Abstract
Epithelial-myoepithelial carcinoma (EMC) is a malignant salivary gland neoplasm comprised of a biphasic arrangement of inner luminal ductal cells and outer myoepithelial cells. Adenoid cystic carcinoma (AdCC) is also a biphasic tumor comprised of ductal and myoepithelial cells, but these components tend to be arranged in a more cribriform pattern. The occurrence of “hybrid carcinomas” that show mixed patterns of EMC and AdCC raises questions about the relationship of these morphologically overlapping but clinically distinct tumors. AdCCs frequently harbor MYB-NFIB gene fusions. Mapping of EMCs (including hybrid forms with an AdCC component) for this fusion could help clarify the true nature of EMC as a distinct entity or simply as some variant form of AdCC.
Twenty-nine cases of EMC were evaluated including 15 classic low-grade EMCs, 7 intermediate-grade EMCs, 2 EMCs with myoepithelial anaplasia, 1 EMC with high-grade transformation and 4 hybrid EMCs with an AdCC component. Break apart fluorescence in situ hybridization (FISH) for MYB was performed, as was MYB immunohistochemistry. For the hybrid carcinomas and those with high-grade transformation, the divergent tumor components were separately analyzed.
A MYB translocation was identified in 5 of 28 (18%) tumors including 3 of 4 (75%) hybrid carcinomas and 2 of 7 (29%) intermediate-grade EMCs. For the positive hybrid carcinomas, the fusion was detected in both the EMC and AdCC components. The MYB fusion was not detected in any of the classic EMCs (0 of 15) or in any of the EMCs with myoepithelial anaplasia (0 of 2) or high-grade transformation (0 of 1). The FISH assay was unsuccessful in 1 case. MYB immunostaining was seen in 5 of 5 fusion-positive cases, and also 9 of 23 fusion-negative tumors.
Classic low-grade EMCs are genetically distinct from AdCCs in that they do not harbor MYB fusions. The presence of a MYB fusion in EMCs showing hybrid features of AdCC or exhibiting highly infiltrative growth points to a subset of these tumors that may well be true AdCCs masquerading as EMCs.
Keywords: epithelial-myoepithelial carcinoma, MYB-NFIB, adenoid cystic carcinoma, hybrid carcinoma
Introduction
Epithelial-myoepithelial carcinoma (EMC) was first described in 1972 by Donath et al.(1) It is histologically characterized by a tightly coupled biphasic arrangement of inner luminal ductal cells and outer myoepithelial cells that often demonstrate cytoplasmic clearing.(2, 3) Classic EMC is a low-grade neoplasm that exhibits bland cellular features and nodular growth with well-circumscribed borders. Since its initial description, the histologic spectrum of EMC has expanded to include variants demonstrating oncocytic/apocrine changes, sebaceous differentiation and myoepithelial cell anaplasia.(4–6) Moreover, it is recognized that EMC can occasionally show features more in keeping with an intermediate-grade or even a high-grade carcinoma, including increased mitotic activity, nuclear atypia, necrosis and a more infiltrative border including perineural or lymphovascular invasion.(5, 7–9)
Like EMC, adenoid cystic carcinoma (AdCC) is a biphasic carcinoma that is comprised of luminal ductal cells and abluminal myoepithelial cells. AdCC is set apart from EMC by its cribriform architecture and highly infiltrative growth, but difficulty in distinguishing these carcinomas can arise, as in those AdCCs with a predominant tubular pattern or those intermediate-grade EMCs with highly infiltrative growth. In some tumors, both components are so well-developed that they are often classified as “hybrid carcinomas.”(10–13) These composite tumors may represent a single entity (i.e., EMC or AdCC) showing divergent differentiation rather than a collision of two distinct tumor types.
Recent studies have shown that an increasingly large group of salivary gland tumors are characterized by specific gene fusions. Indeed, up to 40–65% of adenoid cystic carcinomas (AdCCs) harbor the MYB-NFIB fusion, an alteration that so far appears to be specific for that tumor type.(14–21) These specific genetic alterations are increasingly used as diagnostic adjuncts to help separate salivary gland neoplasms that may share considerable overlap at the morphologic level. Potentially, MYB fusion status could help clarify the relationship of EMC to AdCC, particularly for those EMCs that show some similar morphologic features. The distinction between EMC and AdCC is more than just academic. EMC is relatively indolent, with five-year and ten-year disease-specific survivals of 90–95% and 80–90%, respectively.(5, 22) In contrast, AdCC is infamous for its slow and relentless growth, with a five-year survival of 75–90% but a 15-year survival of as low as 30%.(23–25). The purpose of this study was to determine if MYB fusion status could be useful in distinguishing EMC from AdCC, particularly in those EMCs that deviate from the conventional appearance and take on a more aggressive phenotype.
Methods
Cases
The study was approved by The Johns Hopkins Institutional Review Board (NA0001336). All cases of EMC or cases with hybrid features of EMC and AdCC from 2000 to 2013 with available tissue (unstained slides or tissue block) were retrieved from the surgical pathology archives of The Johns Hopkins Hospital. Demographic information and tumor location were recorded. The cases were reviewed by both study pathologists to confirm the diagnosis, histologic grade, and subtype. The presence and extent of cribriform architecture (i.e., a sieve-like appearance with multiple luminae and/or matrix-filled pseudoluminae within a tumor nest) was noted.
The diagnosis of classic, low-grade EMC was based on the criteria defined by the 2017 WHO classification of head and neck tumors.(2) Specifically, classic EMC was diagnosed for tumors that exhibited a bilayered tumor cell arrangement with eosinophilic ductal cells tightly coupled with polygonal abluminal cells with clear cytoplasm and round nuclei with open chromatin. Classic EMCs demonstrated a nodular, pushing pattern of invasion. EMC was regarded as intermediate-grade if it exhibited the features of classic EMC but with a more infiltrative pattern than typical for EMC including perineural invasion, and an elevated mitotic rate. EMC with high-grade transformation was diagnosed in cases with a component of typical EMC and a component of high-grade adenocarcinoma with necrosis, marked cellular atypia, and a high mitotic rate. The diagnosis of hybrid EMC-AdCC was made in cases with two distinct tumor components, one of which met criteria for EMC and the other of which was diagnostic for AdCC. The diagnosis of an AdCC component was also based on the 2017 WHO classification of head and neck tumors.(2) Specifically, AdCC is highly infiltrative and demonstrates at least focal cribriform growth. AdCC has two cell populations (ductal and myoepithelial), with small, hyperchromatic, angulated nuclei in the myoepithelial cells. A focal cribriform pattern, by itself, in the absence of other histologic features of AdCC (e.g., hyperchromatic, angulated nuclei) was not regarded as an AdCC component.
Fluorescence in situ hybridization
Fluorescence in situ hybridization (FISH) was performed on formalin fixed paraffin embedded sections using a commercially available MYB dual color break apart probe (ZTV-Z-2143-200, ZytoVision, Germany). This probe was designed to detect translocations involving the chromosomal region 6q23.3 harboring the MYB gene. Prior to hybridization the slides were deparaffinized using a VP 2000 processor (Abbott Molecular, Des Plains, IL). The slides and the MYB probe were co-denatured at 80 °C for 7 minutes and allowed to anneal over night at 37 °C in humidified atmosphere. Following incubation the slides were washed in 2 × SSC/0.3% NP-40 for 2 min at 72 °C and for 2 min at room temperature in 2 × SSC. Slides were counterstained with DAPI and a cover slip was applied using Vectashield mounting medium (H-1000, Vector Laboratories, Inc.). Fluorescence microscopy was used to evaluate the probe pattern: cells with two fusion signals of one orange and one green fluorochrome were scored as normal, and considered negative for MYB break-apart (MYB-negative). A signal pattern consisting of one fusion signal and one orange and one green signal at distance from each other indicated one normal 6q23.3 locus and one locus affected by a translocation. Tumors with ≥ 12% cells with split signals were regarded as positive.
Immunohistochemistry
Immunohistochemistry for MYB protein was performed on all available cases using standard manual immunostaining techniques. Briefly, formalin-fixed, paraffin-embedded, 4-micron tissue sections were dried for 2 minutes in a 1200 watt microwave oven, then dried in a rotating oven for 20 minutes. Next, the slides were deparaffinized through immersion in xylene and a series of alcohol solutions. Peroxidase activity was quenched by immersion for 10 minutes in a sodium azide/hydrogen peroxide solution. A Tris-based buffer was used for the MYB (Clone 5E11, Santa Cruz, 1:10 dilution) immunostain with heat induced epitope retrieval using a pressure cooker. The primary antibody was then applied to sections at room temperature for 50 minutes on a rotator. Sections were then incubated with detection complex (Powervision poly HRP anti-mouse for 45 minutes). Finally, slides were counterstained with Surgipath hematoxylin. Slides were then dehydrated, cleared, and mounted with permanent mounting media. Nuclear staining in >10% of tumor cells, was regarded as positive, while staining in >1% but <10% was regarded as focally positive.
Results
The clinical and pathologic findings of the cases are summarized in Table 1. Twenty-nine EMCs were identified from 19 women and 10 men. Eighteen cases were external consultations, and 11 were internal cases. The tumors arose in the parotid gland (n=8), oral cavity (n=6), submandibular gland (n=4), nasal cavity (n=4), maxillary sinus (n=2), oropharynx (n=2), sublingual gland (n=1), epiglottis (n=1) and lung (n=1). Fifteen (52%) were classic EMC (Figure 1), 7 (24%) had intermediate-grade features (Figure 2), 4 (14%) were hybrid carcinomas (with the AdCC component representing 20%, 20%, 40% and 50% of the tumor volume) (Figure 3), 1 (3%) was an EMC with high-grade transformation, with the high-grade carcinoma representing 10% of the tumor volume (Figure 4A), and 2 (7%) exhibited myoepithelial cell anaplasia (Figure 4B).
Table 1.
MYB translocation status for classic and variant forms of epithelial-myoepithelial carcinoma, as well as hybrid epithelial-myoepithelial carcinoma/adenoid cystic carcinomas.
| Age/Sex | Location | EMC type | Cribriform Pattern (%) |
MYB fusion status |
MYB IHC5 |
|
|---|---|---|---|---|---|---|
|
| ||||||
| 1 | 50/F1 | Parotid | Classic | 0 | − | − |
| 2 | 52/F | Submandibular | Classic | 0 | − | − |
| 3 | 60/F | Nasal | Classic | 5 | − | + |
| 4 | 62/F | Parotid | Classic | 0 | − | − |
| 5 | 57/F | Epiglottis | Classic | 0 | − | F+6 |
| 6 | 47/F | Hard palate | Classic | 0 | − | − |
| 7 | 64/F | Lung | Classic | 5 | − | + |
| 8 | 38/F | Parotid | Classic | 10 | failed | − |
| 9 | 74/M2 | Parotid | Classic | 0 | − | − |
| 10 | 59/F | Upper lip | Classic | 5 | − | − |
| 11 | 18/F | Hard palate | Classic | 0 | − | + |
| 12 | 57/M | Parotid | Classic | 0 | − | − |
| 13 | 60/M | Hard palate | Classic | 5 | − | − |
| 14 | 44/F | Sublingual | Classic | 5 | − | − |
| 15 | 46/M | Parotid | Classic | 5 | − | F+ |
|
| ||||||
| 16 | 86/F | Submandibular | High-grade transformation | 5 | − | F+ |
|
| ||||||
| 17 | 65/M | Submandibular | Myoepithelial anaplasia | 0 | − | − |
| 18 | 57/F | Parotid | Myoepithelial anaplasia | 0 | − | F+ |
|
| ||||||
| 19 | 46/F | Base of tongue | Intermediate grade | 5 | − | + |
| 20 | 37/F | Upper lip | Intermediate grade | 10 | + | + |
| 21 | 68/M | Nasal | Intermediate grade | 5 | − | − |
| 22 | 36/F | Nasal | Intermediate grade | 10 | + | F+ |
| 23 | 77/M | Maxillary sinus | Intermediate grade | 5 | − | + |
| 24 | 61/F | Submandibular | Intermediate grade | 0 | − | − |
| 25 | 37/M | Nasal | Intermediate grade | 0 | − | ND7 |
|
| ||||||
| 26 | 60/F | Base of tongue | Hybrid EMC3-AdCC4 | 40 | + | + |
| 27 | 57/F | Tongue | Hybrid EMC-AdCC | 50 | + | + |
| 28 | 82/M | Maxillary sinus | Hybrid EMC-AdCC | 20 | + | + |
| 29 | 69/M | Parotid | Hybrid EMC-AdCC | 20 | − | F+ |
F=female;
M=male;
EMC= epithelial-myoepithelial carcinoma;
AdCC = adenoid cystic carcinoma;
IHC=immunohistochemistry;
F+=focally positive;
ND=not done.
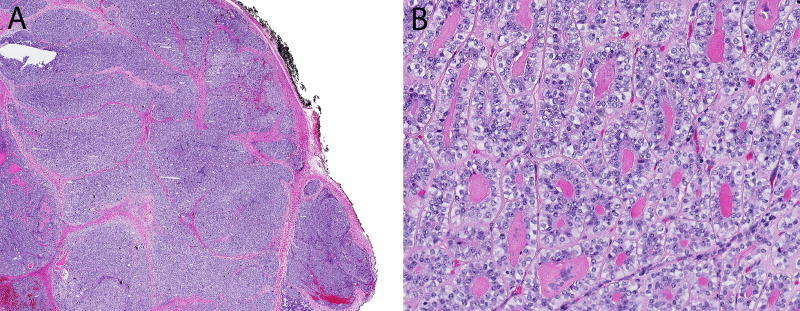
Figure 1.
(A) Classic, low-grade epithelial-myoepithelial carcinoma is relatively well-circumscribed with a nodular tumor edge. (B) Classic epithelial-myoepithelial carcinoma is cytologically bland in both the inner ductal cells and the surrounding rim of clear myoepithelial cells.
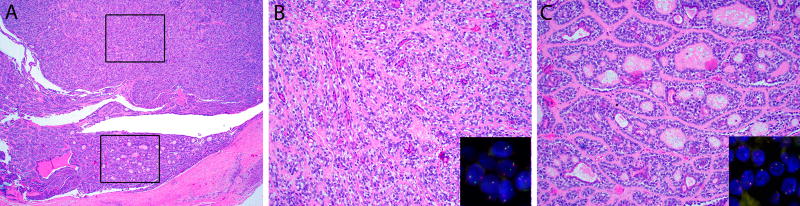
Figure 2.
(A) Occasionally epithelial-myoepithelial carcinoma can show intermediate grade features with prominent perineural invasion, as well as (B) increased cellular atypia and an elevated mitotic rate. Two of 7 cases of intermediate-grade epithelial-myoepithelial carcinoma harbored MYB fusions as demonstrated by split signals on MYB fluorescence in situ hybridization (inset). (C) Both cases of MYB-rearranged epithelial-myoepithelial carcinoma demonstrated focal cribriform “adenoid cystic-like” growth (center).
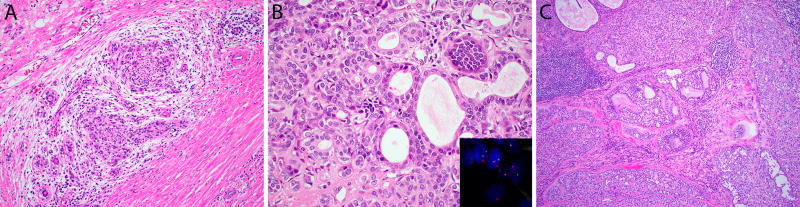
Figure 3.
(A) Hybrid carcinoma with a well-defined component of epithelial-myoepithelial carcinoma (top A, and B) and adenoid cystic carcinoma (bottom A, and C). Three of 4 hybrid carcinomas harbored MYB fusions, which were found in both tumor components (insets).
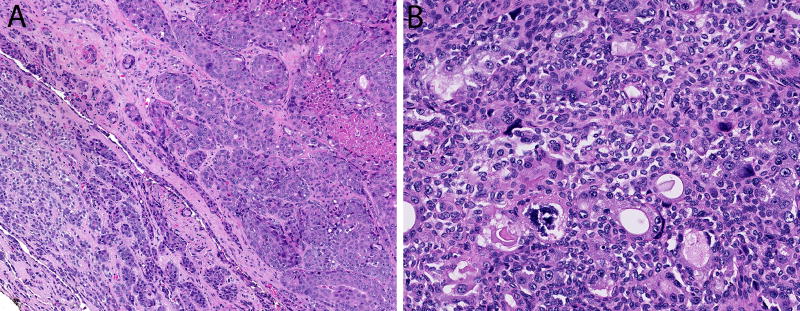
Figure 4.
(A) Epithelial-myoepithelial carcinoma with high-grade transformation has a component of low-grade epithelial-myoepithelial carcinoma (bottom left) and a component of high-grade adenocarcinoma with nuclear pleomorphism and necrosis (top right). (B) Epithelial-myoepithelial carcinomas sometimes exhibit cellular anaplasia of the myoepithelial cell component.
FISH was successful in 28 of 29 (97%) cases. Five (18%) carcinomas demonstrated split signals indicating fusion of the MYB gene. By subtype, 3 of the 4 (75%) hybrid EMC-AdCC carcinomas (Figure 3) and 2 of the 7 (29%) intermediate-grade EMCs harbored the fusion. In the positive intermediate-grade EMCs, the presence of a cribriform architecture was a focal finding (each 10% of tumor volume) (Figure 2). The MYB fusion was not detected in any of the classic EMCs (0 of 15), EMCs with myoepithelial anaplasia (0 of 3), or EMCs with high-grade transformation (0 of 1). For the 4 hybrid carcinomas, the FISH signals were concordant in both the EMC and AdCC components: the EMC and AdCC components were both positive in 3 cases, and both negative in 1 case. Similarly, both the low-grade and high-grade components of the EMC with high-grade transformation demonstrated normal FISH signals.
In effect, the likelihood of finding a MYB gene fusion was statistically much higher for those EMCs that demonstrated AdCC features (i.e. highly infiltrative pattern and/or hybrid morphology) than EMCs without these features (5 of 11 [45%] vs. 0 of 17 [0%], p = 0.0047, Fischer’s exact 2-tailed test).
At least focal cribriform growth was seen in 5 of 5 fusion-positive cases, but also 12 of 23 fusion-negative tumors. The association of some cribriform architecture alone with MYB fusions approached statistical significance (5 of 16 [31%] vs. 0 of 12 [0%], p=0.0525, Fischer’s exact 2-tailed test).
To cross-validate the MYB FISH results, MYB immunohistochemistry was also performed. MYB immunostaining was seen in 15 (6 focal, 9 diffuse) of 28 cases including 5 of 5 fusion-positive cases (100% sensitivity), and also 10 of 22 fusion-negative tumors (45% specificity). Although MYB immunoreactivity was not very specific for the presence of MYB fusions, tumors with MYB immunolabeling were more likely to harbor MYB fusions (5 of 15 [33%] vs. 0 of 12 [0%], p=0.047, Fischer’s exact 2-tailed test).
Discussion
Over the past decade, a growing number of salivary gland tumors have been found to harbor characteristic translocations, including mucoepidermoid carcinoma (CRTC1-MAML2 or CRTC3-MAML2),(26, 27) secretory carcinoma (ETV6-NTRK3),(28) clear cell carcinoma (EWSR1-ATF1)(29), and polymorphous adenocarcinoma (PRKD1-3 partnered with various genes).(30) In addition, up to 40–65% of adenoid cystic carcinomas (AdCCs) of salivary gland and non-salivary sites harbor the MYB-NFIB fusion, an alteration that so far appears to be specific for AdCC.(14–21) In our institutional experience using break apart MYB FISH, 65% of AdCCs harbored MYB fusions.(21)
Epithelial-myoepithelial carcinoma (EMC) is an uncommon salivary gland malignancy that shares morphologic features with AdCC. Both tumors are comprised of a biphasic population of ductal cells surrounded by a rim of myoepithelial cells. There is little data regarding the molecular profile of EMC,(31) and only a handful have been tested for the AdCC-defining MYB fusion. Like our own findings, these previous studies have shown that classic EMCs do not harbor the MYB fusion.(14, 18, 20) The consistent absence of MYB fusions in classic EMC supports the current concept that EMC is genetically distinct from AdCC.
A more problematic and unsettled issue has to do with the true nature of those alleged EMCs that deviate from the low-grade appearance of classic EMC. Some of these tumors show areas that resemble AdCC, even to the point of being regarded as true hybrid carcinomas with mixed EMC and AdCC components. Genetic profiling has been shown to be a valid strategy for discerning the true nature of salivary gland tumors with divergent “hybrid” phenotypes. As one example, Jin et al. used the presence of the EWR1-ATF1 fusion to support the diagnosis of hyalinzing clear cell carcinoma with high-grade transformation in a case that otherwise would have been regarded as a “hybrid clear cell-salivary duct carcinoma.”(33) Similarly, Bundele et al. recently used the presence of MAML2 fusions to highlight mucoepidermoid carcinomas with acinar differentiation that would have otherwise been misclassified as “hybrid acinic cell-mucoepidermoid carcinoma.”(32) Using this same approach, we found that many of those EMCs that demonstrated AdCC-like areas including cribriform architecture and highly infiltrative growth harbored the AdCC-specific MYB fusion. Moreover, the fusion was distributed across all tumor components including the tightly biphasic areas. Based on this finding, hybrid carcinomas and even some EMCs with focal cribriforming and/or highly infiltrative growth may actually represent true AdCCs.
Although the finding of MYB-rearrangement in a hybrid EMC-AdCC may not be entirely unexpected, its presence in non-hybrid EMCs is diagnostically problematic and highlights the tenuous line that separates some EMCs from AdCCs. Although these 2 cases did not demonstrate classic features of AdCC, they did demonstrate more infiltrative growth than typical for AdCC as well as focal cribriform “AdCC-like” areas. Although the sample size is small, these cases suggest that distinguishing intermediate-grade EMC and AdCC is not straightforward and that the features used to make the distinction may need to be reassessed. Focal cribriform architecture may not by itself exclude the diagnosis of EMC, but its presence in an intermediate-grade, highly infiltrative salivary carcinoma should raise suspicion for AdCC no matter how “EMC-like” the tumor may appear. In this scenario, the demonstration of MYB fusion could be essential in confirming a definite diagnosis of AdCC. Reliance on MYB immunostaining alone is not advised, as it suffers from suboptimal specificity (45% in this study, similar to other published reports).(18, 33)
In conclusion, classic, low-grade EMC do not harbor the same MYB fusion that characterizes many AdCCs. At the same time, the presence of the MYB fusion in intermediate-grade and hybrid carcinomas that at least partially exhibit an EMC phenotype may in fact point to an AdCC. The traditional histologic approach to separating EMC and AdCC may be unreliable when dealing with these intermediate-grade salivary carcinomas, and MYB FISH may be useful for facilitating the distinction.
Acknowledgments
Funding: This work was funded in part by the National Institute of Dental and Craniofacial Research (R01 DE013152-11).
References
- 1.Donath K, Seifert G, Schmitz R. Diagnosis and ultrastructure of the tubular carcinoma of salivary gland ducts. Epithelial-myoepithelial carcinoma of the intercalated ducts. Virchows Arch A Pathol Pathol Anat. 1972;356:16–31. [PubMed] [Google Scholar]
- 2.Seethala R, Bell D, Fonseca I, et al. Epithelial-myoepithelial carcinoma. In: el-Naggar AK, Chan JKC, Grandis JR, et al., editors. WHO Classification of Head and Neck Tumours. Lyon, France: IARC Press; 2017. pp. 175–176. [Google Scholar]
- 3.Ellis GLA, P L. AFIP atlas of tumor pathology: tumors of the salivary glands. Washington, D.C.: ARP Press; 2008. Epithelial-myoepithelial carcinoma; pp. 309–322. [Google Scholar]
- 4.Seethala RR, Richmond JA, Hoschar AP, et al. New variants of epithelial-myoepithelial carcinoma: oncocytic-sebaceous and apocrine. Arch Pathol Lab Med. 2009;133:950–959. doi: 10.5858/133.6.950. [DOI] [PubMed] [Google Scholar]
- 5.Seethala RR, Barnes EL, Hunt JL. Epithelial-myoepithelial carcinoma: a review of the clinicopathologic spectrum and immunophenotypic characteristics in 61 tumors of the salivary glands and upper aerodigestive tract. Am J Surg Pathol. 2007;31:44–57. doi: 10.1097/01.pas.0000213314.74423.d8. [DOI] [PubMed] [Google Scholar]
- 6.Seethala RR. Oncocytic and apocrine epithelial myoepithelial carcinoma: novel variants of a challenging tumor. Head Neck Pathol. 2013;7(Suppl 1):S77–84. doi: 10.1007/s12105-013-0461-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lima FJ, Porto DE, Cavalcante JR, et al. Epithelial-myoepithelial carcinoma of high grade transformation: the case report in the buccal mucosa. Open Dent J. 2012;6:111–117. doi: 10.2174/1874210601206010111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang S, Chen X. Epithelial-myoepithelial carcinoma with high grade transformation. Int J Oral Maxillofac Surg. 2012;41:810–813. doi: 10.1016/j.ijom.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 9.Roy P, Bullock MJ, Perez-Ordonez B, et al. Epithelial-myoepithelial carcinoma with high grade transformation. Am J Surg Pathol. 2010;34:1258–1265. doi: 10.1097/PAS.0b013e3181e366d2. [DOI] [PubMed] [Google Scholar]
- 10.Woo JS, Kwon SY, Jung KY, et al. A hybrid carcinoma of epithelial-myoepithelial carcinoma and adenoid cystic carcinoma in maxillary sinus. J Korean Med Sci. 2004;19:462–465. doi: 10.3346/jkms.2004.19.3.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grenko RT, Abendroth CS, Davis AT, et al. Hybrid tumors or salivary gland tumors sharing common differentiation pathways? Reexamining adenoid cystic and epithelial-myoepithelial carcinomas. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;86:188–195. doi: 10.1016/s1079-2104(98)90124-x. [DOI] [PubMed] [Google Scholar]
- 12.Mosqueda-Taylor A, Cano-Valdez AM, Ruiz-Gonzalez JD, et al. Hybrid salivary gland tumor of the upper lip or just an adenoid cystic carcinoma? Case report. Med Oral Patol Oral Cir Bucal. 2010;15:e43–47. doi: 10.4317/medoral.15.e43. [DOI] [PubMed] [Google Scholar]
- 13.Croitoru CM, Suarez PA, Luna MA. Hybrid carcinomas of salivary glands. Report of 4 cases and review of the literature. Arch Pathol Lab Med. 1999;123:698–702. doi: 10.5858/1999-123-0698-HCOSG. [DOI] [PubMed] [Google Scholar]
- 14.Brill LB, 2nd, Kanner WA, Fehr A, et al. Analysis of MYB expression and MYB-NFIB gene fusions in adenoid cystic carcinoma and other salivary neoplasms. Mod Pathol. 2011;24:1169–1176. doi: 10.1038/modpathol.2011.86. [DOI] [PubMed] [Google Scholar]
- 15.Fehr A, Kovacs A, Loning T, et al. The MYB-NFIB gene fusion-a novel genetic link between adenoid cystic carcinoma and dermal cylindroma. J Pathol. 2011;224:322–327. doi: 10.1002/path.2909. [DOI] [PubMed] [Google Scholar]
- 16.Persson M, Andren Y, Mark J, et al. Recurrent fusion of MYB and NFIB transcription factor genes in carcinomas of the breast and head and neck. Proc Natl Acad Sci U S A. 2009;106:18740–18744. doi: 10.1073/pnas.0909114106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Persson M, Andren Y, Moskaluk CA, et al. Clinically significant copy number alterations and complex rearrangements of MYB and NFIB in head and neck adenoid cystic carcinoma. Genes Chromosomes Cancer. 2012;51:805–817. doi: 10.1002/gcc.21965. [DOI] [PubMed] [Google Scholar]
- 18.Mitani Y, Li J, Rao PH, et al. Comprehensive analysis of the MYB-NFIB gene fusion in salivary adenoid cystic carcinoma: Incidence, variability, and clinicopathologic significance. Clin Cancer Res. 2010;16:4722–4731. doi: 10.1158/1078-0432.CCR-10-0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitani Y, Rao PH, Futreal PA, et al. Novel chromosomal rearrangements and break points at the t(6;9) in salivary adenoid cystic carcinoma: association with MYB-NFIB chimeric fusion, MYB expression, and clinical outcome. Clin Cancer Res. 2011;17:7003–7014. doi: 10.1158/1078-0432.CCR-11-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.West RB, Kong C, Clarke N, et al. MYB expression and translocation in adenoid cystic carcinomas and other salivary gland tumors with clinicopathologic correlation. Am J Surg Pathol. 2011;35:92–99. doi: 10.1097/PAS.0b013e3182002777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rettig EM, Tan M, Ling S, et al. MYB rearrangement and clinicopathologic characteristics in head and neck adenoid cystic carcinoma. Laryngoscope. 2015;125:E292–299. doi: 10.1002/lary.25356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vazquez A, Patel TD, D'Aguillo CM, et al. Epithelial-Myoepithelial Carcinoma of the Salivary Glands: An Analysis of 246 Cases. Otolaryngol Head Neck Surg. 2015;153:569–574. doi: 10.1177/0194599815594788. [DOI] [PubMed] [Google Scholar]
- 23.van Weert S, Bloemena E, van der Waal I, et al. Adenoid cystic carcinoma of the head and neck: a single-center analysis of 105 consecutive cases over a 30-year period. Oral Oncol. 2013;49:824–829. doi: 10.1016/j.oraloncology.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 24.Amit M, Binenbaum Y, Sharma K, et al. Analysis of failure in patients with adenoid cystic carcinoma of the head and neck. An international collaborative study. Head Neck. 2014;36:998–1004. doi: 10.1002/hed.23405. [DOI] [PubMed] [Google Scholar]
- 25.Ellington CL, Goodman M, Kono SA, et al. Adenoid cystic carcinoma of the head and neck: Incidence and survival trends based on 1973–2007 Surveillance, Epidemiology, and End Results data. Cancer. 2012;118:4444–4451. doi: 10.1002/cncr.27408. [DOI] [PubMed] [Google Scholar]
- 26.Behboudi A, Enlund F, Winnes M, et al. Molecular classification of mucoepidermoid carcinomas-prognostic significance of the MECT1-MAML2 fusion oncogene. Genes, chromosomes & cancer. 2006;45:470–481. doi: 10.1002/gcc.20306. [DOI] [PubMed] [Google Scholar]
- 27.Seethala RR, Dacic S, Cieply K, et al. A reappraisal of the MECT1/MAML2 translocation in salivary mucoepidermoid carcinomas. Am J Surg Pathol. 2010;34:1106–1121. doi: 10.1097/PAS.0b013e3181de3021. [DOI] [PubMed] [Google Scholar]
- 28.Skalova A, Vanecek T, Sima R, et al. Mammary analogue secretory carcinoma of salivary glands, containing the ETV6-NTRK3 fusion gene: a hitherto undescribed salivary gland tumor entity. Am J Surg Pathol. 2010;34:599–608. doi: 10.1097/PAS.0b013e3181d9efcc. [DOI] [PubMed] [Google Scholar]
- 29.Antonescu CR, Katabi N, Zhang L, et al. EWSR1-ATF1 fusion is a novel and consistent finding in hyalinizing clear-cell carcinoma of salivary gland. Genes Chromosomes Cancer. 2011;50:559–570. doi: 10.1002/gcc.20881. [DOI] [PubMed] [Google Scholar]
- 30.Weinreb I, Zhang L, Tirunagari LM, et al. Novel PRKD gene rearrangements and variant fusions in cribriform adenocarcinoma of salivary gland origin. Genes Chromosomes Cancer. 2014;53:845–856. doi: 10.1002/gcc.22195. [DOI] [PubMed] [Google Scholar]
- 31.Chiosea SI, Miller M, Seethala RR. HRAS mutations in epithelial-myoepithelial carcinoma. Head Neck Pathol. 2014;8:146–150. doi: 10.1007/s12105-013-0506-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bundele MM, Weinreb I, Xu B, et al. Mucoacinar Carcinoma: A Rare Intercalated Duct/Acinar Variant of Mucoepidermoid Carcinoma, Hybrid Tumor, or Distinct Entity? Modern Pathology. 2017;30:322A. (Abstract) [Google Scholar]
- 33.Xu B, Drill E, Ho A, et al. Predictors of Outcome in Adenoid Cystic Carcinoma of Salivary Glands: A Clinicopathologic Study With Correlation Between MYB Fusion and Protein Expression. Am J Surg Pathol. 2017;41:1422–1432. doi: 10.1097/PAS.0000000000000918. [DOI] [PMC free article] [PubMed] [Google Scholar]