Abstract
Background and Aims
In schizophrenia, abnormalities in structural connectivity between brain regions known to contain mirror neurons and their relationship to negative symptoms related to a domain of social cognition are not well understood.
Methods
Diffusion tensor imaging (DTI) scans were acquired in 16 patients with first episode schizophrenia and 16 matched healthy controls. FA and Trace of the tracts interconnecting regions known to be rich in mirror neurons, i.e., anterior cingulate cortex (ACC), inferior parietal lobe (IPL) and premotor cortex (PMC) were evaluated.
Results
A significant group effect for Trace was observed in IPL-PMC white matter fiber track (F (1, 28) = 7.13, p = .012), as well as in the PMC-ACC white matter fiber track (F (1, 28) = 4.64, p = .040). There were no group differences in FA. In addition, patients with schizophrenia showed a significant positive correlation between the Trace of the left IPL-PMC white matter fiber track, and the Ability to Feel Intimacy and Closeness score (rho =.56, p = 0.036), and a negative correlation between the Trace of the left PMC-ACC and the Relationships with Friends and Peers score (rho =.−.54, p = 0.049).
Conclusions
We have demonstrated disrupted white mater microstructure within the white matter tracts subserving brain regions containing mirror neurons. We further showed that such structural disruptions might impact negative symptoms and, more specifically, contribute to the inability to feel intimacy (a measure conceptually related to theory of mind) in first episode schizophrenia;. Further studies are needed to understand the potential of our results for diagnosis, prognosis and therapeutic interventions.
Keywords: Mirror neuron, First-Episode Schizophrenia, White Matter Connectivity, Diffusion tensor imaging
Introduction
Schizophrenia is a severe mental disorder, characterized by a number of impairments that include sensory and cognitive processing abnormalities (Kalkstein et al. 2010). However, in addition to these fairly well examined abnormalities, sometimes called ‘cold cognition’ abnormalities, schizophrenia patients experience a number of impairments in social cognition (Mehta et al. 2014). Indeed, it has been suggested that schizophrenia should be thought of as a ‘social brain’ disorder (Burns 2006). Social cognition has been defined as an ability to communicate feelings and attitudes and to understand signals that express these feelings and attitudes in other people (Adolphs 2010). Social cognition skills are critical to successful functioning in social contexts and, in schizophrenia, their deficits contribute to poor functional outcomes (Pinkham 2014). It is now understood that the social cognition is mediated by an interconnected network of cortical and subcortical brain regions, including orbitofrontal and ventromedial frontal cortex, cingulate cortex, insula, striatum and amygdala (McDonald et al. 2013) (Adolphs 2010).
Mirror neurons, first discovered in the ventral pre-motor cortex of the macaque (area F5) (di Pellegrino et al. 1992);(Rizzolatti et al. 1996) play a unique role in the brain architecture in relation to social cognition skills. These neurons are active not only during an action execution but also when an action of others is observed. Thus, It has been proposed that they may confer not only the capacity to anticipate the actions of others but that they have contributed to the development of the theory of mind and empathy, as well as other important aspects of social cognition skills in non-human primates and humans (Bonini 2016; Ferrari 2014). Following the identification of mirror neurons in the pre-motor cortex, subsequent studies have identified a network of mirror neuron housing regions which include superior temporal sulcus (STS), rostral inferior parietal lobule (rIPL), dorsal premotor cortex (dPMC), medial frontal cortex (MFC), ventrolateral prefrontal cortex (VLPFC) and anterior cingulate gyrus (ACG) (Bonini 2016).
Research on mirror neuron (MN) system abnormalities in schizophrenia is still in infancy (Mehta et al. 2014). However, altered activation in the regions containing mirror neurons have been reported in a number of studies in schizophrenia (Mehta et al. 2014). Most studies have found reductions in activity in MN rich brain regions in tasks that required either observation of socially relevant movement or in tasks that tapped social cognition directly, i.e., in studies using tasks that tested theory of mind, imitation or attributional bias (Mehta et al. 2014). For example, three studies reported an association with social cognition measures: Mehta and colleagues reported an association with theory of mind (Mehta et al. 2014), an association with attributional style and (Horan et al. 2014) reported a relationship with perspective taking. A recent functional MRI study by Quintana et al. suggested that individuals with chronic schizophrenia demonstrate abnormal activation of mirror neuron-related cortical regions while viewing emotional stimuli (Quintana et al. 2001). A study by Thakkar et al. found reduced activity of the mirror neuron system during action observation. The degree of activation was related to symptom severity and social cognition in patients with chronic schizophrenia (Thakkar et al. 2014). Furthermore, some studies reported that reduced MN activation was associated with more negative symptoms, and one study found an association with auditory hallucinations (Mehta et al. 2014).
While there is a limited number of studies examining the activation of brain regions comprising MN in schizophrenia, studies focusing on connections among regions making up the MN system in schizophrenia are even fewer. The one study that explicitly examined white matter connections between regions forming the MN system looked at the third branch of the superior longitudinal fasciculus connecting the inferior frontal gyrus (IFG) and the supramarginal gyrus (SMG) and the callosal fibers connecting IFG and SMG in chronic schizophrenia (Tseng et al. 2015). White matter integrity was found to be reduced in the callosal fibers connecting the SMG and IFG. No relationship to symptoms was found.
We posit that studies exploring the relationship between structural connectivity of regions including MN and schizophrenia symptoms and social cognition skills are important since schizophrenia has been viewed as a disconnection syndrome (Friston and Frith 1995), with evidence suggesting that white matter fiber tracts might be abnormally myelinated (Flynn et al. 2003); (Hakak et al. 2001). Since white matter tracts provide long range neuronal connectivity that is necessary for the brain networks to function properly, it is conceivable that the functional abnormalities related to MN network may be due to not only faulty neuronal activation, but also to the disrupted white matter interconnecting brain regions constituting this network.
Therefore, the aim of this study was to use free-surfer cortical parcellation, and state of the art tractography algorithm (unscented Kalman filter based two-tensor tractography algorithm (Malcolm et al. 2009)) to evaluate the white matter microstructure of the fiber tracks connecting the premotor area, the inferior parietal lobule and the anterior cingulate gyrus in patients diagnosed with a first-episode schizophrenia. We used these regions as approximation for MN hub locations, based on previous functional experiments (Bonini 2016). A secondary aim was to test for an association between measures of white matter microstructure and measures of social cognition. To the extent that social cognition is understood as the ability to communicate feelings and attitudes to other people and to understand these signals by other people, we have selected items from the Anhedonia and Asociality subscale of the Scale for the Assessment of Negative Symptoms (SANS) as best representing skills belonging to the domain of social cognition. We selected this measure for two reasons: 1. it is a subscale of negative symptoms scale and, as described above, negative symptoms have been associated with reduced activity in regions rich in MNs and 2. The two items of the subscale, ‘the ability to feel closeness and intimacy measure’ and the ‘relationships with friends and peers’ represent skills related to the construct of theory of mind, believed to be mediated by regions including MN.
We hypothesized the presence of white matter abnormalities in patients experiencing first-episode schizophrenia in white matter tracks connecting MN rich brain regions. We also postulated that subtle changes in the microstructure of those tracts may be associated with proxy measures of social cognition included in the SANS Anhedonia-Asociality scale, and especially with ‘the ability to feel intimacy and closeness’ and with ‘the relationships with friends and peers’. To the best of our knowledge, this is the first study to investigate the structural connectivity of the brain regions that include mirror neurons in first episode schizophrenia.
Methods and Materials
Participants
Sixteen patients with first episode schizophrenia (mean age (SD), 20.19 years (4.51)) and sixteen healthy controls (mean age (SD), 21.88 years (2.71)) were selected from the larger sample recruited via the Boston CIDAR study (www.bostoncidar.org) (see Table 1 for clinical and demographic information). Patients were recruited from local hospital and outpatient clinics, referrals from clinicians, advertisements, and outreach presentations. Controls were recruited from the general community via advertisements.
Table 1.
Demographic and clinical characteristics of study groups
| Variable | SZ Patients (n = 16) |
Control Subjects (n = 16) |
df | t/χ2/u | P value |
|---|---|---|---|---|---|
| Age (years) | 20.19 (4.51) [14–31] |
21.88 (2.71) [17–26] |
30 | t = 1.29 | .209 |
|
| |||||
| Male/female | 12/4 | 10/6 | 1 | χ2 = 0.582 | .446 |
|
| |||||
| Race | 12 White 2 Black or African 1 Asian, Western 1 Asian, East Southeast |
4 White 3 Black or African American 9 Unknown |
– | – | – |
|
| |||||
| Ethnicity | 14 Not Hispanic or Latino 2 Hispanic or Latino |
6 Not Hispanic or Latino 1 Hispanic or Latino 9 Unknown |
– | – | – |
|
| |||||
| Years of formal schooling | 12.81(3.124) (n=16) |
13.57(1.512) (n=7) |
21 | t =−.606 | .551 |
|
| |||||
| Wechsler Abbreviated Scale of Intelligence (WASI) | 110.29(14.751) (n=14) |
114.86(14.358) (n=7) |
19 | t =−.675 | .508 |
|
| |||||
| Parental Socioeconomic Status (PSES) | 2.31 (1.35) | 1.94 (0.68) | — | u=117.00 | .696 |
|
| |||||
| Wide Range Achievement Test fourth revision | 111.63 (19.21) | 110.71 (21.49) (n=7) |
21 | t =0.101 | .920 |
| (WRAT- 4) | |||||
|
| |||||
| Medication Dosage (CPZ Equivalent)a | 286.04 (378.015) (n=16) |
— | — | — | — |
| Medication Typeb | 10 atypical, 2 both, 4 unmedicated | — | — | — | — |
| Age at Onset (years) | 19.44 (4.69) | — | — | — | — |
| Total global score of SAPS | 4.60 (3.64) (n=15) | — | — | — | — |
| Total global score of SANS | 9.21 (4.54) (n=14) | — | — | — | — |
CPZ, chlorpromazine; SANS, Scale for the Assessment of Negative Symptoms; SAPS, Scale for the Assessment of Positive Symptoms; WRAT-4, Wide Range Achievement Test, fourth revision (premorbid IQ estimate based on Reading subtest) ; WASI, Wechsler Abbreviated Scale of Intelligence; PSES, Parental Socioeconomic Status based on Hollingshead score (1–5 scale, 1 highest)
Medication dosages: patients’ medication dosages at time of clinical interview were converted into chlorpromazine equivalents on the basis of the Lehman, et al. (Lehman and Steinwachs 1998)conversion for typical antipsychotics and the Woods (Woods 2003) conversion for atypical antipsychotics.
Medication type: at the time of interview, 10 were taking atypical antipsychotics (aripiprazole, clozapine, risperidone, quetiapine or olanzapine), 2 patients were taking both typical and atypical antipsychotics (clozapine and haloperidol, aripiprazole and haloperidol), and 4 patients were unmedicated.
Clinical diagnoses were based on interviews with the Structured Clinical Interview for the DSM-IV-TR, Research Version (SCID)(First et al. 2002) for ages >18 as well as information from available medical records. All clinical participants were diagnosed with schizophrenia (DSM-IV-TR criteria), and were assessed with Scale for the Assessment of Positive Symptoms (SAPS) and SANS. Healthy controls were drawn from the same geographic base as the schizophrenia group with comparable age, gender, race and ethnicity, handedness, and parental socioeconomic status (PSES), and were screened for Axis I disorders using the SCID for DSM-IV-TR, Non-patient Edition. No healthy controls met criteria for any current major DSM-IV-TR Axis I disorders, or any history of psychosis, Major Depression (recurrent), Bipolar disorder, Obsessive Compulsive Disorder, Post Traumatic Stress Disorder, or developmental disorders. Healthy controls were also excluded for any history of psychiatric hospitalizations, prodromal symptoms, schizotypal or other Cluster A personality disorders, first degree relatives with psychosis, or any current or past use of antipsychotics (other past psychotropic medication use was acceptable, but the individual must have been off medication for at least 6 months before participating in the study, except for PRN medications such as sleeping or anxiolytic medications). Exclusion criteria for all participants were: sensory-motor handicaps, neurological disorders, medical illnesses that significantly impair neurocognitive function, diagnosis of mental retardation, education <9th grade, not fluent in English, DSM-IV-TR substance abuse in the past month, DSM-IV-TR substance dependence, excluding nicotine, in the past 3 months, current suicidality, a history of ECT within the past five years for patients and a history of ECT ever for controls, or study participation by another family member. Prior to MRI scanning, all subjects were screened for foreign metal in their body, pacemakers, pregnancy, claustrophobia, or any other health risks.
This study was approved by the local institutional review board committees at Beth Israel Deaconess Medical Center, the Veterans Affairs Boston Healthcare System (Brockton campus), Harvard Medical School, Brigham and Women’s Hospital, and Massachusetts General Hospital. Written informed consent was obtained for all subjects.
Image Acquisition
Diffusion weighted image (DWI) scans were acquired on a 3-Tesla GE Echospeed system (General Electric Medical Systems, Milwaukee, WI). A double echo option was used to reduce eddy-current related distortions. Acquisitions included 51 gradient directions with b=900 and eight baseline scans with b=0. The original GE sequence was modified to increase spatial resolution and to further minimize image artifacts. The following scan parameters were used: TR 17000 ms, minima TE (<80 ms), FOV 24 cm, 144×144 encoding steps, 1.7 mm slice thickness. All scans had 85 axial slices parallel to the AC–PC line covering the whole brain. After downloading from the scanner, data were preprocessed using our in-house software. This involved motion correction and eddy current distortion correction.
Two-tensor Tractography
We reconstructed the tracts interconnecting cortical regions believed to be part of the MN network in two steps: we performed tractography of the whole brain first and then filtered only the tracts of interest by selecting those that connected the premotor area (caudal middle frontal gyrus (Desikan et al. 2006)), the inferior parietal lobule and the anterior cingulate gyrus.
In the first step of the process, white matter tracts of the entire brain for each subject were generated using unscented Kalman filter based two-tensor tractography algorithm (Malcolm et al. 2009). This method makes it possible to follow tracts that pass through branching and crossing regions of the brain (Malcolm et al. 2010). Seeding was done in all voxels of the brain where single tensor FA was greater than 0.18. Each voxel was seeded 10 times, and tract was terminated only when FA dropped below 0.15.
In the second step, the tracts of the whole brain were filtered using brain parcellations generated by FreeSurfer software 4.2.0 (http://surfer.nmr.mgh.harvard.edu/) and registered to DTI data using ANTS algorithm (Avants et al. 2011). Tracts were filtered through the three free-surfer provided ROIs that closest correspond to known MN network hubs: caudal middle frontal area, the inferior parietal lobule, and the anterior cingulate gyrus, and they all included both gray and white matter. The mean FA and Trace were calculated for each of the tracts in each subject separately. To assess interrater reliability, two raters (YS and TR), who were blind to the diagnosis, evaluated the left IPL-PMC FA and Trace of the five randomly selected cases. Intraclass correlation coefficients for FA achieved 0.99 and for Trace achieved 1.00.
Statistical Analysis
Statistical analyses were performed using SPSS version 19 (IBM, New York, USA, www.spss.com). To determine group differences in each DTI measure (FA, Trace), a repeated-measures analysis of variance (ANCOVA) was applied for each DTI measure separately with group as a between-subjects factor, side as a within-subjects factor, and age and gender as covariates. To verify our hypotheses regarding white matter tracks connecting MN rich brain regions and their contribution to social cognition we used multiple hierarchical regression models to investigate the association between DTI measures for which significant group differences were found and the items of the Anhedonia-Asociality subscale of the SANS). They were followed by Spearman’s correlations with significant levels set at p < .05 because of the hypothesis driven nature of the correlation analyses. In addition, associations between medication dosage and diffusion measures were evaluated. A p < .05 was considered statistically significant.
Results
Demographic and Behavioral Data
There were no group differences in Parental Socioeconomic Status (PSES), Wide Range Achievement Test fourth revision (WRAT- 4 scores), Years of formal schooling, or Wechsler Abbreviated Scale of Intelligence (WASI) between groups. Twelve patients were treated with antipsychotic medication (n =10 only atypical antipsychotics; n = 2 both typical and atypical antipsychotics). The mean daily antipsychotic medication dosage was 286.04 ±378.015 mg (Chlorpromazine equivalent). Four patients were unmedicated at the time of the scan. The mean of the Total global scores of SAPS and Scale for the Assessment of Negative Symptoms (SANS) were 4.60 ±3.64 (n= 15) and 9.21 ±4.54 (n= 14), respectively (Table 1).
DTI Measures
For the IPL to PMC connections, ANCOVA revealed a significant effect of group on Trace, with higher values in the patients compared with healthy controls (F (1, 28) = 9.308, p = .005). In addition, there was no side-by-group interaction (F (1, 28) = .001, p = .977). For FA, there were no group effects (F (1, 28) = 1.219, p = .279) or side-by-group interactions (F (1, 28) = 0.038, p = .847).
For the PMC to ACC connections, ANCOVA also revealed a significant (but weaker than for the IPL-PMC) effect of group on Trace, again with higher values in the patients compared with healthy controls (F (1, 28) = 4.64, p = .040). In addition, there was no side-by-group interaction (F (1, 28) = .844, p = .366), no significant side-by-gender interaction (F (1, 28) = .723, p = .402). For FA, there were no group effects (F (1, 28) = 3.697, p = .065) or side-by-group interactions (F (1, 28) = 2.161, p = .153) (Figure 1) (Figure 2).
Figure 1.
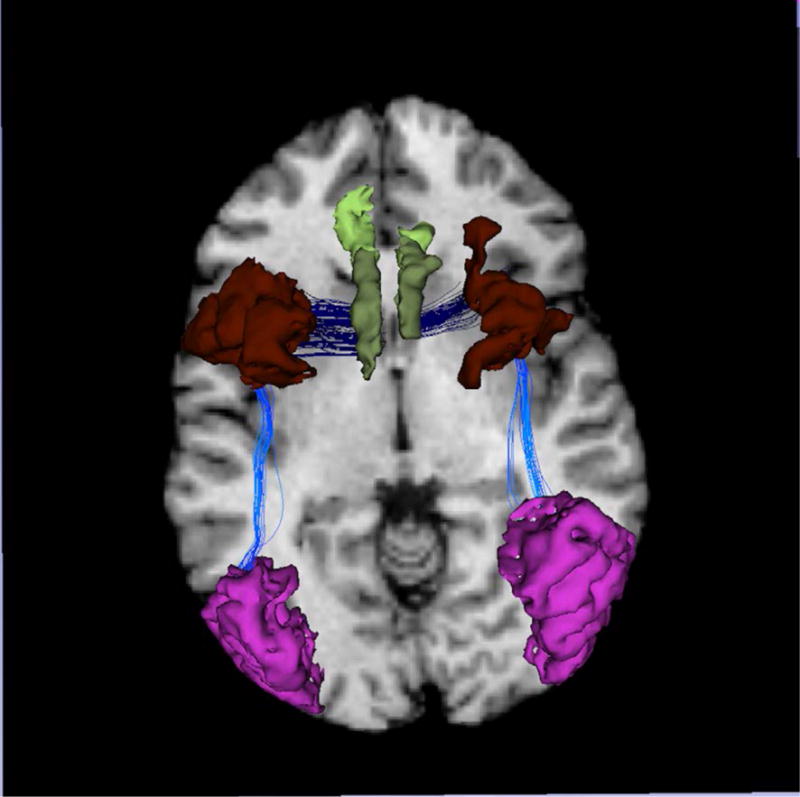
The tracts (dark and bright blue) interconnecting regions known to be rich in mirror neurons, inferior parietal lobule (Red), premotor cortex (Brown), and anterior cingulate cortex (Green).
Figure 2.

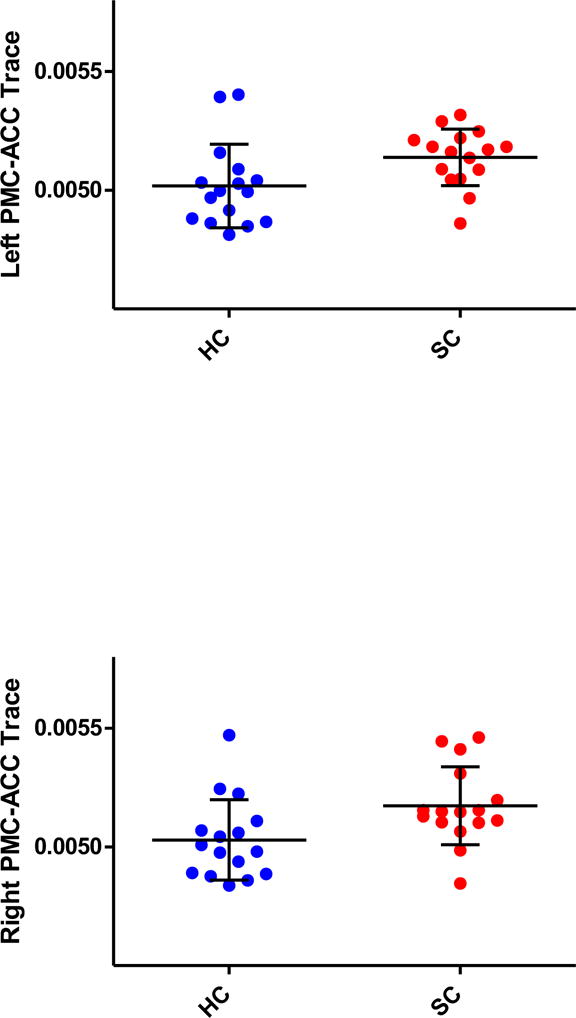
Scatterplots illustrating the variations in the Trace between groups (schizophrenia in red, control subjects in blue)
No direct IPL-ACC connections could be traced with DTI.
There were no statistical group differences between the numbers of fibers of the tracts, IPL-PMC and PMC-ACC, between the patients and the controls (t (30) =.411, p = 0.684) (right IPL-PMC; t (30) =−1.745, p = .091) (left PMC-ACC; t (30) =−.170, p = .866) (right PMC-ACC; t (30) =−.643, p = .525).
Multiple Hierarchical Regressions Models and Correlations of DTI Measures with Clinical
Parameters
For the Trace left IPL-PMC connection, among the hierarchical multiple regression models, the only model that significantly associated this connection with items of the Anhedonia-Asociality subscale was the model including ‘ability to feel intimacy and closeness’ (R2= .32, p<0.036). No other models predicted the association of the left Trace IPL-PMC with items of Anhedonia-Asociality subscale. No multiple regression model predicted an association between Trace right IPL-PMC connection and items of the Anhedonia- Asociality subscale. For the Trace, both left and right PMC-ACC connections, the model that significantly predicted the connections included all items of the Anhedonia-Asociality subscale except for the Global Rating of Anhedonia-Asociality (R2= .47, p<0.04 (for the left PMC-ACC) and R2 = .52, p<0.38). No other model predicted the PMC-ACC connection either on the left or the right. It should be noted though that ‘Relationships with Friends and Peers’ was the item that contributed to a significant association between the left/right PMC-ACC connectivity and the predictors from the Anhedonia-Asociality subscale. The items that resulted in significant multiple regression results were also tested with a correlational approach: in the patient group, a significant positive correlation between Trace of the IPL-PMC connection in the left hemisphere and the Ability to Feel Intimacy and Closeness score (rho =.57, p = .034) (Figure 3-1) was found, and a negative correlation was found between left PMC-ACC and the Relationships with Friends and Peers score (rho=−.54, p =0.049). (Figure 3–2)
Figure 3-1.
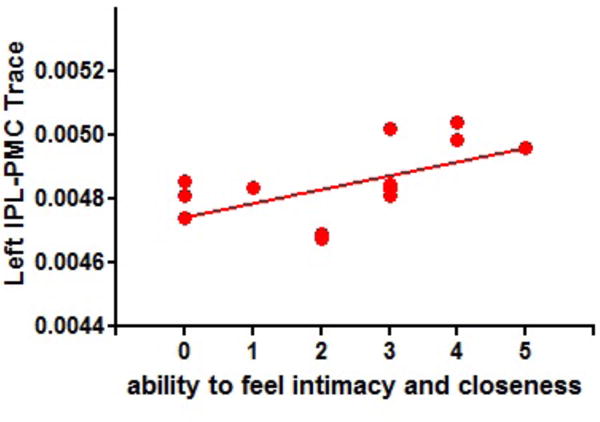
Scatterplot illustrating the relationship between scores on the Ability to Feel Intimacy and Closeness test (an item of the Anhedonia-Asociality subscale of the SANS) and Trace in the left IPL-PMC in first-episode schizophrenia. The solid line represents the best linear fit for the data. (rho =.56, p = .036)
Two patients have the same Trace and the score. Their scale is 2 and their Trace is 0.00468. It shows 2 plots on score 2. But actually there are 3 plots on the score.
Figure 3-2.
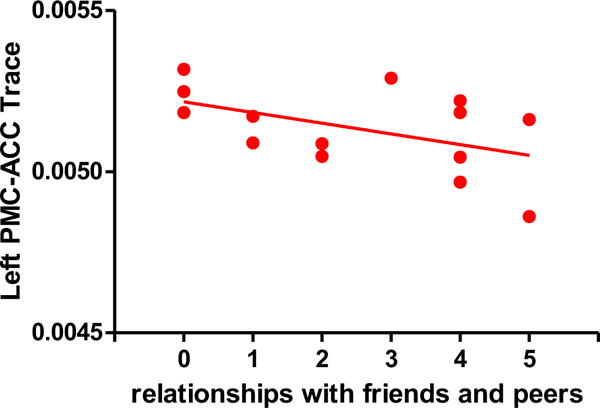
Scatterplot illustrating the relationship between scores on the Relationships with Friends and Peers test (an item of the Anhedonia-Asociality subscale of the SANS) and Trace in the left PMC-ACC in first-episode schizophrenia. The solid line represents the best linear fit for the data. (rho=−.54, p =0.049)
Eleven patients had the illness duration data. There was no correlation between illness duration and trace in the tracts, IPL-PMC and PMC-ACC (left IPL-PMC: rho = .032, p = .926) (right IPL-PMC: rho = .223, p = .509) (left PMC-ACC: rho = .182, p = .593) (right PMC-ACC: rho = .018, p = .958).
There was no correlation between age and trace in the tracts, IPL-PMC and right PMC-ACC in first-episode schizophrenia (left IPL-PMC: rho = −.28, p = .12) (right IPL-PMC: rho = −.09, p = .61) (right PMC- ACC: rho = −.13, p = .47). We have observed linear relationship between left PMC-ACC Trace and age (rho = −.37, p = .038).
Medication dosage showed no association with either left IPL-PMC Trace (rho =, 222, p =.334 or with the Ability to Feel Intimacy and Closeness score (rho =.072, p = .806).
Discussion
The primary findings of this study are that tracts interconnecting areas believed to be part of the MN network that is IPL-PMC and PMC-ACC, showed overall higher diffusivity (Trace) in first episode schizophrenia patients compared to healthy controls. No group differences or clinical associations have been found for FA diffusion measures, suggesting that the Trace measure might serve as an early indicator of abnormal connectivity between regions containing MNs, and possibly may be a target for clinical intervention. Furthermore, using both a multiple regression and correlational approach, the Trace increase in the left IPL-PMC showed statistical association uniquely with the ‘ability to feel intimacy and closeness’ score, a negative symptom measure that is related to the theory of mind construct, suggesting that abnormal IPL-PMC connectivity may contribute to negative symptomatology and poor social function in schizophrenia. In addition, the ‘relationships with friends and peers’ score, another negative symptom and proxy social cognition measure, contributed to significant association with the Trace left PMC-ACC demonstrated via both multiple regression and correlational approach.
The findings of this study, although preliminary, add to the emerging evidence on the anatomy and biological timing of structural connectivity abnormalities, especially as they relate to MN brain regions, and their associations with negative symptoms and social cognition skills impairment in schizophrenia. White matter pathology, as measured by DTI, has been reported in both first-episode as well as chronic schizophrenia.
DTI is an advanced MRI technique to investigate white mater of human brain. The diffusion of water molecule is anisotropic in white matter, limited in the direction perpendicular to the axon. Fractional anisotropy and Trace are the quantitative indices for anisotropy of the water molecule. FA reflects the degree of directionality of water diffusion while Trace measures the overall displacement of the water molecules (Basser 1995) (Kubicki et al. 2007). While majority of schizophrenia studies take FA and Trace differences as support for the dysmyelination theory of schizophrenia (Kubicki et al., 2007), it is worth noting that the diffusion is not only sensitive to myelin changes, but also depends on the axonal packing, axonal size, extracellular water content and tract geometry (Jones et al. 2013).”
Proportionally larger increases in Trace (measure of overall diffusion within tissue (Warach et al. 1992; Basser and Pierpaoli 1996; Pierpaoli et al. 1996; Basser and Pierpaoli 1998; Rowley et al. 1999)), when compared to much smaller and more circumscribed decreases in FA, have been recently reported in early course of schizophrenia (Moriya et al. 2010), as well as in the entire brain in first episode schizophrenia (Lee et al. 2013), when compared to healthy controls. Differences in FA have been taken as support for the dysmyelination theory of schizophrenia (Kubicki et al., 2007). The authors of those investigations concluded that an increase in Trace may represent increased extracellular volume, which could potentially predate desmyelination. In fact, this exact hypothesis has been recently tested by our group on two patient populations representing two different stages of the disease (first episode and chronic schizophrenia). In these studies, Pasternak et al. applied free-water imaging (measure of extracellular water) and found a significant, widespread increase in extracellular volume in first episode schizophrenia, that was much more restricted in chronic phase (Pasternak et al. 2012). The current study adds additional support to this finding and specifically implicates connections that link brain regions containing mirror neurons. In addition, the fact that negative symptomatology related to theory of mind skills has been found to be associated with early pathological changes in white matter connections serving MN network could suggest that disruptions of this fronto-parietal network might be one of the core schizophrenia symptoms. In further support of this idea, Horan et al. reported that impairment in social cognition may already be present at first-episode of schizophrenia (Horan et al. 2012).
As described above, the degree of patients’ ability to feel intimacy and closeness was positively correlated with Trace of the IPL-PMC in the left hemisphere. In addition, a negative correlation was found between Trace of the left PMC-ACC connection in the left hemisphere and ‘relationships with friends and peers’. Brain regions containing MNs tend to be multimodal and bilateral and even motor regions in the human brain can play a role in the processing of multimodal information (Mehta et al. 2014). However, the left hemisphere of the human brain specifically seems to be well suited to the processing of multimodal inputs (Aziz-Zadeh et al. 2004). It has been suggested by many authors that cognition grounded in the analysis of action and its possible consequences has given rise to the development of social cognition skills (Mehta et al. 2014; Bonini 2016). The finding of the associations between abnormal IPL-PMC and PMC-ACC connections in the left hemisphere and proxy measures of social cognition seems to indicate that faulty connectivity, in addition to the possible abnormalities in the activity of the neurons within the MN rich regions, may contribute to social cognition dysfunction in schizophrenia. Further studies are needed to fully understand the functional correlates of laterality in such pathology.
This is the first study to examine the structural connectivity between the brain regions housing MNs in first-episode schizophrenia. As such, it is not free from limitations.
Previously published studies have demonstrated relationship between age and Trace in healthy control subjects (Lebel et al. 2008; Westlye et al. 2010). In addition, white matter has been also shown to be under the influence of sex hormones (Perrin et al. 2009; Herting et al. 2012), and gender related asymmetries are common finding in white matter research (Gur et al. 1999; Good et al. 2001). We thus used age and sex as covariates. DTI is a rather approximate technique(Jones et al. 2013). The fibers are not actual ones strictly. We have to say we have limitation of our results about this. As for the multiple comparisons, we acknowledge that our sample size is small, and that we consider our experiments and analysis exploratory.
It is also worth pointing out that while the subjects underwent rigorous clinical testing that allowed examining their positive and negative symptomatology, explicit social cognition measures have not been used in this investigation. Thus, future studies that explicitly examine both negative symptomatology and relevant social cognition measures that target theory of mind should be designed in order to address the question of the relationship between structural connectivity abnormalities and clinical manifestations and theory of mind measures. Furthermore, the presence of “mirror neurons” has been inferred from previous literature, and not experimentally derived here. Ideally, a functional task engaging brain regions rich in mirror neurons coupled with the analysis of structural connections between these regions would bolster the claims of unique contributions of faulty connectivity to mirror neuron related impairments in social function in schizophrenia. In addition, while connections between IPL, PMC and ACC, based on previous fMRI studies, are believed to serve the MN network, they are also a part of larger white matter bundles (more specifically superior longitudinal fasciculus (SLF) and cingulum bundle (CB), and as such serve other functional networks: thus, anatomical specificity of our experiment is limited. Finally, our patient group was not homogenous regarding antipsychotic medication. While dosage showed no association with any of the diffusion measures, the medication effects could not be ruled out.
Conclusion
This study has demonstrated disrupted white mater microstructure within the white matter connections sub serving mirror neuron network. We further showed that such structural disruptions might have an impact on negative symptomatology related to theory of mind, more specifically, the inability to feel intimacy and relationships with friends and peers, in first episode schizophrenia. Further studies are needed to understand the potential of our results for diagnosis, prognosis and therapeutic interventions.
Table 2.
Significant effect of group on Trace
| White matter tracts | F | P value |
|---|---|---|
| IPL-PMC | F (1, 28) = 9.308 | .005 |
| PMC-ACC | F (1, 28) = 4.64 | .040 |
Abbreviations: IPL, inferior parietal lobe; PMC, premotor cortex; ACC, anterior cingulate cortex
Table 3.
Relationships between Trace and clinical scores.
| White matter tracts | Ability to Feel Intimacy and Closeness score (n=14 patients) | Relationships with Friends and Peers score (n=14 patients) | Duration (n=11 patients) |
|---|---|---|---|
| Left IPL-PMC | rho =.56, p = .036 | rho = .259, p = .371 | rho = .032, p = .926 |
| Right IPL-PMC | rho =.02, p = .95 | rho = − .045, p = .879 | rho = .223, p = .509 |
| Left PMC-ACC | rho =−.052, p = .86 | rho = −.535, p = .049 | rho = .182, p = .593 |
| Right PMC-ACC | rho =−.025, p = .93 | rho = − .325, p = .257 | rho = .018, p = .958 |
Abbreviations: IPL, inferior parietal lobe; PMC, premotor cortex; ACC, anterior cingulate cortex
Acknowledgments
FUNDING
This work was supported in part by a Department of Veteran Affairs Merit Award (MES), and in part by NIH grants including 1P50MH080272 CIDAR (RWM, MES), P41 RR13218 (MES), R01 MH 50740 (MES), NA-MIC (NIH) grant U54 GM072977 (MK), 1R01 AG04252 (MK and OP), R01 MH102377 (MK, MES and OP). R01 MH074794 (OP), 2P41 EB015902-16 (OP, MES), the Commonwealth Research Center (SCDMH82101008006; LJS), NARSAD (ZK and OP), Else Kröner-Fresenius Stiftung, Germany (IK), NIMH R21MH094509 (MAN).
Footnotes
Compliance with ethical standards
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
The Authors Yukiko Saito, Marek Kubicki, Inga Koerte, Tatsui Otsuka, Yogesh Rathi, Ofer Pasternak, Sylvain Bouix, Ryan Eckbo, Zora Kikinis, Christian Clemm von Hohenberg, Tomohide Roppongi, Elisabetta Del Re, Takeshi Asami, Sang-Hyuk Lee, Raquelle I. Mesholam-Gately, Larry J. Seidman, James Levitt, Robert W. McCarley, Martha E. Shenton, and Margaret A. Niznikiewicz have declared that there are no conflicts of interest in relation to the subject of this study.
RESEARCH INVOLVING HUMAN PARTICIPANTS AND/OR ANIMALS
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
INFORMED CONSENT
Informed consent was obtained from all individual participants included in the study.
References
- Adolphs R. Conceptual challenges and directions for social neuroscience. Neuron. 2010;65(6):752–767. doi: 10.1016/j.neuron.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage. 2011;54(3):2033–2044. doi: 10.1016/j.neuroimage.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz-Zadeh L, Iacoboni M, Zaidel E, Wilson S, Mazziotta J. Left hemisphere motor facilitation in response to manual action sounds. [Comparative Study Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.] The European journal of neuroscience. 2004;19(9):2609–2612. doi: 10.1111/j.0953-816X.2004.03348.x. [DOI] [PubMed] [Google Scholar]
- Basser PJ. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images [Review] NMR in biomedicine. 1995;8(7–8):333–344. doi: 10.1002/nbm.1940080707. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. Journal of magnetic resonance Series B. 1996;111(3):209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. A simplified method to measure the diffusion tensor from seven MR images. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 1998;39(6):928–934. doi: 10.1002/mrm.1910390610. [DOI] [PubMed] [Google Scholar]
- Bonini L. The Extended Mirror Neuron Network: Anatomy, Origin, and Functions. Neuroscientist. 2016 doi: 10.1177/1073858415626400. [DOI] [PubMed] [Google Scholar]
- Burns J. The social brain hypothesis of schizophrenia. World Psychiatry. 2006;5(2):77–81. [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] Neuroimage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- di Pellegrino G, Fadiga L, Fogassi L, Gallese V, Rizzolatti G. Understanding motor events: a neurophysiological study. Exp Brain Res. 1992;91(1):176–180. doi: 10.1007/BF00230027. [DOI] [PubMed] [Google Scholar]
- Ferrari PF. The neuroscience of social relations. A comparative-based approach to empathy and to the capacity of evaluating others’ action value. Behaviour. 2014;151(2–3):297–313. doi: 10.1163/1568539X-00003152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Biometrics Research. New York: New York State Psychiatric Institute; 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P) [Google Scholar]
- Flynn SW, Lang DJ, Mackay AL, Goghari V, Vavasour IM, Whittall KP, et al. Abnormalities of myelination in schizophrenia detected in vivo with MRI, and post-mortem with analysis of oligodendrocyte proteins. Mol Psychiatry. 2003;8(9):811–820. doi: 10.1038/sj.mp.4001337. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD. Schizophrenia: a disconnection syndrome? Clin Neurosci. 1995;3(2):89–97. [PubMed] [Google Scholar]
- Good CD, Johnsrude I, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. Cerebral asymmetry and the effects of sex and handedness on brain structure: a voxel-based morphometric analysis of 465 normal adult human brains. Neuroimage. 2001;14(3):685–700. doi: 10.1006/nimg.2001.0857. [DOI] [PubMed] [Google Scholar]
- Gur RC, Turetsky BI, Matsui M, Yan M, Bilker W, Hughett P, et al. Sex differences in brain gray and white matter in healthy young adults: correlations with cognitive performance. J Neurosci. 1999;19(10):4065–4072. doi: 10.1523/JNEUROSCI.19-10-04065.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakak Y, Walker JR, Li C, Wong WH, Davis KL, Buxbaum JD, et al. Genome-wide expression analysis reveals dysregulation of myelination-related genes in chronic schizophrenia. Proc Natl Acad Sci U S A. 2001;98(8):4746–4751. doi: 10.1073/pnas.081071198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting MM, Maxwell EC, Irvine C, Nagel BJ. The impact of sex, puberty, and hormones on white matter microstructure in adolescents. Cereb Cortex. 2012;22(9):1979–1992. doi: 10.1093/cercor/bhr246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan WP, Green MF, DeGroot M, Fiske A, Hellemann G, Kee K, et al. Social cognition in schizophrenia, Part 2: 12-month stability and prediction of functional outcome in first-episode patients. [Research Support, N.I.H., Extramural] Schizophrenia bulletin. 2012;38(4):865–872. doi: 10.1093/schbul/sbr001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan WP, Pineda JA, Wynn JK, Iacoboni M, Green MF. Some markers of mirroring appear intact in schizophrenia: evidence from mu suppression. Cogn Affect Behav Neurosci. 2014;14(3):1049–1060. doi: 10.3758/s13415-013-0245-8. [DOI] [PubMed] [Google Scholar]
- Iacoboni M. Imitation, empathy, and mirror neurons. [Research Support, Non-U.S. Gov’t Review] Annual review of psychology. 2009;60:653–670. doi: 10.1146/annurev.psych.60.110707.163604. [DOI] [PubMed] [Google Scholar]
- Jones DK, Knosche TR, Turner R. White matter integrity, fiber count, and other fallacies: the do’s and don’ts of diffusion MRI. Neuroimage. 2013;73:239–254. doi: 10.1016/j.neuroimage.2012.06.081. [DOI] [PubMed] [Google Scholar]
- Kalkstein S, Hurford I, Gur RC. Neurocognition in schizophrenia. Curr Top Behav Neurosci. 2010;4:373–390. doi: 10.1007/7854_2010_42. [DOI] [PubMed] [Google Scholar]
- Kubicki M, McCarley R, Westin CF, Park HJ, Maier S, Kikinis R, et al. A review of diffusion tensor imaging studies in schizophrenia. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, Non-P.H.S. Review] Journal of psychiatric research. 2007;41(1–2):15–30. doi: 10.1016/j.jpsychires.2005.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage. 2008;40(3):1044–1055. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- Lee SH, Kubicki M, Asami T, Seidman LJ, Goldstein JM, Mesholam-Gately RI, et al. Extensive white matter abnormalities in patients with first-episode schizophrenia: a Diffusion Tensor Iimaging (DTI) study. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, Non-P.H.S.] Schizophrenia research. 2013;143(2–3):231–238. doi: 10.1016/j.schres.2012.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman AF, Steinwachs DM. Translating research into practice: the Schizophrenia Patient Outcomes Research Team (PORT) treatment recommendations. Schizophrenia bulletin. 1998;24(1):1–10. doi: 10.1093/oxfordjournals.schbul.a033302. [DOI] [PubMed] [Google Scholar]
- Malcolm JG, Michailovich O, Bouix S, Westin CF, Shenton ME, Rathi Y. A filtered approach to neural tractography using the Watson directional function. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, Non-P.H.S.] Medical image analysis. 2010;14(1):58–69. doi: 10.1016/j.media.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcolm JG, Shenton ME, Rathi Y. Neural tractography using an unscented Kalman filter. Information processing in medical imaging : proceedings of the … conference. 2009;21:126–138. doi: 10.1007/978-3-642-02498-6_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald S, English T, Randall R, Longman T, Togher L, Tate RL. Assessing social cognition and pragmatic language in adolescents with traumatic brain injuries. J Int Neuropsychol Soc. 2013;19(5):528–538. doi: 10.1017/S1355617713000039. [DOI] [PubMed] [Google Scholar]
- Mehta UM, Thirthalli J, Aneelraj D, Jadhav P, Gangadhar BN, Keshavan MS. Mirror neuron dysfunction in schizophrenia and its functional implications: a systematic review. Schizophr Res. 2014;160(1–3):9–19. doi: 10.1016/j.schres.2014.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriya J, Kakeda S, Abe O, Goto N, Yoshimura R, Hori H, et al. Gray and white matter volumetric and diffusion tensor imaging (DTI) analyses in the early stage of first-episode schizophrenia. Schizophrenia research. 2010;116(2–3):196–203. doi: 10.1016/j.schres.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Pasternak O, Westin CF, Bouix S, Seidman LJ, Goldstein JM, Woo TU, et al. Excessive extracellular volume reveals a neurodegenerative pattern in schizophrenia onset. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, Non-P.H.S.] The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32(48):17365–17372. doi: 10.1523/JNEUROSCI.2904-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin JS, Leonard G, Perron M, Pike GB, Pitiot A, Richer L, et al. Sex differences in the growth of white matter during adolescence. Neuroimage. 2009;45(4):1055–1066. doi: 10.1016/j.neuroimage.2009.01.023. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Di Chiro G. Diffusion tensor MR imaging of the human brain. Radiology. 1996;201(3):637–648. doi: 10.1148/radiology.201.3.8939209. [DOI] [PubMed] [Google Scholar]
- Pinkham AE. Social cognition in schizophrenia. J Clin Psychiatry. 2014;75(Suppl 2):14–19. doi: 10.4088/JCP.13065su1.04. [DOI] [PubMed] [Google Scholar]
- Quintana J, Davidson T, Kovalik E, Marder SR, Mazziotta JC. A compensatory mirror cortical mechanism for facial affect processing in schizophrenia. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, Non-P.H.S. Research Support, U.S. Gov’t, P.H.S.] Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2001;25(6):915–924. doi: 10.1016/S0893-133X(01)00304-9. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fadiga L, Gallese V, Fogassi L. Premotor cortex and the recognition of motor actions. Brain Res Cogn Brain Res. 1996;3(2):131–141. doi: 10.1016/0926-6410(95)00038-0. [DOI] [PubMed] [Google Scholar]
- Rowley HA, Grant PE, Roberts TP. Diffusion MR imaging. Theory and applications. [Review] Neuroimaging clinics of North America. 1999;9(2):343–361. [PubMed] [Google Scholar]
- Thakkar KN, Peterman JS, Park S. Altered Brain Activation During Action Imitation and Observation in Schizophrenia: A Translational Approach to Investigating Social Dysfunction in Schizophrenia. The American journal of psychiatry. 2014;171(5):539–548. doi: 10.1176/appi.ajp.2013.13040498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng CE, Chien YL, Liu CM, Wang HL, Hwu HG, Tseng WY. Altered cortical structures and tract integrity of the mirror neuron system in association with symptoms of schizophrenia. Psychiatry Res. 2015;231(3):286–291. doi: 10.1016/j.pscychresns.2015.01.010. [DOI] [PubMed] [Google Scholar]
- Warach S, Chien D, Li W, Ronthal M, Edelman RR. Fast magnetic resonance diffusion-weighted imaging of acute human stroke. Neurology. 1992;42(9):1717–1723. doi: 10.1212/wnl.42.9.1717. [DOI] [PubMed] [Google Scholar]
- Westlye LT, Walhovd KB, Dale AM, Bjornerud A, Due-Tonnessen P, Engvig A, et al. Life-span changes of the human brain white matter: diffusion tensor imaging (DTI) and volumetry. Cereb Cortex. 2010;20(9):2055–2068. doi: 10.1093/cercor/bhp280. [DOI] [PubMed] [Google Scholar]
- Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S. Review] The Journal of clinical psychiatry. 2003;64(6):663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]


