Abstract
Background
East Liverpool, Ohio, the site of a hazardous waste incinerator and a manganese (Mn) processor, has had air Mn concentrations exceeding United States Environmental Protection Agency reference levels for over a decade. Save Our County, Inc., a community organization, was formed to address community environmental health concerns related to local industry. Researchers from the University of Cincinnati partnered with Save Our County to determine if air Mn had an impact on the neurocognitive function of children in the community.
Methods
Children 7-9 years of age from East Liverpool and its surrounding communities, were enrolled (N=106) in the Communities Actively Researching Exposure Study from between March 2013-June 2014. Blood and hair were analyzed for Mn and lead, and serum was analyzed for cotinine. We used linear regression to assess associations between biological measures and IQ subscale scores.
Results
Geometric mean blood lead (n=67), blood Mn (n=66), hair Mn (n=98), and serum cotinine (n= 69) concentrations were 1.13 ± 1.96 μg/dL, 10.06 ± 1.30 μg/L, and 360.22 ± 2.17 ng/g, 0.76 ± 6.12 μg/L respectively. After adjusting for potential confounders, hair Mn was negatively associated with Full Scale IQ.
Conclusions
Hair Mn was negatively associated with child IQ scores. Community partners were instrumental in the conception and implementation of this study.
Keywords: Manganese, Appalachia, children, environmental justice, community engagement
1. Introduction
The City of East Liverpool sits on the Ohio River in northeastern Ohio. East Liverpool was once the center of the American pottery industry and was coined the “Pottery Capital of the Nation” [1]. It reached its peak in 1970 with a population over 26,000 but rapidly declined along with the pottery industry; 2015 Census population estimate was just under 11,000 (92% Caucasian) [2]. Overall, 7.3% of residents have earned a bachelor's degree or higher compared to 29.3% in the nation; 30.6% of East Liverpool residents are below the federal poverty limit compared to 15.6% in the nation [2]. The East Liverpool School District reported that in 2010, they had a higher percentage of students in special education (19%) as compared to the state of Ohio (13%) [3]. These socioeconomic issues are compounded by potentially significant environmental exposures. An environmental organization, Save Our County, Inc., was formed by East Liverpool residents in 1982 in response to the proposed construction of a hazardous waste incinerator in their community. In 2005, East Liverpool was deemed a potential environmental justice area by the United States Environmental Protection Agency (US EPA).
The Ohio Environmental Protection Agency (Ohio EPA) 2010 All Ohio Air Toxics Report reported that manganese (Mn) concentrations from an air sampling station in East Liverpool were 30 times higher than the US EPA reference concentration of 0.05 μg/m3 [4]. Based on the evaluation of ambient air Mn data, Ohio EPA indicated that S.H. Bell, a warehouse facility which handles and distributes metals, minerals, and semi-finished industrial materials was the primary source of the airborne Mn [5]. Their data indicate that Mn concentrations in East Liverpool are highest when the wind is blowing from the direction of S.H. Bell [6]. Primarily due to elevated Mn levels, Ohio EPA calculated the non-cancer hazard index (HI) in East Liverpool as 34.5; an HI below 1.0 is considered “safe” [4].
Mn is an essential nutrient, but is neurotoxic in excess. Adverse neurological outcomes, such as declines in cognitive and motor function, are associated with occupational exposures as well as environmental exposures among highly exposed adults and children [7-12]. In response to the 2010 reports regarding elevated airborne Mn levels, the East Liverpool Public Schools Superintendent requested that “hair metal level tests” and “follow-up neuropsychological tests” be conducted on school-aged children …by Dr. Erin Haynes” [13]. The first author (E.N.H.) and coauthors (E.N.H, P.K., N.N., K.D.) were already engaged in an ongoing community-based participatory research study, Communities Actively Researching Exposure Study (CARES) in Marietta, Ohio with residents concerned about airborne Mn [14]. Following meetings with East Liverpool residents and the East Liverpool Board of Health, a pilot study was conducted to examine hair Mn and blood Mn concentrations in children [15]. Hair Mn concentrations in that pilot study were nearly double the levels found in children of similar age from the Marietta cohort [15]. The purpose of this study was to respond to the request of the school district Superintendent and investigate the association between Mn exposure and child cognition.
2. Methods
2.1 Study participants
This current study represents an expansion of CARES [12] into East Liverpool, Ohio. Children aged 7, 8, or 9 were recruited to participate if they resided in East Liverpool or the surrounding area throughout their life with no plans to relocate in the coming year (Figure 1). The biological mother must also have resided in the area during her pregnancy with the child. A volunteer sampling strategy was used for recruitment, which included postcards sent home from schools, advertisements aired on local radio and printed in local newspapers, and recruitment information placed in public locations such as libraries. Participant data collection took place March 2013-June 2014.
Figure 1. Map of East Liverpool CARES Participants.
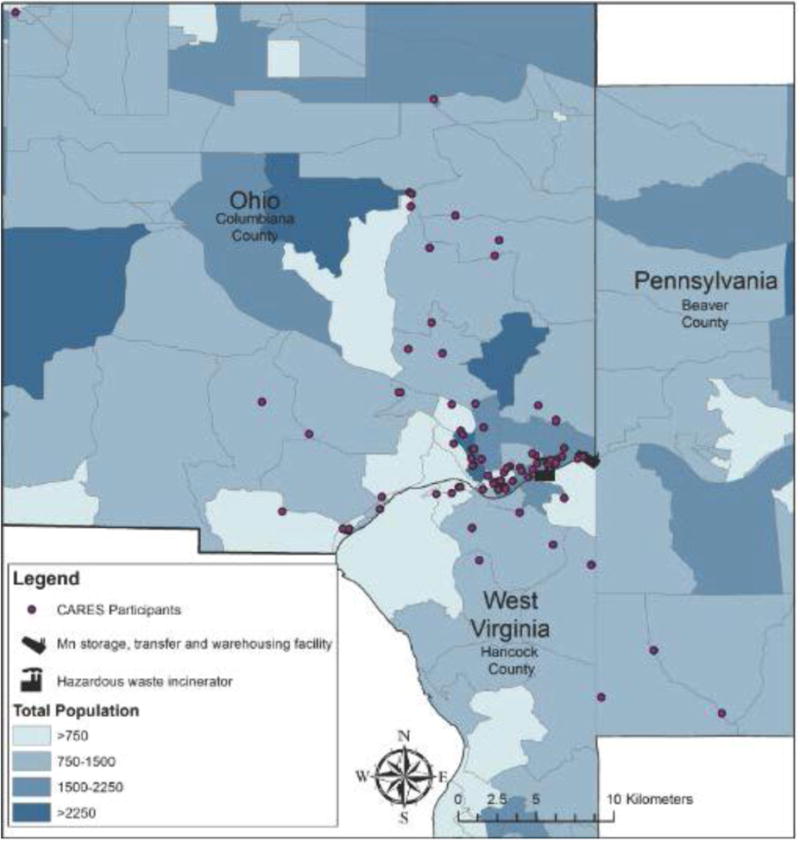
Children with a health condition that could impede their ability to participate in the behavioral assessment testing (i.e. a significant visual, auditory, or motor impairment) were excluded from participation. The University of Cincinnati Institutional Review Board approved this study. All parents signed an informed consent and children signed an informed assent.
High-volume sampling for Total Suspended Particulate (TSP) was conducted by Ohio EPA at three East Liverpool locations: Port Authority, Maryland Avenue and Water Plant (Figure 2). Through a public records request, Ohio EPA provided monthly values since 2003 for each of these locations. The values are a composite result from analysis of five filters for each month.
Figure 2. Location of Ohio EPA air monitoring locations in East Liverpool, Ohio.
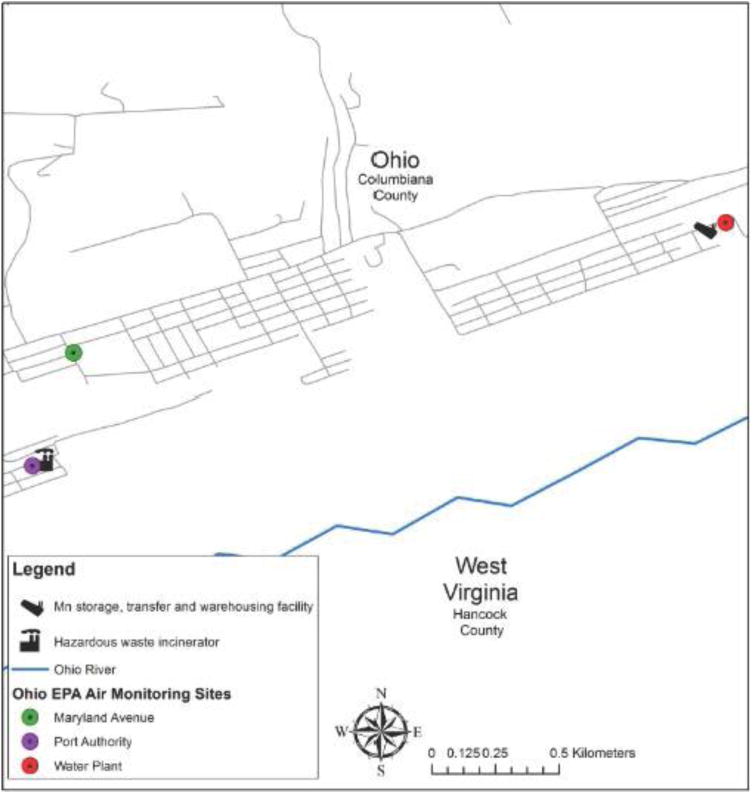
2.2 Specimen collection and analysis
The methods for specimen collection and analysis have been described in detail elsewhere [12, 16]. Approximately 20 strands of hair were collected from the occipital region, cut with ceramic scissors as close to the scalp as possible. Long hair was trimmed to 6 cm and taped towards the non-scalp-side of the hair shaft onto an index card with an arrow pointing in the direction of the scalp end. The hair sample was placed into a pre-labeled envelope and stored at room temperature until shipped. The Channing Trace Metals Laboratory, Brigham and Women's Hospital, Harvard School of Public Health, which processed hair samples for the pilot study, relocated to the Molecular Environmental Health Laboratory at the Mount Sinai Hospital in the interim and was utilized for the current study. The samples were first washed in a 1% (v/v) Triton™ X-100 solution and then digested using concentrated HNO3. Acid digestates were then analyzed by Inductively Coupled Plasma Mass Spectrometry (ICP-MS) using previously described methods. The method detection limit (MDL) for Mn in hair was 2 ng/g. In light of this change in location, five hair samples from the pilot study were re-tested, with the new lab providing slightly higher values, but within 10% of the original lab.
Venous whole blood specimens were collected and shipped to the Laboratory of Inorganic and Nuclear Chemistry at the New York State Department of Health's Wadsworth Center where they were analyzed for Mn via Graphite Furnace Atomic Absorption Spectrometry (GFAAS). The MDL for Mn in blood was 2.1 μg/L. Blood lead (Pb), cadmium (Cd), and mercury (Hg) was determined by ICP-MS. The MDL for blood Pb, Cd, and Hg were 0.069 μg/L, 0.042 μg/L, and 0.33 μg/L respectively. Serum cotinine levels were also measured at the Wadsworth Center. The MDL was 0.05 μg/L cotinine in serum. Child's serum ferritin was measured by the East Liverpool City Hospital.
2.3 Neurocognitive assessment
Neurocognitive assessments of the children were conducted by a registered nurse from East Liverpool after training by an experienced developmental neuropsychologist (K.N.D.). Quality control was maintained via periodic review of videotapes of the assessment sessions. The Wechsler Intelligence Scale for Children-IV (WISC-IV) [17] was administered at the time of biological sample collection.
The WISC-IV provides an overall score (Full Scale IQ) and four major areas of intellectual functioning including Perceptual Reasoning, Processing Speed, Working Memory, and Verbal Comprehension.
2.4 Other covariates
The IQ of the primary caregiver was assessed with the Wechsler Abbreviated Scale of Intelligence (WASI) [18]. The Parenting Relationship Questionnaire (PRQ) [19] was also administered. The PRQ provides T scores for the following domains: Attachment, Communication, Discipline Practices, Involvement, Parenting Confidence, School Satisfaction, and Relational Frustration. The PRQ was administered for each child, including siblings. Parent education was assessed using the Barratt Simplified Measure of Social Status (BSMSS) [20] [21]. Other demographic or socioeconomic factors considered included child age, child sex, child race (white yes/no), child birth weight, tobacco smoke exposure, whether or not the child's home was owned or rented, and parent income.
2.5 Statistical Analysis
The following variables were transformed using the natural logarithm to obtain normal distributions: hair Mn, blood Mn, blood Pb, serum cotinine, and serum ferritin. As a blood sample was unobtainable on some children, there were missing values for the biological measures: 7% hair Mn, 38% blood Mn, 37% blood Pb, 35% serum cotinine, and 40% serum ferritin. Correlations among variables were evaluated using Spearman's rank correlation coefficient. Potential differences between sexes for each biomarker were evaluated using the t-test. Multiple imputation using the Markov chain Monte Carlo method was used to impute missing value [22, 23]. The imputation models included all independent variables of interest and covariates and 10 datasets were imputed. Potential nonlinear associations between log hair and blood Mn and WISC-IV outcomes were examined using penalized splines in generalized additive models with covariates included as linear terms. Since there was insufficient evidence to suggest nonlinear associations, linear regression models were used to evaluate associations with WISC-IV outcomes. The method of generalized estimating equations was employed to account for the correlation between siblings. Covariates that were either statistically significantly associated with the outcome (p<0.05) or resulted in over 10% change in the biomarker variable when removed were retained in the final models. Separate regression models for each biomarker (hair Mn, blood Mn, blood Pb, and serum cotinine) were evaluated. The hair Mn models were stratified by sex. All biomarkers (hair Mn, blood Mn, blood Pb, and serum cotinine) along with covariates were then included in the multiple biomarkers models. The sample size of 106 children provides 80% power to detect a coefficient of slope greater than or equal to 2.52 between biomarker measure and WISC-IV outcome with significance level of 0.05 assuming a coefficient standard error of 0.90.
All statistical analyses were completed using SAS version 9.4 (SAS Institute Inc., Cary, NC) and R version 2.10.1 (The R Foundation for Statistical Computing, www.r-project.org).
3. Results
3.1 Air Sampling
Geometric mean Mn air sampling values from three Ohio EPA monitoring stations in East Liverpool (Figure 2 and 3) exceeded the US EPA reference concentration (RfC) of 0.05 μg/m3 during the current study period: Water Plant (1.04 μg/m3), Port Authority (0.17 μg/m3) and Maryland Avenue (0.09 μg/m3). The values from the Water Plant station were noticeably higher than the other two stations and were twentyfold the RfC. The monthly mean airborne Mn concentrations exceeded the RfC for every month during the study period at the Water Plant and Port Authority, and 69% of the months at the Maryland Avenue site. The geometric mean air concentrations measured from 2003-2014 were similar to the current study period: Water Plant (1.01 μg/m3), Port Authority (0.18 μg/m3) and Maryland Avenue (0.11 μg/m3); all of these values exceeded the RfC.
Figure 3. Monthly Mn total suspended particulate (TSP) concentration (μg/m3) from Ohio EPA monitoring stations in three East Liverpool locations 2003-2014.
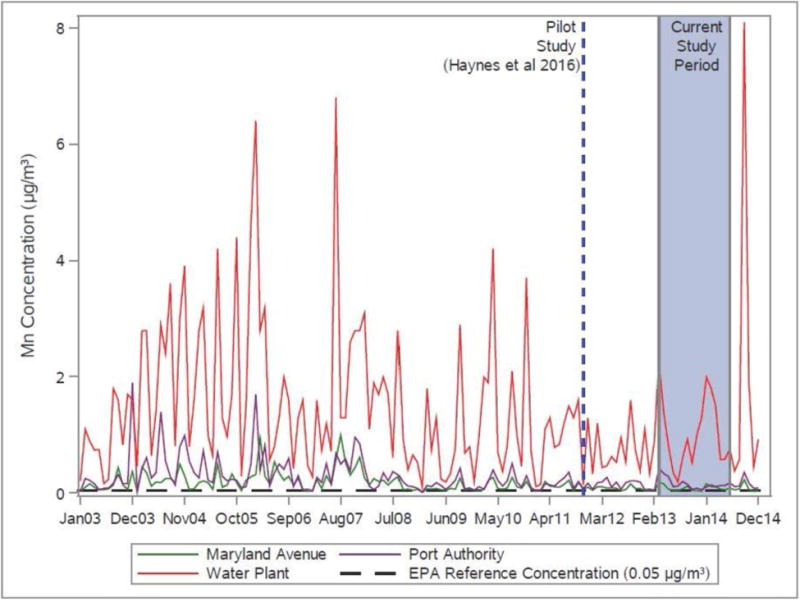
3.2 Descriptive characteristics of participants
Our study participants (n=106) were predominately Caucasian (non-Hispanic white, 83%) and female (61%) with a mean age of 8.4 years (Table 1). Annual household income below $20,000 was reported by 39%, with 81% below $50,000. Most parents (62%) reported having 12 or more years of education. Biological measures of blood Pb, blood Mn and hair Mn (geometric mean ± GSD) were 1.13 ± 1.96 μg/dL, 10.06 ± 1.30 μg/L, and 360.22 ± 2.17 ng/g, respectively (Table 1). Blood Hg and Cd were measured (geometric mean ± GSD were 0.11 ± 2.64 μg/dL, 0.09 ± 1.40 μg/L respectively) (data not shown). The geometric mean of serum cotinine was 0.76 μg/L (GSD ± 7.84 μg/L). Hair Mn was significantly and inversely correlated with blood Mn (r = -0.27, p=0.03) and significantly correlated with serum cotinine (r= 0.37, p<0.01) and blood Pb (r=0.27, p=0.03); blood Pb was significantly correlated with serum cotinine (r=0.44, p<0.001). Males were significantly higher in log blood Mn (p=0.02) while females were significantly higher in log blood Pb (p=0.01). No significant sex differences were identified for log hair Mn, log serum cotinine, and log serum ferritin. Mean performance on our core measures of intellectual attainment [18] were within normal limits, but below national standardized population norms by about 5-20 points or one third to more than one standard deviation.
Table 1. Descriptive characteristics of the study participants.
| Characteristic | East Liverpool Total (n=106) | |
|---|---|---|
| Child Measures | Range | |
| Age (years) [mean ± SD] | 8.40 ± 0.95 | 7.00 – 9.99 |
| Child's sex [n (%) ] | ||
| Male | 41 (39) | |
| Female | 65 (61) | |
| Race/ethnicity [n (%) ] | ||
| Caucasian | 88 (83) | |
| Hispanic | 3 (3) | |
| More than one race | 14 (13) | |
| Birth Weight (g) [mean ± SD] [n = 101] | 3270 ± 590 | 1361 – 4876 |
| Hours per day near someone smoking [mean ± SD] | 1.7 ± 3.3 | 0 – 8 |
| Household measures | Range | |
| Income [n (%) ] | ||
| Less than $20000 | 32 (36) | |
| $20000-$50000 | 37 (41) | |
| More than $50000 | 20 (23) | |
| Parent Education <12 Years [n (%) ] | 39 (44) | |
| Parent IQ [mean ± SD] [n = 89] | 95.98 ± 10.26 | 70 – 122 |
| Biological measures [GM ± GSD] | Range | |
| Hair Mn (ng/g) [n = 98] | 360.22 ± 2.17 | 16.84 – 15967.08 |
| Blood Mn (μg/L) [n = 66] | 10.06 ± 1.30 | 5.9 – 18.4 |
| Blood Pb (μg/dL) [n = 67] | 1.13 ± 1.96 | 0.30 – 6.64 |
| Serum Cotinine (μg/L) [n = 69] | 0.76 ± 6.12 | <DL – 20.59 |
| Serum Ferritin (ng/m) [n = 64] | 30.48 ± 1.72 | 10.7 – 160.6 |
| WISC IV [mean ± SD] | ||
| Verbal Comprehension | 92.71 ± 11.38 | 55 – 119 |
| Perceptual Reasoning | 96.91 ± 13.2 | 63 – 129 |
| Working Memory | 93.5 ± 12.05 | 65 – 123 |
| Processing Speed | 79.66 ± 9.16 | 53 – 106 |
| Full Scale IQ | 88.98 ± 11.34 | 54 – 110 |
WISC IV - Wechsler Intelligence Scale for Children-IV
3.3 Neurocognitive outcomes
Linear associations were investigated. In the single biomarker model, increasing log hair Mn was significantly associated with declines in Full Scale IQ, processing speed and working memory (Table 2, Model 1). In the sex-stratified models (Table 2, Model 2), although the associations between hair Mn and WISC-IV outcomes were not statistically significant, a similar trend was observed between hair Mn concentration and Full Scale IQ for both female (-2.24, 95% CI: −4.88, 0.39) and male (−1.55, 95% CI: −4.20, 1.10). In the multiple biomarkers model (Table 2, Model 3), the association between log hair Mn and WISC-IV outcomes was no longer statistically significant. There was no association between log blood Mn, log blood Pb, log serum cotinine, and WISC-IV outcomes, except log serum cotinine was positively associated with verbal comprehension in the multiple biomarkers model.
Table 2. Linear regression models for Wechsler Intelligence Scale for Children-IV (WISC-IV) outcomes (n=106).
| Full Scale IQa Beta (95% CI) | Perceptual Reasoningb Beta (95% CI) | Processing Speedc Beta (95% CI) | Working Memoryd Beta (95% CI) | Verbal Comprehensione Beta (95% CI) | |
|---|---|---|---|---|---|
| Model 1: Single biomarker models | |||||
| Log Hair Mn | −1.91* (−3.68, −0.14) | −0.88 (−3.08, 1.32) | −1.53 * (−3.03, −0.03) | −2.28 * (−4.31, −0.25) | −1.24 (−3.17, 0.68) |
| Log Blood Mnf | 3.49 (−6.65, 13.63) | 3.46 (−5.88, 12.80) | 2.08 (−5.72, 9.90) | 7.12 (−4.14, 18.37) | −3.37 (−14.09, 7.36) |
| Log Blood Pb | −2.71 (−5.85, 0.44) | −1.66 (−5.25, 1.94) | −2.14 (−4.95, 0.66) | −0.86 (−4.95, 3.22) | −1.90 (−5.50, 1.71) |
| Log Serum Cotinine | −0.42 (−1.57, 0.74) | −0.79 (−2.19, 0.60) | −0.63 (−1.67, 0.41) | −0.67 (−2.17, 0.83) | 0.76 (−0.76, 2.28) |
| Model 2: Log hair Mn sex stratified models | |||||
| Female, Log Hair Mn | −2.24 (−4.88, 0.39) | −2.16 (−5.24, 0.92) | −2.16 (−4.61, 0.29) | −2.18 (−5.22, 0.87) | −0.81 (−3.66, 2.04) |
| Male, Log Hair Mn | −1.55 (−4.20, 1.10) | 0.59 (−2.71, 3.90) | −1.59 (−3.25, 0.07) | −2.70 (−5.51, 0.11) | −0.63 (−3.36, 2.10) |
| Model 3: Multiple biomarkers models | |||||
| Log Hair Mn | −1.49 (−3.48, 0.48) | −0.16 (−2.66, 2.33) | −1.14 (−3.00, 0.72) | −1.94 (−4.52, 0.64) | −1.84 (−4.07, 0.40) |
| Log Blood Mnf | 1.71 (−7.90, 11.32) | 3.36 (−6.35, 13.08) | 0.72 (−7.13, 8.56) | 5.95 (−6.36, 18.24) | −5.37 (−14.79, 4.05) |
| Log Blood Pb | −2.58 (−6.10, 0.94) | −1.02 (−5.08, 3.03) | −1.60 (−4.73, 1.53) | 0.72 (−3.43, 4.87) | −2.80 (−6.63, 1.02) |
| Log Serum Cotinine | 0.20 (−1.09, 1.50) | −0.70 (−2.37, 0.96) | −0.14 (−1.36, 1.07) | −0.54 (−2.18, 1.09) | 1.66* (0.05, 3.26) |
p-value < 0.05
Covariates include parent IQ and Scholastic Satisfaction T score;
Covariates include parent IQ and home ownership;
Covariates include parent IQ, income, birth weight, and Communication T score;
Covariate includes parent IQ;
Covariates include Parent IQ, income, and Scholastic Satisfaction T score;
Serum ferritin also included in models with blood Mn
4. Discussion
During the current study, monthly mean airborne Mn concentrations exceeded US EPA reference concentration of 0.05 μg/m3 at Ohio EPA sampling stations: Water Plant (100% months), Port Authority (100% months), and Maryland Avenue (69% months). The mean value of Mn measured at the Water Plant sampling station also exceeded US EPA reference concentration by twentyfold during the study period. Using Water Plant samples collected in 2011, the Mn fraction of TSP was determined as 0.35 for PM10 (aerodynamic diameter < 10 microns) and 0.037 for PM25 (aerodynamic diameter < 2.5 microns) [24]. Utilizing 2003-2013 Ohio EPA air sampling data from East Liverpool and these size fractions, the estimated respirable (PM10) geometric mean concentration was 0.123 μg/m3 and PM25 was 0.013 μg/m3 [24]. The Pennsylvania Department of Environmental Protection collected TSP and PM10 samples at a location one-mile northeast of the East Liverpool Water Plant from October 26, 2014 through July 5, 2015, after the current study period [25]. The arithmetic mean airborne Mn concentration for TSP was 0.521 μg/m3 (n=40 samples) and PM10 was 0.1452 μg/m3 (n=41 samples).
A number of studies have reported neurological health effects at similar levels of airborne Mn [10, 26–33]. Another Ohio community with environmental Mn exposure demonstrated reduced child IQ at a reported geometric mean airborne Mn PM2.5 exposure level of 0.011 μg/m3 [12, 26]. A Brazilian study with limited PM2.5 samples (n=7) with a mean of 0.15 μg/m3 identified reduced cognitive function among mothers and children [27] [28]. Impaired motor function has been identified among those with environmental Mn exposure measured at 0.13 μg/m3 (PM10 geometric mean) [29] and 0.05 μg/m3 (PM10) [10]. An environmentally exposed Quebec cohort demonstrated neurological health effects with average air Mn measured at 0.022 μg/m3 TSP and 0.013 μg/m3 PM10 [30] [31–33].
Inhaled Mn is capable of crossing the blood brain barrier and can enter the brain through axonal transport from the olfactory bulb to the cerebral cortex [34]. Mn accumulates in iron-rich brain regions of the basal ganglia: caudate, putamen, globus pallidus, substantia nigra, and subthalamic nuclei of the brain [35, 36]. Children may be particularly susceptible to the neurotoxic effects of ambient Mn exposure, as their brains are undergoing a dynamic process of growth, differentiation, pathway direction, and apoptosis. Maturational events in the brain, such as myelination and the formation of synapses, are critical developmental events that influence higher cognitive functions and emotional control [37]. In the current study, after adjusting for covariates, a significant inverse association between hair Mn concentration and Full Scale IQ was identified in this cohort of children. This association was most apparent in the working memory and processing speed WISC-IV subscales. Even when not significant, all subscales demonstrated an inverse relationship. Inverse linear relationships between children's hair Mn and Full Scale IQ have previously been reported [27] [38], [39], [16]. Within the Marietta CARES cohort, Haynes et al. found an inverse U-shaped association between IQ and children's hair Mn, demonstrating an association with both elevated and decreased levels of Mn [12]. In the current study, a nonlinear effect was not observed which could be related to the higher environmental levels of ambient Mn levels in this population shifting the dose response curve to the right, creating a more linear relationship. When the data from the current study were pooled with the Marietta CARES cohort[12], the inverse U-shaped association was observed (data not shown).
The inverse association between hair Mn concentration and Full Scale IQ identified in this cohort of children was observed despite a mean hair Mn concentration (360 ng/g, range 17 to 15967 ng/g) lower than other studies: Menezes-Filho et al. (mean hair Mn 5,830 ng/g, range 100 to 86,680 ng/g), Riojas-Rodriguez et al. (mean 12,130 ng/g, range 4,200 to 48,000 ng/g), Bouchard et al. (mean 700 ng/g, range 10 to 21000 ng/g) [39] [38], [27]. This may be due to differences in hair collection and analysis methodologies [40]. The Marietta CARES cohort had mean hair Mn concentration (416 ng/g) [12] comparable with the current study as well as with a cohort of children aged 11–13 who lived near a hazardous waste site in Oklahoma (mean 471 ng/g, range 89 to 2145 ng/g) [16] using similar hair collection and analysis methodologies [12, 16]. A study of adults in East Liverpool has also found an association between increased environmental Mn exposure and deficits in cognitive function [41].
The mean hair Mn concentration in the current study was approximately half the concentration in the pilot study (716 ng/g) [15]. Many factors could influence hair Mn concentration. As data collection methodology was identical, it is unclear whether differences in hair Mn among the pilot study, other studies and the current study are a reflection of differences in exposure levels, Mn metabolism/excretion levels, or in characteristics of the cohorts such as rates of sequestration of Mn into hair. Mean exposure levels declined slightly from the 6-month period prior to the pilot study compared to those during the current study. However, as seen in Figure 3, there is a great deal of variability in the exposure levels which likely impacted measured hair Mn values, particularly for male participants. The study cohort resided in essentially the same geographic area as the pilot cohort. Although both cohorts had the same number of participants (n=106), only 14 (13.2%) were in both studies. Race and sex differences have been demonstrated in blood Mn, but not hair Mn, levels [42]. Race was similar, with the percent reporting Caucasian at 83% in the current study and 92% in the pilot study. Participants in the current study were more likely to be female and sex has been associated with lower Mn biomarkers [43]. However, no significant difference in hair Mn was observed between the sexes. As the pilot study had no neurocognitive assessment, an exploration of the impact of the difference in these characteristics cannot be pursued. It should be noted that the primary source of exposure in this study was ambient Mn. Presumably, ambient Mn would be found on the outside of the hair shaft. Our laboratory carefully washed each hair sample to remove external Mn but some may have remained. While often considered as a contaminant, presumably this external Mn would theoretically correlate with cumulative ambient Mn exposure. If so, this may explain why an association was found primarily with this biomarker. It could also explain the lack of relationship between sex and hair Mn as well, as internal metabolism/absorption would be impacted.
A lack of correlation between hair Mn and blood Mn has been demonstrated in similar studies [12], [27] and likely reflects differences in exposure timeframes or pathways as measured by the two biomarkers. The current literature suggests that hair Mn may reflect chronic exposure whereas blood Mn may best represent current exposure [40, 44–46]. Figure 3 demonstrates the variability in exposure levels that may contribute to the lack of correlation.
Community engagement in research has long been known to improve the quality and meaningfulness of the research [47] [48] [49] [50]. The role of community partners in the conduct of this research study was critical. Kent State East Liverpool Campus facilitated space for the research, providing a convenient and trusted location for study participants. Community partners provided insight on recruitment strategies and connections with local school districts and local organizations for distribution of study invitation materials. Research team meetings were held weekly with community partners to resolve issues that arose and to ensure consistency between the Marietta and East Liverpool sites.
This study has several limitations. The number of children in this current study was limited, which may have prevented the ability to detect an inverted U-shaped association between hair Mn and child IQ; nonetheless, a linear association was observed. Biomarker measurements may not reflect chronic exposure and declines in IQ may be more related to historic exposures. It is currently unknown what exposure time frame is most closely related to neurodevelopmental health effects of Mn exposure. Also unknown is the relative importance of chronic versus peak exposure. New research is providing insight into the role of various pathways of exposure such as air, soil, indoor dust and water [51] [52].
In conclusion, increasing hair Mn concentration was significantly and inversely associated with child cognition. Mn exposure in this community should be further evaluated in comparison to other pediatric Mn cohorts. Environmental justice issues such as psychosocial stressors should be included in these analyses as they may play a role in neurotoxicity. Furthermore, exposure to multiple environmental toxicants from other local industries should be considered, including hydraulic fracturing and hazardous waste incineration. Community partners assisted in both the conception and the implementation of this study.
EL paper Highlights.
Air manganese levels in East Liverpool, Ohio exceeded EPA reference levels for a decade
An academic-community partnership was essential for the conduct of the study
IQ scores for children aged 7-9 were negatively associated with manganese levels in hair
Acknowledgments
Funding: This research was supported by the NIEHS (R01ES016531, R21ES021106, and P30-ES06096) and NIH/NCRR 8UL1TR000077. The funding agencies were not involved in data collection, analysis, interpretation, or writing of this manuscript.
The authors acknowledge the contributions of Amy Sigley, RN, Delores Silverthorn, RN, Jody Alden, RN, Kent State University East Liverpool Campus, Virgil Reynolds, and Mike Walton.
List of Abbreviations
- BSMSS
Barratt Simplified Measure of Social Status
- CARES
Communities Actively Researching Exposure Study
- Cd
cadmium
- GSD
geometric standard deviation
- Hg
mercury
- HI
hazard index
- ICP-MS
Inductively Coupled Plasma Mass Spectrometry
- MDL
method detection limit
- Mn
Manganese
- Ohio EPA
Ohio Environmental Protection Agency
- Pb
lead
- PM10
particle with aerodynamic diameter < 10 microns
- PM2.5
particle with aerodynamic diameter < 10 microns
- PRQ
Parenting Relationship Questionnaire
- RfC
reference concentration
- TSP
Total Suspended Particulate
- US EPA
United States Environmental Protection Agency
- WASI
Wechsler Abbreviated Scale of Intelligence
- WISC-IV
Wechsler Intelligence Scale for Children-IV
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.East Liverpool Historical Society Webpage. [Accessed August 29];2016 http://www.eastliverpoolhistoricalsociety.org/
- 2.US Census American Fact Finder. [Accessed October 12];2016 http://factfinder.census.gov/faces/nav/jsf/pages/index.xhtml#.
- 3.FY2010 District Profile Report. [Accessed October 12];2016 http://education.ohio.gov/Topics/Finance-and-Funding/Finance-Related-Data/District-Profile-Reports/FY2010-District-Profile-Report.
- 4.All Ohio Air Toxics Report. [Accessed May 23];2016 http://www.epa.ohio.gov/dapc/atu.aspx.
- 5.Director's Final Findings and Orders. S.H. Bell Company; [Accessed October 12]. 2016. http://www.epa.ohio.gov/portals/27/enforcement/year_2010/SHBell_020810.pdf. [Google Scholar]
- 6. [Accessed August 29];Health Consultation - East Liverpool Air Quality. 2016 http://www.atsdr.cdc.gov/HAC/pha/EastLiverpoolHC/EastLiverpoolHealthConsultation11210.pdf.
- 7.Racette BA, Criswell SR, Lundin JI, Hobson A, Seixas N, Kotzbauer PT, et al. Increased risk of parkinsonism associated with welding exposure. NeuroToxicology. 2012;33:1356–1361. doi: 10.1016/j.neuro.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rugless F, Bhattacharya A, Succop P, Dietrich KN, Cox C, Alden J, et al. Childhood exposure to manganese and postural instability in children living near a ferromanganese refinery in Southeastern Ohio. Neurotoxicol Teratol. 2014;41:71–79. doi: 10.1016/j.ntt.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lucchini R, Bergamaschi E, Smargiassi A, Festa D, Apostoli P. Motor function, olfactory threshold, and hematological indices in manganese-exposed ferroalloy workers. Environ Res. 1997;73:175–180. doi: 10.1006/enrs.1997.3702. [DOI] [PubMed] [Google Scholar]
- 10.Lucchini RG, Guazzetti S, Zoni S, Donna F, Peter S, Zacco A, et al. Tremor, olfactory and motor changes in Italian adolescents exposed to historical ferro-manganese emission. Neurotoxicology. 2012;33:687–696. doi: 10.1016/j.neuro.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bowler RM, Gysens S, Diamond E, Nakagawa S, Drezgic M, Roels HA. Manganese exposure: neuropsychological and neurological symptoms and effects in welders. Neurotoxicology. 2006;27:315–326. doi: 10.1016/j.neuro.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 12.Haynes EN, Sucharew H, Kuhnell P, Alden J, Barnas M, Wright RO, et al. Manganese exposure and neurocognitive outcomes in rural school-age children: The Communities Actively Researching Exposure Study. Environ Health Perspect. 2015;123:1066–1071. doi: 10.1289/ehp.1408993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McElwain MD. EL board may get help with health study. The Review. 2010 Dec 21; [Google Scholar]
- 14.Haynes EN, Beidler C, Wittberg R, Meloncon L, Parin M, Kopras EJ, et al. Developing a bidirectional academic-community partnership with an Appalachian-American community for environmental health research and risk communication. Environ Health Perspect. 2011;119:1364–1372. doi: 10.1289/ehp.1003164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haynes EN, Elam S, Burns R, Spencer A, Yancey E, Kuhnell P, et al. Community engagement and data disclosure in environmental health research. Environ Health Perspect. 2015;124:A24–27. doi: 10.1289/ehp.1510411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wright RO, Amarasiriwardena C, Woolf AD, Jim R, Bellinger DC. Neuropsychological correlates of hair arsenic, manganese, and cadmium levels in school-age children residing near a hazardous waste site. Neurotoxicology. 2006;27:210–216. doi: 10.1016/j.neuro.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 17.Wechsler D. Wechsler Intelligence Scale for Children-IV. San Antonio, TX: Harcourt Assessment, Inc.; 2003. [Google Scholar]
- 18.Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Harcourt Assessment, Inc; 1999. [Google Scholar]
- 19.Reynolds CR, Kamphaus RW. Behavior Assessment System for Children. Second. Circle Pines, MN: American Guidance Service; 2004. [Google Scholar]
- 20.Barratt W. The Barratt Simplified Measure of Social Status. Terre Haute: Indiana State University; 2012. [Google Scholar]
- 21.Davis J, Smith T, Hodge R, Nakao K, Treas K. Occupational prestige ratings from the 1989 general social survey. Ann Arbor, MI: Inter-University Consortium for Political and Social Research; 1991. [Google Scholar]
- 22.Bernard J, Rubin DB. Small-sample degrees of freedom with multiple imputations. Biometrika. 1999;86:948–955. [Google Scholar]
- 23.Schafer JL. Analysis of Incomplete Multivariate Data. New York: Chapman and Hall; 1997. [Google Scholar]
- 24.Colledge MA, Julian JR, Gocheva VV, Beseler CL, Roels HA, Lobdell DT, Bowler RM. Characterization of air manganese exposure estimates for residents in two Ohio towns. J Air Waste Manag Assoc. 2015;65:948–957. doi: 10.1080/10962247.2015.1040525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ambient Air Toxics Monitoring Project Summary. Glasgow Borough (Beaver County), Pennsylvania: [Accessed August 29]. 2016. http://files.dep.state.pa.us/RegionalResources/SWRO/SWROPortalFiles/Ambient%20Air%20Toxics%20Monitoring%20Project%20Summary,%20Glasgow%20Borough%20(Beaver%20C….pdf. [Google Scholar]
- 26.Haynes EN, Ryan P, Chen A, Brown D, Roda S, Kuhnell P, et al. Assessment of personal exposure to manganese in children living near a ferromanganese refinery. Sci Total Environ. 2012;427-428:19–25. doi: 10.1016/j.scitotenv.2012.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Menezes-Filho JA, Novaes Cde O, Moreira JC, Sarcinelli PN, Mergler D. Elevated manganese and cognitive performance in school-aged children and their mothers. Environ Res. 2011;111:156–163. doi: 10.1016/j.envres.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Menezes-Filho JA, Paes CR, Pontes AM, Moreira JC, Sarcinelli PN, Mergler D. High levels of hair manganese in children living in the vicinity of a ferro-manganese alloy production plant. Neurotoxicology. 2009;30:1207–1213. doi: 10.1016/j.neuro.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodriguez-Agudelo Y, Riojas-Rodriguez H, Rios C, Rosas I, Sabido Pedraza E, Miranda J, et al. Motor alterations associated with exposure to manganese in the environment in Mexico. Sci Total Environ. 2006;368:542–556. doi: 10.1016/j.scitotenv.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 30.Baldwin M, Mergler D, Larribe F, Belanger S, Tardif R, Bilodeau L, Hudnell K. Bioindicator and exposure data for a population based study of manganese. Neurotoxicology. 1999;20:343–353. [PubMed] [Google Scholar]
- 31.Beuter A, Edwards R, deGeoffroy A, Mergler D, Hundnell K. Quantification of neuromotor function for detection of the effects of manganese. Neurotoxicology. 1999;20:355–366. [PubMed] [Google Scholar]
- 32.Bowler RM, Mergler D, Sassine MP, Larribe F, Hudnell K. Neuropsychiatric effects of manganese on mood. Neurotoxicology. 1999;20:367–378. [PubMed] [Google Scholar]
- 33.Mergler D, Baldwin M, Belanger S, Larribe F, Beuter A, Bowler R, et al. Manganese neurotoxicity, a continuum of dysfunction: results from a community based study. Neurotoxicology. 1999;20:327–342. [PubMed] [Google Scholar]
- 34.Elder A, Gelein R, Silva V, Feikert T, Opanashuk L, Carter J, et al. Translocation of inhaled ultrafine manganese oxide particles to the central nervous system. Environ Health Perspect. 2006;114:1172–1178. doi: 10.1289/ehp.9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bock NA, Paiva FF, Nascimento GC, Newman JD, Silva AC. Cerebrospinal fluid to brain transport of manganese in a non-human primate revealed by MRI. Brain Res. 2008;1198:160–170. doi: 10.1016/j.brainres.2007.12.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uchino A, Noguchi T, Nomiyama K, Takase Y, Nakazono T, Nojiri J, Kudo S. Manganese accumulation in the brain: MR imaging. Neuroradiology. 2007;49:715–720. doi: 10.1007/s00234-007-0243-z. [DOI] [PubMed] [Google Scholar]
- 37.Rakic PBJ, Goldman-Rakic PS. Synaptic development of the cerebral cortex: implications for learning, memory, and mental illness. Prog Brain Res. 1994;102:227–243. doi: 10.1016/S0079-6123(08)60543-9. [DOI] [PubMed] [Google Scholar]
- 38.Riojas-Rodriguez H, Solis-Vivanco R, Schilmann A, Montes S, Rodriguez S, Rios C, Rodriguez-Agudelo Y. Intellectual function in Mexican children living in a mining area and environmentally exposed to manganese. Environ Health Perspect. 2010;118:1465–1470. doi: 10.1289/ehp.0901229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bouchard M. Intellectual impairment in school-age children exposed to manganese from drinking water. Environmental Health Perspectives. 2011;119:138–143. doi: 10.1289/ehp.1002321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eastman RR, Jursa TP, Benedetti C, Lucchini RG, Smith DR. Hair as a biomarker of environmental manganese exposure. Environ Sci Technol. 2013;47:1629–1637. doi: 10.1021/es3035297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bowler RM, Kornblith ES, Gocheva VV, Colledge MA, Bollweg G, Kim Y, et al. Environmental exposure to manganese in air: Associations with cognitive functions. Neurotoxicology. 2015;49:139–148. doi: 10.1016/j.neuro.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oulhote Y, Mergler D, Bouchard M. Sex- and age-differences in blood manganese levels in the U.S. general population: national health and nutrition examination survey 2011-2012. Environ Health. 2014;13:87–98. doi: 10.1186/1476-069X-13-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Finley JW. Manganese absorption and retention by young women is associated with serum ferritin concentration. Am J Clin Nutr. 1999;70:37–43. doi: 10.1093/ajcn/70.1.37. [DOI] [PubMed] [Google Scholar]
- 44.Bouchard M, Laforest F, Vandelac L, Bellinger D, Mergler D. Hair manganese and hyperactive behaviors: pilot study of school-age children exposed through tap water. Environ Health Perspect. 2007;115:122–127. doi: 10.1289/ehp.9504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park RM, Baldwin M, Bouchard MF, Mergler D. Airborne manganese as dust vs. fume determining blood levels in workers at a manganese alloy production plant. Neurotoxicology. 2014;45:267–275. doi: 10.1016/j.neuro.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O'Neal SL, Zheng W. Manganese Toxicity Upon Overexposure: a Decade in Review. Curr Environ Health Rep. 2015;2:315–328. doi: 10.1007/s40572-015-0056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Israel BA. Review of Community-Based Research: Assessing Partnership Approaches to Improve Public Health. Annual Review of Public Health. 1998;19:173–202. doi: 10.1146/annurev.publhealth.19.1.173. [DOI] [PubMed] [Google Scholar]
- 48.Israel BA Methods in Community- Based Participatory Research for Health. San Francisco, CA: Jossey-Bass; 2005. [Google Scholar]
- 49.Macaulay AC, Commanda LE, Freeman WL, Gibson N, McCabe ML, Robbins CM, Twohig PL. Participatory research maximizes community and lay involvement. British Medical Journal. 1999;319:774–778. doi: 10.1136/bmj.319.7212.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O'Fallon LR, Dearry A. Community-based participatory research as a tool to advance environmental health sciences. Environ Health Perspect. 2002;110(2):155–159. doi: 10.1289/ehp.02110s2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fulk F, Succop P, Hilbert TJ, Beidler C, Brown D, Reponen T, Haynes EN. Pathways of inhalation exposure to manganese in children living near a ferromanganese refinery: A structural equation modeling approach. Science of the Total Environment. 2017;579:768–775. doi: 10.1016/j.scitotenv.2016.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lucas EL, Bertrand P, Guazzetti S, Donna F, Peli M, Jursa TP, et al. Impact of ferromanganese alloy plants on household dust manganese levels: Implications for childhood exposure. Environ Res. 2015;138C:279–290. doi: 10.1016/j.envres.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]


