Abstract
Visuospatial attention often improves task performance by increasing signal gain at attended locations and decreasing noise at unattended locations. Attention is also believed to be the mechanism that allows information to enter awareness. In this experiment, we assessed whether orienting endogenous visuospatial attention with cues differentially affects visual discrimination sensitivity (an objective task performance) and visual awareness (the subjective feeling of perceiving) during the same discrimination task. Gabor patch targets were presented laterally, either at low contrast (contrast stimuli) or at high contrast embedded in noise (noise stimuli). Participants reported their orientation either in a 3-alternative choice task (clockwise, counterclockwise, unknown) that allowed for both objective and subjective reports, or in a 2-alternative choice task (clockwise, counterclockwise) that provided a control for objective reports. Signal detection theory models were fit to the experimental data: estimated perceptual sensitivity reflected objective performance; decision criteria, or subjective biases, were a proxy for visual awareness. Attention increased sensitivity (i.e., improved objective performance) for the contrast, but not for the noise stimuli. Indeed, with the latter, attention did not further enhance the already high target signal or reduce the already low uncertainty on its position. Interestingly, for both contrast and noise stimuli, attention resulted in more liberal criteria, i.e., awareness increased. The noise condition is thus an experimental configuration where people think they see the targets they attend to better, even if they do not. This could be explained by an internal representation of their attentional state, which influences awareness independent of objective visual signals.
Keywords: Conscious perception, Objective and subjective vision, Contrast, Noise, Signal detection theory
1. Introduction
Our brains receive a vast amount of information through our sensory apparatus, but only a small portion of this information reaches awareness. Perceptual awareness, or consciousness, can be broadly defined as the subjective feeling of perceiving. Attention has been proposed to be the mechanism by which information is selected to reach awareness (Posner, 1994). Attention is a selective mechanism allowing enhanced processing of attended information and decreased processing of unattended information. Attention and awareness are so entangled that it has been suggested that attention is necessary for awareness, or at least for the ability to report sensory experiences (Dehaene et al., 2006; Lamme, 2003). However, attention is not sufficient for awareness; for example, attention can facilitate the processing of targets that remain otherwise invisible to the participants (Kentridge et al., 2008). Furthermore, some experiments have demonstrated a neural dissociation between attention and awareness, indicating that these processes are distinct. For example, MEG recordings have shown that whether attended or not, consciously seen stimuli induce increased mid-frequency gamma-band activity over the contralateral visual cortex, whereas spatial attention modulates high-frequency gamma-band activity in response to both consciously seen and unseen stimuli (Wyart and Tallon-Baudry, 2008).
Surprisingly, visual awareness (the subjective feeling of perceiving) has not been shown to be systematically increased by attention (Chica et al., 2011; Rahnev et al., 2011). Instead, experiments typically report increases of other aspects of visual perception, such as objective performance on specific tasks (the objective measures of how well one perceives, such as sensitivity in a discrimination task, detection speed in a visual search task, accuracy in a visuomotor reaching task, etc.) The distinction between objective task performance and awareness is best illustrated in cases of blindsight patients, who after a lesion of the striate cortex, deny being aware of stimuli presented in their scotoma, yet still have above-chance objective performance, such as discriminating or pointing to targets in their scotoma (Weiskrantz et al., 1974). Sometimes, their performance could even be superior to that in their intact visual field (Weiskrantz, 2009). This outcome reveals that the brain mechanisms mediating objective performance and awareness may be dissociated. Similarly, the effects of attention on objective performance and awareness might be dissociated as well (Sumner et al., 2006).
Visuospatial attention is the mechanism by which the processing of visual stimuli presented in one spatial location is enhanced compared to other spatial locations. Shifts of visuospatial attention are said to be covert when they occur while maintaining visual fixation (Rizzolatti et al., 1987), and endogenous when voluntarily triggered by the individual or an instruction (Corbetta and Shulman, 2002). To manipulate covert endogenous attention in a typical psychophysics paradigm, a central cue or instruction indicates the location to which the individuals should shift their attention while maintaining fixation. The effect of attention is typically assessed by comparing visual perception at attended vs. unattended locations (for example, on valid vs. invalid trials when attention is oriented by a central cue) (Posner, 1980). A typical way to assess that visuospatial attention has been allocated according to the cue is to examine reaction times: participants respond faster on validly cued than on invalidly cued trials (Posner, 1980).
Using this method, the effect of endogenous visuospatial attention on objective visual task performance has been extensively characterized. It has been shown to often increase objective visual performance, likely by increasing the visual signal strength in the brain at the attended location and/or reducing noise at unattended locations (Carrasco, 2011). For example, endogenous visuospatial attention has been shown to increase the probability of correctly detecting target items in stimulus arrays (Sperling and Melchner, 1978) and to increase accuracy in orientation discrimination tasks (Abrams et al., 2010; Grubb et al., 2013; Herrmann et al., 2010; Lu and Dosher, 1998, 2000).
By comparison, the effect of endogenous visuospatial attention on awareness is less understood and somewhat controversial. There is still no gold standard on how to measure awareness. Many studies have used confidence or visibility ratings following discrimination tasks as a measure of awareness: higher ratings are taken as greater awareness and lower ratings are taken as less awareness (Normal and Price, 2015; Timmermans and Cleeremans, 2015). Awareness has also been related to sensitivity in detection tasks, i.e., the ability to detect a target independent of the tendency to report seeing or not seeing in case of doubts (Pessoa et al., 2005). Alternatively, estimating decision criteria or subjective biases from detection tasks has been used to measure changes in awareness (Rahnev et al., 2011): more liberal responses are taken as increased awareness and more conservative responses are taken as decreased awareness. It has been suggested that attention increases awareness, because observers assign more weight to information extracted from the attended location (Kinchla, 1992). However, experimental studies have reported that endogenous visuospatial attention either weakly increases awareness, has no effect on awareness, or even decreases awareness (Chica et al., 2011; Rahnev et al., 2011). The use of complex designs and specific instructions in these studies may have contributed to these conflicting findings.
In this experiment, we aimed to simultaneously evaluate the effect of endogenous visuospatial attention on visual discrimination sensitivity (a measure of objective performance) and on subjective decision (a measure of visual awareness), as well as the relationship between these two effects using the simplest possible experimental paradigm. First, the participants performed a single orientation discrimination task with three response options that allowed measuring both objective performance and visual awareness, hence avoiding the use of two tasks, which could increase memory load and metacognitive processes (Timmermans and Cleeremans, 2015). Second, the target could appear at only two possible locations, one attended and the other unattended, which allowed participants to form a simple internal representation of the visual environment (Gorea and Sagi, 2000). Third, the visual targets were presented using the same contrast or same level of noise for the attended and unattended locations. This helped preventing unequal physical properties of the targets, which could have been detected by the individuals and used to guide subjective decisions concerning awareness (Rahnev et al., 2011). Fourth, no feedback was given to the participants, which prevented the adoption of rigid criteria (Chica et al., 2011). Lastly, we used signal detection theory (SDT) models with no a priori constraints on either objective or subjective components of visual perception (Stanislaw and Todorov, 1999). Because attention can increase target signal and/or reduce noise, this simple paradigm was employed using two kinds of stimuli: low-contrast targets and high-contrast targets embedded in noise. We predicted that attention would increase visual awareness (participants would become more liberal in their subjective decisions), independent of its effect on visual sensitivity.
2. Methods
2.1. Participants
Twenty healthy adults (age: 20–50 years, mean of 27 ± 8 years; 17 females, 19 right-handed, 16 right-eye dominant) participated in a two-hour study after giving their written informed consent. All participants had normal or corrected-to-normal vision and received monetary compensation for their participation. All procedures were approved by the Institutional Review Board of the National Institute of Mental Health. Five out of twenty participants were excluded since their performance data were considered outliers according to the generalized extreme Studentized deviate (ESD) test (Rosner, 1983) (see details on the analysis below, section “Consideration of outliers”). Thus, all analyses were performed on data from the remaining fifteen participants.
2.2. Materials
Participants were comfortably seated in a dark room with their chin placed on a chin-rest. Their eyes were 57 cm from a computer screen (53 × 29.8 cm), which presented the visual stimuli using MATLAB (Mathworks) and the Psychtoolbox (Brainard, 1997) running on a MacBook Pro laptop computer. To ensure that participants were fixating at the center of the screen throughout the experiment, eye movements were recorded using a monocular ASL Eye-Trac 6000 eye tracker. Eye movements were monitored online and participants received warnings when they occasionally broke fixation.
2.3. Discrimination task
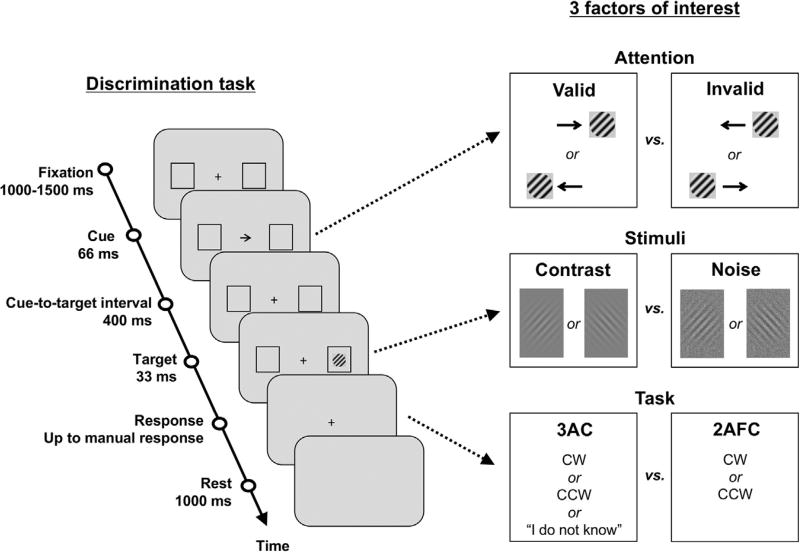
The main aim of the study was to compare the effect of visuospatial attention on discrimination sensitivity and awareness with the same discrimination task. A 2 (stimulus type: contrast vs. noise stimuli) × 2 (attention: valid vs. invalid trials) × 2 (task: 3AC vs. 2AFC tasks) factorial design was used, as explained below.
2.3.1. Stimulus type factor: contrast vs. noise stimuli
The study comprised two sessions of equal duration. In both sessions, the visual targets were Gabor patches (standard deviation of the Gaussian: 2° of visual angle; period of the sinusoid: 1°; inclination relative to the vertical + 45° for the clockwise (CW) target and − 45° for the counterclockwise (CCW) target). In one session, the Gabor patches were low-contrast stimuli with variable levels of contrast. A contrast of 0 corresponds to an entirely gray target (pixel values = 0.5), indistinguishable from the background, whereas a contrast of 1 corresponds to a Gabor made with the full gray scale from black to white (pixel values from 0 to 1). These stimuli are referred to as the contrast stimuli (see Fig. 1). In the other session, the Gabor patches were embedded in noise (Gaussian distribution of pixel values between 0.3 and 0.7, where 0 is black and 1 is white) with variable levels of transparency. A transparency of 0 corresponds to noise only, whereas a transparency of 1 corresponds to the target only. These stimuli are referred to as the noise stimuli (see Fig. 1). The order of the sessions was counterbalanced across subjects.
Fig. 1.
Illustration of a single trial of the experimental paradigm. After a fixation screen, a rightward or leftward arrow appeared for 66 ms, which indicated where to orient one's attention while maintaining fixation; the target appeared at the cued location with an 80% probability (80% valid vs. 20% invalid trials). Then, following a fixed cue-to-target interval of 400 ms, the target appeared for 33 ms in the center of one of the two placeholders. The target was a Gabor patch, either embedded in noise or displayed at a low contrast. The participant then responded, as fast as possible, about the orientation of the target with clockwise (CW), counterclockwise (CCW) and “I do not know” options in the 3AC task or CW and CCW options in the 2AFC task. Such a design allowed the manipulation of 3 factors: attention (valid vs. invalid trials), stimulus type (contrast vs. noise stimuli) and task (3AC and 2AFC tasks).
2.3.2. Attention factor: valid vs. invalid trials
Each trial (see Fig. 1) started with a gray screen (1000 ms), followed by a fixation screen (randomly lasting between 1000 and 1500 ms). A fixation cross (0.5° × 0.5°) was displayed at the center of the screen, along with two lateral placeholders (4.1° × 6°, 8.5° eccentricity). The fixation cross then changed into an arrow with the same dimensions as the fixation cross (66 ms duration), which indicated with an 80% probability the position of the upcoming target (80% valid vs. 20% invalid cue). The participants were informed that the cue was correct most of the time and were encouraged to use it to covertly shift their attention before the target appeared. Then, following a fixed cue-to-target interval (400 ms), the contrast or noise stimulus appeared briefly (33.3 ms) in the center of one of the two placeholders (50% left trials, 50% right trials). The trial ended with the disappearance of the stimulus and of the two lateral placeholders. Participants were instructed to respond as fast as possible when they saw the stimulus, or alternatively when the trial ended, by pressing keys on a computer keyboard. The choice of keys depended on the task (described below).
2.3.3. Task factor: 3AC vs. 2AFC tasks
During the titration procedure (see below) and the first task (3AC, 3-alternative choice), participants chose between the following responses: CW (right arrow), CCW (left arrow) or “I do not know” (space bar) when they could not discriminate the target orientation. The arrow keys were pressed with two fingers of their choice from their right hand and the space bar with a finger of their choice from their left hand. During the second task (2AFC, 2-alternative forced-choice), participants were forced to respond CW or CCW, as fast as possible, with one of the two arrow-keys, irrespective of whether they could or could not discriminate the orientation of the target. The 3AC task allowed us to measure both objective performance (discrimination sensitivity) and subjective decisions about one's own feeling of perception (awareness). By contrast, the 2AFC task only evaluated discrimination sensitivity and was designed as a control to compare to the objective performance measured in the 3AC task.
2.3.4. Titration procedure and organization of a session
Each of the two sessions (one for the contrast stimuli, the other for the noise stimuli) began with a titration procedure, in which the contrast or level of noise was adjusted to perceptual threshold, followed by the 3AC task, and then the 2AFC task.
During the titration procedure, a one-up-one-down algorithm was used to determine the perceptual threshold at which the participants correctly detected the orientation of the target on 50% of the trials (participants were provided with the same response keys as in the 3AC task). During the titration procedure only, a third of the trials were presented with maximum noise or minimum contrast (“catch trials”) as a way to verify that the participants would mostly use the “I do not know” option when not presented with relevant visual information.
Both the 3AC and 2AFC tasks contained 180 trials divided in blocks of 20 trials each. Contrast or levels of noise were readjusted if necessary at the end of each block to maintain orientation discrimination performance at ~ 50% correct in the 3AC task and ~ 75% correct in the 2AFC task. Such values are half-way between the worst performance and the best performance. The worst performance is 0% in the 3AC task (when participants only select “I do not know” responses) and 50% in the 2AFC task (when participants performance is at chance level). For both tasks, the best performance is 100%. All conditions (CCW vs. CW targets, left vs. right target's positions, 80% valid vs. 20% invalid cues) were balanced and randomized within each block.
2.4. Data analysis
2.4.1. Reaction times
For each individual, each type of stimulus, and each task, we excluded trials whose reaction time (RT) was considered an outlier according to the generalized extreme Studentized deviate (ESD) test (Rosner, 1983). On average, 2.3 ± 2.8% of the trials were removed per subject.
To ensure that our paradigm was efficient in orienting visuospatial attention, a three-way ANOVA was performed with the following factors: attention (valid vs. invalid trials), stimulus type (contrast vs. noise stimuli), and task (3AC vs. 2AFC tasks) applied to the RT data. We anticipated that attention would decrease RT, i.e., RTs would be shorter on valid than on invalid trials.
2.4.2. Signal detection theory models
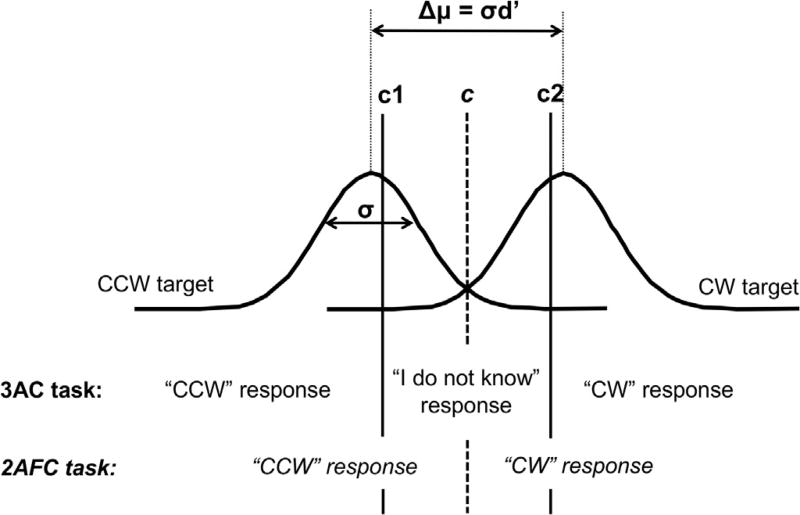
We used signal detection theory (SDT, Stanislaw and Todorov, 1999) to estimate objective performance and visual awareness. SDT is based on modeling the internal representation of the CW and CCW targets with Gaussian distributions, as illustrated in Fig. 2. The intuitive understanding of SDT models is as follows. The abscissa axis can represent any encoding variable in the brain (a simple example being the frequency of discharge of a single neuron). If the two distributions are almost entirely separated, it is easy to distinguish the two signals (CW and CCW); whereas if the two distributions are entirely superimposed, it is impossible to distinguish the two signals. The degree of overlap between the two distribution is called perceptual sensitivity, or d′, and measures objective visual performance. Response criteria are the other parameters defined by SDT. They are adjusted by the participants independently of their objective performance, and reflect their subjective decisions (see vertical bars in Fig. 2: each side of the bar reflects different responses of the participants). For example, for the 2AFC task, some participants may have a response bias in favor of the CW target over the CCW target. In that case, the criterion c would be shifted to the left. At the group level, we do not expect any bias in favor of one target over another; thus, the criterion c is expected to be close to 0 (the center). More importantly, the criteria c1 and c2 for the 3AC task reflect the tendency to select “I do not know” vs. CW or CCW in the range where the internal representations of the two targets overlap. If the two criteria c1 and c2 are far from 0, the participant is said to be conservative because when in doubt, she/he tends to select “I do not know”. By contrast, if the two criteria are close to 0, the participant is said to be liberal because when in doubt, she/he tends to select CW or CCW. In our study, if attention makes participants more conservative (or more liberal), it will indicate that attention decreases (or increases) awareness.
Fig. 2.
Signal Detection Theory (SDT) modeling. The internal representations of the two targets (CW/CCW) are modeled by two partially overlapping Gaussian distributions of the same shape. Increasing objective performance is equivalent to decreasing the overlap between the Gaussians (i.e., increasing d′, either by increasing Δμ, or by decreasing σ, or both). Subjective decisions are assessed by the location of the criteria. In the case of the 2AFC task, when the internal variable is to the left (or right) of the criterion c, the participant answers CCW (or CW). In the case of the 3AC task, the participant answers CCW when the internal variable is to the left of c1, the participant answers CW when the internal variable is to the right of c2, and the participant answers “I do not know” in between. Fitting the model of SDT consists of determining d′ (or Δμ and σ) and the criteria, which minimize the difference between the estimated and experimental response ratios (see text for more details).
Perceptual sensitivity d′ and response criteria c, c1 and c2 (referred to here as relative criterion) are expressed in units of the width of the Gaussian (the widths of the CW and CCW distributions are expected to be the same and modeled by a single parameter, σ). An increase in d′ (i.e., a decrease in the overlap between the two Gaussians) can be explained by two distinct (non-mutually exclusive) mechanisms: 1) an increase in Δμ, the distance between the peaks of the two Gaussians, which can be interpreted as an increase in signal strength or contrast, or 2) a decrease in σ, the width of the Gaussians, which can be interpreted as a decrease in noise. Similarly, a shift in relative criteria can be explained by two distinct (non-mutually exclusive) mechanisms: 1) a shift in the absolute value of the criteria (i.e., not expressed in units of σ; referred to here as absolute criterion) or 2) a change in σ. Note that d′ would be equal to Δμ, and relative criteria would be the same as the absolute criteria, if σ, the width of the Gaussians, was equal to 1. For these reasons, we analyzed our data using two models. In Model 1 we estimated d′ and the relative criteria (c for the 2AFC task and c1 and c2 for the 3AC task) and in Model 2 we estimated Δμ, σ and the two absolute criteria (cabs1 and cabs2) for the 3AC task. Note that the second model could not be fit to the data in the 2AFC task, because it required three degrees of freedom (see more details below).
The model fitting procedure consisted of finding the value of perceptual sensitivity (d′) and relative criteria (Model 1), and Δμ, σ and the absolute criteria (Model 2) that minimized the difference between the experimental and the estimated response ratios. The experimental response ratios were calculated based on the number of trials in which the participant answered CW, CCW or “I do not know” for the 3AC task and CW or CCW for the 2AFC task when the target was CW or CCW (resulting in six ratios covering four degrees of freedom for the 3AC task, and four ratios covering two degrees of freedom for the 2AFC task). The estimated response ratios are the areas under the curves in the SDT models. For example, for the 3AC task, when the target was CW, the ratio of CW responses is the area under the CW Gaussian to the right of c2, the ratio of “I do not know” responses is the area under the CW Gaussian in-between c1 and c2, and the ratio of CCW responses is the area under the CW Gaussian to the left of c1. The models were fit to the experimental data using the fminunc (find minimum of unconstrained multivariable) function available in the MATLAB Optimization Toolbox.
2.4.3. Consideration of outliers
SDT might not apply efficiently to extreme behaviors (e.g., when few false alarms occur in a detection task; in our design, when few errors occurred). More importantly, attention effects might be minimized in participants who were at ceiling performance (and ceiling criteria, see details on the relationship below). The generalized ESD test (Rosner, 1983) applied to our d′ data identified 5 outliers: all had d′ exceeding 3 in at least one of the conditions.
2.4.4. Individual effect of attention on visual sensitivity and awareness
One of the main questions of our study was to assess the effects of visuospatial attention on both visual discrimination sensitivity and visual awareness during the same discrimination task, and to examine if these effects were independent of each other.
Estimating the discrimination sensitivity (d′) and relative criteria (c1 and c2) ideally dissociates the estimation of objective performance from the estimation of subjective decision. This does not mean, however, that the results of the two measures will be unrelated. On the contrary, recall that, for the 3AC task, contrast and level of noise were adjusted so that 50% of the targets were correctly discriminated. Participants using conservative criteria (large half-distance between c1 and c2) often used the “I do not know” option when in doubt about their perception. Such a strategy led to large values of d′ to reach 50% of correct discriminations. By contrast, participants using liberal criteria (smaller half-distance between c1 and c2) more often used the CW and CCW options when in doubt, resulting in smaller values of d′ for the same 50% of correct discriminations. Thus, by design, we expected a correlation across participants between d′ and relative criteria and we assessed this using Pearson's r correlation.
Inasmuch as one goal of our study was to determine whether the effects of attention on discrimination sensitivity [Δatt(d′)] and the effects of attention on awareness [Δatt(criteria half-distance)] were related, we also assessed this correlation with Pearson's r correlation.
2.4.5. Group effect of attention on visual sensitivity and awareness
To further assess the effect of attention on d′, and how the effect of attention on d′ differed across stimulus types and tasks, we performed a three-way ANOVA with the same factors as in the RT analysis: attention (valid vs. invalid trials), stimulus type (contrast vs. noise stimuli), and task (3AC vs. 2AFC tasks). Since different criteria were estimated for the two tasks, separate two-way ANOVAs with attention (valid vs. invalid trials) and stimulus type (contrast vs. noise stimuli) as factors were run on the relative criteria (c, c1 and c2).
Similar ANOVAs were conducted on parameters estimated from the second SDT model for the 3AC task only. The effect of attention (and its potential interaction with stimulus type) on Δμ, σ and the absolute criteria cabs1 and cabs2 were evaluated with two-way ANOVAs with the following factors: attention (valid vs. invalid trials) and stimulus type (contrast vs. noise stimuli).
2.4.6. Complementary estimations of objective performance and awareness
To allow comparisons with previous studies, we also examined simpler measures of objective performance and awareness, namely, the discrimination accuracy (percentage of correctly discriminated targets) and the detection rate (percentage of detected targets), respectively. Note that, unlike d′ and the criteria, such measures are not independent of each other. For both the 2AFC and the 3AC tasks, the discrimination accuracy was calculated as the number of correct CW and CCW responses divided by the total number of CW and CCW responses. For the 3AC task, the detection rate was calculated as the number of correct and incorrect CW and CCW responses divided by the total number of trials. Three-way and two-way ANOVAs were performed on these two measures, similar to the three-way and two-way ANOVAs performed on d′ and the criteria.
2.4.7. Further statistical considerations
Values are indicated as mean ± standard deviation. Significant interactions in ANOVAs were followed by post-hoc two-tailed t-tests. For the correlation analyses, p-values were corrected by the number of correlations performed (Bonferroni correction). The level of significance was set at 0.05.
3. Results
3.1. Effect of attention on RT
The three-way ANOVA of the RT data showed a significant main effect of attention (F(1,14) = 73.88; p < 0.001) and a significant main effect of task (F(1,14) = 5.67; p = 0.032), but no significant main effect of stimulus type, and no significant interactions (all p > 0.05). The main effect of attention showed that participants were faster to respond to valid trials (609 ± 114 ms) than to invalid trials (673 ± 128 ms). This result confirmed that the participants were appropriately using the cue to orient their attention. The main effect of task showed that participants were faster to respond when they had to choose between two options (2AFC; 621 ± 130 ms) than when they had to choose between three options (3AC; 662 ± 118 ms).
3.2. Individual effect of attention on visual sensitivity (d′) and relative criteria
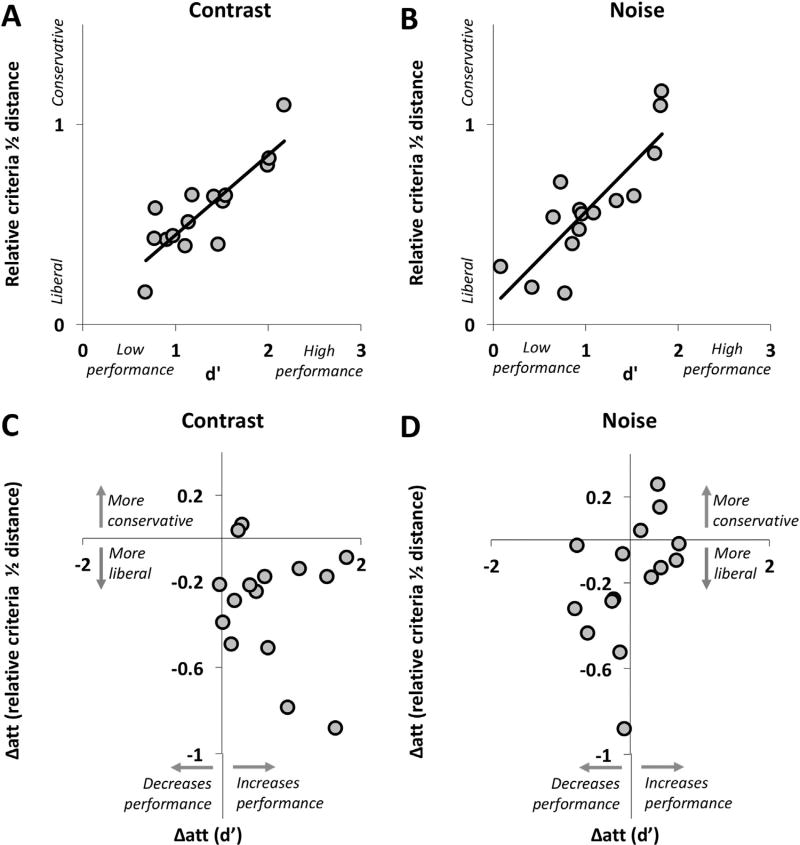
Signal detection theory (SDT) dissociates genuine objective visual abilities from subjective decisions. However, during the 3AC task, contrast and level of noise were adjusted so that about 50% of the targets were correctly discriminated. Thus, by design, we expected a high correlation across participants between discrimination sensitivity and awareness (see fuller explanation in the Methods). Consistent with this expectation, we obtained significant correlations between d′ and the relative criteria half-distance (composite values calculated as follows: 0.8*averaged value for valid trials + 0.2*averaged value for invalid trials) for both the contrast and the noise stimuli (r2 = 0.72 for contrast and r2 = 0.70 for noise, both corrected p < 0.001, see Figs. 3A and B).
Fig. 3.
Relationship between objective performance and criteria for the contrast (A) and noise (B) stimuli. Significant correlations were observed between d′ and the distance between relative criteria for both the contrast and the noise stimuli (expected by design). Relationship between the effect of attention on objective performance and the effect of attention on awareness for contrast (C) and noise (D) stimuli. There was no significant correlation between the effects of attention on d′ and on the distance between relative criteria. In these figures, it is apparent that attention resulted in most participants improving visual performance for the contrast stimuli but not for the noise stimuli, while becoming more liberal for both the contrast and noise stimuli.
The relationship between the effect of attention on d′ and the effect of attention on the relative criteria half-distance was tested in a second analysis, which did not show any significant correlation, for either the contrast or the noise stimuli (r2 = 0.05 for contrast and r2 = 0.19 for noise, both p > 0.1, see Figs. 3C and D). This result points to independent effects of attention on discrimination sensitivity and on awareness.
3.3. Group effect of attention on visual sensitivity (d′) and relative criteria
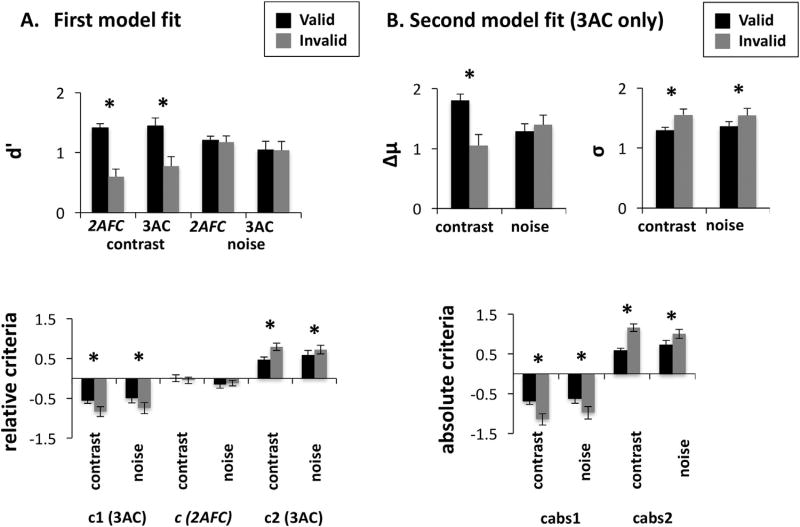
The effect of attention on d′ and on the relative criteria as well as its interactions with task and stimulus type is shown in Fig. 4A. The three-way ANOVA of d′ data showed a significant main effect of attention (F(1,14) = 35.26, p < 0.001) and a significant interaction between attention and stimulus type (F(1,14) = 32.92, p < 0.001). No other main effect or interaction was significant (all p > 0.05). Post-hoc analyses revealed no significant effect (p > 0.25) of attention on d′ for the noise stimuli, but a significant effect (p < 0.001) for the contrast stimuli, such that participants exhibited greater visual sensitivity on valid trials than on invalid trials.
Fig. 4.
Effects of attention on objective performance and visual awareness. A. Group results of the first model fit estimating d′ and the relative criteria across attention conditions (valid vs. invalid trials), stimulus types (contrast vs. noise) and tasks (2AFC vs. 3AC). The results showed that attention increased d′ (i.e., d′ was higher for valid than invalid trials) for the contrast, but not for the noise stimuli. Moreover, attention made the subjective criteria more liberal (i.e., c1 and c2 were closer to 0 for valid than for invalid trials) for both the contrast and the noise stimuli in the 3AC task. For the 2AFC task, the criterion c was close to 0 in all conditions (not favoring CW over CCW responses and vice versa). B. Group results of the second model fit estimating Δμ, σ, and the absolute criteria across attention conditions (valid/invalid trials) and stimulus types (contrast/noise) for the 3AC task. The results showed that attention increased Δμ (i.e., Δμ was higher for valid than invalid trials) for the contrast, but not for the noise stimuli. Moreover, attention decreased σ (i.e., σ was smaller for valid than invalid trials) for both types of stimuli. Finally, attention made the subjective criteria more liberal (i.e., c1 and c2 were closer to 0 for valid than for invalid trials) for both the contrast and the noise stimuli.
The two-way ANOVA on the relative criterion c for the 2AFC task showed no significant main effects or interaction (all p > 0.25), indicating that the participants did not favor the CW response over the CCW response or vice versa. The two-way ANOVAs on the relative criteria c1 and c2 for the 3AC task showed significant main effects of attention on both (c1: F(1,14) = 11.12, p = 0.005; c2: F(1,14) = 13.34, p = 0.003). This analysis revealed that, in the 3AC task, attention made the criteria more liberal (i.e., closer to 0 for valid trials than for invalid trials) for both contrast and noise stimuli. Neither the main effect of stimulus type, nor the interaction were significant (all p > 0.05).
3.4. Group effect of attention on Δμ, σ and absolute criteria
For the 3AC task, the effect of attention, and its interaction with the stimulus type, on Δμ, σ, and the absolute criteria is shown in Fig. 4B. The two-way ANOVA on Δμ showed a significant main effect of attention (F(1,14) = 5.88, p = 0.030) and a significant interaction between attention and stimulus type (F(1,14) = 12.42, p = 0.003). The main effect of stimulus type was not significant (p > 0.25). Post-hoc analysis revealed that for the noise stimuli, the effect of attention on Δμ was not significant (p > 0.25), whereas for the contrast stimuli, it was significant (p = 0.002): Δμ was larger on valid trials than on invalid trials.
The two-way ANOVA on σ showed a significant main effect of attention (F(1,14) = 10.4, p = 0.006). This analysis revealed that σ was smaller for valid trials than for invalid trials. Neither the main effect of stimulus type, nor the interaction was significant (all p > 0.25).
The two-way ANOVAs on absolute criteria showed a significant main effect of attention (for cabs1: F(1,14) = 27.94, p < 0.001; for cabs2: F(1,14) = 35.9, p < 0.001), such that criteria were more liberal (i.e., closer to 0) on valid trials than on invalid trials. Neither the main effect of stimulus type, nor the interaction was significant (all p > 0.05).
3.5. Group effect of attention on the discrimination accuracy and on the detection rate
The three-way ANOVA of the discrimination accuracy showed a significant effect of task (F(1,14) = 7.18, p < 0.018). Indeed, the discrimination accuracy increased when participants had the possibility to select the “I do not know” response (3AC task, 76 ± 13%) compared to when they were forced to answer (2AFC task, 69 ± 8%). Additionally, the ANOVA showed a significant main effect of attention (F(1,14) = 27.97, p < 0.001) and a significant interaction between attention and stimulus type (F(1,14) = 23.38, p < 0.001). Post-hoc analyses revealed no significant effect of attention on discrimination accuracy for the noise stimuli (p > 0.25; valid trials: 73 ± 10%; invalid trials: 74% ± 11%), but a significant effect (p < 0.001) for the contrast stimuli, such that participants exhibited better discrimination accuracy on valid trials (79 ± 8%) than on invalid trials (66 ± 12%). The main effect of stimulus type was not significant and no other interaction was significant (all p > 0.05).
The two-way ANOVAs on the detection rate in the 3AC task revealed a significant main effects of attention (F(1,14) = 19.99, p < 0.001) and a significant interaction between attention and stimulus type (F(1,14) = 15.42, p = 0.002). Post-hoc analysis revealed that attention had a significant effect on the contrast stimuli (p < 0.001; 71 ± 8% for valid and 50 ± 17% for invalid) and, to a lesser extent, on the noise stimuli (p = 0.010; 68 ± 13% for valid and 58 ± 16% for invalid).
This complementary analysis matches the results of the ANOVAs on d′ and the relative criteria c1 and c2 (STD Model 1). First, objective performance (d′ in Model 1; discrimination accuracy here) increased overall for the contrast, but not for the noise stimuli. Second, visual awareness (related to the criteria distance in Model 1; detection rate here) increased overall for both the contrast and the noise stimuli.
4. Discussion
Our experiment aimed to examine whether endogenous visuospatial attention influences objective performance and/or awareness and whether there is a relationship between these two effects. First, we validated that our experimental paradigm was effective in orienting endogenous attention - indeed, attention decreased reaction time on valid compared to invalid trials (Posner, 1980). After this validation, our main results were: (i) Attention increased objective performance, but only for stimuli presented with low contrast and not for stimuli embedded in noise; (ii) The effects of attention on objective performance and awareness were unrelated; (iii) Attention increased visual awareness (i.e., participants became more liberal) for both types of stimuli tested. The implications of these findings are discussed below.
4.1. Attention increased objective performance by increasing signal strength and decreasing spatial uncertainty
For both the contrast and the noise stimuli, allocating attention to where the target appeared reduced internal noise. Additionally, attention increased signal strength Δμ and d′ for contrast stimuli, but failed to do so for noise stimuli. Indeed, for the noise stimuli, for which reduced visibility stemmed from the addition of external noise, the already high target contrast resulted in the lack of effect of attention on objective performance. Thus, our results are compatible with several experiments indicating that endogenous visuospatial attention increases performance by increasing signal strength (Ling and Carrasco, 2006; Lu and Dosher, 1998). Indeed, in our study, attention increased signal with low strength (contrast stimuli), but when signal strength was already high, attention had no effect (noise stimuli).
In addition to the mechanism of increased signal strength, decreased spatial uncertainty may have also contributed to our attentional effect on objective performance. Indeed, empty locations introduce noise that can be confused with the target; attention, by decreasing spatial uncertainty, can in turn enhance performance (for a review see (Carrasco, 2011)). In our experiment, contrast stimuli were in competition with the empty location and attention could act by decreasing this competition. By contrast, the noise stimuli were high-contrast targets embedded in high-contrast noise and participants always saw where the targets appeared, even if discriminating their orientation was difficult. Thus, there was no competition between the target and the empty location and no spatial uncertainty that attention could have decreased.
4.2. Attention increased awareness by integrating several sources of external and internal information
Our study provides two sets of evidence for a dissociation between the effects of attention on objective performance and on awareness. First, we found no correlation between the effect of attention on d′ and its effect on the criteria (neither for the contrast stimuli, nor for the noise stimuli). Second, we found an experimental paradigm (i.e., discrimination task using noise stimuli) for which attention did not modulate d′, but rather made participants more liberal in their responses. Such a dissociation between the effects of attention on objective and subjective components has been shown before, although previous studies have shown attention to either have no effect on or decrease awareness; unlike the increase we found in our study (Chica et al., 2011; Rahnev et al., 2011).
As mentioned in the Introduction, there are clear examples of dissociations between objective performance and awareness in the literature, both in patients (Weiskrantz, 2009) and in healthy participants (Hesselmann et al., 2011; King and Dehaene, 2014; Passingham and Lau, 2017). Such dissociation can be further revealed when TMS is applied to the temporal cortex of healthy participants (Overgaard et al., 2004). Most often, one observes excellent performance when participants claim to be aware and, in some instances, above-chance performance when participants claim not to be aware. This observation, as well as our current findings, are in line with the idea that only a portion of the information present in the brain can be consciously accessed; presumably, attention is the mechanism that allows one to access it (Dehaene et al., 2006).
Such an “attentional gate to awareness” mechanism can explain our results, but fail to account for previous results in the literature, namely, the failure of endogenous attention to increase visual awareness (Chica et al., 2011) or the decreased awareness of attended targets compared to unattended ones (Rahnev et al., 2011). Thus, we propose the existence of a larger framework: awareness is constructed by integrating signals from different sources , including not only visual information present in the brain relevant for the task, but also other visual task-irrelevant information, task instructions, and even knowledge of one's own attentional state. Each of these signals might itself be modulated by several factors, such as attention and statistics of the environment.
In this “integration and modulation” framework, our results might be explained by two non-mutually exclusive phenomena. First, awareness is based on task-relevant information (source signal) modulated by attention (modulating factor); such an explanation is equivalent to the “attentional gate to awareness” mechanism mentioned earlier. Second, participants could have used the knowledge of where their attention was (source signal) to make their awareness decisions; indeed, they were explicitly encouraged to use the attentional cue, which was correct “most of the time”. This explanation is compatible with a recent proposal in which consciousness might be the model one would maintain concerning one's own attentional state (Graziano, 2013).
This “integration and modulation” framework can also explain why attention had only a weak or null impact on awareness in Chica et al. (2011), where task instructions (source signal) could have played a predominant role. Indeed, participants were encouraged not to make any false alarms, which induced highly conservative criteria, thereby limiting the impact of attention on conscious perception. Of interest, when this instruction was not strictly enforced (by warning participants only when their false alarm rate exceeded 55%), attention weakly increased awareness. This framework can also explain why attention decreased awareness in Rahnev et al. (2011). Task-irrelevant visual information (source signal), such as lower contrast for attended than unattended targets, might have been used by participants to make their awareness decision in favor of unattended targets. In addition, task complexity (modulating factor), such as attention simultaneously oriented towards two opposite quadrants of the visual field, may have contributed to rigid absolute criteria (Gorea and Sagi, 2000). By contrast, in our simpler task, criteria were free to be adjusted independently for each attentional condition.
The neural substrate of this integration of signals from multiple sources, and its modulation by attention or environment statistics, is still unknown. Besides the difficulty to measure awareness (the integrated information), it is challenging to dissociate it from its sources and influences (e.g., attention, visual information, etc.) as well as its consequences (de Graaf et al., 2012). Based on previous studies, investigating the neural correlates of consciousness, such integration could occur: (i) in a global neuronal workspace (Baars, 1989) subserved by the attentional fronto-parietal network (Dehaene and Changeux, 2011; see also Passingham and Lau, 2017); (ii) in the ventral visual stream, when local recurrent processing occurs (Lamme, 2006); (iii) in posterior cortical areas which allow differentiation and integration of information (Koch et al., 2016); or (iv) in the superior temporal sulcus and/or temporo-parietal junction, which would be able to track what others and oneself are attending to (Graziano, 2013; Webb et al., 2016). Future studies will be needed to test the predictions of each of these models in a variety of paradigms testing different aspects of awareness.
4.3. Conclusion
In conclusion, we confirmed that endogenous visuospatial attention decreases reaction time on visual discrimination tasks. This is a robust finding, which can be taken as a pre-requisite to ensure that participants indeed use the endogenous cue to orient their attention. We also confirmed that endogenous visuospatial attention does or does not improve objective performance, depending on the characteristics of the visual stimulus. These results are compatible with an attentional mechanism of signal enhancement and an attentional mechanism of spatial uncertainty reduction. Finally, together with previous studies, we showed that attention can also impact awareness independent of objective performance. We suggest that awareness decisions are built on a combination of signals originating from visual processing of task-relevant (for example, the orientation of the target) or task-irrelevant (for example, the contrast or the level of noise) visual information, task instructions, knowledge of one's own attentional state, etc. The weight of these signals is itself modulated by attention and environmental statistics and complexity. In our study, we specifically suggest that (i) attention could have increased the portion of the task-relevant visual information present in the brain that can be consciously accessed; and (ii) participants maintained an internal representation of their attentional state that influenced their awareness independent of visual signals.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute of Mental Health (NCT00001360 – Protocol 93-M-0170 - ZIA MH002035-37). The authors would like to thank John INGEHOLM for help with the technical testing environment and Romain QUENTIN for helpful discussions of data analyses.
References
- Abrams J, Barbot A, Carrasco M. Voluntary attention increases perceived spatial frequency. Atten. Percept. Psychophys. 2010;72:1510–1521. doi: 10.3758/APP.72.6.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baars BJ. In the Theater of Consciousness: The Workspace of the Mind. Cambridge University Press; Cambridge: 1989. [Google Scholar]
- Brainard DH. The psychophysics toolbox. Spat. Vision. 1997;10:433–436. [PubMed] [Google Scholar]
- Carrasco M. Visual attention: the past 25 years. Vision. Res. 2011;51:1484–1525. doi: 10.1016/j.visres.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chica AB, Lasaponara S, Chanes L, Valero-Cabre A, Doricchi F, Lupianez J, Bartolomeo P. Spatial attention and conscious perception: the role of endogenous and exogenous orienting. Atten. Percept. Psychophys. 2011;73:1065–1081. doi: 10.3758/s13414-010-0082-6. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- de Graaf TA, Hsieh PJ, Sack AT. The 'correlates' in neural correlates of consciousness. Neurosci. Biobehav. Rev. 2012;36:191–197. doi: 10.1016/j.neubiorev.2011.05.012. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Changeux JP. Experimental and theoretical approaches to conscious processing. Neuron. 2011;70:200–227. doi: 10.1016/j.neuron.2011.03.018. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Changeux JP, Naccache L, Sackur J, Sergent C. Conscious, preconscious, and subliminal processing: a testable taxonomy. Trends Cogn. Sci. 2006;10:204–211. doi: 10.1016/j.tics.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Gorea A, Sagi D. Failure to handle more than one internal representation in visual detection tasks. Proc. Natl. Acad. Sci. USA. 2000;97:12380–12384. doi: 10.1073/pnas.97.22.12380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano MS. Consciousness and the Social Brain. Oxford University Press; 2013. [Google Scholar]
- Grubb MA, Behrmann M, Egan R, Minshew NJ, Carrasco M, Heeger DJ. Endogenous spatial attention: evidence for intact functioning in adults with autism. Autism Res. 2013;6:108–118. doi: 10.1002/aur.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann K, Montaser-Kouhsari L, Carrasco M, Heeger DJ. When size matters: attention affects performance by contrast or response gain. Nat. Neurosci. 2010;13:1554–1559. doi: 10.1038/nn.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselmann G, Hebart M, Malach R. Differential BOLD activity associated with subjective and objective reports during "blindsight" in normal observers. J. Neurosci. 2011;31:12936–12944. doi: 10.1523/JNEUROSCI.1556-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kentridge RW, Nijboer TC, Heywood CA. Attended but unseen: visual attention is not sufficient for visual awareness. Neuropsychologia. 2008;46:864–869. doi: 10.1016/j.neuropsychologia.2007.11.036. [DOI] [PubMed] [Google Scholar]
- Kinchla RA. Attention. Annu. Rev. Psychol. 1992;43:711–742. doi: 10.1146/annurev.ps.43.020192.003431. [DOI] [PubMed] [Google Scholar]
- King JR, Dehaene S. A model of subjective report and objective discrimination as categorical decisions in a vast representational space. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014;369 doi: 10.1098/rstb.2013.0204. 20130204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch C, Massimini M, Boly M, Tononi G. Neural correlates of consciousness: progress and problems. Nat. Rev. Neurosci. 2016;17:307–321. doi: 10.1038/nrn.2016.22. [DOI] [PubMed] [Google Scholar]
- Lamme VA. Why visual attention and awareness are different. Trends Cogn. Sci. 2003;7:12–18. doi: 10.1016/s1364-6613(02)00013-x. [DOI] [PubMed] [Google Scholar]
- Lamme VA. Towards a true neural stance on consciousness. Trends Cogn. Sci. 2006;10:494–501. doi: 10.1016/j.tics.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Ling S, Carrasco M. Sustained and transient covert attention enhance the signal via different contrast response functions. Vision. Res. 2006;46:1210–1220. doi: 10.1016/j.visres.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu ZL, Dosher BA. External noise distinguishes attention mechanisms. Vision. Res. 1998;38:1183–1198. doi: 10.1016/s0042-6989(97)00273-3. [DOI] [PubMed] [Google Scholar]
- Lu ZL, Dosher BA. Spatial attention: different mechanisms for central and peripheral temporal precues? J. Exp. Psychol. Hum. Percept. Perform. 2000;26:1534–1548. doi: 10.1037//0096-1523.26.5.1534. [DOI] [PubMed] [Google Scholar]
- Normal E, Price MC. In: Measuring consciousness with confience ratings. Overgaard M, editor. Behavioural Methods in Consciousness Research: Oxford University Press; 2015. [Google Scholar]
- Overgaard M, Nielsen JF, Fuglsang-Frederiksen A. A TMS study of the ventral projections from V1 with implications for the finding of neural correlates of consciousness. Brain Cognit. 2004;54:58–64. doi: 10.1016/s0278-2626(03)00260-4. [DOI] [PubMed] [Google Scholar]
- Passingham RE, Lau HC. Acting, seeing, and conscious awareness. Neuropsychologia. 2017 doi: 10.1016/j.neuropsychologia.2017.06. [DOI] [PubMed]
- Pessoa L, Japee S, Ungerleider LG. Visual awareness and the detection of fearful faces. Emotion. 2005;5:243–247. doi: 10.1037/1528-3542.5.2.243. [DOI] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Q. J. Exp. Psychol. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Posner MI. Attention: the mechanisms of consciousness. Proc. Natl. Acad. Sci. USA. 1994;91:7398–7403. doi: 10.1073/pnas.91.16.7398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahnev D, Maniscalco B, Graves T, Huang E, de Lange FP, Lau H. Attention induces conservative subjective biases in visual perception. Nat. Neurosci. 2011;14:1513–1515. doi: 10.1038/nn.2948. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Riggio L, Dascola I, Umilta C. Reorienting attention across the horizontal and vertical meridians: evidence in favor of a premotor theory of attention. Neuropsychologia. 1987;25:31–40. doi: 10.1016/0028-3932(87)90041-8. [DOI] [PubMed] [Google Scholar]
- Rosner B. Percentage points for a generalized Esd many-outlier procedure. Technometrics. 1983;25:165–172. [Google Scholar]
- Sperling G, Melchner MJ. The attention operating characteristic: examples from visual search. Science. 1978;202:315–318. doi: 10.1126/science.694536. [DOI] [PubMed] [Google Scholar]
- Stanislaw H, Todorov N. Calculation of signal detection theory measures. Behav. Res. Methods Instrum. Comput. 1999;31:137–149. doi: 10.3758/bf03207704. [DOI] [PubMed] [Google Scholar]
- Sumner P, Tsai PC, Yu K, Nachev P. Attentional modulation of sensorimotor processes in the absence of perceptual awareness. Proc. Natl. Acad. Sci. USA. 2006;103:10520–10525. doi: 10.1073/pnas.0601974103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmermans B, Cleeremans A. In: How can we measure awareness? An overview of current methods. Overgaard M, editor. Behavioural Methods in Consciousness Research: Oxford University Press; 2015. [Google Scholar]
- Webb TW, Igelstrom KM, Schurger A, Graziano MS. Cortical networks involved in visual awareness independent of visual attention. Proc. Natl. Acad. Sci. USA. 2016;113:13923–13928. doi: 10.1073/pnas.1611505113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiskrantz L. Is blindsight just degraded normal vision? Exp. Brain Res. 2009;192:413–416. doi: 10.1007/s00221-008-1388-7. [DOI] [PubMed] [Google Scholar]
- Weiskrantz L, Warrington EK, Sanders MD, Marshall J. Visual capacity in the hemianopic field following a restricted occipital ablation. Brain. 1974;97:709–728. doi: 10.1093/brain/97.1.709. [DOI] [PubMed] [Google Scholar]
- Wyart V, Tallon-Baudry C. Neural dissociation between visual awareness and spatial attention. J. Neurosci. 2008;28:2667–2679. doi: 10.1523/JNEUROSCI.4748-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]