Abstract
Background
The Women’s Health Initiative (WHI) Life and Longevity after Cancer (LILAC) study offers an important opportunity to advance cancer research by extending the original WHI studies to examine survivorship in women diagnosed with cancer during their participation in WHI.
Methods
The goals of LILAC are to: 1) obtain cancer treatment information and long-term cancer outcomes for women diagnosed with one of eight selected cancers (breast, endometrial, ovarian, lung, and colorectal cancers, melanoma, lymphoma and leukemia); 2) augment the existing WHI biorepository with fixed tumor tissue from the solid tumor sites for cancers diagnosed since 2002; and 3) develop, refine and validate methods to use administrative data to capture treatment and recurrence data. Methods for accomplishing these goals are described, as are results from the initial LILAC participant survey.
Results
A total of 9,934 WHI participants living with cancer were eligible for LILAC participation, of which 78% (n=7,760) agreed to participate. Among the three most prevalent cancer types, 54% are breast cancer survivors, 11% are melanoma survivors, and 10% are survivors of colorectal cancer.
Conclusions
In addition to describing this resource, we present pertinent lessons that may assist other investigators interested in embedding survivorship research into existing large epidemiologic cohorts.
Impact
The LILAC resource offers a valuable opportunity for researchers to study cancer survivorship and issues pertinent to cancer survivors in future studies.
Introduction
Improvements in early detection and treatment have led to a surge in the number of cancer survivors in the US [1] creating a demand for a better understanding of their many, and sometimes unique, health concerns. These needs, recognized by both cancer researchers and cancer care delivery leaders, require new focused research. Cancer survivor health concerns are diverse and complex—arising from heterogeneity among individuals, the cancer with which they have been diagnosed, its treatment and ensuring effects on a wide range of health and social conditions. To effectively examine these concerns in a rigorous fashion, significant new resources need to be developed, including a much more comprehensive assessment on a large sample with longer-term follow-up than is typically available in cancer clinical trials. Such resources are expensive to develop and take considerable time to mature when created.
The Women’s Health Initiative (WHI), initiated in 1993, is a mature cohort with clinical trial and observational study components. Its original goal was to evaluate approaches to prevent heart disease, cancer and osteoporotic fractures in 161,000 post-menopausal women [2, 3]. With the clinical trials concluded nearly 10 years ago, the WHI cohort continues to be a resource to study issues related to aging and health, including cancer. Over 30,000 cancer diagnoses have been confirmed within this cohort, making the WHI a valuable platform in which to study cancer survivorship in older women.
In this report, we describe our efforts to accelerate survivorship research by creating the WHI Cancer Survivor Cohort, called LILAC, for Life and Longevity After Cancer, within the parent study. By building on this large, very well characterized epidemiologic cohort with high-quality follow-up for a broad range of health outcomes and health related conditions, LILAC provides timely opportunities to examine many of the important questions regarding aging women who are living with cancer. Herein we describe the design and implementation of LILAC and discuss both strengths and potential limitations of embedding survivorship research within an existing epidemiologic cohort.
Materials and Methods
Study Objectives
The LILAC survivorship cohort was developed to : 1) collect information on cancer treatment and outcomes in women diagnosed during their WHI participation with eight selected cancers (breast, colorectal, endometrial, lung, ovarian cancers, and melanoma, non-Hodgkin lymphoma, and leukemia), using surveys, Medicare linkage, and direct medical record abstraction; 2) augment the WHI biorepository with formalin-fixed paraffin-embedded (FFPE) tumor tissue from WHI participants diagnosed with selected solid tumors (invasive breast, colorectal, endometrial, lung and ovarian cancers, and in situ and invasive melanoma of the skin); and 3) conduct methodological research testing the ability of electronic administrative data to give reliable estimates of treatment and recurrence. These aforementioned cancers were selected because they represent the most prevalent cancers in the WHI and/or cancers where survivorship research is lacking, and offers the opportunity to create new knowledge. This study was conducted within the Belmont Report recognized ethical guidelines. Written informed consent was obtained from all participants and this study was approved by each institution’s institutional review board (IRB).
Women’s Health Initiative
Overview
The design and implementation of WHI have been published [2, 3]. Briefly, WHI was launched in 1993, with sponsorship from the NIH, to study the risk factors and prevention of the major causes of morbidity and mortality in postmenopausal women including cancer. The WHI included randomized clinical trials testing three primary chronic disease prevention interventions: hormone therapy (HT), a low-fat eating pattern (Dietary Modification or DM) and calcium and vitamin D (CaD) supplements. In all, 68,132 women were randomized into one or more of the clinical trial (CT) components and an additional 93,676 women enrolled into a parallel observational study (OS) with similar data collection procedures (Figure 1) [2, 3].
Figure 1.
Eligibility and participant flow from the main WHI into LILAC. Describes the recruitment and retention of participants from the main WHI study into the LILAC cancer survivorship study.
LILAC Organization
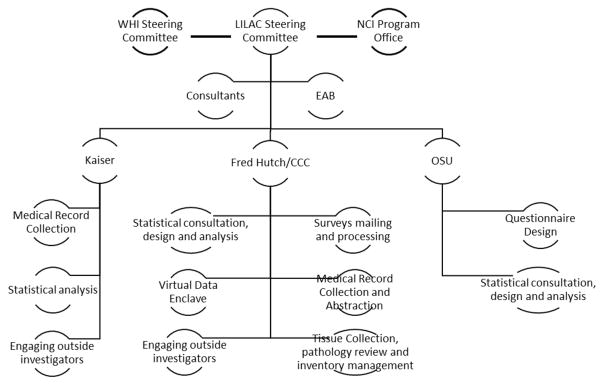
The LILAC database resides at the WHI Clinical Coordinating Center (CCC) at the Fred Hutchinson Cancer Research Center (FHCRC) in Seattle, buts its leadership is spread across three sites from WHI investigators at Kaiser-Permanente Northern California (KPNC) in Oakland and the Ohio State University (OSU) in Columbus. Each site is responsible for specific LILAC functions as well as scientific direction and statistical analyses (Figure 2). The CCC staff conducts all mail-based data collection from participants including informed consent, medical release of information, and annual questionnaires to survivors, performs regular linkages to Medicare and NDI and manages all WHI and LILAC data. The CCC also requests, collects, processes, and stores tumor tissues and performs a confirmatory pathology review. KPNC supervises the WHI field centers in their collection of medical records. The OSU team oversees the development and analyses of the survivorship surveys.
Figure 2.
Organizational structure of the LILAC cohort. Describes the organization of the participating LILAC institutions as well as their responsibilities and duties for the cohort.
Eligibility, recruitment and data collection
Participants with no cancer (other than non-melanoma skin cancer) prior to enrollment in WHI and a confirmed invasive cancer diagnosis during WHI follow-up of one of the eight selected cancers are eligible for LILAC. Because of the close relationship between ovarian, fallopian tube and primary peritoneal cancer as well as the need for larger numbers, hereafter all three cancer sites are defined as EOC for LILAC purposes. Women with diagnoses of in situ melanoma (n=340) were recently added under supplemental funding and are included here for completeness.
Beginning in 2013, all LILAC eligible participants still in active WHI follow-up were sent an invitational mailing including a personalized letter of introduction listing their previously reported qualifying cancer and year of diagnosis, two copies of the consent form, a medical release form, an eight-page LILAC enrollment questionnaire and a self-addressed business-reply return envelope. Invitation mailings were sent by first class mail. Outgoing envelopes carried the WHI logo to ensure study recognition. All questionnaires were printed in optical mark recognition (bubble) format and large font, consistent with WHI practices. The mailings followed the established WHI follow-up protocol with questionnaires returned to CCC for processing. Non-respondents to the first mailing were sent a second mailing after eight weeks. Women who did not respond to these invitations or who indicated they did not want to participate were excluded from further active LILAC data collection. In each subsequent year, WHI participants with newly diagnosed cancers included in LILAC have been approached using the same protocol. Contact information within the WHI database, is updated routinely based on mail forwarding orders, proxy contacts, and self-reports of address changes.
LILAC Data collection
Initial LILAC Survivorship Survey
The first LILAC questionnaire asked about initial cancer treatment including chemotherapy, hormonal/endocrine therapy, radiation therapy, and biological therapies, including type, start dates and providers of these treatments; and reports of new cancer events related to their primary cancer diagnosis. Information about type(s) of surgical procedures was not collected in since this information was available from previously collected operative and pathology reports used to confirm the diagnoses initially. These reports included the date of the first resection procedure, along with the extent of site-specific tissue that was removed, and relevant ancillary procedures such as lymph node dissection. Details regarding specifics of medical record information abstracted for each cancer can be found online at https://www.whi.org/studies/LILAC/Abstraction%20Forms/Forms/AllItems.aspx. Reports of longer-term disabling conditions or effects on quality-of-life common to many cancers or their treatments but not covered by WHI questionnaires (e.g., lymphedema, cardiotoxicity, nephrotoxicity, neurotoxicity), symptoms after treatment completion, pain, depression, anxiety, fatigue, distress, social support, weight and marital status, and questions on insurance coverage were also asked. These items were selected based on their relevance to cancer treatment and outcomes and for their complementarity to those collected in ongoing WHI data collection.
Annual Survivorship Surveys
Each year after enrollment, a questionnaire is mailed to all participants who completed the LILAC baseline questionnaire. This form requests updates on new cancer events and cancer treatments. A small number of additional questions are included each year to support newly funded ancillary studies or other emerging topics related to survivorship. Measures collected in the first annual questionnaire included: current weight; weight at first cancer diagnosis; weight two years after cancer diagnosis; intentional weight loss after cancer diagnosis; current or ever use of selected medications (NSAIDs, statins, metformin, ACE inhibitors, anti-depressants and bisphosphonates); financial toxicity [4]; cancer worry [5];social networks and social support; peripheral neuropathy [6]; participation in cancer support groups’, or online peer support groups; cognitive functioning [7]; physical functioning [8]; exercise [8] body image; symptoms within the past 4 weeks; selected nutrition/diet; and lymphedema [8–11].
Additional measures collected in the second annual follow-up questionnaire included: depression, anxiety, fatigue and distress; and unmet needs of cancer survivors (e.g., pain, physical functioning, memory/concentration, weight changes, end of life planning, etc.).
Cancer Treatment and Outcomes Information
Details of cancer treatment and outcomes are derived from three main sources: Medicare data from the Center for Medicare and Medicaid Services (CMS), direct medical record abstraction, and self-reported data from cancer survivors. Data from these sources vary considerably in terms of the level of detail, timeliness and specificity. Because of the age of the WHI population, LILAC uses Medicare for patients who were diagnosed at age 65 or older to allow inexpensive and efficient collection of information from historical cases. For WHI participants not covered by Medicare, LILAC collects medical records and abstracts selected data items, similar to those collected for those with Medicare, to support analyses that use data from both sources. Analyses may need to be restricted to cases documented through a specific source for certain analyses (e.g., cost analyses will be restricted to the Medicare population, analyses requiring molecular testing results will be done among those with medical records). Documentation of cause and date of death comes from either death certificates, medical records, or National Death Index data.
Use of Medicare Claims to Assess Cancer Treatment and Outcome
The CCC has obtained Medicare files from 1991 through the present [12] allowing us to extract diagnosis, treatment and procedure codes for cancers occurring in women enrolled in fee-for service Medicare at the time of diagnosis (53%) throughout the entire WHI follow-up period.
Medical Records Collection
The goal of LILAC medical records collection is to document the initial course of cancer treatment and identify the first recurrence among women whose information was unobtainable through Medicare (i.e., were not in Medicare at the time of their cancer diagnosis or who received their Medicare benefits through a managed care organization). For WHI participants who died prior to the LILAC baseline, medical records are requested under an IRB-approved partial waiver of consent. Because of the difficulty in obtaining historical records, we are requesting medical records only for those cancers diagnosed since 2000. For the 6,853 eligible cases, we request medical records using the WHI approach for clinical outcomes documentation. A random sample of cases (approximately 125 for each of the targeted tumor sites) from the Medicare linkage set will also be requested to serve to validate claims data on treatment and recurrence data.
Medical Records Review and Coding
Final abstraction, review and coding cancer treatment and recurrence occurs at the CCC. Medical records offer the opportunity to extract many more details than claims data but given LILAC’s heavy reliance on Medicare and the cost of manual abstraction, we limit LILAC abstractions to first course of treatment and evidence of recurrence or last known disease-free time point. We abstract type, extent and dates of initial surgery, molecular testing (indications and results); chemotherapy (recommendations, agents, and timing); radiation therapy (recommendations, modality, timing and total dose); hormonal therapy (regimen, dates, changes over time and reasons for changes), and other selected medical interventions specific to each cancer (e.g., oophorectomy, breast reconstruction, use of bisphosphonates). Site and date of new or recurrent cancer is recorded. The records are maintained centrally so that additional information could be abstracted for specific hypotheses.
When multiple sources are available, we will assume medical records are the most accurate, followed by Medicare and self-report, unless our validation studies and other investigations suggest otherwise. Analyses may be restricted to a specific subpopulation (e.g., cost analyses restricted to the Medicare population in analyses looking at recommended versus receipt of chemotherapy limited to medical records subset).
Tumor Tissue Collection
LILAC collects FFPE tumor tissue from six solid tumor sites (breast, colorectal, endometrial, lung, ovary, skin melanoma). Recognizing the College of American Pathologists minimum standards for retention of diagnostic specimens is 10 years [13] and that many facilities would consider destroying tissue samples after that time, we have limited our requests at the time the study in 2013 was initiated for tissue from cases diagnosed in 2002 or later.
Requesting tumor tissue
Once a release form is received (or under a waiver of consent for decedents), a CCC data coordinator reviews the pathology report in the existing WHI records to determine which specimens (i.e., biopsies, excisions, or more extensive surgical procedures) should be requested and from what institution. Tumors identified for request are greater than 0.3 cm in at least one dimension and atypical histologies are excluded.
To reduce the burden to large institutions, requests for tissues for multiple cases are batched and mailed quarterly. Lack of response within eight weeks generates a repeat request and follow-up phone contact with hospital/laboratory personnel. To address potential concerns about access to specimens for continuing care, we assure the return of any specimen to any location in the US within 48 hours if the specimen is needed for clinical care. For facilities that will not release multiple tumor blocks, we ask for, in order of preference, a single representative block, 10 unstained slides (5 um each), and finally two 0.6–2 mm punches (to be re-embedded in paraffin locally for storage).
Tumor Tissue Review and Storage
Upon receipt of a FFPE tissue specimen, it is logged into the WHI repository database, and labeled with a unique WHI specimen ID barcode. They are batched and sent to the FHCRC Specialized Pathology Shared Resource, accompanied by an electronic transmittal sheet with diagnostic information from the WHI database to be verified and triaged by pathologist single staff pathologist to determine general characteristics of the sample and concordance with the previously collected diagnostic information where applicable. For each block the pathologist 1) confirms the presence or absence of tumor and normal structures; 2) estimates the two dimensional area of the entire tissue block; 3) estimates the amount of available tumor and normal epithelial and non-epithelial structures as a percentage of the area of the tissue section; and 4) ranks the tissue blocks based on the amount of representative tumor. The supervising pathologist performs the secondary pathology review, resolves any discrepancies, and signs off on the pathology review for each case. Disagreements between the pathologists or with the original diagnosis require additional discussion or investigation.
Statistical Considerations
The LILAC study population can be used to support diverse survival and survivorship analyses but the study population will vary with each analysis, based on data availability. Cancer survival can be examined among all WHI participants diagnosed with a specific cancer, since vital status information is nearly complete for the WHI participants. For analyses needing treatment information, several options are available, including pooling data across all sources or restricting analyses to one data source that best supports the study aims. For studies examining participant reported outcomes available only through the LILAC questionnaires, the selection bias associated with survival until the start of LILAC activities is a necessary consideration. To characterize potential selection bias inherent in this design, we provide the WHI baseline characteristics of three groups: all WHI participants with an eligible diagnosis of one of the eight LILAC cancers; all who were eligible for the LILAC enrollment mailing (alive and remaining in active WHI follow-up until LILAC initiation) and finally those who consented to LILAC.
The distribution of treatment, symptom, and quality of life data from the initial LILAC survey were described using contingency tables for categorical variables and mean and standard deviations for continuous variables. Pearson chi-squared tests and two sample t-tests were used to compare the demographic characteristics of LILAC participants to other WHI participants diagnosed with cancer but not enrolled in LILAC to assess representativeness of the LILAC sample.
Accounting for selection bias
Given the retrospective aspects of the LILAC design, it is not surprising that we observe some modest differences in clinical and demographic factors between women participating in the LILAC survey and the other WHI participants diagnosed with cancer (Supplemental Tables 1 and 2). Analyses restricted to this subset of women (e.g., those who are interested in patient reported outcomes assessed only through the LILAC survey) must address the inherent selection bias in this sample. At least two approaches may be used to address the selection bias. For some analysis, it may be reasonable to simply condition on survival to a selected time point. In some analyses, it will be important to use Inverse Probability Weighting (IPW) [14]. In this approach, the probability of inclusion in LILAC is modeled using, as predictors, factors with meaningful differences between LILAC participants and other cancer participants in the WHI. The inverse of these estimated inclusion probabilities is then incorporated as weights in regression models examining relationships between patient outcomes and exposures of interest. When implemented properly, IPW minimizes selection biases by creating a hypothetical population in which the effect of an exposure on an outcome is the same as in the original population [14].
Results
LILAC Participants
As of September 15, 2015, 30,306 incident cancers had been documented among the 161,808 original WHI participants, including 20,784 women with one or more of the designated eight LILAC cancer sites. Of these, 9,934 (48%) participants were still alive and in active WHI follow-up as of the initiation of LILAC in 2013. The remaining had died (n=6,864) or were no longer active in the WHI (n=1,121). After two mailings, 7,760 (78%) of these women consented to LILAC survey participation, including 4,211 breast cancer survivors, 796 colorectal cancer survivors, 406 lung cancer survivors, 663 endometrial cancer survivors, 451 Non-Hodgkin lymphoma survivors, 186 leukemia survivors, 834 participants with melanoma and 213 women diagnosed with EOC cancers (Table 1). A total of 7,760 women consented to participate in the study, but 109 women did not return the baseline survey, leaving 7,651 cancer survivors included in the remaining analyses of the survey data.
Table 1.
Baseline Characteristics and LILAC participation status of WHI women with LILAC designated cancers reported as of September 2015, by cancer site
| Total | Breast | Colorectal | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||||
| All1 | Eligible2 | Consented3 | All1 | Eligible2 | Consented3 | All1 | Eligible2 | Consented3 | ||||||||||
| N | N (%) | N (%) | % of All | N | N (%) | N (%) | % of All | N | N (%) | N (%) | % of All | |||||||
|
| ||||||||||||||||||
| Total | 20784 | 9934 | (48) | 7760 | (78) | 37% | 9305 | 5251 | (56) | 4211 | (80) | 45% | 2499 | 1039 | (42) | 796 | (77) | 32% |
| Age at WHI baseline | ||||||||||||||||||
| 50–59 | 6180 | 3944 | (64) | 3248 | (82) | 53% | 3088 | 2163 | (70) | 1828 | (85) | 59% | 516 | 288 | (56) | 224 | (78) | 43% |
| 60–69 | 9969 | 4780 | (48) | 3697 | (77) | 37% | 4357 | 2481 | (57) | 1959 | (79) | 45% | 1198 | 560 | (47) | 451 | (81) | 38% |
| 70–79 | 4635 | 1210 | (26) | 815 | (67) | 18% | 1860 | 607 | (33) | 424 | (70) | 23% | 785 | 191 | (24) | 121 | (63) | 15% |
| Race | ||||||||||||||||||
| White | 18455 | 8981 | (49) | 7074 | (79) | 38% | 8141 | 4658 | (57) | 3787 | (81) | 47% | 2123 | 907 | (43) | 699 | (77) | 33% |
| Black | 1228 | 455 | (37) | 313 | (69) | 25% | 620 | 297 | (48) | 203 | (68) | 33% | 217 | 63 | (29) | 42 | (67) | 19% |
| Hispanic | 425 | 183 | (43) | 137 | (75) | 32% | 214 | 114 | (53) | 89 | (78) | 42% | 63 | 22 | (35) | 17 | (77) | 27% |
| Other | 676 | 315 | (47) | 236 | (75) | 35% | 330 | 182 | (55) | 132 | (73) | 40% | 96 | 47 | (49) | 38 | (81) | 40% |
| Marital Status | ||||||||||||||||||
| Married /Living as | 13204 | 6816 | (52) | 5395 | (79) | 41% | 6020 | 3610 | (60) | 2930 | (81) | 49% | 1460 | 660 | (45) | 522 | (79) | 36% |
| Previously Married | 6530 | 2609 | (40) | 1975 | (76) | 30% | 2769 | 1346 | (49) | 1048 | (78) | 38% | 916 | 334 | (36) | 240 | (72) | 26% |
| Never married | 983 | 478 | (49) | 369 | (77) | 38% | 485 | 279 | (58) | 221 | (79) | 46% | 114 | 41 | (36) | 31 | (76) | 27% |
| Education | ||||||||||||||||||
| ≤High School | 4047 | 1604 | (40) | 1158 | (72) | 29% | 1696 | 821 | (48) | 617 | (75) | 36% | 580 | 206 | (36) | 153 | (74) | 26% |
| Some College | 7673 | 3407 | (44) | 2614 | (77) | 34% | 3400 | 1794 | (53) | 1414 | (79) | 42% | 988 | 386 | (39) | 295 | (76) | 30% |
| College grad | 2522 | 1334 | (53) | 1069 | (80) | 42% | 1145 | 701 | (61) | 568 | (81) | 50% | 265 | 127 | (48) | 99 | (78) | 37% |
| Post grad | 6410 | 3526 | (55) | 2872 | (81) | 45% | 3005 | 1905 | (63) | 1589 | (83) | 53% | 653 | 315 | (48) | 246 | (78) | 38% |
| Smoking | ||||||||||||||||||
| Never | 9274 | 4773 | (51) | 3737 | (78) | 40% | 4478 | 2544 | (57) | 2026 | (80) | 45% | 1198 | 519 | (43) | 406 | (78) | 34% |
| Former | 9473 | 4479 | (47) | 3492 | (78) | 37% | 4134 | 2362 | (57) | 1911 | (81) | 46% | 1070 | 441 | (41) | 327 | (74) | 31% |
| Current | 1777 | 578 | (33) | 443 | (77) | 25% | 574 | 288 | (50) | 223 | (77) | 39% | 192 | 62 | (32) | 50 | (81) | 26% |
| BMI | ||||||||||||||||||
| ≤24.9 | 7179 | 3587 | (50) | 2872 | (80) | 40% | 3067 | 1818 | (59) | 1500 | (83) | 49% | 796 | 353 | (44) | 270 | (76) | 34% |
| 25–29.9 | 7078 | 3389 | (48) | 2631 | (78) | 37% | 3207 | 1820 | (57) | 1444 | (79) | 45% | 856 | 354 | (41) | 266 | (75) | 31% |
| 30–34.9 | 3873 | 1753 | (45) | 1345 | (77) | 35% | 1809 | 955 | (53) | 753 | (79) | 42% | 501 | 211 | (42) | 168 | (80) | 34% |
| 35+ | 2463 | 1116 | (45) | 835 | (75) | 34% | 1143 | 612 | (54) | 475 | (78) | 42% | 326 | 116 | (36) | 87 | (75) | 27% |
| History of Diabetes4 | 1129 | 357 | (32) | 236 | (66) | 21% | 466 | 184 | (39) | 130 | (71) | 28% | 195 | 58 | (30) | 39 | (67) | 20% |
| History of CVD5 | 2125 | 659 | (31) | 489 | (74) | 23% | 883 | 331 | (37) | 253 | (76) | 29% | 291 | 87 | (30) | 64 | (74) | 22% |
| Lung | Endometrial | Leukemia | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||||
| All6 | Eligible7 | Consented8 | All1 | Eligible2 | Consented3 | All1 | Eligible2 | Consented3 | ||||||||||
| N | N (%) | N (%) | % of All | N | N (%) | N (%) | % of All | N | N (%) | N (%) | % of All | |||||||
|
| ||||||||||||||||||
| Total | 2628 | 534 | (20) | 406 | (76) | 15% | 1509 | 839 | (56) | 663 | (79) | 44% | 690 | 253 | (37) | 186 | (74) | 27% |
| Age at WHI baseline | ||||||||||||||||||
| 50–59 | 568 | 187 | (33) | 145 | (78) | 26% | 528 | 368 | (70) | 311 | (85) | 59% | 170 | 97 | (57) | 77 | (79) | 45% |
| 60–69 | 1395 | 291 | (21) | 221 | (76) | 16% | 682 | 371 | (54) | 292 | (79) | 43% | 353 | 124 | (35) | 85 | (69) | 24% |
| 70–79 | 665 | 56 | (8) | 40 | (71) | 6% | 299 | 100 | (33) | 60 | (60) | 20% | 167 | 32 | (19) | 24 | (75) | 14% |
| Race | ||||||||||||||||||
| White | 2332 | 482 | (21) | 368 | (76) | 16% | 1356 | 772 | (57) | 614 | (80) | 45% | 618 | 234 | (38) | 174 | (74) | 28% |
| Black | 165 | 26 | (16) | 19 | (73) | 12% | 79 | 33 | (42) | 22 | (67) | 28% | 43 | 7 | (16) | 4 | (57) | 9% |
| Hispanic | 37 | 9 | (24) | 7 | (78) | 19% | 27 | 8 | (30) | 4 | (50) | 15% | 11 | 5 | (45) | 3 | (60) | 27% |
| Other | 94 | 17 | (18) | 12 | (71) | 13% | 47 | 26 | (55) | 23 | (88) | 49% | 18 | 7 | (39) | 5 | (71) | 28% |
| Marital Status | ||||||||||||||||||
| Married /Living as | 1448 | 336 | (23) | 255 | (76) | 18% | 989 | 581 | (59) | 463 | (80) | 47% | 458 | 185 | (40) | 136 | (74) | 30% |
| Previously Married | 1076 | 173 | (16) | 135 | (78) | 13% | 421 | 209 | (50) | 157 | (75) | 37% | 204 | 61 | (30) | 44 | (72) | 22% |
| Never married | 95 | 23 | (24) | 15 | (65) | 16% | 98 | 48 | (49) | 42 | (88) | 43% | 25 | 7 | (28) | 6 | (86) | 24% |
| Education | ||||||||||||||||||
| ≤High School | 633 | 110 | (17) | 73 | (66) | 12% | 266 | 125 | (47) | 89 | (71) | 33% | 144 | 54 | (38) | 31 | (57) | 22% |
| Some College | 1069 | 180 | (17) | 138 | (77) | 13% | 494 | 265 | (54) | 203 | (77) | 41% | 243 | 81 | (33) | 53 | (65) | 22% |
| College grad | 278 | 67 | (24) | 51 | (76) | 18% | 190 | 105 | (55) | 85 | (81) | 45% | 91 | 35 | (38) | 29 | (83) | 32% |
| Post grad | 631 | 171 | (27) | 139 | (81) | 22% | 551 | 340 | (62) | 282 | (83) | 51% | 207 | 81 | (39) | 71 | (88) | 34% |
| Smoking | ||||||||||||||||||
| Never | 421 | 121 | (29) | 88 | (73) | 21% | 800 | 455 | (57) | 358 | (79) | 45% | 328 | 128 | (39) | 90 | (70) | 27% |
| Former | 1450 | 304 | (21) | 235 | (77) | 16% | 623 | 345 | (55) | 272 | (79) | 44% | 322 | 115 | (36) | 87 | (76) | 27% |
| Current | 723 | 106 | (15) | 81 | (76) | 11% | 70 | 34 | (49) | 28 | (82) | 40% | 35 | 10 | (29) | 9 | (90) | 26% |
| BMI | ||||||||||||||||||
| ≤24.9 | 1033 | 217 | (21) | 175 | (81) | 17% | 464 | 271 | (58) | 223 | (82) | 48% | 243 | 108 | (44) | 89 | (82) | 37% |
| 25–29.9 | 891 | 186 | (21) | 136 | (73) | 15% | 418 | 238 | (57) | 189 | (79) | 45% | 248 | 83 | (33) | 62 | (75) | 25% |
| 30–34.9 | 440 | 81 | (18) | 65 | (80) | 15% | 310 | 164 | (53) | 126 | (77) | 41% | 124 | 40 | (32) | 22 | (55) | 18% |
| 35+ | 233 | 44 | (19) | 25 | (57) | 11% | 304 | 159 | (52) | 118 | (74) | 39% | 69 | 19 | (28) | 11 | (58) | 16% |
| History of Diabetes9 | 154 | 22 | (14) | 13 | (59) | 8% | 90 | 30 | (33) | 21 | (70) | 23% | 35 | 6 | (17) | 3 | (50) | 9% |
| History of CVD10 | 400 | 52 | (13) | 41 | (79) | 10% | 119 | 43 | (36) | 31 | (72) | 26% | 65 | 16 | (25) | 10 | (63) | 15% |
| Non-Hodgkin Lymphoma | Melanoma11 | EOC12 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||||
| All13 | Eligible14 | Consented15 | All3 | Eligible4 | Consented5 | All3 | Eligible4 | Consented5 | ||||||||||
| N | N (%) | N (%) | % of All | N | N (%) | N (%) | % of All | N | N (%) | N (%) | % of All | |||||||
|
| ||||||||||||||||||
| Total | 1314 | 582 | (44) | 451 | (77) | 34% | 1835 | 1187 | (65) | 834 | (70) | 45% | 1004 | 249 | (25) | 213 | (86) | 21% |
| Age at WHI baseline | ||||||||||||||||||
| 50–59 | 345 | 206 | (60) | 166 | (81) | 48% | 664 | 526 | (79) | 403 | (77) | 61% | 301 | 109 | (36) | 94 | (86) | 31% |
| 60–69 | 622 | 300 | (48) | 232 | (77) | 37% | 855 | 530 | (62) | 352 | (66) | 41% | 507 | 123 | (24) | 105 | (85) | 21% |
| 70–79 | 347 | 76 | (22) | 53 | (70) | 15% | 316 | 131 | (41) | 79 | (60) | 25% | 196 | 17 | (9) | 14 | (82) | 7% |
| Race | ||||||||||||||||||
| White | 1195 | 538 | (45) | 418 | (78) | 35% | 1796 | 1164 | (65) | 819 | (70) | 46% | 894 | 226 | (25) | 195 | (86) | 22% |
| Black | 46 | 16 | (35) | 12 | (75) | 26% | 5 | 3 | (60) | 2 | (67) | 40% | 53 | 10 | (19) | 9 | (90) | 17% |
| Hispanic | 32 | 12 | (38) | 8 | (67) | 25% | 12 | 7 | (58) | 5 | (71) | 42% | 29 | 6 | (21) | 4 | (67) | 14% |
| Other | 41 | 16 | (39) | 13 | (81) | 32% | 22 | 13 | (59) | 8 | (62) | 36% | 28 | 7 | (25) | 5 | (71) | 18% |
| Marital Status | ||||||||||||||||||
| Married /Living as | 854 | 399 | (47) | 322 | (81) | 38% | 1303 | 867 | (67) | 613 | (71) | 47% | 672 | 178 | (26) | 154 | (87) | 23% |
| Previously Married | 419 | 163 | (39) | 116 | (71) | 28% | 431 | 260 | (60) | 184 | (71) | 43% | 294 | 63 | (21) | 51 | (81) | 17% |
| Never married | 38 | 18 | (47) | 11 | (61) | 29% | 92 | 54 | (59) | 35 | (65) | 38% | 36 | 8 | (22) | 8 | (100) | 22% |
| Education | ||||||||||||||||||
| ≤High School | 279 | 114 | (41) | 80 | (70) | 29% | 239 | 130 | (54) | 76 | (58) | 32% | 210 | 44 | (21) | 39 | (89) | 19% |
| Some College | 488 | 198 | (41) | 151 | (76) | 31% | 644 | 423 | (66) | 292 | (69) | 45% | 347 | 80 | (23) | 68 | (85) | 20% |
| College grad | 168 | 80 | (48) | 64 | (80) | 38% | 248 | 171 | (69) | 133 | (78) | 54% | 137 | 48 | (35) | 40 | (83) | 29% |
| Post grad | 373 | 187 | (50) | 153 | (82) | 41% | 688 | 450 | (65) | 326 | (72) | 47% | 302 | 77 | (25) | 66 | (86) | 22% |
| Smoking | ||||||||||||||||||
| Never | 667 | 299 | (45) | 235 | (79) | 35% | 880 | 564 | (64) | 413 | (73) | 47% | 502 | 143 | (28) | 121 | (85) | 24% |
| Former | 570 | 249 | (44) | 193 | (78) | 34% | 874 | 572 | (65) | 387 | (68) | 44% | 430 | 91 | (21) | 80 | (88) | 19% |
| Current | 67 | 28 | (42) | 20 | (71) | 30% | 55 | 37 | (67) | 22 | (59) | 40% | 61 | 13 | (21) | 10 | (77) | 16% |
| BMI | ||||||||||||||||||
| ≤24.9 | 455 | 216 | (47) | 172 | (80) | 38% | 730 | 489 | (67) | 348 | (71) | 48% | 391 | 115 | (29) | 95 | (83) | 24% |
| 25–29.9 | 486 | 224 | (46) | 178 | (79) | 37% | 647 | 413 | (64) | 296 | (72) | 46% | 325 | 71 | (22) | 60 | (85) | 18% |
| 30–34.9 | 242 | 96 | (40) | 66 | (69) | 27% | 272 | 169 | (62) | 110 | (65) | 40% | 175 | 37 | (21) | 35 | (95) | 20% |
| 35+ | 120 | 42 | (35) | 31 | (74) | 26% | 163 | 101 | (62) | 68 | (67) | 42% | 105 | 23 | (22) | 20 | (87) | 19% |
| History of Diabetes16 | 66 | 23 | (35) | 13 | (57) | 20% | 70 | 31 | (44) | 15 | (48) | 21% | 53 | 3 | (6) | 2 | (67) | 4% |
| History of CVD17 | 132 | 37 | (28) | 25 | (68) | 19% | 142 | 76 | (54) | 51 | (67) | 36% | 93 | 17 | (18) | 14 | (82) | 15% |
Participants with reported and centrally confirmed eligible cancers in WHI as of September 30, 2015.
Eligible for LILAC invitation mailing (in active follow-up at LILAC initiation with prior confirmed cancer or subsequently diagnosed with a qualifying cancer).
Provided written consent for LILAC data collection activities.
Self-reported history of diabetes (treated or untreated) other than when pregnant.
Self-reported history of myocardial infarction, stroke, cardiac arrest, congestive heart failure, angina, coronary revascularization, peripheral artery disease, carotid angioplasty, or aortic aneurysm.
Melanoma includes both in situ and invasive cancers.
ovarian, fallopian tube and primary peritoneal cancer
Participants with reported and centrally confirmed eligible cancers in WHI as of September 30, 2015.
Eligible for LILAC invitation mailing (in active follow-up at LILAC initiation with prior confirmed cancer or subsequently diagnosed with a qualifying cancer).
Provided written consent for LILAC data collection activities.
Self-reported history of diabetes (treated or untreated) other than when pregnant.
Self-reported history of myocardial infarction, stroke, cardiac arrest, congestive heart failure, angina, coronary revascularization, peripheral artery disease, carotid angioplasty, or aortic aneurysm.
Eligibility reflects both survival and continuing WHI participation which varied strongly by age. (Table 1). We see a further, though more gradual, decline with age in the consent rates for LILAC (82% in those who were 50–59 years old at WHI baseline declining to 67% in the 70–79 year olds). This pattern is reflected in each of the specific cancer sites although the absolute rates of eligibility vary considerably, reflecting the varying lethality of these cancers. Consent rates among the eligible cases were much more comparable across tumor sites. Black women with cancer were somewhat less likely to be eligible (37%) and less likely to agree to participate (69%) than other race/ethnicity subgroups. Other factors associated with lower eligibility and consent rates to the survey but to a lesser degree included lower education, no longer having a life partner (widowed/separated/divorced), higher body mass index (BMI), and women with a history of diabetes and cardiovascular disease. Current smokers were less likely to be eligible for the survey but were consented (77%) at similar frequency as non-smokers (78%).
Similar strong trends in eligibility and lesser trends for consent are seen with factors measured at the time of diagnosis (Table 2). In particular, we saw a higher proportion of recently diagnosed women consented than those with earlier diagnoses buta lower proportion of women with poorer prognostic factors consented than did those with good prognoses, a pattern that was consistent across all cancer sites. There was no consistent pattern of eligibility and consent trends across sites by age at diagnosis. Increasing age at diagnosis had no major effect on eligibility among breast and endometrial cancer survivors, but showed a trend toward less willingness to consent. Whereas for leukemia, lymphoma and melanoma survivors, older women were no less likely to be eligible but were somewhat less likely to consent to participate. Factors associated with poor prognosis (e.g., stage of disease and tumor size) show strong trends of decreasing eligibility and more modest reductions in consent rates.
Table 2.
Clinical Characteristics and LILAC participation status of WHI women with LILAC designated cancers reported as of September 2015, by cancer site
| Total | Breast | Colorectal | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||||
| All18 | Eligible19 | Consented20 | All1 | Eligible2 | Consented3 | All1 | Eligible2 | Consented3 | ||||||||||
| N | N (%) | N (%) | % of All | N | N (%) | N (%) | % of All | N | N (%) | N (%) | % of All | |||||||
|
| ||||||||||||||||||
| Total | 20784 | 9934 | (48) | 7760 | (78) | 37% | 9305 | 5251 | (56) | 4211 | (80) | 45% | 2499 | 1039 | (42) | 796 | (77) | 32% |
| Year of diagnosis | ||||||||||||||||||
| < 2001 | 5713 | 1896 | (33) | 1451 | (77) | 25% | 2802 | 1161 | (41) | 905 | (78) | 32% | 758 | 203 | (27) | 148 | (73) | 20% |
| 2001–2005 | 6598 | 2623 | (40) | 2019 | (77) | 31% | 3041 | 1490 | (49) | 1183 | (79) | 39% | 801 | 277 | (35) | 208 | (75) | 26% |
| 2006–2010 | 5180 | 2898 | (56) | 2312 | (80) | 45% | 2141 | 1492 | (70) | 1228 | (82) | 57% | 580 | 294 | (51) | 229 | (78) | 39% |
| 2011–2015 | 3293 | 2517 | (76) | 1978 | (79) | 60% | 1321 | 1108 | (84) | 895 | (81) | 68% | 360 | 265 | (74) | 211 | (80) | 59% |
| Age at diagnosis | ||||||||||||||||||
| 50–59 | 1182 | 591 | (50) | 496 | (84) | 42% | 681 | 385 | (57) | 329 | (85) | 48% | 98 | 38 | (39) | 26 | (68) | 27% |
| 60–69 | 6391 | 3257 | (51) | 2597 | (80) | 41% | 3146 | 1864 | (59) | 1526 | (82) | 49% | 658 | 278 | (42) | 217 | (78) | 33% |
| 70–79 | 9452 | 4475 | (47) | 3496 | (78) | 37% | 4101 | 2272 | (55) | 1807 | (80) | 44% | 1135 | 489 | (43) | 384 | (79) | 34% |
| 80–89 | 3558 | 1513 | (43) | 1109 | (73) | 31% | 1318 | 694 | (53) | 524 | (76) | 40% | 554 | 212 | (38) | 154 | (73) | 28% |
| 90+ | 201 | 98 | (49) | 62 | (63) | 31% | 59 | 36 | (61) | 25 | (69) | 42% | 54 | 22 | (41) | 15 | (68) | 28% |
| Stage at diagnosis | ||||||||||||||||||
| In situ | 855 | 578 | (68) | 344 | (60) | 40% | ||||||||||||
| Local | 11235 | 6461 | (58) | 5169 | (80) | 46% | 6951 | 4075 | (59) | 3285 | (81) | 47% | 1107 | 591 | (53) | 458 | (77) | 41% |
| Regional | 4348 | 1932 | (44) | 1522 | (79) | 35% | 2118 | 1113 | (53) | 883 | (79) | 42% | 1021 | 411 | (40) | 308 | (75) | 30% |
| Distant | 2929 | 557 | (19) | 431 | (77) | 15% | 132 | 31 | (23) | 21 | (68) | 16% | 318 | 31 | (10) | 26 | (84) | 8% |
| Unknown | 727 | 153 | (21) | 108 | (71) | 15% | 104 | 32 | (31) | 22 | (69) | 21% | 53 | 6 | (11) | 4 | (67) | 8% |
| Morphology grade | ||||||||||||||||||
| Well differentiated | 3173 | 1906 | (60) | 1496 | (78) | 47% | 2397 | 1512 | (63) | 1210 | (80) | 50% | 185 | 94 | (51) | 66 | (70) | 36% |
| Moderately differentiated | 6538 | 3423 | (52) | 2739 | (80) | 42% | 3853 | 2232 | (58) | 1799 | (81) | 47% | 1514 | 654 | (43) | 512 | (78) | 34% |
| Anaplastic/poorly differentiated | 4568 | 1890 | (41) | 1527 | (81) | 33% | 2253 | 1157 | (51) | 926 | (80) | 41% | 566 | 203 | (36) | 160 | (79) | 28% |
| Unknown | 2666 | 693 | (26) | 527 | (76) | 20% | 802 | 350 | (44) | 276 | (79) | 34% | 234 | 88 | (38) | 58 | (66) | 25% |
| Tumor size, cm | ||||||||||||||||||
| ≤0.5 | 1724 | 1070 | (62) | 836 | (78) | 48% | 1115 | 668 | (60) | 538 | (81) | 48% | 78 | 44 | (56) | 26 | (59) | 33% |
| >0.5–1 | 2810 | 1669 | (59) | 1350 | (81) | 48% | 2376 | 1420 | (60) | 1146 | (81) | 48% | 65 | 37 | (57) | 28 | (76) | 43% |
| >1–2 | 4502 | 2504 | (56) | 2035 | (81) | 45% | 3510 | 2024 | (58) | 1653 | (82) | 47% | 183 | 94 | (51) | 76 | (81) | 42% |
| >2–5 | 4446 | 1889 | (42) | 1489 | (79) | 33% | 1706 | 873 | (51) | 683 | (78) | 40% | 1093 | 451 | (41) | 353 | (78) | 32% |
| >5 | 1467 | 541 | (37) | 433 | (80) | 30% | 247 | 116 | (47) | 89 | (77) | 36% | 542 | 211 | (39) | 169 | (80) | 31% |
| Unknown | 3831 | 1426 | (37) | 980 | (69) | 26% | 351 | 150 | (43) | 102 | (68) | 29% | 538 | 202 | (38) | 144 | (71) | 27% |
| Lung | Endometrial | Leukemia | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||||
| All21 | Eligible22 | Consented23 | All1 | Eligible2 | Consented3 | All1 | Eligible2 | Consented3 | ||||||||||
| N | N (%) | N (%) | % of All | N | N (%) | N (%) | % of All | N | N (%) | N (%) | % of All | |||||||
|
| ||||||||||||||||||
| Total | 2628 | 534 | (20) | 406 | (76) | 15% | 1509 | 839 | (56) | 663 | (79) | 44% | 690 | 253 | (37) | 186 | (74) | 27% |
| Year of diagnosis | ||||||||||||||||||
| < 2001 | 614 | 40 | (7) | 32 | (80) | 5% | 448 | 202 | (45) | 149 | (74) | 33% | 148 | 19 | (13) | 13 | (68) | 9% |
| 2001–2005 | 792 | 77 | (10) | 57 | (74) | 7% | 484 | 226 | (47) | 178 | (79) | 37% | 200 | 62 | (31) | 46 | (74) | 23% |
| 2006–2010 | 773 | 182 | (24) | 151 | (83) | 20% | 365 | 235 | (64) | 188 | (80) | 52% | 216 | 90 | (42) | 64 | (71) | 30% |
| 2011–2015 | 449 | 235 | (52) | 166 | (71) | 37% | 212 | 176 | (83) | 148 | (84) | 70% | 126 | 82 | (65) | 63 | (77) | 50% |
| Age at diagnosis | ||||||||||||||||||
| 50–59 | 73 | 7 | (10) | 6 | (86) | 8% | 94 | 60 | (64) | 51 | (85) | 54% | 16 | 4 | (25) | 3 | (75) | 19% |
| 60–69 | 643 | 115 | (18) | 90 | (78) | 14% | 545 | 317 | (58) | 253 | (80) | 46% | 192 | 80 | (42) | 63 | (79) | 33% |
| 70–79 | 1317 | 277 | (21) | 213 | (77) | 16% | 661 | 363 | (55) | 288 | (79) | 44% | 319 | 114 | (36) | 82 | (72) | 26% |
| 80–89 | 564 | 130 | (23) | 96 | (74) | 17% | 201 | 94 | (47) | 68 | (72) | 34% | 157 | 54 | (34) | 37 | (69) | 24% |
| 90+ | 31 | 5 | (16) | 1 | (20) | 3% | 8 | 5 | (63) | 3 | (60) | 38% | 6 | 1 | (17) | 1 | (100) | 17% |
| Stage at diagnosis | ||||||||||||||||||
| In situ | ||||||||||||||||||
| Local | 688 | 308 | (45) | 249 | (81) | 36% | 1204 | 720 | (60) | 570 | (79) | 47% | ||||||
| Regional | 610 | 142 | (23) | 108 | (76) | 18% | 211 | 97 | (46) | 80 | (82) | 38% | ||||||
| Distant | 1018 | 64 | (6) | 37 | (58) | 4% | 78 | 15 | (19) | 10 | (67) | 13% | ||||||
| Unknown | 312 | 20 | (6) | 12 | (60) | 4% | 16 | 7 | (44) | 3 | (43) | 19% | ||||||
| Morphology grade | ||||||||||||||||||
| Well differentiated | 255 | 110 | (43) | 81 | (74) | 32% | 310 | 179 | (58) | 130 | (73) | 42% | ||||||
| Moderately differentiated | 465 | 152 | (33) | 122 | (80) | 26% | 593 | 350 | (59) | 281 | (80) | 47% | ||||||
| Anaplastic/poorly differentiated | 657 | 109 | (17) | 88 | (81) | 13% | 533 | 272 | (51) | 220 | (81) | 41% | ||||||
| Unknown | 1251 | 163 | (13) | 115 | (71) | 9% | 73 | 38 | (52) | 32 | (84) | 44% | ||||||
| Tumor size, cm | ||||||||||||||||||
| ≤0.5 | 18 | 4 | (22) | 4 | (100) | 22% | 55 | 34 | (62) | 24 | (71) | 44% | ||||||
| >0.5–1 | 93 | 42 | (45) | 38 | (90) | 41% | 50 | 33 | (66) | 26 | (79) | 52% | ||||||
| >1–2 | 431 | 169 | (39) | 124 | (73) | 29% | 176 | 116 | (66) | 94 | (81) | 53% | ||||||
| >2–5 | 1013 | 225 | (22) | 172 | (76) | 17% | 457 | 266 | (58) | 218 | (82) | 48% | ||||||
| >5 | 338 | 62 | (18) | 46 | (74) | 14% | 121 | 57 | (47) | 44 | (77) | 36% | ||||||
| Unknown | 735 | 32 | (4) | 22 | (69) | 3% | 650 | 333 | (51) | 257 | (77) | 40% | ||||||
| Non-Hodgkin Lymphoma | Melanoma24 | EOC25 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||||
| All26 | Eligible27 | Consented28 | All3 | Eligible4 | Consented5 | All3 | Eligible4 | Consented5 | ||||||||||
| N | N (%) | N (%) | % of All | N | N (%) | N (%) | % of All | N | N (%) | N (%) | % of All | |||||||
|
| ||||||||||||||||||
| Total | 1314 | 582 | (44) | 451 | (77) | 34% | 1835 | 1187 | (65) | 834 | (70) | 45% | 1004 | 249 | (25) | 213 | (86) | 21% |
| Year of diagnosis | ||||||||||||||||||
| < 2001 | 291 | 69 | (24) | 52 | (75) | 18% | 372 | 169 | (45) | 123 | (73) | 33% | 280 | 33 | (12) | 29 | (88) | 10% |
| 2001–2005 | 436 | 149 | (34) | 110 | (74) | 25% | 530 | 303 | (57) | 202 | (67) | 38% | 314 | 39 | (12) | 35 | (90) | 11% |
| 2006–2010 | 369 | 202 | (55) | 162 | (80) | 44% | 480 | 320 | (67) | 218 | (68) | 45% | 256 | 83 | (32) | 72 | (87) | 28% |
| 2011–2015 | 218 | 162 | (74) | 127 | (78) | 58% | 453 | 395 | (87) | 291 | (74) | 64% | 154 | 94 | (61) | 77 | (82) | 50% |
| Age at diagnosis | ||||||||||||||||||
| 50–59 | 56 | 15 | (27) | 12 | (80) | 21% | 115 | 68 | (59) | 58 | (85) | 50% | 49 | 14 | (29) | 11 | (79) | 22% |
| 60–69 | 331 | 160 | (48) | 127 | (79) | 38% | 554 | 377 | (68) | 262 | (69) | 47% | 322 | 66 | (20) | 59 | (89) | 18% |
| 70–79 | 635 | 303 | (48) | 235 | (78) | 37% | 826 | 533 | (65) | 380 | (71) | 46% | 458 | 124 | (27) | 107 | (86) | 23% |
| 80–89 | 274 | 93 | (34) | 70 | (75) | 26% | 321 | 192 | (60) | 125 | (65) | 39% | 169 | 44 | (26) | 35 | (80) | 21% |
| 90+ | 18 | 11 | (61) | 7 | (64) | 39% | 19 | 17 | (89) | 9 | (53) | 47% | 6 | 1 | (17) | 1 | (100) | 17% |
| Stage at diagnosis | ||||||||||||||||||
| In situ | 855 | 579 | (68) | 344 | (59) | 40% | ||||||||||||
| Local | 313 | 139 | (44) | 103 | (74) | 33% | 879 | 573 | (65) | 457 | (80) | 52% | 93 | 55 | (59) | 47 | (85) | 51% |
| Regional | 179 | 85 | (47) | 64 | (75) | 36% | 60 | 24 | (40) | 23 | (96) | 38% | 149 | 60 | (40) | 56 | (93) | 38% |
| Distant | 624 | 282 | (45) | 228 | (81) | 37% | 19 | 1 | (5) | 0 | (0) | 0% | 740 | 133 | (18) | 109 | (82) | 15% |
| Unknown | 198 | 76 | (38) | 56 | (74) | 28% | 22 | 10 | (45) | 10 | (100) | 45% | 22 | 1 | (5) | 1 | (100) | 5% |
| Morphology grade | ||||||||||||||||||
| Well differentiated | 26 | 11 | (42) | 9 | (82) | 35% | ||||||||||||
| Moderately differentiated | 113 | 35 | (31) | 25 | (71) | 22% | ||||||||||||
| Anaplastic/poorly differentiated | 559 | 149 | (27) | 133 | (89) | 24% | ||||||||||||
| Unknown | 306 | 54 | (18) | 46 | (85) | 15% | ||||||||||||
| Tumor size, cm | ||||||||||||||||||
| ≤0.5 | 445 | 313 | (70) | 239 | (76) | 54% | 13 | 7 | (54) | 5 | (71) | 38% | ||||||
| >0.5–1 | 209 | 135 | (65) | 110 | (81) | 53% | 17 | 2 | (12) | 2 | (100) | 12% | ||||||
| >1–2 | 161 | 92 | (57) | 80 | (87) | 50% | 41 | 9 | (22) | 8 | (89) | 20% | ||||||
| >2–5 | 81 | 36 | (44) | 31 | (86) | 38% | 96 | 38 | (40) | 32 | (84) | 33% | ||||||
| >5 | 18 | 7 | (39) | 6 | (86) | 33% | 201 | 88 | (44) | 79 | (90) | 39% | ||||||
| Unknown | 921 | 604 | (66) | 368 | (61) | 40% | 636 | 105 | (17) | 87 | (83) | 14% | ||||||
Participants with reported and centrally confirmed eligible cancers in WHI as of September 30, 2015.
Eligible for LILAC invitation mailing (in active follow-up at LILAC initiation with prior confirmed cancer or subsequently diagnosed with a qualifying cancer).
Provided written consent for LILAC data collection activities.
Participants with reported and centrally confirmed eligible cancers in WHI as of September 30, 2015.
Eligible for LILAC invitation mailing (in active follow-up at LILAC initiation with prior confirmed cancer or subsequently diagnosed with a qualifying cancer).
Provided written consent for LILAC data collection activities.
Melanoma includes both in situ and invasive cancers.
ovarian, fallopian tube and primary peritoneal cancer
Participants with reported and centrally confirmed eligible cancers in WHI as of September 30, 2015.
Eligible for LILAC invitation mailing (in active follow-up at LILAC initiation with prior confirmed cancer or subsequently diagnosed with a qualifying cancer).
Provided written consent for LILAC data collection activities.
Characteristics of consented LILAC participants
Among cancer survivors who consented to participate in LILAC, approximately 91% were white, 15% reported a high school education or less, 45% were former smokers and 6% were current smokers when enrolled in the WHI (Supplemental Table 2). Approximately 34% of participants were considered overweight; an additional 17% were considered class I obese and 11% were class II or III obese. Few reported a history of diabetes (3%) or cardiovascular disease (6%) at WHI baseline. Risk factor patterns were consistent across cancer sites with a few expected exceptions. The comparison of WHI baseline characteristics of women with cancer who consented to the LILAC surveys to those who had the same diagnosis but are not participating in LILAC surveys suggested only a modest health advantage in these pre-diagnostic factors. The majority of LILAC survey responders were diagnosed with cancer in the last 10 years (55%) and 60% were 70+ years of age at diagnosis, including 15% who were over 80 years (Supplemental Table 2). Two thirds had localized and 5% had distant disease at diagnosis. EOC cancer survivors were the exception to this trend, as 51% of responders were diagnosed at a distant stage, and most of these women (70%) were diagnosed within the past 10 years.
Self-reported cancer treatment data reflects the pattern of care that one would generally expect in the community during this timeframe (1994–2016), which varies considerably by site (Table 3). (Information on surgeries is being abstracted from existing WHI medical records). Approximately 68% of breast cancer survivors reported treatments with hormone or anti-estrogen pills. In this cohort of survivors, 553 women (7.2%) reported they had survived a cancer recurrence. The highest proportion of those surviving a recurrence was among EOC cancer survivors; 27% reported a cancer recurrence. EOC cancer survivors also reported the highest average number of symptoms after a cancer diagnosis (3.1), compared to other cancer survivors. The highest proportion reporting depression was noted in leukemia survivors, where just over 18% reported often feeling depressed.
Table 3.
Clinical and Psychosocial characteristics of LILAC participants by cancer type (N=7651)
| Variable | Level | Breast (n=4173) |
Colorectal (n=781) |
Lung (n=397) |
Endometrial (n=648) |
Leukemia (n=181) |
Non-Hodgkin Lymphoma (n=442) |
Melanoma (n=821) |
EOC (n=208) |
|---|---|---|---|---|---|---|---|---|---|
| Chemotherapy | No | 2937 (71%) | 510 (66%) | 276 (71%) | 555 (87%) | 106 (60%) | 133 (30%) | 792 (98%) | 20 (10.0%) |
| Yes | 1185 (29%) | 262 (34%) | 115 (29%) | 83 (13%) | 72 (40%) | 304 (70%) | 13 (2%) | 181 (90.0%) | |
| Radiation | No | 1247 (30%) | 715 (92%) | 292 (75%) | 439 (69%) | 177 (98%) | 324 (74%) | 786 (98%) | 195 (96%) |
| Yes | 2874 (70%) | 58 (7%) | 97 (25%) | 196 (31%) | 3 (2%) | 111 (25%) | 17 (2%) | 8 (4%) | |
| Hormone or anti-estrogen pills | No | 1301 (32%) | 758 (99%) | 386 (99%) | 596 (96%) | 179 (100%) | 425 (99%) | 807 (100%) | 190 (95%) |
| Yes | 2743 (68%) | 6 (1%) | 3 (1%) | 25 (4%) | 0 (0%) | 4 (1%) | 1 (0%) | 9 (4%) | |
| Recurrence | No | 3867 (94%) | 728 (95%) | 326 (86%) | 613 (96%) | 167 (95%) | 372 (86%) | 726 (92%) | 149 (73%) |
| Yes | 249 (6%) | 34 (4%) | 53 (14%) | 26 (4%) | 8 (5%) | 61 (14%) | 67 (8%) | 55 (27%) | |
| Number of symptoms after cancer diagnosis | Mean (SD) | 2.1 (2.5) | 1.6 (2.3) | 2.2 (2.7) | 1.3 (2.0) | 1.9 (2.3) | 2.4 (2.6) | 0.4 (1.3) | 3.1 (2.6) |
| Number of symptoms after cancer dx- categorized | None | 1263 (33%) | 319 (48%) | 118 (35%) | 279 (49%) | 51 (42%) | 106 (29%) | 591 (81%) | 31 (16%) |
| 1–2 | 1352 (36%) | 182 (28%) | 106 (31%) | 178 (31%) | 33 (27%) | 111 (31%) | 97 (13%) | 60 (31%) | |
| 3+ | 1182 (31%) | 157 (24%) | 112 (33%) | 116 (20%) | 36 (30%) | 146 (40%) | 40 (5%) | 101 (53%) | |
| Often feel depressed | No | 3250 (85%) | 591 (85%) | 308 (83%) | 511 (86%) | 128 (81%) | 332 (84%) | 703 (91%) | 163 (84%) |
| Yes | 581 (15%) | 104 (15%) | 61 (16%) | 82 (14%) | 29 (18%) | 61 (15%) | 68 (9%) | 30 (15%) | |
| Rate pain in last 24 hours | Mean (SD) | 1.7 (2.3) | 1.8 (2.4) | 1.6 (2.3) | 1.7 (2.2) | 1.3 (2.1) | 1.5 (2.1) | 1.2 (1.9) | 1.7 (2.3) |
| Anxiety past week | Mean (SD) | 1.9 (2.1) | 2.0 (2.1) | 2.2 (2.2) | 2.0 (2.1) | 2.1 (2.1) | 2.0 (2.0) | 1.7 (1.9) | 2.2 (2.1) |
| Social support construct | Mean (SD) | 74.9 (23.8) | 76.7 (22.5) | 73.8 (24.5) | 75.9 (22.1) | 77.5 (21.4) | 77.2 (22.8) | 75.7 (23.2) | 81.7 (20.1) |
Discussion
In developing the LILAC cohort, our goal was to create a resource to support a broad range of cancer survivorship studies among older women, an understudied population where large gaps exist in knowledge about care needs, multiple coexisting diseases, side effects from treatment, and need for social support. In addition, we sought to provide a resource that could be highly complementary to data derived from the conduct of cancer therapy trials. By embedding this resource within a large, high-quality epidemiologic cohort, analyses can take advantage of several unusual features. First, analyses of LILAC cancers can access the comprehensive WHI database of prospectively collected pre-diagnostic information of participant characteristics, lifestyle, disease risk factors and co-morbidities. These data can be used to look at the impact of these factors on cancer outcomes directly and on access to treatment and treatment efficacy. Second, LILAC tumor tissue can be paired with the existing repository of blood products collected prior to diagnosis, to study biomarkers of risk and early detection. The high quality information on non-cancer outcomes (e.g., cardiovascular disease) will provide opportunities to look at risk of other conditions among cancer survivors, and in particular, the very unusual chance to compare post-cancer experiences (e.g., symptoms, quality of life, and conditions associated with aging) with those of similar participants who remained free of cancer. Overall, survey response was high, as 77% of women who were eligible to participate in the survey returned the baseline survey, which demonstrates the generalizability of our survey data to other populations of older women living with cancer.
Based on a recent study which investigated the types of information collected by cancer survivorship cohorts, most cancer survivorship cohort studies focus their research on survivorship issues amongst breast cancer survivors [15]. The LILAC cohort is a unique resource in that, not only does the cohort collect information from breast cancer survivors, but also leukemia, lymphoma, colorectal, lung, melanoma, endometrial and EOC cancer survivors as well. Therefore, the LILAC cohort is filling a valuable gap in cancer survivorship research by including survivors from cancer sites that are currently understudied. The average age of participants was 79 years with a range of 65–97 years, which is similar to the age distribution of female cancer survivors in the United States [16]. Thus, this cohort can contribute greatly to our understanding of survivorship among older women diagnosed with these cancers. In addition to understudied cancer survivors, unmet social needs such as access to social support, is information not commonly collected from cancer survivors. The LILAC cohort also fills this research gap by including measures of social support within the annual surveys. Lastly, only around half of current cancer survivorship cohorts collect biospecimen and biomarker data from cancer survivors. The LILAC cohort has the opportunity to further fill this gap in survivorship research by utilizing tumor tissue data collected from cancer survivors to understand the relationships between biomarkers and survivorship outcomes such as side effects, symptoms, and survival.
The design of LILAC includes prevalent cases (cancers diagnosed prior to LILAC initiation) and thus requires retrospective collection of treatment and recurrence data. The primary advantage of this design is that it allows us access to a larger population of cancer survivors for whom multiple years of follow-up are already available, thereby telescoping the timeline to analyses.
There are several limitations of this design. First, there is noteworthy survival bias in the self-reported information, particularly among women whose cancers were diagnosed several years before the start of LILAC. Our data collection methods partially address this by accessing multiple sources (Medicare and medical records) to obtain treatment and recurrence data, some of which are independent of participant survival. Some cancers were diagnosed so many years previously that medical records collection is very difficult and expensive; accordingly, we are limiting this effort to cases diagnosed in 2000 or later. For participant reported outcomes, we have elected to focus on late and long-term effects of treatments, since our ability to capture acute effects is compromised by this design. Analyses of these data will need to account for selection bias through approaches that are conditional on survival, or adjust the known biases through inverse probability weighting methods. Another potential limitation is the fact that some treatments administered in the early part of this century may soon be obsolete. While this dilutes the value of this resource for newly treated cancers, the information developed here would remain relevant for the many millions of patients living with a cancer diagnosis now.
The LILAC cohort lacks the racial/ethnic diversity needed to fully address the cancer survivorship needs of the US population. Approximately 18% of the original WHI study population self-identified as women of color (African American, Hispanic, Asian/Pacific Islander, Native American) which was similar to the larger population of that age-group at the time of enrollment, but these subgroups represent a smaller fraction (11.2%) of women with a LILAC cancer diagnosis and only 8.8%of those who consented. Women of color were more likely than white women to stop the WHI participation or be lost to follow-up. As such, future studies utilizing this data resource must be cautious in drawing conclusions regarding this population, as results may not be generalizable to racial minorities and non-white populations.
The reliance on multiple sources of treatment data, including Medicare, introduces complexity into the data analyses but it has allowed us to expand our reach to cases diagnosed years before LILAC initiation, reduce the cost of data collection and rapidly create a database with mature cancer outcomes. We have incorporated validation samples into the design to allow us to look at the correspondence between Medicare, medical records and self-reported treatment and recurrence data. With the increasing emphasis on reusing existing data, the results of these validation studies will provide insight into their strengths and weaknesses.
Tumor tissue is a highly valued resource that creates the potential to do novel molecular studies, including better phenotyping and biomarker studies. Our efforts to develop the LILAC tumor tissue repository are ongoing but have so far yielded tissue provided by pathology facilities for just over 61.4% of tissues requested, which is somewhat less than anticipated but higher for cancers diagnosed within the last 10 years were received. The primary barriers to receiving tissue for cases where the participant has given consent are that institutions do not agree to provide them, they report having destroyed them, or the institution will not allow us to keep tissue blocks for long-term storage. Because LILAC tissue request materials guarantee the pathology providers return of the tissue within 48 hours of a request, most facilities were willing to release blocks at least on a temporary basis, even for recent diagnoses and those at risk for recurrence (there was marginally higher tissue receipt for living participants relative to deceased—the latter of which are no longer at risk for recurrence). Additionally, providing the option to send unstained slides, rather than tumor blocks, increased our yield of tissues provided by approximately 13 percentage points. Many institutions will kindly provide these specimens at no charge and many others charge only a modest processing fee. The exorbitant fees charged by a few institutions likely represent a reluctance to share tissues based on their own research needs or agreements.
In summary, the LILAC study is an excellent resource to study survival and survivorship among older women diagnosed with eight different cancers with varying time since diagnosis. The resource has treatment data, clinical information and tissue (on a large sample of women), with data on issues of survivorship obtained from those still alive. In addition, data within the large WHI can be used to study various interventions to improve survivorship. Researchers internal and external to WHI can utilize this resource, using previously developed methods and policies within the WHI. Given that over seven million cancers survivors living in the US are over the age of 70, the demographics of LILAC participants, their willingness to contribute to research, and the richness and maturity of the WHI and LILAC data and biospecimen resources makes LILAC exceedingly well-positioned to address the survivorship questions that account for a large and growing segment of US health care demands.
Supplementary Material
Acknowledgments
The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts N01WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118-32119, 32122, 42107-26, 42129-32, and 44221; The WHI Life and Longevity after Cancer (LILAC) study is funded by UM1 CA173642.
Footnotes
The authors have no conflicts of interest to declare.
References
- 1.de Moor JS, et al. Cancer survivors in the United States: prevalence across the survivorship trajectory and implications for care. Cancer Epidemiol Biomarkers Prev. 2013;22(4):561–70. doi: 10.1158/1055-9965.EPI-12-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Design of the Women’s Health Initiative clinical trial and observational study. The Women’s Health Initiative Study Group. Control Clin Trials. 1998;19(1):61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 3.Anderson GL, et al. Implementation of the Women’s Health Initiative study design. Ann Epidemiol. 2003;13(9 Suppl):S5–17. doi: 10.1016/s1047-2797(03)00043-7. [DOI] [PubMed] [Google Scholar]
- 4.Zafar SY, et al. The financial toxicity of cancer treatment: a pilot study assessing out-of-pocket expenses and the insured cancer patient’s experience. Oncologist. 2013;18(4):381–90. doi: 10.1634/theoncologist.2012-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Custers JA, et al. The Cancer Worry Scale: detecting fear of recurrence in breast cancer survivors. Cancer Nurs. 2014;37(1):E44–50. doi: 10.1097/NCC.0b013e3182813a17. [DOI] [PubMed] [Google Scholar]
- 6.Huang HQ, et al. Validation and reduction of FACT/GOG-Ntx subscale for platinum/paclitaxel-induced neurologic symptoms: a gynecologic oncology group study. Int J Gynecol Cancer. 2007;17(2):387–93. doi: 10.1111/j.1525-1438.2007.00794.x. [DOI] [PubMed] [Google Scholar]
- 7.Wagner LI, et al. Chemotherapy-related cognitive deficits: development of the FACT-Cog instrument. Ann Behav Med. 2004;27(S10) [Google Scholar]
- 8.Woods NF, et al. Aging Well: Observations From the Women’s Health Initiative Study. J Gerontol A Biol Sci Med Sci. 2016;71(Suppl 1):S3–S12. doi: 10.1093/gerona/glv054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cormier JN, et al. Measuring quality of life in patients with melanoma: development of the FACT-melanoma subscale. J Support Oncol. 2005;3(2):139–45. [PubMed] [Google Scholar]
- 10.Lloyd-Jones DM, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. 2010;121(4):586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 11.Yost KJ, et al. Development and validation of a self-report lower-extremity lymphedema screening questionnaire in women. Phys Ther. 2013;93(5):694–703. doi: 10.2522/ptj.20120088. [DOI] [PubMed] [Google Scholar]
- 12.Anderson GL, et al. Use of administrative data to increase the practicality of clinical trials: Insights from the Women’s Health Initiative. Clin Trials. 2016;13(5):519–26. doi: 10.1177/1740774516656579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.College of American Pathologists (CAP) Retention of Laboratory Records and Materials. Available from: http://www.ncleg.net/documentsites/committees/PMC-LRC2011/December%205,%202012/College%20of%20American%20Pathologist%20Retention%20Policy.pdf.
- 14.Hernan MA, Hernandez-Diaz S, Robins JM. A structural approach to selection bias. Epidemiology. 2004;15(5):615–25. doi: 10.1097/01.ede.0000135174.63482.43. [DOI] [PubMed] [Google Scholar]
- 15.Krok-Schoen JL, BB, et al. An environmental scan of cancer survivor epidemiology cohort studies. 2016 [Google Scholar]
- 16.Society, A.C. Cancer Treatment & Survivorship Facts & Figures 2014–2015. Facts & Figures. 2014 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.