Abstract
To test the efficacy of a community-based intervention, Empowering Communities for Life (EC4L), designed to increase colorectal cancer (CRC) screening through fecal occult blood test (FOBT) in rural underserved communities in a randomized controlled trial. Participants were randomized into 3 groups (2 interventions and 1 control). Interventions were delivered by community lay health workers or by academic health professionals. The main outcome of interest was return rate of FOBT screening kit within 60 days. Participants (n=1050) included 330 screening-eligible adults. The return rate of FOBT kits within 60 days was 32%. The professional group (Arm 2) had the highest proportion of returned FOBTs within 60 days at 42% (n=46/110), a significantly higher return rate than the lay group (Arm 1) [28%(n=29/103);P=0.0422] or control group (Arm 3) [25%(n=29/117);P=0.0099]. Thus, one arm (Arm 2) of our intervention produced significantly higher CRC screening through FOBT. Community-based participation partnered with academic health professionals enhanced CRC screening among rural and poor-resourced communities.
Keywords: Community-based participatory research, health care delivery, early detection of cancer, health care disparities, rural health
1. Introduction
1.1 Colorectal Cancer Screening and Disparities
Colorectal cancer (CRC) is the third leading cause of cancer and the second most common cause of cancer-related deaths in the US.1–3 Every year, 136,000 people are diagnosed and more than 50,000 die from the disease.4 CRC is preventable through screening, and early detection greatly improves patient outcomes across the continuum of care.5 One main clinical benefit of screening to “cancer-free” patients is that precancerous lesions can be identified and removed before they develop into cancer.6 For patients who already started to develop the disease, CRC early detection program participants can experience an earlier shift in stage of diagnosis when treatment is more effective. Yet, while the overall death rate for colorectal cancer has declined over the past two decades, disparities remain among underserved populations, such as those in rural areas.7;8 African Americans (AA), for example, have a significantly higher mortality rate compared to other enthnicities.9;10 Screening and early detection are an important conduit to saving lives, but both are often underutilized or unavailable to these populations.11 Therefore, to improve health care delivery and outcomes for the communities most at need, we need to determine the barriers and facilitators to effective screening and use this information to develop interventions and models for use in rural and poor-resourced communities.
1.2 Community-Based Participatory Research (CBPR)
Such interventions include community based participatory research (CBPR) as a useful model in addressing community needs.12 As defined by the Evidence-based Practice Centers (EPC) from the Agency for Health care Research and Quality,13 CBPR “is a collaborative research approach that is designed to ensure and establish structures for participation by communities affected by the issue being studied, representatives of organizations, and researchers in all aspects of the research process to improve health and well-being through taking action, including social change”.14 This partnership in research involves community members, their representatives, and academic researchers working together in the process that includes research design, implementation, evaluation and dissemination. Israel et al.,14 a noted expert in CBPR defines the key principles of CBPR which recognizes that the community is central to the program; the program builds on strengths and resources within the community; the program facilitates involvement of community leaders in the research; and it integrates knowledge and intervention for the mutual benefit of all partners. Other principles are the co-learning and empowering processes that can be derived by the community in solving their problems. It is a cyclical, iterative process that recognizes not only physical, mental, and social wellbeing, but also biomedical, economic, cultural, and political factors as important elements for health. It involves a long term process and commitment. Our work with CBPR is based on several of these principles. In looking at approaches that utilize CBPR for cancer screening, investigators have found that community approaches can greatly increase cancer screening. In 2006, Gellert et al15 used a community-based approach to overcome the healthcare gap Native Hawaiians faced when searching for culturally attractive and convenient cancer services.15 The program was a yearlong project that recognized and addressed this minority population’s insufficient knowledge of screening procedures, limited access to health services, and poor financial status. Researchers then utilized culture- and community-based strategies to find solutions. The program appealed to the target population due to the researchers’ approach, which incorporated CBPR methods like including community members as equal partners and culturally tailoring the educational materials and curricula. Overall, the program increased colorectal and other cancer screenings in the population, suggesting that a CBPR model can be effective in a rural and poor-resourced community.15
1.3 Empowering Communities for Life (EC4L) Program
Based on the success of studies such as Gellert et al, we developed a model to deliver a CBPR program in two of Arkansas’ most medically underserved and poor-resourced communities: Mississippi County and St. Francis County, see Table 1. The objectives of our EC4L program were to increase colorectal cancer (CRC) screening through fecal-occult blood testing (FOBT), and to study the outcomes of a CBPR model in a rural and poor-resourced setting using a randomized controlled trial. The program established community partners and developed community-based interventions that identified and addressed health disparities in colorectal cancer screening in two rural and poor-resourced communities.
Table 1.
Colorectal Cancer Age-Adjusted Incidence and Mortality, 2009–2013 Rates per 100,000*
| Incidence (2009–2013) | Mortality (2009–2011) | |||
|---|---|---|---|---|
|
| ||||
| African American | White | African American | White | |
| Healthy People 2020 Goal# | 39.9 | 39.9 | 14.5 | 14.5 |
|
| ||||
| US | 47.5 | 37.9 | 21.1 | 14.6 |
| Arkansas | 52.0 | 42.5 | 24.0 | 17.2 |
| Mississippi County | 80.3 | 56.6 | 47.0 | 28.6 |
| St. Francis County | 46.9 | 45.6 | ** | ** |
NCI SEER, Arkansas Cancer Registry
Rates/Counts are suppressed if less than 11 cases were reported.
Health People 2020 Objective for incidence and mortality.
2. Material and methods
2.1 Design overview
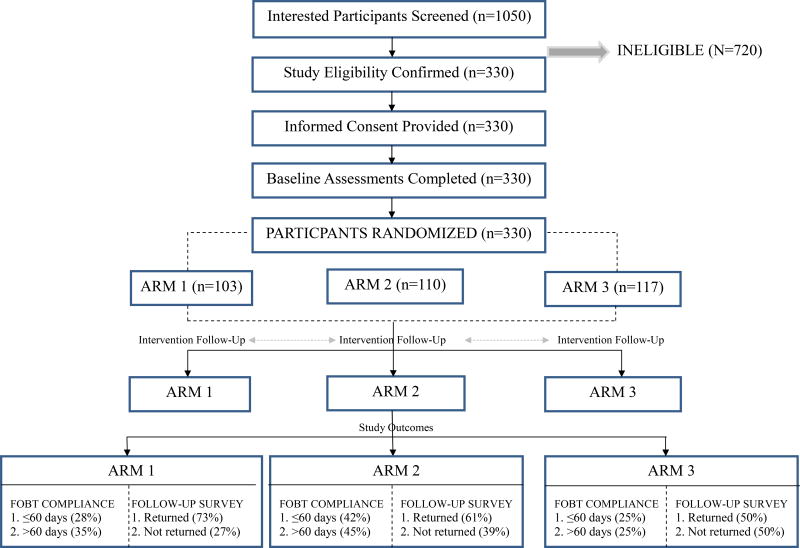
The research conducted was approved by the university’s Institutional Review Board. From 2008–2014, we conducted a randomized controlled trial to assess the efficacy of the EC4L intervention designed to increase CRC screening through FOBT. EC4L was a five-year intervention that originally planned to screen a pool of 1344 Mississippi or St. Francis County residents with the goal of identifying 1050 participants. We collaborated directly with community partners to develop strategies to increase CRC screening by disseminated FOBT kits in the target community. Participants were randomized into 3 groups (2 intervention groups (Arms 1–2) and 1 control group (Arm 3)), see Figure 1. The recruitment, assignment, and empowering process are detailed in the sections below. Outcomes were summarized using proportions, and the differences between those were assessed with a chi-square test. Pair-wise comparisons were conducted using simple logistic regression models. The main outcome of interest was the return rate of FOBT screening kit within 60 days and secondary outcomes included greater than 60 days and follow-up survey.
Figure 1.
Study Schematic
2.2 Community partnership and the CBPR process
Community partners in two underserved and impoverished counties in the Mississippi Delta of Arkansas (Mississippi and St. Francis) held trainings and community-capacity development meetings with academic staff to design and implement EC4L, a CBPR study that developed, implemented, and evaluated a culturally appropriate and community relevant intervention. Our novel approach partnered the community with academia in all aspects of the CBPR study. Grounded in health behavior theory, our study evaluated a previously unexplored combination of health education and provision of FOBT kits, instruction cards, and reminders to increase CRC screening rates.
2.3 Description of intervention
At the onset of the intervention phase of the project, senior leadership at the academic institution identified community investigators (CIs) in targeted counties. CIs subsequently hired Community Lay Health Workers (CLHWs) in each county and developed a protocol for inviting county residents to a recruitment meeting, followed by an intervention meeting. CIs contacted participants, scheduled and conducted approximately 50 meetings at local sites they selected in an effort to enhance attendance. A combination of academic health professionals (AHPs) and CLHWs conducted all intervention meetings. Procedures for each of the three intervention conditions included the presentation of information by designated personnel (APH or CLHW), followed by an opportunity for participants to ask questions and discuss any concerns about issues addressed in the meeting. The duration of each presentation at the intervention meetings was about 45 minutes.
2.4 Group (Arm) assignment procedures
Eligible, consenting participants were categorized by a single condition—whether they adhered to the screening guidelines for cancer. If they had been screened for cancer as recommended, they were considered adherent and did not take part in the randomized controlled trial phase of the study.
The statistician assigned all consenting participants who were not adherent to colorectal screening guidelines at the date of the recruitment/assessment meeting, at random, to one of three groups (arms). The first two groups received the intervention (Arm 1, Arm 2), and the third group acted as the control group. The intervention arms included Arm 1, a tailored colorectal risk reduction condition presented by a CLHW; and Arm 2, a general risk reduction intervention presented by an AHP. The non-intervention arm, Arm 3, had an alternate-treatment control condition (cardiovascular disease risk reduction) presented by an AHP, see Table 2. In an effort to limit bias, eligible participants that were adherent to the screening guidelines on the date of the recruitment/assessment were automatically assigned to the control condition group (cardiovascular disease risk reduction, Arm 3) to recruit a representative sample that did not only include participants interested specifically in cancer control. The content of each arm is detailed further in the sections, below.
Table 2.
Comparison of Intervention Components.
| ARM 1: Tailored Cancer Risk Reduction |
ARM 2:General Cancer Risk Reduction |
ARM 3: Cardiovascular Disease Risk Reduction (no treatment control) |
|
|---|---|---|---|
|
| |||
| Presenter | Local role model who has had abnormal colorectal cancer screen results and colonoscopy with polyp removal | Academic health professional | Academic health professional |
|
|
|||
| Educational Content | 1. Information about racial disparities in colorectal cancer incidence and mortality in the Mississippi River Delta region of Arkansas | 1. National rates for colorectal cancer incidence and mortality | 1. National data for cardiovascular disease incidence and mortality |
| 2. Information about screening to reduce cancer incidence and mortality | 2. Information about screening to reduce cancer incidence and mortality | 2. Information about measuring blood pressure to monitor cardiovascular risk | |
| 3. Information about screening rates in the Mississippi River Delta region of Arkansas | 3. Information about national screening rates | 3. Information about prevalence of high blood pressure | |
| 4. Individual colorectal cancer risk feedback (from baseline questionnaire) and screening guidelines | 4. General information about risk factors for colorectal cancer and screening guidelines | 4. General information about risk factors for cardiovascular disease and screening guidelines | |
2.4.1 Arm 1: Tailored colorectal cancer risk reduction intervention group
Arm 1 included participants that had not followed screening guidelines. A CLHW presented information that was community-centered and driven by personal stories and individual assessments. Examples of presented information included data about racial disparities in CRC incidence and mortality in the Arkansas Delta; information about screening to reduce incidence; information about screening rates in the area; and individual CRC risk and screening guidelines. A local role model presented a personal story about their experience with the disease, the screening process, and polyp removal. The CLHW demonstrated appropriate use of the FOBT screening cards and discussed potential barriers to screening and resolution strategies to promote self-efficacy for screening and follow-up. Materials provided to the participants included an FOBT kit to be completed at home and mailed to the academic health center lab for analysis and a brochure that summarized information presented during the intervention meeting. In the two weeks following the meeting, cards were mailed with stamped, pre-addressed return envelopes.
2.4.2 Arm 2: General cancer risk reduction intervention group
Arm 2, like Arm 1, was comprised of participants that had not followed screening guidelines. Unlike Arm 1, this arm presented information concerning national statistics and general information, rather than community-focused or tailored. To emphasize the potential influence of systematic informed professional advice, an AHP led the presentation, which included information concerning national rates for CRC incidence and mortality; national screening information and rates; general information about risk factors for CRC and screening guidelines. The AHP provided instructions for the appropriate use of FOBT screening cards but did not demonstrate their use. Materials provided to the participants included an FOBT kit to be completed at home and mailed to the academic health center lab for analysis and a brochure that summarized information presented during the intervention meeting. In the two weeks following the meeting, cards were mailed with stamped, pre-addressed return envelopes.
2.4.3 Arm 3: Cardiovascular disease risk reduction group (control)
Arm 3 included both those who had and had not adhered to screening guidelines. Information was presented by an AHP, focused on cardiovascular risk and monitoring. To emphasize the potential influence of systematic informed professional advice, an AHP led the presentation, which was modeled after the General Colorectal Risk Reduction Group (Arm 2) to minimize any potential general influence of tailored strategies on preventive health behaviors. This session included national data for cardiovascular disease and mortality; information about measuring blood pressure to gauge cardiovascular risk; information about incidence of high blood pressure; and general information about risk factors for cardiovascular disease and screening guidelines. Similar to Arms 1 and 2, materials were provided to the participants, but the brochure was replaced with a brochure addressing cardiovascular disease. These participants also received FOBT kits, but no instruction or demonstration on completing the kit. Colorectal cancer was not addressed and those who had questions were instructed to contact their PCP.
2.4 Community involvement in intervention
CIs in the targeted counties played an active role in planning and implementing the interventions. To ensure CBPR principles were maintained, training and development meetings with CIs, CLHWs, and AHPs and staff were held bi-monthly within each community prior to implementation. CIs devoted more than 80% effort to the project over the five-year study period. Their duties included: overseeing implementation of the project activities, coordinating with partner organizations, liaising with federal agencies (NIH), planning of training, training, conducting meetings, and coordinating with others involved with cancer control prevention (e.g., University of Arkansas for Medical Sciences College of Public Health, American Cancer Society, and Arkansas Cancer Coalition). They also worked with the academic health center staff to develop study methods, conduct pilot studies, recruit participants, and collect follow-up data. Community partners played an active role in developing relationships with community groups to promote the project. Consistent with a CBPR approach, there was a community subcontract with each county, which provided funding for community investigators and other project-related costs.
3. Results
3.1 Intervention efficacy
Measured outcomes consisted of participant completion of follow-up calls and return of the FOBT for lab testing. The general cancer risk reduction intervention group (Arm 2) led by an AHP (n=110) had the highest percent of return (42%). This was significantly higher than the cardiovascular disease risk reduction group (Arm 3) (P=0.0099). Our data found that of the 105 FOBT kits returned from the three arms, 45, or nearly 42%, were from participants in the general cancer risk reduction group (Arm 2) in comparison to the 35 tests (34%) returned by participants of the tailored cancer risk reduction group (Arm 1) and 25 tests (24%) from cardiovascular disease risk reduction group (Arm 3) participants.
3.2 Target population
The proposed recruitment pool of 1050 participants represented 2% of the population in Mississippi and St. Francis counties that met eligibility criteria. The targeted enrollment was 96% non-Hispanic and 3% Hispanic in both counties combined. Planned enrollment by race was as follows: white 57%, AA 43%, and 0% for American Indian/Alaska Native, Asian, and Native Hawaiian or other Pacific Islander. At the point of study design, targeted enrollment for Mississippi County was 64.4% white, 32.7% AA, and 2.9% other. Racial composition for St. Francis County was 48.4% white, 49% AA, and about 2.6% other.
During the enrollment phase, census estimates showed a decline in population in both counties, with the steepest decline in the white population. It is possible that the decrease in this racial group had a direct effect on the number of white participants, accounting for the lower-than-projected racial composition. The total number of participants who consented and completed the intervention was 330. The actual population enrolled in the intervention is as follows: AA (n=231) 70%, white (n=46) 14% and other (n=4) 1%. Forty-nine (about 15%) of the enrolled participants did not report their race/ethnicity.
3.3 Summary outcomes and retention rates
Study outcomes among rural participants are reported in Table 3. The overall return rate of FOBT kits within 60 days was 32%. The AHP-led general cancer risk reduction group (Arm 2) had the highest proportion of returned FOBTs within 60 days at 42%, which was a significantly higher return rate than those of the tailored cancer risk reduction group (Arm 1) (28%; P=0.0422) or control group (Arm 3) (25%; P=0.0099). FOBT returns for time periods over 60 days were again highest for the general risk reduction group (Arm 2) at 45%. This was significantly higher than the control group (Arm 3) (25%; P=0.0029), but not significantly different from the tailored cancer risk reduction group (Arm 1) (35%; P=0.178). Thus one arm (Arm 2) of our intervention produced significantly higher CRC screening through FOBT in our sample.
Table 3.
Summary outcomes by study group among rural participants in a RCT.
| (%) | Arm 1 (n=103) |
Arm 2 (n=110) |
Arm 3 (n=117) |
p-value |
|---|---|---|---|---|
| FOBT ≤ 60 days | 28 | 42 | 25 | 0.0206 |
| FOBT > 60 days | 35 | 45 | 25 | 0.0107 |
| Follow Up Survey | 73 | 61 | 50 | 0.0043 |
We measured our retention by offering each of the 330 participants the opportunity to complete a follow up survey at the end of the project. In total, 201 completed the survey. The overall participant follow-up/retention rate was 61%. The highest follow-up rate was achieved within Arm 1 at 73%, which was significantly higher than the control group (Arm 3) at 50% (P=0.0011) and approaching significance when compared to the AHP-led group (Arm 2) (61%; P=0.0735). Results are reported in Table 3. Pair-wise comparisons were conducted using simple logistic regression models and results are shown in Table 4.
Table 4.
Pairwise comparisons of study outcomes.
| FOBT ≤ 60 days | OR | 95%CI | p-value |
|
| |||
| Arm 1 vs Arm 3 | 1.2 | 0.6–2.2 | 0.6380 |
| Arm 2 vs Arm 3 | 2.1 | 1.2–3.8 | 0.0099 |
| Arm 1 vs Arm 2 | 0.5 | 0.3–1.0 | 0.0422 |
| FOBT > 60 days | OR | 95%CI | p-value |
|
| |||
| Arm 1 vs Arm 3 | 1.6 | 0.9–3.0 | 0.1117 |
| Arm 2 vs Arm 3 | 2.4 | 1.4–4.3 | 0.0029 |
| Arm 1 vs Arm 2 | 0.7 | 0.4–1.2 | 0.1780 |
| Follow Up Survey | OR | 95%CI | p-value |
|
| |||
| Arm 1 vs Arm 3 | 2.6 | 1.5–4.7 | 0.0011 |
| Arm 2 vs Arm 3 | 1.5 | 0.9–2.6 | 0.1213 |
| Arm 1 vs Arm 2 | 1.7 | 0.9–3.1 | 0.0735 |
This study showed that a well-designed CBPR program can lead to an increase in cancer knowledge and screening rates in underserved communities. Our project, EC4L, reduced participant barriers by providing relatively inexpensive FOBT kits and health-related education at the community level.
4. Discussion
The most effective programs have been those tailored to the needs of individual populations, those with navigators to help patients understand and use the medical system,16 and those with cancer coalition programs in which local leaders work within their communities to increase screening to improve quality of life.17;18
When reviewing the literature, several programs that increased CRC screening rates can be found. The effectiveness of education to increase CRC by FOBT kits was demonstrated in a community-based program in Palo Alto, California. The education received by patients was physician led, and 17% of the 11,000 participating in the study had positive FOBT results and were later diagnosed through colonoscopy with advanced colonic neoplastic lesions.19 Earlier research explored the ability of cancer-related education to improve CRC rates, and mailing was a favored mode of communication.19 Programs utilizing education and programs utilizing mailing of FOBT kits with reminders exist in the literature and showed an associated increase in screening. A combination of direct mailing of FOBT kits and education had not been investigated and thus, E4CL sought to determine if screening rates could improve further.
By combining a tailored message about colorectal screening with both instructions and the FOBT card for screening, the EC4L program has effectively increased CRC screening beyond the increase seen when simply providing FOBT cards. The importance of collaboration was demonstrated by the results that indicated that the AHP involvement leads to higher initial participation while the CLHW involvement increased retention rates. Developing relationships with community leaders provided inroads for our academic health center to connect with medically underserved communities and residents who would have otherwise gone unscreened. Future enhancements could include implementation of this model in a mobile unit that targets community health fairs and senior centers. Further analysis of the role of navigation and connection with an academic health center to follow-up on positive FOBT results is suggested.
5. Conclusions
A recent study describes the Lower Mississippi Delta, which included the targeted counties in this study, as “hotspots”.20 This area has the highest rates of CRC deaths from 1970 to 2011, and provided information as to where CRC screening interventions would be most effective. The literature supports the conclusion that effective interventions must engage communities. Our program, EC4L, used CBPR principles to do so and achieved positive results.
We intend for EC4L to be used as a model for other community healthcare providers to implement in an effort to increase CRC screening in communities that tend to be rural, poor-resourced, underserved, socially disengaged, disadvantaged, and have higher rates of colorectal cancer. To achieve the aims of this study, a CBPR approach was implemented to increase CRC screening through FOBT in the targeted counties. The 3-card FOBT was the choice of screening test based on the time period of this study. Since this study was conducted, we have transitioned to the fecal immunochemical test as our screening modality. We believe our CBPR program was effective, partly, because it eliminated participant barriers by providing FOBT kits and health-related education at the community level. Dissemination of this culturally congruent evidence-based approach will ultimately decrease mortality rates by increasing early detection.
Community-based participation partnered with academic health professionals enhanced CRC screening among rural and poor-resourced participants. With the introduction of the Affordable Care Act, responsive public health systems require strategies to determine which policies, systems, and administrative strategies are most effective in increasing preventive health service utilization and reducing health disparities.
Acknowledgments
We thank the support of the East Arkansas Enterprise Community; Mississippi County Arkansas Economic Opportunity Commission; our community research team Priscilla Johnson, Janell French, Ron Rasdon; and the UAMS research team Gemessia Hudson, Desiree Burroughs-Ray, Chara Stewart-Abrams, Danny Carter, Shannon Langhorn, Brandon Watson, Kimberly Enoch, Dale Gray, Christopher Ezika, and Eric Flowers. A special thanks to the UAMS College of Medicine Department of Surgery; UAMS College of Public Health researchers Drs. Karen Yeary and Paul Greene; the UAMS Translational Research Institute; and the UAMS Winthrop P. Rockefeller Cancer Institute. This manuscript was edited by Madison Hedrick, MA, of the Science Communication Group at the University of Arkansas for Medical Sciences.
Support was provided by the National Institutes of Health/National Center on Minority Health and Health Disparities grant no. R24MD002805 and Arkansas Cancer Plan Implementation Fund agreement no. 46000 39029.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the supporting agency.
There are no financial conflicts of interest to disclose.
References
- 1.Green BB, Coronado GD, Devoe JE, Allison J. Navigating the murky waters of colorectal cancer screening and health reform. Am J Public Health. 2014;104:982–986. doi: 10.2105/AJPH.2014.301877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Preston M. Health Care Reform: Colorectal Cancer Screening Expansion, Before and After the Affordable Care Act (ACA) [abstract]Preston M. Keeneland Conference 2014 Annual Meeting. 2014 [Google Scholar]
- 3.Cole AM, Jackson JE, Doescher M. Colorectal cancer screening disparities for rural minorities in the United States. J Prim Care Community Health. 2013;4:106–111. doi: 10.1177/2150131912463244. [DOI] [PubMed] [Google Scholar]
- 4.American Cancer Society. Cancer Facts & Figures 2014. Atlanta, GA: 2014. 3-12-2014. Ref Type: Online Source. [Google Scholar]
- 5.Chien C, Morimoto LM, Tom J, Li CI. Differences in colorectal carcinoma stage and survival by race and ethnicity. Cancer. 2005;104:629–639. doi: 10.1002/cncr.21204. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention (CDC) Vital signs: colorectal cancer screening test use--United States, 2012. MMWR Morb Mortal Wkly Rep. 2013;62:881–888. [PMC free article] [PubMed] [Google Scholar]
- 7.American Cancer Society. Cancer Facts & Figures 2013. 2013 Ref Type: Report. [Google Scholar]
- 8.Baquet CR, Commiskey P. Colorectal cancer epidemiology in minorities: a review. J Assoc Acad Minor Phys. 1999;10:51–58. [PubMed] [Google Scholar]
- 9.Berry J, Caplan L, Davis S, et al. A black-white comparison of the quality of stage-specific colon cancer treatment. Cancer. 2010;116:713–722. doi: 10.1002/cncr.24757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Polite BN, Dignam JJ, Olopade OI. Colorectal cancer model of health disparities: understanding mortality differences in minority populations. J Clin Oncol. 2006;24:2179–2187. doi: 10.1200/JCO.2005.05.4775. [DOI] [PubMed] [Google Scholar]
- 11.American Cancer Society. Cancer Facts & Figures 2009. 2009 Ref Type: Online Source. [Google Scholar]
- 12.Israel BA, Schulz AJ, Parker EA, Becker AB. Review of community-based research: assessing partnership approaches to improve public health. Annu Rev Public Health. 1998;19:173–202. doi: 10.1146/annurev.publhealth.19.1.173. [DOI] [PubMed] [Google Scholar]
- 13.United States Department of Health & Human Services. Agency for Healthcare Research and Quality. 2007 Ref Type: Online Source. [Google Scholar]
- 14.Israel BA, Schulz AJ, Parker EA, Becker AB. Community-based participatory research: policy recommendations for promoting a partnership approach in health research. Educ Health (Abingdon) 2001;14:182–197. doi: 10.1080/13576280110051055. [DOI] [PubMed] [Google Scholar]
- 15.Gellert K, Braun KL, Morris R, Starkey V. The 'Ohana Day Project: a community approach to increasing cancer screening. Prev Chronic Dis. 2006;3:A99. [PMC free article] [PubMed] [Google Scholar]
- 16.Sohl SJ, Moyer A. Tailored interventions to promote mammography screening: a meta-analytic review. Prev Med. 2007;45:252–261. doi: 10.1016/j.ypmed.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Battaglia TA, Roloff K, Posner MA, Freund KM. Improving follow-up to abnormal breast cancer screening in an urban population. A patient navigation intervention. Cancer. 2007;109:359–367. doi: 10.1002/cncr.22354. [DOI] [PubMed] [Google Scholar]
- 18.Rutledge W, Gibson R, Siegel E, et al. Arkansas Special Populations Access Network perception versus reality--cancer screening in primary care clinics. Cancer. 2006;107:2052–2060. doi: 10.1002/cncr.22147. [DOI] [PubMed] [Google Scholar]
- 19.Church TR, Yeazel MW, Jones RM, et al. A randomized trial of direct mailing of fecal occult blood tests to increase colorectal cancer screening. J Natl Cancer Inst. 2004;96:770–780. doi: 10.1093/jnci/djh134. [DOI] [PubMed] [Google Scholar]
- 20.Siegel RL, Sahar L, Robbins A, Jemal A. Where can colorectal cancer screening interventions have the most impact? Cancer Epidemiol Biomarkers Prev. 2015;24:1151–1156. doi: 10.1158/1055-9965.EPI-15-0082. [DOI] [PubMed] [Google Scholar]