Abstract
Background
Many studies have reported a higher prevalence of ADHD among disadvantaged populations, but few have considered how parental history of ADHD might modify that relationship. We evaluated whether the prevalence of ADHD varies by socioeconomic status (SES) and parental history of ADHD in a population-sample of elementary school children age 6–14 years.
Methods
We screened all children in grades 1–5 in 17 schools in one North Carolina (U.S.) county for ADHD using teacher rating scales and 1160 parent interviews, including an ADHD structured interview (DISC). We combined parent and teacher ratings to determine DSM-IV ADHD status. Data analysis was restricted to 967 children with information about parental history of ADHD. Socioeconomic status (SES) was measured by family income and respondent education.
Results
We found an interaction between family income and parental history of ADHD diagnosis (P=.016). The SES gradient was stronger in families without a parental history and weaker among children with a parental history. Among children without a parental history of ADHD diagnosis, low income children had 6.2 times the odds of ADHD (95% C.I. 3.4–11.3) as high income children after adjusting for covariates. Among children with a parental history, all had over 10 times the odds of ADHD as high income children without a parental history but the SES gradient between high and low income children was less pronounced (OR=1.4, 95% 0.6–3.5).
Conclusions
Socioeconomic status and parental history of ADHD are each strong risk factors for ADHD that interact to determine prevalence. More research is needed to dissect the components of SES that contribute to risk of ADHD. Future ADHD research should evaluate whether the strength of other environmental risk factors vary by parental history. Early identification and interventions for children with low SES or parental histories of ADHD should be explored.
Keywords: ADHD, Epidemiology, Family History, Social Class, Prevalence
Introduction
For many years, Attention-Deficit/Hyperactivity Disorder (ADHD) was believed to equally affect children of all social classes; that is, risk was not believed to vary by socioeconomic status (SES) (Polanczyk and Jensen, 2008, Barkley, 1998). This perception has persisted despite strong evidence that ADHD is more common among the poor, which has been documented in clinical samples (Biederman et al., 2002, Counts et al., 2005), population-based epidemiologic studies (Scahill et al., 1999, Lambert et al., 1978, Szatmari et al., 1989b, St Sauver et al., 2004), meta-analyses (Russell et al., 2016), and large national surveys like the National Health Interview Study (CDC/NCHS, 2007), and the National Survey of Children’s Health (Larson et al., 2011).
Given the strength of the evidence, it is puzzling that controversy exists; but two factors may play a role. First, low-income children without health care coverage are less likely to be treated for ADHD (Froehlich et al., 2007), which skews the pool of clinically identified patients toward more affluent children. The second factor is historical; the authors of two influential population-based studies of ADHD minimized SES in the etiology of ADHD when interpreting their results (Lambert et al., 1978, Szatmari et al., 1989a). Citing these two studies, a leading textbook concluded that the impact of SES on the prevalence of ADHD remains an unresolved issue (Barkley, 2015).
In one of those studies, researchers sampled 5200 children in 191 classrooms (Lambert et al., 1978). They reported that hyperactive children (the term for ADHD at the time) were disproportionately from poor families when they identified cases using parent and teacher ratings, but this association weakened when they required an additional physician diagnosis. The authors concluded that the diagnosis depended on the source of the diagnostic information (Lambert et al., 1978). However, by requiring physician diagnosis, they may have obscured the SES effect because physician access is strongly influenced by ability to pay.
The second study evaluated over 2500 children and reported a 2.8 fold increase in the odds of ADHD for participants on welfare (Szatmari et al., 1989a). However, after adjusting for developmental delays and comorbidity, the poverty effect disappeared for boys but persisted for girls. This discrepancy was difficult to explain and the authors discounted the SES finding in their conclusions. However, developmental delays are a common precursor to ADHD and comorbidity is concurrent with ADHD in about two-thirds of cases (Larson et al., 2011). By statistically controlling for these two risk factors on the causal pathway, the researchers may have obscured some of the SES effect. In both the epidemiology and the psychology literature, methodologists have cautioned against simple statistical adjustment without careful thought about the underlying causal model (Weinberg, 1993) (Beauchaine et al., 2010).
Two recent studies, and an accompanying editorial, have challenged the perception that ADHD affects all children equally, and prompted a reevaluation of the relationship between social class and ADHD risk (Russell et al., 2014, Larsson et al., 2014, Nigg and Craver, 2014). Larsson et al. found an inverse relationship between SES level and ADHD risk in a population-based cohort of over 800,000 Swedish children after accounting for co-occurring risk among siblings and cousins to assess “unmeasured familial confounding” (Larsson et al., 2014). Russell et al. reported even stronger SES effects among a cohort of 19,000 British children using multiple measures of poverty (Russell et al., 2014). They also ruled out reverse causation; families who had a child with ADHD were no more likely than families without a child with ADHD to drop in their socioeconomic position over time. Finally, Russell et al. presented evidence that parental attachment or family conflict partially mediates the association between SES and ADHD risk. These two papers have refocused attention on the possible importance of SES in the etiology of ADHD but not all observers consider the matter settled (Faraone et al., 2015).
Neither the Larsson nor the Russell paper accounted for parental history of ADHD, even though the estimated heritability of ADHD is as high as 71–90% (Thapar et al., 2013) and family history is an established risk factor for ADHD. A study using the Swedish National registry system reported that the incidence of externalizing and internalizing disorders was higher in high-deprivation neighborhoods but familial effects accounted for nearly eight times the total variation in externalizing disorders as neighborhood-level effects (Sundquist et al., 2015). One clinic-based study reported a 2.8 fold increased odds of ADHD in children whose parents had ADHD as children (Faraone et al., 2005), and another reported odds ratios of between 2.5–4.6 for fathers with childhood ADHD and between 15–23 for mothers with childhood ADHD (Chronis et al., 2003). More recently, Musser et al. used a population-based sample to evaluate the risk of ADHD among children of mothers with ADHD (Musser et al., 2014). (Data on fathers were not available.) They reported the odds of having a child diagnosed with ADHD were five-fold greater (OR=5.0, 95% C.I. 3.7–6.8; P<.001) among mothers with a prior ADHD diagnosis.
A recent study of 132 children collected histories of low birthweight and prenatal smoking, adverse life events, deviant peer affiliation, parental history of ADHD, and socio-economic status in three groups : ADHD, ADHD with oppositional behavior, and controls (Noordermeer et al., 2017). The researchers reported that adverse life events and parental history of ADHD were important risk factors for ADHD but low SES was not, after adjusting for the other cofactors. However, the inclusion in a single statistical model of low SES, adverse life events, perinatal adversities and deviant peer affiliation, which may all be on the same causal pathway, make the results difficult to interpret.
Relatively few studies have explored how family history of ADHD might modify risk factors for ADHD, an idea with new importance based on recent advances in understanding of gene-environment interactions and epigenetics (Meaney, 2010, Faraone et al., 2015). One study reported that perinatal risk factors for ADHD were strongest among women without a family history of ADHD (Sprich-Buckminster et al., 1993). A recent study reported that levels of cognitive impairment were higher among youth with ADHD who had multiple family members with ADHD (Oerlemans et al., 2015). In one of the few studies to look at the joint effects of parental history of ADHD and child adversity on the risk of ADHD, the authors reported that the relationship between maternal ADHD and child ADHD was stronger in families with less adversity (Breaux et al., 2017). Each of these studies suggests that a child’s family history of ADHD may modify the impact of other risk factors. By exploring the joint effects of SES and family history, we hope to gain insight into how risk factors may combine to shape the complex etiology of ADHD.
Here we use a population-based sample of elementary school children in Johnston County, North Carolina, to examine the joint effects of socioeconomic status and family history of ADHD on the prevalence of ADHD. We focus on these risk factors because of the controversy about the role of SES, the strength of family history as a risk factor for ADHD, and the few studies suggesting that family history of ADHD may modify risk factors for ADHD. We hypothesized that ADHD would be more common among the poor and among those with a family history of ADHD but we did not have enough information to hypothesize the direction of the joint effects.
Methods
Participants
In 1998 and 1999, we screened all children in grades 1–5 in 17 public elementary schools in Johnston County, North Carolina for ADHD. Their age range was 6–14 years. Here we briefly summarize our methods.
Sampling and Screening Procedure
The NIEHS IRB approved the original study protocol and subsequent data analysis was approved by the University of New Mexico IRB. Our overall sampling goal was to screen children for ADHD in two stages: first by identifying potential cases using teacher ratings and later by parent telephone interview. We then combined parent and teacher ratings to determine case status.
There were 7,847 children enrolled in grades 1–5. After excluding children with severe developmental disabilities in self-contained classrooms or severe health problems, 7,587 children were eligible. Parents or guardians of 6,139 (81%) children gave written permission, and teachers completed a behavioral checklist to rate ADHD symptoms and impairment (Rowland et al., 2007) on 6,072 children. After excluding 411 children with severe medical disabilities or low English proficiency, there were 5661 children eligible for the parent interview. We identified 1,414 children as potential cases; these were children taking medication to treat ADHD or who often exhibited at least 3 DSM-IV hyperactive/impulsive or inattentive behaviors and impairment at school on teacher ratings. We also selected a random sample of the eligible children (N=706) as potential controls. These two groups had 169 children in common. At this stage, before parental interviews had been completed, both groups contained some children who would later screen positive and become ADHD cases. (A detailed explanation and sampling diagram is available.) (Rowland et al., 2013)
Of the 1,951 potential cases and potential controls, we attempted to interview parents of 1,619 children (randomly eliminating 332 to meet budget constraints) and completed 1,160 telephone parental interviews (71.6%). The parental interview included the ADHD module of The NIMH Diagnostic Interview Schedule for Children (DISC-2.3) (Shaffer et al., 1996).
After the parent interview, we combined parent and teacher reports to determine final case status using full DSM-IV criteria of six hyperactive/impulsive symptoms or six inattentive symptoms, impairment in two settings and severe impairment in at least one setting (Rowland et al., 2013). In combining parent and teacher ratings, we required six or more symptoms in all with three or more symptoms from the teacher screener and three or more symptoms from the parent interview; symptoms reported by both informants were only counted once.
We did not use the DSM-IV ADHD age 7 age-at-onset criterion because of difficulty establishing an accurate age at onset, particularly for the Predominately Inattentive Subtype (Barkley and Biederman, 1997), which may have inflated our prevalence estimates slightly. Youth taking stimulant medication were treated as cases if they met DSM criteria while taking medication or if their parents reported they met symptom and impairment criteria the year prior to beginning medication treatment.
Children in the random sample of potential controls, who met ADHD case criteria after the phone interview, were classified as cases. After removing these cases, those remaining were our controls; they represent a random sample of the entire population who did not meet ADHD case criteria.
Some of the children eligible for parental interview were siblings. We randomly selected an index child from each family with multiple siblings. We collected a full interview from the parent on the index child but, to reduce respondent burden, only an abbreviated interview on other siblings.
Of the 1160 completed parental interviews, we had 967 full interviews from birth mothers and 127 interviews from non-birth parents (i.e., foster parents, step-parents and grandparents), and 66 children with abbreviated parental interviews. We restricted our sample to the 967 index children living with their birth mothers because only for these children had respondents been asked about parental history of ADHD. The final sample contained 392 ADHD cases, 393 controls, and 182 children who had been classified as potential cases based on the teacher rating, but did not meet case criteria after the parent interview, and were not in the random sample of potential controls.
Measures
Validation of ADHD Case definition: We validated our case definition using three approaches. First, we compared our ADHD prevalence estimate to the estimate obtained from a pilot study the previous year on our same population; very similar estimates were obtained (Rowland et al., 2001, Rowland et al., 2013). Second, we compared our prevalence estimate to estimates reported in two population-based studies of the prevalence of ADHD in South Carolina and in Oklahoma (Wolraich et al., 2012). When we used similar assumptions about counting ADHD medication use and age of onset, our prevalence estimate of 9.3% (Rowland et al., 2013) fell between the estimates of those two studies. Finally, we conducted a validation study of 34 cases comparing our epidemiologic case definition to a clinical consensus diagnosis process that included a semi-structured clinical interview (KSADS-PL) with parent and child, as well as rating scales and extra testing. Three cases identified as ADHD by our epidemiologic method were found to have been misidentified (Rowland et al., 2013), indicating a false positive rate near 9%.
Parental History of ADHD: We measured parental history of ADHD in two ways.
Parental history of ADHD diagnosis
Birth mothers were asked “Has a doctor or psychologist ever said that the Child’s birth father had ADHD, ADD or hyperactivity?” and “Has a doctor or other health provider ever told you that you had Attention Deficit Disorder or Attention Deficit Hyperactivity Disorder (ADD or ADHD)?” If they answered yes to either question, their child was considered to have a positive parental history of ADHD.
Parental history of ADHD symptoms
Birth mothers were asked four questions: 1. “When you were a child, did you often have trouble paying attention or concentrating in school?” 2. “When you were a child, did you often have problems because you were overactive, fidgety or impulsive (that is doing things that might be dangerous or get you into trouble without thinking about them first)?” The next questions are about Child’s birth father’s activity and attention. 3. “As far as you know, when Child’s father was a child, did he often have trouble paying attention or concentrating in school?” 4. “As far as you know, when Child’s father was a child, did he often have problems because he was overactive, fidgety or impulsive (that is doing things that might be dangerous or get him into trouble without thinking about them first)?” If respondents answered yes to any of these four questions, their child was considered to have a positive parental history of ADHD symptoms.
Socioeconomic Status (SES)
Our primary SES measure was based on annual family income; we also ran additional models using respondent education. We asked, “Which of the following categories best describes your total yearly income from all sources? This includes salaries, tips, Social Security, child support, retirement and other sources of money for this past year.” Later we grouped responses into- <$20,000, $20,000–$49,999 and $50,000 or higher. For education, we asked about the highest grade completed and grouped responses into less than high school, high school, or some college or higher. The income and education distribution for Johnston County was similar to that of North Carolina and the United States at the time of the study. Thirty percent of the Johnston County population had incomes less than $25,000 year, compared to 31% of the North Carolina population and 29% of the U.S. population. Similarly 76% of the Johnston County population had a high school degree or higher, compared to 78% of the North Carolina population and 80% of the United States population (U.S. Census Bureau, 2000).
Statistical Analysis
Because our sampling design oversampled potential cases, we used sampling weights to be able to adjust prevalence of ADHD cases back to the population eligible for parental interview (5,661). Weighting methods are commonly used with health surveys to account for sampling design and for non-response (Korn and Graubard, 1991). We calculated sampling weights as the inverse of the sampling fraction for each of five strata over each year (1998,1999) when data were collected. Children in each of these strata had different probabilities of entering our sample and of becoming ADHD cases. The strata were based on the main aspects of our sampling design (potential case status, whether they were in the random sample of potential controls, and ADHD medication use). The five strata were 1) potential cases taking ADHD medication in the random sample, 2) potential cases not taking ADHD medication in the random sample 3) potential cases taking ADHD medication, not in the random sample 4) potential cases, not taking ADHD medication, not in the random sample and 5) children who had screened negative on the teacher screen (non-potential cases) (Rowland et al., 2013).
Survey procedures for complex design in STATA were used to incorporate sampling weights, primary sampling units and strata into our logistic regression models. We used the STATA “subpop” command to restrict data analysis to the 967 birth mothers. We parameterized our models to estimate ORs in each parental-history-by-SES-cell compared to a referent cell (without parental history of ADHD, high income). To assess statistical interaction under this parameterization, we compared a full model including an interaction term with a reduced main effects model using an adjusted Wald test. We calculated area under the ROC curve (AUC) to assess the prediction power of the fitted models.
The main purpose of our weighted, multivariate, logistic regression analysis was to look at the combined impact of parental history of ADHD and SES (whether measured by family income or respondent education) on the odds of ADHD after controlling for age, gender and race/ethnicity. The comparison group in all models was youth from high SES families without a parental history of ADHD since this group had the lowest prevalence of ADHD.
Results
Analyses of SES and parental history separately
In our data analysis sample, the weighted prevalence of ADHD was 14.7% (95% C.I. 13.7–15.8) (Table 1). In unadjusted (bivariate) analyses, the prevalence of ADHD was highest among children with parents with less than a high school education (OR 6.0 (95% C.I. 3.3–10.7) compared to parents with at least some college, and highest among children from families with household incomes of less than $20,000 (OR 4.0 95% C.I. 2.6–6.0) compared to children with family incomes of $50,000 or higher (Table 1).
Table 1.
Weighted1 prevalence of demographic factors and unadjusted odds ratio of ADHD
| N=967 | Weighted Prevalence % (95% CI) | Association with ADHD | ||
|---|---|---|---|---|
| Unadjusted odds ratio (95% CI) | P | |||
| ADHD Status | ||||
| ADHD | 392 | 14.7 (13.7–15.8) | - | |
| Potential cases who did not become cases | 182 | 7.0 (6.1–7.9) | - | |
| Controls | 393 | 78.3 (77.4–79.2) | - | |
| Annual Family Income | ||||
| Low (<$20,000) | 219 | 15.7 (13.1–18.6) | 4.0 (2.6–6.0) | <.001 |
| Middle ($20,000–$49,999) | 442 | 46.5 (42.3–50.7) | 1.6 (1.2–2.3) | <.001 |
| High (>= $50,000) | 292 | 37.8 (33.8–42.0) | 1.0 | |
| Missing | 14 | |||
| Respondent Education | ||||
| Less than high school | 111 | 8.3 (6.4–10.7) | 6.0 (3.3–10.7) | <.001 |
| High school | 688 | 68.7 (64.6–72.4) | 3.3 (2.2–5.0) | <.001 |
| More than high school | 161 | 23.0 (19.6–26.9) | 1.0 | |
| Missing | 7 | |||
| Parental History of ADHD Diagnosis | ||||
| With Parental History of ADHD diagnosis | 237 | 15.7 (13.2–18.6) | 4.3 (3.0–6.1) | <.001 |
| Without Parental History of ADHD diagnosis | 730 | 84.3 (81.4–86.8) | 1.0 | |
| Parental History of ADHD Symptoms | ||||
| With Parental History of ADHD Symptoms | 444 | 34.1 (30.5–38.0) | 4.4 (3.2–5.9) | <.001 |
| Without Parental History of ADHD Symptoms | 523 | 65.9 (62.0–69.5) | 1.0 | |
| Gender | ||||
| Male | 614 | 51.4 (47.3 – 55.5) | 4.6 (3.3–6.4) | <.001 |
| Female | 353 | 48.6 (44.5–52.7) | 1.00 | |
| Race/ethnicity | ||||
| Nonwhite | 216 | 19.2 (16.2–22.5) | 1.1 (0.8–1.6) | 0.51 |
| White | 751 | 80.8 (77.5–83.8) | 1.0 | |
| Age group | ||||
| 11–14 | 213 | 21.7 (18.4–25.3) | 1.1 (0.8–1.6) | 0.61 |
| 9–10 | 387 | 38.6 (34.6–42.7) | 1.3 (1.0–1.8) | .08 |
| 6–8 | 367 | 39.8 (35.7–43.9) | 1.0 | |
The weighted prevalence is an estimate of the prevalence of the characteristic in the population of children eligible for parental interviews (N=5661) based on our sample of children with birth mother interviews (N=967).
Because the DSM-IV definition of ADHD is complex, to better understand our data, we examined the impact of SES using several simple alternative definitions of ADHD: current ADHD medication use, parental report of previous ADHD diagnosis, and combined teacher and parent ratings of 6 or more hyperactive/impulsive symptoms or 6 or more inattentive symptoms. There was only a weak gradient between SES (either income or education) and current ADHD medication use; but, for the other alternative measures, there was a strong inverse relationship between family income or education and the prevalence of the other ADHD measures (Table S1 and S2). This suggests that the SES effect that we observed was not a methodological artifact of our epidemiologic study definition of ADHD.
In the unadjusted (bivariate) analysis, parental history of ADHD diagnosis was a strong predictor of ADHD (OR=4.3, 95% C.I. 3.0–6.1 P<.001) (Table 1). Among cases, parental history of ADHD symptoms (64.0%) was more common than a parental history of ADHD diagnosis (36.9%) and was also a strong predictor of ADHD (OR=4.4, 95% C.I.3.2–5.9; P<.001). The overlap between the two parental history measures is presented in Table S3.
Joint analysis of family income and parental history of ADHD diagnosis
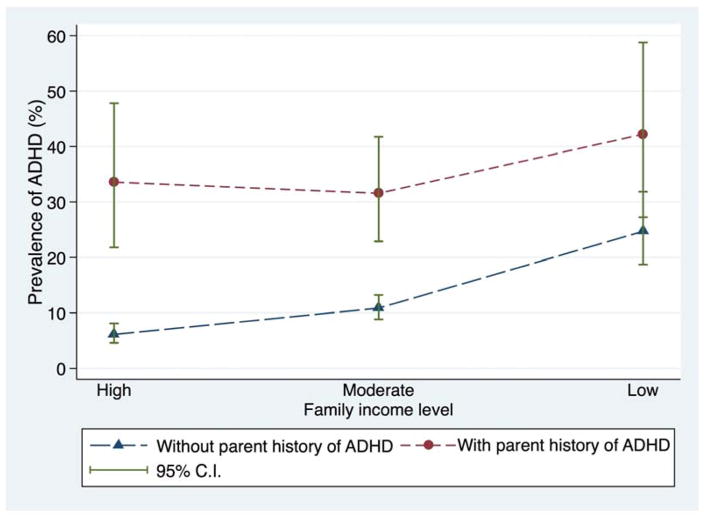
Both family income and parental history of ADHD diagnosis were strong predictors of the prevalence of ADHD (Figure 1). Among children with a parental history of ADHD diagnosis, between 31.5% and 42.2% of the children met the study case definition for ADHD, regardless of family income level. We saw a similar pattern with respondent education. Among children with a parental history of ADHD, between 21.1% and 46.8% had ADHD, regardless of family education level (Figure S1). Children with low SES and a parental history of ADHD diagnosis had the highest prevalence of ADHD -- 42.2% for low income and 46.8% for low education.
Figure 1. Prevalence of ADHD by Family Income and Parental History of ADHD Diagnosis.
Figure represents the prevalence of ADHD after accounting for sampling weights but without covariate adjustment.
We found a statistically significant interaction between family income and parental history of ADHD diagnosis (F2,1149=4.3, P=.016) (Table 2, Model 1). The corresponding interaction parameters, expressed on the OR scale, were 0.36 (95% CI 0.14–0.92) and 0.23 (95% CI 0.08–0.65) for parental history of diagnosis interacting with moderate and low income levels, respectively. These values less than one indicate that the proportional increase in ADHD odds associated with a parental ADHD diagnosis was smaller at both moderate and low income levels than at the high-income level.
Table 2.
Two logistic models of the relationship between parental history of ADHD and family income
| Model 11 | N 953 | Adjusted OR2 | 95% C.I. | P | Model 2 | N 953 | Adjusted OR | 95% C.I. | P |
|---|---|---|---|---|---|---|---|---|---|
| With parental history of ADHD diagnosis | With parental history of ADHD symptoms | ||||||||
| Low family income3 | 59 | 16.7 | 7.8–35.5 | .0164 | Low family income | 115 | 21.6 | 11.0–42.5 | .082 |
| Moderate family income | 112 | 10.2 | 5.4–19.1 | Moderate family income | 202 | 12.0 | 6.8–21.4 | ||
| High family income | 66 | 12.0 | 5.7–24.9 | High family income | 121 | 8.3 | 4.4–15.6 | ||
| Without Parental history of ADHD diagnosis | Without Parental history of ADHD symptoms | ||||||||
| Low family income | 160 | 6.2 | 3.4–11.3 | Low family income | 104 | 7.5 | 3.7–15.4 | ||
| Moderate family income | 330 | 2.3 | 1.5–3.7 | Moderate family income | 240 | 2.6 | 1.5–4.5 | ||
| High family income | 226 | 1.0 | - | High family income | 171 | 1.0 | |||
| | | | | | | | | | |
| Race ethnicity | Race ethnicity | ||||||||
| Non-White | 212 | 0.78 | 0.5–1.2 | 0.30 | Non-White | 212 | 0.75 | 0.5–1.2 | 0.25 |
| White | 741 | 1.0 | - | White | 741 | 1.0 | |||
| Gender | Gender | ||||||||
| Male | 603 | 5.8 | 4.0–8.5 | <.001 | Male | 603 | 5.7 | 3.9–8.2 | <.001 |
| Female | 350 | 1.0 | - | Female | 350 | 1.0 | |||
| Age group | Age group | ||||||||
| Age 11–14 | 208 | 1.1 | 0.7–1.8 | 0.59 | Age 11–14 | 208 | 1.2 | 0.8–2.0 | 0.41 |
| Age 9–10 | 382 | 1.4 | 1.0–2.1 | .06 | Age 9–10 | 382 | 1.6 | 1.1–2.4 | .015 |
| Age 6–8 | 363 | 1.0 | 1.0 | Age 6–8 | 363 | 1.0 |
Both Model 1 and Model 2 include family income, parental history, race/ethnicity, gender and age. The only difference in the two models is how parental history was defined.
The group with the Odds Ratio of 1.0 is the comparison group in each case. For the interaction model which combines parental history and family income, the comparison group is high family income without a parental history of ADHD.
Family income was defined as low if <$20,000 per year, medium $20–49,999 per year, and high >$50,000 per year
P values for interactions (P=.016 and P=.08) are for a 2 degree-of-freedom adjusted Wald test comparing a model with a multiplicative interaction term for family income and parental history of ADHD with a main effects model. The remaining P values in the table are based on the main effects of the other covariates.
Both income and parental history of ADHD diagnosis were strong predictors of ADHD. Compared to children from high income families without a parental history of ADHD, children from low income families without a parental history had over six times the odds of ADHD (OR= 6.2, 95% C.I. 3.4–11.3; P<.001), after adjusting for the child’s age, race/ethnicity, and gender. Parental history of ADHD diagnosis was also a powerful predictor of ADHD. Compared to children from high income families without a parental history, low income children with a parental history of ADHD diagnosis had 16.7 times the odds of ADHD (95% C.I. 7.8–35.5; P<.001). In fact, all children with a parental history of ADHD diagnosis had adjusted odds of ADHD over 10 compared to children from high SES families without a parental history (Table 2, Model 1). This statistical model had good explanatory power – the area under the curve was 0.79 (LaValley, 2008).
The interaction model suggested that the relationship between income and ADHD was strongly modified by parental history of ADHD diagnosis; specifically, the relationship between family income and childhood ADHD was stronger among children without a parental history of ADHD and weaker among children with a parental history of ADHD diagnosis. Among children without a parental history of ADHD diagnosis there was over a six-fold difference in the odds of ADHD between children from low income and high income families (OR=6.2, 95% C.I. 3.4–11.3; P <.001). But among children with a parental history of ADHD diagnosis, there was a 1.4- fold difference in the odds of ADHD between children from low income and high income families; OR=1.4, 95% 0.6–3.5, P=0.48 (data not shown), a comparison between the 16.7 odds ratio and the 12.0 odds ratio (Table 2).
Joint analysis of family income and parental history of ADHD symptoms
Over time, the diagnostic procedures for ADHD have changed. In the past, many children with ADHD went undiagnosed. Practically, this suggests that many studies may have too few children with a parental history of ADHD diagnosis to use this variable in analysis. Therefore, we also tested a model using a parental history of ADHD symptoms instead of ADHD diagnosis (Table 2, Model 2). Results were similar. The P value for the interaction term was .08 (F2,1149=2.54) with corresponding interaction parameters of 0.56 (95% CI 0.25–1.25) and 0.34 (95% CI 0.13–0.88) for parental history of symptoms interacting with moderate and low income levels, respectively.
There was a clear SES gradient in both children with, and without, a parental history of ADHD symptoms. As was true with the model using parental diagnosis, for the model using parental history of ADHD symptoms the impact of family income on the odds of ADHD was more pronounced in the group without a parental history. Among those without a parental history of ADHD symptoms, the gradient between low income and high income was 7.5 (95% C.I. 3.7–15.4) but among those with a parental history of ADHD symptoms, the gradient between low and high income was 2.6 (95% C.I. 1.3–5.1;P=.006). We also ran a model using respondent education instead of family income to measure SES and again the gradient between low education and high education was strongest among children without a parental history of ADHD (Table S4).
Discussion
One of the most important questions in mental health epidemiology is why rates of psychopathology are higher among the poor (McLaughlin, 2016, Costello et al., 2003). For many years, ADHD was considered an exception because many mental health researchers believed that ADHD equally impacted rich and poor. Our data supports the hypothesis that ADHD is inversely related to socioeconomic status. Understanding this relationship is of considerable public health importance because ADHD is a common developmental disorder of childhood that is often accompanied by psychiatric comorbidity and substantial impairment in day-to-day functioning (Larson et al., 2011). Understanding that the risk of ADHD is higher among the poor can help us reevaluate where the burden of this disorder lies and where resources are needed. If ADHD symptoms are more common among poor children but those children have less access to effective ADHD treatment, the concept of ADHD as a health disparity becomes apparent. However, controversy remains about the role of SES in the etiology of ADHD based, in part, on how to interpret statistical models which adjust for covariates on the same causal pathway or adjust for the comorbid conditions which accompany ADHD (Noordermeer et al., 2017, Szatmari et al., 1989a, Barkley, 2015).
Our data also indicate that the prevalence of ADHD is modified by parental history of ADHD. Although parental history is a well-established risk factor for ADHD on an individual level, surprisingly few epidemiologic studies have included it. We are unaware of other studies that have included parental history of ADHD in their prevalence estimates.
The powerful combined effects of SES and parental history are noteworthy because they suggest that both social and biological risk factors are involved in the etiology of ADHD, a paradigm that is gaining increasing acceptance (Nigg et al., 2010). Epigenetic research on how a person’s genes and their social environment interact to shape their risk of psychopathology (Kofink et al., 2013, Meaney, 2010) suggests a compelling avenue of research for understanding the etiology of ADHD and its comorbidity.
We evaluated the impact of family income and respondent education as measures of SES in our statistical models. Because the overall patterns were similar using either measure, we will discuss income and education together as SES. The gradient in risk between low SES and high SES varied by whether a child had a parental history of ADHD. The SES gradient was stronger in families without a parental history and weaker among children with a parental history of ADHD. Our observation is consistent with another recent report that the effect of adversity was stronger among those without a maternal history of ADHD and weaker among those with a maternal history (Breaux et al., 2017).
What does this mean? One interpretation, though speculative, is that environmental and genetic risk factors work differently. Among children without a strong genetic vulnerability, environmental risk factors become important; however, among children with a substantial genetic vulnerability, the impact of environmental risk factors becomes less important. If replicated, these findings suggest that studying etiologic risk factors for ADHD without accounting for parental history of ADHD may create biased estimates. More research is needed to evaluate whether other environmental risk factors might differ by parental history.
Researchers have begun to evaluate the role of epigenetics in the etiology of ADHD (van Mil et al., 2014, Dadds et al., 2016). SES can be considered an environmental exposure or a series of environmental exposures like adverse childhood events (ACES), that might influence gene expression (Needham et al., 2015, Uddin et al., 2013). Our data seems to be consistent with a process of biologic embedding through epigenetic mechanisms for low SES children (Cunliffe, 2016), but only when there was not a strong familial risk.
A caveat in interpreting the interaction between SES and parental history, however, is that parental history carries both a genetic (heritable) and an environmental component; it should not be interpreted only as the effects of genes. There is considerable evidence that parents with ADHD often exhibit parenting deficits, which place children at increased risk of behavioral problems (Johnston et al., 2012).
Because SES and parental history of ADHD are such powerful risk factors, we think it is important to include both measures in studies of ADHD. However, because parental history of a formal ADHD diagnosis was less common in the past, many studies may not be large enough to evaluate the combined impact of parental ADHD diagnosis with SES. But a history of ADHD symptoms is more common and therefore, could be used in smaller samples. In our data, 15.7 % of the children had a parental history of ADHD diagnosis but 34.1% of children had a parental history of ADHD symptoms. When we looked at parental history of ADHD, we found that the SES gradient almost disappeared in a model that relied on a parental diagnosis of ADHD by a health professional (OR=1.4, 95% 0.6–3.5, P=0.48) but reemerged in a model that used a parental history of ADHD symptoms (OR= 2.6, 95% C.I. 1.3–5.1 =.006.) This suggests we may have encountered the same problem as Lambert et al. where an SES effect disappeared when physician diagnosis was used as part of the study definition because access to physicians varies greatly by SES (Lambert et al., 1978). It is worth noting that collecting reliable parental histories of ADHD symptoms remains a challenge and more work is needed on how to collect it most effectively in epidemiologic studies.
Socioeconomic status, however measured, is a broad term associated with many behaviors like harsh parenting, adverse childhood experiences or food insecurity, which affect child development. Accepting that SES is related to risk of psychopathology is only a first step, we need to understand the components of risk better. Future research should try to distinguish which of those related behaviors most contribute to risk to identify modifiable and preventable risk factors for ADHD.
Limitations
This study was conducted in one county in North Carolina, which might limit its generalizability to other populations. However, because our sample was population-based, we think that our data has applicability to many similar counties across the U.S. The income distribution in Johnston County at the time of our study was similar to much of North Carolina and the U.S.
We measured SES with family income and with education but we do not know if our results would have been different if we used occupational status to define SES.
Another limitation of these data is that we were only able to collect parental histories by interviewing the birth mother. We did not collect a parental history of ADHD on children who were not living with their birth mothers. It is possible that the relationship between SES and parental history may have been different for children growing up without their birth mothers. We also used a non-validated scale to assess parental history of ADHD symptoms. More research is needed to produce a validated scale of ADHD symptoms for epidemiologic studies that can be collected with just a few questions. An additional limitation was that we did not collect a full psychiatric history so we are unable to rule out the possibility that other parental psychiatric symptoms may have explained some of our findings.
Conclusion
We conclude that the prevalence of ADHD is inversely related to socioeconomic status. ADHD therefore fits, and is not an exception from, the general pattern of most psychopathology, where disease is more frequent among the poor. Parental history of ADHD is also a strong risk factor that interacts with SES to modify its impact. The SES gradient was stronger in families without a parental history and weaker among children with a parental history. Epidemiologic studies of ADHD should include both SES and parental history when possible and evaluate their potential interaction. Future ADHD research should also evaluate whether the strength of other environmental risk factors vary by parental history. Interventions should be explored to early identify and offer extra assistance to children with ADHD symptoms from low SES backgrounds or with parental histories of ADHD.
Supplementary Material
Table S1. Unadjusted relationship between family income and alternative measures of ADHD.
Table S2. Unadjusted relationship between respondent education and alternative measures of ADHD.
Table S3. Cross tabulation of respondent reported parental diagnosis of ADHD and respondent reported parental symptoms of ADHD.
Table S4. Two logistic models of the relationship between parental history of ADHD and education.
Figure S1. Prevalence of ADHD by respondent level of education.
Key points
We found an interaction between SES and parental history of ADHD. The SES gradient was stronger in families without a parental history and weaker among children with a parental history.
Epidemiologic studies of ADHD should collect information on both risk factors and evaluate their interaction. Investigators should also check whether the effects of other environmental risk factors vary by family history of ADHD.
Future ADHD research should try to isolate which components of SES most contribute to risk in order to identify modifiable risk factors.
Interventions should be explored to proactively identify and offer extra assistance to children with symptoms of ADHD from low SES backgrounds or with parental histories of ADHD.
Acknowledgments
This research was supported in part by Intramural Research Programs of the NIH, NIEHS. Data analysis was supported by 5 R01 MH071563-01 from NIMH, UNM CTSC grant UL1TR001449 and UNM Mountain West CTRIN grant 1U54GM104944. Donna Baird and Dale Sandler helped design the study and Lilian Stallone supervised data collection. Dale Sandler and Kathleen Wayland provided comments which improved the manuscript. Dr. Rabiner owns stock in C8 Sciences, received sponsorship in SOARNC, and is a consultant for Attention Point. None of the other authors reported any financial interests or potential conflicts of interest.
Abbreviations
- SES
socioeconomic status
Footnotes
Conflict of interest statement: No conflicts declared.
Additional Supporting Information may be found in the online version of this article:
References
- BARKLEY R. Attention-Deficit Hyperactivity Disorder : A Handbook for Diagnosis and Treatment. Guilford Press; 2015. [Google Scholar]
- BARKLEY RA. Attention-deficit hyperactivity disorder: a handbook for diagnosis and treatment. New York: Guilford; 1998. [Google Scholar]
- BARKLEY RA, BIEDERMAN J. Toward a broader definition of the age-of-onset criterion for attention-deficit hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 1997;36:1204–1210. doi: 10.1097/00004583-199709000-00012. [DOI] [PubMed] [Google Scholar]
- BEAUCHAINE T, HINSHAW S, PANG K. Comorbidity of Attention-Deficit/Hyperactivity Disorder and early-onset conduct disorder: biological, environmental and developmental mechanisms. Clinical Psychology: Science and Practice. 2010;17:327–336. [Google Scholar]
- BIEDERMAN J, FARAONE SV, MONUTEAUX MC. Differential effect of environmental adversity by gender: Rutter’s index of adversity in a group of boys and girls with and without ADHD. Am J Psychiatry. 2002;159:1556–1562. doi: 10.1176/appi.ajp.159.9.1556. [DOI] [PubMed] [Google Scholar]
- BREAUX RP, BROWN HR, HARVEY EA. Mediators and Moderators of the Relation between Parental ADHD Symptomatology and the Early Development of Child ADHD and ODD Symptoms. J Abnorm Child Psychol. 2017;45:443–456. doi: 10.1007/s10802-016-0213-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC/NCHS, N. H. I. S. Diagnosed attention deficit/hyperactivity disorder (ADHD) and learning disability among children 6–17 years of age, by selected characteristics: United States: 2004–2006. 2007:10. [Google Scholar]
- CHRONIS AM, LAHEY BB, PELHAM WE, JR, KIPP HL, BAUMANN BL, LEE SS. Psychopathology and substance abuse in parents of young children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2003;42:1424–1432. doi: 10.1097/00004583-200312000-00009. [DOI] [PubMed] [Google Scholar]
- COSTELLO EJ, COMPTON SN, KEELER G, ANGOLD A. Relationships between poverty and psychopathology: a natural experiment. Jama. 2003;290:2023–2029. doi: 10.1001/jama.290.15.2023. [DOI] [PubMed] [Google Scholar]
- COUNTS CA, NIGG JT, STAWICKI JA, RAPPLEY MD, VON EYE A. Family adversity in DSM-IV ADHD combined and inattentive subtypes and associated disruptive behavior problems. J Am Acad Child Adolesc Psychiatry. 2005;44:690–698. doi: 10.1097/01.chi.0000162582.87710.66. [DOI] [PubMed] [Google Scholar]
- CUNLIFFE VT. The epigenetic impacts of social stress: how does social adversity become biologically embedded? Epigenomics. 2016;8:1653–1669. doi: 10.2217/epi-2016-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DADDS MR, SCHOLLAR-ROOT O, LENROOT R, MOUL C, HAWES DJ. Epigenetic regulation of the DRD4 gene and dimensions of attention-deficit/hyperactivity disorder in children. Eur Child Adolesc Psychiatry. 2016;25:1081–1089. doi: 10.1007/s00787-016-0828-3. [DOI] [PubMed] [Google Scholar]
- FARAONE S, ASHERSON P, BANASCHEWSKI T, BIEDERMAN J, BUITELAAR J, RAMOS-QUIROGA JA, ROHDE LA, SONUGA-BARKE E, TANNOCK R, FRANKE B. Attention-deficit/hyperactivity disorder. Nature reviews | disease primers. 2015;1:1–23. doi: 10.1038/nrdp.2015.20. [DOI] [PubMed] [Google Scholar]
- FARAONE SV, PERLIS RH, DOYLE AE, SMOLLER JW, GORALNICK JJ, HOLMGREN MA, SKLAR P. Molecular genetics of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1313–1323. doi: 10.1016/j.biopsych.2004.11.024. [DOI] [PubMed] [Google Scholar]
- FROEHLICH TE, LANPHEAR BP, EPSTEIN JN, BARBARESI WJ, KATUSIC SK, KAHN RS. Prevalence, recognition, and treatment of attention-deficit/hyperactivity disorder in a national sample of US children. Arch Pediatr Adolesc Med. 2007;161:857–864. doi: 10.1001/archpedi.161.9.857. [DOI] [PubMed] [Google Scholar]
- JOHNSTON C, MASH EJ, MILLER N, NINOWSKI JE. Parenting in adults with attention-deficit/hyperactivity disorder (ADHD) Clin Psychol Rev. 2012;32:215–228. doi: 10.1016/j.cpr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOFINK D, BOKS MP, TIMMERS HT, KAS MJ. Epigenetic dynamics in psychiatric disorders: Environmental programming of neurodevelopmental processes. Neurosci Biobehav Rev. 2013;37:831–845. doi: 10.1016/j.neubiorev.2013.03.020. [DOI] [PubMed] [Google Scholar]
- KORN EL, GRAUBARD BI. Analysis of Health Surveys. John Wiley & Sons; 1991. 1999. [Google Scholar]
- LAMBERT NM, SANDOVAL J, SASSONE D. Prevalence of hyperactivity in elementary school children as a function of social system definers. American Journal of Orthopsychiatry. 1978;48:446–463. doi: 10.1111/j.1939-0025.1978.tb01334.x. [DOI] [PubMed] [Google Scholar]
- LARSON K, RUSS SA, KAHN RS, HALFON N. Patterns of comorbidity, functioning, and service use for US children with ADHD, 2007. Pediatrics. 2011;127:462–470. doi: 10.1542/peds.2010-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LARSSON H, SARIASLAN A, LANGSTROM N, D’ONOFRIO B, LICHTENSTEIN P. Family income in early childhood and subsequent attention deficit/hyperactivity disorder: a quasi-experimental study. J Child Psychol Psychiatry. 2014;55:428–435. doi: 10.1111/jcpp.12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAVALLEY MP. Logistic regression. Circulation. 2008;117:2395–2399. doi: 10.1161/CIRCULATIONAHA.106.682658. [DOI] [PubMed] [Google Scholar]
- MCLAUGHLIN KA. Future Directions in Childhood Adversity and Youth Psychopathology. J Clin Child Adolesc Psychol. 2016:1–22. doi: 10.1080/15374416.2015.1110823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEANEY MJ. Epigenetics and the biological definition of gene x environment interactions. Child Dev. 2010;81:41–79. doi: 10.1111/j.1467-8624.2009.01381.x. [DOI] [PubMed] [Google Scholar]
- MUSSER ED, HAWKEY E, KACHAN-LIU SS, LEES P, ROULLET JB, GODDARD K, STEINER RD, NIGG JT. Shared familial transmission of autism spectrum and attention-deficit/hyperactivity disorders. J Child Psychol Psychiatry. 2014;55:819–827. doi: 10.1111/jcpp.12201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEEDHAM BL, SMITH JA, ZHAO W, WANG X, MUKHERJEE B, KARDIA SL, SHIVELY CA, SEEMAN TE, LIU Y, DIEZ ROUX AV. Life course socioeconomic status and DNA methylation in genes related to stress reactivity and inflammation: The multi-ethnic study of atherosclerosis. Epigenetics. 2015;10:958–969. doi: 10.1080/15592294.2015.1085139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIGG J, NIKOLAS M, BURT SA. Measured gene-by-environment interaction in relation to attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2010;49:863–873. doi: 10.1016/j.jaac.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIGG JT, CRAVER L. Commentary: ADHD and social disadvantage: an inconvenient truth? - a reflection on Russell et al. () and Larsson et al. () J Child Psychol Psychiatry. 2014;55:446–447. doi: 10.1111/jcpp.12237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOORDERMEER SD, LUMAN M, WEEDA WD, BUITELAAR JK, RICHARDS JS, HARTMAN CA, HOEKSTRA PJ, FRANKE B, HESLENFELD DJ, OOSTERLAAN J. Risk factors for comorbid oppositional defiant disorder in attention-deficit/hyperactivity disorder. Eur Child Adolesc Psychiatry. 2017 doi: 10.1007/s00787-017-0972-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OERLEMANS AM, HARTMAN CA, DE BRUIJN YG, FRANKE B, BUITELAAR JK, ROMMELSE NN. Cognitive impairments are different in single-incidence and multi-incidence ADHD families. J Child Psychol Psychiatry. 2015;56:782–791. doi: 10.1111/jcpp.12349. [DOI] [PubMed] [Google Scholar]
- POLANCZYK G, JENSEN P. Epidemiologic considerations in attention deficit hyperactivity disorder: a review and update. Child Adolesc Psychiatr Clin N Am. 2008;17:245–260. vii. doi: 10.1016/j.chc.2007.11.006. [DOI] [PubMed] [Google Scholar]
- ROWLAND AS, SKIPPER BJ, UMBACH DM, RABINER DL, CAMPBELL RA, NAFTEL AJ, SANDLER DP. The Prevalence of ADHD in a Population-Based Sample. J Atten Disord. 2013 doi: 10.1177/1087054713513799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROWLAND AS, UMBACH DM, BOHLIG EM, STALLONE L, SANDLER DP. Modifying the response labels of an ADHD teacher rating scale: psychometric and epidemiologic implications. J Atten Disord. 2007;11:384–397. doi: 10.1177/1087054707305082. [DOI] [PubMed] [Google Scholar]
- ROWLAND AS, UMBACH DM, CATOE KE, STALLONE L, LONG S, RABINER D, NAFTEL AJ, PANKE D, FAULK R, SANDLER DP. Studying the epidemiology of attention-deficit hyperactivity disorder: screening method and pilot results. Can J Psychiatry. 2001;46:931–940. doi: 10.1177/070674370104601005. [DOI] [PubMed] [Google Scholar]
- RUSSELL AE, FORD T, WILLIAMS R, RUSSELL G. The Association Between Socioeconomic Disadvantage and Attention Deficit/Hyperactivity Disorder (ADHD): A Systematic Review. Child Psychiatry Hum Dev. 2016;47:440–458. doi: 10.1007/s10578-015-0578-3. [DOI] [PubMed] [Google Scholar]
- RUSSELL G, FORD T, ROSENBERG R, KELLY S. The association of attention deficit hyperactivity disorder with socioeconomic disadvantage: alternative explanations and evidence. J Child Psychol Psychiatry. 2014;55:436–445. doi: 10.1111/jcpp.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCAHILL L, SCHWAB-STONE M, MERIKANGAS KR, LECKMAN JF, ZHANG H, KASL S. Psychosocial and clinical correlates of ADHD in a community sample of school-age children. J Am Acad Child Adolesc Psychiatry. 1999;38:976–984. doi: 10.1097/00004583-199908000-00013. [DOI] [PubMed] [Google Scholar]
- SHAFFER D, FISHER P, DULCAN MK, DAVIES M, PIACENTINI J, SCHWAB-STONE ME, LAHEY BB, BOURDON K, JENSEN P, BIRD HR, CANINO G, REGIER DA. The NIMH diagnostic interview schedule for children version 2.3 (DISC-2.3): description, acceptability, prevalence rates, and performance in the meca study. J Am Acad Child Adolesc Psychiatry. 1996;35:865–877. doi: 10.1097/00004583-199607000-00012. [DOI] [PubMed] [Google Scholar]
- SPRICH-BUCKMINSTER S, BIEDERMAN J, MILBERGER S, FARAONE SV, LEHMAN BK. Are perinatal complications relevant to the manifestation of ADD? Issues of comorbidity and familiality. J Am Acad Child Adolesc Psychiatry. 1993;32:1032–1037. doi: 10.1097/00004583-199309000-00023. [DOI] [PubMed] [Google Scholar]
- ST SAUVER JL, BARBARESI WJ, KATUSIC SK, COLLIGAN RC, WEAVER AL, JACOBSEN SJ. Early life risk factors for attention-deficit/hyperactivity disorder: a population-based cohort study. Mayo Clin Proc. 2004;79:1124–1131. [PubMed] [Google Scholar]
- SUNDQUIST J, LI X, OHLSSON H, RASTAM M, WINKLEBY M, SUNDQUIST K, KENDLER KS, CRUMP C. Familial and neighborhood effects on psychiatric disorders in childhood and adolescence. J Psychiatr Res. 2015;66–67:7–15. doi: 10.1016/j.jpsychires.2015.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SZATMARI P, OFFORD DR, BOYLE MH. Correlates, associated impairments and patterns of service utilization of children with attention deficit disorder: findings from the Ontario Child Health Study. J Child Psychol Psychiatry. 1989a;30:205–217. doi: 10.1111/j.1469-7610.1989.tb00235.x. [DOI] [PubMed] [Google Scholar]
- SZATMARI P, OFFORD DR, BOYLE MH. Ontario child health study: prevalence of attention deficit disorder with hyperactivity. Journal of Child Psychology and Psychiatry and Allied Disciplines. 1989b;30:219–230. doi: 10.1111/j.1469-7610.1989.tb00236.x. [DOI] [PubMed] [Google Scholar]
- THAPAR A, COOPER M, EYRE O, LANGLEY K. What have we learnt about the causes of ADHD? J Child Psychol Psychiatry. 2013;54:3–16. doi: 10.1111/j.1469-7610.2012.02611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. CENSUS BUREAU. North Carolina Quickfacts. Washington, D.C: U.S. Census Bureau, Department of Commerce; 2000. Johnston County North Carolina. [Google Scholar]
- UDDIN M, GALEA S, CHANG SC, KOENEN KC, GOLDMANN E, WILDMAN DE, AIELLO AE. Epigenetic signatures may explain the relationship between socioeconomic position and risk of mental illness: preliminary findings from an urban community-based sample. Biodemography Soc Biol. 2013;59:68–84. doi: 10.1080/19485565.2013.774627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN MIL NH, STEEGERS-THEUNISSEN RP, BOUWLAND-BOTH MI, VERBIEST MM, RIJLAARSDAM J, HOFMAN A, STEEGERS EA, HEIJMANS BT, JADDOE VW, VERHULST FC, STOLK L, EILERS PH, UITTERLINDEN AG, TIEMEIER H. DNA methylation profiles at birth and child ADHD symptoms. J Psychiatr Res. 2014;49:51–59. doi: 10.1016/j.jpsychires.2013.10.017. [DOI] [PubMed] [Google Scholar]
- WEINBERG CR. Toward a clearer definition of confounding. Am J Epidemiol. 1993;137:1–8. doi: 10.1093/oxfordjournals.aje.a116591. [DOI] [PubMed] [Google Scholar]
- WOLRAICH ML, MCKEOWN RE, VISSER SN, BARD D, CUFFE S, NEAS B, GERYK LL, DOFFING M, BOTTAI M, ABRAMOWITZ AJ, BECK L, HOLBROOK JR, DANIELSON M. The Prevalence of ADHD: Its Diagnosis and Treatment in Four School Districts Across Two States. J Atten Disord. 2012 doi: 10.1177/1087054712453169. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Unadjusted relationship between family income and alternative measures of ADHD.
Table S2. Unadjusted relationship between respondent education and alternative measures of ADHD.
Table S3. Cross tabulation of respondent reported parental diagnosis of ADHD and respondent reported parental symptoms of ADHD.
Table S4. Two logistic models of the relationship between parental history of ADHD and education.
Figure S1. Prevalence of ADHD by respondent level of education.