Abstract
Tuberculosis (TB) remains as a major threat to human health worldwide despite of the availability of standardized antibiotic therapy. One of the characteristic of pathogenic Mycobacterium tuberculosis (Mtb), the causative agent of tuberculosis is its ability to persist in the host in a dormant state and develop latent infection without clinical signs of active disease. However, the mechanisms involved in bacterial persistence and the establishment of latency is not well understood. Adipose tissue is emerging as an important niche that favors actively replicating as well as dormant Mtb during acute and latent infection. This also suggests that Mtb can disseminate from the lungs to adipose tissue during aerosol infection and/or from adipose tissue to lungs during reactivation of latent infection. In this study, we report the interplay between key adipokine levels and the dynamics of Mtb pathogenesis in the lungs and adipose tissue using a rabbit model of pulmonary infection with two clinical isolates that produce divergent outcome in disease progression. Results show that markers of adipocyte physiology and function were significantly altered during Mtb infection and distinct patterns of adipokine expression were noted between adipose tissue and the lungs. Moreover, these markers were differentially expressed between active disease and latent infection. Thus, this study highlights the importance of targeting adipocyte function as potential target for developing better TB intervention strategies.
Keywords: Tuberculosis, Latency, adipose tissue, adiponectin, inflammation
1. INTRODUCTION
Tuberculosis (TB), caused by Mycobacterium tuberculosis (Mtb), is a top infectious disease worldwide. According to the World Health Organization (WHO, 2016), 10.4 million people contracted TB (new cases) and 1.4 million died from the disease in 2015 [1]. About one-third of the world’s population has latent TB (LTBI), which is asymptomatic and non-communicable [2]. Nevertheless, in these individuals the pathogen is alive and over 10% of this population ultimately develop active and communicable TB. Reactivation occurs under conditions of immune system compromise, which explains why individuals infected with HIV and those with type 2 diabetes (T2DM) [3–5] are at exceptionally high risk for reactivation [6–8]. The WHO estimates that by 2030 there will be a substantial proportion of TB reactivation cases attributable to HIV or T2DM comorbidity [5]. Therefore, understanding the mechanisms involved in the pathogenesis of TB reactivation is of prime importance to control TB reactivation.
Mtb has shown a remarkable ability to persist in the infected host in a non/semi-replicating dormant stage [9]. Recent studies suggest that the dormant bacteria most likely exist in host cells at both pulmonary and extra-pulmonary sites [10]. Adipose tissue, a nutritionally rich organ, provides a suitable environment for dormant Mtb [11–13]. Many pathogens, including Mtb and Trypanosoma cruzi, Rickettsia, HIV and SIV utilize adipose tissue as a reservoir for their survival [10–17]. Recent findings have shown the presence of Mtb in various adipose tissue depots during acute and chronic phases of Mtb aerosol infection [10–12] suggesting that Mtb disseminates from lungs to distant adipose depots. Similar to the observations made in T. cruzi infection, Mtb can disseminate to the lungs from adipose depots [10–15].
Adipose tissue is not only a storage site for triglycerides, but also acts as an endocrine organ contributing to energy homeostasis, inflammation and immune response to infection. It constitutes 15–25% of the total body mass and is broadly distributed throughout the body [18, 19]. Adipose tissue is composed of various cell types including fibroblasts, endothelial cells, leukocytes, skeletal, and smooth muscle cells in addition to adipocytes [15]. Mtb infection and persistence may have a dynamic effect on adipose tissue physiology and pathology which regulate metabolic and energy homeostasis [18, 19].
We have established a rabbit model of pulmonary Mtb infection using clinical strains HN878 and CDC1551 that mimic most of the pathological features in humans with active TB or latent infection (LTBI) [20]. Although the rabbit model is more expensive and has stringent regulatory and facility requirements compared to mouse and guinea pig models, it is an excellent animal model to study host-pathogen interactions during LTBI and active disease. Aerosol infection of rabbits with the hypervirulent clinical Mtb isolate HN878 leads to progressive, active pulmonary TB marked with elevated bacterial growth, inflammation and formation of granulomas that undergo central necrosis, caseation/liquefaction; some of these granulomas ultimately develop cavitation [21]. In contrast, pulmonary infection of rabbits with the hyperimmunogenic clinical Mtb isolate CDC1551 results in protracted bacillary growth in the lungs early during infection that is controlled effectively upon the onset of adaptive immunity, resulting in significant reduction in bacillary load until no viable bacteria could be cultured from the lung homogenates. The kinetics of bacillary growth is consistent with the loss of disease pathology in the lungs [22]. Thus, infection with CDC1551 results in non-progressive latent infection (LTBI) in rabbits. Importantly, upon immune suppression treatment, these rabbits can reactive bacillary growth and disease pathology in the lungs [22].
Here, we investigated the effect of Mtb infection on the key adipokine levels using our rabbit model of pulmonary active TB and LTBI to elucidate a link between adipose tissue physiology and the lung pathology during TB infection. We hypothesize that adipokine levels are differentially altered in LTBI and active TB, which distinctly affect respective lung TB pathogenesis.
2. MATERIALS AND METHODS
2.1 Ethics statement
All rabbit procedures were performed in accordance with Animal Welfare Act guidelines and approved by the Institutional Animal Care and Use and Institutional Biosafety Committees of Rutgers University.
2.2 Bacteria and Chemicals
Mtb CDC1551 and HN878 were obtained from Dr. Shinnick at the Centers for Disease Control and Prevention (CDC), Atlanta, GA and Dr. Musser at Houston Methodist, TX, respectively. Stock Mtb cultures were prepared by growing the bacilli in Middlebrook 7H9 medium with supplements (BD, Sparks, MD) as described earlier and banked frozen at −80°C [23]. To prepare bacterial inoculum for infection, frozen cultures were thawed just before use as previously described [23]. All chemicals were obtained from Sigma-Aldrich (St Louis, MO), unless otherwise mentioned.
2.3 Aerosol infection of rabbits
Female New Zealand White rabbits (Covance Inc, MI, USA) weighing between 2.2 and 2.5 kg (n=16) were exposed to HN878 or CDC1551 aerosols, as described [22]. Uninfected rabbits served as controls (n=4). Briefly, rabbits were exposed to Mtb-containing aerosols using a nose-only delivery system (CH Technologies, NJ). At 3 hours after exposure, a group (n = 4) of rabbits was euthanized, and serial dilutions of the lung homogenates were cultured on Middlebrook 7H11 (Difco BD, Franklin Lakes, NJ) agar plates to enumerate the number of initial (time = 0) bacterial CFUs implanted in the lungs. At 12 weeks after infection (p.i.), groups of infected rabbits (n = 4) were euthanized and lung and visceral white adipose tissues (WAT) were harvested for protein analysis and total RNA isolation. As expected, we observed a significant decrease in the body weight of HN878-infected, compared CDC 1551-infected, rabbits at this time point (data not shown).
2.4 Mtb infection of cultured adipocytes
3T3-L1 murine preadipocytes purchased from Zen-bio inc., were propagated and differentiated to adipocytes in culture plates as previously described [24]. Cells were used between day 8 and 12 post-induction of differentiation. The cultured adipocytes were infected by Mtb (HN878 or CDC1551) at a multiplicity of infection of 3:1 (bacteria:adipocyte) for 48h as described previously [25].
2.5 Preparation of cell and tissue protein lysates
After 48h post infection, the adipocytes were washed four times with phosphate buffered saline (pH 7.4) and lysed in 1 ml lysis buffer containing 50 mmol/l Tris pH 7.5, 1% NP-40, and 150 mmol/l sodium chloride plus protease inhibitor cocktail. Adipose tissues were homogenized in TNET buffer lacking Triton X-100 (150 mm NaCl, 5 mm EDTA, 50 mm Tris-HCl, pH 7.5), supplemented with complete protease inhibitor cocktail and phosphatase inhibitor cocktail (Thermofisher). This was followed by low-speed centrifugation (3000 × g at 4° C) to remove the fat cake from the top of the tube. Triton X-100 was added to the homogenate for a final concentration of 1%, and the extract was cleared at 20,000 × g for 15 min at 4° C and mixed with 2× Laemmli sample buffer. The lung tissues were lysed in RIPA buffer (150 mm NaCl, 0.1% SDS, 1% triton X-100, 2 mM EDTA, 1% sodium deoxycholate, Tris-HCl pH 7.5) supplemented with complete protease inhibitor cocktail and phosphatase inhibitor cocktail (Thermofisher) and centrifuged at 12,000 rpm for 15 min at 4° C.
2.6 Immunoblot analysis
Protein lysates were resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis on 10% acrylamide gels and transferred to BA85 nitrocellulose (Schleicher & Schuell, Florham Park, NJ) for immunoblot analysis. Blots were probed with adiponectin (1:1000 Cell Signaling C45B10), PPAR-γ (1:1000 Cell Signaling C26H12) and TNF-α (1:1000 Abcam ab6671). Horseradish peroxidase-conjugated goat anti-mouse immunoglobulin (1:2000 dilution, Amersham Biosciences, Piscataway, NJ) or horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin (1:5000 dilution, Amersham Biosciences) were used to detect specific protein bands (explained in Figure Legends) using a chemiluminescence system [26]. Guanosine nucleotide dissociation inhibitor (GDI, 1: 10000 dilution, 71–0300, and rabbit polyclonal, Invitrogen, CA) and a secondary antibody horseradish peroxidase conjugated goat anti-rabbit (1:2000 dilution, Amersham Biosciences) was used to normalize protein loading. Quantitative analysis of the protein bands was performed using Image Studio Lite Ver (5.2) software and represented the data as Box and Whisker plots.
2.7 Real time PCR quantification
Total host RNA from the lungs and white adipose tissue (WAT) of Mtb-infected rabbits (n = 4) and matched uninfected control animals (n = 4) at 12 weeks p.i. was isolated, using the Trizol reagent (Invitrogen, Carlsbad, CA) as previously described [25]. Isolated RNA was purified by on-column digestion of the contaminating DNA using DNase I, as described earlier [25]. The quality and quantity of the purified RNA were assessed by formaldehyde–agarose gel electrophoresis and a NanoDrop instrument (NanoDrop Products, Wilmington, DE), as previously described [25]. RNA was reverse transcribed from 100 ng of total RNA using All-in-One cDNA Synthesis SuperMix (Biotool) according to the manufacturer’s protocol. The primers used for the amplification of quantitative PCR (qPCR) of adiponectin, PPAR-γ and cytokines (TNFα, IL-6 and IL-10) and HPRT (Hypoxanthine-guanine phosphoribosyltransferase) genes are listed in Supplementary Table S1 online. The qPCR was run using Power SYBR™ Green PCR Master Mix (Thermo Fisher Scientific) following the manufacturer’s protocol. To normalize gene expression and to calculate fold change mRNA expression of the housekeeping gene, HPRT was measured. For each sample, both the housekeeping and target genes were amplified in triplicate using the reaction condition and analytic parameters described previously [26].
2.8 Statistical Analysis
Statistical analysis were performed to determine the changes in protein levels between the experimental groups using Kruskal Wallis test (StataCorp 13). Statistical analyses were performed to determine fold change significance for qPCR analyses using a Student’s t-test (Microsoft Excel) as appropriate and significance differences were determined as p values between < 0.05 and <0.001 as appropriate.
3. RESULTS
3.1 Mtb infection alters adipose tissue physiology
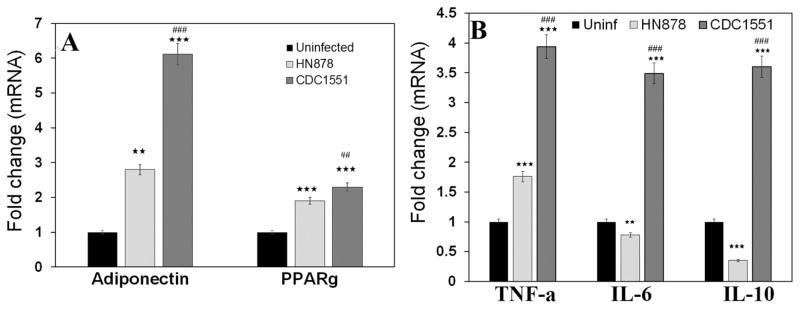
Adipose tissue physiology is determined by the expression levels of adipokines, such as adiponectin, leptin, PPARγ, and TNFα, which regulate fat metabolism and immune homeostasis at local and systemic levels. We examined the alterations in adipose tissue physiology in white adipose tissue (WAT) samples from the rabbit model of LTBI or active TB (i.e, by qPCR and protein analysis. mRNA analysis demonstrated increased expression of adiponectin and peroxisome proliferator activated receptor (PPARγ) in the WAT of rabbits with LTBI vs active TB at 12 weeks (Fig. 1A). Adiponectin levels were 2 fold greater in the WAT of LTBI compared to active TB rabbits (Fig. 1A). Consistent with adiponectin and PPARγ regulating inflammatory cytokines [27–29], mRNA levels of TNF-α, IL-6, and IL-10 were significantly higher (greater than 2, 5 and 6 folds respectively, p≤ 0.001) in the WAT of rabbits with LTBI, compared to active TB (Fig. 1B).
Figure 1. qPCR analysis demonstrated different pattern of adipokines mRNA expression levels in the WAT of rabbits aerosol infected with HN878 (active TB) and CDC1551 (latent TB).
The fold changes in the mRNA levels were calculated after comparing to control rabbits and normalizing to housekeeping HPRT mRNA levels (n=4, 12 weeks post infection). (A) Fold change in the levels of adiponectin and PPARγ. (B) Fold change in the levels of adipocytokines TNFα, IL-6 and IL-10.
Significance represent mean values of the data with Standard Error of the mean (SEM) as vertical lines. (The error bars represent standard error of the mean. ** p ≤ 0.01 or *** p ≤ 0.001 compared to uninfected rabbits; ##p ≤ 0.01 or ###p ≤ 0.001 compared to HN878 infected rabbits).
3.2 Mtb infection of cultured adipocytes alters the adipogenic proteins
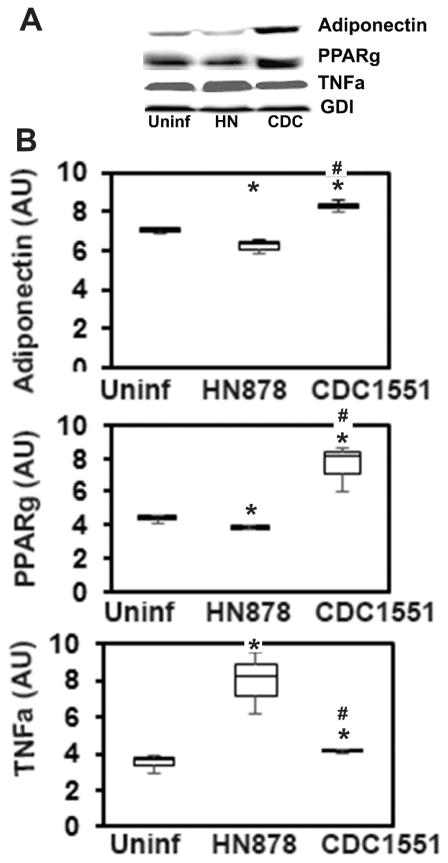
Immunoblot analysis of proteins from Mtb infected cultured adipocytes demonstrated a significantly altered levels of adipogenic factors such as adiponectin and PPAR-γ at 48 h post infection (Fig. 2). To evaluate the effect of different strains of Mtb on the adipogenic factors of adipocytes, we infected 3T3L1 cultured adipocytes with Mtb HN878 or CDC1551strains at MOI of 3 (bacteria/adipocytes) for 48h. These Mtb strains cause active TB or LTBI, respectively, in our rabbit model. Immunoblot analysis (p≤ 0.05) normalized to GDI) showed that HN878 infection significantly reduced the levels of adiponectin and PPAR-γ (whereas, CDC1551 infection induced significantly higher levels of adiponectin and PPAR-γ compared to uninfected adipocytes (Fig. 2B). Adiponectin and PPAR-γ negatively regulate the inflammatory response in adipocytes [30]. Consistently, adipocytes infected with Mtb HN878 had significantly increased (p≤ 0.05) TNF-α levels compared to uninfected cells. However, no significant change in the levels of TNF-α was observed in CDC1551 infected cells compared to uninfected adipocytes.
Figure 2. Immunoblot analysis demonstrated a significant alteration in the levels of adiponectin, PPARγ and TNFα in 3T3-l1 adipocytes infected with different strains of Mtb (MOI 3:1) for 48h.
(A) Protein analysis of adipogenic factors (adiponectin and PPARγ) and proinflammatory TNFα levels in the adipocytes infected with Mtb strains HN878 or CDC1551 compared to uninfected cells. (B) Band intensity was measured using Image J software and normalized to GDI, and represented as Box-Whisker plots (n=3, experiment was duplicated). (The error bars represent standard error of the mean. *p ≤ 0.05 compared to uninfected rabbits; #p ≤ 0.05 compared to HN878 infected rabbits).
3.3 Adiponectin protein levels inversely correlated between the adipose tissue and lungs in TB infection
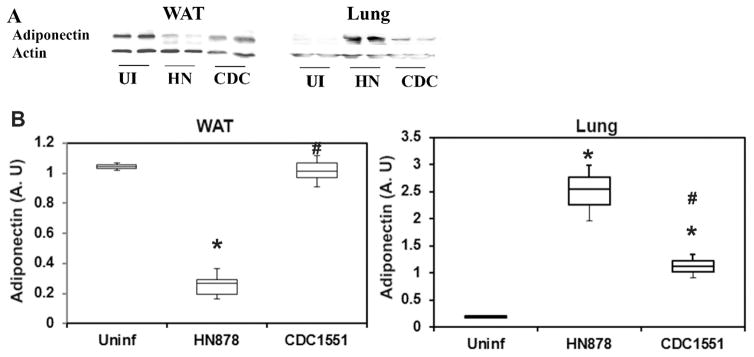
Adiponectin levels were measured in WAT and lungs of rabbits with LTBI or active TB (Fig. 3). Immunoblot-based analysis showed a significant decrease (p≤ 0.05) in adiponectin levels (4 fold) in the WAT of rabbits with active TB compared to uninfected rabbits (Fig. 3). The levels of adiponectin significantly (p≤ 0.5) increased in the WAT of latently infected rabbits (4 fold) compared to those with active TB (Fig. 3). Immunoblot analysis demonstrated an inverse relation in the levels of adiponectin between WAT and the lungs of infected rabbits. Adiponectin levels were significantly increased (p≤ 0.05) in the lungs of Mtb (HN878 or CDC1551) infected rabbits, compared to uninfected rabbits (Fig. 3). About 12.5 fold and 5.75 fold increase in the lung adiponectin levels was noted in rabbits with active TB and LTBI, respectively, compared to uninfected rabbits. Among the Mtb infected rabbits, the level of adiponectin was significantly higher in the lungs of active TB, compared to LTBI rabbits (No significant difference).
Figure 3. Immunoblot analysis of adiponectin in WAT and lungs during Mtb infection in rabbit models.
(A) Protein levels of adiponectin in Wat and Lungs of rabbits infected with Mtb strains HN878 or CDC1551 compared to uninfected rabbits. (B) Band intensity was measured using Image J software and normalized to GDI, and represented as Box-Whisker plots. (n=3) (The error bars represent standard error of the mean. * p ≤ 0.05 or $p ≤ 0.5 compared to uninfected rabbits; #p ≤ 0.05 compared to HN878 infected rabbits).
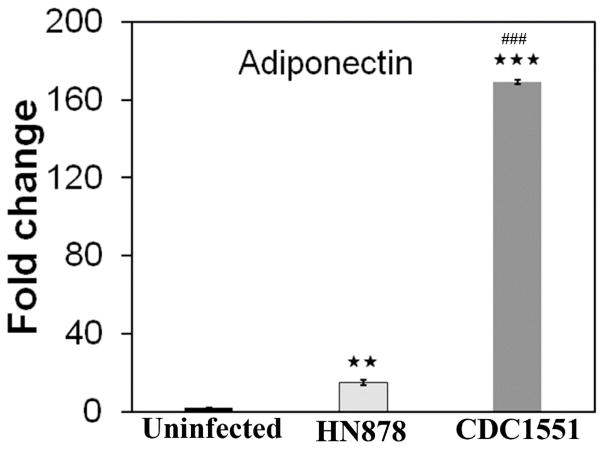
To investigate whether adiponectin is indeed expressed by the lungs in Mtb infected rabbits, we measured adiponectin mRNA levels in the lungs by qPCR and found them significantly increased (p≤ 0.01) compared to uninfected rabbits (Fig. 4). Rabbits with active TB and LTBI - showed 15 and 170 fold increase, respectively, in the lung adiponectin mRNA levels compared to uninfected rabbits (Fig. 4). These data show that the lungs can express adiponectin at higher levels during Mtb infection.
Figure 4. qPCR analysis demonstrated increased adiponectin mRNA expression in the lungs during Mtb infection in rabbit models infected with HN878 (active TB) and CDC1551 (latent TB) Mtb strains.
The fold change in the mRNA levels was calculated after comparing to control rabbits and normalizing to housekeeping HPRT mRNA levels. (n=4, 12 weeks post infection. The error bars represent standard error of the mean. ** p ≤ 0.01 or *** p ≤ 0.001 compared to uninfected rabbits; ##p ≤ 0.01 or ###p ≤ 0.001 compared to HN878 infected rabbits).
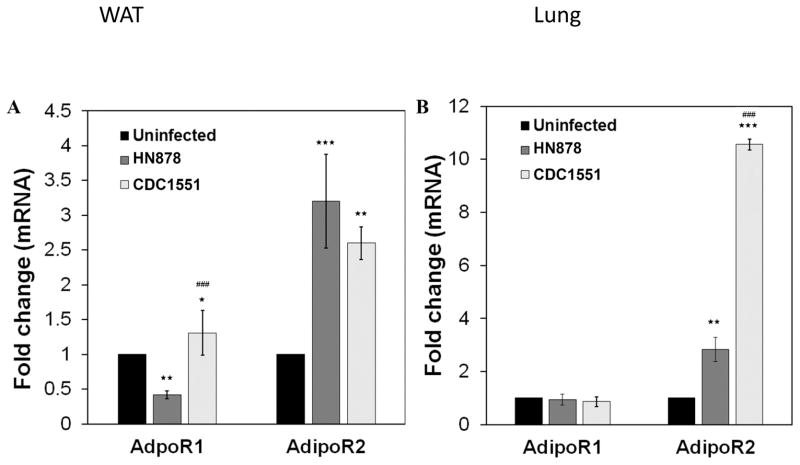
3.5 Differential expression of adiponectin receptors in adipose tissue and lungs in Mtb infected rabbits
Adiponectin receptors ADIPOR1 and ADIPOR2 serve as receptors for full-length adiponectin and mediate the downstream signaling of adiponectin such as increased AMP-activated protein kinase, peroxisome proliferator-activated receptor-alpha ligand activities, and glucose uptake and fatty-acid oxidation. qPCR analysis demonstrated a significant alteration in the levels of ADIPOR1/ADIPOR2 in WAT and lungs during Mtb infection in rabbits. mRNA levels of ADIPOR1 significantly increased (3.1 fold, p≤ 0.05) in the WAT of rabbits with LTBI, compared to those with active TB (Fig. 5A). Whereas, ADIPOR2, slightly increased (1.2 fold) in the WAT of active TB rabbits compared to LTBI (Fig. 5A). In the lungs, the mRNA levels of ADIPOR1 reduced in both LTBI and active TB rabbits compared to uninfected with no significant difference observed between LTBI and active TB. However, the levels of ADIPOR2 significantly increased (p≤ 0.01) in the lungs of infected rabbits compared to uninfected (Fig. 5B). ADIPOR2 levels were significantly down regulated (3.5 fold, (p≤ 0.001)) in the lungs of active TB rabbits compared to LTBI (Fig. 5B). The levels of ADIPOR1 in WAT and ADIPOR2 in the lungs of rabbits with LTBI were significantly increased compared to active TB and a reversed expression profile was observed in the WAT and lungs of active TB rabbits compared to LTBI. These observations suggest that ADIPOR1 and R2 may play a differential role in the WAT and the lungs during latent and active TB pathogenesis.
Figure 5. qPCR analysis demonstrated different pattern of adiponectin receptors R1 and R2 mRNA expression levels in the WAT (A) and lungs (B) of rabbits aerosol infected with HN878 (active TB) and CDC1551 (latent TB).
The fold changes in the mRNA levels were calculated after comparing to control rabbits and normalizing to housekeeping HPRT mRNA levels (n=4, 12 weeks post infection). (The error bars represent standard error of the mean. * p≤ 0.05, ** p ≤ 0.01 or *** p ≤ 0.001 compared to uninfected rabbits; ##p ≤ 0.01 or ###p ≤ 0.001 compared to HN878 infected rabbits).
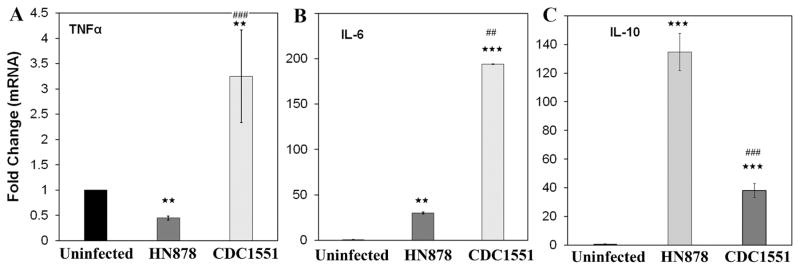
3.6 Expression of inflammatory mediators differs in the lungs of latent and active TB infected rabbits
Full length adiponectin regulates inflammatory signaling via TNF-α, IL-10, and IL-6 [27–29]. qPCR analysis of the pro-inflammatory (TNF-α and IL-6) and anti-inflammatory (IL-10) genes in the lungs of Mtb infected rabbits demonstrated a significant difference between active TB and LTBI (Fig. 6). The mRNA levels of TNFα and IL6 were significantly increased (7 (p≤ 0.001) and 6.5 folds p≤ 0.01 respectively) in the lungs of LTBI rabbits compared to active TB rabbits (Fig. 6 A&B). However, the mRNA levels of IL-10 significantly increased in the lungs of active TB (3.5 fold, p≤ 0.001) compared to LTBI rabbits (Fig. 6C). This suggests that increased adiponectin protein levels in the lungs of active TB rabbits positively correlate to the levels anti-inflammatory cytokines.
Figure 6. qPCR analysis demonstrated different pattern of inflammatory cytokines mRNA expression levels in the lungs of rabbits aerosol infected with HN878 (active TB) and CDC1551 (latent TB).
The fold changes in the mRNA levels of pro-inflammatory cytokines (A) TNFα and (B) IL-6 and anti-inflammatory cytokine (C) IL-10 in the lungs of rabbits infected with HN878 or CDC1551 compared to uninfected. The fold changes were calculated after comparing to control rabbits and normalizing to housekeeping HPRT mRNA levels (n=4, 12 weeks post infection). (The error bars represent standard error of the mean. ** p ≤ 0.01 or *** p ≤ 0.001 compared to uninfected rabbits; ##p ≤ 0.01 or ###p ≤ 0.001 compared to HN878 infected rabbits).
4. DISCUSSION
In the recent years adipose tissue has gained more attention for its significant role in the pathogenesis of many infectious and metabolic diseases [10–18]. In addition to its metabolic and immunologic functions, adipose tissue serves as a reservoir for many pathogens including parasites and bacteria [10–18]. Earlier studies have demonstrated that Mtb infects adipocytes and survive in adipose tissue in TB patients [10]. It has been shown that Mtb infection evokes immune response in adipocytes [31]. In this study, using rabbit models of LTBI and active TB, we have shown for the first time that Mtb infection alters adipokine levels, including adiponectin, in both white adipose tissue and lungs, and differentially affects immune signaling during latent infection and active disease. Previously, our group as well as others, has shown Mtb strain-specific host responses in animal models of pulmonary TB. Particularly, MtbHN878, a strain of the hypervirulent W-Beijing lineage, causes more destructive disease pathology, rapid bacillary replication and induces Type I interferon production in mice and rabbits (20–22), whereas, the hyperimmunogenic strain MtbCDC1551, elicites a strong and early proinflammatory response that contributes to effective control of bacterial growth and pathology. Although differences in cell wall components and regulation of gene expression has been suggested as potential mechanism for their variable pathogenicity and/or virulence in different host systems, the specific characteristics of Mtb HN878 and CDC1551, that caused active disease versus latent infection in the rabbit model remains to be determined. Specific mycobacterial components that contribute to differential host responses, such as induction of adipokine or tissue specific cytokine/chemokine expression, have not been fully explored.
Adipose tissue physiology is determined by the expression levels of adipokines, such as adiponectin, leptin, PPARγ, and TNFα, which regulate fat metabolism and immune homeostasis at local and systemic levels. We demonstrate that key adipokines such as adiponectin, PPARγ and TNFα (mRNA and adiponectin protein) levels are differentially regulated in LTBI and active TB models. Infection with the Mtb strain that causes latent TB infection (CDC1551) in rabbits showed significantly greater levels of adipogenic factors such as adiponectin and PPARγ in the adipose tissue compared to rabbits infected with the Mtb strain that causes active TB (HN878). Adiponectin protein levels were significantly higher in LTBI models compared to ATBI (Fig. 3). However, in the lungs of LTBI rabbits, the protein levels of adiponectin were significantly reduced compared to active TB rabbits (Fig. 3). This observation is interesting since adiponectin is known to be secreted by adipose tissue into circulation; in the active TB rabbit model, the level of adiponectin protein as well as mRNA is significantly decreased. Previously we reported the growth kinetics of Mtb in rabbit lungs with active and latent TB (21, 22). While rabbits with active TB had a higher number of Mtb, those with LTBI had no cultivable bacteria in the lungs at 12 weeks post infection. However, no significant association was observed between the differential lung bacterial loads and the metabolic/immunologic markers, including adipokine and pro-inflammatory cytokines, measured in rabbits with active TB or LTBI in this study. It has been previous reported that the Mtb strains that produce active TB (HN878) or LTBI (CDC1551) in rabbits elicit distinct host immune response (REF:PMID-22280836; 15322056).
To verify whether the lungs themselves express adiponectin and contribute to the increased pulmonary levels of adiponectin, we performed qPCR analysis. Our data suggest that the lungs express adiponectin and contribute to the increased pulmonary adiponectin levels. These data are consistent with previous reports showing that under pathological conditions, non-adipose tissues (e.g. the heart) can express adiponectin, furthermore provide the first evidence demonstrating that adiponectin is expressed by the lungs during TB [26]. The increased adiponectin protein levels in the lungs during active TB infection compared to LTBI may play an immunometabolic role in the pathogenesis of pulmonary TB infection and TB reactivation. Clinical studies also demonstrated increased serum adiponectin levels in TB patients and correlated to TB severity [32, 33]. Hence, further studies are warranted to understand the role of adiponectin played in Mtb infection.
WAT is the major body repository of energy in mammals and regulates whole body energy homeostasis [19]. Along with its endocrine functions, adipose tissue contributes to local and systemic immune responses via expression and secretion of adipokines such as adiponectin, leptin, resistin, cytokines, and chemokines [18]. The active adiponectin hexamers have shown to positively regulate anti-inflammatory signaling in various systems by either decreasing the levels of pro-inflammatory cytokines such as TNFα, IFNγ, IL-6 or by increasing the levels of anti-inflammatory cytokines such as IL-10 [28, 29]. AdipoR1 and R2 serve as the predominant receptors for adiponectin in vivo and play important roles in the regulation of glucose and lipid metabolism, inflammation, and oxidative stress in vivo [34]. qPCR analysis demonstrated different levels of adipoR1 and R2 expression in both adipose tissue and lung of rabbits infected with latent and active TB causing Mtb strains (Fig. 5). The mRNA levels of IL-10 in the adipose tissue of LTBI rabbits significantly increased compared to active TB rabbits which coordinated to the levels of adiponectin suggesting that increased adiponectin exerts anti-inflammatory effect via IL-10. Similarly but in contrast to the WAT, IL-10 levels were significantly higher along with the levels of adiponectin proteins in the lungs of active TB rabbits compared to LTBI rabbits. This suggests that increased levels of adiponectin in the lungs of active TB rabbits may inhibit pro-inflammatory immune signaling via IL-10 induction which is necessary for the clearance of pathogen. The data also suggest that the latent and active TBI causing Mtb strains differently affect adipose tissue physiology and adiponectin levels. These observations were also supported by the in vitro studies using Mtb infected cultured adipocytes (Fig. 2). Infection of adipocytes with the Mtb strain that caused LTBI in rabbits increased adiponectin levels whereas, the infection with the Mtb strain that caused active TB in rabbits decreased adiponectin levels after 48h infection similar to the observations made in the adipose tissues of rabbit LTBI and active TB models. The levels of TNFα in the infected adipocytes inversely correlate to adiponectin levels. These data suggest that adipocytes/adipose tissue respond differently to different strains of Mtb and can contribute to lung pathogenesis in latent and active/reactive TB infection. Further mechanistic studies need to be conducted to evaluate causal relationship between adiponectin expression and cytokine production in the lungs.
In summary, using the rabbit model of latent and active TB, we have found that decreased adipogenesis and altered adipose tissue physiology during active TB infection influences lung adipogenic factors. It is known that the metabolic and immunologic signaling of adipocytes/adipose tissue is associated with a number of pathological conditions. These alterations result in dyslipidemia – an abnormal amount of lipids (e.g. triglycerides, cholesterol and/or fat phospholipids) in the blood – a known risk factor for TB activation/reactivation [35]. Our studies demonstrated that adipose tissue physiology is altered in Mtb infection and lung adiponectin levels increased in active TB rabbits, suggesting that adipose tissue physiology is linked to lung pathology and the immune-modulatory levels of lung adiponctin levels in TB activation/reactivation.
Supplementary Material
Acknowledgments
This study was supported by grants from the National Institutes of Health HL-RO1A122866 (JNJ), R21AI110335 and R03AI119619 (SS) and 5K23A1102639 (CV).
Footnotes
6. CONFLICT OF INTEREST STATEMENT
None of the authors have conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Global tuberculosis report. http://www.who.int/tb/publications/global_report/gtbr15_main_text.pdf.
- 2.http://www.who.int/tb/publications/global_report/gtbr2016_main_text.pdf?ua=1
- 3.Selwyn PA, Hartel D, Wasserman W, Drucker E. Impact of the AIDS epidemic on morbidity and mortality among intravenous drug users in a New York City methadone maintenance program. Am J Public Health. 1989;79:1358–62. doi: 10.2105/ajph.79.10.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oni T, Stoever K, Wilkinson RJ. Tuberculosis, HIV, and type 2 diabetes mellitus: a neglected priority. Lancet Respir Med. 2013;1:356–58. doi: 10.1016/S2213-2600(13)70116-4. [DOI] [PubMed] [Google Scholar]
- 5.Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103:137–49. doi: 10.1016/j.diabres.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Spranger J, Kroke A, Möhlig M, Hoffmann K, Bergmann MM, Ristow M, et al. Inflammatory cytokines and the risk to develop type 2 diabetes results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes. 2003;52:812–17. doi: 10.2337/diabetes.52.3.812. [DOI] [PubMed] [Google Scholar]
- 7.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11:98–7. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- 8.Lonnroth K, Roglic G, Harries AD. Improving tuberculosis prevention and care through addressing the global diabetes epidemic: from evidence to policy and practice. Lancet Diabetes Endocrinol. 2014;2:730–39. doi: 10.1016/S2213-8587(14)70109-3. [DOI] [PubMed] [Google Scholar]
- 9.Bacon J, Alderwick LJ, Allnutt JA, Gabasova E, Watson R, Hatch KA, et al. Non-replicating Mycobacterium tuberculosis elicits a reduced infectivity profile with corresponding modifications to the cell wall and extracellular matrix. PloS one. 2014;9:e87329. doi: 10.1371/journal.pone.0087329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neyrolles O, Hernández-Pando R, Pietri-Rouxel F, Fornès P, Tailleux L, Payán JAB, et al. Is adipose tissue a place for Mycobacterium tuberculosis persistence? PloS one. 2006;1:e43. doi: 10.1371/journal.pone.0000043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agarwal P, Khan SR, Verma SC, Beg M, Singh K, Mitra K, et al. Mycobacterium tuberculosis persistence in various adipose depots of infected mice and the effect of anti-tubercular therapy. Microbes Infect. 2014;16:571–80. doi: 10.1016/j.micinf.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 12.Agarwal P, Pandey P, Sarkar J, Krishnan MY. Mycobacterium tuberculosis can gain access to adipose depots of mice infected via the intra-nasal route and to lungs of mice with an infected subcutaneous fat implant. Microb Pathog. 2016;93:32–37. doi: 10.1016/j.micpath.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 13.Rastogi S, Agarwal P, Krishnan MY. Use of an adipocyte model to study the transcriptional adaptation of Mycobacterium tuberculosis to store and degrade host fat. Int J Mycobacteriol. 2016;5:92–98. doi: 10.1016/j.ijmyco.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Ferreira AV, Segatto M, Menezes Z, Macedo AM, Gelape C, de Oliveira Andrade L, Nagajyothi F, et al. Evidence for Trypanosoma cruzi in adipose tissue in human chronic Chagas disease. Microbes Infect. 2011;13:1002–05. doi: 10.1016/j.micinf.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Combs TP, Nagajyothi, Mukherjee S, de Almeida CJ, Jelicks LA, Schubert W, et al. The adipocyte as an important target cell for Trypanosoma cruzi infection. J Biol Chem. 2005;280:24085–94. doi: 10.1074/jbc.M412802200. [DOI] [PubMed] [Google Scholar]
- 16.Bechah Y, Paddock CD, Capo C, Mege JL, Raoult D. Adipose tissue serves as a reservoir for recrudescent Rickettsia prowazekii infection in a mouse model. PloS one. 2010;5:e8547. doi: 10.1371/journal.pone.0008547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Damouche A, Lazure T, Avettand-Fenoël V, Huot N, Dejucq-Rainsford N, Satie AP, et al. Adipose tissue is a neglected viral reservoir and an inflammatory site during chronic HIV and SIV infection. PLoS Pathog. 2015;11:e1005153. doi: 10.1371/journal.ppat.1005153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choe SS, Huh JY, Hwang IJ, Kim JI, Kim JB. Adipose tissue remodeling: its role in energy metabolism and metabolic disorders. Front Endocrinol (Lausanne) 2016;7:30. doi: 10.3389/fendo.2016.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nascimento CM, Ribeiro EB, Oyama LM. Metabolism and secretory function of white adipose tissue: effect of dietary fat. An Acad Bras Cienc. 2009;81:453–66. doi: 10.1590/s0001-37652009000300010. [DOI] [PubMed] [Google Scholar]
- 20.Subbian S, Karakousis PC, Kaplan G. Rabbit model of tuberculosis. In: Mukundan H, Waters RW, Chambers MA, Larsen MH, editors. Tuberculosis, Leprosy, and other Mycobacterial diseases of man and animals: the many hosts of Mycobacteria. Chapter 22. CAB International Publications; Wallingford, UK: 2015. pp. 402–18. [Google Scholar]
- 21.Subbian S, Tsenova L, Yang G, O’Brien P, Parsons S, Peixoto B, et al. Chronic pulmonary cavitary tuberculosis in rabbits: a failed host immune response. Open Biol. 2011;4:110016. doi: 10.1098/rsob.110016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Subbian S, Tsenova L, O’Brien P, Yang G, Kushner NL, Parsons S, et al. Spontaneous latency in a rabbit model of pulmonary tuberculosis. Am J Pathol. 2012;181:1711–24. doi: 10.1016/j.ajpath.2012.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Subbian S, Tsenova L, O’Brien P, Yang G, Koo MS, Peixoto B, et al. Phosphodiesterase-4 inhibition alters gene expression and improves isoniazid–mediated clearance of Mycobacterium tuberculosis in Rabbit Lungs. PLoS Pathog. 2011;7:e1002262. doi: 10.1371/journal.ppat.1002262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagajyothi F, Desruisseaux MS, Thiruvur N, Weiss LM, Braunstein VL, Albanese C, et al. Trypanosoma cruzi infection of cultured adipocytes results in an inflammatory phenotype. Obesity (Silver Spring) 2008;16:1992–97. doi: 10.1038/oby.2008.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koo MS, Subbian S, Kaplan G. Strain specific transcriptional response in Mycobacterium tuberculosis infected macrophages. Cell Commun Signal. 2012;10:2. doi: 10.1186/1478-811X-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagajyothi, Weiss LM, Zhao D, Koba W, Jelicks LA, Cui MH, et al. High fat diet modulates Trypanosoma cruzi infection associated myocarditis. PLoS Negl Trop Dis. 2014;8:e3118. doi: 10.1371/journal.pntd.0003118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maeda N, Takahashi M, Funahashi T, Kihara S, Nishizawa H, Kishida K, et al. PPARγ ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes. 2001;50:2094–99. doi: 10.2337/diabetes.50.9.2094. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Wang X, Lau WB, Yuan Y, Booth D, Li JJ, et al. Adiponectin inhibits tumor necrosis factor-α–induced vascular inflammatory response via caveolin-mediated ceramidase recruitment and activation. Circ res. 2014;114:792–05. doi: 10.1161/CIRCRESAHA.114.302439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolf AM, Wolf D, Rumpold H, Enrich B, Tilg H. Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochem Biophys Res Commun. 2004;323:630–35. doi: 10.1016/j.bbrc.2004.08.145. [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto K, Kiyohara T, Murayama Y, Kihara S, Okamoto Y, Funahashi T, et al. Production of adiponectin, an anti-inflammatory protein, in mesenteric adipose tissue in Crohn’s disease. Gut. 2005;54:789–96. doi: 10.1136/gut.2004.046516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim JS, Ryu MJ, Byun EH, Kim WS, Whang J, Min KN, et al. Differential immune response of adipocytes to virulent and attenuated Mycobacterium tuberculosis. Microbes Infect. 2011;13:1242–51. doi: 10.1016/j.micinf.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 32.Elnemr GM, Elnashar MA, Elmargoushy NM, Elnashar NA, Elnashar MA. Adiponectin levels as a marker of inflammation in pulmonary tuberculosis. Egypt JHM. 2015;59:208–14. [Google Scholar]
- 33.Keicho N, Matsushita I, Tanaka T, Shimbo T, Le Hang NT, Sakurada S, et al. Circulating levels of adiponectin, leptin, fetuin-A and retinol-binding protein in patients with tuberculosis: markers of metabolism and inflammation. PLoS one. 2012;7:e38703. doi: 10.1371/journal.pone.0038703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, et al. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med. 2007;13:332. doi: 10.1038/nm1557. [DOI] [PubMed] [Google Scholar]
- 35.Martens GW, Arikan MC, Lee J, Ren F, Vallerskog T, Kornfeld H. Hypercholesterolemia impairs immunity to tuberculosis. Infect Immun. 2008;76:3464–72. doi: 10.1128/IAI.00037-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.