Abstract
Background
Executive cognitive functions, including working memory, cognitive flexibility, and inhibition, are impaired in schizophrenia. Executive functions rely on coordinated information processing between the prefrontal cortex (PFC) and thalamus, particularly the mediodorsal (MD) nucleus. This raises the possibility that anatomical connectivity between the PFC and MD thalamus may be: 1) reduced in schizophrenia; and 2) related to deficits in executive function. The current investigation tested these hypotheses.
Methods
45 healthy subjects and 62 individuals with a schizophrenia spectrum disorder completed a battery of tests of executive function and underwent diffusion-weighted imaging (DWI). Probabilistic tractography was used to quantify anatomical connectivity between six cortical regions, including the PFC, and the thalamus. Thalamocortical anatomical connectivity was compared between healthy subjects and schizophrenia using region-of-interest and voxel-wise approaches, and the association between PFC-thalamic anatomical connectivity and severity of executive function impairment was examined in patients.
Results
Anatomical connectivity between the thalamus and PFC was reduced in schizophrenia. Voxel-wise analysis localized the reduction to areas of the MD thalamus connected to lateral PFC. Reduced PFC-thalamic connectivity in schizophrenia correlated with impaired working memory, but not cognitive flexibility and inhibition. In contrast to reduced PFC-thalamic connectivity, thalamic connectivity with somatosensory and occipital cortices was increased in schizophrenia.
Conclusions
The results are consistent with models implicating dysrupted PFC-thalamic connectivity in the pathophysiology of schizophrenia and mechanisms of cognitive impairment. PFC-thalamic anatomical connectivity may be an important target for pro-cognitive interventions. Further work is needed to determine the implications of increased thalamic connectivity with sensory cortex.
Keywords: Thalamus, Cortex, Anatomical, Connectivity, Diffusion, Schizophrenia
INTRODUCTION
Executive cognitive functions, which collectively encompasses working memory, cognitive flexibility/set-shifting, and inhibition (1), are essential for functioning in dynamic environments. Schizophrenia is characterized by cognitive impairment, including prominent deficits in executive function (2–6). Cognitive impairment, including deficits in executive function, is an important predictor of functional outcome making it a critical target for interventions (7). The pro-cognitive effects of existing pharmacological and behavioral interventions are modest (8–10). The development of more effective interventions is hampered by an incomplete understanding of the neural basis of neuropsychological impairment.
Executive functions are supported by a distributed set of brain regions that includes the prefrontal cortex (PFC) and thalamus, particularly the mediodorsal (MD) nucleus which is reciprocally connected to the PFC. Lesions to the MD nucleus often result in deficits in executive function that mimic those observed following damage to the PFC (11,12). Neuroimaging investigations indicate that the MD thalamus is a key sub-cortical node in a fronto-cingulo-parietal, “executive control” network that supports working memory, cognitive flexibility, and inhibition (13). Animal models have illuminated the mechanisms of executive function. Rodent electrophysiological investigations in particular have clarified the mechanisms of executive function by demonstrating the importance of functional coupling between the PFC and MD nucleus to working memory, attention, and cognitive flexibility (14–18).
There is compelling evidence that the PFC and thalamus, are abnormal in schizophrenia. Key findings confirmed by meta-analysis include reduced grey matter volume and reduced activity during cognition (19,20). Abnormal PFC-thalamic circuitry is further supported by resting-state fMRI functional connectivity studies that consistently find reduced functional connectivity between the PFC and thalamus (for review see 21). Several disease models are based, in part, on these findings including the ‘cognitive dysmetria’ model which hypothesizes that the cognitive deficits and clinical features of schizophrenia result from a core defect in cortical-subcortical circuitry (22–26).
Irrespective of the model, critical knowledge gaps remain. First, while altered PFC-thalamic activity and functional connectivity is well-established, considerably less is known about the integrity of anatomical connectivity between the PFC and thalamus. A small number of diffusion-weighted imaging (DWI) studies have examined thalamocortical anatomical connectivity; however, they have yielded inconclusive results, likely due to small sample sizes in some cases (27–29). Second, the cognitive correlates of PFC-thalamic structural connectivity, especially executive function, are poorly understood. Finally, the integrity of structural connections linking other cortical areas to the thalamus has received little attention. The current study was undertaken to address these knowledge gaps and limitations of prior studies. The primary aim of this investigation was to test the hypothesis that PFC-MD thalamus anatomical connectivity is: 1) reduced in schizophrenia; and 2) related to deficits in executive function. A secondary aim of this investigation was to characterize connectivity disturbances in other anatomical networks linking the cortex to thalamus.
METHODS AND MATERIALS
Study Participants
47 healthy subjects and 67 individuals with a schizophrenia spectrum disorder (i.e. schizophreniform disorder, schizophrenia, schizoaffective disorder) that participated in an on-going study of thalamocortical networks in psychotic disorders were screened for inclusion in this study. All schizophrenia spectrum patients, hereafter referred to as ‘schizophrenia,’ were recruited through the Psychotic Disorders Program at the Vanderbilt Psychiatric Hospital. Healthy individuals were recruited from Nashville and surrounding area via advertisement and word-of-mouth. This study was approved by the Vanderbilt University Institutional Review Board and all subjects provided written informed consent prior to participating. Psychiatric diagnoses were confirmed in patients, and ruled out in healthy subjects, using the Structured Clinical Interview for Diagnosing DSM-IV Disorders (SCID: 30). The Positive and Negative Syndrome Scale (PANSS: 31) was administered to patients to quantify the severity of clinical symptoms of psychosis. The Wechsler Test of Adult Reading (WTAR: 32) was administered to all subjects to provide an estimate of pre-morbid intellectual quotient (IQ). Exclusion criteria included age less than 16 or greater than 55; estimated pre-morbid IQ less than 70; presence of a systemic medical illness (e.g. diabetes, cardiovascular disease) or central nervous system disorder (e.g. multiple sclerosis, epilepsy) that would affect study results; history of significant head trauma; psychotropic drug use (healthy subjects only); active substance abuse, based on SCID criteria, within the past 3 months (or lifetime history of substance abuse/dependence in healthy subjects); and MRI contra-indicators (e.g. metal implants, claustrophobia).
Neuropsychological Testing
All study participants completed a brief neuropsychological assessment. Given our a-priori focus on executive function and PFC-thalamic circuitry, testing focused on assessing the three domains of executive function identified by Miyake et al. (1): working memory, set-shifting/cognitive flexibility, and inhibition. The Wechsler Memory Scale-III (WMS-III: 33) digit and spatial span subtests, which comprise the Working Memory Index, Wisconsin Card Sorting Test- 64 Card Version (WCST: 34), and AX-Continuous Performance Test (AX-CPT: 35) were administered to quantify working memory, set-shifting, and inhibition, respectively. The WMS-III Working Memory Index, WCST total errors, d-prime measure from the AX-CPT, served as the dependent variables. The Screen for Cognitive Impairment in Psychiatry (SCIP: 36) was also administered to quantify overall neuropsychological functioning.
Neuroimaging Data Acquisition, Preprocessing, and Probabilistic Tractography
Neuroimaging data were acquired on a 3T Philips Intera Achieva scanner (32 channel received head coil, single-band imaging) located at the Vanderbilt Institute for Imaging Sciences (VUIIS). A T1-weighted structural scan (1 mm isotropic resolution) and high-angular radial diffusion-weighted imaging (HARDI) scan (2.5 mm isotropic resolution, 60 directions, b-value=2000 s/mm, 5 bo images) were collected on each subject in a single imaging session. Of note, the HARDI data were collected with a SENSE factor of 2.2 to reduce echo time and EPI distortions.
Each subject’s structural T1-weighted MRI was automatically segmented using the program Multi Atlas developed by author BL (37). Briefly, Multi Atlas uses a statistical fusion framework built on an a priori model comprised of a manually-traced dataset to automatically label 133 cortical and subcortical brain structures. Selected cortical structures were combined to generate 6 bilateral cortical regions-of-interest (ROIs) along with the thalamus were used as targets and the seed, respectively, for probabilistic tractography. The six cortical ROIs corresponded to the prefrontal cortex (PFC), motor cortex/supplementary motor area, somatosensory cortex, posterior parietal cortex, temporal cortex, and occipital cortex (see Supplemental Material for an example segmentation and list of cortical structures included in each cortical ROI). The thalamus ROI was manually edited by a single individual (author MGC), blind to diagnostic status, to include the lateral and medial geniculate nuclei. In addition, each subject’s T1-weighted anatomical image was segmented into grey matter, white matter, and CSF using the Voxel-based Morphometry 8 (VBM8) toolbox (http://www.neuro.uni-jena.de/vbm/download/) which employs the Diffeomorphic Anatomical Registration using Exponential Lie algebra (DARTEL) algorithm for high-dimensional spatial normalization to Montreal Neurological Institute (MNI) space (38). Prior to analysis, all HARDI data underwent quality assurance (QA) using an automated QA pipeline developed by our group that includes visualization of HARDI data, and extraction of mean head translation and rotation in the X, Y, and Z directions and average number of voxels (in percent) rejected per gradient due to poor diffusion tensor imaging (DTI) model fitting (39).
Following QA, HARDI data preprocessing and probabilistic tractography was performed using FMRIB’s Diffusion Toolbox (FDT) for FSL v5.0.6 software package (http://www.fmrib.ox.ac.uk/fsl/). Data preprocessing and probabilistic tractography are described in detail in the Supplemental Material. Briefly, seed-to-target tractography analyses were run using probtrackx2 (40) in order to quantify anatomical connectivity between the thalamus (i.e. seed) and each of the six cortical ROIs (i.e. targets). The left and right hemispheres were analyzed separately. From each thalamic voxel, 5000 samples were sent through the probability distributions on principle fibre direction. From probtrackx2, seed-to-target voxel-wise images were generated in which the value of each voxel within the seed mask (i.e. thalamus) represents the number of samples seeded from that voxel reaching the relevant target mask. The connectivity of each cortical region with the thalamus was calculated by dividing the number of samples reaching that region, summed across all voxels in the thalamus, by the total number of samples within the thalamus reaching all cortical regions (29). This is a measure of total tractography-defined connectivity from the thalamus to a particular cortical area, independent of where the tract originated from inside the thalamus and after controlling for overall connectivity of the thalamus. These values, expressed as “total connectivity” (in percent), served as the dependent variables for the primary analysis described below. In addition, voxel-wise probability maps were generated by dividing the number of samples from each voxel that arrived at the corresponding target by the total number of samples from that voxel reaching any cortical region. These probability maps were used in the voxel-wise analysis described below.
Statistical Analysis
Group differences in dichotomous and continuous demographic and cognitive variables were examined using chi-square and independent groups t-tests, respectively. For the analysis of total cortical-thalamic connectivity, left and right hemisphere connectivity values were averaged since we did not have any a-priori hypotheses regarding laterality and to maximize statistical power. This resulted in 6 dependent variables per subject, one for each cortical target ROI. Total percent connectivity values were analyzed using independent groups t-tests. Given our a-priori hypothesis that prefrontal-thalamic connectivity would be reduced in schizophrenia, no correction was applied to the critical alpha for this contrast. Significance was set to p=.01 (i.e. Bonferroni corrected) for the remaining 5 cortical target ROIs. The primary analysis described above was complemented with a voxel-wise analysis to further localize potential group differences in thalamocortical structural connectivity (see Supplemental Material). Voxel-wise analyses were thresholded at the cluster-level p-value (family-wise error (FWE) corrected)=.05 for voxel-wise p(uncorrected)=.001. Finally, to test the hypothesis that PFC-thalamic anatomical connectivity is related to cognitive impairment in schizophrenia, average PFC-thalamic connectivity was correlated with each measure of executive function (WMS-III Working Memory Index, WCST total errors, AX-CPT d-prime) using partial correlation analysis controlling for age and sex. Similarly, average connectivity was extracted from significant clusters identified in the PFC-thalamic voxel-wise analyses and correlated with each of the executive cognitive measures using partial correlation analysis controlling for age and sex.
RESULTS
7 subjects (2 healthy individuals and 5 patients) were excluded due to presence of neuroimaging data artifacts based on visual inspection. Thus, the final sample included 45 healthy subjects and 62 individuals with schizophrenia (see Table 1). With the exception of education, the groups did not differ on demographic variables. No significant differences in HARDI QA metrics were detected between groups, including mean head translation and rotation in X, Y, and Z directions, and percentage of outlier voxels per gradient with poor fitting DTI (see Supplemental Material). With the exception of 12 individuals, all patients were taking an antipsychotic medication. Antipsychotic dosage was unavailable for 2 patients.
Table 1.
Sample Demographics
| Healthy Subjects | Schizophrenia | Statistics | |||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| n=45 | n=62 | t/x2 | df | p | |||
| Sex (M:F) | 29:16 | 43:19 | 0.29 | 1 | .593 | ||
| Race (W:B:O) | 32:9:4 | 44:16:2 | 1.87 | 2 | .393 | ||
|
| |||||||
| Mean | SD | Mean | SD | ||||
|
| |||||||
| Age | 27.8 | 7.0 | 27.0 | 8.6 | 0.53 | 105 | .595 |
| Education | 16.3 | 2.0 | 13.6 | 2.1 | 6.96 | 105 | <.001 |
| Parental Education | 15.0 | 2.1 | 14.8 | 2.9 | 0.45 | 104 | .653 |
| Premorbid IQ | 113.0 | 10.2 | 102.6 | 13.8 | 4.24 | 104 | <.001 |
| SCIP Z-Score | 0.39 | 0.56 | −0.73 | 0.77 | 8.30 | 105 | <.001 |
| WMS-III WM Index | 107.5 | 12.7 | 95.0 | 12.1 | 5.16 | 104 | <.001 |
| WCST total errors | 11.1 | 6.6 | 19.6 | 10.6 | 4.62 | 103 | <.001 |
| AX-CPT d-prime | 3.54 | 0.63 | 2.70 | 1.15 | 4.47 | 105 | <.001 |
| Age at Illness Onset | -- | -- | 21.8 | 5.7 | -- | -- | -- |
| Duration of Illness | -- | -- | 5.7 | 6.4 | -- | -- | -- |
| APD dose (CPZ equivalents) | -- | -- | 353.6 | 224.0 | -- | -- | -- |
| PANSS Positive | -- | -- | 13.7 | 7.2 | -- | -- | -- |
| PANSS Negative | -- | -- | 14.2 | 5.7 | -- | -- | -- |
| PANSS General | -- | -- | 27.4 | 7.4 | -- | -- | -- |
Abbreviations: APD=Antipsychotic Drug; AX-CPT=Continuous Performance Test-AX version; B=Black; df=degrees of freedom; CPZ=Chlorpromazine; F=Female; M=Male; O=Other; PANSS=Positive and Negative Syndrome Scale; SCIP=Screen for Cognitive Impairment in Psychiatry; White=W; WCST=Wisconsin Card Sorting Test; WMS-III=Wechsler Memory Scale-III; WM=Working Memory
Thalamocortical Anatomical Connectivity
Total percent connectivity of each cortical ROI with the thalamus in healthy individuals and patients with schizophrenia is presented in Figure 1. Complete statistical results are presented in the Supplemental Material. Consistent with our hypothesis, anatomical connectivity between the PFC and thalamus was reduced in schizophrenia (t(105)=3.06, p=.003). In contrast to the reduction in PFC-thalamic connectivity, schizophrenia patients demonstrated significantly increased thalamic connectivity with somatosensory (t(105)=2.69, p=.008) and occipital cortices (t(105)=2.80, p=.006). Follow-up analyses revealed that PFC-thalamic anatomical connectivity was lower in both left and right hemispheres; although only the left hemisphere reached statistical significance (t(105)=3.73, p<.001). Increased thalamic connectivity with the occipital cortex was also more prominent in the left hemisphere, whereas somotasensory connectivity was increased bilaterally. Of note, total volume of the thalamus and cortical ROIs used as seed/target masks for probabilistic tractography did not differ between groups (see Supplemental Material). Additionally, average daily dose of antipsychotic medication (in 11 chlorpromazine equivalents) was unrelated to thalamocortical connectivity (all r’s<|.22|, p>.142).
Figure 1.
Anatomical connectivity between the cortex and thalamus in healthy subjects and individuals with schizophrenia.
Thalamocortical Anatomical Connectivity: Voxel-wise Analysis
Voxel-wise analyses are presented in Figure 2. As shown in Figure 2B and 2C, the connectivity patterns appeared qualitatively similar in both groups, and consistent with the anatomy of the thalamus and prior neuroimaging investigations (41,42). Each cortical region was anatomically connected to distinct, largely non-overlapping regions of the thalamus. The between group analyses revealed several differences in connectivity between healthy subjects and schizophrenia patients (see Figure 2D). Consistent with our hypotheses, and the primary analysis presented above, anatomical connectivity between the thalamus and PFC was decreased in schizophrenia in two clusters located in the medial dorsal thalamus at MNI coordinates -6 -18 10 (cluster size=24 voxels, pFWE-corr= .022) and -6 -12 2 (cluster size=18 voxels, pFWE-corr= .028). To determine what areas of the PFC these regions are connected to, we performed a separate probabilistic tractography analysis using these clusters as seeds and the PFC as the target ROI (see Supplemental Material). This analysis revealed that the two thalamic clusters are connected to the lateral PFC, including superior, middle, and inferior frontal gyri (see Supplemental Material). Qualitatively, there was less overlap across individuals in the schizophrenia group in the paths linking PFC to the two thalamic clusters.
Figure 2.
Thalamocortical anatomical connectivity in schizophrenia: results of voxel-wise analysis. Panel A: Each individual’s cortex was partitioned into 6 regions-of-interest (ROIs) that were used as targets to quantify anatomical connectivity between the thalamus (i.e. seed) and cortex. Panels B and C: Voxel-wise probability maps indicating probability of connectivity of thalamus voxels with each cortical ROI in healthy subjects and individuals with schizophrenia. Panel D: Group differences in anatomical connectivity between the cortex and thalamus (thresholded at cluster-level corrected p(FWE)=.05 for voxel-wise p(uncorrected)=.001). Axial images shown in neurological format (i.e. right side of image=right hemisphere).
Additionally, the voxel-wise analysis revealed group differences in thalamic connectivity with motor cortex, sensorimotor cortex, posterior parietal cortex, and occipital lobe. Specifically, clusters of both decreased and increased connectivity in schizophrenia were detected for motor-thalamic connectivity, whereas increased thalamic connectivity was observed for somatosensory, posterior parietal, and occipital cortex (see Figure 2D). Voxel-wise results are detailed in the Supplemental Material.
PFC-thalamic Anatomical Connectivity and Cognitive Impairment in Schizophrenia
The schizophrenia group performed worse than healthy subjects on all tests of executive function (see Table 1). To test the hypothesis that impaired executive function in schizophrenia is related to PFC-thalamic anatomical connectivity, the three executive function measures; WMS-III Working Memory Index, WCST total errors, and AX-CPT d-prime, were correlated with total PFC-thalamic connectivity and average PFC-thalamic connectivity extracted from the two clusters identified in the voxel-wise analysis. None of the measures of executive cognitive function correlated with total PFC-thalamic connectivity. Similar, none of the measures of executive function correlated with PFC-thalamic total connectivity in healthy individuals (one healthy subject that had an exceptionally high WMS-III Working Memory Index score of 151 was excluded from the analysis).
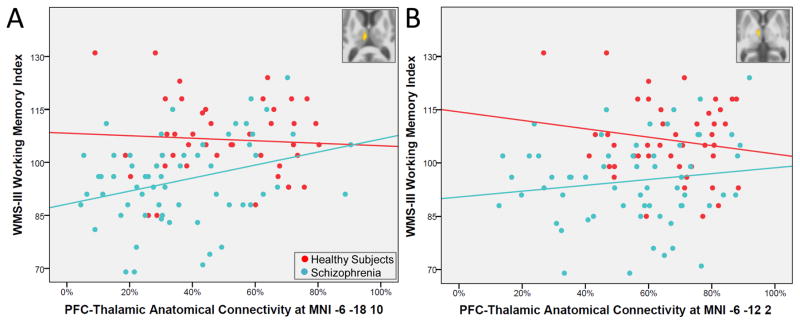
With respect to the voxel-wise analysis, neither thalamic cluster that demonstrated reduced connectivity with the PFC was related to impaired AX-CPT and WCST performance in patients. However, average connectivity within one of the clusters (MNI -6 -18 10) positively correlated with WMS-III Working Memory Index (r=.30, p=.020) indicating that higher PFC-thalamic structural connectivity was associated with better working memory (see Figure 3). The same correlation did not reach significance in healthy subjects (r=−.05, p=.778). A regression analysis with age, sex, group, and group x PFC connectivity (at MNI -6 -18 10) interaction term entered as predictors of WMS-III Working Memory Index revealed that the association between working memory and connectivity at the MNI cluster -6 -18 10 did not differ between groups (group x connectivity interaction term: t(104)=1.41, p=.161). PFC connectivity at the other cluster identified in the between groups analysis (MNI -6 -12 2) was unrelated to WMS-III Working Memory Index in both schizophrenia patients and healthy subjects (partial r’s<|.18|, p>.261).
Figure 3.
Prefrontal cortex (PFC) anatomical connectivity with the thalamus and working memory in schizophrenia. Panel A: Anatomical connectivity in the cluster located at MNI -6 -18 10 which demonstrated reduced structural connectivity in schizophrenia (inset) correlated with Wechsler Memory Scale-III Working Memory Index scores in schizophrenia (r=0.30, p=.020). The same correlation was not significant in healthy subjects (r=−.05, p=.778). Panel B: Anatomical connectivity in a cluster located at MNI -6 -12 2 which demonstrated reduced structural connectivity in schizophrenia (inset) did not correlate with Wechsler Memory Scale-III Working Memory Index scores in schizophrenia (r=.15, p=.264) and healthy subjects (r=−.18, p=.261).
To assess the relative specificity of the association between PFC-thalamic connectivity and cognition in schizophrenia for the cluster located at MNI -6 -18 10, we examined the correlation between SCIP scores and average connectivity within this cluster. The SCIP is composed of several subtests, including a version of the Auditory Consonant Trigrams test of working memory, a word list learning test of verbal learning and delayed recall, phonemic verbal fluency, and a measure of psychomotor processing speed (see 36,43). Of these subtests, only the verbal working memory subtest correlated with PFC-thalamic anatomical connectivity in the schizophrenia group (partial r=.27, p=.036; all remaining partial rs<|.14|, p>.304).
Finally, to explore the potential cognitive correlates of hyper-connectivity, average connectivity was extracted from the clusters that demonstrated hyper-connectivity with motor, somatosensory, posterior parietal, and occipital cortex in the between groups voxel-wise analysis and correlated with executive function measures. This analyses revealed that occipital hyper-connectivity with the cluster located at MNI -8 -28 2 inversely correlated with AX-CPT d-prime (r=−.31, p=.018), and somatosensory hyper-connectivity with thalamic cluster located at MNI 12 -20 6 correlated positively with WMS-III Working Memory Index (r=.27, p=.042).
Thalamocortical Anatomical Connectivity and Clinical Symptoms in Schizophrenia
Although no relationships were hypothesized a-priori, associations between thalamocortical anatomical connectivity and clinical symptoms in patients (i.e. PANSS positive, negative, and general scores) were examined using partial correlations controlling for age and sex. Total connectivity measures were unrelated to PANSS scores (all partial r’s<|.24|, p>.060). With respect to the voxel-wise analysis, both thalamic clusters that demonstrated reduced connectivity with the PFC in schizophrenia correlated inversely with PANSS general symptoms indicating that severity of general symptoms was related to lower PFC-thalamic connectivity (cluster at MNI -6 -12 2: r=− .32, p=.012; cluster at MNI -6 -18 10: r=−.27, p=.039). Additionally, hyper-connectivity of the occipital cortex at thalamus cluster located at MNI -8 -28 8 and motor cortex at the thalamic cluster located at MNI -8 -6 0 positively correlated with general symptoms (r=.29, p=.027) and positive symptoms (r=.26, p=.043), respectively.
DISCUSSION
Motivated by evidence implicating PFC-thalamic circuitry in the pathophysiology of schizophrenia and mechanisms of cognitive impairment, we tested the hypothesis that anatomical connectivity between the PFC and thalamus is: 1) reduced in schizophrenia; and 2) related to impaired executive function. Our first hypothesis was supported; total connectivity of the PFC with the thalamus was significantly reduced in schizophrenia. Voxel-wise analyses confirmed that the reduction in connectivity localized to the medial dorsal aspect of the thalamus where paths linking the thalamus to lateral PFC originate from. Our second hypothesis was partially supported. While total PFC connectivity with the thalamus did not correlate with any measure of executive function, average connectivity within a cluster located in the medial dorsal thalamus that demonstrated reduced connectivity in patients correlated with working memory. Similar associations were not found for other measures of executive function and overall neuropsychological functioning.
The findings add to the modest literature on thalamocortical anatomical connectivity in schizophrenia that so far has produced mixed findings. Our results are consistent with a prior study of 15 schizophrenia patients by Marenco and colleagues (29) which, using almost identical methods, found that PFC-thalamic connectivity is reduced in schizophrenia. Marenco and colleagues also reported a correlation between lateral PFC-thalamic connectivity and working memory in subset of 9 patients that, while not statistically significant, was very similar to the current findings (r=.38 vs. r=.30). However, both studies are at odds with two recent investigations that found reduced connectivity with the orbitofrontal cortex (OFC), but not lateral and medial PFC (27,28). The inconsistent findings may be due to differences in sample sizes, the current investigation included substantially more patients than prior studies; differences across studies in the methods used to quantify anatomical connectivity and define cortical targets; difficulties imaging the OFC and possibly reduced reliability of OFC-thalamic connectivity (e.g. 29); and heterogeneity across patient samples. Interestingly, average connectivity within the two clusters in the thalamus that demonstrated reduced PFC-thalamic connectivity in schizophrenia also correlated with severity of general clinical symptoms. This finding was not hypothesized a-priori and should be interpreted cautiously given the exploratory nature of the analysis. Nonetheless, it suggests that PFC-thalamic pathology may underlie some dimensions of clinical symptoms and may also account for the heterogeneous findings across studies.
The results support thalamocortical network models of schizophrenia and cognitive impairment (22,24,26,44,45). They are also consistent with functional imaging studies, particularly resting-state fMRI investigations, that consistently find reduced functional connectivity between the PFC and thalamus in schizophrenia (reviewed in 21). Whether dysrupted connectivity results from pathology in the PFC and/or thalamus is an unresolved question that is especially difficult to address with neuroimaging due to the reciprocal nature of anatomical connections between the thalamus and cortex. Recent electrophysiology studies in rodents highlight the importance of resolving this question, not only for understanding the nature of cognitive impairment in schizophrenia, but also developing pro-cognitive interventions. Specifically, Bolkan et al. (15) found that MD thalamus-PFC coupling is essential for sustaining working memory delay-related activity in the PFC, whereas PFC-thalamic functional coupling is critical during the choice selection phase of working memory. Similarly, Schmitt et al. (18) found that MD thalamus stimulation enhances functional connectivity within the PFC and improves performance on forced-choice tests of attentional control, in contrast to direct stimulation of the PFC which impairs task performance. Combined, the results indicate that pathology within specific nodes of the executive control network may lead to differential cognitive impairment and, by extension, suggest that targeting different nodes may improve specific aspects of behavior. Interestingly, a recent clinical trial found that cognitive remediation training (CRT) improves PFC-thalamic functional connectivity which, in turn, correlates with the degree of improvement in overall cognitive functioning (46); findings that are consistent with the broader literature on the effect of CRT on PFC and thalamic activity (47). While agnostic with respect to the primary site of dysfunction (i.e. PFC, thalamus, or both), they provide compelling evidence that PFC-thalamic circuitry may be an important target for improving cognitive function in schizophrenia.
While the focus of our study was on PFC-thalamic connectivity, we also examined the integrity of thalamic connections with other cortical areas. In contrast to the PFC, anatomical connectivity with other cortical areas, primarily somatosensory and occipital cortex, and to a lesser extent motor and posterior parietal cortex, was increased in schizophrenia. Again, the findings are reminiscent of resting-state fMRI investigations which consistently find that PFC-thalamic hypo-connectivity in schizophrenia is accompanied by thalamic hyper-connectivity with sensory and motor cortical areas (48–59). However, unlike functional dysconnectivity, which could result from alterations in direct or indirect connections, abnormalities in connectivity inferred from DWI presumably reflects dysruption of direct anatomical connections (60). It’s possible that anatomical hyper-connectivity reflects novel anatomical connections and/or a failure to prune some connections during development. In humans, segregation of neurons into thalamic nuclei begins 10–14 weeks into gestation and continues until 40 weeks (61,62). The development of thalamocortical anatomical connections is similarly prolonged and relies on a complex interplay between genes and intrinsic neuronal signaling (63,64). Consequently, alterations in the expression and timing of certain genes, possibly in combination with extrinsic factors, could theoretically lead to abnormalities in the patterning of connections between the cortex and thalamus. Very little is known about the postnatal development of thalamic nuclei and anatomical connectivity. The limited available evidence suggests that connections between the thalamus and association cortical areas, especially the PFC, mature later than connections linking the thalamus with primary sensory and motor areas (65,66). Interestingly, some of the thalamic regions that demonstrated hyper-connectivity with areas outside the PFC correlated with severity of clinical symptoms and cognitive impairment. While these findings suggest there may be functional consequences of anatomical hyper-connectivity and, therefore, may merit further investigation, they should also be interpreted cautiously given the lack of a-priori hypotheses and the number of correlations performed.
It’s also possible that the abnormalities in anatomical connectivity reflect differences in the relative volume of thalamic nuclei and/or dysruption of postnatal brain development. For instance, the combination of reduced PFC-thalamic and elevated sensory-thalamic anatomical connectivity may result from relative volume loss of the MD nucleus and expansion of ventral posterior nuclei and pulvinar. Reduced thalamic volume is a consistent finding in clinical neuroimaging studies (19); however, most conventional structural imaging methods are not able to delineate thalamic nuclei due to lack of contrast and, in some cases, limited resolution (67). One exception is proton density imaging which can be used to resolve the LGN and MGN. Unfortunately, we did not collect proton density images; an important limitation of the study given our finding of increased occipital-thalamic connectivity in schizophrenia. Similarly, it is also important to note that DWI is an indirect method for inferring white matter integrity and that the reliability of thalamocortical connectivity derived from probabilistic tractography was not confirmed in the current investigation. However, prior studies suggest that test-retest reliability of thalamocortical connectivity is reasonable and probabilistic tractography is useful for tracking disease progression in white matter (29,68)
In conclusion, PFC-anatomical connectivity is reduced in schizophrenia and correlated with the severity of working memory impairment. In addition to illuminating the pathophysiology of schizophrenia, the present results suggest that PFC-thalamic connectivity may be a useful neural target for pro-cognitive interventions.
Supplementary Material
Supplemental Figure S1. Cortical regions-of-interest (ROIs) and thalamus for one subject. Segmentations derived from multi atlas were used to create subject-specific ROIs for the thalamus (purple) and 6 cortical subdivisions: prefrontal cortex (blue), motor cortex/supplementary motor area (red), somatosensory cortex (cyan), temporal cortex (green), posterior parietal cortex (yellow), and occipital cortex (violet). The thalamus and cortical ROIs were used as seed and targets, respectively, to quantify thalamocortical structural connectivity using probabilistic tractography. The lateral surface renderings in the top panel were generated by projecting the cortical ROIs onto the central surface of the cortical mantle.
Supplemental Figure S2. Paths from thalamic clusters identified in the between groups voxel-wise analysis. These paths were generated by performing probabilistic tractography using the clusters that demonstrated lower probability of connectivity to the prefrontal cortex (PFC) in schizophrenia as seeds and the prefrontal cortex (PFC) as the target. This generated a connectivity distribution image (fdt_paths) for each individual showing connectivity between the seed (i.e. thalamic cluster) and PFC. These images were thresholded, binarized, normalized to MNI space, and averaged across subjects within each group. The images above show the overlap across subjects in the paths linking each thalamic cluster to the PFC. Brightly colored voxels (yellow) indicate that there is more overlap across subjects in the paths than darker voxels.
Supplemental Table S1. HARDI data quality metrics.
Supplemental Table S2. Thalamocortical structural connectivity in schizophrenia.
Supplemental Table S3. Thalamocortical structural connectivity in schizophrenia: voxel-wise analysis results.
Supplemental Table S4. Volume of Seed/Target ROIs used in Probabalistic Tractography Analysis*.
Supplemental Table S5. Structures Included in Cortical Target ROIs.
Acknowledgments
This research was supported by funding from the NIMH (5 R01 MH102266 awarded to NDW) and the Vanderbilt Institute for Clinical and Translational Research (1-UL1-RR024975 NCRR/NIH). The authors thank the individuals who participated in the study. The authors would also like to thank Kristan Armstrong, Erin Brosey, Molly Boyce, and Yasmeen Iqbal for their assistance recruiting and screening subjects for participation in the study.
Footnotes
Disclosures: The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: a latent variable analysis. Cogn Psychol. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- 2.Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- 3.Palmer BW, Heaton RK, Paulsen JS, Kuck J, Braff D, Harris MJ, et al. Is it possible to be schizophrenic yet neuropsychologically normal? Neuropsychology. 1997;11:437–446. doi: 10.1037//0894-4105.11.3.437. [DOI] [PubMed] [Google Scholar]
- 4.Wilk CM, Gold JM, McMahon RP, Humber K, Iannone VN, Buchanan RW. No, it is not possible to be schizophrenic yet neuropsychologically normal. Neuropsychology. 2005;19:778–786. doi: 10.1037/0894-4105.19.6.778. [DOI] [PubMed] [Google Scholar]
- 5.Weickert TW, Goldberg TE, Gold JM, Bigelow LB, Egan MF, Weinberger DR. Cognitive impairments in patients with schizophrenia displaying preserved and compromised intellect. Arch Gen Psychiatry. 2000;57:907–913. doi: 10.1001/archpsyc.57.9.907. [DOI] [PubMed] [Google Scholar]
- 6.Dickinson D, Ragland JD, Gold JM, Gur RC. General and specific cognitive deficits in schizophrenia: Goliath defeats David? Biol Psychiatry. 2008;64:823–827. doi: 10.1016/j.biopsych.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lepage M, Bodnar M, Bowie CR. Neurocognition: clinical and functional outcomes in schizophrenia. Can J Psychiatry. 2014;59:5–12. doi: 10.1177/070674371405900103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woodward ND, Purdon SE, Meltzer HY, Zald DH. A meta-analysis of neuropsychological change to clozapine, olanzapine, quetiapine, and risperidone in schizophrenia. Int J Neuropsychopharmacol. 2005;8:457–472. doi: 10.1017/S146114570500516X. [DOI] [PubMed] [Google Scholar]
- 9.Woodward ND, Purdon SE, Meltzer HY, Zald DH. A meta-analysis of cognitive change with haloperidol in clinical trials of atypical antipsychotics: dose effects and comparison to practice effects. Schizophr Res. 2007;89:211–224. doi: 10.1016/j.schres.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 10.Simons DJ, Boot WR, Charness N, Gathercole SE, Chabris CF, Hambrick DZ, et al. Do “Brain-Training” Programs Work? Psychol Sci Public Interest. 2016;17:103–186. doi: 10.1177/1529100616661983. [DOI] [PubMed] [Google Scholar]
- 11.de WL, Brouns R, Kavadias D, Engelborghs S, De Deyn PP, Marien P. Cognitive, affective and behavioural disturbances following vascular thalamic lesions: a review. Cortex. 2011;47:273–319. doi: 10.1016/j.cortex.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Van der Werf YD, Scheltens P, Lindeboom J, Witter MP, Uylings HB, Jolles J. Deficits of memory, executive functioning and attention following infarction in the thalamus; a study of 22 cases with localised lesions. Neuropsychologia. 2003;41:1330–1344. doi: 10.1016/s0028-3932(03)00059-9. [DOI] [PubMed] [Google Scholar]
- 13.Niendam TA, Laird AR, Ray KL, Dean YM, Glahn DC, Carter CS. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn Affect Behav Neurosci. 2012;12:241–268. doi: 10.3758/s13415-011-0083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Block AE, Dhanji H, Thompson-Tardif SF, Floresco SB. Thalamic-prefrontal cortical-ventral striatal circuitry mediates dissociable components of strategy set shifting. Cereb Cortex. 2007;17:1625–1636. doi: 10.1093/cercor/bhl073. [DOI] [PubMed] [Google Scholar]
- 15.Bolkan SS, Stujenske JM, Parnaudeau S, Spellman TJ, Rauffenbart C, Abbas AI, et al. Thalamic projections sustain prefrontal activity during working memory maintenance. Nat Neurosci. 2017 doi: 10.1038/nn.4568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parnaudeau S, O’Neill PK, Bolkan SS, Ward RD, Abbas AI, Roth BL, et al. Inhibition of mediodorsal thalamus disrupts thalamofrontal connectivity and cognition. Neuron. 2013;77:1151–1162. doi: 10.1016/j.neuron.2013.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parnaudeau S, Taylor K, Bolkan SS, Ward RD, Balsam PD, Kellendonk C. Mediodorsal thalamus hypofunction impairs flexible goal-directed behavior. Biol Psychiatry. 2015;77:445–453. doi: 10.1016/j.biopsych.2014.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmitt LI, Wimmer RD, Nakajima M, Happ M, Mofakham S, Halassa MM. Thalamic amplification of cortical connectivity sustains attentional control. Nature. 2017;545:219–223. doi: 10.1038/nature22073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glahn DC, Laird AR, Ellison-Wright I, Thelen SM, Robinson JL, Lancaster JL, et al. Meta-analysis of gray matter anomalies in schizophrenia: application of anatomic likelihood estimation and network analysis. Biol Psychiatry. 2008;64:774–781. doi: 10.1016/j.biopsych.2008.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry. 2009;66:811–822. doi: 10.1001/archgenpsychiatry.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giraldo-Chica M, Woodward ND. Review of thalamocortical resting-state fMRI studies in schizophrenia. Schizophr Res. 2017;180:58–63. doi: 10.1016/j.schres.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andreasen NC. The role of the thalamus in schizophrenia. Can J Psychiatry. 1997;42:27–33. doi: 10.1177/070674379704200104. [DOI] [PubMed] [Google Scholar]
- 23.Andreasen NC, Paradiso S, O’Leary DS. “Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr Bull. 1998;24:203–218. doi: 10.1093/oxfordjournals.schbul.a033321. [DOI] [PubMed] [Google Scholar]
- 24.Jones EG. Cortical development and thalamic pathology in schizophrenia. Schizophr Bull. 1997;23:483–501. doi: 10.1093/schbul/23.3.483. [DOI] [PubMed] [Google Scholar]
- 25.Swerdlow NR. Integrative circuit models and their implications for the pathophysiologies and treatments of the schizophrenias. Curr Top Behav Neurosci. 2010;4:555–583. doi: 10.1007/7854_2010_48. [DOI] [PubMed] [Google Scholar]
- 26.Cronenwett WJ, Csernansky J. Thalamic pathology in schizophrenia. Curr Top Behav Neurosci. 2010;4:509–528. doi: 10.1007/7854_2010_55. [DOI] [PubMed] [Google Scholar]
- 27.Cho KI, Shenton ME, Kubicki M, Jung WH, Lee TY, Yun JY, et al. Altered Thalamo-Cortical White Matter Connectivity: Probabilistic Tractography Study in Clinical-High Risk for Psychosis and First-Episode Psychosis. Schizophr Bull. 2016;42:723–731. doi: 10.1093/schbul/sbv169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kubota M, Miyata J, Sasamoto A, Sugihara G, Yoshida H, Kawada R, et al. Thalamocortical disconnection in the orbitofrontal region associated with cortical thinning in schizophrenia. JAMA Psychiatry. 2013;70:12–21. doi: 10.1001/archgenpsychiatry.2012.1023. [DOI] [PubMed] [Google Scholar]
- 29.Marenco S, Stein JL, Savostyanova AA, Sambataro F, Tan HY, Goldman AL, et al. Investigation of Anatomical Thalamo-Cortical Connectivity and fMRI Activation in Schizophrenia. Neuropsychopharmacology. 2011 doi: 10.1038/npp.2011.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders. Clinical Version (SCID-CV) Washington, D.C: American Psychiatric Press Inc; 1996. [Google Scholar]
- 31.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 32.Wechsler D. Wechsler Test of Adult Reading. San Antonio, TX: Pearson; 2001. [Google Scholar]
- 33.Wechsler D. Wechsler Memory Scale. 3. The Psychological Corporation; 1997. [Google Scholar]
- 34.Kongs sk, Thompson LL, Iverson GL, Heaton RK. Wisconsin Card Sorting Test-64 Card Version. Odessa, FL: Psychological Assessment Resources; 2000. [Google Scholar]
- 35.Carter CS, Minzenberg M, West R, Macdonald A., III CNTRICS imaging biomarker selections: Executive control paradigms. Schizophr Bull. 2012;38:34–42. doi: 10.1093/schbul/sbr114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Purdon SE. The Screen for Cognitive Impairment in Psychiatry (SCIP): Administration Manual and Normative Data. Edmonton, Alberta: PNL Inc; 2005. [Google Scholar]
- 37.Asman AJ, Landman BA. Hierarchical performance estimation in the statistical label fusion framework. Med Image Anal. 2014;18:1070–1081. doi: 10.1016/j.media.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 39.Lauzon CB, Asman AJ, Esparza ML, Burns SS, Fan Q, Gao Y, et al. Simultaneous analysis and quality assurance for diffusion tensor imaging. PLoS One. 2013;8:e61737. doi: 10.1371/journal.pone.0061737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW. Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? Neuroimage. 2007;34:144–155. doi: 10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Behrens TE, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CA, Boulby PA, et al. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci. 2003;6:750–757. doi: 10.1038/nn1075. [DOI] [PubMed] [Google Scholar]
- 42.Johansen-Berg H, Behrens TE, Sillery E, Ciccarelli O, Thompson AJ, Smith SM, et al. Functional-anatomical validation and individual variation of diffusion tractography-based segmentation of the human thalamus. Cereb Cortex. 2005;15:31–39. doi: 10.1093/cercor/bhh105. [DOI] [PubMed] [Google Scholar]
- 43.Woodward ND, Rogers B, Heckers S. Functional resting-state networks are differentially affected in schizophrenia. Schizophr Res. 2011;130:86–93. doi: 10.1016/j.schres.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Andreasen NC, O’Leary DS, Cizadlo T, Arndt S, Rezai K, Ponto LL, et al. Schizophrenia and cognitive dysmetria: a positron-emission tomography study of dysfunctional prefrontal-thalamic-cerebellar circuitry. Proc Natl Acad Sci U S A. 1996;93:9985–9990. doi: 10.1073/pnas.93.18.9985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lisman JE, Pi HJ, Zhang Y, Otmakhova NA. A thalamo-hippocampal-ventral tegmental area loop may produce the positive feedback that underlies the psychotic break in schizophrenia. Biol Psychiatry. 2010;68:17–24. doi: 10.1016/j.biopsych.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramsay IS, Nienow TM, MacDonald AW., III Increases in Intrinsic Thalamocortical Connectivity and Overall Cognition Following Cognitive Remediation in Chronic Schizophrenia. Biol Psychiatry Cogn Neurosci Neuroimaging. 2017;2:355–362. doi: 10.1016/j.bpsc.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ramsay IS, MacDonald AW., III Brain Correlates of Cognitive Remediation in Schizophrenia: Activation Likelihood Analysis Shows Preliminary Evidence of Neural Target Engagement. Schizophr Bull. 2015;41:1276–1284. doi: 10.1093/schbul/sbv025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anticevic A, Haut K, Murray JD, Repovs G, Yang GJ, Diehl C, et al. Association of Thalamic Dysconnectivity and Conversion to Psychosis in Youth and Young Adults at Elevated Clinical Risk. JAMA Psychiatry. 2015;72:882–891. doi: 10.1001/jamapsychiatry.2015.0566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anticevic A, Cole MW, Repovs G, Murray JD, Brumbaugh MS, Winkler AM, et al. Characterizing thalamo-cortical disturbances in schizophrenia and bipolar illness. Cereb Cortex. 2014;24:3116–3130. doi: 10.1093/cercor/bht165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anticevic A, Yang G, Savic A, Murray JD, Cole MW, Repovs G, et al. Mediodorsal and visual thalamic connectivity differ in schizophrenia and bipolar disorder with and without psychosis history. Schizophr Bull. 2014;40:1227–1243. doi: 10.1093/schbul/sbu100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Woodward ND, Heckers S. Mapping Thalamocortical Functional Connectivity in Chronic and Early Stages of Psychotic Disorders. Biol Psychiatry. 2016;79:1016–1025. doi: 10.1016/j.biopsych.2015.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Woodward ND, Karbasforoushan H, Heckers S. Thalamocortical dysconnectivity in schizophrenia. Am J Psychiatry. 2012;169:1092–1099. doi: 10.1176/appi.ajp.2012.12010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cheng W, Palaniyappan L, Li M, Kendrick KM, Zhang J, Luo Q, et al. Voxel-based, brain-wide association study of aberrant functional connectivity in schizophrenia implicates thalamocortical circuitry. NPJ Schizophr. 2015;1:15016. doi: 10.1038/npjschz.2015.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Atluri G, Steinbach M, Lim KO, Kumar V, Macdonald A., III Connectivity cluster analysis for discovering discriminative subnetworks in schizophrenia. Hum Brain Mapp. 2015;36:756–767. doi: 10.1002/hbm.22662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lerman-Sinkoff DB, Barch DM. Network community structure alterations in adult schizophrenia: identification and localization of alterations. Neuroimage Clin. 2016;10:96–106. doi: 10.1016/j.nicl.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tu PC, Lee YC, Chen YS, Hsu JW, Li CT, Su TP. Network-specific cortico-thalamic dysconnection in schizophrenia revealed by intrinsic functional connectivity analyses. Schizophr Res. 2015;166:137–143. doi: 10.1016/j.schres.2015.05.023. [DOI] [PubMed] [Google Scholar]
- 57.Klingner CM, Langbein K, Dietzek M, Smesny S, Witte OW, Sauer H, et al. Thalamocortical connectivity during resting state in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2014;264:111–119. doi: 10.1007/s00406-013-0417-0. [DOI] [PubMed] [Google Scholar]
- 58.Wang HL, Rau CL, Li YM, Chen YP, Yu R. Disrupted thalamic resting-state functional networks in schizophrenia. Front Behav Neurosci. 2015;9:45. doi: 10.3389/fnbeh.2015.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Skatun KC, Kaufmann T, Brandt CL, Doan NT, Alnaes D, Tonnesen S, et al. Thalamo-cortical functional connectivity in schizophrenia and bipolar disorder. Brain Imaging Behav. 2017 doi: 10.1007/s11682-017-9714-y. [DOI] [PubMed] [Google Scholar]
- 60.Honey CJ, Sporns O, Cammoun L, Gigandet X, Thiran JP, Meuli R, et al. Predicting human resting-state functional connectivity from structural connectivity. Proc Natl Acad Sci U S A. 2009;106:2035–2040. doi: 10.1073/pnas.0811168106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.DEKABAN A. Human thalamus; an anatomical, developmental and pathological study. II. Development of the human thalamic nuclei. J Comp Neurol. 1954;100:63–97. doi: 10.1002/cne.901000105. [DOI] [PubMed] [Google Scholar]
- 62.Mojsilovic J, Zecevic N. Early development of the human thalamus: Golgi and Nissl study. Early Hum Dev. 1991;27:119–144. doi: 10.1016/0378-3782(91)90033-y. [DOI] [PubMed] [Google Scholar]
- 63.Krsnik Z, Majic V, Vasung L, Huang H, Kostovic I. Growth of Thalamocortical Fibers to the Somatosensory Cortex in the Human Fetal Brain. Front Neurosci. 2017;11:233. doi: 10.3389/fnins.2017.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lopez-Bendito G, Molnar Z. Thalamocortical development: how are we going to get there? Nat Rev Neurosci. 2003;4:276–289. doi: 10.1038/nrn1075. [DOI] [PubMed] [Google Scholar]
- 65.Fama R, Sullivan EV. Thalamic structures and associated cognitive functions: Relations with age and aging. Neurosci Biobehav Rev. 2015;54:29–37. doi: 10.1016/j.neubiorev.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fair DA, Bathula D, Mills KL, Dias TG, Blythe MS, Zhang D, et al. Maturing thalamocortical functional connectivity across development. Front Syst Neurosci. 2010;4:10. doi: 10.3389/fnsys.2010.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tourdias T, Saranathan M, Levesque IR, Su J, Rutt BK. Visualization of intra-thalamic nuclei with optimized white-matter-nulled MPRAGE at 7T. Neuroimage. 2014;84:534–545. doi: 10.1016/j.neuroimage.2013.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ciccarelli O, Behrens TE, Altmann DR, Orrell RW, Howard RS, Johansen-Berg H, et al. Probabilistic diffusion tractography: a potential tool to assess the rate of disease progression in amyotrophic lateral sclerosis. Brain. 2006;129:1859–1871. doi: 10.1093/brain/awl100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure S1. Cortical regions-of-interest (ROIs) and thalamus for one subject. Segmentations derived from multi atlas were used to create subject-specific ROIs for the thalamus (purple) and 6 cortical subdivisions: prefrontal cortex (blue), motor cortex/supplementary motor area (red), somatosensory cortex (cyan), temporal cortex (green), posterior parietal cortex (yellow), and occipital cortex (violet). The thalamus and cortical ROIs were used as seed and targets, respectively, to quantify thalamocortical structural connectivity using probabilistic tractography. The lateral surface renderings in the top panel were generated by projecting the cortical ROIs onto the central surface of the cortical mantle.
Supplemental Figure S2. Paths from thalamic clusters identified in the between groups voxel-wise analysis. These paths were generated by performing probabilistic tractography using the clusters that demonstrated lower probability of connectivity to the prefrontal cortex (PFC) in schizophrenia as seeds and the prefrontal cortex (PFC) as the target. This generated a connectivity distribution image (fdt_paths) for each individual showing connectivity between the seed (i.e. thalamic cluster) and PFC. These images were thresholded, binarized, normalized to MNI space, and averaged across subjects within each group. The images above show the overlap across subjects in the paths linking each thalamic cluster to the PFC. Brightly colored voxels (yellow) indicate that there is more overlap across subjects in the paths than darker voxels.
Supplemental Table S1. HARDI data quality metrics.
Supplemental Table S2. Thalamocortical structural connectivity in schizophrenia.
Supplemental Table S3. Thalamocortical structural connectivity in schizophrenia: voxel-wise analysis results.
Supplemental Table S4. Volume of Seed/Target ROIs used in Probabalistic Tractography Analysis*.
Supplemental Table S5. Structures Included in Cortical Target ROIs.