G184S) in Gαi that blocks the RGS/Gαi interactions. As expected, this mutation causes enhanced platelet aggregation and increased platelet accumulation after vascular injury[48]. Platelets express several RGS proteins. The most abundant are RGS18 and RGS10[11], which have GTPase-activating protein activity for Gαi and Gαq[49,50]. RGS2, which is much less abundant, could be regulating Gαs[51], while RGS16 negatively regulates the chemokine Gi-coupled receptor CXCR4[52]. In mice genetic ablation of either Rgs10[53] or Rgs18[54,55] shortens bleeding as well as thrombus occlusion times in vivo and enhances agonist-induced platelet responses in vitro. However, since Gα subunits possess an intrinsic GTPase activity that is retained even in the absence of RGS proteins the hyper-responsiveness is evident only at suboptimal doses of agonist stimulation. In either one of these mouse models there is no evidence of premature platelet clearance. Rgs10−/− mice have a normal platelet count/size[53] and Rgs18−/− mice display a mild thrombocytopenia (~ 15% reduction) due to defects in megakaryocyte function[54,55]. One possible explanation for the mild in vivo phenotype is of course the redundancy between RGS isoforms. Another plausible cause may be that, like PECAM-1 and unlike G6B-B, their primary function is to dampen thrombus growth at the site of injury, while they are not crucial to maintain circulating cells in a non-adhesive state. The function of RGS18 and RGS10 is tightly controlled by scaffold proteins that sequester them and hinder their inhibitory function. In unstimulated platelets both RGS10 and RGS18 form a complex with spinophilin (SPL) and the tyrosine phosphatase, SHP-1[56]. Both PGI2 treatment[57] or thrombin/TxA2 stimulation[56] induce the dissociation of the complex to allow RGS proteins to quench platelet activation in circulation and at sites of injury. Consistently, SPL deficiency in mice causes a loss of function by making more RGS available to inhibit Gq and Gi protein signaling[56]. However, the defect is not profound because in agonist-stimulated platelets RGS18 is also regulated by the reversible interaction with 14-3-3γ that is controlled by PKA/PKG-dependent phosphorylation of RGS18[58,59].
The GPCR signal intensity and duration is also modulated by receptor desensitization, a reversible process that controls the density of agonist receptors on the platelet surface. Desensitization is initiated by agonist-induced phosphorylation of the receptor as a protective measure to prevent excessive platelet accumulation and vessel occlusion[60,61].
Checkpoints at the level of intracellular Ca2+ elevation
The second important signaling hub controls the levels of cytosolic Ca2+ concentrations ([Ca2+]cyt) (Figure 2). Platelets express numerous Ca2+-sensitive effector proteins and a rise of [Ca2+]cyt is critical to evoke cytoskeleton remodeling, integrin activation, granule secretion, TxA2 generation and phosphatidylserine exposure. If not properly regulated, however, changes in [Ca2+]cyt can also lead to unwanted platelet activation in circulation and excessive thrombus formation at sites of injury.
Quiescent platelets maintain a low cytosolic Ca2+ concentration estimated to be around 50 nM. Active transporters are in place at the plasma membrane and on the dense-tubular system to counteract the leakage of Ca2+ through the membranes and to restore resting [Ca2+]cyt after unwanted elevations, due to shear stress for example. Upon platelet activation, [Ca2+]cyt can increase up to μM levels in a matter of milliseconds. The near-immediate increase of [Ca2+]cyt is stimulated by agonist-induced PLC activation and IP3 generation, that in turn evokes Ca2+ release from the dense tubular system through the IP3R. Depletion of intracellular stores is sensed by stromal interaction molecule 1 (STIM1) that leads to the activation of the plasma membrane Ca2+ channel ORAI-1, which allows entry from the extracellular space (store-operated Ca2+ entry). Additionally, platelets express on the plasma membrane the receptor-operated channel P2X1, that enables Ca2+ entry in response to ATP, and second-messenger operated channels of the TRPC family, that respond to DAG. The best characterized inhibitory signaling pathways regulating Ca2+ levels are stimulated by the endothelial-derived platelet antagonists, PGI2 and NO, and are mediated by PKA and PKG[17]. Early studies demonstrated that PKA-mediated phosphorylation increases the activity of the plasma membrane Ca2+-ATPase (PMCA)[62], which transports Ca2+ from the cytosol to the extracellular space, and that PGI2 and NO inhibit Ca2+ release from the intracellular stores through the direct phosphorylation of IP3R[63]. More recently it was shown that a major target of NO/PKG signaling is IRAG (also known as MRVI1), an IP3R-associated protein. In a mouse model expressing a mutant form of IRAG unable to bind IP3-R, platelets were very weakly inhibited by NO donors and NO inhibition of agonist-induced aggregation was impaired[64]. Moreover, systemic deletion of IRAG in mice lead to platelet hyper-reactivity[65]. A recent proteomic study[16] characterizing the phosphorylation patterns of platelets treated with Iloprost, the synthetic analogue of PGI2, confirmed that IRAG and the IP3RI are major PKA/PKG targets, and, in addition, identified PKA phosphorylation sites on TRPC6 and STIM1, and detected the de-phosphorylation of ORAI1. Thus PKA/PKG control all levels of Ca2+ signaling, including the removal of Ca2+ from the cytosol, the release of Ca2+ from the stores and the entry of Ca2+ from the extracellular space, producing the net effect of inhibiting the rise of [Ca2+]cyt.
A distinct, but less well understood, mechanism regulating [Ca2+]cyt is mediated by PKCs. Even though PKCs are best known for their positive role on platelet activation, broad spectrum PKC inhibitors reduce [Ca2+]cyt [66]. This paradox has been solved by analyzing isoform-specific knockout mice, which revealed that individual isoforms have distinct functions on specific platelet responses, including Ca2+ signaling[4,67,68]. In particular, while PKCα and PKCβ positively regulate Ca2+ signaling, PKCθ negatively regulates Ca2+ signaling and thereby inhibits phosphatidylserine exposure and platelet pro-coagulant activity[68]. The PKC targets mediating these effects are not entirely clear. In GPVI stimulated platelets PKCθ inhibits store-independent Ca2+ entry[69] and a similar mechanism could be in place downstream of the thrombin receptor PAR1[66]. In addition, a recent study employing pharmacological inhibitors suggests that PKC may be accelerating the removal of Ca2+ from the cytosol through the sarco-endoplasmic reticulum Ca2+-ATPases (SERCA) and the Na+/Ca2+ exchangers[70].
Checkpoints at the level of RAP1 activation
A central signaling node controlling integrin activation and platelet adhesiveness is at the level of the small GTPase RAP1 (Figure 2). Our ongoing studies demonstrate that concomitant deletion of the two most abundant RAP isoforms expressed in platelets[11], RAP1A and RAP1B, impairs integrin activation almost completely and inhibits in vivo thrombus formation in a manner almost comparable to that of conditional TALIN1-deficient mice (unpublished observations). The activation state of RAP1 proteins is tightly regulated by two kinetically-distinct pathways and directly correlates with platelet adhesiveness[7]. Rapid and reversible RAP1 activation is dependent on the Ca2+-regulated RAP-GEF, CALDAG-GEFI (RASGRP2), which is critical for the initial accumulation of platelets at the site of injury. The sustained activation of RAP1, required to achieve stable platelet adhesion and formation of shear-resistant thrombi, depends on the secretion of ADP from storage granules and signaling through the Gi-coupled receptor P2Y12[71].
The main inhibitor of RAP1 is the GTPase-activating protein RASA3[72]. RASA3 is catalytically active in circulating platelets. It is strategically positioned at the plasma membrane, where RAP1-GTP recruits TALIN to activate the integrins, in order to avoid any spurious RAP1 activation and maintain platelets in a quiescent/non-adhesive state. Consistently, genetic ablation of Rasa3 in mice results in platelet pre-activation and a virtually complete thrombocytopenia due to increased platelet clearance. Transgenic mice expressing a Rasa3 variant with a missense mutation (H794L) are also strongly thrombocytopenic (~95% platelet count reduction), but have enough platelets to survive through development. Their residual circulating platelets exhibit increased activation of RAP1 and αIIbβ3 integrin in unstimulated and agonist-stimulated conditions. The platelet hyper-reactivity, the elevated clearance rate and the consequent thrombocytopenia are almost completely reversed by crossing the RASA3 mutant mice with mice lacking CALDAG-GEFI, confirming that RASA3 is an important negative regulator of RAP1 activation in circulating platelets. In addition, RASA3 is also necessary to inhibit sustained platelet adhesion at sites of injury to avoid the risk of excessive platelet accumulation. Consequently, lack of functional RASA3 enhances agonist-induced integrin activation and leads to the formation of more stable thrombi in less time. However, to allow sustained RAP1 activation required for hemostatic plug formation, the inhibitory signal of RASA3 needs to be temporally quenched in proximity of the injury. To do so platelets locally release ADP, a short-lived agonist that inhibits RASA3 through stimulation of the P2Y12 receptor and PI3K-mediated generation of PIP3. Furthermore, integrin engagement itself promotes the perpetuation of RAP1 activation by inducing RASA3 inhibition in a PI3K-dependent manner[73] (see below for details). Consistent with this, RASA3 mutant platelets are insensitive to P2Y12 and PI3K inhibitors both in vivo and in vitro[72].
In addition to the direct inhibition mediated by RASA3, RAP1 function is regulated by the endothelial-derived platelet antagonists at many levels. Two independent studies demonstrated that PKA phosphorylates the RAP activator CALDAG-GEFI on Serine587, Serine116 and Serine117 and thereby prevents Ca2+/CALDAG-GEFI-dependent RAP1 activation[74,75]. A recent proteomic study confirmed the Serine587 phosphorylation site and showed that it is inversely regulated by Iloprost and ADP[76]. Another important target of PKA and PKG is RAP1GAP2 [77], a GTPase activating protein expressed in human (but not in mouse) platelets in relatively low amounts. In agonist-stimulated platelets RAP1GAP2 is phosphorylated on Serine9 and binds 14-3-3 which inhibits its function. In PGI2/NO-stimulated platelets, PKA/PKG phosphorylate Serine7 and induce the dissociation of 14-3-3, therefore allowing the GAP activity and contributing to RAP1 inhibition[78]. Notably, PKA also phosphorylates RAP1B itself[79] on Serine179. The phosphorylation does not affect RAP1B GTP-loading and has a slower kinetics compared to the kinetics of RAP1 activation[80]. However, studies in platelets and in other cells support the notion that this modification induces the translocation of RAP1 from the plasma membrane to the cytosol, thereby separating RAP1 from the integrin receptors[79,81].
Checkpoints at the level of integrin outside-in signaling
Ligand binding to activated integrins induces a cascade of signaling events, collectively known as outside-in signaling, which promote the amplification of platelet activation and mediate cytoskeletal changes required for spreading, clot retraction and ultimately thrombus stabilization (for review see [12]). Thus, also this process must be negatively regulated to avoid excessive thrombus growth and vessel occlusion (Figure 2).
Integrin outside-in signaling is initiated by c-SRC and, like ITAM signaling, relies on the sequential activation of multiple protein and lipid kinases. An important protein phosphatase that counteracts the kinases downstream of integrin αIIbβ3 is PP2A. PP2A associates with integrin αIIbβ3 in resting platelets and its phosphatase activity negatively regulates integrin-dependent adhesion by inhibiting ERK signaling. Upon integrin engagement, the PP2A activity associated to the integrin is reduced and integrin-mediated ERK signaling is unleashed[82]. Interestingly, the lipid phosphatase SHIP1, which has a positive role during agonist-induced platelet activation[83], is activated by c-SRC-mediated phosphorylation and acts as a negative regulator of outside-in signaling. When activated SHIP1 downregulates the stability of integrin αIIbβ3-fibrinogen adhesive bonds, leading to a decrease in the proportion of platelets forming shear-resistant adhesion contacts[84]. SHIP1 dephosphorylates PIP3 (see above), a lipid messenger generated during outside-in signaling. A recent study demonstrated that the activity of the RAP1-GAP, RASA3, is downregulated in a PI3K/PIP3-dependent manner during integrin outside-in signaling[73]. Thus, the inhibitory effect of SHIP1 may be due to its ability to prevent RASA3 inactivation required for sustained RAP1 signaling.
One of the best characterized negative regulators of outside-in signaling is the Junctional adhesion molecule-A (JAM/A). JAM/A is an adhesion molecule of the cortical thymocyte marker of the Xenopus (CTX) family that in epithelial and endothelial cells functions as a tight junction protein. In resting platelets, JAM/A is tyrosine phosphorylated and associates with integrin αIIbβ3 and with the inhibitory kinase CSK. Because of JAM/A, CSK is strategically positioned to inhibit β3-bound c-SRC and prevent the initiation of outside-in signaling[85]. Upon platelet stimulation, dephosphorylation of JAM/A determines the dissociation of CSK from the integrin/c-SRC complex and allows c-SRC to activate and trigger outside-in signaling[85]. Genetic ablation of Jam/A in mice results in a pro-thrombotic phenotype and a shortened bleeding time in vivo. Consistent with its role in outside-in signaling, integrin activation, granule secretion and TxA2 generation are normal, but spreading and clot retraction are enhanced in Jam/A mutant mice[86]. ESAM, another member of the CTX family of adhesion molecules, also contributes to outside-in signaling. Similar to Jam/A knockout mice, genetic deletion of Esam leads to enhanced thrombus formation and more stable hemostatic plugs in vivo[87]. Surprisingly, however, clot retraction is delayed in Esam−/− mice, suggesting that JAM/A and ESAM may have different regulatory functions. In fact, differently from JAM/A, ESAM is localized in α granules in resting platelets and translocates to the platelet surface only after agonist-induced secretion[87]. However, the exact mechanisms by which ESAM deficiency leads to a prothrombotic phenotype are not clear.
Another class of proteins implicated in the negative regulation of outside-in signaling are the adaptor proteins of the DOK family. Platelets express DOK1-3, which are phosphorylated upon platelet spreading on fibrinogen[88]. Both DOK1 and DOK2 have been shown to bind integrin αIIbβ3 and negatively regulate outside-in signaling. Dok1 deficiency in mice results in normal inside-out signaling responses but increased spreading and clot retraction, which result in shortened bleeding times and accelerated thrombus formation[89]. Similarly, Dok2 deficient mice form larger and more stable thrombi in less time. From the more detailed analysis of these mice it appears that the increased adhesiveness of Dok2−/− platelets is not due to increased αIIbβ3 affinity but is associated with increased αIIbβ3 bond stability in conditions of shear stress[90]. DOK proteins contain a phospho-tyrosine binding domain that can bind the NPXY motives of integrins and a proline- and tyrosine-rich carboxyl-terminal region, that can support the binding with SH2 and SH3-domain containing proteins. Thus, their inhibitory function could be mediated by interfering with the signaling of c-SRC-SYK-PLCγ2 or by directly competing with TALIN or KINDLIN3 for the binding to the integrin NPXY motif.
Although significant progress has been made in understanding outside-in signaling, it remains to be understood how these inhibitory mechanisms integrate with each other and it is still unclear how molecules that supposedly interact directly with the integrin like JAM/A can control integrins that are much more abundant (13,300 copies of JAM/A vs 80,000 copies of αIIbβ3[11]).
Conclusions
Extensive studies in transgenic mice and other model systems established a number of key signaling molecules, both inhibitory or activatory, which control platelet adhesiveness in circulation and at sites of vascular injury. Dysregulation of their antagonistic balance can cause (1) impaired platelet activation and bleeding, or (2) unwanted activation and clearance of platelets and/or an increased risk of thrombosis. In humans, we have identified patients with bleeding complications due to variants in several of these activatory signaling molecules[91]. Less is known about mutations in negative regulators of platelet activation. One example is a common polymorphism of the β3 integrin (PlA2 Proline33/Leucine33 PlA1) associated with coronary events, arterial thrombosis and sudden cardiac death, which correlates with increased PP2A activity and reduced ERK signaling when expressed in CHO cells[92]. Moreover, a recent study demonstrated that a nonsense mutation at the codon for residue Cysteine108 of human G6b-B underlies a severe autosomal recessive thrombocytopenia associated with splenomegaly, an increased number of megakaryocytes, and fibrosis in bone marrow biopsies[93]. Unfortunately, studies on the lifespan of platelets in circulation are more difficult to be performed in humans, which is why these thrombocytopenias are often explained by impaired platelet production rather than a defect in platelet survival. This shortcoming should be addressed in future studies.
Acknowledgments
This work was supported by the American Heart Association (14EIA18910004) and NIH grants HL121650 and HL130404 (W.B.) and the Young Researchers Program Rita Levi Montalcini (L.S.).
Footnotes
Disclosure
W. Bergmeier reports grants from Merck outside the submitted work.
Authors report no conflicts of interests.
References
- 1.Aarts PA, van den Broek SA, Prins GW, Kuiken GD, Sixma JJ, Heethaar RM. Blood platelets are concentrated near the wall and red blood cells, in the center in flowing blood. Arteriosclerosis. 1988;8:819–24. doi: 10.1161/01.atv.8.6.819. [DOI] [PubMed] [Google Scholar]
- 2.Ruggeri ZM. Platelet adhesion under flow. Microcirculation. 2009;16:58–83. doi: 10.1080/10739680802651477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmaier AA, Zou Z, Kazlauskas A, Emert-Sedlak L, Fong KP, Neeves KB, Maloney SF, Diamond SL, Kunapuli SP, Ware J, Brass LF, Smithgall TE, Saksela K, Kahn ML. Molecular priming of Lyn by GPVI enables an immune receptor to adopt a hemostatic role. Proc Natl Acad Sci USA. 2009;106:21167–72. doi: 10.1073/pnas.0906436106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heemskerk JWM, Harper MT, Cosemans JMEM, Poole AW. Unravelling the different functions of protein kinase C isoforms in platelets. FEBS Lett. 2011;585:1711–6. doi: 10.1016/j.febslet.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 5.Iwig JS, Vercoulen Y, Das R, Barros T, Limnander A, Che Y, Pelton JG, Wemmer DE, Roose JP, Kuriyan J. Structural analysis of autoinhibition in the Ras-specific exchange factor RasGRP1. eLife. 2013;2:e00813. doi: 10.7554/eLife.00813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crittenden JR, Bergmeier W, Zhang Y, Piffath CL, Liang Y, Wagner DD, Housman DE, Graybiel AM. CalDAG-GEFI integrates signaling for platelet aggregation and thrombus formation. Nat Med. 2004;10:982–6. doi: 10.1038/nm1098. [DOI] [PubMed] [Google Scholar]
- 7.Stolla M, Stefanini L, Roden RC, Chavez M, Hirsch J, Greene T, Ouellette TD, Maloney SF, Diamond SL, Poncz M, Woulfe DS, Bergmeier W. The kinetics of αIIbβ3 activation determines the size and stability of thrombi in mice: implications for antiplatelet therapy. Blood. 2011;117:1005–13. doi: 10.1182/blood-2010-07-297713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cifuni SM, Wagner DD, Bergmeier W. CalDAG-GEFI and protein kinase C represent alternative pathways leading to activation of integrin alphaIIbbeta3 in platelets. Blood. 2008;112:1696–703. doi: 10.1182/blood-2008-02-139733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kato H, Nakazawa Y, Kurokawa Y, Kashiwagi H, Morikawa Y, Morita D, Banno F, Honda S, Kanakura Y, Tomiyama Y. Human CalDAG-GEFI deficiency increases bleeding and delays αIIbβ3 activation. Blood. 2016;128:2729–33. doi: 10.1182/blood-2016-03-704825. [DOI] [PubMed] [Google Scholar]
- 10.Han J, Lim CJ, Watanabe N, Soriani A, Ratnikov B, Calderwood DA, Puzon-McLaughlin W, Lafuente EM, Boussiotis VA, Shattil SJ, Ginsberg MH. Reconstructing and deconstructing agonist-induced activation of integrin alphaIIbbeta3. Current Biology. 2006;16:1796–806. doi: 10.1016/j.cub.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 11.Burkhart JM, Vaudel M, Gambaryan S, Radau S, Walter U, Martens L, Geiger J, Sickmann A, Zahedi RP. The first comprehensive and quantitative analysis of human platelet protein composition allows the comparative analysis of structural and functional pathways. Blood. 2012;120:e73–82. doi: 10.1182/blood-2012-04-416594. [DOI] [PubMed] [Google Scholar]
- 12.Durrant TN, van den Bosch MT, Hers I. Integrin αIIbβ3 outside-in signaling. Blood. 2017 doi: 10.1182/blood-2017-03-773614. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Geet C, Izzi B, Labarque V, Freson K. Human platelet pathology related to defects in the G-protein signaling cascade. J Thromb Haemost. 2009;7(Suppl 1):282–6. doi: 10.1111/j.1538-7836.2009.03399.x. [DOI] [PubMed] [Google Scholar]
- 14.Dangel O, Mergia E, Karlisch K, Groneberg D, Koesling D, Friebe A. Nitric oxide-sensitive guanylyl cyclase is the only nitric oxide receptor mediating platelet inhibition. J Thromb Haemost. 2010;8:1343–52. doi: 10.1111/j.1538-7836.2010.03806.x. [DOI] [PubMed] [Google Scholar]
- 15.Walsh MT, Kinsella BT. Regulation of the human prostanoid TPalpha and TPbeta receptor isoforms mediated through activation of the EP(1) and IP receptors. British Journal of Pharmacology. 2000;131:601–9. doi: 10.1038/sj.bjp.0703624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beck F, Geiger J, Gambaryan S, Veit J, Vaudel M, Nollau P, Kohlbacher O, Martens L, Walter U, Sickmann A, Zahedi RP. Time-resolved characterization of cAMP/PKA-dependent signaling reveals that platelet inhibition is a concerted process involving multiple signaling pathways. Blood. 2014;123:e1–e10. doi: 10.1182/blood-2013-07-512384. [DOI] [PubMed] [Google Scholar]
- 17.Smolenski A. Novel roles of cAMP/cGMP-dependent signaling in platelets. J Thromb Haemost. 2012;10:167–76. doi: 10.1111/j.1538-7836.2011.04576.x. [DOI] [PubMed] [Google Scholar]
- 18.Patil S, Newman DK, Newman PJ. Platelet endothelial cell adhesion molecule-1 serves as an inhibitory receptor that modulates platelet responses to collagen. Blood. 2001;97:1727–32. doi: 10.1182/blood.v97.6.1727. [DOI] [PubMed] [Google Scholar]
- 19.Hu M, Zhang H, Liu Q, Hao Q. Structural Basis for Human PECAM-1-Mediated Trans-homophilic Cell Adhesion. Sci Rep. 2016;6:38655. doi: 10.1038/srep38655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paddock C, Zhou D, Lertkiatmongkol P, Newman PJ, Zhu J. Structural basis for PECAM-1 homophilic binding. Blood. 2016;127:1052–61. doi: 10.1182/blood-2015-07-660092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Falati S, Patil S, Gross PL, Stapleton M, Merrill-Skoloff G, Barrett NE, Pixton KL, Weiler H, Cooley B, Newman DK, Newman PJ, Furie BC, Furie B, Gibbins JM. Platelet PECAM-1 inhibits thrombus formation in vivo. Blood. 2006;107:535–41. doi: 10.1182/blood-2005-04-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahooti S, Graesser D, Patil S, Newman P, Duncan G, Mak T, Madri JA. PECAM-1 (CD31) Expression Modulates Bleeding Time in Vivo. The American Journal of Pathology. 2010;157:75–81. doi: 10.1016/S0002-9440(10)64519-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quek LS, Pasquet JM, Hers I, Cornall R, Knight G, Barnes M, Hibbs ML, Dunn AR, Lowell CA, Watson SP. Fyn and Lyn phosphorylate the Fc receptor gamma chain downstream of glycoprotein VI in murine platelets, and Lyn regulates a novel feedback pathway. Blood. 2000;96:4246–53. [PubMed] [Google Scholar]
- 24.Ming Z, Ming Z, Hu Y, Hu Y, Xiang J, Xiang J, Polewski P, Polewski P, Newman PJ, Newman DK. Lyn and PECAM-1 function as interdependent inhibitors of platelet aggregation. Blood. 2011;117:3903–6. doi: 10.1182/blood-2010-09-304816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paddock C, Lytle BL, Peterson FC, Holyst T, Newman PJ, Volkman BF, Newman DK. Residues within a lipid-associated segment of the PECAM-1 cytoplasmic domain are susceptible to inducible, sequential phosphorylation. Blood. 2011;117:6012–23. doi: 10.1182/blood-2010-11-317867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tourdot BE, Brenner MK, Keough KC, Holyst T, Newman PJ, Newman DK. Immunoreceptor tyrosine-based inhibitory motif (ITIM)-mediated inhibitory signaling is regulated by sequential phosphorylation mediated by distinct nonreceptor tyrosine kinases: a case study involving PECAM-1. Biochemistry. 2013;52:2597–608. doi: 10.1021/bi301461t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moraes LA, Barrett NE, Jones CI, Holbrook LM, Spyridon M, Sage T, Newman DK, Gibbins JM. Platelet endothelial cell adhesion molecule-1 regulates collagen-stimulated platelet function by modulating the association of phosphatidylinositol 3-kinase with Grb-2-associated binding protein-1 and linker for activation of T cells. J Thromb Haemost. 2010;8:2530–41. doi: 10.1111/j.1538-7836.2010.04025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Senis YA. Protein-tyrosine phosphatases: a new frontier in platelet signal transduction. J Thromb Haemost. 2013;11:1800–13. doi: 10.1111/jth.12359. [DOI] [PubMed] [Google Scholar]
- 29.Jones CI, Garner SF, Moraes LA, Kaiser WJ, Rankin A, Ouwehand WH, Goodall AH, Gibbins JM Consortium B. PECAM-1 expression and activity negatively regulate multiple platelet signaling pathways. FEBS Lett. 2009;583:3618–24. doi: 10.1016/j.febslet.2009.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rathore V, Stapleton MA, Hillery CA, Montgomery RR, Nichols TC, Merricks EP, Newman DK, Newman PJ. PECAM-1 negatively regulates GPIb/V/IX signaling in murine platelets. Blood. 2003;102:3658–64. doi: 10.1182/blood-2003-06-1888. [DOI] [PubMed] [Google Scholar]
- 31.Crockett J, Newman DK, Newman PJ. PECAM-1 functions as a negative regulator of laminin-induced platelet activation. J Thromb Haemost. 2010;8:1584–93. doi: 10.1111/j.1538-7836.2010.03883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones CI, Sage T, Moraes LA, Vaiyapuri S, Hussain U, Tucker KL, Barrett NE, Gibbins JM. Platelet endothelial cell adhesion molecule-1 inhibits platelet response to thrombin and von Willebrand factor by regulating the internalization of glycoprotein Ib via AKT/glycogen synthase kinase-3/dynamin and integrin αIIbβ3. Arterioscler Thromb Vasc Biol. 2014;34:1968–76. doi: 10.1161/ATVBAHA.114.304097. [DOI] [PubMed] [Google Scholar]
- 33.Macaulay IC, Tijssen MR, Thijssen-Timmer DC, Gusnanto A, Steward M, Burns P, Langford CF, Ellis PD, Dudbridge F, Zwaginga J-J, Watkins NA, van der Schoot CE, Ouwehand WH. Comparative gene expression profiling of in vitro differentiated megakaryocytes and erythroblasts identifies novel activatory and inhibitory platelet membrane proteins. Blood. 2007;109:3260–9. doi: 10.1182/blood-2006-07-036269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Senis YA, Tomlinson MG, García Á, Dumon S, Heath VL, Herbert J, Cobbold SP, Spalton JC, Ayman S, Antrobus R, Zitzmann N, Bicknell R, Frampton J, Authi KS, Martin A, Wakelam MJO, Watson SP. A Comprehensive Proteomics and Genomics Analysis Reveals Novel Transmembrane Proteins in Human Platelets and Mouse Megakaryocytes Including G6b-B, a Novel Immunoreceptor Tyrosine-based Inhibitory Motif Protein. Mol Cell Proteomics. 2007;6:548–64. doi: 10.1074/mcp.D600007-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mazharian A, Wang Y-J, Mori J, Bem D, Finney B, Heising S, Gissen P, White JG, Berndt MC, Gardiner EE, Nieswandt B, Douglas MR, Campbell RD, Watson SP, Senis YA. Mice lacking the ITIM-containing receptor G6b-B exhibit macrothrombocytopenia and aberrant platelet function. Science Signaling. 2012;5(248):ra78. doi: 10.1126/scisignal.2002936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mori J, Pearce AC, Spalton JC, Grygielska B, Eble JA, Tomlinson MG, Senis YA, Watson SP. G6b-B inhibits constitutive and agonist-induced signaling by glycoprotein VI and CLEC-2. J Biol Chem. 2008;283:35419–27. doi: 10.1074/jbc.M806895200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mazharian A, Mori J, Wang Y-J, Heising S, Neel BG, Watson SP, Senis YA. Megakaryocyte-specific deletion of the protein-tyrosine phosphatases Shp1 and Shp2 causes abnormal megakaryocyte development, platelet production, and function. Blood. 2013;121:4205–20. doi: 10.1182/blood-2012-08-449272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Newland SA, Macaulay IC, Floto AR, de Vet EC, Ouwehand WH, Watkins NA, Lyons PA, Campbell DR. The novel inhibitory receptor G6B is expressed on the surface of platelets and attenuates platelet function in vitro. Blood. 2007;109:4806–9. doi: 10.1182/blood-2006-09-047449. [DOI] [PubMed] [Google Scholar]
- 39.Wong C, Liu Y, Yip J, Chand R, Wee JL, Oates L, Nieswandt B, Reheman A, Ni H, Beauchemin N, Jackson DE. CEACAM1 negatively regulates platelet-collagen interactions and thrombus growth in vitro and in vivo. Blood. 2009;113:1818–28. doi: 10.1182/blood-2008-06-165043. [DOI] [PubMed] [Google Scholar]
- 40.Alshahrani MM, Yang E, Yip J, Ghanem SS, Abdallah SL, deAngelis AM, O’Malley CJ, Moheimani F, Najjar SM, Jackson DE. CEACAM2 negatively regulates hemi (ITAM-bearing) GPVI and CLEC-2 pathways and thrombus growth in vitro and in vivo. Blood. 2014;124:2431–41. doi: 10.1182/blood-2014-04-569707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fan X, Shi P, Dai J, Lu Y, Chen X, Liu X, Zhang K, Wu X, Sun Y, Wang K, Zhu L, Zhang C-C, Zhang J, Chen G-Q, Zheng J, Liu J. Paired immunoglobulin-like receptor B regulates platelet activation. Blood. 2014;124:2421–30. doi: 10.1182/blood-2014-03-557645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coxon CH, Geer MJ, Senis YA. ITIM receptors: more than just inhibitors of platelet activation. Blood. 2017;129:3407–18. doi: 10.1182/blood-2016-12-720185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Washington AV, Gibot S, Acevedo I, Gattis J, Quigley L, Feltz R, La Mota De A, Schubert RL, Gomez-Rodriguez J, Cheng J, Dutra A, Pak E, Chertov O, Rivera L, Morales J, Lubkowski J, Hunter R, Schwartzberg PL, McVicar DW. TREM-like transcript-1 protects against inflammation-associated hemorrhage by facilitating platelet aggregation in mice and humans. J Clin Invest. 2009;119:1489–501. doi: 10.1172/JCI36175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wee JL, Jackson DE. The Ig-ITIM superfamily member PECAM-1 regulates the “outside-in” signaling properties of integrin alpha(IIb)beta3 in platelets. Blood. 2005;106:3816–23. doi: 10.1182/blood-2005-03-0911. [DOI] [PubMed] [Google Scholar]
- 45.Yip J, Alshahrani M, Beauchemin N, Jackson DE. CEACAM1 regulates integrin αIIb β3-mediated functions in platelets. Platelets. 2015;27:168–77. doi: 10.3109/09537104.2015.1064102. [DOI] [PubMed] [Google Scholar]
- 46.Alshahrani MM, Kyriacou RP, O’Malley CJ, Heinrich G, Najjar SM, Jackson DE. CEACAM2 positively regulates integrin αIIbβ3-mediated platelet functions. Platelets. 2016;27:743–50. doi: 10.3109/09537104.2016.1171834. [DOI] [PubMed] [Google Scholar]
- 47.Gardiner EE, Andrews RK. Transfusion Medicine Reviews. Vol. 28. Elsevier Inc; 2014. Platelet Receptor Expression and Shedding: Glycoprotein Ib-IX-V and Glycoprotein VI; pp. 56–60. [DOI] [PubMed] [Google Scholar]
- 48.Signarvic RS, Cierniewska A, Stalker TJ, Fong KP, Chatterjee MS, Hess PR, Ma P, Diamond SL, Neubig RR, Brass LF. RGS/Gi2alpha interactions modulate platelet accumulation and thrombus formation at sites of vascular injury. Blood. 2010;116:6092–100. doi: 10.1182/blood-2010-05-283846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nagata Y, Oda M, Nakata H, Shozaki Y, Kozasa T, Todokoro K. A novel regulator of G-protein signaling bearing GAP activity for Galphai and Galphaq in megakaryocytes. Blood. 2001;97:3051–60. doi: 10.1182/blood.v97.10.3051. [DOI] [PubMed] [Google Scholar]
- 50.Gagnon AW, Murray DL, Leadley RJ. Cloning and characterization of a novel regulator of G protein signalling in human platelets. Cell Signal. 2002;14:595–606. doi: 10.1016/s0898-6568(02)00012-8. [DOI] [PubMed] [Google Scholar]
- 51.Noé L, Di Michele M, Giets E, Thys C, Wittevrongel C, De Vos R, Overbergh L, Waelkens E, Jaeken J, Van Geet C, Freson K. Platelet Gs hypofunction and abnormal morphology resulting from a heterozygous RGS2 mutation. J Thromb Haemost. 2010;8:1594–603. doi: 10.1111/j.1538-7836.2010.03885.x. [DOI] [PubMed] [Google Scholar]
- 52.Karim ZA, Alshbool FZ, Vemana HP, Conlon C, Druey KM, Khasawneh FT. CXCL12 regulates platelet activation via the regulator of G-protein signaling 16. Biochim Biophys Acta. 2016;1863:314–21. doi: 10.1016/j.bbamcr.2015.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hensch NR, Karim ZA, Druey KM, Tansey MG, Khasawneh FT. RGS10 Negatively Regulates Platelet Activation and Thrombogenesis. In: Schulz C, editor. PLoS ONE. Vol. 11. 2016. p. e0165984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Delesque-Touchard N, Pendaries C, Volle-Challier C, Millet L, Salel V, Hervé C, Pflieger A-M, Berthou-Soulie L, Prades C, Sorg T, Herbert J-M, Savi P, Bono F. Regulator of G-protein signaling 18 controls both platelet generation and function. In: Cox D, editor. PLoS ONE. Vol. 9. 2014. p. e113215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alshbool FZ, Karim ZA, Vemana HP, Conlon C, Lin OA, Khasawneh FT. The regulator of G-protein signaling 18 regulates platelet aggregation, hemostasis and thrombosis. Biochem Biophys Res Commun. 2015;462:378–82. doi: 10.1016/j.bbrc.2015.04.143. [DOI] [PubMed] [Google Scholar]
- 56.Ma P, Cierniewska A, Signarvic R, Cieslak M, Kong H, Sinnamon AJ, Neubig RR, Newman DK, Stalker TJ, Brass LF. A newly identified complex of spinophilin and the tyrosine phosphatase, SHP-1, modulates platelet activation by regulating G protein-dependent signaling. Blood. 2012;119:1935–45. doi: 10.1182/blood-2011-10-387910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ma P, Ou K, Sinnamon AJ, Jiang H, Siderovski DP, Brass LF. Modulating platelet reactivity through control of RGS18 availability. Blood. 2015;126:2611–20. doi: 10.1182/blood-2015-04-640037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gegenbauer K, Elia G, Blanco-Fernandez A, Smolenski A. Regulator of G-protein signaling 18 integrates activating and inhibitory signaling in platelets. Blood. 2012;119:3799–807. doi: 10.1182/blood-2011-11-390369. [DOI] [PubMed] [Google Scholar]
- 59.Gegenbauer K, Nagy Z, Smolenski A. Cyclic nucleotide dependent dephosphorylation of regulator of G-protein signaling 18 in human platelets. In: Freson K, editor. PLoS ONE. Vol. 8. 2013. p. e80251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hardy AR, Conley PB, Luo J, Benovic JL, Poole AW, Mundell SJ. P2Y1 and P2Y12 receptors for ADP desensitize by distinct kinase-dependent mechanisms. Blood. 2005;105:3552–60. doi: 10.1182/blood-2004-07-2893. [DOI] [PubMed] [Google Scholar]
- 61.Shapiro MJ, Weiss EJ, Faruqi TR, Coughlin SR. Protease-activated receptors 1 and 4 are shut off with distinct kinetics after activation by thrombin. J Biol Chem. 2000;275:25216–21. doi: 10.1074/jbc.M004589200. [DOI] [PubMed] [Google Scholar]
- 62.Dean WL, Chen D, Brandt PC, Vanaman TC. Regulation of platelet plasma membrane Ca2+-ATPase by cAMP-dependent and tyrosine phosphorylation. J Biol Chem. 1997;272:15113–9. doi: 10.1074/jbc.272.24.15113. [DOI] [PubMed] [Google Scholar]
- 63.Cavallini L, Coassin M, Borean A, Alexandre A. Prostacyclin and sodium nitroprusside inhibit the activity of the platelet inositol 1,4,5-trisphosphate receptor and promote its phosphorylation. J Biol Chem. 1996;271:5545–51. doi: 10.1074/jbc.271.10.5545. [DOI] [PubMed] [Google Scholar]
- 64.Antl M, Brühl von M-L, Eiglsperger C, Werner M, Konrad I, Kocher T, Wilm M, Hofmann F, Massberg S, Schlossmann J. IRAG mediates NO/cGMP-dependent inhibition of platelet aggregation and thrombus formation. Blood. 2007;109:552–9. doi: 10.1182/blood-2005-10-026294. [DOI] [PubMed] [Google Scholar]
- 65.Schinner E, Salb K, Schlossmann J. Signaling via IRAG is essential for NO/cGMP-dependent inhibition of platelet activation. Platelets. 2011;22:217–27. doi: 10.3109/09537104.2010.544151. [DOI] [PubMed] [Google Scholar]
- 66.Harper MT, Poole AW. PKC inhibition markedly enhances Ca2+ signaling and phosphatidylserine exposure downstream of protease-activated receptor-1 but not protease-activated receptor-4 in human platelets. J Thromb Haemost. 2011;9:1599–607. doi: 10.1111/j.1538-7836.2011.04393.x. [DOI] [PubMed] [Google Scholar]
- 67.Strehl A, Munnix ICA, Kuijpers MJE, van der Meijden PEJ, Cosemans JMEM, Feijge MAH, Nieswandt B, Heemskerk JWM. Dual role of platelet protein kinase C in thrombus formation: stimulation of pro-aggregatory and suppression of procoagulant activity in platelets. J Biol Chem. 2007;282:7046–55. doi: 10.1074/jbc.M611367200. [DOI] [PubMed] [Google Scholar]
- 68.Gilio K, Harper MT, Cosemans JMEM, Konopatskaya O, Munnix ICA, Prinzen L, Leitges M, Liu Q, Molkentin JD, Heemskerk JWM, Poole AW. Functional divergence of platelet protein kinase C (PKC) isoforms in thrombus formation on collagen. J Biol Chem. 2010;285:23410–9. doi: 10.1074/jbc.M110.136176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Harper MT, Poole AW. Protein kinase Ctheta negatively regulates store-independent Ca2+ entry and phosphatidylserine exposure downstream of glycoprotein VI in platelets. J Biol Chem. 2010;285:19865–73. doi: 10.1074/jbc.M109.085654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lever RA, Hussain A, Sun BB, Sage SO, Harper AGS. Conventional protein kinase C isoforms differentially regulate ADP- and thrombin-evoked Ca2+ signalling in human platelets. Cell Calcium. 2015;58:577–88. doi: 10.1016/j.ceca.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 71.Stefanini L, Bergmeier W. RAP1-GTPase signaling and platelet function. J Mol Med. 2016;94:13–9. doi: 10.1007/s00109-015-1346-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stefanini L, Paul DS, Robledo RF, Chan ER, Getz TM, Campbell RA, Kechele DO, Casari C, Piatt R, Caron KM, Mackman N, Weyrich AS, Parrott MC, Boulaftali Y, Adams MD, Peters LL, Bergmeier W. RASA3 is a critical inhibitor of RAP1-dependent platelet activation. J Clin Invest. 2015;125:1419–32. doi: 10.1172/JCI77993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Battram AM, Durrant TN, Agbani EO, Heesom KJ, Paul DS, Piatt R, Poole AW, Cullen PJ, Bergmeier W, Moore SF, Hers I. The Phosphatidylinositol 3,4,5-trisphosphate (PI(3,4,5)P3) Binder Rasa3 Regulates Phosphoinositide 3-kinase (PI3K)-dependent Integrin αIIbβ3 Outside-in Signaling. J Biol Chem. 2017;292:1691–704. doi: 10.1074/jbc.M116.746867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Subramanian H, Zahedi RP, Sickmann A, Walter U, Gambaryan S. Phosphorylation of CalDAG-GEFI by protein kinase A prevents Rap1b activation. J Thromb Haemost. 2013;11:1574–82. doi: 10.1111/jth.12271. [DOI] [PubMed] [Google Scholar]
- 75.Guidetti GF, Manganaro D, Consonni A, Canobbio I, Balduini C, Torti M. Phosphorylation of the guanine-nucleotide-exchange factor CalDAG-GEFI by protein kinase A regulates Ca 2+-dependent activation of platelet Rap1b GTPase. Biochem J. 2013;453:115–23. doi: 10.1042/BJ20130131. [DOI] [PubMed] [Google Scholar]
- 76.Beck F, Geiger J, Gambaryan S, Solari FA, Dell’Aica M, Loroch S, Mattheij NJ, Mindukshev I, Pötz O, Jurk K, Burkhart JM, Fufezan C, Heemskerk JWM, Walter U, Zahedi RP, Sickmann A. Temporal quantitative phosphoproteomics of ADP stimulation reveals novel central nodes in platelet activation and inhibition. Blood. 2017;129:e1–e12. doi: 10.1182/blood-2016-05-714048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schultess J, Danielewski O, Smolenski AP. Rap1GAP2 is a new GTPase-activating protein of Rap1 expressed in human platelets. Blood. 2005;105:3185–92. doi: 10.1182/blood-2004-09-3605. [DOI] [PubMed] [Google Scholar]
- 78.Hoffmeister M, Riha P, Neumüller O, Danielewski O, Schultess J, Smolenski AP. Cyclic Nucleotide-dependent Protein Kinases Inhibit Binding of 14-3-3 to the GTPase-activating Protein Rap1GAP2 in Platelets. J Biol Chem. 2008;283:2297–306. doi: 10.1074/jbc.M706825200. [DOI] [PubMed] [Google Scholar]
- 79.Lapetina EG, Lacal JC, Reep BR, Molina y Vedia L. A ras-related protein is phosphorylated and translocated by agonists that increase cAMP levels in human platelets. Proc Natl Acad Sci USA. 1989;86:3131–4. doi: 10.1073/pnas.86.9.3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kawata M, Kawata M, Kikuchi A, Kikuchi A, Hoshijima M, Hoshijima M, Yamamoto K, Yamamoto K, Hashimoto E, Hashimoto E, Yamamura H, Yamamura H, Takai Y. Phosphorylation of smg p21, a ras p21-like GTP-binding protein, by cyclic AMP-dependent protein kinase in a cell-free system and in response to prostaglandin E1 in intact human platelets. J Biol Chem. 1989;264:15688–95. [PubMed] [Google Scholar]
- 81.Hata Y, Kaibuchi K, Kawamura S, Hiroyoshi M, Shirataki H, Takai Y. Enhancement of the actions of smg p21 GDP/GTP exchange protein by the protein kinase A-catalyzed phosphorylation of smg p21. J Biol Chem. 1991;266:6571–7. [PubMed] [Google Scholar]
- 82.Gushiken FC, Patel V, Liu Y, Pradhan S, Bergeron AL, Peng Y, Vijayan KV. Protein phosphatase 2A negatively regulates integrin alpha(IIb)beta(3) signaling. J Biol Chem. 2008;283:12862–9. doi: 10.1074/jbc.M708804200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Severin S, Gratacap M-P, Lenain N, Alvarez L, Hollande E, Penninger JM, Gachet C, Plantavid M, Payrastre B. Deficiency of Src homology 2 domain–containing inositol 5-phosphatase 1 affects platelet responses and thrombus growth. J Clin Invest. 2007;117:944–52. doi: 10.1172/JCI29967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Maxwell MJ, Yuan Y, Anderson KE, Hibbs ML, Salem HH, Jackson SP. SHIP1 and Lyn Kinase Negatively Regulate Integrin α IIbβ 3Signaling in Platelets. J Biol Chem. 2004;279:32196–204. doi: 10.1074/jbc.M400746200. [DOI] [PubMed] [Google Scholar]
- 85.Naik MU, Caplan JL, Naik UP. Junctional adhesion molecule-A suppresses platelet integrin αIIbβ3 signaling by recruiting Csk to the integrin-c-Src complex. Blood. 2014;123:1393–402. doi: 10.1182/blood-2013-04-496232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Naik MU, Stalker TJ, Brass LF, Naik UP. JAM-A protects from thrombosis by suppressing integrin αIIbβ3-dependent outside-in signaling in platelets. Blood. 2012;119:3352–60. doi: 10.1182/blood-2011-12-397398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stalker TJ, Wu J, Morgans A, Traxler EA, Wang L, Chatterjee MS, Lee D, Quertermous T, Hall RA, Hammer DA, Diamond SL, Brass LF. Endothelial cell specific adhesion molecule (ESAM) localizes to platelet-platelet contacts and regulates thrombus formation in vivo. J Thromb Haemost. 2009;7:1886–96. doi: 10.1111/j.1538-7836.2009.03606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Senis YA, Antrobus R, Severin S, Parguina AF, Rosa I, Zitzmann N, Watson SP, Garcia A. Proteomic analysis of integrin alphaIIbbeta3 outside-in signaling reveals Src-kinase-independent phosphorylation of Dok-1 and Dok-3 leading to SHIP-1 interactions. J Thromb Haemost. 2009;7:1718–26. doi: 10.1111/j.1538-7836.2009.03565.x. [DOI] [PubMed] [Google Scholar]
- 89.Niki M, Nayak MK, Jin H, Bhasin N, Plow EF, Pandolfi PP, Rothman PB, Chauhan AK, Lentz SR. Dok-1 negatively regulates platelet integrin αIIbβ3 outside-in signalling and inhibits thrombosis in mice. Thromb Haemost. 2016;115:969–78. doi: 10.1160/TH15-05-0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hughan SC, Spring CM, Schoenwaelder SM, Sturgeon S, Alwis I, Yuan Y, McFadyen JD, Westein E, Goddard D, Ono A, Yamanashi Y, Nesbitt WS, Jackson SP. Dok-2 Adaptor Protein Regulates the Shear-dependent Adhesive Function of Platelet Integrin α IIbβ 3in Mice. J Biol Chem. 2014;289:5051–60. doi: 10.1074/jbc.M113.520148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nurden AT, Nurden P. Inherited disorders of platelet function: selected updates. J Thromb Haemost. 2015;13(Suppl 1):S2–9. doi: 10.1111/jth.12898. [DOI] [PubMed] [Google Scholar]
- 92.Wang H, Yan B, Satterwhite LL, Ma Q, Goldschmidt-Clermont PJ. Increased activity of phosphatase PP2A in the presence of the PlA2 polymorphism of αIIbβ3. Biochem Biophys Res Commun. 2008;367:72–7. doi: 10.1016/j.bbrc.2007.12.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Melhem M, Abu-Farha M, Antony D, Madhoun AA, Bacchelli C, Alkayal F, AlKhairi I, John S, Alomari M, Beales PL, Alsmadi O. Novel G6B gene variant causes familial autosomal recessive thrombocytopenia and anemia. Eur J Haematol. 2017;98:218–27. doi: 10.1111/ejh.12819. [DOI] [PubMed] [Google Scholar]