Summary
Evidence on the relationship between the vitamin D pathway and outcomes in melanoma is growing, although it is not always clear. We investigated the impact of measured levels of sun exposure at diagnosis on associations of vitamin D receptor gene (VDR) polymorphisms and melanoma-death in 3336 incident primary melanoma cases.
Interactions between six SNPs and a common 3′ end haplotype were significant (p<0.05). These SNPs, and a haplotype, had a statistically significant association with survival among subjects exposed to high UVB in multivariable regression models, and exerted their effect in the opposite direction among those with low UVB. SNPs rs1544410/BsmI and rs731236/TaqI remained significant after adjustment for multiple testing.
These results suggest that the association between VDR and melanoma-specific survival is modified by sun exposure around diagnosis, and require validation in an independent study. Whether the observed effects are dependent or independent of vitamin D activation, remains to be determined.
Significance
High UVB increases risk of skin cancer and sun avoidance practices are necessary. However, vitamin D insufficiency is prevalent among melanoma patients, and new data suggest that VDR SNPs and haplotypes interact with measured sun exposure/UVB (surrogate of skin vitamin D) around diagnosis to modify survival. This pathway and area of research merit further consideration.
Keywords: VDR, UVB, SNP, melanoma, survival, interaction, polymorphism, haplotype, exposure
Introduction
Sun exposure is a recognized carcinogen and intermittent exposure to UV is a well known risk factor for developing melanoma. However, sun exposure has not been associated with death in these individuals. Studies that addressed the effect of a ‘presumed’ sun exposure have provided mixed results (Boniol et al., 2006; Garland et al., 2003; Jemal et al., 2000). Few and more robust investigations included individual-level measured data and utilized information such as anatomic site, sunburns, intermittent sun exposure, solar elastosis, sunny vacations (Berwick et al., 2005; Berwick et al., 2014; Heenan et al., 1991; Rosso et al., 2008). Using a comprehensive set of variables measured across the lifespan, we found a protective effect of sunburns (HR 0.27, 95% CI 0.09-0.85) within the decade of diagnosis (Berwick et al., 2014). Overall, the evidence suggests that UV exposure may exhibit a restraining effect on melanoma. Sun exposure might improve survival through production of epidermal vitamin D, a hormone with an anti-proliferative effect on melanoma cells in vitro and in xenografts (Colston et al 1981, Yudoh et al 1999, Danielsson et al 1998, Eisman et al 1987, Evans et al 1996, Ishibashi et al 2012). Meta-analyses have demonstrated a benefit of vitamin D supplementation on survival in cancer patients (Keum and Giovanucci 2014, Schotker et al. 2014; Hu et al 2017). In relation to melanoma, a few epidemiologic studies have investigated the relationship of serum vitamin D and disease specific outcomes (Bade et al., 2014; Fang et al., 2016; Gambichler et al., 2013; Newton-Bishop et al., 2009; Nurnberg et al., 2009; O'Shea et al., 2016; Randerson-Moor et al., 2009) higher vitamin D levels have been associated with thinner tumors (Bade et al., 2014; Gambichler et al., 2013; Randerson-Moor et al., 2009), although this effects may also be due to greater surveillance. Higher vitamin D levels have been associated with longer overall and melanoma specific survival (Bade et al., 2014; Fang et al., 2016; Newton-Bishop et al., 2009; O'Shea et al., 2016).
Vitamin D exerts its effects through the vitamin D receptor (VDR) and VDR expression has been associated with less aggressive disease or to better outcome in several cancers (Hendrickson et al., 2011; Jóźwicki et al., 2015; Ahearn et al., 2016; Ferrer-Mayorga et al., 2017). In melanoma, VDR expression has been linked to less advanced stages and more favorable tumor characteristics (Brozyna et al., 2014). The VDR expression and function can be affected by genetic and epigenetic events (Saccone et al., 2015; Uitterlinden et al., 2004). We and others have previously reported significant associations between VDR polymorphisms and melanoma survival (Orlow et al., 2016). Here we investigate whether these associations are modified by UVB exposure.
Results
Of the 3336 incident primary cases with sun exposure, genetics, and outcomes data available for analysis, 238 (7.1%) died of the disease, and 286 (8.6%) died of other causes. The average follow up time was 7.6 years (range 0.4-10.6). Main effect associations of SNPs and survival in GEM have been recently described (Orlow et al., 2016). Table 1 describes demographic, clinical and histologic characteristics of the participants.
Table 1. Selected characteristics of GEM study participants (N= 3336).
| Variable | N (%) | N-melanoma deaths | N-non melanoma deaths1 |
|---|---|---|---|
| Sun Exposure | |||
| Low UVB (Q1) | 833 (25.0) | 59 | 55 |
| High UVB (Q2-Q4) | 2503 (75.0) | 179 | 230 |
| Gender | |||
| Male | 1868 (56.0) | 171 | 216 |
| Female | 1468 (44.0) | 67 | 69 |
| Site | |||
| Head/neck | 521 (15.6) | 71 | 66 |
| Trunk/pelvis | 1483 (44.5) | 100 | 138 |
| Arms | 621 (18.6) | 32 | 48 |
| Legs | 711 (21.3) | 35 | 33 |
| Breslow thickness | |||
| 0.01-1.00 | 2084 (62.5) | 41 | 149 |
| 1.01-2.00 | 666 (20.0) | 73 | 62 |
| 2.01-4.00 | 333 (10.0) | 71 | 41 |
| >4.00 | 169 (5.1) | 49 | 24 |
| missing | 84 (2.5) | 4 | 9 |
| Histology | |||
| SSM | 2148 (64.4) | 101 | 144 |
| NM | 314 (9.4) | 64 | 43 |
| LM | 336 (10.1) | 16 | 55 |
| NOS | 463 (13.9) | 38 | 35 |
| other | 75 (2.2) | 19 | 8 |
| Ulceration | |||
| No | 2343 (70.2) | 133 | 199 |
| Yes | 246 (7.4) | 66 | 39 |
| missing | 747 (22.4) | 39 | 47 |
| Mitosis | |||
| Absent | 1443 (43.3) | 39 | 121 |
| Present | 1156 (34.7) | 161 | 117 |
| missing | 737 (22.1) | 38 | 47 |
| Cases | |||
| Single primary | 2198 (65.9) | 140 | 157 |
| Multiple primary | 1138 (34.1) | 98 | 128 |
Number of deaths due to other non-melanoma causes
We found significant interactions (p-value <0.05) between 6 VDR SNPs located in the promoter (rs1989969) and the coding region (rs12370156, rs2238140, rs7305032, rs1544410, rs731236) and UVB exposure (Table 2, Table S1). Competing risk models revealed that for the minor alleles (rs1544410, rs731236) and the major alleles (rs1989969, rs12370156, rs2238140, rs7305032), the risk of melanoma-specific death was significantly reduced among those with high UVB exposure in the decade of diagnosis, after adjustment for covariates (Table 2, Model A; Table S1). In this high exposure group, the per-allele effect on risk of death ranged from 25 to 33%. Two SNPs (rs1544410/BsmI, rs731236/TaqI) remained significant after adjustment for multiple testing (p-value <0.001). Among those in the low UVB group, we observed the opposite effect, although associations did not reach statistical significance. We also analyzed the effect of sun exposure on the associations of VDR SNPs and survival by dividing data into quartiles of sun exposure levels. Although not significant, the subdistribution hazard ratios confirmed a distinct effect among those in the highest quartile (Q1) (Table S2).
Table 2. Effect of VDR SNPs on melanoma death according to levels of sun exposure around time of diagnosis after accounting for competing risk of death from other causes.
| Low UVB (Q1) | High UVB (Q2-Q3-Q4) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Position1 | RefSeq | Allele2 | N total/N deaths3 | Per allele sHR4 (95% CI) | p-value | N total/N deaths3 | Per allele sHR4 (95% CI) | N total/N deaths3 | p-value5 |
| Model A | |||||||||
| Promoter region | |||||||||
| 19 | rs1989969 | T | 831/59 | 0.75 (0.50-1.12) | 0.16 | 2482/176 | 1.25 (1.02-1.54) | 0.036 | 0.024 |
| Coding region | |||||||||
| 29 | rs12370156 | T | 827/59 | 1.28 (0.89-1.84) | 0.19 | 2472/175 | 0.73 (0.60-0.91) | 0.004 | 0.011 |
| 30 | rs2238140 | T | 831/59 | 1.30 (0.90-1.88) | 0.16 | 2474/175 | 0.73 (0.59-0.90) | 0.003 | 0.009 |
| 31 | rs7305032 | A | 773/53 | 1.19 (0.84-1.70) | 0.33 | 2350/171 | 0.75 (0.60-0.93) | 0.008 | 0.042 |
| 32 | rs1544410 (BsmI) | A (B) | 819/59 | 1.17 (0.79-1.71) | 0.43 | 2446/172 | 0.67 (0.53-0.84) | <0.001 | 0.016 |
| 33 | rs731236 (TaqI) | C (t) | 820/58 | 1.13 (0.77-1.65) | 0.54 | 2447/171 | 0.68 (0.54-0.85) | <0.001 | 0.032 |
|
| |||||||||
| Model B | |||||||||
| Promoter region | |||||||||
| 19 | rs1989969 | T | 831/59 | 0.83 (0.54-1.28) | 0.41 | 2482/176 | 1.19 (0.95-1.48) | 0.13 | 0.22 |
| Coding region | |||||||||
| 29 | rs12370156 | T | 827/59 | 1.32 (0.89-1.96) | 0.16 | 2472/175 | 0.80 (0.64-1.00) | 0.045 | 0.06 |
| 30 | rs2238140 | T | 831/59 | 1.34 (0.91-1.99) | 0.14 | 2474/175 | 0.79 (0.63-0.99) | 0.038 | 0.049 |
| 31 | rs7305032 | A | 773/53 | 1.16 (0.79-1.71) | 0.45 | 2350/171 | 0.79 (0.63-1.00) | 0.05 | 0.19 |
| 32 | rs1544410 (BsmI) | A (B) | 819/59 | 1.21 (0.79-1.86) | 0.37 | 2446/172 | 0.69 (0.54-0.88) | 0.0031 | 0.026 |
| 33 | rs731236 (TaqI) | C (t) | 820/58 | 1.26 (0.84-1.88) | 0.26 | 2447/171 | 0.70 (0.55-0.89) | 0.0041 | 0.015 |
Relative 5′ to 3′ position within the VDR gene Refseq, reference sequence.
Effect reported for minor alleles for SNPs rs1989969, rs1544410, and rs731236, and for major alleles for SNPs rs12370156, rs2238140, and rs7305032.
N total and N deaths refer to the total number of subjects and to the number of melanoma-specific deaths within each category of sun exposure, respectively.
Subdistribution hazard ratio (sHR) for melanoma-specific death and 95% Confidence Intervals (95%CI).
Interaction p-value.
Model A: adjusted for age at diagnosis of the first primary melanoma, sex, study center, anatomic site of the deepest primary melanoma, presence of multiple primary melanomas, and time dependent crossover status (patients who entered the study with single primary melanoma and developed a subsequent melanoma during follow up). Model B: adjusted for age at diagnosis of the first primary melanoma, sex, study center, anatomic site and Breslow thickness of the deepest primary melanoma, presence of multiple primary melanomas, and time dependent crossover status (patients who entered the study with single primary melanoma and developed a subsequent melanoma during follow up). Allele frequencies and N total/N deaths for each individual genotype can be found in the Supporting Table S1. Allele frequencies and N total/N deaths for each individual genotype can be found in the Supporting Table S1.
Five significant SNPs, located in the 3′ end of the VDR, are in high LD (Figure S1). The haplotype T-T-A-A(B)-C(t) was inversely associated with melanoma death after accounting for competing risk of other causes of death among individuals exposed to high UVB (subdistribution hazard ratio, sHR 0.64, 95% CI 0.50-0.81) (Table 3, Model A). We observed the opposite effect among individuals exposed to low UVB levels (sHR 1.26), although the effect was not statistically significant. The global interaction was statistically significant (p=0.04). Figure 1 compares the main effect and the effect of genotypes and haplotype according to UVB levels in relation to melanoma death.
Table 3. Effect of VDR haplotype on melanoma death according to levels of sun exposure at diagnosis after accounting for competing risk of death from other causes.
| Haplotypes 1 | Frequency | Low UVB | High UVB | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| sHR2 | 95% CI | p-value | sHR2 | 95% CI | p-value | ||
| Model A | |||||||
| CCGGT | 0.42 | 1 | 1 | ||||
| TTAAC | 0.38 | 1.26 | 0.83-1.90 | 0.281 | 0.64 | 0.50-0.81 | 0.0003 |
| TTAGT | 0.11 | 1.38 | 0.81-2.34 | 0.234 | 1.01 | 0.73-1.42 | 0.939 |
| CCAGT | 0.05 | 0.80 | 0.28-2.28 | 0.681 | 0.62 | 0.35-1.11 | 0.109 |
| Other/rare | 0.04 | 0.18 | 0.03-1.24 | 0.083 | 0.95 | 0.61-1.47 | 0.808 |
| interaction global p-value 0.041 | |||||||
|
| |||||||
| Model B | |||||||
| CCGGT | 0.42 | 1 | 1 | ||||
| TTAAC | 0.38 | 1.32 | 0.85-2.05 | 0.212 | 0.67 | 0.52-0.88 | 0.0034 |
| TTAGT | 0.11 | 1.34 | 0.75-2.42 | 0.323 | 1.22 | 0.85-1.76 | 0.276 |
| CCAGT | 0.05 | 0.64 | 0.19-2.10 | 0.459 | 0.61 | 0.32-1.14 | 0.123 |
| Other/rare | 0.04 | 0.12 | 0.01-1.02 | 0.052 | 0.98 | 0.62-1.55 | 0.936 |
| interaction global p-value 0.041 | |||||||
Haplotypes correspond to SNPs rs12370156, rs2238140, rs7305032, rs1544410 (BsmI), and rs731236 (TaqI) haplotypes with frequencies below 0.01 were combined into one category.
Per haplotype subdistribution hazard ratio (sHR) and 95% Confidence Intervals (95%CI); the reference group corresponds to the most common haplotype.
Model A: adjusted for age at diagnosis of the first primary melanoma, sex, study center, anatomic site of the deepest primary melanoma, presence of multiple primary melanomas, and time dependent crossover status. Model B: adjusted for age at diagnosis of the first primary melanoma, sex, study center, anatomic site of the deepest primary melanoma, presence of multiple primary melanomas, and time dependent crossover status.
Figure 1.
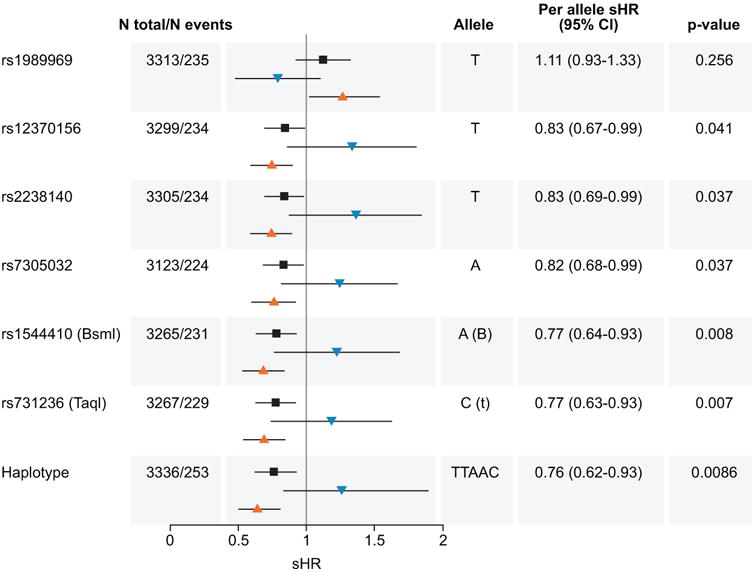
Effect of sun exposure levels and VDR genotypes and 3′ haplotype on melanoma death. Subdistribution Hazard Ratios (sHR) and 95% Confidence Intervals (95% CI) for six significant VDR SNPs on melanoma death according to levels of sun exposure (high levels, orange triangles pointing up; low levels, blue triangles pointing down) after accounting for competing risk of death from other causes. By comparison, note the main effect when sun exposure is not considered (black squares). N total represents the total number of participants included in this study and N events, the total number of melanoma specific deaths. The per allele HRs, 95% CI, and p-values correspond to the main effect regardless of levels of sun exposure (table). The per haplotype sHR, 95% CI, and p-values correspond to the 3′ UTR haplotype block formed by rs12370156, rs2238140, rs7305032, rs1544410 (BsmI), and rs731236 (TaqI).
In secondary analysis, after including Breslow in the competing risk model we found that the per-allele effects on melanoma-death were similar to those observed in the main analysis for the significant SNPs (Table 2, Model B). The interaction with high UVB remained significant for 3 SNPs (rs2238140, rs1544410, rs731236) and borderline significant for one SNP (rs12370156). The haplotype T-T-A-A(B)-C(t) remained significantly associated with melanoma death among individuals exposed to high UVB (per haplotype subdistribution hazard ratio, sHR 0.67, 95% CI 0.52-0.88; global interaction, p=0.041) (Table 3, Model B).
Discussion
In this prospective, population-based cohort of 3336 incident primary melanoma cases we found that six VDR SNPs (rs1989969, rs12370156, rs2238140, rs7305032, rs1544410, rs731236), and a haplotype located in the 3′-end of the VDR gene interacted with sun exposure levels in the decade of diagnosis to exert an effect on melanoma-specific death, and the associations of two SNPs within the high UVB group (rs1544410/BsmI, rs731236/TaqI) remained significant after correction for multiple testing. Among individuals exposed to high levels of UVB, we also observed a moderate protective effect for a common haplotype derived from five SNPs (including rs1544410 and rs731236). The opposite effect was noticed among those exposed to low UVB levels, albeit the association was not statistically significant, possibly due to reduced power to detect the association in this smaller group. These results provide additional evidence implicating the vitamin D pathway in melanoma survival and suggest that UVB can modify the effect of some common VDR polymorphisms. Our results are plausible considering that sun exposure is the main source of vitamin D, and that this hormone exerts several tumor suppressor effects by binding to its receptor (Feldman et al., 2014).
Five significant SNPs located in the 3′-end of the VDR are non-coding, and while no data is yet available regarding their function in melanocytes, supporting in vitro data varies according to other cell types (Uitterlinden et al., 2004). The significant rs1989969 is located in an evolutionary conserved region upstream of the translation start, and T-alleles are responsible for the differential binding of the transcription factor (TF) GATA-1 to the VDR gene.33 GATA-1 is generally regarded as a negative TF, and although specific information for melanomas is lacking, in esophageal adenocarcinomas the rs1989969-T allele (responsible for greater risk of melanoma-death in our study among those with high UVB) was linked to reduction in VDR expression (Janmaat et al., 2015).
Only one other study has addressed the potential VDR gene-environment interaction in relation to melanoma outcomes. A prospective investigation of 872 melanoma cases found increased risk for relapse in carriers of the BsmI-A allele in patients with circulating vitamin D <20.2 ng/mL (Newton-Bishop et al., 2009). Similarly, we noted a non-significant increased risk associated with BsmI-A (sHR 1.17, 95% CI 0.79-1.71) among those with low sun exposure, but among those with high sun exposure risk for melanoma death associated with this allele was reduced. An interaction between VDR SNPs and post-operative vitamin D levels has been observed in relation to colorectal cancer survival (Zgaga et al., 2014), suggesting that greater amounts of ligand are required to overcome ‘defective’ VDR alleles.
The evidence on the relationship between the vitamin D pathway and outcomes in melanoma is growing (Fang et al., 2016; Newton-Bishop et al., 2009; Newton-Bishop et al., 2015; Orlow et al., 2016; O'Shea et al., 2016; Sondak et al., 2016; Yin et al., 2016), although it is not always clear (Saiag et al., 2015). Fang et al. (2016) recently demonstrated that vitamin D levels were significantly associated with overall, disease-free, and melanoma-specific survival. Results from a recent meta-analysis (Yin et al., 2016) found a significant effect on melanoma survival for polymorphisms in the vitamin D ‘carrier’ (VDBP) and retinoid X receptor (RXRA) which forms heterodimers with VDR that subsequently bind to vitamin D response elements (Feldman et al., 2014; Holick, 2007). These results build on prior data which showed that several VDR polymorphisms were associated with disease-specific survival (Orlow et al., 2016). Compared to their main effects on melanoma death, here we noticed a somewhat greater effect in presence of high sun exposure. For example, each additional rs1989969-effect allele showed a non-statistically significant overall 11% elevated risk of melanoma death (sHR 1.11, 95% CI, 0.93-1.33) which increased to 25% among those with high UVB (Table 2). Similarly, BsmI showed an overall 23% reduced risk of death (sHR 0.77, 95% CI, 0.64-0.93) while here we noticed a 32% per-allele reduction among those with high UVB. Three additional polymorphisms that were previously reported in association to melanoma survival (rs7299460, rs3782905 and rs2239182) were found to be associated with melanoma survival among those with high UVE. However, the effect of these variants on progression is not appreciably different according to UVB levels, resulting in interaction p-values that do not reach statistical significance. The reported effect on survival for the functional SNP rs2239182 (Orlow et al 2016) may be driven mainly by the group of cases exposed to high levels of UVB. Similar to our prior report and to findings reported by Newton-Bishop et al. (2009), we found no effect for rs2228570 (FokI).
To our knowledge, this is the first study to date to investigate interactions between VDR polymorphisms and sun exposure and their effects on melanoma-survival. The strengths of this study include its size, population-based accrual, comprehensive measures of UVB exposures, plus the inclusion of a substantial number of VDR polymorphisms. In addition, our results can be likely generalized due to the geographically diverse population. The lack of published equivalent cohorts with detailed UV exposure data and extended follow-up hampered replication of our findings. Additional limitations to our work include potential misclassification of the UVB measure, which uses self-reported hours in combination with actual measured sun exposure but its misclassification is likely to bias the results towards the null. Other unmeasured variables include serum vitamin D and body mass index, a host factor that can limit the bioavailability of vitamin D (Holick, 2007). We have observed no evidence for an association between VDR SNPs and tumor characteristics (Orlow et al., 2016), and have not included these variables in our main model. It is possible that other vitamin D-independent mechanisms such as healthier lifestyles are also at play (Juzeniene and Moan, 2012), although preliminary data on physical activity in this cohort suggest a null effect on outcomes (Schwitzer et al., 2017). Finally, one can also consider that melanomas arising in people exposed to higher UVB are biologically different.
Even though there is some evidence of a beneficial effect of sun exposure in other conditions (Juzeniene and Moan, 2012), our results should be considered cautiously, first and foremost because high UVB is a well-known skin cancer risk factor (Gandini et al., 2005). Second, although there is some evidence of a protective effect of vitamin D in other cancers (Feldman et al., 2014; Zgaga et al., 2014), evidence from randomized controlled clinical trials of vitamin D use through supplements or diet and melanoma-specific survival is not yet available and would be necessary before considering use of vitamin D to improve melanoma outcome. Nevertheless, considering our results in the context of sun avoidance practices among individuals diagnosed with melanoma and the prevalence of vitamin D insufficiency in the general population and among melanoma patients (Fang et al., 2016), this area of research merits further attention.
In conclusion, we have found that a significant interaction between VDR SNPs and personal UVB exposure around the time of diagnosis modifies melanoma-specific survival after controlling for other potential causes of death. Our results warrant further replication in comparable longitudinal studies. Whether the observed effect on survival is due to the direct activation of vitamin D, to other downstream effectors, or to factors that are vitamin D independent, remains to be determined.
Methods
Study subjects and data collection
Subjects were recruited (1998 to 2003) through an international multi-center study of melanoma, the Genes, Environment and Melanoma Study (GEM). The GEM population, identified in eight population-based registries in Australia, Canada, Italy and USA, and one hospital center in the US, consists of 2,372 single primary and 1,206 multiple primary melanoma cases, and includes 3,523 (98.5%) white Caucasians, 36 (1%) non-Caucasians, and 19 (0.5%) participants with unknown race. Details of the study design and its rationale have been published (Begg et al., 2006; Orlow et al., 2016). The human research oversight committees at each of the GEM sites approved the study protocol. All the participants signed written informed consent. After removing non-white participants and those with missing sun exposure or genotyping data, a total of 3336 incident primary cases were included in this analysis.
Sun exposure
We selected UVB dose as our sun exposure measure because its wavelength (280-315nm) induces production of vitamin D (Holick, 2007). UVB dose was obtained by coding individual residential histories by latitude, longitude and altitude from birth to age at diagnosis. Ambient UVB exposure was calculated for the decade of age corresponding to melanoma diagnosis (the most recent lesion for those with multiple primaries) by extracting UVB-specific information from satellite measures of irradiance at the earth's surface (Berwick et al., 2014; Thomas et al., 2010). For the same decade, ambient UVB was multiplied by the self-reported time spent outdoors on weekends and weekdays. Because in most individuals vitamin D synthesis occurs within few minutes of sun exposure, we dichotomized exposure levels into two unequal categories: low (lowest quartile of the UVB dose level – Q1) and high (if >Q1, or Q2-3-4). We performed sensitivity analysis by splitting data into four quartiles and testing for significant linear trends of increase or decrease in the sHRs across the four quartiles (Table S2).
Genotyping
The selection of 38 VDR SNPs, genotyping, and quality control procedures have been described elsewhere (Orlow et al., 2012; Orlow et al., 2016). Briefly, we included SNPs with known or suspected impact on the transcription, stability, and/or VDR activity SNPs reported as significant in other association studies and a set of tag SNPs described among Caucasians. For most SNPs, we arbitrarily considered the most common (i.e. major) allele as the reference allele and the least frequent (i.e. minor) allele as the effect allele. For rs12370156, rs2238140, and rs7305032 the most common allele is reported as the effect allele due to the region's pattern of linkage disequilibrium (LD).
Outcomes
A thorough search for deaths and their causes was completed and information on vital status (alive at the end of follow up, dead of disease, dead of other causes) was obtained for all the participants. Follow-up for vital status ended on December 31, 2007 across all centers, except for British Columbia and Turin, where follow-up period ended on December 31, 2008. Date and cause of death were obtained from National Death Indexes, cancer registries and municipal records. Cases were considered alive if not in the National Death Index.
Statistical analysis
Our main analysis focused on the interaction between VDR SNPs/haplotypes and sun exposure and their effect on melanoma-specific death within strata based on UVB exposure.
Previous literature have reported biased estimates when Cox proportional hazards models were used to describe the risk of disease outcomes in the presence of other competing risk events (Southern et al. 2006; Wolbers et al. 2009). The amount of bias depends on frequency and distribution of competing events. To reduce this bias, we used the Fine and Gray model (Fine and Gray, 1999) to assess the SNP effects on the cumulative incidence of melanoma death adjusting for other non-melanoma causes of death, which was demonstrated the ability to provide more refined estimates of an individual's risk of experiencing the event of interest (Lau et al. 2009). The proportional hazards assumptions for the Fine and Gray model was assessed using score tests employing the Schoenfeld residuals adapted to competing risks data (Zhou et al. 2013) as well as Schoenfeld residual plots against the time variable. We found no evidence of violation from the assumption of proportional subdistribution hazards for the evaluated SNPs.
Statistical significance was assessed with the Wald test. The endpoint was either date of melanoma-death, date of death from non-melanoma causes, or end of follow-up for the censored patients. There was no loss of follow-up. Survival time was accumulated from the diagnosis date of the index lesion (date of the first primary for patients with single primaries and the date of the more recent lesion for patients with multiple primaries). We adjusted for potential confounding factors including age, sex, anatomic site (site of the thickest lesion for those with multiple primaries), as well as study center, single and multiple primary melanoma status, and a time-dependent covariate for individuals with single primaries who developed subsequent melanoma during follow up. We previously reported lack of evidence of association between tumor characteristics and VDR in our cohort (Orlow et al., 2016). Based on existing in vitro evidence, we hypothesize that vitamin D or UVB exposure impacts melanoma outcomes through reduced cell proliferation that might manifest as thinner melanomas. If true, tumor thickness acts as an intermediate marker on the causal chain from sun exposure to better melanoma survival. Because adjusting for thickness when examining the association of sun exposure with melanoma survival could underestimate the prognostic effects of sun exposure and VDR SNPs, we adjusted for melanoma thickness only in secondary analyses.
Per-allele interactions between SNPs and sun exposure were examined using an additive genetic model where genotypes were coded as ordinal (0,1,2) variables, and sun exposure levels were dichotomized as high vs low based on the lowest quartile UVB dose cutoff p-values were calculated for each of the interactions.
To adjust for multiple testing, the joint distribution of the test statistics for the SNPs was approximated by an efficient Monte Carlo simulation and this joint distribution was then used to determine a multiple testing threshold that appropriately controls the family-wise error rate. This adjustment properly accounts for the LD between SNPs and is slightly less conservative than the traditional Bonferroni correction method (He et al., 2013; Lin, 2005).
The haplotype blocks in strong LD (upper 95% confidence bounds of D′>0.95) were estimated using the confidence interval method (Gabriel et al., 2002) in Haploview 4.2. We investigated the effect of the haplotype for SNPs in high LD located in the 3′ end of the VDR gene (rs12370156-rs2238140-rs7305032-rs1544410-rs731236) with PHASE version 2.1 software to infer haplotype frequencies (Stephens and Scheet, 2005). Haplotypes with frequency <0.01 were combined into one category. The haplotype associations with survival were assessed in a competing-risks regression framework by relating inferred individual haplotype probabilities to the survival outcome. This method effectively took into account the haplotype phase uncertainty and reduced bias (Zaykin et al., 2002).
All the analyses were performed using statistical software R (version 3.2.2) and SAS (version 9.4).
Supplementary Material
Figure S1. The figure depicts the LD plot and the position of the studied polymorphisms in relation to the genomic organization of the VDR gene. The vertical bars represent promoters 1F – 1C (blue bars) and exons 2 – 9 (green bars). The grey box represents the 3′ untranslated region (UTR). The SNPs are numbered 1 to 38 in relation to their 5′ to 3′ position (Table 2 and Table S1). Haplotype blocks are depicted with inverted triangles, and the inset depicts block 8, containing five of the six significant SNPs in this study. Numbers in squares are R2 values between SNPs.
Table S1. Effect of 38 VDR SNPs on melanoma death according to levels of sun exposure at diagnosis after accounting for competing risk of death from other causes.
Table S2. Effect of VDR SNPs on melanoma death according to quartiles of sun exposure at diagnosis after accounting for competing risk of death from other causes
Acknowledgments
This work was supported by the National Cancer Institute [UO183180, CA112524, and CA112524-S1 to M.B.], [CA112243 and CA112243-05S1 to N.T.], [P30-CA008748 to MSK]. The MSK Sequenom facility was supported by the Anbinder Fund. The study was conducted by the GEM Study Group. Coordinating Center, Memorial Sloan Kettering Cancer Center, New York, NY (USA): Marianne Berwick (PI, currently at the University of New Mexico), Colin Begg, Ph.D. (co-PI), Irene Orlow, Ph.D., M.S. (co-Investigator), Klaus J. Busam, M.D. (Dermatopathologist), Pampa Roy, Ph.D. (Senior Laboratory Technician), Himali Patel, M.S. (Senior Laboratory Technician), Sarah Yoo, M.S. (Senior Laboratory Technician), Anne Reiner, M.S. (Biostatistician); University of New Mexico, Albuquerque: Marianne Berwick, M.P.H., Ph.D. (PI), Li Luo, Ph.D. (Biostatistician), Susan Paine, M.P.H. (Data Manager). Study Centers: The University of Sydney and The Cancer Council New South Wales, Sydney, Australia: Anne Cust, Ph.D. (PI), Bruce K. Armstrong M.D. Ph.D. (former PI), Anne Kricker Ph.D., (former Co-PI) Menzies Institute for Medical Research University of Tasmania, Hobart, Australia: Alison Venn (current PI), Terence Dwyer (PI, currently at University of Oxford, United Kingdom), Paul Tucker (Dermatopathologist) British Columbia Cancer Research Centre, Vancouver, Canada: Richard P. Gallagher, M.A. (PI), Cancer Care Ontario, Toronto, Canada: Loraine D. Marrett, Ph.D. (PI), Lynn From, M.D. (Dermatopathologist) CPO, Center for Cancer Prevention, Torino, Italy: Roberto Zanetti, M.D (PI), Stefano Rosso, M.D., M.S. (co-PI) University of California, Irvine, CA: Hoda Anton-Culver, Ph.D. (PI) University of Michigan, Ann Arbor, MI: University of Michigan, Ann Arbor: Stephen B. Gruber, M.D., M.P.H., Ph.D. (PI, currently at University of Southern California, Los Angeles, CA), University of North Carolina, Chapel Hill, NC: Nancy E. Thomas, M.D., Ph.D. (PI), David W. Ollila, M.D. (co-Investigator), Pamela A. Groben, M.D. (Dermatopathologist) University of Pennsylvania, Philadelphia, PA: Timothy R. Rebbeck, Ph.D. (former PI), Peter A. Kanetsky, M.P.H., Ph.D. (PI, currently at H. Lee Moffitt Cancer Center & Research Institute, Tampa, Florida) UV data consultants: Julia Lee Taylor, Ph.D. and Sasha Madronich, Ph.D., National Centre for Atmospheric Research, Boulder, CO.
References
- Ahearn TU, Tchrakian N, Wilson KM, Lis R, Nuttall E, Sesso HD, Loda M, Giovannucci E, Mucci LA, Finn S, et al. Calcium-Sensing Receptor Tumor Expression and Lethal Prostate Cancer Progression. J Clin Endocrinol Metab. 2016;101:2520–7. doi: 10.1210/jc.2016-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bade B, Zdebik A, Wagenpfeil S, Graber S, Geisel J, Vogt T, Reichrath J. Low serum 25-hydroxyvitamin d concentrations are associated with increased risk for melanoma and unfavourable prognosis. PloS one. 2014;9:e112863. doi: 10.1371/journal.pone.0112863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg CB, Hummer AJ, Mujumdar U, Armstrong BK, Kricker A, Marrett LD, Millikan RC, Gruber SB, Culver HA, Zanetti R, et al. A design for cancer case-control studies using only incident cases: experience with the GEM study of melanoma. International journal of epidemiology. 2006;35:756–64. doi: 10.1093/ije/dyl044. [DOI] [PubMed] [Google Scholar]
- Berwick M, Armstrong BK, Ben-Porat L, Fine J, Kricker A, Eberle C, Barnhill R. Sun exposure and mortality from melanoma. Journal of the National Cancer Institute. 2005;97:195–9. doi: 10.1093/jnci/dji019. [DOI] [PubMed] [Google Scholar]
- Berwick M, Reiner AS, Paine S, Armstrong BK, Kricker A, Goumas C, Cust AE, Thomas NE, Groben PA, From L, et al. Sun exposure and melanoma survival: a GEM study. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2014;23:2145–52. doi: 10.1158/1055-9965.EPI-14-0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boniol M, Armstrong BK, Dore JF. Variation in incidence and fatality of melanoma by season of diagnosis in new South Wales, Australia. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2006;15:524–6. doi: 10.1158/1055-9965.EPI-05-0684. [DOI] [PubMed] [Google Scholar]
- Brozyna AA, Jozwicki W, Slominski AT. Decreased VDR expression in cutaneous melanomas as marker of tumor progression: new data and analyses. Anticancer research. 2014;34:2735–43. [PMC free article] [PubMed] [Google Scholar]
- Colston K, Colston MJ, Feldman D. 1,25-dihydroxyvitamin D3 and malignant melanoma: the presence of receptors and inhibition of cell growth in culture. Endocrinology. 1981;108:1083–6. doi: 10.1210/endo-108-3-1083. [DOI] [PubMed] [Google Scholar]
- Danielsson C, Fehsel K, Polly P, Carlberg C. Differential apoptotic response of human melanoma cells to 1 alpha,25-dihydroxyvitamin D3 and its analogues. Cell Death Differ. 1998;5:946–52. doi: 10.1038/sj.cdd.4400437. [DOI] [PubMed] [Google Scholar]
- Eisman JA, Barkla DH, Tutton PJ. Suppression of in vivo growth of human cancer solid tumor xenografts by 1,25-dihydroxyvitamin D3. Cancer research. 1987;47:21–5. [PubMed] [Google Scholar]
- Evans SR, Houghton AM, Schumaker L, Brenner RV, Buras RR, Davoodi F, Nauta RJ, Shabahang M. Vitamin D receptor and growth inhibition by 1,25-dihydroxyvitamin D3 in human malignant melanoma cell lines. The Journal of surgical research. 1996;61:127–33. doi: 10.1006/jsre.1996.0092. [DOI] [PubMed] [Google Scholar]
- Fang S, Sui D, Wang Y, Liu H, Chiang YJ, Ross MI, Gershenwald JE, Cormier JN, Royal RE, Lucci A, et al. Association of Vitamin D Levels With Outcome in Patients With Melanoma After Adjustment For C-Reactive Protein. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2016;34:1741–7. doi: 10.1200/JCO.2015.64.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman D, Krishnan AV, Swami S, Giovannucci E, Feldman BJ. The role of vitamin D in reducing cancer risk and progression. Nature reviews Cancer. 2014;14:342–57. doi: 10.1038/nrc3691. [DOI] [PubMed] [Google Scholar]
- Ferrer-Mayorga G, Gomez-Lopez G, Barbachano A, Fernandez-Barral A, Pena C, Pisano DG, Cantero R, Rojo F, Munoz A, Larriba MJ. Vitamin D receptor expression and associated gene signature in tumour stromal fibroblasts predict clinical outcome in colorectal cancer. Gut. 2017;66:1449–1462. doi: 10.1136/gutjnl-2015-310977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- Gabriel SB, Schaffner SF, Nguyen H, Moore JM, Roy J, Blumenstiel B, Higgins J, Defelice M, Lochner A, Faggart M, et al. The structure of haplotype blocks in the human genome. Science. 2002;296:2225–9. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- Gambichler T, Bindsteiner M, Hoxtermann S, Kreuter A. Serum 25-hydroxyvitamin D serum levels in a large German cohort of patients with melanoma. The British journal of dermatology. 2013;168:625–8. doi: 10.1111/j.1365-2133.2012.11212.x. [DOI] [PubMed] [Google Scholar]
- Gandini S, Sera F, Cattaruzza MS, Pasquini P, Picconi O, Boyle P, Melchi CF. Meta-analysis of risk factors for cutaneous melanoma: II. Sun exposure. European journal of cancer. 2005;41:45–60. doi: 10.1016/j.ejca.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Garland CF, Garland FC, Gorham ED. Epidemiologic evidence for different roles of ultraviolet A and B radiation in melanoma mortality rates. Annals of epidemiology. 2003;13:395–404. doi: 10.1016/s1047-2797(02)00461-1. [DOI] [PubMed] [Google Scholar]
- He Q, Avery CL, Lin DY. A general framework for association tests with multivariate traits in large-scale genomics studies. Genetic epidemiology. 2013;37:759–67. doi: 10.1002/gepi.21759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heenan PJ, English DR, Holman CD, Armstrong BK. Survival among patients with clinical stage I cutaneous malignant melanoma diagnosed in Western Australia in 1975/1976 and 1980/1981. Cancer. 1991;68:2079–87. doi: 10.1002/1097-0142(19911101)68:9<2079::aid-cncr2820680940>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Hendrickson WK, Flavin R, Kasperzyk JL, Fiorentino M, Fang F, Lis R, Fiore C, Penney KL, Ma J, Kantoff PW, et al. Vitamin D receptor protein expression in tumor tissue and prostate cancer progression. J Clin Oncol. 2011;29:2378–85. doi: 10.1200/JCO.2010.30.9880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick MF. Vitamin D deficiency. The New England journal of medicine. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- Hu K, Callen DF, Li J, Zheng H. Circulating Vitamin D and Overall Survival in Breast Cancer Patients: A Dose-Response Meta-Analysis of Cohort Studies. Integr Cancer Ther. 2017 doi: 10.1177/1534735417712007. 1534735417712007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi M, Arai M, Tanaka S, Onda K, Hirano T. Antiproliferative and apoptosis-inducing effects of lipophilic vitamins on human melanoma A375 cells in vitro. Biological & pharmaceutical bulletin. 2012;35:10–7. doi: 10.1248/bpb.35.10. [DOI] [PubMed] [Google Scholar]
- Janmaat VT, Van De Winkel A, Peppelenbosch MP, Spaander MC, Uitterlinden AG, Pourfarzad F, Tilanus HW, Rygiel AM, Moons LM, Arp PP, et al. Vitamin D Receptor Polymorphisms Are Associated with Reduced Esophageal Vitamin D Receptor Expression and Reduced Esophageal Adenocarcinoma Risk. Molecular medicine. 2015;21:346–54. doi: 10.2119/molmed.2012.00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A, Devesa SS, Fears TR, Hartge P. Cancer surveillance series: changing patterns of cutaneous malignant melanoma mortality rates among whites in the United States. Journal of the National Cancer Institute. 2000;92:811–8. doi: 10.1093/jnci/92.10.811. [DOI] [PubMed] [Google Scholar]
- Jozwicki W, Brozyna AA, Siekiera J, Slominski AT. Expression of Vitamin D Receptor (VDR) Positively Correlates with Survival of Urothelial Bladder Cancer Patients. International journal of molecular sciences. 2015;16:24369–86. doi: 10.3390/ijms161024369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juzeniene A, Moan J. Beneficial effects of UV radiation other than via vitamin D production. Dermato-endocrinology. 2012;4:109–17. doi: 10.4161/derm.20013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keum N, Giovannucci E. Vitamin D supplements and cancer incidence and mortality: a meta-analysis. British journal of cancer. 2014;111:976–80. doi: 10.1038/bjc.2014.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. Am J Epidemiol. 2009;170:244–56. doi: 10.1093/aje/kwp107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin DY. An efficient Monte Carlo approach to assessing statistical significance in genomic studies. Bioinformatics. 2005;21:781–7. doi: 10.1093/bioinformatics/bti053. [DOI] [PubMed] [Google Scholar]
- Newton-Bishop JA, Beswick S, Randerson-Moor J, Chang YM, Affleck P, Elliott F, Chan M, Leake S, Karpavicius B, Haynes S, et al. Serum 25-hydroxyvitamin D3 levels are associated with breslow thickness at presentation and survival from melanoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:5439–44. doi: 10.1200/JCO.2009.22.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton-Bishop JA, Davies JR, Latheef F, Randerson-Moor J, Chan M, Gascoyne J, Waseem S, Haynes S, O'donovan C, Bishop DT. 25-Hydroxyvitamin D2 /D3 levels and factors associated with systemic inflammation and melanoma survival in the Leeds Melanoma Cohort. International journal of cancer. 2015;136:2890–9. doi: 10.1002/ijc.29334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurnberg B, Graber S, Gartner B, Geisel J, Pfohler C, Schadendorf D, Tilgen W, Reichrath J. Reduced serum 25-hydroxyvitamin D levels in stage IV melanoma patients. Anticancer research. 2009;29:3669–74. [PubMed] [Google Scholar]
- Orlow I, Roy P, Reiner AS, Yoo S, Patel H, Paine S, Armstrong BK, Kricker A, Marrett LD, Millikan RC, et al. Vitamin D receptor polymorphisms in patients with cutaneous melanoma. International journal of cancer. 2012;130:405–18. doi: 10.1002/ijc.26023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlow I, Reiner AS, Thomas NE, Roy P, Kanetsky PA, Luo L, Paine S, Armstrong BK, Kricker A, Marrett LD, et al. Vitamin D receptor polymorphisms and survival in patients with cutaneous melanoma: a population-based study. Carcinogenesis. 2016;37:30–8. doi: 10.1093/carcin/bgv157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'shea SJ, Davies JR, Newton-Bishop JA. Vitamin D, vitamin A, the primary melanoma transcriptome and survival. The British journal of dermatology. 2016;175 Suppl 2:30–34. doi: 10.1111/bjd.14919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randerson-Moor JA, Taylor JC, Elliott F, Chang YM, Beswick S, Kukalizch K, Affleck P, Leake S, Haynes S, Karpavicius B, et al. Vitamin D receptor gene polymorphisms, serum 25-hydroxyvitamin D levels, and melanoma: UK case-control comparisons and a meta-analysis of published VDR data. European journal of cancer. 2009;45:3271–81. doi: 10.1016/j.ejca.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso S, Sera F, Segnan N, Zanetti R. Sun exposure prior to diagnosis is associated with improved survival in melanoma patients: results from a long-term follow-up study of Italian patients. European journal of cancer. 2008;44:1275–81. doi: 10.1016/j.ejca.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Saccone D, Asani F, Bornman L. Regulation of the vitamin D receptor gene by environment, genetics and epigenetics. Gene. 2015;561:171–80. doi: 10.1016/j.gene.2015.02.024. [DOI] [PubMed] [Google Scholar]
- Saiag P, Aegerter P, Vitoux D, Lebbe C, Wolkenstein P, Dupin N, Descamps V, Aractingi S, Funck-Brentano E, Autier P, et al. Prognostic Value of 25-hydroxyvitamin D3 Levels at Diagnosis and During Follow-up in Melanoma Patients. J Natl Cancer Inst. 2015;107:djv264. doi: 10.1093/jnci/djv264. [DOI] [PubMed] [Google Scholar]
- Schwitzer E, Orlow I, Zabor EC, Begg CB, Berwick M, Thomas NE, Kanetsky PA, Jones LW GEM Study Group. No Association between Pre-diagnosis Exercise and Survival in Patients with High-Risk Primary Melanoma: A Population-Based Study. Pigment Cell Melanoma Res. 2017 Apr 11; doi: 10.1111/pcmr.12594. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schottker B, Jorde R, Peasey A, Thorand B, Jansen EH, Groot L, Streppel M, Gardiner J, Ordonez-Mena JM, Perna L, et al. Vitamin D and mortality: meta-analysis of individual participant data from a large consortium of cohort studies from Europe and the United States. Bmj. 2014;348:g3656. doi: 10.1136/bmj.g3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sondak VK, Mciver B, Kanetsky PA. Vitamin D and Melanoma: What Do We Tell Our Patients? Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2016;34:1713–4. doi: 10.1200/JCO.2016.66.5240. [DOI] [PubMed] [Google Scholar]
- Southern DA, Faris PD, Brant R, Galbraith PD, Norris CM, Knudtson ML, Ghali WA, Investigators A. Kaplan-Meier methods yielded misleading results in competing risk scenarios. J Clin Epidemiol. 2006;59:1110–4. doi: 10.1016/j.jclinepi.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Stephens M, Scheet P. Accounting for decay of linkage disequilibrium in haplotype inference and missing-data imputation. American journal of human genetics. 2005;76:449–62. doi: 10.1086/428594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas NE, Kricker A, From L, Busam K, Millikan RC, Ritchey ME, Armstrong BK, Lee-Taylor J, Marrett LD, Anton-Culver H, et al. Associations of cumulative sun exposure and phenotypic characteristics with histologic solar elastosis. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2010;19:2932–41. doi: 10.1158/1055-9965.EPI-10-0686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uitterlinden AG, Fang Y, Van Meurs JB, Pols HA, Van Leeuwen JP. Genetics and biology of vitamin D receptor polymorphisms. Gene. 2004;338:143–56. doi: 10.1016/j.gene.2004.05.014. [DOI] [PubMed] [Google Scholar]
- Wolbers M, Koller MT, Witteman JC, Steyerberg EW. Prognostic models with competing risks: methods and application to coronary risk prediction. Epidemiology. 2009;20:555–61. doi: 10.1097/EDE.0b013e3181a39056. [DOI] [PubMed] [Google Scholar]
- Yin J, Liu H, Yi X, Wu W, Amos CI, Fang S, Lee JE, Han J, Wei Q. Genetic variants in the vitamin D pathway genes VDBP and RXRA modulate cutaneous melanoma disease-specific survival. Pigment cell & melanoma research. 2016;29:176–85. doi: 10.1111/pcmr.12437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudoh K, Matsuno H, Kimura T. 1alpha,25-dihydroxyvitamin D3 inhibits in vitro invasiveness through the extracellular matrix and in vivo pulmonary metastasis of B16 mouse melanoma. The Journal of laboratory and clinical medicine. 1999;133:120–8. doi: 10.1016/s0022-2143(99)90004-5. [DOI] [PubMed] [Google Scholar]
- Zaykin DV, Westfall PH, Young SS, Karnoub MA, Wagner MJ, Ehm MG. Testing association of statistically inferred haplotypes with discrete and continuous traits in samples of unrelated individuals. Human heredity. 2002;53:79–91. doi: 10.1159/000057986. [DOI] [PubMed] [Google Scholar]
- Zgaga L, Theodoratou E, Farrington SM, Din FV, Ooi LY, Glodzik D, Johnston S, Tenesa A, Campbell H, Dunlop MG. Plasma vitamin D concentration influences survival outcome after a diagnosis of colorectal cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32:2430–9. doi: 10.1200/JCO.2013.54.5947. [DOI] [PubMed] [Google Scholar]
- Zhou B, Fine J, Laird G. Goodness-of-fit test for proportional subdistribution hazards model. Stat Med. 2013;32:3804–11. doi: 10.1002/sim.5815. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. The figure depicts the LD plot and the position of the studied polymorphisms in relation to the genomic organization of the VDR gene. The vertical bars represent promoters 1F – 1C (blue bars) and exons 2 – 9 (green bars). The grey box represents the 3′ untranslated region (UTR). The SNPs are numbered 1 to 38 in relation to their 5′ to 3′ position (Table 2 and Table S1). Haplotype blocks are depicted with inverted triangles, and the inset depicts block 8, containing five of the six significant SNPs in this study. Numbers in squares are R2 values between SNPs.
Table S1. Effect of 38 VDR SNPs on melanoma death according to levels of sun exposure at diagnosis after accounting for competing risk of death from other causes.
Table S2. Effect of VDR SNPs on melanoma death according to quartiles of sun exposure at diagnosis after accounting for competing risk of death from other causes


