To the Editor
Little is known about the natural history of eosinophilic esophagitis (EoE). It remains unknown if patients with eosinophilic esophagitis can achieve complete clinical tolerance to food triggers, and if so, what is the likelihood that this will occur? In Kagwalla et al, 5 of the original 46 patients on elimination diet were followed out to 4-years’ time and rechallenged.1 Only one patient was found to have outgrown EoE to wheat and peanut, but continued to react to milk.1
We sought to investigate the rate of occurrence of complete clinical tolerance in eosinophilic esophagitis patients with the reintroduction of all foods removed in elimination diet therapy. We conducted a retrospective chart review of children one month to 18 years of age followed in Center for Pediatric Eosinophilic Disorders Clinic from 2005 to 2016. Subjects were included into this database following a definitive diagnosis of EoE, which was made following consensus clinical criteria including EoE symptoms and abnormal esophagogastroduodenoscopy with biopsy with more than 15 eosinophils per high powered field on esophageal biopsies.2 1,812 charts of children with EoE were reviewed for inclusion in this study and had similar ethnic and gender distribution as previously described.3
Patients were included in this cohort if: (1) initial diagnosis was consistent with EoE while on proton pump inhibitor, (2) management with removal of food(s) from their diet resulted in normalization of their esophageal biopsies, (3) patient subsequently re-introduced all foods removed for EoE management back into the diet with normal esophageal biopsies, (4) patient continued to include all foods in diet with no recurrence of EoE symptoms and no subsequent abnormal biopsies. Patients avoiding foods due to IgE-mediated food allergy were not excluded.
In total, we found 9 patients (Table 1) from the database of 1,812 eosinophilic esophagitis patients who achieved complete clinical tolerance to all removed foods, which represents an overall frequency of 0.5% of our EoE cohort. These patients presented with typical symptoms of EoE with dysphagia (44%) and regurgitation/vomiting (44%) as the most common presenting symptoms. Patients’ initial diagnostic biopsies had median eosinophil count of 30 (interquartile range 30–40), and all of these patients had been compliant with maximum dose of PPI for 6–8 weeks at the time of EoE diagnosis. Additionally, we observed a high co-occurrence of atopic comorbidities (Table 1). Allergic rhinitis was observed most frequently in 88% of the cohort with asthma in 55%, which is a slightly higher than general EoE. Additionally, there is a significantly higher proportion of female patients (44%) compared to 25% seen previously.3 Interestingly, Patient 6 was noted to have some seasonal EoE exacerbations which improved on maintenance doses of environmental allergen immunotherapy. Patient 9 had IgE-mediated food allergy to peanut and tree nuts and these foods were not reintroduced.
Table 1.
Summary of patient cohort demographics, presenting characteristics and management. Abbreviations used: M= male, F=female, AR= allergic rhinitis, OAS= oral allergy syndrome, IgE-FA= IgE mediated food allergy, AD=atopic dermatitis.
| # | Gender | Age at Diagnosis (years) | Race | Comorbid conditions | Presenting Symptoms | Initial Biopsy (eos/hpf) | Management | Duration on elimination diet (years) | % time biopsy clear while on elimination diet | # of clear follow-up endoscopy with biopsies after open diet | Follow-up interval after open diet (years) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 8 | Caucasian | AR | Dysphagia, impaction | 40 | Elimination diet | 9.1 | 43.5 | 3 | 4.3 |
| 2 | M | 4 | Caucasian | Asthma, AR | Regurgitation, vomiting | 60 | Elemental formula, then diet reintroduction | 10.1 | 51.2 | 3 | 1 |
| 3 | F | 1.5 | Caucasian | Asthma, AR | Regurgitation, vomiting | 25 | Elimination diet | 2.9 | 82.6 | 4 | 9.7 |
| 4 | M | 6 | Caucasian | AR | Dysphagia | 30 | Elimination diet | 3.4 | 20.7 | 2 | 2.7 |
| 5 | M | 11 | Caucasian | AR, ADHD | Dysphagia | 45 | Elimination diet | 1.7 | 58.8 | 1 | 6.4 |
| 6 | F | 10 | Caucasian | AR, OAS | Abdominal pain, vomiting | 50 | Elimination diet + environmental allergen immunotherapy | 4.5 | 70.4 | 2 | 2.5 |
| 7 | M | 3 | Caucasian | Asthma, AR | Regurgitation, reflux | 20 | Elimination diet +1 month swallowed steroids | 10.1 | 58.3 | 2 | 0.5 |
| 8 | M | 5 | Caucasian | Asthma, AR | Food aversion, slow eating | 25 | Elimination diet + swallowed steroids | 4.8 | 82.0 | 1 | 0.75 |
| 9 | F | 7 | Asian | IgE-FA, AD, Asthma | Dysphagia | 30 | Elimination diet | 2.1 | 17.9 | 4 | 1.6 |
All patients in this cohort were initially managed with targeted food elimination diet with the exception of patient 4 who was initially placed empirically on an elemental diet for less than one year and added back foods beginning with fruits and vegetables. Swallowed steroids were added into the regimens of two patients when food elimination was insufficient to control EoE (Table 1). This is a significant contrast to our overall EoE database in which 49% of patients have used swallowed steroids at some point during their management. Patient 7 required only a brief initial course of swallowed steroids and returned to elimination diet management alone. Patient 8 was on swallowed steroids for several years however these were tapered and discontinued over one year prior to the last scope on open diet.
78% of patients in this cohort removed more than one food from the diet (Figure 1A), and all but one patient had removed milk from the diet. Two patients (22%) were managed with removal of milk exclusively. Although rice and corn were triggers in this cohort, wheat was not a significant trigger for EoE in this cohort. All patients reintroducing multiple foods were managed by sequential food reintroduction. Across the cohort, the timing of endoscopy with was variable across the cohort at a mean of 9.5 months prior to the last food introduction (min 2.5, max 17.5 months). All patients had a quiescent biopsy result immediately prior to proceeding to the final food reintroduction. The mean age of EoE diagnosis was 5.9±3.9 years and the mean age patients reached clinical tolerance to all foods was 11.0±3.9 years (Figure 1B). Across the cohort, the mean duration of food avoidance was 4.9±3.3 years (Table 1, minimum 1.7 years and maximum 10.1 years). All patients had normal endoscopies with no eosinophils seen on their last biopsies following food reintroduction, and had at least one post-scope clinic visit with no recurrence of eosinophilic esophagitis symptoms while continuing full, open diet. The mean duration of follow-up after initiation of open diet across the cohort was 3.3±3.0 years (Table 1, minimum 6 months, maximum 7 years).
Figure 1.
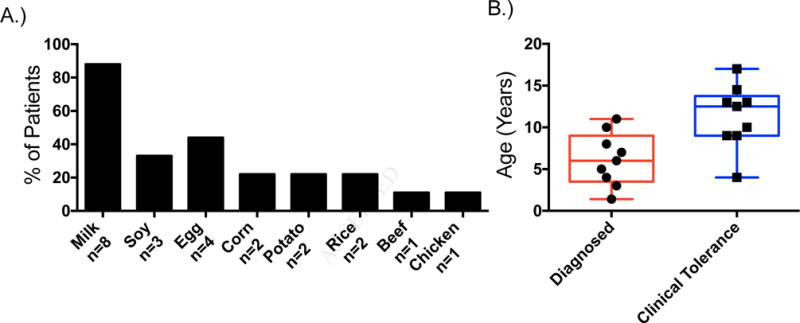
A.) Foods which were removed and ultimately reintroduced into the cohort of clinically tolerant eosinophilic esophagitis patients’ diets, along the x-axis numbers of patients per group are represented in text. B.) Age of diagnosis (red, mean age 5.9±3.9 years) and age reaching clinical tolerance to all foods (blue, mean age 11±3.9 years) of the cohort of clinically tolerant eosinophilic esophagitis patients.
It is unclear why this small group of patients experienced a period of clinical tolerance in the course of their EoE. Five of these patients had previously been enrolled in genome-wide association studies performed at our institution.4 Their inheritance of alleles associated with risk of EoE are similar to what we saw in our overall EoE population within this GWAS study. Therefore, we conclude that the occurrence of clinical tolerance in these patients is not the result of the exclusive inheritance of protective alleles.
There are several limitations to our study. Inherent to its retrospective design, there may be differences in patients’ management and follow-up which unknowingly affect our data. Further, our study examines a pediatric population and therefore may underestimate the rate of tolerance achieved due exclusion of adults. In addition, we limited the scope of this study to patients who had liberalized to an open diet with successful follow-up biopsy, and therefore this study does not provide information about patients who have partially added foods back into the diet, which based on unpublished data is closer to 10–20%.
In summary, in this retrospective study, we observe that 9 out of 1,812 total EoE patients reintroduced all foods excluded for EoE management, representing a frequency of 0.5% of EoE patients achieving complete clinical tolerance. Our study contributes knowledge about the natural history of a subset of patients followed at one US tertiary care center. Although very uncommon, we conclude that some patients with EoE can have significant periods of complete clinical tolerance. There is evidence from previous studies to indicate that after a period of avoidance a higher proportion of children are successful at adding some foods back into the diet.1,5 In keeping with this, we have observed 5 of 30 children with normal endoscopy on milk reintroduction during the prospective enrollment phase of NCT #02579876. Therefore, the process of sequential food reintroduction remains important as it opens the diet and improves quality of life for patients with EoE. Follow-up of EoE patients after food reintroduction remains essential due to risk of relapse, and should include screening for EoE symptoms and prompt endoscopy if symptoms occur. This cohort has an overrepresentation of patients managed with dietary elimination compared to our general database population, and demonstrates that in a cohort of patients achieving clinical tolerance after a period of dietary elimination the most common trigger is milk. Additional studies are needed to determine if the type of therapy chosen impacts tolerance outcome, or if there are subsets of EoE patients which may be more likely to achieve clinical tolerance than others.
Acknowledgments
Funding Sources: Support from: MAR is funded by NIH T32-HD043021, AC funded by 2015 AAAAI ART and APFED Awards, JMS is funded by Stuart Starr Endowed Chair, and in part by the Consortium of Eosinophilic Gastrointestinal Disease Researchers (CEGIR). CEGIR (U54 AI117804) is part of the Rare Diseases Clinical Research Network (RDCRN), an initiative of the Office of Rare Diseases Research (ORDR), NCATS, and is funded through collaboration between NIAID, NIDDK, and NCATS. CEGIR is also supported by patient advocacy groups including APFED, CURED, and EFC.
Abbreviations Used
- IgE
immunoglobulin E
- EoE
eosinophilic esophagitis
- M
male
- F
female
- AR
allergic rhinitis
- OAS
oral allergy syndrome
- IgE-FA
IgE mediated food allergy
- AD
atopic dermatitis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
ORCID ID INFO:
Melanie A Ruffner: 0000-0002-2943-9944
Chris A Liacouras: 0000-0002-6125-7243
Jonathan M Spergel: 0000-0002-4658-5353
Conflicts of Interest: none
Clinical Trial Registration: None
References
- 1.Kagalwalla AF, Shah A, Li BU, Sentongo TA, Ritz S, Manuel-Rubio M, et al. Identification of specific foods responsible for inflammation in children with eosinophilic esophagitis successfully treated with empiric elimination diet. J Pediatr Gastroenterol Nutr. 2011;53:145–9. doi: 10.1097/MPG.0b013e31821cf503. [DOI] [PubMed] [Google Scholar]
- 2.Liacouras CA, Furuta GT, Hirano I, Atkins D, Attwood SE, Bonis PA, et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol. 2011;128:3–20 e6. doi: 10.1016/j.jaci.2011.02.040. quiz 1–2. [DOI] [PubMed] [Google Scholar]
- 3.Spergel JM, Brown-Whitehorn TF, Beausoleil JL, Franciosi J, Shuker M, Verma R, et al. 14 years of eosinophilic esophagitis: clinical features and prognosis. J Pediatr Gastroenterol Nutr. 2009;48:30–6. doi: 10.1097/MPG.0b013e3181788282. [DOI] [PubMed] [Google Scholar]
- 4.Sleiman PM, Wang ML, Cianferoni A, Aceves S, Gonsalves N, Nadeau K, et al. GWAS identifies four novel eosinophilic esophagitis loci. Nat Commun. 2014;5:5593. doi: 10.1038/ncomms6593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spergel JM, Brown-Whitehorn TF, Cianferoni A, Shuker M, Wang ML, Verma R, Liacouras C. Identification of causative foods in children with eosinophilic esophagitis treated with an elimination diet. J Allergy Clin Immunology. 2012;130:461–7 e5. doi: 10.1016/j.jaci.2012.05.021. [DOI] [PubMed] [Google Scholar]


