Abstract
Background
T-cell depletion (TCD) effectively reduces severe graft-versus-host disease in recipients of HLA-mismatched allografts. However, TCD is associated with delayed immune recovery and increased infections. We hypothesized that specific depletion of CD45RA+ naïve T cells, rather than broad depletion of CD3+ T cells, can preserve memory-immunity in the allografts, and confer protection against important viral infections in the early post-transplant period.
Methods
Sixty-seven patients who received TCD haploidentical donor transplantation for hematologic malignancy on three consecutive trials were analyzed.
Results
Patients receiving CD45RA-depleted donor grafts had 2000-fold more donor T cells infused, significantly higher T-cell counts at Day +30 post transplant (550/μL vs 10/μL; P< .001), and higher T-cell diversity by Vbeta spectratyping at Day +100 (P < .001). Importantly these recipients experienced a significant reduction in both the incidence (P = .002) and duration (P = .02) of any viremia (cytomegalovirus, Epstein-Barr virus, or adenovirus) in the first 6 months post transplant. Specifically, recipients of CD3-depleted grafts were more likely to experience adenovirus viremia (27% vs 4%, P = .02).
Conclusion
CD45RA-depletion provided a large number of donor memory T cells to the recipients, and was associated with enhanced early T-cell recovery and protection against viremia.
Keywords: CD45RA depletion, haploidentical donors, hematopoietic cell transplantation, memory T cells, T-cell depletion
1 INTRODUCTION
T-cell depletion (TCD) is important for haploidentical donor (haplo) hematopoietic cell transplantation (HCT) to reduce graft-versus-host disease (GVHD). TCD can be ex vivo in which T cells are removed from the allografts before infusion, or in vivo where anti-lymphocyte antibodies or immunosuppressive drugs such as high dose cyclophosphamide are given to kill donor T cells after infusion.1,2 One early successful technique for haploHCT gave an anti-thymocyte globulin (ATG)-containing preparative regimen with immunomagnetic enrichment of CD34+ cells.3 This indirectly depleted T cells and other donor immune cells extensively. The use of high-intensity conditioning, ATG, and megadose progenitor cells was able to overcome the high incidence of rejection previously seen.3 However, the absence of adoptively transferred immune cells led to high risk of relapse and infection.
A recent strategy for providing more donor T-cells in TCD haploHCT provided 1–2 ×106 haploidentical conventional T cells/kg recipient weight with donor regulatory T cell co-infusion.4 Using this approach, more robust donor T-cell reconstitution occured with CD4+ and CD8+ T cells reaching a count of 200/μL by approximately Day +60 after transplantation. Selective TCD has also been used, such as immunomagnetic depletion of TCRαβ+ cells.5–7 This method provides large doses of TCRγδ+ T cells, which may facilitate engraftment, protect against infection, and deliver some graft-versus-leukemia effect.8–10
Novel methods have been developed to selectively deplete naïve T cells and preserve memory T cells through CD45RA-depletion.11–14 The alloreactivity of CD45RA– T-cell fraction seems to be less durable than that of CD45RA+ T-cells.15–17 Therefore, it is possible that large numbers of haploidentical donor CD45RA– T cells could be provided to the recipient without significantly increasing the risk of steroid-refractory acute GVHD and severe chronic GVHD. We have previously shown that the depletion of CD45RA+ cells provide robust early recovery of diverse memory T-cell populations in haploHCT utilizing a total lymphoid irradiation-based conditioning regimen without the need for serotherapy such as ATG, alemtuzumab (Campath 1H), or muromonab-CD3 (OKT3).18 We hypothesize that the observed memory T-cell reconstitution may confer protection against virulent viruses and more rapid lymphocyte reconstitution in the first 6 months after transplantation when compared to previous haploHCT strategies using non-selective TCD. Herein, we describe the results of 3 consecutive therapeutic haploHCT trials, and compare the results from two trials that used broad CD3-depletion ex vivo and in vivo, vs the current trial that utilizes specific CD45RA-depletion without serotherapy.
2 PATIENTS AND METHODS
2.1 Patients and regimen
All patients who received initial allogeneic HCT on 3 consecutive Institutional Revuew Board-approved haploHCT trials at St. Jude Children’s Research Hospital were reviewed. Forty-one received CD3-depleted (CD3dep-recipients) progenitor cell products (HPC,A) on two consecutive trials (Trial1: n = 10, 2005–2006; Trial2: n = 31, 2007–2012). Twenty-six received CD45RA-depleted HPC,A (CD45dep-recipients) on the current trial (2013–2015); this includes 17 patients for whom early immune reconstitution was previously described.18 This trial is registered at ClinicalTrials.gov, Identifier: NCT01807611. All patients received similar preparative backbone consisting of fludarabine (150–200 mg/m2), thiotepa (10 mg/kg), and melphalan (120–140 mg/m2). CD3dep-recipients additionally received OKT3 (n = 21) or alemtuzumab (n = 20) when OKT3 was no longer commercially available, and all received rituximab on Day 0 as Epstein-Barr virus (EBV) prophylaxis. CD45RAdep-recipients (n = 26) did not receive antibody therapy, but received total lymphoid irradiation (8Gy), a single dose of cyclophosphamide (60 mg/kg), and no EBV prophylaxis. CD3dep-recipients typically received 2 HPC,A on consecutive days, both CD3-depleted by CliniMACS.19 Patients on Trial1 received T-cell add back with a goal of 0.15×106/kg (minimum 0.1×106/kg); whereas patients on Trial2 had a maximum T-cell dose of 0.1×106/kg. CD45RAdep-recipients received 2 HPC,A on consecutive days, the first enriched for CD34+ cells, the second CD45RA-depleted by CliniMACS, and donor natural killer (NK) cell infusion on Day +6 as previously described.18 All CD3dep-recipients and the majority (n = 17) CD45RAdep-recipients received a short (<60 days) course of mycophenolate mofetil as the sole pharmacologic post-transplant GVHD prophylaxis, the remaining 9 patients received a short course of sirolimus. Acyclovir was the standard cytomegalovirus (CMV) prophylaxis for all patients. Following graft failure in the second-to-last patient on Trial 2 that was thought to be precipitated by human herpesvirus-6 (HHV6) reactivation, all subsequent patients received HHV6 polymerase chain reaction (PCR) testing and immediate empiric intravenous (i.v.) ganciclovir (GCV) for fever and rash occurring prior to engraftment.
2.2 Virus surveillance
Quantitative PCR of blood samples for CMV, EBV, and adenovirus (ADV) was performed weekly until Day +100, then as indicated. Viremia was defined as any PCR detection of the virus in the first 180 days post transplant. Duration was defined as time from first of two consecutive positive tests until the first of two consecutive negative tests for that virus, without subsequent consecutive positives in the next 6 weeks. Testing for ADV in the stool by qualitative PCR was performed when diarrhea was present. PCR for CMV was initially assayed as copy/mL whole blood, later the assay was changed to international units (IU)/mL plasma. Quality assurance testing demonstrated similar detection limits in both assays. The peak viral load was determined using the IU/mL assay in only 1 patient (CD45RAdep-recipeint with peak viral load at the detection limit, <2.14 log10IU/mL). Similarly, EBV PCR was initially assayed as copy/ug DNA from blood mononuclear cells and later the assay was changed to IU/mL whole blood. Quality assurance testing again demonstrated similar detection limits. Thus, preemptive viral treatment strategies for these 3 viruses were not changed during the entire study period (2005–2015). Preemptive treatment for CMV was initiated when a 1 log increase in viral load was detected. Preemptive treatment for ADV was commenced for any detectible viral load in the blood, and was allowed at physician discretion for the presence of ADV in the stool based on the clinical perceived risk of dissemination.
2.3 Immune monitoring
Blood was collected monthly for the first 6 months post transplant for CLIA-certified flow cytometric quantification of lymphocyte subsets in all recipients. Blood was collected in the two most recent trials from donors prior to mobilization and recipients at Day +100 and Day +180 for Vbeta spectratyping and T-cell receptor excision circles (TREC), as previously described.18
2.4 Clinical outcome evaluations
Treatment related mortality (TRM) was defined as any death not due to malignant progression. GVHD grading was performed according to standardized criteria.20 Comprehensive audit of medical records and research data was performed by internal and extramural experts on all 31 patients on Trial2 and all 26 patients on the current trial to verify the incidence of grade III-IV acute GVHD. The data presented here includes any modifications to the research record following these audits.
2.5 Statistical analysis
Two sample t-tests or Wilcoxon Mann-Whitney tests were used to compare groups of CD3dep-recipients and CD45RAdep-recipients for continuous variables, depending on normality, while chi-squared tests (Fisher’s exact test for small samples) were used for categorical variables. Linear mixed models were conducted to examine the kinetics of immune reconstitution; the correlation structure and random effects were chosen as the model with the lowest Akaike Information Criteria. Cumulative incidence rates for CMV reactivation were estimated using the Kalbfleisch-Prentice method with death as a competing risk and comparison using Gray’s test.21,22 All analyses were for the first 180 days post transplantation. All reported P-values are two-sided and considered significant if < .05. SAS software version 9.4 (Cary, NC USA) and GraphPad Prism version 6.07 (La Jolla, CA USA) were used for statistical analyses and figure generation, respectively.
3 RESULTS
3.1 Patients
All 67 patients were under 21 years of age at the time of transplant. Table 1 summarizes patient characteristics stratified by TCD methods. Most patients had acute leukemia, with 37% not in remission at the time of HCT. A higher proportion of refractory disease (induction failure, active relapse) was noted in CD45RAdep-recipients. CMV exposure was similar in both groups, as a majority of patients were serologically positive and/or had serologically positive donors.
TABLE 1.
Patient summary
| CD3-depleted (n=41) | CD45RA-depleted (n=26) | P-value | |
|---|---|---|---|
| Age Median (range); years | 15.0 (3.5–20.4) | 10.5 (0.6–20.7) | .038 |
| Weight Median (range); kg | 57.2 (15.2–137) | 31.2 (6.4–158) | .023 |
| Gender | .870 | ||
| Male | 26 (63%) | 17 (65%) | |
| Female | 15 (37%) | 9 (35%) | |
| Race/Ethnicity | .267 | ||
| Caucasian | 27 (66%) | 17 (65%) | |
| Hispanic | 13 | 6 | |
| Non-Caucasian | 14 (34%) | 9 (35%) | |
| African American | 12 | 5 | |
| Multiple Race/Other | 1 | 3 | |
| Asian | 1 | 1 | |
| Disease | .581 | ||
| Acute lymphoblastic leukemia | 17 (41%) | 11 (42%) | |
| B cell | 13 | 8 | |
| T cell | 4 | 3 | |
| Acute myeloid leukemia | 11 (27%) | 11 (42%) | |
| Mixed lineage leukemia | 2 (5%) | 2 (8%) | |
| Myelodysplastic syndrome | 7 (17%) | 1 (4%) | |
| Lymphoma | 2 (5%) | 1 (4%) | |
| Chronic myeloid leukemia | 2 (5%) | 0 | |
| Disease stage | .021 | ||
| CR1 | 13 (32%) | 6 (23%) | |
| Subsequent CR | 16 (39%) | 7 (27%) | |
| Active relapse | 2 (5%) | 6 (23%) | |
| Induction failure | 1 (2%) | 5 (19%) | |
| Refractory anemia | 5 (12%) | 1 (4%) | |
| RAEB | 2 (5%) | 0 | |
| Chronic phase | 2 (5%) | 0 | |
| Untreated | 0 | 1 (4%) | |
| HLA match status | .62 | ||
| 4 of 8 | 25 (61%) | 19 (73%) | |
| 5 of 8 | 12 (29%) | 6 (23%) | |
| 6 of 8 | 4 (10%) | 1 (4%) | |
| Donor | .33 | ||
| Mother | 20 (49%) | 17 (65%) | |
| Father | 18 (44%) | 7 (27%) | |
| Sibling | 3 (7%) | 2 (8%) | |
| CMV status (Donor/Recipient) | .201 | ||
| Negative/Negative | 6 (15%) | 3 (12%) | |
| Positive/Positive | 24 (56%) | 11 (42%) | |
| Positive/Negative | 7 (17%) | 4 (15%) | |
| Negative/Positive | 4 (10%) | 8 (31%) |
CR, complete remission; RAEB, refractory anemia with excess blasts; HLA, human leukocyte antigen; CMV, cytomegalovirus.
Cumulative cell doses of all donor grafts infused is detailed in Table 2. CD3dep-recipients received a median of 40 thousand T cells/kg, while CD45RAdep-recipients received a median of 80 x106 T cells/kg. CD45RA+ T cells were routinely measured only in CD45RA-depleted products and the median dose was 11 thousand/kg. In the CD3dep-recipients, the median CD45RA+ T-cell dose was an estimated 22 thousand/kg (50% of total T cells). CD34+ cell dose was a median of 9 x106/kg in CD3dep-recipients and 15.6 x106/kg in CD45RAdep-recipients, in association with a lower median recipient age and weight.
TABLE 2.
Cell quantities infused, cumulative for all products
| Median (range) | CD3-depleted | CD45RA-depleted | P-value |
|---|---|---|---|
| TNC (x108/kg) | 5.3 (1.7–28.2) | 4.5 (0.91–19.4) | .437 |
| CD34+ (x106/kg) | 9.0 (1.7–47.9) | 15.6 (2.9–67.5) | .005 |
| CD3+ (x106/kg) | 0.043 (0.010–0.15) | 80.0 (16.1–528.6) | <.001 |
| CD3+ CD45RA+ (x106/kg)* | 0.022 (0.005–0.075) | 0.011 (0.002–0.13) | .102 |
| CD19+ (x106/kg) | 47.1 (19.8–225) | 0.465 (0.054–5.79) | <.001 |
| CD3–CD56+ (x106/kg) | 14.4 (1.5–422.5) |
CD3+ CD45RA+ cells were measured directly in CD45RA-depleted grafts, and estimated in CD34+ enriched, CD3-depleted, and NK-cell grafts by 0.5 x CD3+ count.
CD3-CD56+ doses were not measured in the grafts provided to the CD3-depleted cohort.
3.2 Viremia
Thirty-one (76%) CD3dep-recipients had detectible viremia in the first 180 days post transplant, compared to 9 (35%) CD45RAdep-recipients (P = .002; Figure 1A). Prolonged viremia (>60 days) occurred in 18 of 31 (58%) viremic CD3dep-recipients, compared to 1 of 9 (11%) viremic CD45RAdep-recipient (P = .02).
FIGURE 1.
Incidence of viremia. (A) Incidence and duration of any cytomegalovirus (CMV), Epstein-Barr virus (EBV), or adenovirus (ADV) detection in the blood of recipients of CD3-depleted and CD45RA-depleted grafts (B) Incidence of detection of each virus independently. (C) Cumulative incidence of CMV virus detection in the blood over time for recipients of CD3-depleted products (black) and recipients of CD45RA-depleted products (gray)
CD3dep-recipients were more likely to experience CMV viremia – 23 (56%) vs 5 (19%) (P = .005; Figure 1B). Protection from CMV viremia primarily occurred in CMV seropositive recipient (R+) and seropositive donor (D+) pairs, as CMV was detected in 22 of 24 (92%) D+/R+ CD3dep-recipients compared to 4 of 11 (36%) D+/R+ CD45RAdep-recipients (P = .001). Cumulative incidence of CMV viremia was lower in the CD45RAdep-recipients when analyzed at Day +30 (P = .01) and Day +180 (P = .003; Figure 1C). Although the mean peak viral load between the two cohorts was not different (P = .078), many CD3dep-recipients had peak viral loads several-fold higher than the maximum viral load seen in CD45RAdep-recipients (Figure 2A). All CD3dep-recipients with CMV received treatment, including GCV in 9, foscarnet in 7, and both in 7. Eight (36%) also received donor lymphocyte infusion (DLI) for CMV. By contrast, CMV treatment was required for only 3 of the 5 CD45RAdep-recipients, with GCV in 1, foscarnet and GCV in 1, and valganciclovir in 1. Two patients had resolution without intervention. No patient received DLI for CMV. Despite aggressive i.v. and cellular antiviral treatment, median duration of CMV viremia in CD3dep-recipients was 81 days, vs 23 days in CD45RAdep-recipients. Protracted viremia (>30 days) was more common in CD3dep-recipeints – 18 of 23 (78%) vs 1 of 5 (20%) (P = .026). Some patients received GCV or foscarnet23 without detectible CMV. This was most commonly due to routine testing and immediate preemptive treatment of HHV6 in the constellation of fever and rash in the recent cohort, a measure prompted by prior cases of HHV6-induced graft rejection. Because testing for HHV6 was not routinely done in earlier era, only 15% of CD3dep-recipients received GCV or foscarnet in the absence of detectible CMV, compared to 88% of CD45RAdep-recipients who received brief therapy – median duration 14 days (range 3 – 141), with 5 (19%) recipients receiving 4 weeks or more.
FIGURE 2.
Peak viral load. The peak viral load of (A) cytomegalovirus (CMV) and (B) adenovirus
No difference was seen in the incidence of EBV detection in the first 180 days post transplant between the two groups – 7 of 41 (17%) vs 5 of 26 (19%). Four of 7 (57%) EBV+ CD3dep-recipients received treatment rituximab for rising EBV viral load, including one whose PTLD was successfully treated with rituximab, chemotherapy, and DLI. No CD45RAdep-recipient received EBV-directed therapy.
Adenovirus viremia was more frequent in CD3dep-recipients - 11 (27%) vs 1 (4%) (P = .02; Figure 1B); and the peak viral load was often higher (Figure 2B). In the lone CD45RAdep-recipient with detectible ADV, the viral load was below the limit of quantification (2.0 log10copies/mL = 100 copies/mL). Nine of 11 (82%) CD3dep-recipients with ADV were treated with cidofovir, and 4 (36%) also received DLI as treatment. The CD45RAdep-recipient was treated with cidofovir without DLI. Despite aggressive i.v. and cellular antiviral treatment, the median duration of ADV viremia in recipients of CD3-depleted products was 42 days, whereas the CD45RAdep-recipient cleared ADV in 7 days. Some patients received cidofovir without detectible ADV in blood, most commonly caused by BK polomavirus-associated hemorrhagic cystitis and/or adenoviral colitis. Seventeen (41%) CD3dep-recipients received i.v. cidofovir without ADV in blood. Only 6 (23%) CD45RAdep-recipients received cidofovir without ADV in blood (one was later switched to brincidofovir). No other patient received brincidofovir. PCR testing of the stool for ADV was done as needed based on symptoms.25 Sixteen (39%) CD3dep-recipients were positive for ADV in stool, and 11 of these 16 (69%) disseminated to blood. Conversely, 5 (19%) CD45RAdep-recipients were positive for ADV in stool, and only 1 of those 5 (20%) disseminated to blood.
3.3 Immune reconstitution
T-cell recovery was faster in CD45RAdep-recipients based on linear mixed models, with a median T-cell count at Day +30 of 550 T cells/μL vs 10 T cells/μL in CD3dep-recipients (15/μL in OKT3 recipients, 0/μL in alemtuzumab recipients). Quantitative differences were apparent in both CD4+ and CD8+ T-cell fractions (all P < .001). Median CD4+ T-cell counts were 140/μL vs 0/μL, and median CD8+ T-cell counts were 315/μL vs 0/μL at Day +30 (Figure 3A and 3B, respectively). There was a paucity of B cells in both cohorts at Day +30, however quantitative B cell recovery occurred earlier in CD45RAdep-recipients, who had higher B cell counts by Day +90 (P = .009; Figure 3C). NK cell recovery was generally quantitatively normal to supra-normal at Day +30 and remained normal throughout the first 6 months in both cohorts, but was quantitatively higher in CD3dep-recipients despite the NK cell graft provided to CD45RAdep-recipients (P <.001).
FIGURE 3.
Quantitative lymphocyte reconstitution. The peripheral blood cell counts of (A) CD4+ T cells, (B) CD8+ T cells, and (C) B cells are shown as the median cell count and interquartile range at each time point, stratified by TCD methods
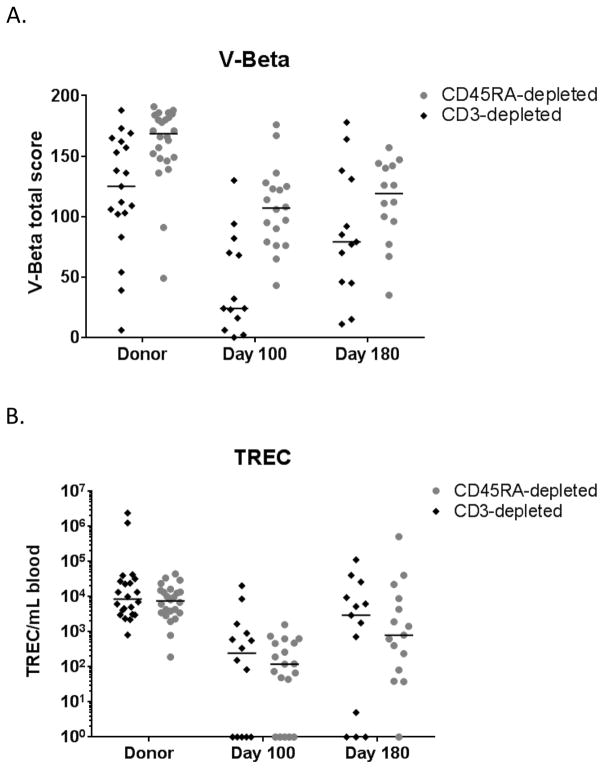
The median TCR V-Beta total score was higher in CD45RAdep-recipients at Day +100 (24 vs 107) (P < .001; Figure 4A). TREC analysis showed very low quantities in both cohorts at Day +100 (median of 247 and 120 TREC/mL blood respectively; Figure 4B).
FIGURE 4.
T-cell diversity. The (A) V-beta total score and (B) T-cell receptor excision circles (TREC) counts per mL of blood are presented for donor samples obtained prior to mobilization/collection and in recipients at Day +100 and +180
3.4 Clinical outcomes
Within the first 180 days after transplantation, 9 (22%) CD3dep-recipients and 4 (15.4%) CD45RAdep-recipients died of treatment related causes. Primary cause of death was frequently bacterial or fungal infection, pulmonary hemorrhage/failure, and multi-organ failure. Viral infection was not listed as the primary cause of death in any patient. However, three CD3dep-recipients died with active CMV viremia. No CD45RAdep-recipient had detectible virus at the time of death.
Acute GVHD Grade III-IV occurred in 6 of 10 (60%) recipients on Trial1, therefore that trial was halted and T-cell add-back was no longer utilized for CD3dep-recipients. Acute GVHD grade III-IV occurred in 7 of 31 (22.6%) CD3dep-recipients on Trial2, and 6 (23.1%) CD45RAdep-recipients.
4 DISCUSSION
CD45RA-depleted allografts have few naïve-T cells but abundant memory-T cells. Thus, we hypothesize that GVHD potential is reduced, and that large memory-T cell doses, which may facilitate engraftment without the need for serotherapy, can be given. Collectively, this novel approach may facilitate rapid immune reconstitution and prevent infection. We previously published a detailed analysis of early immune reconstitution in the first 17 CD45RAdep-recipients, and found that T-cell reconstitution was a direct recapitulation of the CD45RA-depleted graft content, included robust recovery of central memory and effector memory T cells in CD4+ and CD8+ T-cell fractions, and demonstrated early proliferative responses to antigens such as CMV and HSV.18 Here, we observed that CD3dep-recipients who needed OKT3 or alemtuzumab for engraftment had significantly lower median T-cell count compared to CD45RAdep-recipients as early as Day +30 (10/μL vs 550/μL, P< .001). Furthermore, there was substantial T-cell diversity at Day +100 in CD45RAdep-recipients even before TREC normalization, indicating successful adoptive transfer of memory T cells and not de novo T-cell production.
Because memory T cells retain potent virus-specific activity, we hypothesized that recipients of CD45RA-depleted grafts and serotherapy-free conditioning would have better virus control. In line with this hypothesis, the incidence of viremia, the peak viral loads observed, and the duration of viremia were all substantially decreased when compared with CD3dep-recipients. Importantly, memory T-cell recipients who developed viremia generally required less aggressive treatment.
Our data suggest that the newer CD45RA-depletion protocol may confer specific protection against adenoviral colitis and subsequent dissemination. CD3dep-recipients developed adenoviral colitis more often and then also more frequently experienced dissemination to viremia than CD45RAdep-recipients. The ADV outcome among CD45RAdep-recipients compared favorably to that of a recently published diverse allogeneic transplant population from our institution that had an incidence of adenoviral colitis of 33%, with 28% becoming viremic.25 Additionally, protection against adenoviremia was present in CD45RAdep-recipients despite reduced use of cidofovir in that cohort.
While the protective effect of CD45RA-depleted graft against ADV is evident, its effect against CMV viremia is unclear, because many CD45RAdep-recipients received short-course GCV in the early post-transplant period despite the absence of CMV for preemptive treatment of HHV6 in the setting of fever and rash. Although the brief duration of preemptive HHV6 treatment (median only 14 days) was not likely fully protective against CMV reactivation (which typically required treatment through Day+100), 26 five recipients received 4 weeks or more of GCV; therefore, a time-dependent bias in cumulative incidence analysis is possible.
Overall, EBV reactivation was not common and only 1 of 67 developed PTLD. This is likely a result of the efficacy of rituximab as EBV prophylaxis in CD3dep-recipients and the efficacy of ex vivo B-cell depletion with CD45RA-depletion (~2.5 log depletion).18 However, perhaps because rituximab has a long half-life and was not needed in CD45RAdep-recipients, B-cell recovery occurs earlier in those patients. Alternatively, adoptive memory T-cell transfer may facilitate de novo B-cell recovery in CD45RAdep-recipients.
Another advantage of CD45RA-depletion is independence from serotherapy for conditioning. Previous studies of broad TCD in haploHCT typically required serotherapy such as ATG, OKT3, or alemtuzumab for high rate of engraftment. Use of serotherapy in the CD3dep-recipients might further contribute to the slow T-cell recovery. However, OKT3 has a very short half-life, and no statistical difference was seen in immune reconstitution in OKT3 recipients vs alemtuzumab recipients, except for a quantitatively greater NK cell recovery at Day +30 in OKT3 recipients (data not shown). Therefore, serotherapy given to CD3dep-recipients was unlikely the sole explanation for slower T-cell reconstitution. Although NK cells have antiviral properties, recovery was quantitatively higher at Day +30 in CD3dep-recipients, indicating that the observed reduction in viremia in CD45RAdep-recipients was more likely due to the addition of memory T cells than additional NK cells.
Although it was not statistically significant, the TRM in CD45RAdep-recipients compared favorably to CD3dep-recipients at Day +180. Furthermore, the incidence of severe acute GVHD in CD45RAdep-recipients was similar to CD3dep-recipients on Trial2 after both trials underwent an extensive audit looking for any potential grade III-IV acute GvHD. Therefore, there is no apparent increase in severe acute GVHD from the provision of large numbers of donor memory cells, unlike that seen with the add-back of comparatively small numbers of unfractionated conventional T-cells to CD3dep-recipients on Trial1.
In summary, CD45RA-depletion allows for the first time a large memory T-cell dose, which results in enhanced T-cell reconstitution as early as Day +30, protection from adenoviremia, and elimination of EBV prophylaxis. Critically, these benefits were achieved with no increase in the incidence of severe acute GVHD and a favorable early TRM in this high risk cohort which includes a large subset of patients with active refractory hematologic malignancy. Accrual to this therapeutic trial is ongoing to wholly assess important clinical outcomes such as full characterization of acute and chronic GVHD, disease control, hospital cost, and survival. Additional trials utilizing CD45RA-depletion in older patients and other disease groups will be needed to test the broad applicability of this selective T-cell depletion technique.
Acknowledgments
Funding: This work is supported in part by the National Institutes of Health Cancer Center Support (CORE) grant P30 CA021765, the Assisi Foundation of Memphis, the Press On Fund, and the American Lebanese Syrian Associated Charities (ALSAC).
The authors would like to thank our colleagues for data collection and clinical management. Our thanks also go out to the many patients and families who participated in the transplantation and cellular therapy research program.
Footnotes
Conflict-of-interest statement
W.L. is currently an employee of Miltenyi Biotech. The other authors have no financial relationships or other conflicts of interest to disclose relevant to this article. No author received an honorarium, grant, or other form of payment to produce the article.
Author Contributions:
B.M.T. and W.L. designed the research, analyzed and interpreted data, and wrote the article; B.M. collected and analyzed data; Y.L., S.J.C., W.J., J.C., and R.T.H. provided data for analysis and contributed to interpretation of the data; B.M.T., L.C., E.M., D.R.S., A.S., and W.L. provided study material and patient information and contributed to the interpretation of the data; G.K. and J.M. provided statistical analysis; and all authors contributed to the revisions of the draft and approval of the final article.
References
- 1.Or-Geva N, Reisner Y. The evolution of T-cell depletion in haploidentical stem-cell transplantation. Br J Haematol. 2016;172:667–684. doi: 10.1111/bjh.13868. [DOI] [PubMed] [Google Scholar]
- 2.Robinson TM, O’Donnell PV, Fuchs EJ, Luznik L. Haploidentical bone marrow and stem cell transplantation: Experience with post-transplantation cyclophosphamide. Semin Hematol. 2016;53:90–97. doi: 10.1053/j.seminhematol.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aversa F, Tabilio A, Velardi A, et al. Treatment of high-risk acute leukemia with T-cell-depleted stem cells from related donors with one fully mismatched HLA haplotype. N Engl J Med. 1998;339:1186–1193. doi: 10.1056/NEJM199810223391702. [DOI] [PubMed] [Google Scholar]
- 4.Martelli MF, Di Ianni M, Ruggeri L, et al. HLA-haploidentical transplantation with regulatory and conventional T-cell adoptive immunotherapy prevents acute leukemia relapse. Blood. 2014;124:638–644. doi: 10.1182/blood-2014-03-564401. [DOI] [PubMed] [Google Scholar]
- 5.Chaleff S, Otto M, Barfield RC, et al. A large-scale method for the selective depletion of alphabeta T lymphocytes from PBSC for allogeneic transplantation. Cytotherapy. 2007;9:746–754. doi: 10.1080/14653240701644000. [DOI] [PubMed] [Google Scholar]
- 6.Lang P, Feuchtinger T, Teltschik HM, et al. Improved immune recovery after transplantation of TCRalphabeta/CD19-depleted allografts from haploidentical donors in pediatric patients. Bone Marrow Transplant. 2015;50(Suppl 2):S6–10. doi: 10.1038/bmt.2015.87. [DOI] [PubMed] [Google Scholar]
- 7.Bertaina A, Merli P, Rutella S, et al. HLA-haploidentical stem cell transplantation after removal of alphabeta+ T and B cells in children with nonmalignant disorders. Blood. 2014;124:822–826. doi: 10.1182/blood-2014-03-563817. [DOI] [PubMed] [Google Scholar]
- 8.Girardi M. Immunosurveillance and immunoregulation by gammadelta T cells. J Invest Dermatol. 2006;126:25–31. doi: 10.1038/sj.jid.5700003. [DOI] [PubMed] [Google Scholar]
- 9.Godder KT, Henslee-Downey PJ, Mehta J, et al. Long term disease-free survival in acute leukemia patients recovering with increased gammadelta T cells after partially mismatched related donor bone marrow transplantation. Bone Marrow Transplant. 2007;39:751–757. doi: 10.1038/sj.bmt.1705650. [DOI] [PubMed] [Google Scholar]
- 10.Perko R, Kang G, Sunkara A, Leung W, Thomas PG, Dallas MH. Gamma delta T cell reconstitution is associated with fewer infections and improved event-free survival after hematopoietic stem cell transplantation for pediatric leukemia. Biol Blood Marrow Transplant. 2015;21:130–136. doi: 10.1016/j.bbmt.2014.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teschner D, Distler E, Wehler D, et al. Depletion of naive T cells using clinical grade magnetic CD45RA beads: A new approach for GVHD prophylaxis. Bone Marrow Transplant. 2014;49:138–144. doi: 10.1038/bmt.2013.114. [DOI] [PubMed] [Google Scholar]
- 12.Touzot F, Neven B, Dal-Cortivo L, et al. CD45RA depletion in HLA-mismatched allogeneic hematopoietic stem cell transplantation for primary combined immunodeficiency: A preliminary study. J Allergy Clin Immunol. 2015;135:1303–1309. e1301–1303. doi: 10.1016/j.jaci.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 13.Shook DR, Triplett BM, Eldridge PW, Kang G, Srinivasan A, Leung W. Haploidentical stem cell transplantation augmented by CD45RA negative lymphocytes provides rapid engraftment and excellent tolerability. Pediatr Blood Cancer. 2015;62:666–673. doi: 10.1002/pbc.25352. [DOI] [PubMed] [Google Scholar]
- 14.Bleakley M, Heimfeld S, Loeb KR, et al. Outcomes of acute leukemia patients transplanted with naive T cell-depleted stem cell grafts. J Clin Invest. 2015 doi: 10.1172/JCI81229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen BJ, Deoliveira D, Cui X, et al. Inability of memory T cells to induce graft-versus-host disease is a result of an abortive alloresponse. Blood. 2007;109:3115–3123. doi: 10.1182/blood-2006-04-016410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng H, Matte-Martone C, Li H, et al. Effector memory CD4+ T cells mediate graft-versus-leukemia without inducing graft-versus-host disease. Blood. 2008;111:2476–2484. doi: 10.1182/blood-2007-08-109678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng H, Matte-Martone C, Jain D, McNiff J, Shlomchik WD. Central memory CD8+ T cells induce graft-versus-host disease and mediate graft-versus-leukemia. J Immunol. 2009;182:5938–5948. doi: 10.4049/jimmunol.0802212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Triplett BM, Shook DR, Eldridge P, et al. Rapid memory T-cell reconstitution recapitulating CD45RA-depleted haploidentical transplant graft content in patients with hematologic malignancies. Bone Marrow Transplant. 2015;50:1012. doi: 10.1038/bmt.2015.139. [DOI] [PubMed] [Google Scholar]
- 19.Gordon PR, Leimig T, Mueller I, et al. A large-scale method for T cell depletion: Towards graft engineering of mobilized peripheral blood stem cells. Bone Marrow Transplant. 2002;30:69–74. doi: 10.1038/sj.bmt.1703619. [DOI] [PubMed] [Google Scholar]
- 20.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 21.Prentice RL, Kalbfleisch JD, Peterson AV, Jr, Flournoy N, Farewell VT, Breslow NE. The analysis of failure times in the presence of competing risks. Biometrics. 1978;34:541–554. [PubMed] [Google Scholar]
- 22.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Statistics. 1988;16:1141–1154. [Google Scholar]
- 23.Reusser P, Einsele H, Lee J, et al. Randomized multicenter trial of foscarnet versus ganciclovir for preemptive therapy of cytomegalovirus infection after allogeneic stem cell transplantation. Blood. 2002;99:1159–1164. doi: 10.1182/blood.v99.4.1159. [DOI] [PubMed] [Google Scholar]
- 24.Peggs KS, Preiser W, Kottaridis PD, et al. Extended routine polymerase chain reaction surveillance and pre-emptive antiviral therapy for cytomegalovirus after allogeneic transplantation. Br J Haematol. 2000;111:782–790. [PubMed] [Google Scholar]
- 25.Srinivasan A, Klepper C, Sunkara A, et al. Impact of adenoviral stool load on adenoviremia in pediatric hematopoietic stem cell transplant recipients. Pediatr Infect Dis J. 2015;34:562–565. doi: 10.1097/INF.0000000000000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burns LJ, Miller W, Kandaswamy C, et al. Randomized clinical trial of ganciclovir vs acyclovir for prevention of cytomegalovirus antigenemia after allogeneic transplantation. Bone Marrow Transplant. 2002;30:945–951. doi: 10.1038/sj.bmt.1703770. [DOI] [PubMed] [Google Scholar]