Summary
GATA2 deficiency is a recently described genetic disorder affecting hematopoietic stem cells and is associated with immunodeficiency, hematologic malignancy, and various cutaneous pathologies including cutaneous tumors. To explore the incidence and clinical course of melanoma in patients with germline GATA2 deficiencies, we conducted a retrospective chart review of 71 such patients and identified two with invasive melanoma. One melanoma was diagnosed early because it was associated with pruritus due to a graft-versus-tumor effect following bone marrow transplantation. The other one, a lentigo maligna melanoma, was locally excised but progressed to widespread metastasis and death several years later. Our observations and published studies of melanoma biology suggest an association between decreased GATA2 expression and melanoma progression. These findings suggest that GATA2 deficient patients may have an increased risk of melanoma and should be observed closely for new or changing skin lesions.
Keywords: GATA2 deficiency, immunodeficiency, melanoma, skin cancer, graft-versus-tumor
GATA2 is a zinc finger transcription factor critical for hematopoietic cell regulation.1,2 Haploinsufficiency of GATA2 is a rare condition associated with immunodeficiency, susceptibility to recurrent infections, and predisposition to hematologic malignancies.1,2 In addition, patients with GATA2 deficiency may have mucocutaneous manifestations including severe human papilloma virus (HPV) infection, herpes simplex virus (HSV) infection, cutaneous non-tuberculous mycobacterial (NTM) infection, panniculitis, lymphedema, gingival hypertrophy, glossitis, and granulomatous facial lesions.2,3 There are also reports of cutaneous malignancies such as basal cell carcinoma, squamous cell carcinoma, Merkel cell carcinoma, and melanoma.2,4
The incidence of melanoma is known to be elevated in immunocompromised individuals. Patients with HIV/AIDS, those who receive solid organ transplants, chemotherapy recipients, and those with hereditary immunodeficiency or chronic lymphocytic leukemia (CLL) have an increased risk of melanoma ranging from two to six-fold greater than expected.5–9 Immunosuppression is also associated with worse melanoma outcomes. For example, organ transplant recipients who develop melanoma in the post-transplant setting have decreased overall survival. In addition, CLL patients with melanoma have worse overall and melanoma-specific survival.10 The relationship between melanoma and GATA2-related immunodeficiency is poorly understood. To estimate the incidence and describe the clinical course of melanoma in patients with GATA2 deficiency we conducted a retrospective chart review.
Since the discovery of GATA2 deficiency, we have diagnosed 103 probands with GATA2 mutations at the National Institutes of Health (NIH) Clinical Center (Bethesda, MD). Among these patients, 71 probands were subsequently followed at the NIH and had full demographic information available. A chart review of these patients was conducted to identify those diagnosed with melanoma during follow-up. All patients in the study were enrolled with informed consent on protocols approved by the NIAID IRB (protocols 07-I-0033, 13-I-0157, 93-I-0119 or 01-I-0202).Among 71 probands with GATA2 deficiency, 67.6% were female. The median age at presentation was 30 years with a range of 2–66 years. These patients were followed for a total of 253 patient-years. Two GATA2 deficiency patients were diagnosed with cutaneous melanoma during follow-up. These included a young patient whose melanoma was heralded by a graft-versus-tumor reaction following bone marrow transplant, and a second patient with lethal metastatic melanoma. Details of the presentation and course of the GATA2 deficiency melanomas are as follows.
The first patient was a Caucasian woman with a familial heterozygous mutation (c. 1017+572C>T) within the enhancer region of GATA2 intron 5 who had been followed since age 28. She manifested several hallmarks of GATA2 deficiency including recurrent infections and hematologic abnormalities. She reported recurrent herpes labialis since childhood, and as an adult developed extensive genital HPV-related disease. At age 18, she was diagnosed with mild pancytopenia and myelodysplastic syndrome (MDS). At age 29, the MDS progressed with severe monocytopenia and new cytogenetic abnormalities including trisomy 1q. At that time, she received a standard conditioning regimen with busulfan and fludarabine for four days followed by an allogeneic bone marrow transplant from her HLA-matched brother. Prophylaxis for graft-versus-host disease (GvHD) included methotrexate and tacrolimus.
Four weeks post-transplant, the patient noted a newly-pruritic, 4 mm, skin-colored nevus on the right central back. Prior to transplant the nevus had been asymptomatic and unchanged for years. The patient had numerous cutaneous nevi and a history of multiple blistering sunburns during childhood. There was no family history of melanoma. The nevus was biopsied, and histopathological examination revealed a nodular melanoma with a nevoid pattern, mitotic figures, and no ulceration (Fig. 1a). The Breslow depth was 2.85 mm with Clark level IV invasion. The patient underwent wide local excision of the melanoma. No tumor was detected in the two sentinel lymph nodes biopsied. Fluorescence in situ hybridization (FISH) for the X/Y-chromosomes revealed a dense, donor-derived inflammatory infiltrate associated with the melanoma (Fig. 1b), demonstrating that a graft-versus-tumor (GvT) response likely caused the pruritus that heralded the diagnosis of melanoma in an otherwise benign-appearing nevus.
Fig. 1.
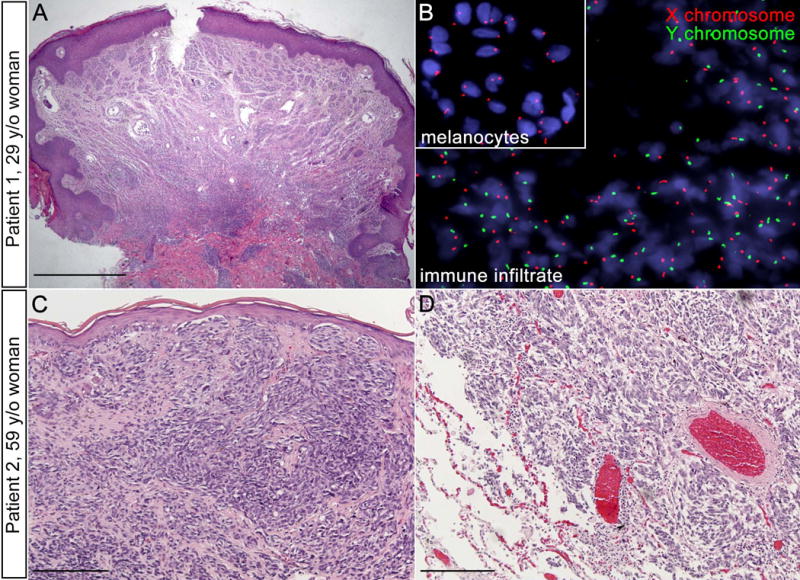
The second patient was a Caucasian woman with GATA2 deficiency due to uniallelic expression of GATA2 (patient 7.I.1 from Hsu et al.1) who had been followed since age 53. Her clinical course was characterized by chronic disseminated NTM infections, large granular lymphocytic leukemia, MDS with trisomy 8, central demyelinating disease, pulmonary alveolar proteinosis, and multiple non-melanoma skin cancers. At age 59, she was diagnosed with lentigo maligna melanoma of the left upper arm with a Breslow thickness of 0.80 mm. The patient underwent a wide local excision (Fig. 1c), with subsequent re-excision due to positive margins. Sentinel lymph node biopsy was negative for tumor. Surveillance CT scans of the head, chest, abdomen, and pelvis until age 63 were negative for evidence of melanoma metastasis. The patient passed away unexpectedly at age 64, and autopsy revealed extensive metastatic melanoma involving the liver, lung, spleen, bone marrow, pericardium, mediastinum, and pancreas (Fig. 1d).
Individuals with GATA2 deficiency present with diverse clinical manifestations, including increased risk for hematologic and solid malignancies. Although observing two melanomas among 71 GATA2 deficiency patients followed for 253 patient years suggests a higher incidence of melanoma compared to the general population, making a definitive melanoma risk determination from a retrospective chart review of such a small group of patients is not possible. To estimate the standardized incidence ratio (SIR) for melanoma, we calculated an expected number of melanoma cases for our cohort using cancer incidence rates from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) cancer registry program. Rates were stratified on sex, age, and calendar year. Because we did not know the race/ethnicity of all of the GATA2 deficient patients, we calculated rates assuming all were non-Hispanic white (to yield a conservatively high expected count). Based on this approach, we estimate that only 0.055 melanoma cases would have been expected, corresponding to an SIR of 36 compared with the general population (95% confidence interval 4.4–130, P = 0.003). However, we caution that this calculation should be interpreted as a provisional estimate of risk and that formal cohort studies of patients with GATA2 deficiency should be conducted.
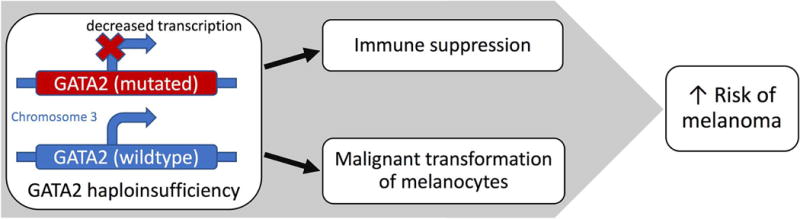
Although defects in cellular immunity found in these patients likely contribute to an increased risk for melanoma,10 there is also evidence to suggest that GATA2 deficiency may directly contribute to the molecular pathology of melanoma (Fig. 2). Several studies suggest that GATA2 is downregulated in melanoma cells, and low GATA2 expression may be associated with a more aggressive phenotype. Bonazzi et al. demonstrated that GATA2 is one of the most frequently silenced genes by DNA methylation in both melanoma cell lines and tumor samples.11 Epigenetic silencing of GATA2 in melanoma is functionally similar to the primary defect in GATA2 deficiency. Another study identified GATA2 as a predicted target for hsa-miR-27, a microRNA found to be differentially expressed in metastatic melanoma tumors compared to primary cutaneous melanoma tumors.12 Similarly, a study comparing the RNA expression profiles of two well-characterized melanoma cell lines found that cells with strong metastatic potential (FEMX-I) had an over 50-fold decrease in GATA2 transcription compared to cells that have no metastatic potential (FEMX-V).13 Interestingly, the second patient described in our study developed highly aggressive, distant melanoma metastases within 5 years of her original diagnosis despite having only a 7.1% predicted 15-year melanoma mortality rate based on her age, tumor thickness, sentinel lymph node status, ulceration status, histology, and tumor location.14 Taken together, these studies suggest that reduced GATA2 expression is associated with the initiation or progression of melanoma and thus, germline GATA2 deficiency may promote the malignant transformation of melanocytes or result in a more aggressive phenotype in transformed melanocytes.
Fig. 2.
A role for immune suppression contributing to melanoma risk is suggested by the clinical presentation of the first patient. The patient had multiple nevi, yet only one melanocytic lesion became inflamed after allogeneic bone marrow transplant. This suggests the patient’s myelosuppression (related to GATA2 deficiency and further aggravated by transplant conditioning and GvHD prophylaxis) was permissive to melanocyte transformation, and an anti-tumor immune response was subsequently conveyed by the donor-derived immune system. A GvT effect following allogeneic stem cell transplant has been recognized in several cancers, including melanoma, and is often seen in association with development of acute or chronic GvHD.15,16 Whereas prior reports describe the GvT effect contributing to regression of advanced malignancies, our patient is a novel example of a GvT effect prompting the early detection and diagnosis of melanoma. This emphasizes the importance of assessing inflamed nevi in the post-transplant setting.
Our observed cases of melanoma in GATA2 deficiency patients and published studies of melanoma biology support an association between reduced GATA2 expression and melanoma progression. Although it is not possible to make definitive conclusions about the exact risk for melanoma based on a population of 71 patients, our data suggest that patients with GATA2 deficiency may be at increased risk for melanoma. Considering the multiple types of cutaneous malignancies and skin infections reported in patients with GATA2 deficiency, they should be observed closely for new or changing skin lesions with a low threshold to biopsy lesions suspicious for malignancy.
Significance.
Melanoma incidence is elevated in immunocompromised individuals, typically two to six-fold higher than expected. In our cohort of 71 patients with GATA2 deficiency, we identified two cases of melanoma. Observing two melanomas among the small number of relatively young GATA2 deficiency patients suggests an increased melanoma risk in this patient population. While immune suppression alone contributes to melanoma risk, decreased GATA2 expression may also have direct effects on melanocyte transformation and melanoma progression. In addition, we report a novel example of a graft-versus-tumor effect heralding the diagnosis of invasive melanoma, emphasizing the importance of assessing inflamed nevi in the post-transplant setting. Our findings emphasize that GATA2 deficient patients should be closely monitored for new or changing skin lesions.
Acknowledgments
This research was supported by the NIH Intramural Research Program, Center of Cancer Research, National Cancer Institute. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
This work was funded by the National Institutes of Health. All authors do not have conflicts of interest to disclose.
Footnotes
MS. JANNETT NGUYEN (Orcid ID : 0000-0002-1523-7452)
References
- 1.Hsu AP, Johnson KD, Falcone EL, et al. GATA2 haploinsufficiency caused by mutations in a conserved intronic element leads to MonoMAC syndrome. Blood. 2013;121(19):3830–3837. S3831–3837. doi: 10.1182/blood-2012-08-452763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spinner MA, Sanchez LA, Hsu AP, et al. GATA2 deficiency: a protean disorder of hematopoiesis, lymphatics, and immunity. Blood. 2014;123(6):809–821. doi: 10.1182/blood-2013-07-515528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polat A, Dinulescu M, Fraitag S, et al. Skin manifestations among GATA2-deficient patients. Br J Dermatol. 2017 doi: 10.1111/bjd.15548. [DOI] [PubMed] [Google Scholar]
- 4.Crall C, Morley KW, Rabinowits G, Schmidt B, Dioun Broyles A, Huang JT. Merkel cell carcinoma in a patient with GATA2 deficiency: a novel association with primary immunodeficiency. Br J Dermatol. 2016;174(1):169–171. doi: 10.1111/bjd.14062. [DOI] [PubMed] [Google Scholar]
- 5.Robbins HA, Clarke CA, Arron ST, et al. Melanoma Risk and Survival among Organ Transplant Recipients. J Invest Dermatol. 2015;135(11):2657–2665. doi: 10.1038/jid.2015.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370(9581):59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 7.Greene MH, Young TI, Clark WH. Malignant melanoma in renal-transplant recipients. Lancet. 1981;1(8231):1196–1199. doi: 10.1016/s0140-6736(81)92359-x. [DOI] [PubMed] [Google Scholar]
- 8.Laing ME, Moloney FJ, Comber H, Conlon P, Murphy GM. Malignant melanoma in renal transplant recipients. Br J Dermatol. 2006;155(4):857. doi: 10.1111/j.1365-2133.2006.07395.x. [DOI] [PubMed] [Google Scholar]
- 9.Lanoy E, Dores GM, Madeleine MM, Toro JR, Fraumeni JF, Jr, Engels EA. Epidemiology of nonkeratinocytic skin cancers among persons with AIDS in the United States. AIDS. 2009;23(3):385–393. doi: 10.1097/QAD.0b013e3283213046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kubica AW, Brewer JD. Melanoma in immunosuppressed patients. Mayo Clin Proc. 2012;87(10):991–1003. doi: 10.1016/j.mayocp.2012.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonazzi VF, Nancarrow DJ, Stark MS, et al. Cross-platform array screening identifies COL1A2, THBS1, TNFRSF10D and UCHL1 as genes frequently silenced by methylation in melanoma. PLoS One. 2011;6(10):e26121. doi: 10.1371/journal.pone.0026121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qi M, Huang X, Zhou L, Zhang J. Identification of differentially expressed microRNAs in metastatic melanoma using next-generation sequencing technology. Int J Mol Med. 2014;33(5):1117–1121. doi: 10.3892/ijmm.2014.1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xi Y, Riker A, Shevde-Samant L, et al. Global comparative gene expression analysis of melanoma patient samples, derived cell lines and corresponding tumor xenografts. Cancer Genomics Proteomics. 2008;5(1):1–35. [PMC free article] [PubMed] [Google Scholar]
- 14.Michaelson J. Melanoma Outcome Calculator. http://www.lifemath.net/cancer/melanoma/outcome/index.php. Accessed July 26, 2017.
- 15.Demirer T, Barkholt L, Blaise D, et al. Transplantation of allogeneic hematopoietic stem cells: an emerging treatment modality for solid tumors. Nat Clin Pract Oncol. 2008;5(5):256–267. doi: 10.1038/ncponc1104. [DOI] [PubMed] [Google Scholar]
- 16.Kasow KA, Handgretinger R, Krasin MJ, Pappo AS, Leung W. Possible allogeneic graft-versus-tumor effect in childhood melanoma. J Pediatr Hematol Oncol. 2003;25(12):982–986. doi: 10.1097/00043426-200312000-00016. [DOI] [PubMed] [Google Scholar]


