Abstract
Objective
Previous research has found that preschoolers with hearing loss have worse visual attention and elevated rates of behavior problems when compared to typically hearing peers (Barker et al., 2009). However, little is known about these deficits in school-age children with cochlear implants (CIs). We evaluated visual selective attention in school-age children with CIs and hearing peers and examined the link between visual attention and behavior problems.
Method
Data were drawn from the Childhood Development after Cochlear Implantation (CDaCI) study, the largest longitudinal, multi-site study of children with CIs. Visual attention was measured using d prime (d’) on a continuous performance test (The Gordon CPT), which requires participants to watch a stream of digits and hit a button after seeing a certain target (a 9 following a 1). The CPT captures the probability of a hit (pressing button for a target) vs a false alarm (pressing the button for a non-target) while accounting for chance responding. In addition, predictors of visual attention, including IQ (using Processing Speed and Perceptional Reasoning on the WISC-IV), age at implantation, gender, and device management were examined. Externalizing problems were assessed using parent-report on the BASC-2. Data were drawn from 60 months post-implantation.
Results
Children with CIs (n = 106) showed significantly worse visual selective attention than hearing peers. The difference in d’ was driven by higher rates of false alarms. In the CI group, the Processing Speed Index on the WISC was correlated with total omissions, total commissions and d’. Within the CI group, d’ significantly predicted parent-reported externalizing behavior problems. This finding was primarily driven by elevated Hyperactivity in the CI group.
Conclusion
Children with CIs continue to display deficits in visual attention when compared to their hearing peers. Despite improvements in oral language, these problems have critical implications for academic performance and social competence. Currently, cochlear implant teams do not focus on these other dimensions of development and thus, may not be positioned to address them. Assessment of attention and behavior should be incorporated into routine, annual visits soon after implant surgery, and remediation of these deficits should be included in early intervention programs.
1. Introduction
The prevalence of moderate to profound hearing loss among children and adolescents in the US ranges from 14.5%–19.5% (Niskar et al., 1998; Van Naarden Braun et al., 2015). Pediatric hearing loss is associated with delays in multiple domains, including spoken language, cognitive abilities, and externalizing behavior problems (e.g., hyperactivity, aggression)(Barker et al., 2009; Mitchell & Quittner, 1996; Smith, Quittner, Osberger, & Miyamoto, 1998). In addition, previous research has indicated there are developmental links between the visual and auditory systems, which likely contribute to the delays mentioned above (Chen & Westermann, 2012; Lewkowicz & Flom, 2014).
This intrinsic coupling of the visual and auditory systems suggests that impairments in one domain can have cascading effects on the other, and that significant hearing loss may lead to deficits in visual attention. Therefore, a child with deficits in auditory information (e.g., inconsistent signals, hard to interpret auditory information) may experience additional effects on their visual system due to this developmental coupling, which could have concomitant effects on other domains. Studying children with hearing loss provides an opportunity to examine the relationship between vision and hearing when one modality is impaired. Importantly, previous research has found delays in selective and sustained visual attention among children with hearing loss vs. typically hearing peers (Horn, Davis, Pisoni, & Miyamoto, 2005; Mitchell & Quittner, 1996; Smith et al., 1998). These delays in visual attention are most likely related to poor auditory skills. However, more research is needed to understand the relationship between visual and auditory system development in children with cochlear implants (CIs).
These deficits in visual attention are important because they are related to several critical outcomes, including problems with oral language, cognition, behavior, and social skills (Barker et al., 2009; Guillon, Hadjikhani, Baduel, & Roge, 2014; Hunnius, 2007; Klenberg, Korkman, & Lahti-Nuuttila, 2001; Quittner, Smith, Osberger, Mitchell, & Katz, 1994). For example, previous studies have shown that young, deaf children tend to monitor the environment visually, because they cannot rely on auditory cues (Quittner et al., 1994). Quittner and colleagues have published a series of studies on the Division of Labor hypothesis which has been supported empirically ((Mitchell & Quittner, 1996; Quittner et al., 1994; Smith et al., 1998). Challenges with visual selective and sustained attention have been associated with descriptions of these children’s behavior as “impulsive” and “hyperactive.” Hearing loss may also lead to challenges in allocating visual attention to accomplish various tasks that require focusing in and selecting the relevant target (Mitchell & Quittner, 1996; Smith et al., 1998). Interestingly, there is evidence that improvements in visual attention are among the earliest benefits for toddlers who receive CIs (Quittner et al., 2007). However, less is known about the development of visual attention in school-age children with CIs and the longitudinal effects of CI usage. It is also unclear which audiological and cognitive factors affect the development of visual attention in this age range and how visual attention is related to behavior problems, such as distractibility and impulsivity. Thus, the purpose of this study was to evaluate sustained and selective visual attention in school-age children with CIs in comparison to their hearing peers, using data from the largest, longitudinal study of children with CIs ever conducted (Niparko et al., 2010).
1.1 Continuous performance tests
Visual attention is a multidimensional construct that includes sustained attention (vigilance), selective discrimination of relevant from nonessential information, and inhibition of responses to nonessential targets. This construct can be measured using questionnaires or neuropsychological tests which assess performance. Questionnaires, such as the Child Behavior Checklist (CBCL) (Achenbach & Edelbrock, 1983), have significant limitations, including rater bias, a focus on specific components of attention (i.e. impulsivity), and an inability to identify the underlying processes that give rise to these behaviors.
In contrast, measuring visual attention using continuous performance tests (CPTs) addresses these limitations by using a standardized task and evaluating multiple components of attention (i.e., sustained, selective attention). This prevents rater bias and identifies potential targets for remediation by allowing for identification of specific attention processes that may be impaired (i.e., ignoring irrelevant information, sustaining attention, and/or attending to important information). CPTs have also been shown to discriminate between clinical and non-clinical levels of attention problems, are easy to administer, and are scored automatically. Depending on the task, they can also measure visual attention without reliance on auditory or linguistic skills, which removes a major confounding factor for children with hearing loss. This facilitates more accurate comparisons of performance between hearing-impaired and typically hearing children. In this study, we used the Gordon CPT, a computerized task that requires the participant to press a button when a pre-specified target appears (i.e., simple digits), while inhibiting responses to non-targets (Gordon & Mettelman, 1987). We selected this task because it does not use an auditory signal and generates 5 outcome variables for analysis, which can be used to examine different components of selective and sustained visual attention.
1.2 The “Division of Labor Hypothesis”
Using CPTs to assess visual attention provides an opportunity to test the “Division of Labor” hypothesis in children with hearing loss (Quittner et al., 2007; Quittner et al., 1994; Smith et al., 1998). According to this hypothesis, children with typical hearing can use auditory information to monitor their environment and focus on a specific task. In contrast, children with hearing loss, who have no auditory input, cannot simultaneously monitor their environment and focus on a specific task or event, but instead, must rely on vision to accomplish both goals. Thus, these children monitor the surrounding environment visually, while attempting to complete tasks that require selective and sustained attention (e.g., reading a book in class). This can lead to worse performance and has important implications for accommodating children with CIs in mainstreamed classrooms. Several studies have observed that deaf children often appear impulsive and distractible because they cannot focus on the task at hand (Mitchell & Quittner, 1996). Based on our Division of Labor hypothesis, children with hearing loss were expected to perform more poorly on CPT tasks than typically hearing peers. These issues may also have cascading effects on the development of oral language and behavioral regulation.
1.3 Comparisons of visual attention in children with hearing loss and hearing peers
The first study comparing selective visual attention in deaf and hearing children using a CPT recruited 3 groups: children with hearing aids (n = 28), CIs (n = 28), and typically hearing peers (n = 25) (Quittner et al., 1994). All participants completed the Gordon CPT. In the younger age range (6–8 years; n = 14), both deaf groups had significantly worse d’ and false alarm scores than the hearing children. In the older age range (9–13 years), children with CIs performed significantly better than children with hearing aids on both attention measures and were comparable to the hearing controls. A significant effect was found for chronological age, with older children performing better than younger children across all groups. A second experiment showed that children with CIs achieved a greater rate of improvement on the CPT task than children with hearing aids. The authors concluded that greater access to auditory information via a CI facilitated the development of selective visual attention; further, the results supported the Division of Labor hypothesis. However, these relationships have not been tested in a large, diverse sample of children with CI who were implanted relatively early in development. The current study provides a large, national cohort of children who were implanted between the ages of 5 months to 5 years.
A follow-up study examined selective visual attention in children ages 6–14 (N = 64) using the Gordon CPT (Mitchell & Quittner, 1996). Children were categorized into 3 age groups: 6–7, 8–9, and 12–14 years. Results indicated that a majority of children with hearing loss scored in the borderline/clinically elevated range (59%–85%). The hearing-impaired group also made significantly fewer correct responses and significantly more false alarms than their hearing peers. Finally, children with hearing loss showed an increase in false alarms across the task, which was likely due to impulsive behaviors. In contrast, the typically hearing group did not show these behaviors over time.
In a longitudinal study of selective and sustained visual attention in deaf and hearing children ages 6 to 13 (N = 153; n = 51 with CIs), Smith and colleagues (1998) examined changes in the development of visual attention over one year. Results showed that all children made the largest gains in visual attention between the ages of 7–9, with the CI and hearing aid users making the greatest gains about 1 year after the hearing group, between ages 8–9. These findings suggested that regardless of access to sound, children have the most rapid improvements in visual attention during this developmental period. However, at the time the study was conducted, universal newborn hearing screening had not begun, which delayed diagnosis, cochlear implant technology was less advanced, and very few children were implanted early in development (prior to age two). In contrast, the current study examines visual attention and the Division of Labor hypothesis in a large, representative sample of deaf children identified soon after birth, who were implanted by age two, on average, with more advanced implant technology.
In contrast to previous findings, Tharpe, Ashmead, and Rothpletz (2002) found no difference between groups when comparing CPT results in school-age children with CIs, hearing aids, and normal hearing. However, these results should be interpreted with caution due to the small sample size (N=28, n=9 children with CIs and 9 children with hearing aids). Furthermore, they utilized a different visual attention task (i.e., letter cancellation), which appeared to produce ceiling effects across all groups, based on the high d’.
In sum, studies evaluating visual attention in children with CIs vs. hearing peers have consistently demonstrated delays in selective visual attention. However, there is less evidence on sustained attention between those with and without hearing loss. Next steps include identifying the predictors of both selective and sustained visual attention.
1.4 Predictors of CPT performance
Horn and colleagues (2005) examined the relationships among IQ, oral language, audiological markers, and performance on the Gordon CPT in two retrospective studies: children implanted between ages 2.5 to 8.9 years who were tested after age 6 and children implanted between ages 3.4 to 9.5 who were tested prior to age 6. In their first experiment using the school-age CPT, 41 prelingually deaf children replicated the findings of Quittner et al. (1994) and Smith et al. (1998), finding worse visual selective and sustained attention in the CI group compared to the normative sample. Only length of CI use predicted CPT performance. However, other variables were not predictive, including: cause of hearing loss, ear of implantation, pure-tone average, number of active electrodes, age at implantation, communication mode, nonverbal IQ, open-set speech perception, receptive language, expressive language, or speech intelligibility scores. In comparisons of language and CPT outcomes, only one relationship was statistically significant: β was a predictor of receptive language scores using the Reynell Language Development Scales (Reynell & Gruber, 1990). Thus, children with higher rates of impulsivity, which was measured by β,had lower levels of receptive language. Note that despite the number of variables being tested, there were limited specific hypotheses regarding the relationship between audiological and medical variables and CPT outcomes. In addition, there was no correction for Type I error rate; thus, the one significant relationship may have been due to chance.
In their second experiment, a sample of 47 younger deaf children were tested prior to age 6, using a simplified, preschool version of the CPT. Again, worse selective and sustained attention were found when comparing the CI group to normative data, which supported the Division of Labor hypothesis. Among the predictors listed above, only length of CI use and the number of active electrodes correlated with CPT performance. Results from this study should be interpreted with caution because the participants as old as 6 were tested with the task, which was developed for children up to age 5. Furthermore, mean age of implantation was 4.8 years, which is fairly late and not reflective of current implantation practices.
Only one other study has examined age at implantation in relation to CPT performance in school-age children (Yucel & Derim, 2008). This study compared children implanted before vs. after age 4 and found no differences between the two implanted groups on hits, false alarms, commissions, or omissions. However, correlational analyses showed a significant relationship between commissions and age at implantation, which led the authors to conclude that children implanted at a later age tended to respond more impulsively. In addition, using age as a categorical variable, rather than a continuous variable, reduced their power to detect the effects of age at implantation. In sum, more research is needed to identify the factors that predict selective visual attention and to increase our understanding of the relationships between attention and language. Given our large sample size, we were able to test several key predictors of visual attention (e.g., age, gender, IQ, oral language).
1.5 Visual attention and behavior problems in children with hearing loss
Despite consistent findings demonstrating poor selective visual attention in children with CIs, less is known about how these findings correlate with behaviors at home and in school. To date, only two studies have examined the relationship between selective visual attention, using a CPT, and behavior problems. In the Mitchell and Quittner study (1996), 39 parents and teachers of children with hearing loss (ages 6–14 years old, m = 9.8 years) completed the CBCL, while children completed the CPT. As noted above, the hearing-impaired group made significantly fewer correct responses and more false alarms than their hearing peers. Based on parent ratings of behavior, 48% of children with hearing loss scored above the clinical cutoff on the externalizing behaviors subscale and 56% were above the cutoff on the hyperactivity subscale. Similarly, teacher ratings indicated that 32% had elevated rates of externalizing behavior problems and 35% had clinically elevated levels of inattention. The authors found a significant correlation between false alarms on the Gordon CPT vigilance task and parent-reported Hyperactivity (r = .65) and Externalizing Behaviors (r = .52) scales. In addition, d’ was negatively correlated with teacher-reported inattentive behaviors (r = −.55), externalizing problems (r = −.48), and overall total behavior problems (r = −.38). Thus, these results suggested that inattentive behaviors on the CPT were associated with externalizing behavior problems at home and school. However, this study did not include children with CIs, only those with hearing aids.
Tharpe et al. (2002) also examined correlations between parent and teacher ratings on the CBCL and CPT performance in 28 children (9 children with CIs, 9 with hearing aids, 10 with typical hearing). Overall, children with hearing loss were rated by parents as having significantly more behavior problems than hearing children, as measured using the Total Problems scale,. There were no significant differences on parent ratings of behavior problems between children with CIs vs hearing aids However, teachers did not report any behavioral differences between groups. Importantly, a significant correlation was found between false alarms and teacher-rated Inattention (r = .44), supporting the findings of Mitchell and Quittner (1996).
In addition to the associations between selective visual attention and externalizing behavior problems, one study also found a relationship between sustained visual attention and behavior problems. Barker et al. (2009) examined the correlations among language, sustained visual attention, and behavior problems in a large, nationally representative sample of 116 children with CIs and 69 hearing peers ages 1.5–5 years. Sustained attention was measured by coding videotapes of on- and off-task behaviors during structured and unstructured parent-child interaction tasks. This included a free play period, a problem-solving task (completing an age-appropriate easy and challenging puzzle), and an art gallery task, which consisted of discussing child-friendly posters. This methodology allowed for observation of the child’s sustained attention in a more naturalistic setting. Children with CIs displayed more language, attention, and behavioral difficulties than their hearing peers, and a significant relationship was found between sustained attention and behavior problems, reported by parents on the CBCL and the Parenting Stress Index (Abidin, 1990). Thus, it appears that poor sustained attention can affect parent-child interactions and increase behavioral difficulties in children with CIs.
Findings from these studies of visual attention and behavior provide consistent evidence that children with hearing loss have significant deficits in visual selective attention, and that these problems are related to real-world attention and behavioral difficulties observed at home and in the classroom. These findings also provide support for the external validity of the CPT task. However, visual attention and its relationship to externalizing behavior problems have not been explored in a large sample of school-age children with CIs.
1.6 Specific aims
This study had five specific aims. Aim 1 examined descriptive data on the means and standard deviations of CPT scores on the Gordon task in the CI and hearing groups, including measures of sustained attention (comparisons of hits and false alarms across 3 blocks), omissions, commissions, response criterion, and d’. The distribution of the Gordon CPT scores was also analyzed for ceiling effects. These data were used to determine if the CPT task was challenging enough to produce meaningful variation within groups.
For Aim 2, we examined correlations between all CPT scores and gender, age at implantation, chronological age, type of device, and IQ. We hypothesized that age at implantation, chronological age, and IQ would be significantly related to these attention variables, with younger age at implantation, older chronological age, and higher IQ related to better performance. No differences were expected for type of device. We also examined whether age at implantation predicted CPT outcomes, controlling for maternal education.
In Aim 3, we compared CPT outcomes the CI group to their typically hearing peers and the normative sample on the Gordon CPT. It was hypothesized that children with CIs would score significantly lower on all of the CPT outcome variables than the hearing control group, and normative data.
Aim 4 examined relationships between visual attention and language development independently for each group. Aim 4a examined the effects of language ability at 48 months post-implant on CPT scores at 60 months post-implant. We did not expect to find a significant relationship between language a year earlier and performance on this attention task, given previous results and our Division of Labor hypothesis. Aim 4b examined changes in language scores in the 12 months preceding administration of the CPT task as a predictor of attention outcomes. Rate of change in receptive and expressive language was expected to be positively related to visual attention on the CPT task.
Aim 5 explored the relationships between CPT scores and externalizing behavior problems rated by parents. We hypothesized that children with worse scores on the CPT would be rated as having more externalizing behavior problems. This hypothesis was evaluated in the CI and hearing groups separately.
2. Material and methods
2.1 Participants
Data were drawn from the Childhood Development after Cochlear Implantation (CDaCI; NIDCD R01 DC004797) study, which is a multi-center, national cohort investigation of the effectiveness of CIs in deaf children in relation to their hearing peers (Fink et al., 2007). The larger, parent study examined a variety of outcomes in children before and after cochlear implantation, including expressive and receptive language, quality of parent-child interactions, joint attention, psychosocial development, and health-related quality of life (Niparko et al., 2010; Quittner et al., 2012).
Participants were recruited from 6 CI centers and 2 preschools that enrolled hearing children. Inclusion criteria for deaf children were: 1) age under 5 years, 2) severe to profound sensorineural hearing loss, and 3) a commitment to raise the child using spoken English. Participants who failed a cognitive screener using the Bayley Psychomotor Index or the Leiter International Performance Scale – Revised (Roid & Miller, 1997) were excluded. Children in the same age range with normal hearing were also enrolled. A total of 188 children with CIs (mean age at baseline = 2.2 years) and 97 hearing peers (mean age at baseline = 2.3 years) were enrolled. See Fink et al. (2007) for a more detailed description of the sample. All participants were assessed at baseline, prior to implantation for those in the CI group, and every 6 months for 3 years. Following that, participants were assessed annually for the next 5 years.
For this study, data were drawn from the 60-month post-implantation assessment point to control for length of CI use. A total of 106 children with CIs (mean age at 60 months post-implantation = 6.98 years) and 66 hearing controls (mean age = 7.17 years) completed all of the visual attention tasks (see Table 1 for demographic data). Both groups were comparable on gender and minority status. The hearing group had significantly higher levels of maternal education, and therefore, all analyses controlled for this variable. Among the CI sample, age of onset of hearing loss ranged from birth to 44 months (M = 1.99; SD = 6.70). All children had severe-to-profound hearing loss, with mean age at diagnosis of 8.42 months (SD = 9.21). For a majority of children in the CI group, the onset of hearing loss was at birth (n = 86; 81.1%). When this subsample was compared to those in the full CDaCI cohort, no significant differences were found in maternal education, child gender, or age at implantation, suggesting there was no systematic dropout. This study was approved by the Institutional Review Boards at all sites, and all parents gave written, informed consent, and children gave assent, when appropriate, prior to completing any study procedures.
Table 1.
Participant demographics
| Category | CI (n=106) | Hearing (n=66) |
|---|---|---|
| Gender: | ||
| Male | 54 (50.94%) | 26 (39.39%) |
| Female | 52 (49.06%) | 40 (60.61%) |
| Age in Years (SD): | 6.98 (0.89) | 7.17 (0.96) |
| Race: | ||
| White | 83 (78.30%) | 54 (81.8%) |
| African American | 9 (8.49%) | 6 (9.09%) |
| Asian | 3 (2.83%) | 1 (1.52%) |
| Other | 11 (10.38%) | 5 (7.58%) |
| Ethnicity: | ||
| Hispanic | 18 (16.98%) | 8 (12.12%) |
| Non-Hispanic | 88 (83.02%) | 58 (87.88%) |
| Cause of Hearing Loss: | ||
| Sudden | 7 (6.60%) | N/A |
| Progressive | 29 (27.36%) | N/A |
| Congenital | 66 (62.26%) | N/A |
| Unknown | 4 (3.77%) | N/A |
| Age of Onset in Months (SD) | 1.99 (6.70) | N/A |
| Age at Diagnosis in Months (SD) | 8.42 (9.21) | N/A |
| Unaided Pure Tone Average in Better Ear* | 107.43 (16.04) | 15.91 (6.27) |
| Age at Implantation in Years (SD) | 1.92 (0.89) | N/A |
| Device Manufacturer | ||
| Advanced Bionics | 37 (34.91%) | N/A |
| Cochlear | 50 (47.17%) | N/A |
| Med-El | 19 (17.92%) | N/A |
| Perceptual Reasoning (SD)* | 99.93 (15.73) | 115.92 (13.72) |
| Processing Speed (SD)* | 96.28 (13.84) | 102.38 (13.92) |
| CASL composite score (SD)* | 78.21 (22.82) | 117.08 (16.09) |
| Maternal Education*: | ||
| Completed High School or Less | 18 (16.98%) | 4 (6.06%) |
| Some College | 31 (29.25%) | 3 (4.55%) |
| College or more | 57 (53.77%) | 59 (89.39%) |
| Family Income*: | ||
| Less than $29,999 | 19 (17.92%) | 3 (4.55%) |
| $30,000–49,999 | 23 (21.70%) | 1 (1.52%) |
| $50,000–$74,999 | 22 (20.75%) | 13 (19.70%) |
| $75,000–$99,999 | 17 (16.04%) | 10 (15.15%) |
| Greater than $100,000 | 18 (16.98%) | 33 (50.00%) |
| Declined to answer | 7 (6.60%) | 6 (9.09%) |
indicates significant difference between groups (p < 0.05)
2.2 Procedure
Study visits were completed in 1or 2 days, depending on family schedules and travel burden. Families in the study were given honoraria for each year of their participation and gift cards after each completed visit. Families in the CI group were also provided with a 2-year extension of their child’s CI processor warranty after completing 3 years of follow-up. A large assessment battery was administered, including measures of receptive and expressive language, psychosocial functioning, cognitive/executive functioning, and visual attention.
2.3 Measures
Visual attention
Visual attention was measured using the Gordon Diagnostic System Continuous Performance Task (Gordon & Mettelman, 1987). It is administered using a child-friendly computer with an LCD display and a large blue button for responses. During the task, participants were shown a stream of digits that appeared on a red LED display and were instructed to “press the blue button every time you see a 9 that comes right after a 1.” If the 9 came after any other number, they were instructed not to press the button. A practice test was administered to ensure that the child understood the task. The experimental test took 9 minutes to complete.
This task was specifically chosen for this study because it is based on visual stimuli that do not require any auditory or linguistic skills. This eliminates language skill as a confounding variable for children in the CI group. Throughout the task, a total of 540 digits appeared at a rate of 1 per second. The digits were classified into 3 blocks (180 digits each) with the target (a 1 followed by a 9) occurring 15 times per block.
The Gordon CPT generates 5 outcome variables: 1) omissions, 2) commissions, 3) sustained attention, 4) β, and 5) d’. First, omission errors occur when a participant fails to press the button after the target appears. Second, commission errors occur when a participant presses the button for a non-target. These are also referred to as “false alarms.” The total number of omissions and commissions in each block were summed for total omission and commission scores. Third, sustained attention is measured by calculating the change in hit and false alarm rates throughout the task. This was calculated by comparing changes in hits and false alarms across the 3 blocks of the task. For correlational analyses, changes across the blocks were combined into a composite variable. As mentioned above, each block contains an equal number of targets and digits, meaning that they have the same potential hits and false alarms. Thus, a decline in hit rate or increase in false alarm rate was conceptualized as a failure to sustain attention throughout the task.
Fourth, β is a measure of the participant’s likelihood to press the button for both targets and non-targets and is considered a measure of impulsivity. β was calculated by generating the z-score of the hit rate and false alarm rates, squaring each, and calculating the natural log of −1*(Z(hit rate)2-Z(false alarm rate)2*.5), as suggested by Wixted (2007). A high β indicates a less impulsive approach to the task and strong differentiation between the target and non-target stimuli. In contrast, a participant with a low β is conceptualized as having more trouble discriminating between the target and non-target stimuli, and may be responding impulsively.
Finally, d’ is a global measure of visual selective attention that combines total hits and false alarms. Thus, it captures the probability of a hit vs. a false alarm, and takes into account the possibility of chance responding. d’ was calculated by weighing the probability of a hit vs. the probability of a false alarm, and these scores were generated by subtracting the false alarm rate z-score from the hit rate z-score (d’ = Z(hit rate) – Z(false alarm rate)). This formula penalizes participants who have both high hit and false alarm rates, which likely reflects impulsively because the child is pressing the button regardless of the stimuli. A high d’ indicates that the participant is more proficient at detecting the target, while avoiding false alarms. For participants with a false alarm rate of 0 and/or a hit rate of 1, corrections were made to generate d’ scores, based on suggestions from Wixted (2007). For false alarms, Wixted recommended using 1/(2N), where N equals the total number of potential false alarms. Thus, false alarm rates of 0 were converted to 1/(2*495) = 0.003. Using the same logic, hit rates of 1 were converted to 1 – 1/(2N), where N is the number of hits. Therefore, hit rates of 1 were changed to 1 – 1/(2*45) = 0.967.
Oral language
Oral language was measured using the Comprehensive Assessment of Spoken Language (CASL) (Carrow-Woolfolk, 1999). The CASL was developed to assess a wide range of receptive and expressive language skills for children ages 3–22. This study utilized the core composite score, which consisted of 4 or 5 subtests, depending on the child’s age. The core composite is comprised of the subtests that theoretically and developmentally best represent language skills at a given age and is designed to capture the underlying skills required for spoken language, which can be divided into 4 structural categories (Lexical/Semantic, Syntactic, Supralinguistic, and Pragmatic). Raw scores from the core subtests are summed and converted to an overall standard score based on the age-normative standardization sample, with higher scores representing better oral language. Standard scores from 85–115 fall within the average range (mean = 100, SD = 15). Language scores from the 48- and 60-month post implantation follow-up assessment were utilized. Language change scores were calculated by subtracting individualized standard scores at 48 months from those at 60 months. Therefore, children who demonstrated growth in oral language scores above and beyond expected development in one year had higher language change scores. These change scores ranged from a −18 points to 24 points in the CI group. Within the Hearing group, scores ranged from −22 points to 27 points.
Intelligence
Cognitive abilities were measured using the fourth edition of the Wechsler Intelligence Scale for Children (WISC-IV) (Wechsler, 2003). All raw scores were summed and converted into scaled scores for each subtest. Subtest scaled scores were summed and converted into standard scores (mean = 100, SD = 15) for index scores. For this study, intelligence was measured using two indices: Perceptual Reasoning and Processing Speed. The Perceptual Reasoning Index measures nonverbal concept formation, visual perception and organization, simultaneous processing, visual-motor coordination, and the ability to separate figure and ground in visual stimuli. The Processing Speed Index measures the ability to mentally process simple or routine information without making errors, which includes quickly comprehending novel information. The Verbal Comprehension and Working Memory indices were not included because they require oral language skills, which can penalize children with hearing loss.
Externalizing Behavior Problems
Parents completed the Behavior Assessment System for Children – Second Edition (BASC-2) (Reynolds & Kemphaus, 2004), a multidimensional assessment system that examines a broad range of clinical and adaptive behaviors for children and young adults ages 2 to 25. The parent form consists of short statements that describe how the child behaves, and are rated using response options of “never,” “sometimes,” “often,” or “almost always.” Responses to individual items are summed and converted into T-scores. Those T-scores are combined to form subscales and composite scales. Higher T-scores indicated more difficulty, with those 60 and above considered “At-Risk” and those 70 or higher considered “Clinically Significant.” To measure behavior problems, scores from the Externalizing Problems composite scale were utilized, which consists of three subscales: Hyperactivity, Aggression, and Conduct Problems. In addition, scores on each subscale were examined. The Hyperactivity subscale assesses disruptive, impulsive, and uncontrollable behaviors, and includes items such as “cannot wait to take a turn,” “interrupts others when speaking,” and “has poor self-control.” The Aggression subscale assesses aggressive behaviors elevated for children of that age, which includes items such as “hits other children,” “loses temper too easily,” and “calls other children names.” Finally, the Conduct Problems scale assesses rule-breaking behaviors, including cheating, deception, and/or stealing. Sample items include “disobeys,” “breaks the rules,” and “gets into trouble.” “=
2.4 Statistical analysis plan
All comparisons between the CI and typically hearing groups were conducted controlling for maternal education due to demographic differences between groups. For Aim 1, descriptive statistics, distributions, means, and standard deviations were calculated using SPSS. In addition, the CPT outcome variables were examined to determine if there were ceiling effects. Furthermore, paired t-tests were used to compare hit and false alarm rates between Block 1 to Block 2, Block 2 to Block 3, and Block 1 to Block 3 for both groups, to measure sustained attention. For Aim 2, which examined the correlations between CPT scores and demographic variables, pairwise correlations were calculated. To examine age at implantation as a predictor of CPT performance, a linear regression model was run.
For Aim 3, a MANOVA was conducted to compare the CI to the hearing group, as well as the normative Gordon CPT sample. To control for Type I error rate, Hotelling’s t-tests were used to assess significance, followed by univariate ANOVAs. Aim 4 examined the effects of language ability, using the CASL, at 48 months post-implantation, as well as changes in language scores from 48 to 60 months post-CI as predictors of CPT performance in the CI group only. These analyses were completed using separate regression models. Aim 5 examined the correlations between parent-reported behavior problems using the BASC Externalizing Behavior Problems composite and the CPT outcome variables for each group. We also examined correlations between the individual subscales of the Externalizing Behavior Problems composite and CPT outcomes.
3. Results
For Aim 1, the means and standard deviations of the outcome variables on the Gordon CPT were analyzed separately for each group (See Table 2) to determine any ceiling effects. In the CI group, β scores ranged from −0.31 to 2.37 with a mean of 0.81 (SD = 0.39); d’ scores ranged from 0.87–4.25 with a mean of 2.51 (SD = 0.81). The Hearing group had β scores that ranged from 0.19–1.21 with a mean of 0.71 (SD = 0.25); d’ scores ranged from 0.70–4.58 with a mean of 3.05 (SD = 0.89). Both the average d’ and its range in the CI group indicated there were no ceiling effects (Horn et al., 2005). Of note, the standard deviations for total omissions, sustained attention, β, and d’ were similar between groups. However, the standard deviation for total commission errors was significantly larger in the CI vs hearing groups (CI SD = 23.18; NH SD = 10.79), which suggested greater heterogeneity in the CI sample. When analyzing sustained attention, the rate of false alarms did not significantly increase when comparing blocks within either group (see Table 3). However, within both groups, the number of hits significantly decreased from Block 1 to Block 2, Block 2 to Block 3, and Block 1 to Block 3. In conclusion, the CPT task was challenging enough to avoid ceiling effects for both groups and served as a strong measure of visual attention.
Table 2.
Comparison of CI vs. Hearing groups on CPT outcomes
| CI (n=106) | Hearing (n=66) | p-value | Cohen's d | |
|---|---|---|---|---|
| Omissions Block 1 | 2.53 (2.68) | 2.14 (2.29) | 0.52 | N/A |
| Omissions Block 2 | 3.02 (2.97) | 2.92 (2.94) | 0.96 | N/A |
| Omissions Block 3 | 3.79 (3.55) | 3.70 (3.60) | 0.94 | N/A |
| Total Omissions | 9.35 (8.06) | 8.76 (8.23) | 0.63 | N/A |
| Commissions Block 1* | 5.67 (7.29) | 2.68 (3.10) | 0.01 | −0.53 |
| Commissions Block 2* | 6.44 (9.17) | 3.07 (3.94) | 0.01 | −0.48 |
| Commissions Block 3 | 5.85 (8.48) | 3.75 (5.25) | 0.19 | N/A |
| Total Commissions* | 17.96 (23.18) | 9.51 (10.79) | 0.03 | −0.47 |
| β Block 1 | 0.75 (0.36) | 0.65 (0.25) | 0.08 | N/A |
| β Block 2 | 0.79 (0.47) | 0.72 (0.31) | 0.37 | N/A |
| β Block 3 | 0.89 (0.48) | 0.76 (0.32) | 0.09 | N/A |
| Total β | 0.81 (0.39) | 0.71 (0.27) | 0.11 | N/A |
| d' Block 1* | 2.62 (0.86) | 3.23 (0.85) | <0.01 | −0.71 |
| d' Block 2* | 2.52 (0.91) | 3.07 (0.97) | <0.01 | −0.57 |
| d' Block 3* | 2.38 (0.95) | 2.87 (1.09) | <0.01 | −0.47 |
| Total d'* | 2.51 (0.81) | 3.05 (0.89) | <0.01 | −0.63 |
Adjusted for maternal education;
p < 0.05
Table 3.
Sustained attention analyses
| CI group | p-value | Cohen's d | |
|---|---|---|---|
|
| |||
| Hits | mean difference (SD) | ||
| Block 1 vs Block 2* | 0.51 (2.41) | 0.03 | 0.42 |
| Block 2 vs Block 3* | 0.78 (2.57) | 0.02 | 0.61 |
| Block 1 vs Block 3* | 1.29 (2.81) | <0.01 | 0.92 |
| False Alarms | |||
| Block 1 vs Block 2 | −0.83 (5.56) | 0.13 | N/A |
| Block 2 vs Block 3 | 0.51 (4.05) | 0.20 | N/A |
| Block 1 vs Block 3 | −0.33 (6.55) | 0.61 | N/A |
|
| |||
| Hearing group | p-value | Cohen's d | |
|
| |||
| Hits | mean difference (SD) | ||
| Block 1 vs Block 2* | 0.76 (1.64) | <0.01 | 0.93 |
| Block 2 vs Block 3* | 0.71 (2.08) | <0.01 | 0.69 |
| Block 1 vs Block 3* | 1.47 (2.28) | <0.01 | 1.3 |
| False Alarms | |||
| Block 1 vs Block 2 | −0.29 (2.68) | 0.39 | N/A |
| Block 2 vs Block 3 | −0.79 (3.75) | 0.09 | N/A |
| Block 1 vs Block 3 | −1.05 (4.51) | 0.06 | N/A |
p < 0.05
For Aim 2, we examined the correlations between CPT scores (sustained attention, β, d’) and IQ, age at implantation, gender and device manufacturer after controlling for maternal education. We hypothesized that IQ and age at implantation would be significantly related to these attention variables, with younger age at implantation and higher IQ related to better performance. In the CI group, the Processing Speed Index on the WISC was correlated with total omissions, (r = −0.20, p<0.05), total commissions (r = −0.22, p<0.05) and d’ (r = 0.40, p<0.01), suggesting that participants with faster cognitive processing speed made fewer errors and performed better on the CPT than those with slower processing speed. In contrast, the Perceptual Reasoning Index was not correlated with any CPT outcome variables. In the Hearing group, we found that better Processing Speed was significantly related to total Omissions, (r = −0.26, p<0.05), Total Commissions (r = −0.29, p<0.05) and d’ (r = 0.32, p<0.05), which was similar to the CI group. Again, the Perceptual Reasoning Index was not correlated with any CPT outcome variables.
In addition, results on age at implantation fully supported our hypothesis that children implanted at a younger age would be less impulsive and have better selective visual attention than those implanted later. Age at implantation was significantly related to total β (β = −0.48, t(101) = −5.50, p <0.01) and d’ (β = 0.20, t(101) = 2.09, p =0.04). In addition, age at implantation accounted for 23% of the variance in total β and 5% of the variance in d’. Our hypothesis that older vs younger children would perform better on the CPT was also supported. Chronological age predicted both β and d’ in the CI and hearing groups (CI group β: β = −0.44, t(101) = −5.00, p <0.01, CI Group d’: β = 0.21, t(101) = 2.13, p =.04) (Hearing group β: β = −0.49, t(64) = −4.52, p <0.01, Hearing Group d’: β = 0.55, t(64) = 5.29, p <.01). With regards to other demographic predictors, there was no relationship between CPT outcomes and gender in both the CI and Hearing groups, suggesting that boys and girls performed comparable on the task (See Table 4 for all correlations). Similarly, there was no relationship between CPT outcomes and device manufacturer (e.g., Advanced Bionics, Cochlear, and MED-EL) for children with CIs.
Table 4.
Partial Correlations
| CI group | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| 1) Omissions | 1 | .40** | .20* | −.66** | .25* | .13 | −.09 | −.20* | −.01 |
| 2) Commissions | .59** | 1 | −.56** | −.55** | −.25* | −.04 | −.09 | −.22* | .02 |
| 3) β | .50** | .04 | 1 | −.25* | .37** | .17 | .04 | .01 | −09 |
| 4) d’ | −.76** | −.76** | −.63** | 1 | −.06 | −.01 | .10 | .40** | .04 |
| 5) Sustained Attention | −.18 | −.48** | .15 | .21 | 1 | .18 | .05 | −.04 | .01 |
| 6) Gender | −.10 | −.05 | .−3 | .03 | .12 | 1 | −.04 | .22* | .03 |
| 7) PRI (WISC) | −.05 | −.08 | −.04 | .08 | .15 | −.15 | 1 | .32** | −.02 |
| 8) PSI (WISC) | 0.26* | −.29* | −.22 | .32* | .20 | −.01 | .12 | 1 | −.03 |
| 9) Device Manufacturer | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 1 |
|
| |||||||||
| Hearing group | |||||||||
Note: All correlations controlled for maternal education
Aim 3 compared the CI and Hearing groups on the Gordon CPT outcomes (see Table 2). Our hypothesis that children with CIs would perform significantly worse than their hearing peers on all CPT outcomes was partially supported. Children CIs performed significantly worse than their hearing peers within each block and on total d’ (mean d’ CI group = 2.51 (SD = 0.81); mean d’ Hearing group = 3.05 (SD = 0.89); Cohen’s d = −0.63). Importantly, these effects were in the medium to large range and were likely driven by significant differences in commissions between groups, with the CI group committing more false alarms than the Hearing group in Blocks 1 and 2 and on Total Commission errors (mean commission errors CI group = 17.96 (SD = 23.18); mean commission errors Hearing group = 9.51 (SD = 10.79); Cohen’s d = −0.47). In contrast, the CI and Hearing groups performed similarly on β, which suggests that both groups of children had a similar likelihood of identifying targets vs. non-targets (CI mean β = 0.81, SD = 0.39; NH mean β = 0.71, SD = 0.27).
We also found strong support for our hypothesis that the CI group would perform worse than the normative data on the CPT task. Comparisons of scores in the CI vs normative samples showed that children in the CI group identified significantly fewer targets from non-targets (CI mean total correct = 29.38, SD =8.25; Normative mean total correct = 36.0, SD = 6.9; t(1,362) = 7.84; p <0.01; Cohen’s d =0.82). Children in the CI group committed a significantly larger number of commission errors in comparison to the normative sample (CI mean total commissions = 17.96, SD = 23.18; Normative mean total commissions = 7.8, SD = 8.3; t(1,362) = 6.15; p <0.01; Cohen’s d =0.65). Therefore, the CI group performed significantly worse on this CPT task than our typically hearing sample and the normative data. Due to a lack of normative data on the standard deviations for β and d’, these outcomes could not be compared.
Aim 4a examined the effects of language ability at 48-months post-implant on CPT scores at 60 months post-implant. As hypothesized, there was no significant relationship between language scores one year prior and performance on this attention task for β (β = −0.14, t(90) = −1.38, p =0.17) or d’ (β = −0.15, t(90) = −1.39, p=0.17). However, it should be noted that children in the CI group were significantly delayed in oral language development (mean CASL composite score = 78.00, SD =22.06). Aim 4b examined change in language scores in the 12 months preceding administration of the CPT task. Results did not support our hypothesis; rate of change in receptive and expressive language was not related to β (β = −0.18, t(90) = −1.62, p <0.18) or d’ (β = −0.09, t(90) = −1.49, p <0.13). These findings suggested that visual attention in school-aged children is not language-based.
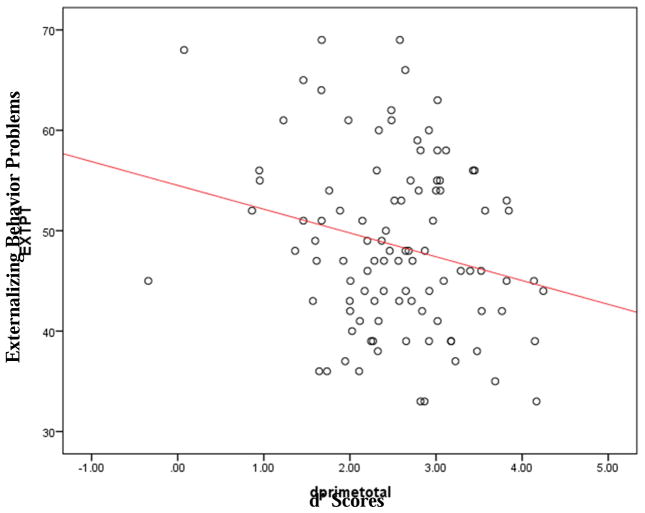
Aim 5 tested the relationship between CPT scores and externalizing behavior problems, rated by parents. We hypothesized that children who performed poorly on the CPT would be rated as having more externalizing behavior problems. This hypothesis received strong support in the CI group. β significantly predicted behavior problems (β = 0.20, F(1,98) = 4.14, p < 0.05) and accounted for 4% of the variance (See Figure 1). Follow-up analyses showed that this finding was driven by the relationship between β and the Aggression subscale ((β = 0.27, F(1,98) = 7.74, p < 0.01). In addition, total d’ scores also significantly predicted behavior problems (β = −0.23, F(1,98) = 5.37, p < 0.05) and accounted for 5% of the variance in the model. This was driven by a significant relationship between d’ and the Hyperactivity scale (β = −0.26, F(1,98) = 7.47, p < 0.01). In contrast, performance on the CPT did not predict behavior problems in the Hearing group; β (β = −0.16, F(1,58) = 1.57, p= 0.22), d’ (β = 0.16, F(1,58) = 1.23, p = 0.21).
Figure 1.
d’ scores predicting Externalizing Behavior Problems
This was likely due to the smaller variability in parent-reported behavior problems in the typically hearing group. (only 6.06% scored in the “At-Risk” or Clinically Elevated” range, as opposed to 11.30% in the CI group).
4. Discussion
This study evaluated visual selective attention 60-months post-implantation in the largest sample of school-age children with CIs in the US. We also examined associations among visual attention, oral language, and externalizing behavior problems. Overall, results showed that children with CIs have deficits in selective visual attention, which are related to externalizing behavior problems, but not to oral language. Note that, as expected, the CI group was delayed in language development (mean language standard score = 78.21; Hearing group mean language standard score = 117.08). These findings also supported the Division of Labor hypothesis, which theorizes that children with hearing loss cannot simultaneously monitor their environment and focus on a specific task-at-hand, but instead must rely on vision to accomplish both goals (Quittner et al., 2007; Quittner et al., 2004; Smith et al., 1998). This leads to poor performance on visual tasks, such as the CPT, but also suggests that these children may have higher levels of impulsive and distractible behavior because they are required to monitor their environment visually while attempted to focus on certain tasks. Note that there was considerable variability in the timing of implantation in this sample (from 5 months to 5 years), which was correlated with d’, the major selective attention index. Thus, this could partially explain why visual selective attention is still worse in CI vs. hearing children. Even after 5 years of CI experience, deaf children exhibited deficits in visual attention, which had cascading effects on other domains of development, such as externalizing behavior problems. This has substantive implications for how these children perform in a variety of learning environments, including school and other social contexts. Children with visual attention delays can be given additional supports in school that have been found to be efficacious, including placement at the front of the class, more frequent breaks, and diverse teaching modalities (Sattler & Ryan, 2009).
No relationships were found between visual attention and gender, device manufacturer, or perceptual reasoning. In contrast, we did find significant, positive relationships between Processing Speed Index and total omissions, total commissions, and d’. Thus, the ability to do simple tasks quickly and easily was associated with discriminating targets from non-targets on this attention task. Our results contradict those of Horn et al., (2005), who found no relationship between nonverbal IQ and CPT outcomes. However, they had a limited sample for which data was available (n=19 in Experiment 1, n=37 in Experiment 2).
Importantly, our results showed that age of implantation was significantly related to d’ scores, indicating that earlier assess to auditory information to the brain has substantial benefits for the development of visual attention. This aligns with previous studies (Yucel & Derim, 2008), but contrasts with those of Horn and colleagues (2005). Further, this key relationship between earlier implantation and a host of important outcomes variables, such as oral language development, novel noun learning, and social skills suggests that we need to redouble our efforts in cochlear implant programs nationally to push for earlier implantation (Hoffman, Quittner, & Cejas, 2014, 2015; Niparko et al., 2010).
We found no associations between visual attention and oral language skills. Although research has linked development of the visual and auditory systems in infants and toddlers (Chen & Westermann, 2012; Lewkowicz & Flom, 2014), it is possible that oral language and visual attention function as somewhat separate systems in school-age children. There may also be a “threshold effect,” in which a minimal level of language is required to allow for continued development of visual attention. However, given that oral language was below average in our CI sample (approximately 1.5 standard deviations below the mean), this threshold would have to be quite low. It is also possible that this particular CPT task, which does not use any sort of auditory cues, may “mask” the effects of language on visual attention performance. Perhaps a CPT task which invokes auditory/linguistic skills might show a relationship between these two domains of functioning. However, more research is needed to understand why these two areas of development are not related.
The lack of association between visual attention and oral language skills is a critical finding, given that most CI programs focus intensively on language learning. Our findings suggest that another key focus should be on training attentional skills, such as attention-shifting. These poor attentional skills have also been shown to be correlated with social skills deficits (Bunford, Evans, Becker, & Langberg, 2015), another area of delayed functioning in school-age children with CIs (Hoffman et al., 2014). Thus, providing interventions that target both oral language and visual attention skills could have positive effects on the development of cognition, behavioral regulation, and social skills. These interventions may include extra time for class assignments and tests, placement in a structured classroom environment, breaking complicated tasks into simple steps, and use of multimodal learning styles (i.e., combining visual and auditory teaching methods) (Sattler & Ryan, 2009).
Our results also showed that visual attention was significantly related to parent-reported externalizing behavior problems in children with CIs. Follow-up analyses showed that children who were reported by parents as hyperactive had worse d’. This is consistent with previous studies that have examined the relationship CPT performance and hyperactivity symptoms among children with ADHD (Epstein et al., 2003; Huang-Pollock, et al., 2012). Thus, deficits in selective visual attention were linked to real-world attention and behavioral difficulties observed at home and at school. Currently, little attention is given to these children’s attentional difficulties, which appear to affect their ability to focus attention, inhibit impulsive responding and control aggressive behavior, all areas of neurological functioning that are related to activation in the prefrontal cortex (Barkley, 1997; Christakou et al., 2013). These findings support the need for clinical psychologists as part of CI teams and early intervention programs, to facilitate the assessment and remediation of attention and behavioral skills. These interventions should not only target oral language development, but include a focus on directing and focusing attention, behavioral regulation, cognition, and social skills--all areas psychologists are well-trained to address. The goal of early intervention should be development of the “whole child” rather than an exclusive focus on audiological and language skills (Cejas & Quittner, In press). Thus, CI clinics should consider a more multidisciplinary approach.
4.1 Limitations
This study had several limitations. First, CPT data used were cross-sectional, which prevented us from evaluating how CPT performance changes over time. We did, however, track language development over the year preceding the CPT test and established that changes in language were not related to attention skills. Second, our analyses comparing the CI and hearing groups controlled for maternal education due to differences between groups. There may be other factors associated with family education and income that we did not measure, which may have affected visual attention in the CI group. However, as noted above, previous studies have consistently documented these deficits in visual attention across a range of families with hearing loss (Quittner et al., 1994; Smith et al., 1998). It is also possible that there are other cognitive factors that could explain differences in visual attention when comparing children with CIs to hearing peers, including short-term memory, working memory, and/or familiarity with digits. Unfortunately, data regarding short-term memory were not available. In addition, participants in this study were not required to utlize the numeric values of the digits in any way (i.e., perform calculations), which minimizes the potential advantage of strong mathematic abilities. Finally, in terms of parent-reported behavior problems, it is possible that these two groups of parents have different expectations and biases that influenced their ratings. The fact that there is also teacher-reported data suggesting these elevations in externalizing problems (Mitchell & Quittner, 1996) lends more credibility to our findings.
4.2 Strengths and Clinical Implications
This study also had several strengths. These data were drawn from a large, longitudinal multisite study designed specifically to include families who were diverse in race and ethnicity, increasing the generalizability of the findings. We were also able to compare performance in the CI group to a cohort of hearing peers moving through development at the same time. We also compared their performance to the normative data on this task. Thus, these findings, taken together with previous research, indicate that these findings are robust and compelling. We also examined a range of predictors of visual attention skills, including age at implantation, gender, cochlear implant device manufacturer, IQ, and language scores. Importantly, earlier age at implantation, but not language ability, was predictive of visual attention. Finally, this was the first study to document the strong link between deficits in visual attention and externalizing behavior problems in children with CIs.
These results have significant clinical implications for cochlear implant programs nationally. First, cochlear implantation alone does not “fix” the problem of childhood deafness given that is affects the “whole child” (Barker et al., 2009; Cejas & Quittner, In press; Guillon et al., 2014; Hunnius, 2007; Klenberg et al., 2001). Severe-to-profound hearing loss, even in the era of cochlear implantation, leads to a cascading set of challenges for young children, including problems with visual attention and development 5of behavioral control. These issues likely affect children’s daily functioning at home and at school, and their overall health-related quality of life (Hoffman, Cejas, & Quittner, 2016). Currently, cochlear implant teams do not focus on these other dimensions of development and thus, may not be positioned to address them. One possibility is that the assessment of attention and behavior should be incorporated into routine, annual visits soon after implant surgery, and remediation of these deficits should be included in early intervention programs.
Supplementary Material
Highlights.
Children with CIs showed significantly worse visual attention than hearing peers
These differences were caused by higher false alarm rates in the CI vs typically hearing group
Visual attention scores predicted parent-reported behavior problems in the CI group
Assessment of attention and behavior should be included in medical visits after implant surgery
Remediation of visual attention should be included in early intervention programs
Acknowledgments
The Childhood Development after Cochlear Implantation (CDaCI) Investigative Team was supported by Grant R01DC004797 from the National Institute on Deafness and Other Communication Disorders. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Deafness and Other Communication Disorders or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abidin RR. Parenting Stress Index-Manual. 3. Charlottesville, VA: Pediatric Psychology Press; 1990. [Google Scholar]
- Achenbach TM, Edelbrock CS. Manual for the Child Behaviorr Checklist and Revised Child Behavior Profile. Burlington, Vermont: University of Vermont, Department of Psychology; 1983. [Google Scholar]
- Barker DH, Quittner AL, Fink NE, Eisenberg LS, Tobey EA, Niparko JK. Predicting behavior problems in deaf and hearing children: The influences of language, attention, and parent–child communication. Developmental Psychopathology. 2009;21(02):373–392. doi: 10.1017/S0954579409000212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychological Bulletin. 1997;121(1):65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Bunford N, Evans SW, Becker SP, Langberg JM. Attention-deficit/hyperactivity disorder and social skills in youth: A moderated mediation model of emotion dysregulation and depression. Journal of Abnormal Child Psychology. 2015;43(2):283–296. doi: 10.1007/s10802-014-9909-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrow-Woolfolk E. Comprehensive assessment of spoken language (CASL) Circle Pines, MN: American Guidance Service; 1999. [Google Scholar]
- Cejas I, Quittner AL. Effects of family variables on spoken language in children with cochlear implants. In: Marschark M, Knoors H, editors. Educating Deaf Learners: New Perspectives. Oxford, England: Oxford University Press; In press. [Google Scholar]
- Chen YC, Westermann G. Twelve-month-old infants learn crossmodal associations between visual objects and natural sounds in ecologically valid situations. Seeing and Perceiving. 2012;25:117. [Google Scholar]
- Christakou A, Murphy C, Chantiluke K, Cubillo A, Smith A, Giampietro V, … Murphy D. Disorder-specific functional abnormalities during sustained attention in youth with attention deficit hyperactivity disorder (ADHD) and with autism. Molecular Psychiatry. 2013;18(2):236–244. doi: 10.1038/mp.2011.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein JN, Erkanli A, Conners CK, Klaric J, Costelle JE, Angold A. Relations between Contiuous Performance Test performance measures and ADHD behaviors. Journal of Abnormal Child Psychology. 2003;31(5):543–554. doi: 10.1023/a:1025405216339. [DOI] [PubMed] [Google Scholar]
- Fink NE, Wang NY, Visaya J, Niparko JK, Quittner A, Eisenberg LS, Tobey EA. Childhood development after cochlear implantation (CDaCI) study: Design and baseline characteristics. Cochlear Implants International. 2007;8(2):92–116. doi: 10.1179/cim.2007.8.2.92. [DOI] [PubMed] [Google Scholar]
- Gordon M, Mettelman B. Technical guide to the Gordon Diagnostic System (GDS) DeWitt, NY: Gordon Systems; 1987. [Google Scholar]
- Guillon Q, Hadjikhani N, Baduel S, Roge B. Visual social attention in autism spectrum disorder: Insights from eye tracking studies. Neuroscience & Behavioral Reviews. 2014;42(1):279–297. doi: 10.1016/j.neubiorev.2014.03.013. [DOI] [PubMed] [Google Scholar]
- Hoffman MF, Cejas I, Quittner AL. Development of school-age and parent-proxy health-related quality of life instruments for children with cochlear implants. Paper presented at the The International Conference on Cochlear Implants; Toronto, Canada. 2016. [Google Scholar]
- Hoffman MF, Quittner AL, Cejas I. Longitudinal trajectories of social competence in children with cochlear implants and normal hearing. Paper presented at the the 14th Symposium on Cochlear Implants in Children; Nashville, TN. 2014. [Google Scholar]
- Hoffman MF, Quittner AL, Cejas I. Comparisons of social competence in young children with and without hearing loss: A dynamic systems framework. Journal of Deaf Studies and Deaf Education. 2015;20(2):115–124. doi: 10.1093/deafed/enu040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn DL, Davis RA, Pisoni DB, Miyamoto RT. Development of visual attention skills in prelingually deaf children who use cochlear implants. Ear and Hearing. 2005;26(4):389–408. doi: 10.1097/00003446-200508000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang-Pollock CL, Karalunas SL, Tam H, Moore AN. Evaluating vigilance deficits in ADHD: A meta-analysis of CPT performance. Journal of Abnormal Psychology. 2012;121(2):360–371. doi: 10.1037/a0027205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunnius S. The early development of visual attention and its implications for social and cognitive development. Progress in Brain Research. 2007;164(1):187–209. doi: 10.1016/S0079-6123(07)64010-2. [DOI] [PubMed] [Google Scholar]
- Klenberg L, Korkman M, Lahti-Nuuttila P. Differential development of attention and executive functions in 3 to 12 years-old Finnish children. Developmental Neuropsychology. 2001;20(1):407–428. doi: 10.1207/S15326942DN2001_6. [DOI] [PubMed] [Google Scholar]
- Lewkowicz DJ, Flom R. The audiovisual temporal binding window narrows in early childhood. Child Development. 2014;85(2):685–694. doi: 10.1111/cdev.12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell TV, Quittner AL. Multimethod study of attention and behavior problems in hearing-impaired children. Journal of Clinical Child Psychology. 1996;25(1):83–96. [Google Scholar]
- Niparko JK, Tobey EA, Thal DJ, Eisenberg LS, Wang NY, Quittner AL … Team CI. Spoken language development in children following cochlear implantation. JAMA. 2010;303(15):1498–1506. doi: 10.1001/jama.2010.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niskar A, Kieszak S, Holmes A, Esteban E, Rubin CDB. Prevalence of hearing loss among children 6 to 19 years of age: The third national health and nutrition examination survey. JAMA. 1998;279(14):1071–1075. doi: 10.1001/jama.279.14.1071. [DOI] [PubMed] [Google Scholar]
- Quittner AL, Barker DH, Snell C, Cruz I, McDonald LG, Grimley ME … Investigative Team C. Improvements in visual attention in deaf infants and toddlers after cochlear implantation. Audiological Medicine. 2007;5(4):242–249. [Google Scholar]
- Quittner AL, Leibach P, Marciel K. The impact of cochlear implants on young deaf children - New methods to assess cognitive and behavioral development. Archives of Otolaryngology-Head & Neck Surgery. 2004;130(5):547–554. doi: 10.1001/archotol.130.5.547. [DOI] [PubMed] [Google Scholar]
- Quittner AL, Sawicki GS, McMullen A, Rasouliyan L, Pasta DJ, Yegin A, Konstan MW. Erratum to: Psychometric evaluation of the cystic fibrosis questionnaire-revised in a national, US sample. Quality of Life Research. 2012;21(7):1279–1290. doi: 10.1007/s11136-011-0091-5. [DOI] [PubMed] [Google Scholar]
- Quittner AL, Smith LB, Osberger MJ, Mitchell TV, Katz DB. The impact of audition on the development of visual-attention. Psychological science. 1994;5(6):347–353. [Google Scholar]
- Reynell JK, Gruber CP. Reynell Developmental Language Scales - Second Revision. Los Angeles, CA: Western Psychological Services; 1990. [Google Scholar]
- Reynolds CR, Kamphaus RW. Behavior assessment system for children (BASC-2) Circle Pines, MN: American Guidance Service; 2004. [Google Scholar]
- Roid GH, Miller LJ. Leiter International Performance Scale-Revised (Leiter-R) manual. Wood Dale, IL: Stoelting; 1997. [Google Scholar]
- Sattler JM, Ryan JJ. Assessment with the WAIS-IV. Jerome M Sattler Publisher; 2009. [Google Scholar]
- Smith LB, Quittner AL, Osberger MJ, Miyamoto R. Audition and visual attention: The developmental trajectory in deaf and hearing populations. Developmental Psychology. 1998;34(5):840–850. doi: 10.1037//0012-1649.34.5.840. [DOI] [PubMed] [Google Scholar]
- Tharpe A, Ashmead D, Rothpletz A. Visual attention in children with normal hearing, children with hearing aids, and children with cochlear implants. Journal of Speech, Language, and Hearing Research. 2002;45(2):403–413. doi: 10.1044/1092-4388(2002/032). [DOI] [PubMed] [Google Scholar]
- Van Naarden Braun K, Christensen D, Doernberg N, Schieve L, Rice C, Wiggins L, … Yeargin-Allsopp M. Trends in the prevalence of autism spectrum disorder, cerebral palsy, hearing loss, intellectual disability, and vision impairment, metropolitan atlanta, 1991–2010. Plos One. 2015;10(4):e0124120. doi: 10.1371/journal.pone.0124120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children. 4. San Antonio, TX: Harcourt Association; 2003. [Google Scholar]
- Wixted JT. Dual-process theory and signal-detection theory of recognition memory. Psychological Review. 2007;114(1):152–176. doi: 10.1037/0033-295X.114.1.152. [DOI] [PubMed] [Google Scholar]
- Yucel E, Derim D. The effect of implantation age on visual attention skills. Otorhinolarynology. 2008;72(6):869–877. doi: 10.1016/j.ijporl.2008.02.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.