Abstract
Adenosine A2a receptor (A2aR) stimulation promotes the synthesis of collagens I and III, and we have recently demonstrated that there is crosstalk between the A2aR and WNT/β-catenin signaling pathway. In in vitro studies, A2aR signaling for collagen III expression was mediated by WNT/β-catenin signaling in human dermal fibroblasts; we further verified whether the crosstalk between A2aR and Wnt/β-catenin signaling was involved in diffuse dermal fibrosis in vivo. Wnt-signaling reporter mice (Tcf/Lef:H2B-GFP) were challenged with bleomycin and treated with the selective A2aR antagonist istradefylline (KW6002) or vehicle. Dermal fibrosis was quantitated and nuclear translocation of β-catenin in fibroblasts was assessed by double-staining for Green fluorescent protein or dephosphorylated β-catenin or β-catenin phosphorylated at Ser552, and vimentin. KW6002 significantly reduced skin thickness, skinfold thickness, breaking tension, dermal hydroxyproline content, myofibroblast accumulation, and collagen alignment in bleomycin-induced dermal fibrosis. Also, there was increased expression of Tcf/Lef:H2B-GFP reporter in bleomycin-induced dermal fibrosis, an effect that was diminished by treatment with KW6002. Moreover, KW6002 significantly inhibited nuclear translocation of Tcf/Lef:H2B-GFP reporter, as well as dephosphorylated β-catenin and β-catenin phosphorylated at Ser552. Our work supports the hypothesis that pharmacologic blockade of A2aR inhibits the WNT/β-catenin signaling pathway, contributing to its capacity to inhibit dermal fibrosis in diseases such as scleroderma.
Dermal fibrosis is a pathologic hallmark of several disorders, including scleroderma, hypertrophic scarring, and keloid, and arises from excessive deposition of collagen and other extracellular matrix components released by pathologically activated fibroblasts.1, 2 Despite a growing effort to understand and target the mechanisms involved in fibrosis, the choice and efficacy of treatment are limited, and the development of new antifibrotic therapies awaits the elucidation of better targets.3
Adenosine, a small molecule generated extracellularly from adenine nucleotides after direct cellular stimulation, hypoxia, injury, or metabolic stress,4, 5 acts via a family of classic seven transmembrane G protein–coupled receptors (A1, A2a, A2b, and A3) that are expressed in a cell- and tissue-specific manner. Adenosine A2a receptor (A2aR), the subtype most relevant to dermal fibrosis, signals via Gs proteins, which activate adenylyl cyclase, leading to an increase in intracellular cAMP. The rise in cAMP, in turn, activates downstream targets such as protein kinase A and the exchange factor directly activated by cAMP proteins 1/2, which results in fibroblast activation and collagen synthesis. Our previous studies demonstrated that A2aR, but not A1R or A2bR, stimulates dermal fibroblasts to produce collagens I and III (COL1A1 and COL3A1) and down-regulates the expression of matrix metalloproteinases 2, 9, and 14, which are involved in collagen breakdown.6 Although the capacity to generate adenosine from ATP is necessary for bleomycin to induce dermal fibrosis,7 prior studies have also shown that signaling via A2aR is required for bleomycin to induce fibrosis, as A2aR knockout mice and A2aR pharmacologic blockade in wild-type mice prevented bleomycin-induced dermal fibrosis6 and radiation-induced dermal injury.8 The inability to generate adenosine from ATP [due to deficiency of ecto-5′-nucleotidase (CD73) and ectonucleoside triphosphate diphosphohydrolase-1 (CD39)] also prevents bleomycin-induced dermal fibrosis.7 Moreover, deficiency in adenosine deaminase, the principal catabolic enzyme for adenosine in vivo, leads to elevated adenosine levels and spontaneous dermal fibrosis in mice, which is also significantly prevented by the administration of an A2aR antagonist.9
The wingless-type mouse mammary tumor virus integration site family (WNT)/β-catenin signaling pathway has been found to regulate cell-fate decisions during normal organ development and tissue homeostasis.10 Accumulating studies have demonstrated that an aberrant WNT/β-catenin signaling pathway plays an important role in wound healing and fibrosis.11, 12, 13 In the absence of WNT signals, the β-catenin–destruction complex, which includes the key regulatory enzyme glycogen synthase kinase 3β, constitutively phosphorylates β-catenin, which promotes subsequent degradation of β-catenin. Binding of WNTs to their frizzled receptors, however, disrupts the destruction complex, and thus prevents the degradation of β-catenin. Once stabilized, cytoplasmic β-catenin accumulates and subsequently translocates to the nucleus, where it binds to members of the T-cell factor/lymphoid enhancer factor (TCF/LEF) family of proteins and forms β-catenin–TCF/LEF transcription factor complexes to activate target genes,10 including profibrotic genes (eg, fibronectin, matrix metalloproteinase-7, plasminogen activator inhibitor-1, twist, and snail).13 Besides canonical activation, β-catenin is also phosphorylated on the indicated sites by diverse kinases such as protein kinase A, protein kinase B (AKT), and janus kinase 2, leading to β-catenin stabilization and the activation of downstream genes.14
We recently reported that A2aR stimulation promotes COL3A1 synthesis via β-catenin activation in normal human dermal fibroblasts.15 We found that stimulation with the A2aR agonist CGS21680 rapidly (within 15 minutes) increases cellular β-catenin, dephosphorylated β-catenin (de-phospho β-catenin; canonical β-catenin activation) and β-catenin phosphorylated at Ser552 (p-Ser552 β-catenin; noncanonical β-catenin activation, site of β-catenin activation by AKT) levels in normal human dermal fibroblasts, resulting in nuclear translocation of β-catenin, an effect blocked by the A2aR selective antagonist SCH58261. β-Catenin knockdown abrogates the A2aR-stimulated increments in COL3A1 synthesis, but not COL1A1 expression.15
This study aimed to further verify the crosstalk between A2aR and Wnt signaling in the pathogenesis of dermal fibrosis in vivo. Using Wnt-signaling reporter mice (Tcf/Lef:H2B-GFP), we report on reporter gene expression in dermal fibroblasts in bleomycin-induced dermal fibrosis used in this murine model of scleroderma, and the impact of A2aR antagonist istradefylline (KW6002) on this effect. Moreover, we report the effects of A2aR pharmacologic blockade on nuclear translocation of Tcf/Lef:H2B-GFP reporter, de-phospho β-catenin, and p-Ser552 β-catenin.
Materials and Methods
Reagents
Bleomycin was purchased from Fresenius Kabi (Grand Island, NY). KW6002 was purchased from Tocris Bioscience (Ellisville, MO). Rabbit polyclonal antibody to de-phospho β-catenin (Ser33/37/Thr41) and p-Ser552 β-catenin were purchased from Cell Signaling Technology (Danvers, MA). Mouse monoclonal antibodies to vimentin and α-smooth muscle actin (α-SMA) were purchased from Abcam (Cambridge, MA). Rabbit monoclonal antibody to vimentin, DAPI mounting medium, goat anti-rabbit IgG (whole-molecule)–fluorescein isothiocyanate secondary antibody, bovine serum albumin, hydroxyproline, chloramine-T, and Ehrlich solution were purchased from Sigma-Aldrich (St. Louis, MO). Mouse monoclonal antibody to Green fluorescent protein (GFP), donkey anti-mouse IgG (H+L)–Alexa Fluor 555 conjugate secondary antibody, fetal bovine serum, and propanol were purchased from Thermo Fisher Scientific (Rochester, NY).
Animal Model
Wnt-signaling reporter mice (Tcf/Lef:H2B-GFP) were purchased from The Jackson Laboratory (Bar Harbor, ME). Transgene induction was performed as described previously.16 Male Tcf/Lef:H2B-GFP mice age 13 weeks (25 to 30 g) in this murine model of scleroderma were treated with the A2aR antagonist KW6002 (10 mg/kg once per day i.p., administered in vehicle consisting of 8% Tween 80 in saline to a total injection volume of 10 μL/kg) or vehicle alone, starting 3 days before dermal fibrosis induction with bleomycin (1.5 mg/mL, 0.1 mL s.c. in a 1.0-cm2 area on the upper back, every other day for 3 weeks). Mice injected with 0.1 mL of phosphate buffered saline (PBS) s.c. were used as negative controls. Male mice were studied exclusively because skin thickness and collagen content are more uniform in male than in female mice.17
The dose and route of KW6002 administration were chosen based on previously published data on the pharmacokinetic properties of this compound.18, 19, 20 The dosing regimen was chosen for achieving drug concentrations that were sufficient for antagonizing the A2aR but that still maintain specificity of A2aR in mice. KW6002 25 mg/kg i.p. administered in C57BL/6J mice reaches a maximum peak of serum concentration of 1030 ng/mL at 1.5 hours; the half-life of KW6002 is 11.6 hours. KW6002 is a selective A2aR antagonist in mice, with a binding affinity of 1.87 nmol/L for A2aR compared with 105.02 nmol/L for A1R.20 KW6002 has a binding affinity of 151.8 nmol/L for A2aR compared with 11,169 nmol/L for A1R, 2701 nmol/L for A2bR, and 1939 nmol/L for A3R when assessed in human receptors overexpressed in CHO and HEK293 cells.18 KW6002 administration at 10 mg/kg would result in an effective concentration of approximately 1 nmol/L, a concentration highly specific for A2aR.20
Mice were randomized to one of three treatment groups, which included negative controls (PBS), bleomycin plus vehicle (BLM; positive controls), and bleomycin plus KW6002 (BLM + KW6002). On day 24, the mice were sacrificed and the skinfold thickness was measured on the treated skin using skin calipers. The skin was shaved, excised, bisected, and fixed in 4% paraformaldehyde for routine histologic processing, or homogenized for the hydroxyproline assay. The number of mice per group was chosen based on the following power analysis: For >90% power for detecting a 70% reduction in the change in skin scores and numbers of cells per high-power field using an analysis of variance with repeated measures, with an α error probability of 0.05 and three groups, a minimum of five animals was needed for each condition. All protocols were approved by the New York University School of Medicine Institutional Animal Care and Use Committee.
Morphometric Dermal Measurements
Skin thickness was measured using a skin caliper on 12-mm punch biopsy samples obtained from the upper back, away from previous sites of injection. Skinfold (pinch) thickness was measured using a skin caliper on the same area over the middle to upper back of the mice. Breaking strength of the skin was measured using a forceps clamped to one side of the 12-mm skin biopsy sample and a tensiometer (Mark-10 Series EG Digital Force Gauge; Mark-10 Corp., Copiague, NY) coupled to another forceps clamped to the furthermost extreme of the biopsy sample. The point of maximal stress before tearing of the sample was recorded. All measurements were undertaken in a blinded manner (J.Z.).6
Dermal Hydroxyproline Assay
Skin tissue specimens were hydrolyzed in 12N HCl at 120°C for 24 hours. After evaporation, 200 μL of dextran-charcoal solution (1% charcoal and 0.05% dextran 500T) was mixed with 800 μL of water and centrifuged at 400 × g for 10 minutes. A volume of 100 mL of a 1:2 dilution of the supernatant was mixed with 250 μL of chloramine solution (1.3% chloramine-T, 10% propanol, and 80% citrate-acetate buffer) over 20 minutes at room temperature, followed by the addition of 250 μL of Ehrlich solution and incubation at 60°C for 20 minutes. Absorbance was measured at 550 nm. Standard curves (0 to 1 mg/mL) were generated for each experiment using reagent hydroxyproline as a standard. Results were expressed as micrograms of hydroxyproline per milligram of tissue.6
Histologic Examination, Immunofluorescence, and Image Analysis
Paraffin-embedded sections of skin were cut into serial sections of 5-μm thickness and stained with hematoxylin and eosin, picrosirius red (method of Puchtler).21
Immunofluorescence was performed on slides of paraffin-embedded skin. After deparaffination and rehydration of tissue sections, antigen retrieval was performed for 20 minutes at 98°C with 10 mmol/L citrate buffer pH 6.0. To reduce the staining background of immunofluorescence, the slides were incubated for 30 minutes with 2 mmol/L glycine. To block nonspecific binding, the slides were incubated for 1 hour with 5% fetal bovine serum in PBS–bovine serum albumin 3% Triton X-100 1%. Primary antibodies were incubated for 1 hour at room temperature. To identify nuclear accumulation of β-catenin in fibroblasts, skin sections were double-stained for GFP (1:200) or p-Ser552 β-catenin (1:200) or de-phospho β-catenin (1:200) and the fibroblast marker vimentin (mouse monoclonal antibody to vimentin, 1:800; rabbit monoclonal antibody to vimentin, 1:200). To identify myofibroblasts, skin sections were stained for α-SMA (1:200). Concentration-matched and species-specific immunoglobulins (Vector Laboratories, Burlingame, CA) were used as control antibodies. Secondary antibodies were labeled with anti-rabbit IgG–fluorescein isothiocyanate (green) and/or anti-mouse IgG–Alexa Fluor 555 (red) for 1 hour at room temperature. Nuclei were counterstained with DAPI mounting medium.
For hematoxylin and eosin–stained tissue, images were acquired using an SCN400 Digital Slide Scanner (Leica Microsystems, Wetzlar, Germany). For picrosirius red, polarized images were collected with a DS-Fi1 camera (Nikon, Tokyo, Japan), and images were quantified with SigmaScan Pro software version 5 (Systat, Chicago, IL) in a blinded manner (J.Z.). The same yellow and red (hue 0, saturation 84, to hue 50, saturation 100) or green (hue 11, saturation 1, to hue 80, saturation 25) color threshold was applied to fields at 100-fold magnification for all of the images, and the area of each color was then calculated and normalized to PBS control as described.8 For immunofluorescence, images were collected with an Eclipse NI-U fluorescence upright microscope (Nikon). For the measurement of fluorescence intensity, all images were captured at the same exposure times, contrast settings, and intensity. For the quantification of fibroblasts with nuclear translocation of Tcf/Lef:H2B-GFP reporter or de-phospho β-catenin or p-Ser552 β-catenin, the percentage of nuclei stained for Tcf/Lef:H2B-GFP reporter or p-Ser552 β-catenin or de-phospho β-catenin, DAPI, and vimentin in the total number of single, spindle-shaped, DAPI-, and vimentin-positive fibroblastic cells was calculated in six randomly chosen high-power fields at 400-fold magnification in a blinded manner (J.Z.) as described.11 For the quantification of myofibroblasts in the dermis, single, spindle-shaped cells positive for α-SMA were counted in six randomly chosen high-power fields at 200-fold magnification in a blinded manner (J.Z.) as described.8
Statistical Analysis
Results are represented as means ± SEM of separate determinations. Data were analyzed by one- or two-way analysis of variance, as appropriate, followed by Bonferroni post hoc test if F achieved P < 0.05 and there was no significant variance in homogeneity. All statistical analyses were performed with Prism software version 6.0 (GraphPad Software, La Jolla, CA). The α nominal level was set at 0.05 in all cases. A P value of <0.05 was considered significant. All data and statistical analysis complied with the recommendations on experimental design and analysis set forth by The American Journal of Pathology.
Results
A2aR Pharmacologic Blockade Prevents Bleomycin-Induced Dermal Fibrosis
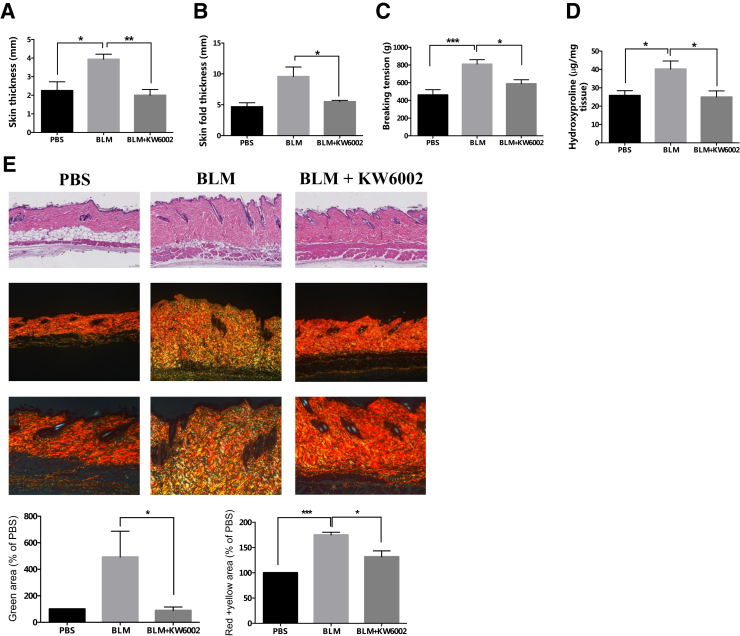
Our previous studies demonstrated that A2aR knockout and A2aR pharmacologic blockade (ZM241385) in wild-type mice prevent bleomycin-induced dermal fibrosis6 and radiation-induced dermal injury.8 Consistent with previous results, Wnt-signaling reporter mice treated with the A2aR antagonist (KW6002) were also protected from bleomycin-induced dermal fibrosis. We found that KW6002-treated Tcf/Lef:H2B-GFP mice displayed significantly reduced skin thickness (2.0 ± 0.3 mm versus 3.9 ± 0.3 mm, P < 0.01, n = 5) (Figure 1A) and skinfold thickness (5.5 ± 0.2 mm versus 9.6 ± 1.5 mm, P < 0.05, n = 5) (Figure 1B) compared with that in vehicle-treated mice after bleomycin challenge. These results were consistent with the higher dermal tensile strength, measured as breaking tension (810.5 ± 49.9 g versus 588.8 ± 44.7 g, P < 0.05, n = 5) (Figure 1C), and also higher dermal hydroxyproline content (40.3 ± 4.3 μg/mg versus 25.0 ± 3.3 μg/mg, P < 0.05, n = 5) (Figure 1D) in the vehicle-treated mice compared with KW6002-treated mice when challenged with bleomycin. Similarly, there was excessive accumulation of collagen and other extracellular matrix components, as seen in the hematoxylin and eosin–stained skin sections (Figure 1E), which was attenuated with KW6002 treatment. We further analyzed the collagen content of skin sections by histochemistry staining with picrosirius red, which, under polarizing microscopy, imparts intense yellow to red birefringence to thicker, more densely packed fibrils and weaker greenish birefringence to more loosely packed, thin fibrils, which may be immature collagen fibers.22, 23 There was a marked increase in greenish birefringence (492% ± 195% versus 89% ± 27% PBS, P < 0.05, n = 5) and yellow to red birefringence (175% ± 5% versus 132% ± 12% PBS, P < 0.05, n = 5) in bleomycin-induced dermal fibrosis, which were both significantly decreased with KW6002 treatment (to 89% ± 27% and 132% ± 12% PBS, respectively, in treated mice, both P < 0.05) (Figure 1E). These results confirm that pharmacologic blockade of A2aR attenuates the development of bleomycin-induced dermal fibrosis in the transgenic mice used in this murine model of scleroderma.
Figure 1.
Tcf/Lef:H2B-GFP reporter mice treated with the adenosine A2a receptor antagonist are protected against bleomycin-induced dermal fibrosis. A–D: Differences in skin thickness (A), skinfold thickness (B), breaking tension (C), and hydroxyproline content (D) between istradefylline (KW6002) or vehicle-treated mice after injections of bleomycin (BLM) or phosphate buffered saline (PBS) for 3 weeks. E: Skin sections for histology were stained with hematoxylin and eosin (top row) and picrosirius red, viewed under polarized microscopy (middle and bottom rows). Representative photomicrographs are shown for each group. Green, red, and yellow areas were calculated as described in Materials and Methods. Data are expressed as means ± SEM. n = 5 mice per group. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001. Original magnification: ×10 (E, top and middle rows); ×20 (E, bottom row).
A2aR Pharmacologic Blockade Prevents Myofibroblast Accumulation in Bleomycin-Induced Dermal Fibrosis
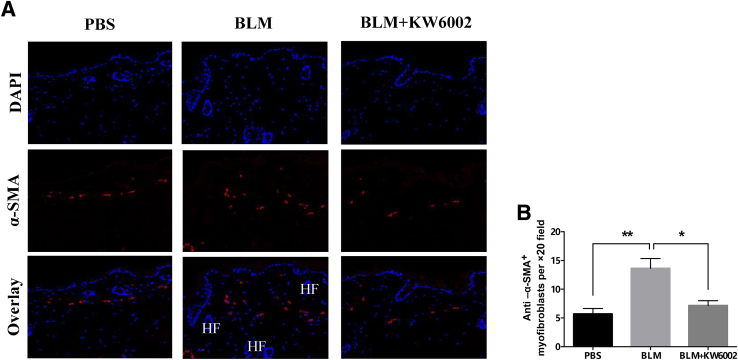
It is well documented that fibroblast/myofibroblast transition plays a pivotal role in the accumulation of excessive collagen and the contraction of the extracellular matrix associated with fibrosis.24 During wound healing and progressive fibrotic diseases, fibroblasts transform into myofibroblasts, acquiring smooth muscle features, most notably the expression of α-SMA, the most widely used myofibroblast marker, and synthesis of mesenchymal cell–related matrix proteins.25, 26 We observed an increase in the α-SMA positive myofibroblast population in bleomycin-induced dermal fibrosis, which was decreased with KW6002 treatment (13.6 ± 1.7 versus 7.2 ± 0.9 α-SMA+ cells/×20 field, P < 0.05, n = 5) (Figure 2). These results indicate that A2aR pharmacologic blockade partially prevents myofibroblast accumulation in bleomycin-induced dermal fibrosis, a finding that is in agreement with previously published results obtained from studies of radiation-induced dermal injury and scarring, and thioacetamide- and CCl4-induced and ethanol-exacerbated liver fibrosis.8, 20, 22, 27, 28
Figure 2.
Adenosine A2a receptor antagonist prevents myofibroblast accumulation in bleomycin (BLM)-induced dermal fibrosis. A: Anti–α-smooth muscle actin (α-SMA) immunofluorescence was performed on skin histologic sections from three groups. Nuclei are shown in blue (DAPI) and α-SMA in red. B: Numbers of α-SMA+ cells in skin sections were counted. Data are expressed as means ± SEM. n = 5 mice per group. ∗P < 0.05, ∗∗P < 0.01. Original magnification: ×20. HF, hair follicle; PBS, phosphate buffered saline.
Pharmacologic Blockade of A2aR Inhibits the Activation of β-Catenin in Bleomycin-Induced Dermal Fibrosis
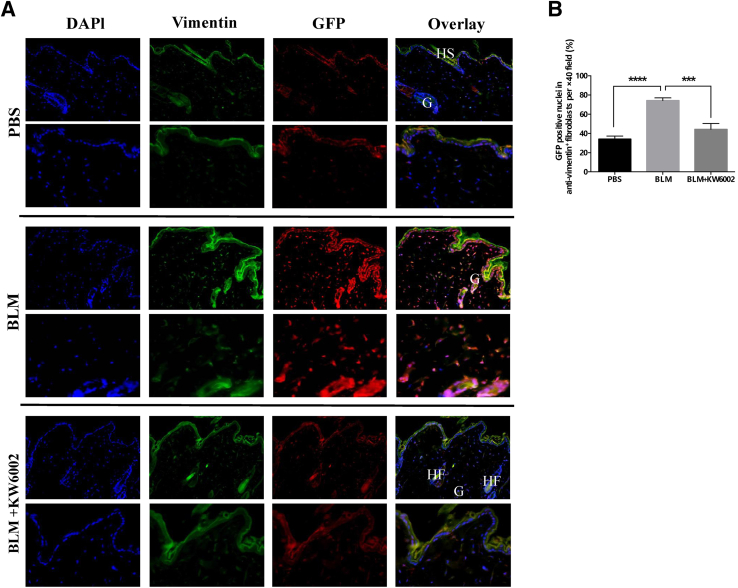
To better detect Wnt/β-catenin signaling activity in skin in vivo, we used Tcf/Lef:H2B-GFP mice, in which six copies of a Tcf/Lef response element and a heat shock protein 68 minimal promoter drive the expression of a fluorescent fusion protein comprising human histone H2B to GFP.16, 29 Sensitivity of the Tcf/Lef:H2B-GFP reporter is supported by its activity at the sites of Wnt/β-catenin signaling, confirmed through genetic analysis.16 The number and intensity of reporter gene expression were increased, indicating enhanced Wnt/β-catenin signaling activity—not only in the fibroblasts of the dermis but also in the epidermis and eccrine glands of the dermis after bleomycin challenge—which was diminished with KW6002 treatment (Figure 3A). We further investigated the effect of the A2aR antagonist KW6002 on nuclear translocation of β-catenin. Indeed, KW6002 treatment diminished the number of fibroblasts with nuclear translocation of Tcf/Lef:H2B-GFP reporter in bleomycin-challenged mice by 40% (44% ± 6% versus 74% ± 39% of vimentin+ fibroblasts with GFP+ nuclei, P < 0.001, n = 5) (Figure 3B). Moreover, it was found that bleomycin induced nuclear translocation of Tcf/Lef:H2B-GFP reporter in epidermal keratinocytes and eccrine glands of the dermis, which was prevented with KW6002 treatment. Taken together, A2aR pharmacologic blockade effectively inhibited the activation of β-catenin in vivo, which, at least in part, likely contributes to preventing bleomycin-induced dermal fibrosis in this murine model of scleroderma.
Figure 3.
Adenosine A2a receptor antagonist prevents nuclear translocation of Tcf/Lef:H2B-GFP reporter in bleomycin (BLM)-induced dermal fibrosis. A: Immunofluorescence images show staining against nuclei (DAPI, blue), Green fluorescent protein (GFP) (red), and vimentin (green) as well as overlay of these three stains in skin sections of three groups. B: The percentage of nuclei stained for GFP, DAPI, and vimentin in the total number of single, spindle-shaped, DAPI- and vimentin-positive fibroblasts were calculated in skin sections. Data are expressed as means ± SEM. n = 5 mice per group. ∗∗∗P < 0.001, ∗∗∗∗P < 0.0001. Original magnification: ×20 (A, top rows); 40× (A, bottom rows). G, gland; HF, hair follicle; HS, hair shaft; PBS, phosphate buffered saline.
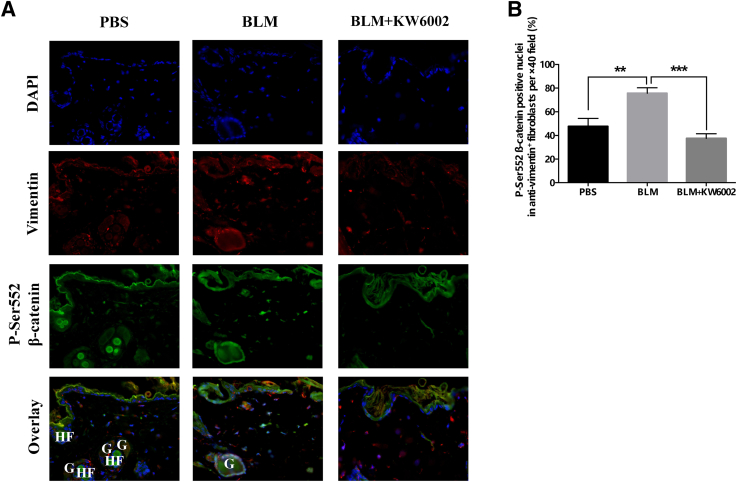
A2aR Pharmacologic Blockade Inhibits Both Canonical and Akt Activation of β-Catenin in Bleomycin-Induced Dermal Fibrosis
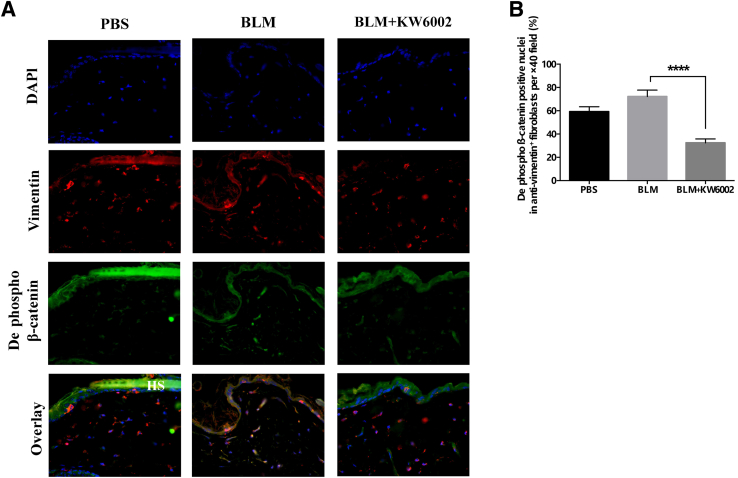
Because it was found that A2aR stimulation increases cellular β-catenin levels via both canonical activation and AKT activation in vitro,15 the expression of de-phospho β-catenin and p-Ser552 β-catenin after bleomycin challenge in vivo were also examined. In agreement with previous findings,15 the number and intensity of the expression of de-phospho β-catenin (Figure 4A and Supplemental Figure S1) and p-Ser552 (Figure 5A and Supplemental Figure S2) were increased in bleomycin-induced dermal fibrosis; these effects were diminished with KW6002 treatment. Moreover, fibroblasts with nuclear translocation of de-phospho β-catenin and p-Ser552 β-catenin were reduced by 55% (fibroblasts with de-phospho β-catenin–positive nuclei: BLM, 72% ± 5%; BLM + KW6002, 32% ± 3%; P < 0.0001, n = 5) (Figure 4B) and by 50% (fibroblasts with p-Ser552 β-catenin–positive nuclei: BLM, 76% ± 5%; BLM + KW6002, 38% ± 4%; P < 0.001, n = 5) (Figure 5B), respectively. Taken together, these results indicate that A2aR pharmacologic blockade inhibits canonical and Akt activation of β-catenin in vivo, which, at least in part, contributes to the prevention of bleomycin-induced dermal fibrosis in this murine model of scleroderma.
Figure 4.
Adenosine A2a receptor antagonist prevents nuclear translocation of dephosphorylated (dephospho) β-catenin in bleomycin (BLM)-induced dermal fibrosis. A: Immunofluorescence images show staining against nuclei (DAPI, blue), de-phospho β-catenin (green), and vimentin (red) as well as overlay of these three stains in skin sections from three groups. B: The percentages of nuclei stained for de-phospho β-catenin, DAPI, and vimentin in the total numbers of single, spindle-shaped, DAPI-, and vimentin-positive fibroblasts were calculated. Data are expressed as means ± SEM. n = 5 mice per group. ∗∗∗∗P < 0.0001. Original magnification, ×40. HS, hair shaft; PBS, phosphate buffered saline.
Figure 5.
Adenosine A2a receptor antagonist prevents nuclear translocation of phosphorylated (p)-Ser552 β-catenin in bleomycin (BLM)-induced dermal fibrosis. A: Immunofluorescence images show staining against nuclei (DAPI, blue), p-Ser552 β-catenin (green), and vimentin (red) as well as overlay of these three stains in skin sections from three groups. B: The percentages of nuclei stained for p-Ser552 β-catenin, DAPI, and vimentin in the total numbers of single, spindle-shaped, DAPI-, and vimentin-positive fibroblasts were calculated. Data are expressed as means ± SEM. n = 5 mice per group. ∗∗P < 0.01, ∗∗∗P < 0.001. Original magnification, ×40. G, gland; HF, hair follicle; PBS, phosphate buffered saline.
Discussion
Previous studies have shown that treatment with an A2aR antagonist (ZM241385) suppressed bleomycin-induced dermal fibrosis in wild-type C57Bl/6 mice.6 Here, we confirmed this finding using a different A2aR antagonist, KW6002, in a different transgenic mouse strain, Tcf/Lef:H2B-GFP reporter mice, and found that KW6002 significantly reduced the severity of bleomycin-induced dermal fibrosis, as assessed by both histologic and immunohistochemistry staining. Additionally, there was increased expression of Tcf/Lef:H2B-GFP reporter in fibroblasts in bleomycin-induced dermal fibrosis, which was diminished with KW6002. Moreover, A2aR blockade significantly inhibited nuclear translocation of Tcf/Lef:H2B-GFP reporter, as well as de-phospho and p-Ser552 β-catenin, in dermal fibroblasts. These findings confirm that there is crosstalk between A2aR and Wnt/β-catenin signaling in vivo in this murine model of bleomycin-induced dermal fibrosis.
Activation of the WNT/β-catenin pathway is often a general feature of fibrotic diseases. Tissue samples from humans with fibrotic diseases, such as systemic sclerosis, idiopathic pulmonary fibrosis, and liver cirrhosis, showed enhanced expression of WNT proteins and decreased expression of Dickkopf-1, a secreted antagonist of WNT/β-catenin signaling.30 Nuclear accumulation of β-catenin in dermal fibroblasts was also observed in scleroderma patients and in animal models of scleroderma, including bleomycin-induced dermal fibrosis and tight skin-1 mice.11, 30 Our findings in Tcf/Lef:H2B-GFP reporter mice in this model of bleomycin-induced dermal fibrosis are in accord with those from previous studies.11, 30
Crosstalk between WNT/β-catenin signaling and the key mediator of fibrosis, transforming growth factor (TGF)-β, has been well documented.30, 31 WNT/β-catenin signaling has been shown to up-regulate the expression of TGF-β1, and TGF-β1 promotes β-catenin activation.30, 31 Mice lacking Smad3, a downstream target of Tgf-β1 signaling, demonstrate reduced β-catenin activation after wounding, and Catnb knockout fibroblasts have reduced Tgf-β–induced proliferation.31
Adenosine is released in response to tissue damage and can trigger a fibrotic cascade.1 In particular, in previous studies, adenosine, released in response to bleomycin-induced generation of oxygen radicals in the skin, promoted dermal fibrosis via occupancy of A2aR.7, 9 A2aR activation increases collagen synthesis in dermal fibroblasts via a pathway involving cAMP and AKT, which is independent of SMAD2/3.32 AKT, an activated downstream target of epidermal growth factor receptor signaling, can directly phosphorylate β-catenin at Ser552 in vitro and in vivo.33 Activated AKT stabilizes β-catenin through inhibition of glycogen synthase kinase 3β and/or directly phosphorylates β-catenin, resulting in its accumulation in the nucleus, which in turn stimulates β-catenin–TCF/LEF transcriptional activity.33 Consistent with these observations, our recent data have demonstrated that A2aR activation increases cellular β-catenin levels in normal human dermal fibroblasts via both canonical activation and AKT activation. In vivo, we found that the A2aR antagonist KW6002 inhibited nuclear translocation of β-catenin, including glycogen synthase kinase 3β–mediated de-phospho β-catenin and Akt-activated p-Ser552 β-catenin, contributing to the prevention of bleomycin-induced dermal fibrosis.
Of note, we observed enhanced Wnt/β-catenin signaling activity and nuclear translocation of β-catenin not only in the fibroblasts of the dermis but also in the keratinocytes and eccrine glands of the dermis after bleomycin challenge. β-Catenin has a dual role in cells: In addition to the WNT-signaling pathway, β-catenin also acts as a structural protein in connection with E-cadherin to form cellular adherens junctions, and participates in the process of epithelial–mesenchymal transition (EMT) in fibrosis.34 During fibrogenic EMT, epithelial cells lose their polarity and cell–cell contact by down-regulating the expression of E-cadherin and releasing β-catenin from contacts, change their morphology to highly motile fibroblastoid mesenchymal cells, and express vimentin or α-SMA. The process of EMT is frequently accompanied by activation of the β-catenin signaling cascade. Activated β-catenin translocates from cytoplasm to nucleus and induces the transcription of EMT genes, such as those encoding vimentin, α-SMA, snail, slug, and twist, which in turn promotes the onset and progression of EMT.35, 36 Thus, a significant increment of nuclear β-catenin in epidermis and epithelial appendages after bleomycin challenge hints at the existence of EMT in bleomycin-induced dermal fibrosis and likely reinforces WNT/β-catenin signaling due to the promotion of EMT.37 We speculate that A2aR blockade may inhibit WNT/β-catenin signaling activity and α-SMA accumulation in fibroblasts in bleomycin-induced dermal fibrosis, in part by turning off the EMT process.
In the present study, we also observed that A2aR blockade prevented fibroblast/myofibroblast transition, as reflected by α-SMA accumulation, in vivo. Nonetheless, we failed to detect nuclear translocation of β-catenin in α-SMA+ myofibroblasts (data not shown). Several studies have addressed the relationship between α-SMA and β-catenin translocation, but the results to date have been controversial. Thus, TGF-β1 induces α-SMA expression via glycogen synthase kinase 3β inhibition and stimulates nuclear β-catenin translocation in human lung fibroblasts,37 but in dermal fibroblasts, β-catenin signaling negatively regulates TGF-β1–induced myofibroblast transition.38 Yet another study reported increased expression of stabilized β-catenin in skin fibroblasts, but this finding was not correlated with the presence of α-SMA+ myofibroblasts in stabilized β-catenin–induced skin fibrosis.39 Our data also suggest that myofibroblast transformation may be independent of WNT signaling and β-catenin activation, although an A2aR antagonist inhibits both WNT/β-catenin signaling activity and α-SMA accumulation in bleomycin-induced dermal fibrosis. Because the α-SMA promoter does not contain TCF/LEF binding motifs, we also cannot rule out the possibility that the induction of α-SMA is secondary to the expression of other β-catenin–dependent genes.37
A2aR and A2bR, the adenosine receptor subtypes most relevant to fibrosis, both signal via a Gs-mediated increase in cAMP, leading to the activation of downstream targets such as protein kinase A and exchange protein directly activated by cAMP 1/2. In human dermal fibroblasts and human hepatic stellate cells, cAMP-dependent signaling mediates the increase in collagen production as inhibition or knockdown of signaling proteins downstream of cAMP suppresses the A2aR-mediated increase in collagen production.1, 6, 15, 32, 40 In contrast, A2bR stimulation also increases cAMP levels, but stimulation of this receptor inhibits collagen production in cardiac fibroblasts.41, 42, 43 It is possible that downstream signaling proteins involved in collagen production differ between cardiac and dermal or hepatic fibroblasts. However, it is more likely that cells stimulated by these two receptor subtypes differ with regard to the levels or locations of increases in cAMP. But the regulatory actions of the receptors likely involve multiple interconnected pathways; one aspect of this regulation is the opposing effect of the different levels of cAMP generated. Perez-Aso et al32, 40 reported that forskolin, an agent that directly stimulates adenylate cyclase and which is often used to increase cAMP level in studies of signaling mechanisms, stimulates much more intracellular cAMP accumulation than does A2aR and also results in inhibition of collagen production by fibroblasts. Although not tested directly, these findings are consistent with the hypothesis that quantitative or geographic differences in intracellular cAMP generation resulting from A2aR and A2bR stimulation dictate different downstream signaling effects and the differing effects on collagen production.
Conclusion
We report here that the A2aR antagonist KW6002 inhibits the Wnt/β-catenin signaling pathway in vivo, which contributes to preventing bleomycin-induced dermal fibrosis. The use of KW6002, which improves motor function in animal models of Parkinson disease and in patients with Parkinson disease, has been approved in Japan as the first A2aR selective antagonist for clinical application.44 Our results provide evidence supporting off-label use of this drug and represents a potential novel therapeutic modality for the treatment and prevention of dermal fibrosis.
Acknowledgments
We thank Dr. Luis Chiriboga, (Immunohistochemistry Core, NYU–Langone Medical Center) and Dr. Cynthia Loomis (Experimental Pathology, NYU–Langone Medical Center), for their technical advice and help with immunohistochemistry analysis and immunofluorescence procedures.
Footnotes
Supported by NIH grants R01 AR056672 and R01 AR068593 (B.C.), New York University–Health and Hospitals Corporation Clinical and Translational Science Institute grant UL1 TR001445, and the Arthritis Foundation (B.C.).
Disclosures: B.C. holds patents on the use of adenosine A2a receptor agonists for promoting wound healing and the use of A2a receptor antagonists for inhibiting dermal fibrosis. B.C. has served as a consultant for AstraZeneca and Bristol-Myers Squibb, and has received grant support from Celgene and AstraZeneca.
Supplemental material for this article can be found at http://dx.doi.org/10.1016/j.ajpath.2017.05.005.
Supplemental Data
Adenosine A2a receptor antagonist prevents nuclear translocation of dephosphorylated (de-phospho) β-catenin in bleomycin (BLM)-induced dermal fibrosis. Immunofluorescence images show staining against nuclei (DAPI, blue), de-phospho β-catenin (green), and vimentin (red), as well as overlay of these three stains in skin sections of three groups. Original magnification, ×20. G, gland; HF, hair follicle; PBS, phosphate buffered saline.
Adenosine A2a receptor antagonist prevents nuclear translocation of phosphorylated (p)-Ser552 β-catenin in bleomycin-induced dermal fibrosis. Immunofluorescence images show staining against nuclei (DAPI, blue), p-Ser552 β-catenin (green), and vimentin (red), as well as overlay of these three stains in skin sections of three groups. Original magnification, ×20. HF, hair follicle; PBS, phosphate buffered saline.
References
- 1.Shaikh G., Cronstein B. Signaling pathways involving adenosine A2A and A2B receptors in wound healing and fibrosis. Purinergic Signal. 2016;12:191–197. doi: 10.1007/s11302-016-9498-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wynn T.A. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sakkas L.I., Simopoulou T., Katsiari C., Bogdanos D., Chikanza I.C. Early systemic sclerosis-opportunities for treatment. Clin Rheumatol. 2015;34:1327–1331. doi: 10.1007/s10067-015-2902-5. [DOI] [PubMed] [Google Scholar]
- 4.Eltzschig H.K., Eckle T. Ischemia and reperfusion–from mechanism to translation. Nat Med. 2011;17:1391–1401. doi: 10.1038/nm.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borea P.A., Gessi S., Merighi S., Varani K. Adenosine as a multi-signalling guardian angel in human diseases: when, where and how does it exert its protective effects? Trends Pharmacol Sci. 2016;37:419–434. doi: 10.1016/j.tips.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Chan E.S., Fernandez P., Merchant A.A., Montesinos M.C., Trzaska S., Desai A., Tung C.F., Khoa D.N., Pillinger M.H., Reiss A.B., Tomic-Canic M., Chen J.F., Schwarzschild M.A., Cronstein B.N. Adenosine A2A receptors in diffuse dermal fibrosis: pathogenic role in human dermal fibroblasts and in a murine model of scleroderma. Arthritis Rheum. 2006;54:2632–2642. doi: 10.1002/art.21974. [DOI] [PubMed] [Google Scholar]
- 7.Fernandez P., Perez-Aso M., Smith G., Wilder T., Trzaska S., Chiriboga L., Franks A., Jr., Robson S.C., Cronstein B.N., Chan E.S. Extracellular generation of adenosine by the ectonucleotidases CD39 and CD73 promotes dermal fibrosis. Am J Pathol. 2013;183:1740–1746. doi: 10.1016/j.ajpath.2013.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perez-Aso M., Mediero A., Low Y.C., Levine J., Cronstein B.N. Adenosine A2A receptor plays an important role in radiation-induced dermal injury. FASEB J. 2016;30:457–465. doi: 10.1096/fj.15-280388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandez P., Trzaska S., Wilder T., Chiriboga L., Blackburn M.R., Cronstein B.N., Chan E.S. Pharmacological blockade of A2A receptors prevents dermal fibrosis in a model of elevated tissue adenosine. Am J Pathol. 2008;172:1675–1682. doi: 10.2353/ajpath.2008.070952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 11.Beyer C., Schramm A., Akhmetshina A., Dees C., Kireva T., Gelse K., Sonnylal S., de Crombrugghe B., Taketo M.M., Distler O., Schett G., Distler J.H. beta-catenin is a central mediator of pro-fibrotic Wnt signaling in systemic sclerosis. Ann Rheum Dis. 2012;71:761–767. doi: 10.1136/annrheumdis-2011-200568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheon S.S., Wei Q., Gurung A., Youn A., Bright T., Poon R., Whetstone H., Guha A., Alman B.A. beta-catenin regulates wound size and mediates the effect of TGF-beta in cutaneous healing. FASEB J. 2006;20:692–701. doi: 10.1096/fj.05-4759com. [DOI] [PubMed] [Google Scholar]
- 13.Guo Y., Xiao L., Sun L., Liu F. Wnt/beta-catenin signaling: a promising new target for fibrosis diseases. Physiol Res. 2012;61:337–346. doi: 10.33549/physiolres.932289. [DOI] [PubMed] [Google Scholar]
- 14.Verheyen E.M., Gottardi C.J. Regulation of Wnt/beta-catenin signaling by protein kinases. Dev Dyn. 2010;239:34–44. doi: 10.1002/dvdy.22019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shaikh G., Zhang J., Perez-Aso M., Mediero A., Cronstein B. Adenosine A2A receptor promotes collagen type III synthesis via beta-catenin activation in human dermal fibroblasts. Br J Pharmacol. 2016;173:3279–3291. doi: 10.1111/bph.13615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferrer-Vaquer A., Piliszek A., Tian G., Aho R.J., Dufort D., Hadjantonakis A.K. A sensitive and bright single-cell resolution live imaging reporter of Wnt/ss-catenin signaling in the mouse. BMC Dev Biol. 2010;10:121. doi: 10.1186/1471-213X-10-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Markova M.S., Zeskand J., McEntee B., Rothstein J., Jimenez S.A., Siracusa L.D. A role for the androgen receptor in collagen content of the skin. J Invest Dermatol. 2004;123:1052–1056. doi: 10.1111/j.0022-202X.2004.23494.x. [DOI] [PubMed] [Google Scholar]
- 18.Yang M., Soohoo D., Soelaiman S., Kalla R., Zablocki J., Chu N., Leung K., Yao L., Diamond I., Belardinelli L., Shryock J.C. Characterization of the potency, selectivity, and pharmacokinetic profile for six adenosine A2A receptor antagonists. Naunyn Schmiedebergs Arch Pharmacol. 2007;375:133–144. doi: 10.1007/s00210-007-0135-0. [DOI] [PubMed] [Google Scholar]
- 19.Borgkvist A., Marcellino D., Fuxe K., Greengard P., Fisone G. Regulation of DARPP-32 phosphorylation by Delta9-tetrahydrocannabinol. Neuropharmacology. 2008;54:31–35. doi: 10.1016/j.neuropharm.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 20.Chiang D.J., Roychowdhury S., Bush K., McMullen M.R., Pisano S., Niese K., Olman M.A., Pritchard M.T., Nagy L.E. Adenosine 2A receptor antagonist prevented and reversed liver fibrosis in a mouse model of ethanol-exacerbated liver fibrosis. PLoS One. 2013;8:e69114. doi: 10.1371/journal.pone.0069114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heap B.J., Kiernan J.A. Mast cells and gastric secretion in the rat. Q J Exp Physiol Cogn Med Sci. 1975;60:307–313. doi: 10.1113/expphysiol.1975.sp002324. [DOI] [PubMed] [Google Scholar]
- 22.Perez-Aso M., Chiriboga L., Cronstein B.N. Pharmacological blockade of adenosine A2A receptors diminishes scarring. FASEB J. 2012;26:4254–4263. doi: 10.1096/fj.12-209627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whittaker P., Kloner R.A., Boughner D.R., Pickering J.G. Quantitative assessment of myocardial collagen with picrosirius red staining and circularly polarized light. Basic Res Cardiol. 1994;89:397–410. doi: 10.1007/BF00788278. [DOI] [PubMed] [Google Scholar]
- 24.McAnulty R.J. Fibroblasts and myofibroblasts: their source, function and role in disease. Int J Biochem Cell Biol. 2007;39:666–671. doi: 10.1016/j.biocel.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 25.Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol. 2007;127:526–537. doi: 10.1038/sj.jid.5700613. [DOI] [PubMed] [Google Scholar]
- 26.Barnes J.L., Gorin Y. Myofibroblast differentiation during fibrosis: role of NAD(P)H oxidases. Kidney Int. 2011;79:944–956. doi: 10.1038/ki.2010.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan E.S., Montesinos M.C., Fernandez P., Desai A., Delano D.L., Yee H., Reiss A.B., Pillinger M.H., Chen J.F., Schwarzschild M.A., Friedman S.L., Cronstein B.N. Adenosine A(2A) receptors play a role in the pathogenesis of hepatic cirrhosis. Br J Pharmacol. 2006;148:1144–1155. doi: 10.1038/sj.bjp.0706812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peng Z., Fernandez P., Wilder T., Yee H., Chiriboga L., Chan E.S., Cronstein B.N. Ecto-5'-nucleotidase (CD73) -mediated extracellular adenosine production plays a critical role in hepatic fibrosis. FASEB J. 2008;22:2263–2272. doi: 10.1096/fj.07-100685. [DOI] [PubMed] [Google Scholar]
- 29.Choi Y.S., Zhang Y., Xu M., Yang Y., Ito M., Peng T., Cui Z., Nagy A., Hadjantonakis A.K., Lang R.A., Cotsarelis G., Andl T., Morrisey E.E., Millar S.E. Distinct functions for Wnt/beta-catenin in hair follicle stem cell proliferation and survival and interfollicular epidermal homeostasis. Cell Stem Cell. 2013;13:720–733. doi: 10.1016/j.stem.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akhmetshina A., Palumbo K., Dees C., Bergmann C., Venalis P., Zerr P., Horn A., Kireva T., Beyer C., Zwerina J., Schneider H., Sadowski A., Riener M.O., MacDougald O.A., Distler O., Schett G., Distler J.H. Activation of canonical Wnt signalling is required for TGF-beta-mediated fibrosis. Nat Commun. 2012;3:735. doi: 10.1038/ncomms1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou B., Liu Y., Kahn M., Ann D.K., Han A., Wang H., Nguyen C., Flodby P., Zhong Q., Krishnaveni M.S., Liebler J.M., Minoo P., Crandall E.D., Borok Z. Interactions between beta-catenin and transforming growth factor-beta signaling pathways mediate epithelial-mesenchymal transition and are dependent on the transcriptional co-activator cAMP-response element-binding protein (CREB)-binding protein (CBP) J Biol Chem. 2012;287:7026–7038. doi: 10.1074/jbc.M111.276311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perez-Aso M., Fernandez P., Mediero A., Chan E.S., Cronstein B.N. Adenosine 2A receptor promotes collagen production by human fibroblasts via pathways involving cyclic AMP and AKT but independent of Smad2/3. FASEB J. 2014;28:802–812. doi: 10.1096/fj.13-241646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fang D., Hawke D., Zheng Y., Xia Y., Meisenhelder J., Nika H., Mills G.B., Kobayashi R., Hunter T., Lu Z. Phosphorylation of beta-catenin by AKT promotes beta-catenin transcriptional activity. J Biol Chem. 2007;282:11221–11229. doi: 10.1074/jbc.M611871200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kalluri R., Weinberg R.A. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bose S.K., Meyer K., Di Bisceglie A.M., Ray R.B., Ray R. Hepatitis C virus induces epithelial-mesenchymal transition in primary human hepatocytes. J Virol. 2012;86:13621–13628. doi: 10.1128/JVI.02016-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Polimeni M., Gulino G.R., Gazzano E., Kopecka J., Marucco A., Fenoglio I., Cesano F., Campagnolo L., Magrini A., Pietroiusti A., Ghigo D., Aldieri E. Multi-walled carbon nanotubes directly induce epithelial-mesenchymal transition in human bronchial epithelial cells via the TGF-beta-mediated Akt/GSK-3beta/SNAIL-1 signalling pathway. Part Fibre Toxicol. 2016;13:27. doi: 10.1186/s12989-016-0138-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caraci F., Gili E., Calafiore M., Failla M., La Rosa C., Crimi N., Sortino M.A., Nicoletti F., Copani A., Vancheri C. TGF-beta1 targets the GSK-3beta/beta-catenin pathway via ERK activation in the transition of human lung fibroblasts into myofibroblasts. Pharmacol Res. 2008;57:274–282. doi: 10.1016/j.phrs.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 38.Liu J., Wang Y., Pan Q., Su Y., Zhang Z., Han J., Zhu X., Tang C., Hu D. Wnt/beta-catenin pathway forms a negative feedback loop during TGF-beta1 induced human normal skin fibroblast-to-myofibroblast transition. J Dermatol Sci. 2012;65:38–49. doi: 10.1016/j.jdermsci.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 39.Hamburg-Shields E., DiNuoscio G.J., Mullin N.K., Lafyatis R., Atit R.P. Sustained beta-catenin activity in dermal fibroblasts promotes fibrosis by up-regulating expression of extracellular matrix protein-coding genes. J Pathol. 2015;235:686–697. doi: 10.1002/path.4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perez-Aso M., Mediero A., Cronstein B.N. Adenosine A2A receptor (A2AR) is a fine-tune regulator of the collagen1:collagen3 balance. Purinergic Signal. 2013;9:573–583. doi: 10.1007/s11302-013-9368-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Insel P.A., Murray F., Yokoyama U., Romano S., Yun H., Brown L., Snead A., Lu D., Aroonsakool N. cAMP and Epac in the regulation of tissue fibrosis. Br J Pharmacol. 2012;166:447–456. doi: 10.1111/j.1476-5381.2012.01847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu D., Aroonsakool N., Yokoyama U., Patel H.H., Insel P.A. Increase in cellular cyclic AMP concentrations reverses the profibrogenic phenotype of cardiac myofibroblasts: a novel therapeutic approach for cardiac fibrosis. Mol Pharmacol. 2013;84:787–793. doi: 10.1124/mol.113.087742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu D., Insel P.A. Hydrolysis of extracellular ATP by ectonucleoside triphosphate diphosphohydrolase (ENTPD) establishes the set point for fibrotic activity of cardiac fibroblasts. J Biol Chem. 2013;288:19040–19049. doi: 10.1074/jbc.M113.466102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stayte S., Vissel B. Advances in non-dopaminergic treatments for Parkinson's disease. Front Neurosci. 2014;8:113. doi: 10.3389/fnins.2014.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Adenosine A2a receptor antagonist prevents nuclear translocation of dephosphorylated (de-phospho) β-catenin in bleomycin (BLM)-induced dermal fibrosis. Immunofluorescence images show staining against nuclei (DAPI, blue), de-phospho β-catenin (green), and vimentin (red), as well as overlay of these three stains in skin sections of three groups. Original magnification, ×20. G, gland; HF, hair follicle; PBS, phosphate buffered saline.
Adenosine A2a receptor antagonist prevents nuclear translocation of phosphorylated (p)-Ser552 β-catenin in bleomycin-induced dermal fibrosis. Immunofluorescence images show staining against nuclei (DAPI, blue), p-Ser552 β-catenin (green), and vimentin (red), as well as overlay of these three stains in skin sections of three groups. Original magnification, ×20. HF, hair follicle; PBS, phosphate buffered saline.