Abstract
Objective
To examine the impact of interventions to optimize medication use on adverse drug reactions (ADRs) in older adults.
Design
Systematic review and meta-analysis. EMBASE, PubMed, OVID, Cochrane Library, Clinicaltrials.gov and Google Scholar was searched through April 30, 2017.
Setting
Randomized controlled trials.
Participants
Adults (mean age >65 years) or older taking medications.
Measurements
Two authors independently extracted relevant information and assessed studies for risk of bias. Discrepancies were resolved in consensus meetings. The outcomes were any and serious ADRs. Random-effects models were used to combine the results of multiple studies and create summary estimates.
Results
A total of 13 randomized controlled trials involving 6198 older adults were included. The studies employed a number of different interventions that were categorized as pharmacist-led interventions (8 studies), other health professional-led interventions (3 studies), a brief educational session (1 study) and a technology intervention (1 study). In comparison to the pooled control group, the intervention group was 21% less likely to experience any ADR (odds ratio 0.79; 95% confidence interval 0.62–0.99). In the six studies that examined serious ADRs, the intervention group was 36% less likely than the pooled control group to experience a serious ADR (OR = 0.64, 95% CI = 0.42–0.98).
Conclusion
Interventions designed to optimize medication use reduced the risk of any and serious ADRs in older adults. Implementation of these successful interventions in health care systems may improve medication safety in older patients.
Keywords: aged, adverse drug reaction, meta-analysis, randomized controlled trials
INTRODUCTION
Adverse drug events (ADEs), defined as “an injury due to a medication”, are a major public health problem for older adults.[1] Adverse drug reactions (ADRs), the most common subset of ADEs, are defined as “a response to a drug that is noxious and unintended and occurs at doses normally used in man for the prophylaxis, diagnosis or therapy of disease, or for modification of physiological function” and excludes therapeutic failure and adverse drug withdrawal events.[2,3] A recent meta-analysis determined that nearly 9% of hospital admissions are due to ADRs in older adults. [4] Moreover, ADRs occur frequently in community dwelling older adults (10–35% yearly), especially during transitions from higher levels of care such as the period following hospital discharge.[5,6] Polypharmacy is a consistent risk factor for ADRs.[7] In addition, inappropriate prescribing and monitoring of medications further predispose older adults to ADRs.[8,9]
Prevention of ADRs in older adults is necessary as they worsen quality of life and unnecessarily increase health care system costs. Further evidence of their importance is the current federal initiative focused on ADR prevention efforts for the high risk medication classes of anticoagulants, diabetes agents, and opioids.[10] However, there is a gap in the current literature regarding the effect that rigorously designed intervention studies have on reducing ADRs in older adults. A recent Cochrane review by Cooper et al. examined the impact of various interventions on inappropriate polypharmacy in older patients.[11] Unfortunately, only 3 of the 12 included studies measured ADRs as secondary outcomes which precluded conducting a meta-analysis. [11] A group from Ireland recently published two separate meta-analyses of pharmacist interventions on prescribing quality in older adults. [12,13]. The one by Riordan et al. focused on 5 studies conducted in primary care but only one study examined ADRs as a secondary outcome. [12] The second by Walsh et al. focused on 4 interventions conducted in the inpatient setting of which only two studies examined ADRs as a secondary outcome. [13] The only meta-analysis in which ADRs were the primary outcome was published by Nuckols et al. [14]. This meta-analysis is limited in that none of the 6 included studies used a randomized controlled design, only one type of intervention was examined (in-hospital computerized order entry), and the impact in older patients was not examined.
Given this background, the objective of this study is to examine the impact of interventions to optimize medication use on ADRs in older adults.
METHODS
Search Strategy
To identify relevant studies, we performed a systematic review of the literature using EMBASE, PubMed, OVID, Cochrane Library, Clinicaltrials.gov and Google Scholar through April 30, 2017. The search terms included a combination of the following key words: aged, adverse drug events or reaction, randomized controlled trials and English language. Supplementary Table S1 provides an example of the EMBASE database search strategy utilized. We also examined the citations from seminal studies, reviews, book chapters as well as the authors' own files.
Study Selection
Two reviewers (SLG and JTH), first examined the titles and then the abstracts of studies to identify those using unifaceted or multifaceted interventions aimed at reducing medication errors in any setting compared with usual care. Further, we looked at the full manuscripts for these studies and included only those studies where the average age of participants was 65 years of age or higher, that used a randomized controlled trial design, and measured ADRs as a primary outcome or as part of an overall assessment of drug-related problems.
Data Extraction
A standardized data collection form based in part on the PRISMA guidelines was created by two of the authors (SLG and JTH), piloted and revised with input from the biostatistician, two other research pharmacists (LAH, TPS) and a geriatrician (KES).[15,16] Elements of the final data collection form included author name, date of publication, country, sample size, mean age of each study arm, setting (e.g., nursing home), study intervention type (e.g., pharmacist-led), follow-up time and rate of any and serious ADRs by group status. Details about ADR assessment included: process for detecting ADRs (e.g., chart review, patient interview); method for assessing causality (e.g., whether a formal causality algorithm was used[17]); and number and background of the evaluators. Study data extraction was independently performed by two reviewers (SLG and JTH). Discrepancies were resolved in consensus meetings. Because the terms ADEs and ADRs were used interchangeably by published studies, when necessary for clarification the authors of the primary studies were contacted to assure the study outcome of interest for this meta-analysis, ADRs, were addressed.
Assessment of Study Quality
Two authors (SLG, LAH) independently assessed the methodological quality of the studies using the Cochrane Collaboration tool for assessing risk of bias. [18] The domains considered addressed selection bias (random sequence generation, allocation concealment), performance bias (blinding of participants and personnel), detection bias (blinding of outcome assessment), attrition bias (incomplete outcome data) and reporting bias (selective reporting). Discrepancies were resolved in consensus meetings.
Outcomes
Any ADR was the primary outcome for this meta-analysis.[2,3] The secondary outcome was rate of serious ADRs defined as those that were associated with death, hospitalization, prolonged hospitalization, permanent disability, or need for an intervention to prevent permanent impairment.[19]
Statistical Analysis
The incidence of ADRs was presented in different formats for the individual studies including numbers of participants, numbers of events, proportions and rates per person time. Prior to analysis, we converted all ADR event information from studies to estimate the numbers of persons experiencing an ADR out of the total numbers of persons exposed, assuming a Poisson distribution for the events. Odds ratios were computed for each study, representing the intervention versus control group difference. We fitted fixed effects models for combining the log odds ratios from the studies, and used Cochran’s Q-statistic and Higgins I2 statistic for assessing the extent of heterogeneity.[20] Generally, an I2 of 25% is considered to be low, 50% as moderate and 75% high heterogeneity. Upon observing high level of heterogeneity, we used a random effects model for pooling the individual log odds ratios and obtaining an overall estimate that incorporates between-study heterogeneity.[21] We performed a series of leave-one-out-at-a-time meta-analyses to identify the most influential studies, and a cumulative meta-analysis to examine the accumulation of evidence over time, as well as a post-hoc analysis that was restricted to studies of pharmacist-led interventions. We constructed forest plots to present the results graphically, and created a funnel plot and performed Egger’s test to examine possible publication bias.[22] For serious ADRs, as they were sufficiently rare, we assumed the exposed persons would at most have one event, and repeated the above analysis. Comprehensive Meta-Analysis® version 2 software (Biostat, Inc., Englewood, New Jersey) was used for all analyses.
RESULTS
Characteristics of Included Studies
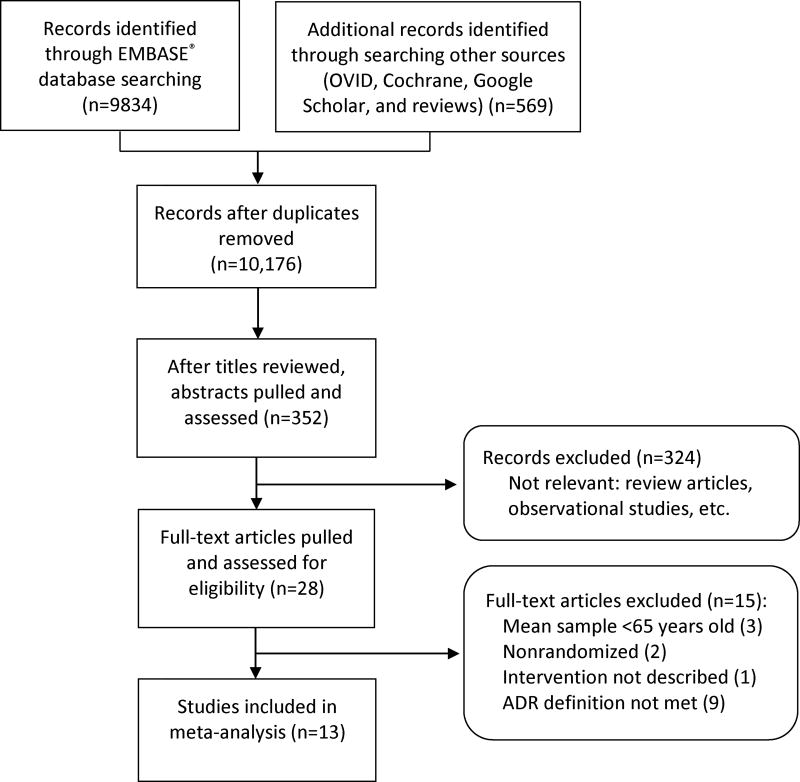
Of the retrieved 10,176 citations, 13 studies involving 6198 patients were eligible for inclusion in this review (Figure 1). Three study authors provided specific requested raw data to allow for their study inclusion. The study characteristics are presented in Table 1. The studies were conducted in Europe (7 studies),[23–29] North America (5 studies),[19,30–33] and Australia (1 study)[34] between 1996 and 2016. Studies were conducted in a variety of clinical settings, including hospitals,[19,23–26, 28] outpatient clinics,[19,27,30,31] long-term care facilities,[32–34] and community pharmacies.[29] Two studies evaluated the effects of inpatient pharmacist-led interventions on outcomes post hospital discharge.[23,28] The total follow-up time ranged from two to 12 months for studies that examined ADRs in non-hospital settings.[19,23,27–34]
Figure 1.
PRISMA flow diagram of the selection of eligible studies.
Table 1.
Randomized Controlled Trials Evaluating Interventions to Reduce Adverse Drug Reactions in Older Adults
| Author, Year |
Country | Setting | Sample Size, N |
ADR Method | Follow-Up Time | |
|---|---|---|---|---|---|---|
| Detection | ADR Causality Assessment | |||||
| Pharmacist-Led Interventions | ||||||
| Crotty, 2004 | Australia | Hospital to long-term care facility | 110 | Chart review | Causality assessment method and number and type of reviewers not reported | 8 weeks |
| Gillespie, 2009 | Sweden | Hospital and homea | 400 | Chart review | DRP definition for ADR; 2 pharm reviewersv | 12 months |
| Hanlon, 1996 | US | Veterans Affairs clinic | 208 | Patient survey | Known effect as per two pharmacology texts; 1 pharm reviewer | 12 months |
| Kwint, 2011 | Netherlands | Community pharmacies | 125 | Chart review | DRP definition for ADR; Consensus of 2 pharm reviewers | 6 months |
| Lenander, 2014 | Sweden | Primary care center | 209 | Patient interview | DRP definition for ADR; 1 pharm reviewer | 12 months |
| Touchette, 2012 | US | Outpatient clinics | 637 | Patient interview | Naranjo algorithm; 1 pharm reviewer | 6 months |
| Willoch, 2012 | Norway | Hospital rehabilitation ward | 77 | Chart review, patient questionnaire | DRP definition for ADR; number and type of reviewers not reported | 3 months |
| O’Sullivan, 2016 | Ireland | Hospital | 737 | Chart review using trigger tool | WHO Uppsala Monitoring Centre algorithm; 2 pharm reviewersb | 7–10 days post-admission or discharge |
| Other Health Professional-Led Interventions | ||||||
| Field, 2011 | US | Nursing homes | 435 | Chart and lab review | IOM definition for ADR; Consensus of pairs of 3 MD reviewers for warfarin bleed | 1 year |
| O’Connor, 2016 | Ireland | Hospital | 732 | Chart review using trigger tool | WHO Uppsala Monitoring Centre algorithm; 1 MD reviewer | 7 to 10 days post-admission or discharge |
| Schmader, 2004 | US | Veterans Affairs hospitals | 834 | Chart review using trigger tool | Naranjo algorithm; Consensus of pharm-MD pairs | 10–11 days |
| Schmader, 2004c | US | Veterans Affairs clinics | 808 | Chart review using trigger tool, patient interview | Naranjo algorithm; Consensus of pharm-MD pairs | 12 months |
| Brief Educational Session | ||||||
| Trivalle, 2010 | France | Hospital (rehabilitation centers) | 576 | Chart review using check-list | Causality assessment method not reported; 4 multidisciplinary (MD and pharm) reviewersb | 2 weeks |
| Technology Interventions | ||||||
| Gurwitz, 2008 | Canada and US | Long-term care facilities | 1,118 | Chart review | IOM definition for ADR; Consensus of pairs of 5 MD reviewers | 6 months for one site; 1 year for other site |
Intervention began in the hospital and spanned hospital discharge and 2 months follow-up;
Whether consensus was reached among multiple reviewers not stated.
Study was a 2×2 factorial design; following. ADR=Adverse drug reaction; DRP=drug related problem; IOM=Institute of Medicine; MD=physician; Pharm=pharmacists; WHO=World Health Organization
The most common type of intervention was pharmacist-led (8 studies) with a core component of medication review that involved a number of implicit structured methods to identify drug-related problems[23,27,28,30,31,34] or a combination of explicit and implicit approaches.[25,29] One study used clinical decision support software (CDSS) to support the pharmacists’ medication review process.[25] Different modes of communication were used to relay recommendations to the prescriber. In most studies, the recommendations were made in-person to the prescriber,[23,28–30,34] however, other studies primarily communicated recommendations through the medical record or facsimile.[25,27,31] Pharmacists also provided education directly to patients in some studies.[23,27,28,30,31] Five studies used other interventions to optimize medication use to reduce ADRs: other health professional-led interventions;[19,24,32] use of clinical decision support added to computerized order entry,[33] and a one-time educational session for physicians.[26] The majority of the studies (n=11) utilized interventions to improve overall prescribing rather than focusing specifically on reducing ADRs and one study focused on improving safety of warfarin.[32] Details of the interventions are provided in Supplementary Table S2.
Most studies used medical record review to detect potential ADRs,[19,23–26,28,29,32–34] with only two studies using more than 1 method.[19,28] Some studies used rigorous assessment of ADRs as determined by using 2 or more reviewers[19,23,25,26,29,32,33] and/or using an ADR causality algorithm.[19,24,25,31]
Methodological Quality of Studies
Assessment of the risk of bias is summarized in Supplementary Table S3. All studies were at high risk for performance bias as personnel and/or participants could not be blinded because of the nature of the intervention, and thus this domain was not reported. Most studies had low risk of detection bias. The allocation concealment was unclear for 10 studies.[19,24–31,33]
Outcomes
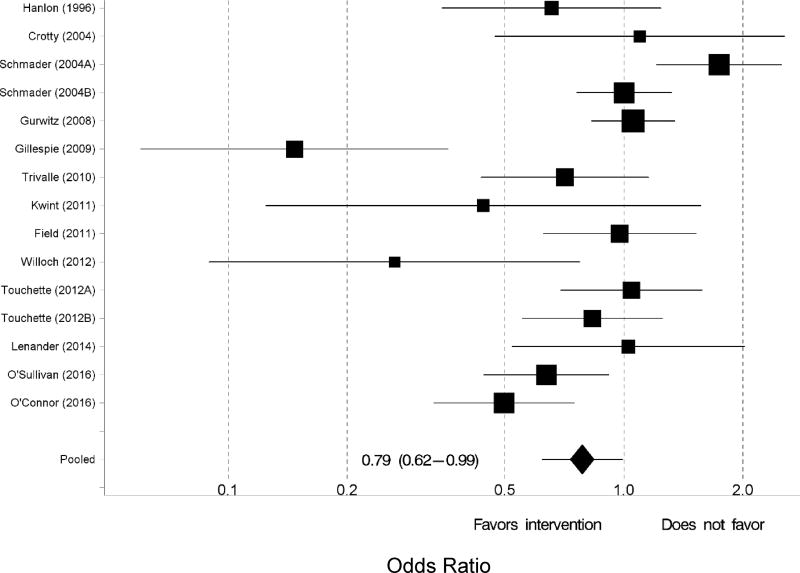
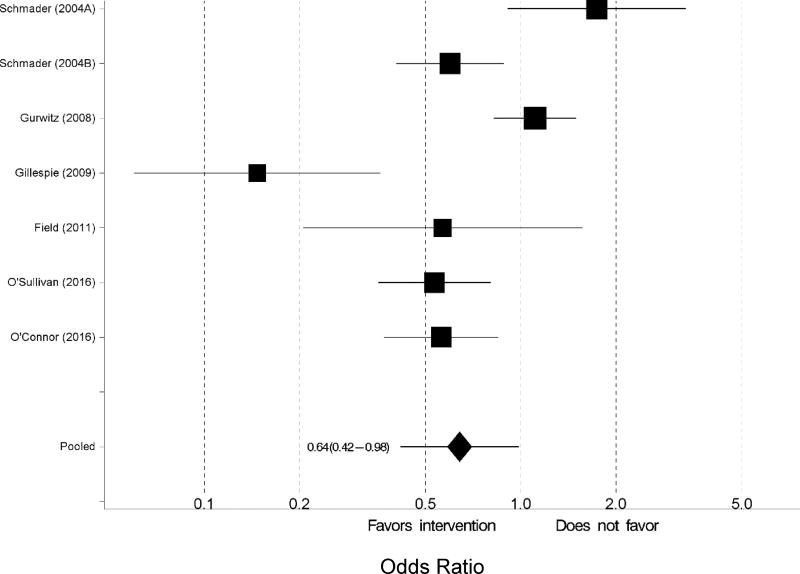
Of the 13 studies that were included in the meta-analysis, two studies reported the outcomes for two interventions.[19,31] Thus, 15 interventions were included in the meta-analysis. Upon observing substantial heterogeneity for the outcome of any ADR (I2 =72.8%), we used a random effects model. Compared to the control group, those randomized to the intervention group were 21% less likely to experience an ADR (odds ratio [OR] 0.79, 95% confidence interval [CI] 0.62 to 0.99; Figure 2). When the analysis was restricted to only the pharmacist-led interventions, the intervention group were 35% less likely to experience an ADR (OR=0.65; CI=0.46–0.92). With all 15 intervention arms, Egger’s test for publication bias was statistically significant (p=0.0449) (Supplementary Figure S1). One-at-a-time removal of studies in the meta-analysis did not materially change the pooled odds ratio, but the study by Gillespie et al. was the most influential (data not shown).[23] The cumulative meta-analysis over time showed the accumulating evidence started favoring interventions around 2009 by magnitude and around 2016 by statistical significance (data not shown). Six studies examined the outcome of serious ADRs.[19,23–25,32,33] There was substantial heterogeneity (I2=80.8%) for serious ADRs. From a random effects model, participants that received the intervention were 36% less likely to experience a serious ADR (OR 0.64, 95% CI 0.42–0.98; Figure 3) compared to the control group.
Figure 2.
Forest plot of the intervention effects on the likelihood of experiencing any adverse drug reaction. Pooled estimates (diamond) calculated by the random effects model for pooling the individual log odds ratios and obtaining an overall estimate that incorporates between-study heterogeneity.
Figure 3.
Forest plot of the intervention effects on the likelihood of experiencing a serious adverse drug reaction. Pooled estimates (diamond) calculated by the random effects model for pooling the individual log odds ratios and obtaining an overall estimate that incorporates between-study heterogeneity.
DISCUSSION
To the best of our knowledge, this is the first meta-analysis to examine the effectiveness of interventions to reduce ADRs in older adults. We identified 13 different studies evaluating 15 intervention arms to include in this meta-analysis. Three previous meta-analyses on interventions to improve prescribing and health outcomes in older adults only found 4 unique studies that evaluated ADRs.[12–14] Interventions to optimize medication use were associated with lower risk of ADRs compared to usual care. Based on the point estimates, there was clear evidence that the intervention was effective in reducing ADRs for 8 of the 15 study arms, whereas 1 intervention arm had a point estimate suggesting an increased risk for ADRs. Furthermore, we observed a significant reduction in serious ADRs. Based on the point estimates, there was clear evidence that the intervention was effective in reducing serious ADRs for 5 of the 6 study arms, whereas 1 intervention arm had a point estimate suggesting an increased risk for serious ADRs. Evaluating serious ADRs is important as shown by the study by Field et al., where the intervention reduced serious ADRs but not any ADR.[32] These findings have important implications as ADR prevention is a national priority for improving patient safety.[10]
This review demonstrates that a variety of interventions were successful in reducing the risk of any ADRs. Most studies utilized a pharmacist led-intervention, and among these studies we found a 35% reduction in odds of ADRs. However, even among these studies, the interventions varied considerably with regard to intensity, mode of communication with providers, provision of patient education, as well as setting. For example, the study by Gillespie et al. evaluated a comprehensive intervention utilizing a pharmacist integrated into the ward team to optimize medication use throughout the hospitalization and provide discharge counselling and a follow-up phone call 2 months following discharge. [23] In contrast, Lenander et al. evaluated a less intensive intervention in primary care that involved a medication review prior to a scheduled physician appointment, with recommendations entered into the medical chart and provided to the patient.[27] Only the study by Touchette et al. compared two different interventions to usual care and found that only the high intensity intervention was effective.[31] Interestingly, O’Sullivan et al. found a significant reduction in ADRs (absolute risk reductionof 6.8%) with an intervention that utilized CDSS to assist the pharmacist in identifying potential drug-related problems in hospitalized patients.[25] Further studies are warranted to determine whether technology can assist with reducing ADRs. Unfortunately, we were unable to separately examine effectiveness of other types of interventions (e.g, other health professional-led interventions) in reducing ADRs due to the small number of studies.
Like any meta-analysis, the present analysis has potential limitations. We found a statistically significant publication bias, likely caused by non-publication of small negative studies, as commonly observed in meta-analyses. It is possible that we missed non-English language randomized controlled intervention trials. Bias may have been introduced through the data abstraction and evaluation process. To reduce this possibility, we used a standardized abstraction form approved by the research team and piloted prior to use in the study. Moreover, we used two reviewers and disagreements were discussed and resolved by a consensus meeting. Regarding generalizability, there were too few studies to conduct analyses specific to type of health care setting or country. Most studies were conducted at a single center; therefore, it is unclear how easily the interventions can be replicated in other health care systems. Finally, we had to use several reasonable strategies for harmonizing the effects reported using different scales in different studies. However, these are common limitations in meta-analyses and are not particularly unique to ours.
Despite these potential limitations, we conclude that interventions designed to optimize medication use reduce the risk of any and serious ADRs in older adults. Successful implementation of these interventions in health care systems may improve medication safety in older patients. Research is needed to identify which interventions and components thereof may be most cost-effective for implementation in specific practice settings. Further, questions remain regarding how best to disseminate these models and provide optimal integration into varied health care systems. Some of these questions may be answered by the completion of planned and on-going studies in primary care (PRIMA-eDS trial)[35] and hospital settings (SENATOR and OPERAM trials).[36]
Supplementary Material
Supplementary Figure S1: Assessment of Publication Bias for Any Adverse Drug Reaction
Supplementary Table S1. Search Strategy Utilized
Supplementary Table S2: Summary of Interventions
Supplementary Table S3. Risk of Bias Assessment for Included Studies
Acknowledgments
Funding Sources: This work was supported in part by National Institute on Aging Grants (P30AG024827, U13AG047008, P30 AG028716, 2U01AG006781 and 3R24 AG045050), an Agency for Health Research and Quality grant (R18 HS023779), and a VA Health Services Research and Development Service Merit Awards (IIR 12–379, IIR 14–297, IIR 14–306, IIR 15–115). This paper was presented in part at the ACC/AGS/NIA Workshop on Pharmacotherapy in Older Adults with CVD, Washington, DC. February 6, 2017.
Role of the Sponsors: The sponsors did not play a role in design and conduct of the study; collection, management, analysis, and interpretation of the data; or in preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Footnotes
| Elements of Financial/Personal Conflicts |
SG | LH | SP | KS | ||||
|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | |
| Employment or Affiliation | X | X | X | X | ||||
| Grants/Funds | X | X | X | X | ||||
| Honoraria | X | X | X | X | ||||
| Speaker Forum | X | X | X | X | ||||
| Consultant | X | X | X | X | ||||
| Stocks | X | X | X | X | ||||
| Royalties | X | X | X | X | ||||
| Expert Testimony | X | X | X | X | ||||
| Board Member | X | X | X | X | ||||
| Patents | X | X | X | X | ||||
| Personal Relationship | X | X | X | X | ||||
| Elements of Financial/Personal Conflicts |
TS | JH | ||
|---|---|---|---|---|
| Yes | No | Yes | No | |
| Employment or Affiliation | X | X | ||
| Grants/Funds | X | X | ||
| Honoraria | X | X | ||
| Speaker Forum | X | X | ||
| Consultant | X | X | ||
| Stocks | X | X | ||
| Royalties | X | X | ||
| Expert Testimony | X | X | ||
| Board Member | X | X | ||
| Patents | X | X | ||
| Personal Relationship | X | X | ||
Authors’ contributions: all authors contributed to study conception, design and interpretation of the data; SLG, LH and JTH drafted the manuscript; all authors revised the manuscript for critical intellectual content; SP conducted statistical analyses; and JTH obtained funding. All authors had full access to all of the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis.
We certify that this work is novel.
A statement about what this research specifically adds to the literature: Although other studies have examined whether various interventions were effective in reducing adverse drug reactions in older adults, with mixed results, we are the first to perform a meta-analysis and found that interventions, especially pharmacist-led, were effective in reducing any and serious adverse drug reactions.
References
- 1.Institute of Medicine. Preventing medication errors. Washington, DC: National Academies Press; 2006. [Google Scholar]
- 2.Karch FE, Lasagna L. Adverse drug reactions. A critical review. JAMA. 1975;234(12):1236–1241. [PubMed] [Google Scholar]
- 3.Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet. 2000;356(9237):1255–1259. doi: 10.1016/S0140-6736(00)02799-9. [DOI] [PubMed] [Google Scholar]
- 4.Oscanoa TJ, Lizaraso F, Carvajal A. Hospital admissions due to adverse drug reactions in the elderly. A meta-analysis. Eur J Clin Pharmacol. 2017;73:759–770. doi: 10.1007/s00228-017-2225-3. [DOI] [PubMed] [Google Scholar]
- 5.Gray SL, Mahoney JE, Blough DK. Adverse drug events in elderly patients receiving home health services following hospital discharge. Ann Pharmacother. 1999;33(11):1147–1153. doi: 10.1345/aph.19036. [DOI] [PubMed] [Google Scholar]
- 6.Naples JN, Handler SM, Maher R, et al. Geriatric Pharmacotherapy And Polypharmacy. In: Fillit H, Rockwood K, Young J, editors. Brocklehurst’s Textbook of Geriatric Medicine. 8. London, UK: Churchill Livingstone; 2016. pp. 849–54. [Google Scholar]
- 7.Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13(1):57–65. doi: 10.1517/14740338.2013.827660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spinewine A, Schmader KE, Barber N, et al. Appropriate prescribing in elderly people: how well can it be measured and optimised? Lancet. 2007;370(9582):173–184. doi: 10.1016/S0140-6736(07)61091-5. [DOI] [PubMed] [Google Scholar]
- 9.Steinman MA, Handler SM, Gurwitz JH, et al. Beyond the prescription: medication monitoring and adverse drug events in older adults. J Am Geriatr Soc. 2011;59(8):1513–1520. doi: 10.1111/j.1532-5415.2011.03500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Action Plan for Adverse Drug Event Prevention. Washington, DC: 2014. U.S. Department of Health and Human Services, Office of Disease Prevention and Health Promotion; p. 11. [Google Scholar]
- 11.Cooper JA, Cadogan CA, Patterson SM, et al. Interventions to improve the appropriate use of polypharmacy in older people: a Cochrane systematic review. BMJ Open. 2015;5(12):e009235. doi: 10.1136/bmjopen-2015-009235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riordan DO, Walsh KA, Galvin R, et al. The effect of pharmacist-led interventions in optimising prescribing in older adults in primary care: A systematic review. SAGE Open Med. 2016;4:1–18. doi: 10.1177/2050312116652568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walsh KA, Riordan DO, Kearney PM, et al. Improving the appropriateness of prescribing in older patients: a systematic review and meta-analysis of pharmacists' interventions in secondary care. Age Ageing. 2016;45:201–209. doi: 10.1093/ageing/afv190. [DOI] [PubMed] [Google Scholar]
- 14.Nuckols TK, Smith-Spangler C, Morton SC, et al. The effectiveness of computerized order entry at reducing preventable adverse drug events and medication errors in hospital settings: a systematic review and meta-analysis. Syst Rev. 2014;3:56. doi: 10.1186/2046-4053-3-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W65–94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. W64. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 17.Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239–245. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 18.Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmader KE, Hanlon JT, Pieper CF, et al. Effects of geriatric evaluation and management on adverse drug reactions and suboptimal prescribing in the frail elderly. Am J Med. 2004;116(6):394–401. doi: 10.1016/j.amjmed.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 20.Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10(1):101–129. [Google Scholar]
- 21.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 22.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gillespie U, Alassaad A, Henrohn D, et al. A comprehensive pharmacist intervention to reduce morbidity in patients 80 years or older: a randomized controlled trial. Arch Intern Med. 2009;169(9):894–900. doi: 10.1001/archinternmed.2009.71. [DOI] [PubMed] [Google Scholar]
- 24.O'Connor MN, O'Sullivan D, Gallagher PF, et al. Prevention of hospital-acquired adverse drug reactions in older people using screening tool of older persons' prescriptions and screening tool to alert to right treatment criteria: A cluster randomized controlled trial. J Am Geriatr Soc. 2016;64(8):1558–1566. doi: 10.1111/jgs.14312. [DOI] [PubMed] [Google Scholar]
- 25.O'Sullivan D, O'Mahony D, O'Connor MN, et al. Prevention of adverse drug reactions in hospitalised older patients using a software-supported structured pharmacist intervention: A cluster randomised controlled trial. Drugs Aging. 2016;33(1):63–73. doi: 10.1007/s40266-015-0329-y. [DOI] [PubMed] [Google Scholar]
- 26.Trivalle C, Cartier T, Verny C, et al. Identifying and preventing adverse drug events in elderly hospitalised patients: a randomised trial of a program to reduce adverse drug effects. J Nutr Health Aging. 2010;14(1):57–61. doi: 10.1007/s12603-010-0010-4. [DOI] [PubMed] [Google Scholar]
- 27.Lenander C, Elfsson B, Danielsson B, et al. Effects of a pharmacist-led structured medication review in primary care on drug-related problems and hospital admission rates: a randomized controlled trial. Scand J Prim Health Care. 2014;32(4):180–186. doi: 10.3109/02813432.2014.972062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Willoch K, Blix HS, Pedersen-Bjergaard AM, et al. Handling drug-related problems in rehabilitation patients: a randomized study. Int J Clin Pharm. 2012;34(2):382–388. doi: 10.1007/s11096-012-9623-5. [DOI] [PubMed] [Google Scholar]
- 29.Kwint HF, Faber A, Gussekloo J, et al. Effects of medication review on drug-related problems in patients using automated drug-dispensing systems: a pragmatic randomized controlled study. Drugs Aging. 2011;28(4):305–314. doi: 10.2165/11586850-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 30.Hanlon JT, Weinberger M, Samsa GP, et al. A randomized, controlled trial of a clinical pharmacist intervention to improve inappropriate prescribing in elderly outpatients with polypharmacy. Am J Med. 1996;100(4):428–437. doi: 10.1016/S0002-9343(97)89519-8. [DOI] [PubMed] [Google Scholar]
- 31.Touchette DR, Masica AL, Dolor RJ, et al. Safety-focused medication therapy management: a randomized controlled trial. J Am Pharm Assoc (2003) 2012;52(5):603–612. doi: 10.1331/JAPhA.2012.12036. [DOI] [PubMed] [Google Scholar]
- 32.Field TS, Tjia J, Mazor KM, et al. Randomized trial of a warfarin communication protocol for nursing homes: an SBAR-based approach. Am J Med. 2011;124(2):179, e1–7. doi: 10.1016/j.amjmed.2010.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gurwitz JH, Field TS, Rochon P, et al. Effect of computerized provider order entry with clinical decision support on adverse drug events in the long-term care setting. J Am Geriatr Soc. 2008;56(12):2225–2233. doi: 10.1111/j.1532-5415.2008.02004.x. [DOI] [PubMed] [Google Scholar]
- 34.Crotty M, Rowett D, Spurling L, et al. PA. Does the addition of a pharmacist transition coordinator improve evidence-based medication management and health outcomes in older adults moving from the hospital to a long-term care facility? Results of a randomized, controlled trial. Am J Geriatr Pharmacother. 2004;2(4):257–264. doi: 10.1016/j.amjopharm.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 35.Sonnichsen A, Trampisch US, Rieckert A, et al. Polypharmacy in chronic diseases-Reduction of Inappropriate Medication and Adverse drug events in older populations by electronic Decision Support (PRIMA-eDS): study protocol for a randomized controlled trial. Trials. 2016;17:57. doi: 10.1186/s13063-016-1177-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Mahony D. Pharmacists and prevention of inappropriate prescribing in hospital. Age Ageing. 2016;45(2):181–183. doi: 10.1093/ageing/afw006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1: Assessment of Publication Bias for Any Adverse Drug Reaction
Supplementary Table S1. Search Strategy Utilized
Supplementary Table S2: Summary of Interventions
Supplementary Table S3. Risk of Bias Assessment for Included Studies