Abstract
BACKGROUND
Abnormally elevated levels of γ-aminobutyric acid (GABA) in the medial prefrontal cortex (MPFC) have been reported in antipsychotic-free patients with schizophrenia. Whether such GABA elevations are also present in other brain regions and persist after antipsychotic treatment has not been previously investigated.
METHODS
Twenty-eight antipsychotic-naive first-episode psychosis (FEP) patients and 18 healthy control subjects completed the study. Following baseline proton magnetic resonance spectroscopy scans targeting the MPFC and a second region, the dorsal caudate, FEP patients were treated with oral risperidone for 4 weeks, at an initial dose of 1mg/day that was titrated as necessary based on clinical judgment. After the 4-week treatment period, both groups were brought back to undergo outcome MRS scans, which were identical to the scans conducted at baseline.
RESULTS
At baseline, higher GABA levels were found both in the MPFC and in the dorsal caudate of FEP patients than in healthy control subjects. Following 4 weeks of antipsychotic treatment, GABA levels in FEP patients decreased relative to baseline in the MPFC, while decreasing only at the trend-level relative to baseline in the dorsal caudate. For either brain region, GABA levels at 4 weeks or post-treatment did not differ between FEP patients and healthy control subjects.
CONCLUSIONS
The results of the present study have documented elevations of GABA levels in both the MPFC and, for the first time, in the dorsal caudate of antipsychotic-naive FEP patients, which normalized in both regions following 4 weeks of antipsychotic treatment.
Keywords: GABA, psychosis, first-episode, schizophrenia, magnetic resonance spectroscopy, antipsychotic treatment
Introduction
Dysregulations of γ-aminobutyric acid (GABA), the major inhibitory amino acid neurotransmitter, have been described in schizophrenia (1–4). GABAergic neurotransmission plays a critical role in the coordination of pyramidal cell activity, and its abnormalities have been linked to neurocognitive deficits in schizophrenia (5). Neuroimaging studies using proton magnetic resonance spectroscopy (1H MRS) to measure GABA levels in vivo in schizophrenia have yielded variable results, with most studies in antipsychotic-treated, stable schizophrenia patients showing lower or normal GABA levels in the anterior cingulate cortex (6), the medial prefrontal cortex (MPFC) (7–11), the dorsolateral prefrontal cortex (8), the dorsal anterior cingulate cortex (9, 12), parieto-occipital cortices (7, 11, 13, 14), and the basal ganglia (7, 14) compared to healthy subjects, while studies in antipsychotic-free patients found lower GABA levels in the occipital cortex (15), normal levels in the dorsal anterior cingulate (12), or elevations in the ventromedial prefrontal cortex (16) and in the MPFC (8). Moreover, in the first study that measured GABA levels in both the MPFC and the dorsolateral prefrontal cortex of the same antipsychotic-free and antipsychotic-treated patients (8), GABA elevations were found in the MPFC of antipsychotic-free patients compared to both the medicated patients and control subjects, while no differences were found among the three groups in the dorsolateral prefrontal cortex. In addition, we recently reported elevated GABA levels in the MPFC and dorsal caudate of antipsychotic-naive individuals at ultra-high risk (UHR) for psychosis (17). These findings suggest that the discrepancies in the in vivo 1H MRS measures of GABA in schizophrenia or psychotic illness reported to date may reflect differences in the targeted brain regions, as well as in the medication status of the cohorts.
The objective of the present study was to assess the effects of antipsychotic medication and brain region on 1H MRS measures of GABA levels using a study design that minimized within-subjects or cohort variability as a potential confound to enhance methodological rigor. A within-subjects study design and 1H MRS were used to measure prefrontal and striatal GABA levels in antipsychotic-naïve first-episode psychosis (FEP) patients, before and then again after 4 weeks of antipsychotic treatment, in comparison with a group of healthy control subjects at the same time points. Based on our prior studies in medicated and unmedicated patients with schizophrenia (8) and in UHR subjects (17), we hypothesized that GABA levels in antipsychotic-naïve FEP patients will be higher than in control subjects at baseline in both the prefrontal and striatal regions, and that the effect of 4 weeks of antipsychotic treatment will be to decrease or normalize GABA levels in both brain regions. Our overall expectation was that the results of the present within-subjects study would offer the opportunity to clarify the nature of the discrepancy in the in vivo 1H MRS measures of GABA levels reported to date because two different brain regions will be targeted in the same subjects, and only the medication status of the patient cohort will differ between the baseline and 4-week assessments.
Methods and Materials
Participants
Twenty-eight right-handed patients were recruited during their first non-affective psychosis episode, through the inpatient or outpatient services of the Instituto Nacional de Neurología y Neurocirugía (INNN) in Mexico City. For inclusion in the study, which was approved by the Ethics and Scientific Committees of the INNN, participants were assessed using the Structured Clinical Interview for DSM-IV and, if found eligible, they were enrolled into the study after providing informed written consent, which was obtained from both parents for participants under 18 years of age. FEP participants were required to be antipsychotic-naive. Exclusion criteria consisted of concomitant medical or neurological illness, history of head trauma with loss of consciousness, current substance abuse or history of substance dependence (excluding nicotine), comorbidity with any other Axis I disorder, active suicidal risk, and psychomotor agitation. Eighteen right-handed healthy subjects were recruited to serve as the normal control group. Participants in this group were assessed in the same manner as the patients, and those with a history of psychiatric illness or family history of schizophrenia were excluded. All participants were screened for drugs of abuse (cannabis, cocaine, heroin, opioids, and benzodiazepines) using Instant-View 5 Panel urine toxicology test, (American Screening Corporation, Shreveport, LA) at inclusion and at 1 hour prior to the 1H MRS scans.
After the baseline 1H MRS scan, patients were treated with oral risperidone for 4 weeks, with an open titration schedule based on clinical judgment, starting with 1mg/daily. Concomitant medications, including benzodiazepines, mood stabilizers and antidepressants, were not allowed for the duration of the study. Treatment response was defined clinically as a reduction of at least 25% in the total Positive and Negative Syndrome Scale (PANSS) score after 4 weeks of treatment. This reduction is above the 20% that can be clinically detected (18) and in accordance with the current consensus of treatment response (19).
Magnetic Resonance Neuroimaging Data Acquisition Procedures
All the neuroimaging studies were conducted at the INNN on a 3.0-T whole-body MRI system (Signa Excite HDxt; GE Healthcare) using an 8-channel phased-array receive-only head coil and a body-transmit coil. To ensure reproducible prescription of the voxels of interest and minimize head motion, each participant’s head was positioned along the cantho-meatal line and then immobilized with a forehead strap. Standardized high-resolution T1-weighted spoiled gradient-recalled (SPGR) echo imaging series (echo time, 5 milliseconds; repetition time, 12 milliseconds; inversion time, 450 milliseconds; flip angle, 20°; field of view, 25.6 cm; 256 × 256 matrix; 186 slices; slice thickness,1 mm), oriented above and parallel to the anterior-posterior commissures line, was acquired, and then reformatted to sagittal and coronal views to use for optimal 1H MRS voxel placement. The same volumetric SPGR images were used for brain tissue segmentation.
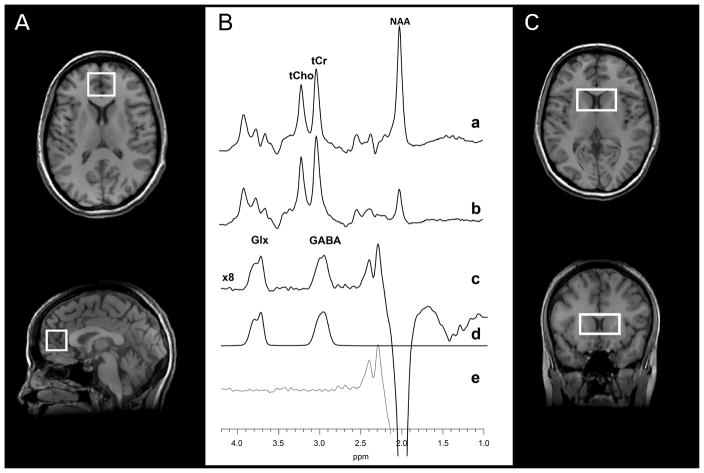
To obtain the brain GABA data, the standard J-edited spin echo difference method (20) was implemented as fully described recently (21) to target a 3.0×2.5×2.5-cm3 MPFC voxel (including portions of Brodmann areas 24, 32 and 10, and the pregenual anterior cingulate cortex), and a 4.5×2.5×2.0-cm3 voxel prescribed to include primarily the dorsal caudate bilaterally, with the inferior edge of the voxel positioned 3 mm dorsal to the anterior commissure (Figure 1). Each spectrum was recorded in 13.4 minutes using 256 interleaved excitations (512 total), with the editing pulse on or off (echo time, 68 milliseconds; repetition time, 1500 milliseconds). The static magnetic field homogeneity was optimized to an unsuppressed water resonance full-width at half maximum (FWHM) of 12 Hz or less. Reproducible positioning of the 1H MRS voxels following treatment to minimize variability between the duplicate scans was based on images that captured each subject’s voxel positioning at baseline.
Figure 1.
Voxel placement in two regions of interest: (A) the medial prefrontal cortex and (C) the bilateral dorsal caudate. (B) Representative J-editing spectra obtained from the medial prefrontal cortex using volume-selective point-resolved spectroscopy with the editing radiofrequency pulse (a) on and (b) off. The difference of the spectra in (a) and (b), showing (c) the edited γ-aminobutyric acid (GABA) and combined resonance of glutamate and glutamine (Glx) peaks, with (d) best-fit model spectrum of (c), and (e) the residuals of the difference between the edited (c) and best-fit (d) spectra. NAA, N-acetyl-aspartate; tCho, total choline; tCr, total creatine.
1H MRS Data Analysis
Details of the 1H MRS data quality assessment criteria to retain or reject spectra for inclusion in group analyses can be found in supplementary online material provided recently (21). To obtain GABA levels, spectra that met those quality assessment criteria were modeled as a linear combination of pseudo-Voigt lineshape functions and then fitted in the frequency domain (Figure 1C) by one of the investigators (X.M.), blinded to diagnosis and clinical status, using a highly optimized public-domain Levenberg–Marquardt nonlinear least squares minimization routine in a data analysis package, XsOSNMR, written in Interactive Data Language (IDL, Exelis Visual, Boulder, Co) at Weill Cornell Medicine (21). The resulting peak areas were then expressed semi-quantitatively as ratios relative to the area of synchronously acquired and similarly fitted unsuppressed voxel water signal. Peak areas of the other metabolites, including the combined resonance of glutamate and glutamine in the edited spectra (Glx), N-acetylaspartate (NAA), total choline (tCho), and total creatine (tCr) in subspectra acquired with the editing pulse turned off, were similarly obtained by spectral fitting (Figure 1C). In-house software developed in MATLAB (MathWorks, Natick, MA) was then used to create a segmentation mask for each voxel, from which the proportions of grey matter, white matter, and cerebrospinal fluid content were determined. In case of significant differences in the proportions of tissue types between groups, these would be included in the statistical model as covariates.
Statistical analysis
Demographic and clinical characteristics were compared between the FEP and control groups using independent-sample t tests. Frequency data were analyzed using χ2 or Fisher’s exact tests. Levels of GABA, the metabolite of primary interest, were analyzed using repeated-measures analyses of variance (RMA), with time (2 levels: baseline and 4 weeks after) as within-subject factors, and group (2 levels: FEP and healthy controls) as the between-subject factor. Additionally, we carried out similar RMA tests using GABA peak area relative to tCr for comparison with prior studies that used tCr rather than the unsuppressed voxel tissue water signal as the intensity reference. Greenhouse-Geisser corrections were applied whenever Mauchly's tests of sphericity were significant for any factor, and for the RMA, we explored the influence of age, gender, and tobacco use as covariates. Independent-samples t tests and paired-samples t tests were used for within-groups post-hoc comparisons, for the rest of the metabolites, tissue composition, and spectral quality measures. The level of significance of all tests was set at p<0.05.
Pearson’s product-moment correlations were used to examine potential associations between clinical scores (PANSS subscales - Positive, Negative, and General psychopathology) and GABA levels in each region in the FEP group, using a statistical threshold of p<.016 (p<.05/3). Lastly, potential relationships between GABA levels, time point (baseline and at 4 weeks) and regions (MPFC and dorsal caudate) were explored. The statistical threshold for these exploratory correlations was set at p<.012 (p<.05/4).
Results
Demographic and Clinical Characteristics of the Study Participants
The level of education was higher in the control group than in the FEP group (t(44)=2.68, p=.01). Also, the FEP group had a higher frequency of tobacco use (χ2=6.23, p=.01). DSM-IV diagnoses of the FEP patients were as follows: schizophrenia, n=14, schizophreniform disorder, n=10, and brief psychotic disorder, n=4. The FEP group had a mean (SD) duration of untreated psychosis of 52 (71) weeks (range, 1–312 weeks). In the aggregate, all but 2 FEP patients had experienced psychotic symptoms for less than 2 years. The FEP and control groups were similar on age, gender, handedness, and cannabis use. The mean (SD) PANSS total scores for the FEP group were 104.4 (15.0) at baseline and 66.6 (17.0) after 4 weeks of antipsychotic treatment (t(27)=11.72, p<.001; mean [SD] decrease, 36% [14%]). The mean (SD) daily dose of risperidone used during the study was 2.89 (1.62) mg (Table 1). After 4 weeks of treatment, 23 patients (82%) showed a clinical response to treatment, while 5 (18%) were classified as non-responders.
Table 1.
Demographic and Clinical Characteristics of the Sample at Baseline
| FEP | Healthy Controls | |
|---|---|---|
| Age (±SD) years | 23 ± 6.1 (range 14–43) | 23. ± 3.8 (range 19–34) |
| Education (±SD) years | 11. ± 2.3 | 14.2 ± 4.0* |
| Gender (male/female) | 20/8 | 9/9 |
| Handedness (right/left) | 28/0 | 18/0 |
| Tobacco (Ever Used) | 7 out of 28 | 0 out of 18* |
| Cannabis (Ever Used) | 1 out of 28 | 0 |
| Duration of untreated psychosis (±SD) weeks | 51. ± 71.0 (range 1–312) | NA |
| PANSS Positive Symptoms | 26.9 ± 5.1 | NA |
| PANSS Negative Symptoms | 25.5 ± 5.7 | NA |
| PANSS General Symptoms | 52.0 ± 7.1 | NA |
Abbreviations: FEP, First-episode psychosis patients; NA, not applicable.
p < 0.05
Voxel Tissue Composition and spectral quality
The proportions of gray matter, white matter, or cerebrospinal fluid in the MPFC or the caudate voxels did not differ between groups. Moreover, no group differences in the unsuppressed reference tissue water signal (W), FWHM or signal-to-noise ratio values were found in either voxel, at baseline or at 4 weeks. Therefore, all metabolite ratios relative to tissue W will be referred to henceforth without the W in the denominator (e.g. GABA/W as simply GABA) unless otherwise specified. The voxel tissue composition and spectral quality control data are summarized in Table 2.
Table 2.
Means (±SD) for each metabolite, tissue composition, and spectral quality in the two regions of interest in first-episode psychosis and healthy control groups
| Mean (SD) | ||||
|---|---|---|---|---|
| Baseline | At 4 weeks | |||
| First-episode psychosis group n = 45 | Healthy control group n = 47 | First-episode psychosis group n = 46 | Healthy control group n = 48 | |
| Medial prefrontal cortex metabolites | ||||
| GABA/W | 2.36 (0.40) × 10−3 a | 2.05 (0.30) × 10−3 | 2.13 (0.43) × 10−3 | 2.09 (0.31) × 10−3 |
| Glx/W | 1.91 (0.35) × 10−3 a | 1.66 (0.27) × 10−3 | 1.64 (0.30) × 10−3 | 1.64 (0.23) × 10−3 |
| NAA/W | 1.80 (0.17) × 10−2 | 1.80 (0.20) × 10−2 | 1.76 (0.11) × 10−2 | 1.77 (0.15) × 10−2 |
| tCho/W | 1.18 (0.17) × 10−2 | 1.15 (0.22) × 10−2 | 1.14 (0.15) × 10−2 | 1.11 (0.16) × 10−2 |
| tCr/W | 1.31 (0.13) × 10−2 | 1.23 (0.15) × 10−2 | 1.26 (0.11) × 10−2 | 1.20 (0.13) × 10−2 |
| Internal water | 1.58 (0.14) × 1012 | 1.68 (0.27) × 1012 | 1.69 (0.31) × 1012 | 1.72 (0.32) × 1012 |
| Spectral Quality | ||||
| FWHM | 11.63 (3.14) | 11.72 (2.75) | 11.63 (1.97) | 12.27 (3.13) |
| SNR | 18.77 (1.29) | 19.75 (1.70) | 18.73 (1.22) | 19.67 (1.71) |
| Dorsal caudate metabolites | ||||
| GABA/W | 2.09 (0.52) × 10−3 a | 1.78 (0.23) × 10−3 | 1.87 (0.37) × 10−3 | 1.80 (0.25) × 10−3 |
| Glx/W | 1.62 (0.32) × 10−3 a | 1.41 (0.20) × 10−3 | 1.49 (0.33) × 10−3 | 1.39 (0.24) × 10−3 |
| NAA/W | 1.51 (0.22) × 10−2 | 1.51 (0.11) × 10−2 | 1.52 (0.23) × 10−2 | 1.46 (0.15) × 10−2 |
| tCho/W | 1.11 (0.14) × 10−2 | 1.11 (0.14) × 10−2 | 1.10 (0.16) × 10−2 | 1.10 (0.16) × 10−2 |
| tCr/W | 1.12 (0.22) × 10−2 | 1.11 (0.15) × 10−2 | 1.12 (0.24) × 10−2 | 1.11 (0.15) × 10−2 |
| Internal water | 1.72 (0.12) × 1012 | 1.83 (0.39) × 1012 | 1.83 (0.45) × 1012 | 1.96 (0.60) × 1012 |
| Spectral Quality | ||||
| FWHM | 7.25 (1.77) | 6.53 (1.55) | 7.43 (2.06) | 6.74 (1.84) |
| SNR | 19.17 (2.51) | 18.01 (2.03) | 19.52 (3.01) | 18.30 (2.66) |
| Voxel Composition, % volume Medial prefrontal cortex voxel | ||||
| Grey matter | 0.50 (0.04) | 0.50 (0.03) | 0.51 (0.04) | 0.50 (0.03) |
| White matter | 0.28 (0.05) | 0.29 (0.05) | 0.28 (0.04) | 0.29 (0.05) |
| Cerebrospinal fluid | 0.21 (0.03) | 0.20 (0.02) | 0.20 (0.04) | 0.21 (0.03) |
| Dorsal caudate voxel | ||||
| Grey matter | 0.35 (0.02) | 0.35 (0.03) | 0.35 (0.03) | 0.35 (0.03) |
| White matter | 0.45 (0.05) | 0.45 (0.04) | 0.45 (0.05) | 0.44 (0.05) |
| Cerebrospinal fluid | 0.19 (0.05) | 0.20 (0.05) | 0.19 (0.06) | 0.20 (0.05) |
SD, standard deviation; n, number of the spectra analyzed; GABA, γ-aminobutyric acid; Glx, Glutamate + glutamine; NAA, N-acetyl-aspartate; tCho, total choline; tCr, total creatine; W, water signal; FWHM, full-width at half maximum of the unsuppressed water resonance; SNR, signal-to-noise ratio of the NAA resonance in the spectra with the editing pulse turned off.
p<0.05
GABA Levels
1H MRS data for 6 participants (MPFC data for 2 FEP and 1 healthy control at baseline, MPFC data for 1 FEP at 4 weeks, and dorsal caudate data for 1 FEP and 1 healthy control at baseline) were rejected from all analyses due to poor quality per our established quality assessment criteria (21).
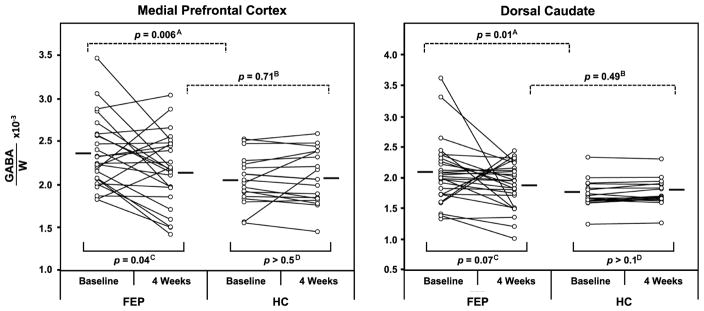
There were significant GABA level decreases between the baseline and 4-week 1H MRS scans (F(1,38)=5.9, p=.02), with a significant interaction of group by time (F(1,38)=5.96, p=.02). Post hoc pairwise comparisons revealed higher GABA levels in both the MPFC and the dorsal caudate in patients than in control subjects at the baseline scan (t(41)=2.9, p=.006; t(42)=2.66, p=.01, respectively). Following 4 weeks of antipsychotic treatment, MPFC GABA levels in the FEP group decreased relative to baseline (t(24)=2.14, p=.04), while there was a trend-level decrease in the dorsal caudate GABA, also relative to baseline (t(26)=1.89, p=.07). After the 4-week treatment period, GABA levels in FEP patients did not differ from those in control subjects either in the MPFC (t(43)=.36, p=.71) or in the dorsal caudate (t(44)=.69, p=.49) (Figure 2). On the other hand, analysis of GABA levels relative to tCr revealed a trend-level interaction of group by time (p=.08).
Figure 2.
Scattergrams depicting γ-aminobutyric acid (GABA) levels in the medial prefrontal cortex (left) and bilateral dorsal caudate (right) for individual first episode-psychosis (FEP) patients and healthy control (HC) subjects at baseline and at 4 weeks. Within- and between-group comparisons are depicted as follows: downward-facing dashed-line brackets represent comparisons between the FEP and HC groups [A] at baseline and [B] at 4 weeks for the two brain regions, whereas upward-facing solid-line brackets represent baseline vs. 4-week comparisons [C] within the FEP group and [D] within the HC group for the two brain regions.
Lastly, we explored the influence of age, gender, and tobacco use as covariates in all analyses. Since no main effects were observed for any of the variables (p>0.1 in all cases), none were included in analyses as covariates.
Since 5 FEP patients did not respond to the 4-week antipsychotic treatment (Total PANSS score decrease <25% after 4 weeks), it was of interest to explore differences in GABA levels between these non-responders and subjects who responded. At baseline, GABA levels were significantly higher in the MPFC of the non-responders than the responders (U=17, p=.02). By contrast, no group differences were found in the dorsal caudate at baseline (U=44, p=.52), or in either region at 4 weeks (MPFC: U=35, p=.23; dorsal caudate: U=53, p=.81, respectively).
Other metabolites
At baseline, Glx levels were higher in FEP patients than in control subjects in both the MPFC (t(41)=2.62, p=.012) and the dorsal caudate (t(42)=2.67, p=.011). After 4 weeks of antipsychotic treatment, the group differences in Glx vanished in both regions compared to controls (MPFC, t(43)=.03, p=.98; dorsal caudate, t(44)=1.12, p=.26). Trend-level higher tCr were found in both the MPFC (t(41)=1.74, p=.09) and the dorsal caudate (t(41)=1.75, p=.08) in the FEP group compared to control subjects at baseline. At 4 weeks, the trend-level higher tCr levels relative to control subjects seen at baseline remained in the MPFC (t(43)=1.73, p=.09), but vanished in the dorsal caudate (t(44)=1.53, p=.13).
No differences in NAA or tCho levels were observed between the FEP and control groups in any region or under any condition (Table 2).
Relationships with Clinical Measures
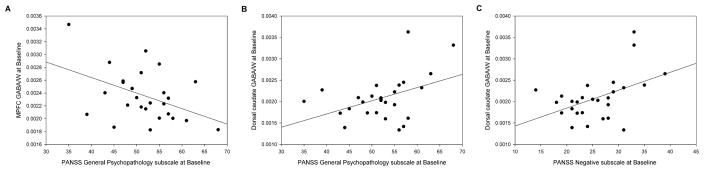
Within the FEP group, there was a negative correlation between baseline MPFC GABA levels and the PANSS General Psychopathology subscale scores (r(26)=−.44, p=.02), and positive correlations between baseline dorsal caudate GABA levels and both General Psychopathology subscale scores (r(27)=.43, p=.03) and the Negative subscale scores (r(27)=.46, p=.02) (Figure 3). However, these correlations did not survive correction for multiple comparisons, although they retained a strong trend toward statistical significance (p=.02 and .03 compared to a statistical threshold p<0.016 for multiple comparisons). Exploratory correlations between changes in GABA levels and changes in clinical measures (PANSS Total score and Positive, Negative, and General Psychopathology subscales) revealed no significant association for either region.
Figure 3.
The relationship between A) γ-aminobutyric acid (GABA) levels in the medial prefrontal cortex and the PANSS General Psychopathology subscale at baseline; B) GABA levels in the dorsal caudate and the PANSS General Psychopathology subscale at baseline; C) GABA levels in the dorsal caudate and the PANSS Negative subscale.
Discussion
The present study is, to the best of our knowledge, the first to investigate the effects of antipsychotic medication on GABA levels in two brain regions simultaneously, in antipsychotic-naive FEP patients using a pre-/post-treatment study design that minimized within-subjects temporal variability and cohort effects. The derived results support elevations of GABA levels at baseline in FEP patients in both the MPFC and the dorsal caudate, which normalized in both brain regions following 4 weeks of treatment. In addition, exploratory analyses revealed higher baseline GABA in non-responders compared to responders, and significant associations between GABA levels and clinical symptoms.
Effects of Antipsychotic Treatment on GABA Levels
The present finding of elevated baseline GABA levels in antipsychotic-naive patients agrees with our two prior cross-sectional studies (8, 17). In one study, we measured GABA levels in the MPFC and the dorsolateral prefrontal cortex of unmedicated patients with schizophrenia (8) and found elevations in the MPFC compared to both control subjects and medicated patients with schizophrenia, while no differences between the latter two groups were found in the MPFC or between any of the groups in the dorsolateral prefrontal cortex. Not only did those results support elevations of GABA in unmedicated patients and decreased or normal levels in medicated patients, they also suggested regional selectivity, since within the same patient group differences were found only in the MPFC and not in the dorsolateral prefrontal cortex. In the second study, which was conducted in drug-naive subjects at UHR for psychosis (17) and measured GABA levels in the MPFC and dorsal caudate, significant elevations compared to control subjects were found in both regions. However, there appears to be a regional specificity to the GABA elevations in unmedicated patients, since prior studies found no group differences in the dorsolateral prefrontal cortex (8), in the dorsal anterior cingulate cortex (12), or in the occipital cortex (15) despite having studied unmedicated patients. In addition, although at baseline in the present study we found elevated MPFC GABA levels in five non-responders compared to responders, suggesting that the elevations observed in unmedicated patients at baseline may be driven by the non-responders, this interpretation must be viewed as preliminary since it is based on a small sample size. With the exception of one study that found elevated GABA levels in treated patients (22) (likely confounded by to the effects of anticonvulsants), the normalization of GABA levels after 4 weeks of antipsychotic treatment in both the MPFC and dorsal caudate found in the present study is in agreement with and likely explains the results of previous 1H MRS studies that reported no differences between medicated patients with schizophrenia and heathy control subjects (6–14, 23).
The accumulating evidence of elevated GABA in drug-naive and drug-free patients with schizophrenia in vivo appears to be inconsistent with postmortem studies (24), which have generally suggested a prefrontal GABA deficit in schizophrenia relative to control subjects, based on the finding of decreased density of the GABA-synthesizing enzyme, glutamic acid decarboxylase 67 kD isoform (GAD67). Potential sources of this apparent discrepancy between in vivo 1H MRS and postmortem data could be that: a) GABA elevations may be present only in the early stages of the disease, since it has been suggested that GABA levels decline more with age in schizophrenia patients than in healthy subjects (10), although such an “age effect” on GABA levels would not be intrinsically inconsistent with a “medication effect”, shown here to decrease GABA levels, since older patients would generally have had many years of antipsychotic medication exposure; b) the detection of GAD67 protein concentration and GAD67 mRNA expression in postmortem brain are confounded by long periods of exposure to antipsychotic medication treatment; c) postmortem deficits in the expression of GAD67 mRNA (25) may be compensated for in vivo by other unimpaired classes of GABA interneurons (8); d) postmortem and in vivo 1H MRS studies may measure different pools of GABA; and e) the need to maintain homeostatic balance between the inhibitory and excitatory neurotransmitter systems stimulates GABA release to match in vivo glutamate elevations (26).
Abnormalities in other metabolites
The Glx elevations found in this study in both the MPFC and dorsal caudate of drug-naive FEP patients are in agreement with previous 1H MRS studies in antipsychotic-free patients with schizophrenia (27), minimally treated patients with schizophrenia (28), antipsychotic-naïve subjects at UHR who later transitioned to psychosis (29), as well as major finding of a recent meta-analysis (30) that found glutamatergic compounds to be generally elevated in unmedicated cohorts. Moreover, the present results replicate those in our previous reports, obtained using a different 1H MRS technique, of increased glutamatergic compounds in the dorsal caudate (31, 32) and in the MPFC of unmedicated schizophrenia patients (8, 33, 34), with the decrease of these initially elevated levels after antipsychotic treatment (35, 36).
In the present study, tCr showed trend-level elevations in both the MPFC and dorsal caudate at baseline and in the MPFC at 4 weeks in the FEP group. These findings, which are in agreement with previous studies that have reported creatine abnormalities in schizophrenia (37–40), lend support to the emerging cautionary tale (40, 41) about the potential pitfall of using tCr as a reference signal for normalizing metabolite levels across groups, especially in cases of relatively small or trend-level changes in absolute metabolite levels that are opposite to those of tCr (41). Such small but opposite changes can be artificially magnified as ratios to tCr to reach statistical significance even when individual absolute metabolite changes are too small to detect and, thus, can confound interpretation (41).
Associations with clinical measures
Our exploratory assessments of potential associations between GABA and PANSS subscale scores as measures of clinical symptoms in FEP patients revealed only three significant correlations for both regions at baseline (Figure 3), which did not survive corrections for multiple comparisons. This is analogous to our prior study in patients with schizophrenia (8), which also found a correlation between MPFC GABA and PANSS Positive Symptoms that did not survive Bonferroni correction. That these correlations vanished when corrected for multiple comparisons is common when assessing correlations involving clinical symptoms that consist of a multitude of subscale scores, like the PANSS. Sample sizes are generally too small for most such correlations to survive corrections for multiple comparisons. Nevertheless, the present limited data (Figure 3) provide preliminary evidence of potential correlations between GABA levels and clinical symptoms that we postulate would survive multiple comparison corrections in larger studies.
Study limitations
This study has several limitations. First, 1H MRS is unable to differentiate neurotransmitter or vesicular and metabolic pools of GABA, which limits interpretation. Second, because antipsychotic serum levels were not measured to confirm treatment compliance, it is uncertain whether the lack of a clinical response in 5 patients was not due to non-compliance with the treatment protocol. Third, we did not include cognitive evaluations, limiting the ability to assess the effect of GABA levels on cognition. Fourth, MRS data acquisition techniques, including the J-edited spin echo difference method used in the present study, require relatively large voxels for reliable quantification of the metabolites. Although we did not find differences in tissue composition of both voxels between groups, the tissue heterogeneity of the dorsal caudate voxel (with large white matter and cerebrospinal fluid proportions) could confound the interpretation of the results. Finally, the reported GABA levels were not corrected for the contribution of the macromolecule signal that is known to co-edit with GABA (42) and is thus a potential confound (21).
Conclusions
The results of the present study have documented baseline elevations of GABA levels in the MPFC and the dorsal caudate of antipsychotic-naive FEP patients, which decreased or normalized following four weeks of antipsychotic treatment in both regions. This finding further supports our prior view (8, 17) that the effect of antipsychotic treatment is to decrease or normalize GABA levels. If replicated in larger cohort studies, this finding could establish 1H MRS measures of GABA levels as noninvasive biomarkers of therapeutic response, not only to antagonists or partial agonists of the dopamine D2 receptors, but also to novel glutamate or GABA-modulating treatments.
Acknowledgments
The authors thank INNN’s Neuroimaging Department, especially Jesus Taboada, MD, and Oscar Marrufo, PhD, for facilities for the development of this study, and Rafael Favila, MSc, from GE Healthcare for technical assistance. This work was supported by Consejo Nacional de Ciencia y Tecnología (CONACyT, Mexico) research grant 182279 to Camilo de la Fuente-Sandoval and Ariel Graff-Guerrero, and 261895 research grant to Camilo de la Fuente-Sandoval; CONACyT scholarship to Francisco Reyes-Madrigal and Pablo León-Ortiz; CONACyT’s Sistema Nacional de Investigadores to Camilo de la Fuente-Sandoval, Helgi Jung-Cook, Rodolfo Solis-Vivanco, and Ariel Graff-Guerrero; and National Institutes of Health R01 MH075895 grant to Dikoma C. Shungu. The funding sources were not involved in the study design, in the collection, analysis, and interpretation of data, in the writing of the report, or in the decision to submit the manuscript for publication.
Footnotes
Financial Disclosure
Camilo de la Fuente-Sandoval has served as a consultant for Janssen (Johnson & Johnson), and Francisco Reyes-Madrigal has served as a speaker for AstraZeneca. The rest of the authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kimoto S, Zaki MM, Bazmi HH, Lewis DA. Altered Markers of Cortical gamma-Aminobutyric Acid Neuronal Activity in Schizophrenia: Role of the NARP Gene. JAMA Psychiatry. 2015;72:747–756. doi: 10.1001/jamapsychiatry.2015.0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akbarian S, Kim JJ, Potkin SG, Hagman JO, Tafazzoli A, Bunney WE, Jr, et al. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry. 1995;52:258–266. doi: 10.1001/archpsyc.1995.03950160008002. [DOI] [PubMed] [Google Scholar]
- 3.Benes FM, Berretta S. GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology. 2001;25:1–27. doi: 10.1016/S0893-133X(01)00225-1. [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez-Burgos G, Cho RY, Lewis DA. Alterations in cortical network oscillations and parvalbumin neurons in schizophrenia. Biol Psychiatry. 2015;77:1031–1040. doi: 10.1016/j.biopsych.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tse MT, Piantadosi PT, Floresco SB. Prefrontal cortical gamma-aminobutyric acid transmission and cognitive function: drawing links to schizophrenia from preclinical research. Biol Psychiatry. 2015;77:929–939. doi: 10.1016/j.biopsych.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 6.Tayoshi S, Nakataki M, Sumitani S, Taniguchi K, Shibuya-Tayoshi S, Numata S, et al. GABA concentration in schizophrenia patients and the effects of antipsychotic medication: a proton magnetic resonance spectroscopy study. Schizophr Res. 2010;117:83–91. doi: 10.1016/j.schres.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 7.Goto N, Yoshimura R, Moriya J, Kakeda S, Ueda N, Ikenouchi-Sugita A, et al. Reduction of brain gamma-aminobutyric acid (GABA) concentrations in early-stage schizophrenia patients: 3T Proton MRS study. Schizophr Res. 2009;112:192–193. doi: 10.1016/j.schres.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 8.Kegeles LS, Mao X, Stanford AD, Girgis R, Ojeil N, Xu X, et al. Elevated prefrontal cortex gamma-aminobutyric acid and glutamate-glutamine levels in schizophrenia measured in vivo with proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 2012;69:449–459. doi: 10.1001/archgenpsychiatry.2011.1519. [DOI] [PubMed] [Google Scholar]
- 9.Rowland LM, Kontson K, West J, Edden RA, Zhu H, Wijtenburg SA, et al. In vivo measurements of glutamate, GABA, and NAAG in schizophrenia. Schizophr Bull. 2013;39:1096–1104. doi: 10.1093/schbul/sbs092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rowland LM, Krause BW, Wijtenburg SA, McMahon RP, Chiappelli J, Nugent KL, et al. Medial frontal GABA is lower in older schizophrenia: a MEGA-PRESS with macromolecule suppression study. Mol Psychiatry. 2016;21:198–204. doi: 10.1038/mp.2015.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marsman A, Mandl RC, Klomp DW, Bohlken MM, Boer VO, Andreychenko A, et al. GABA and glutamate in schizophrenia: a 7 T (1)H-MRS study. Neuroimage Clin. 2014;6:398–407. doi: 10.1016/j.nicl.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marenco S, Meyer C, Kuo S, van der Veen JW, Shen J, DeJong K, et al. Prefrontal GABA Levels Measured With Magnetic Resonance Spectroscopy in Patients With Psychosis and Unaffected Siblings. Am J Psychiatry. 2016;173:527–534. doi: 10.1176/appi.ajp.2015.15020190. [DOI] [PubMed] [Google Scholar]
- 13.Yoon JH, Maddock RJ, Rokem A, Silver MA, Minzenberg MJ, Ragland JD, et al. GABA concentration is reduced in visual cortex in schizophrenia and correlates with orientation-specific surround suppression. J Neurosci. 2010;30:3777–3781. doi: 10.1523/JNEUROSCI.6158-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thakkar KN, Rosler L, Wijnen JP, Boer VO, Klomp DW, Cahn W, et al. 7T Proton Magnetic Resonance Spectroscopy of Gamma-Aminobutyric Acid, Glutamate, and Glutamine Reveals Altered Concentrations in Patients With Schizophrenia and Healthy Siblings. Biol Psychiatry. 2017;81:525–535. doi: 10.1016/j.biopsych.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 15.Kelemen O, Kiss I, Benedek G, Keri S. Perceptual and cognitive effects of antipsychotics in first-episode schizophrenia: the potential impact of GABA concentration in the visual cortex. Prog Neuropsychopharmacol Biol Psychiatry. 2013;47:13–19. doi: 10.1016/j.pnpbp.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 16.Yang Z, Zhu Y, Song Z, Mei L, Zhang J, Chen T, et al. Comparison of the density of gamma-aminobutyric acid in the ventromedial prefrontal cortex of patients with first-episode psychosis and healthy controls. Shanghai Arch Psychiatry. 2015;27:341–347. doi: 10.11919/j.issn.1002-0829.215130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de la Fuente-Sandoval C, Reyes-Madrigal F, Mao X, Leon-Ortiz P, Rodriguez-Mayoral O, Solis-Vivanco R, et al. Cortico-Striatal GABAergic and Glutamatergic Dysregulations in Subjects at Ultra-High Risk for Psychosis Investigated with Proton Magnetic Resonance Spectroscopy. Int J Neuropsychopharmacol. 2015;19:pyv105. doi: 10.1093/ijnp/pyv105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leucht S, Kane JM, Etschel E, Kissling W, Hamann J, Engel RR. Linking the PANSS, BPRS, and CGI: clinical implications. Neuropsychopharmacology. 2006;31:2318–2325. doi: 10.1038/sj.npp.1301147. [DOI] [PubMed] [Google Scholar]
- 19.Howes OD, McCutcheon R, Agid O, de Bartolomeis A, van Beveren NJ, Birnbaum ML, et al. Treatment-Resistant Schizophrenia: Treatment Response and Resistance in Psychosis (TRRIP) Working Group Consensus Guidelines on Diagnosis and Terminology. Am J Psychiatry. 2017;174:216–229. doi: 10.1176/appi.ajp.2016.16050503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rothman DL, Petroff OA, Behar KL, Mattson RH. Localized 1H NMR measurements of gamma-aminobutyric acid in human brain in vivo. Proc Natl Acad Sci U S A. 1993;90:5662–5666. doi: 10.1073/pnas.90.12.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shungu DC, Mao X, Gonzales R, Soones TN, Dyke JP, van der Veen JW, et al. Brain gamma-aminobutyric acid (GABA) detection in vivo with the J-editing (1) H MRS technique: a comprehensive methodological evaluation of sensitivity enhancement, macromolecule contamination and test-retest reliability. NMR Biomed. 2016;29:932–942. doi: 10.1002/nbm.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ongur D, Prescot AP, McCarthy J, Cohen BM, Renshaw PF. Elevated gamma-aminobutyric acid levels in chronic schizophrenia. Biol Psychiatry. 2010;68:667–670. doi: 10.1016/j.biopsych.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rowland LM, Summerfelt A, Wijtenburg SA, Du X, Chiappelli JJ, Krishna N, et al. Frontal Glutamate and gamma-Aminobutyric Acid Levels and Their Associations With Mismatch Negativity and Digit Sequencing Task Performance in Schizophrenia. JAMA Psychiatry. 2016;73:166–174. doi: 10.1001/jamapsychiatry.2015.2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 25.Rocco BR, Lewis DA, Fish KN. Markedly Lower Glutamic Acid Decarboxylase 67 Protein Levels in a Subset of Boutons in Schizophrenia. Biol Psychiatry. 2016;79:1006–1015. doi: 10.1016/j.biopsych.2015.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tritsch NX, Ding JB, Sabatini BL. Dopaminergic neurons inhibit striatal output through non-canonical release of GABA. Nature. 2012;490:262–266. doi: 10.1038/nature11466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kraguljac NV, White DM, Reid MA, Lahti AC. Increased hippocampal glutamate and volumetric deficits in unmedicated patients with schizophrenia. JAMA Psychiatry. 2013;70:1294–1302. doi: 10.1001/jamapsychiatry.2013.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bustillo JR, Rowland LM, Mullins P, Jung R, Chen H, Qualls C, et al. 1H-MRS at 4 tesla in minimally treated early schizophrenia. Mol Psychiatry. 2010;15:629–636. doi: 10.1038/mp.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de la Fuente-Sandoval C, Leon-Ortiz P, Azcarraga M, Favila R, Stephano S, Graff-Guerrero A. Striatal glutamate and the conversion to psychosis: a prospective 1H-MRS imaging study. Int J Neuropsychopharmacol. 2013;16:471–475. doi: 10.1017/S1461145712000314. [DOI] [PubMed] [Google Scholar]
- 30.Merritt K, Egerton A, Kempton MJ, Taylor MJ, McGuire PK. Nature of Glutamate Alterations in Schizophrenia: A Meta-analysis of Proton Magnetic Resonance Spectroscopy Studies. JAMA Psychiatry. 2016;73:665–674. doi: 10.1001/jamapsychiatry.2016.0442. [DOI] [PubMed] [Google Scholar]
- 31.de la Fuente-Sandoval C, Leon-Ortiz P, Favila R, Stephano S, Mamo D, Ramirez-Bermudez J, et al. Higher levels of glutamate in the associative-striatum of subjects with prodromal symptoms of schizophrenia and patients with first-episode psychosis. Neuropsychopharmacology. 2011;36:1781–1791. doi: 10.1038/npp.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Plitman E, de la Fuente-Sandoval C, Reyes-Madrigal F, Chavez S, Gomez-Cruz G, Leon-Ortiz P, et al. Elevated Myo-Inositol, Choline, and Glutamate Levels in the Associative Striatum of Antipsychotic-Naive Patients With First-Episode Psychosis: A Proton Magnetic Resonance Spectroscopy Study With Implications for Glial Dysfunction. Schizophr Bull. 2016;42:415–424. doi: 10.1093/schbul/sbv118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bartha R, Williamson PC, Drost DJ, Malla A, Carr TJ, Cortese L, et al. Measurement of glutamate and glutamine in the medial prefrontal cortex of never-treated schizophrenic patients and healthy controls by proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 1997;54:959–965. doi: 10.1001/archpsyc.1997.01830220085012. [DOI] [PubMed] [Google Scholar]
- 34.Theberge J, Bartha R, Drost DJ, Menon RS, Malla A, Takhar J, et al. Glutamate and glutamine measured with 4.0 T proton MRS in never-treated patients with schizophrenia and healthy volunteers. Am J Psychiatry. 2002;159:1944–1946. doi: 10.1176/appi.ajp.159.11.1944. [DOI] [PubMed] [Google Scholar]
- 35.Theberge J, Williamson KE, Aoyama N, Drost DJ, Manchanda R, Malla AK, et al. Longitudinal grey-matter and glutamatergic losses in first-episode schizophrenia. Br J Psychiatry. 2007;191:325–334. doi: 10.1192/bjp.bp.106.033670. [DOI] [PubMed] [Google Scholar]
- 36.de la Fuente-Sandoval C, Leon-Ortiz P, Azcarraga M, Stephano S, Favila R, Diaz-Galvis L, et al. Glutamate levels in the associative striatum before and after 4 weeks of antipsychotic treatment in first-episode psychosis: a longitudinal proton magnetic resonance spectroscopy study. JAMA Psychiatry. 2013;70:1057–1066. doi: 10.1001/jamapsychiatry.2013.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bustillo JR, Rowland LM, Lauriello J, Petropoulos H, Hammond R, Hart B, et al. High choline concentrations in the caudate nucleus in antipsychotic-naive patients with schizophrenia. Am J Psychiatry. 2002;159:130–133. doi: 10.1176/appi.ajp.159.1.130. [DOI] [PubMed] [Google Scholar]
- 38.Wood SJ, Berger G, Velakoulis D, Phillips LJ, McGorry PD, Yung AR, et al. Proton magnetic resonance spectroscopy in first episode psychosis and ultra high-risk individuals. Schizophr Bull. 2003;29:831–843. doi: 10.1093/oxfordjournals.schbul.a007049. [DOI] [PubMed] [Google Scholar]
- 39.Tibbo PG, Bernier D, Hanstock CC, Seres P, Lakusta B, Purdon SE. 3-T proton magnetic spectroscopy in unmedicated first episode psychosis: a focus on creatine. Magn Reson Med. 2013;69:613–620. doi: 10.1002/mrm.24291. [DOI] [PubMed] [Google Scholar]
- 40.Ongur D, Prescot AP, Jensen JE, Cohen BM, Renshaw PF. Creatine abnormalities in schizophrenia and bipolar disorder. Psychiatry Res. 2009;172:44–48. doi: 10.1016/j.pscychresns.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weiduschat N, Mao X, Hupf J, Armstrong N, Kang G, Lange DJ, et al. Motor cortex glutathione deficit in ALS measured in vivo with the J-editing technique. Neurosci Lett. 2014;570:102–107. doi: 10.1016/j.neulet.2014.04.020. [DOI] [PubMed] [Google Scholar]
- 42.Behar KL, Rothman DL, Spencer DD, Petroff OA. Analysis of macromolecule resonances in 1H NMR spectra of human brain. Magn Reson Med. 1994;32:294–302. doi: 10.1002/mrm.1910320304. [DOI] [PubMed] [Google Scholar]