Abstract
Aims
Metformin is used to treat type 2 diabetes, polycystic ovary syndrome associated infertility, and gestational diabetes. This study aims to evaluate the safety of metformin in early pregnancy.
Method
We evaluated the risk of major birth defects and pregnancy losses in a cohort of pregnant women exposed to metformin during the first trimester for different indications relative to a matched unexposed reference group.
Results
The risk of major birth defects was 5.1% (20/392) in pregnancies exposed to metformin during the first trimester and 2.1% (9/431) in the reference group [adjusted odds ratio (OR) 1.70; 95% CI 0.70–4.38]. Among metformin users, this risk was 7.8% (17/219) in patients with pre‐gestational diabetes and 1.7% (3/173) in those without this diagnosis. Compared to the unexposed reference, the OR for metformin user with diabetes was 3.95 (95% CI 1.77–9.41) and for metformin with other indications it was 0.83 (95% CI 0.18–2.81). The risk of pregnancy losses (spontaneous abortions and stillbirths) was 20.8% in women on metformin during the first trimester and 10.8% in the reference group [adjusted hazard ratio (HR) 1.57; 95% CI 0.90–2.74]. The risks for women on metformin with and without pre‐gestational diabetes were 24.0% and 16.8% respectively, with adjusted HR of 2.51 (95% CI 1.44–4.36) and 1.38 (95% CI 0.74–2.59) when compared to the reference.
Conclusion
Pregnant women with pre‐gestational diabetes on metformin are at a higher risk for adverse pregnancy outcomes than the general population. This appears to be due to the underlying diabetes since women on metformin for other indications do not present meaningfully increased risks.
Keywords: diabetes, metformin, observational study, pregnancy, spontaneous abortion, teratogen
What is Already Known about this Subject
Metformin is considered as the first line of treatment for type 2 diabetes in the general population.
The lack of safety data in early pregnancy has discouraged its use in this population.
What this Study Adds
Results from the first study with a large sample size including women with and without pre‐gestational diabetes allowing to disentangle the potential risks linked to metformin use during early pregnancy from risks linked to diabetes.
Evidence that the higher risks for adverse pregnancy outcomes observed in pregnant women taking metformin for pre‐gestational diabetes are not due to metformin but diabetes.
Introduction
Metformin (N,N‐dimethylbiguanide) is a biguanide hypoglycaemic agent exerting its therapeutic action primarily by reducing hepatic glucose production and, to a lesser extent, by improving glucose uptake and use by peripheral tissues 1. It is the most widely used first line treatment for type 2 diabetes in the general population 2. It does not induce hypoglycaemia or weight gain and is usually well tolerated 2. Metformin is also an alternative to insulin therapy for gestational diabetes mellitus (GDM) 3, 4, 5, 6. Besides its use for diabetes, metformin is an effective treatment for polycystic ovary syndrome (PCOS) associated infertility.
Metformin controls glycaemia in pregnant women with GDM, and it has been shown to prevent adverse maternal and neonatal outcomes associated with hyperglycaemia 7. However, evidence regarding its safety and effectiveness to achieve glycaemic targets in the management of type 2 diabetes in pregnancy is limited 8, 9. Moreover, since metformin, like most other drugs, crosses the placental barrier 10, embryological and fetal risks need to be considered when exposure occurs early in pregnancy. Animal studies did not find metformin to have a teratogenic effect 11, 12. In humans, the few studies that assessed the risk of congenital anomalies showed no evidence of any major teratogenic effect 13, 14. Less is known regarding the potential risk of miscarriages and stillbirths. Further studies are warranted to confirm the safety of metformin when used in early pregnancy.
To better characterize the safety of metformin use early in pregnancy we conducted an observational cohort study and evaluated the risk of birth defects and pregnancy losses after first trimester exposure to metformin. We took advantage of the several potential indications to disentangle the effect of metformin from the known effects of diabetes on pregnancy outcomes.
Methods
Study design and participants
Twelve participating Teratology Information Services (TIS), members of the European Network of Teratology Information Services (ENTIS), and 17 French pharmacovigilance centres collected data for this multicenter, prospective, observational cohort study. The TIS provide information on the safety and risks of medications before and during pregnancy 15. For each patient seeking counselling directly or through their healthcare provider, structured information on the exposure (medication, time of exposure, dose), maternal demographics, as well as medical and obstetric history is collected in order to study drug safety with respect to developmental toxicity 15, 16. Enrolment occurs at the time of first contact, which can be at any time during pregnancy. Follow‐up information is gathered after the estimated date of birth, with inquiry as to pregnancy outcome, gestational age at delivery, birth weight, congenital anomalies and neonatal complications through structured telephone interviews and/or mailed questionnaires sent to the mothers or their healthcare providers 15. Women are enrolled using the same methodology, as all ENTIS members use similar documentation and follow‐up methodology 17, 18, 19. Pregnant women considered as lost to follow‐up were not included in the study (overall rate of loss to follow‐up across centres was 10–30% and is expected to be similar in both exposed and reference groups as they were matched for centre). Gestational age throughout pregnancy was estimated using last menstrual period (LMP) or first trimester ultrasound dating, and provided in completed weeks.
The ENTIS scientific committee approved the study protocol. This observational study contained only anonymous health data, so did not require ethics committee approval in most participating centres; otherwise, approval was received from appropriate authorities.
Exposed and reference groups
Eligible pregnant women for the exposure group were those using metformin (Anatomical Therapeutic Chemical A10BA02) at any time during pregnancy (i.e. any time between conception to week 42 after LMP). A subset of randomly selected pregnant women matched to the exposed women with a ratio of 1:1 according to centre, year of counselling, maternal age, and week of gestational age at enrolment was used as reference group. Eligible pregnant women for the reference group were those that at no time during pregnancy used metformin, insulin or any other hypoglycaemic agent.
For the primary outcomes (pregnancy losses and major birth defects), we considered as exposed only women on metformin during the first trimester of pregnancy. For secondary outcomes expected to have a period of susceptibility beyond the first trimester (i.e., delivery mode, preterm birth, birth weight and macrosomia), we considered metformin exposure after the first trimester of pregnancy. The same unexposed reference group was used for primary and secondary outcomes. Within the exposed group we distinguished two categories, women on metformin with a pre‐gestational diabetes diagnosis and women on metformin without this indication.
Women were excluded from both groups if they were (a) exposed to any of the following known major teratogens or major fetotoxicants: acitretin, isotretinoin, mycophenolate, thalidomide, valproic acid, angiotensin‐II receptor blockers (only when used in second or third trimester), ACE inhibitors (only when used in second or third trimester), or (b) following treatment with indications coded: malignancies [MedDRA code: malignant or unspecified tumours (SMQ 20000091), ICD‐10: C00‐D09)] or malignancy related conditions [MedDRA: (SMQ 20000092), ICD‐10: C00‐D09].
Outcomes
The primary outcomes of interest were the risk of major birth defects (MBD) and the risk of spontaneous pregnancy losses including abortions (i.e. pregnancy loss before 20 completed weeks of pregnancy) (SAB) and stillbirths (i.e. pregnancy loss after 20 completed weeks of pregnancy). Birth defects were classified as major or minor by two independent specialists blinded to exposure using two standard classifications (DB, EA) 20, 21. In case of disagreement, consensus was reached through discussion. Estimation of MBD and pregnancy loss risks was restricted to participants enrolled before the end of the first trimester. Estimation of MBD was restricted to participants with live births or with known results after appropriate pathology exam on the product of fetal loss in case of pregnancy losses (i.e. known birth defect outcome) in both exposed and reference groups.
Secondary outcomes were the risk of elective pregnancy termination (ETOP), medical pregnancy termination (MTOP) – a procedure performed when the pregnancy endangers the mother's health or when the fetus has a condition incompatible with normal life – as well as gestational weeks at birth, birth weight, and delivery mode. Neonatal outcomes such as preterm birth (<37 weeks of gestational age), low birth weight (≤2500 g) (LBW), and macrosomia (≥4500 g) were also assessed as secondary endpoints. This latter assessment was restricted to a subset of patients with available information for these outcomes.
Statistical analysis
The proportion of MBD for each exposure group and the 95% confidence interval (95% CI) were estimated using a binomial exact method. The association between metformin exposure and the risk of MBD and other complications later in pregnancy was evaluated using multivariate logistic regression analysis to estimate odds ratios (OR) with 95% CI and adjust for confounding factors [maternal age, pre‐pregnancy body mass index (BMI), hypertension, use of other medications and study centre]. BMI was categorized (<26, 26–30,>30), with an additional category for the missing values.
Spontaneous losses (SAB and stillbirths) and pregnancy terminations (ETOP and MTOP) were considered competing risks and their frequency is presented as cumulative incidence functions. Women were followed from the gestational age at enrolment (start date) 22. While pregnancy outcome was available for all participants, gestational age at pregnancy outcome was missing for about 6% of the women. These missing values were imputed according to the observed distribution of gestational age at pregnancy outcome for the three possible pregnancy outcomes (i.e., losses, terminations and live births) and within exposure category (diabetes exposed to metformin, non‐diabetes exposed to metformin, control group), and conditionally on the imputed gestational age at pregnancy outcome being larger than the gestational age at which the women entered the study. The imputation process was repeated 100 times and the cumulative incidences obtained were averaged over these 100 analyses. Besides calculating cumulative incidences, a Cox regression model was used to estimate the adjusted hazard ratios (HRadj) of these pregnancy outcomes associated with metformin exposure during the first trimester 23.
Statistical analyses were performed using SAS version 9.4 (AS Institute, Cary, NC) and R version 3.2.1.
Evaluation of confounding factors
Since very few women in the unexposed group would have the indications for metformin, confounding by pre‐gestational diabetes was assessed by stratification of the exposed group based on the presence or absence of pre‐gestational diabetes diagnosis, and on severity criteria defined as the presence of any abnormal glucose test (glycated haemoglobin HbA1c/fasting blood glucose test/2 h oral glucose tolerance test/other) or concomitant use of other oral diabetic drugs or insulin. Important imbalances between the metformin group and the reference group were considered in the regression models.
Results
Cohort size, exposure and maternal characteristics
Between 1993 and 2015, the number of enrolled pregnant women was 471 in the metformin exposed group and 479 in the reference group. Most of the patients started metformin before pregnancy and were exposed during the first trimester (n = 458; 97%), with a median dose of 1325 mg [interquartile range (IQR): 850–1700 mg]. Treatment indications reported for metformin were pre‐gestational diabetes (63%; median dose = 1325 mg, IQR 850–1850 mg), PCOS (12%; median dose = 1325 mg, IQR 1000–1500 mg) and other (i.e., obesity, ovary stimulation, insulin resistance, glucose intolerance, or hyperglycemia) (23%; median dose = 1000 mg, IQR 450–1500); with few missing values (3%). A majority discontinued treatment during the first trimester (335/458; 73%). Treatment indication reported for metformin discontinuers was pre‐gestational diabetes (58%), PCOS (12%), other (27%) or missing (3%).
As shown in Table 1, important differences between the two groups were observed. More patients in the metformin group had a BMI above 30 (23% vs. 4%), pre‐gestational diabetes (57% vs. <1%), hypertension (13% vs. 2%), exposure to >1 medication (77% vs. 44%), >1 prior delivery (33% vs. 22%) and ≥1 previous SAB (22% vs. 16%).
Table 1.
Maternal characteristics. Metformin study from European Teratology Information Services (TIS) 1993–2015
| Characteristics | Metformin group (n = 458) | Reference group (n = 479) | ||
|---|---|---|---|---|
| Maternal age (yr) median (IQR) | 35 | (31–39) | 35 | (31–38) |
| Body mass index (BMI) n (%) | ||||
| Missing | 226 | (49) | 310 | (65) |
| BMI ≤ 30 | 126 | (28) | 152 | (32) |
| BMI > 30 | 106 | (23) | 17 | (4) |
| Comorbidities and co‐medication n (%) | ||||
| ≥ 1 comorbidities (except diabetes) | 178 | (39) | 114 | (24) |
| Pre‐gestational diabetes | 261 | (57) | 2 | (0.4) |
| Hypertension | 60 | (13) | 8 | (2) |
| Psychiatric disorders | 20 | (4) | 34 | (7) |
| Polycystic ovary syndrome | 25 | (5) | 1 | (0.2) |
| Hyperlipidaemia | 21 | (5) | 2 | (0.4) |
| Any concomitant medication | 352 | (77) | 212 | (44) |
| Other oral antidiabetic drugs | 106 | (23) | 0 | (0) |
| Insulin | 123 | (27) | 0 | (0) |
| Gestational age at first contact | ||||
| Median (IQR) [wk] | 9 | (6–13.5) | 8 | (6–14) |
| Smoking n (%) | ||||
| Missing | 124 | (27) | 142 | (30) |
| No | 285 | (62) | 298 | (62) |
| ≤5 cigarettes/day | 11 | (2) | 16 | (3) |
| >5 cigarettes/day | 38 | (8) | 23 | (5) |
| Alcohol n (%) | ||||
| Missing | 146 | (32) | 167 | (34) |
| <1 drink/day | 295 | (64) | 285 | (60) |
| ≥1 drink/day | 17 | (4) | 27 | (6) |
| Folic acid n (%) | ||||
| Missing | 230 | (50) | 259 | (54) |
| Yes | 173 | (38) | 198 | (41) |
| No | 55 | (12) | 22 | (5) |
| Previous pregnancies and deliveries n (%) | ||||
| Missing | 33 | (7) | 41 | (9) |
| Primigravida | 119 | (26) | 167 | (35) |
| Primiparous | 115 | (25) | 116 | (24) |
| Multiparous | 154 | (33) | 104 | (22) |
| Previous SABs n (%) | ||||
| Missing | 70 | (15) | 92 | (19) |
| 0 | 287 | (63) | 313 | (65) |
| 1 | 64 | (14) | 57 | (12) |
| ≥2 | 37 | (8) | 17 | (4) |
| Previous children with birth defects n (%) | ||||
| Missing | 228 | (50) | 243 | (51) |
| Yes | 11 | (2) | 9 | (2) |
| No | 219 | (48) | 227 | (47) |
IQR, interquartile range; SAB, spontaneous abortion
Birth defects
The proportion of offspring with birth defects is presented in Table 2. Among women enrolled and exposed to metformin during the first trimester, 392 (84%) pregnancies were live births or pregnancy losses with known results after appropriate pathology exam on the product of fetal loss (i.e. with available information on the presence of birth defects); 14 of the 79 pregnancy losses had known results after pathology exam. The corresponding number of informative pregnancies in the reference group was 431 (83%); 10 of the 58 pregnancy losses had known results after appropriate pathology exam on the product of fetal loss.
Table 2.
Risk of birth defects in pregnancies exposed to metformin during the first trimester compared to the matched unexposed reference group. Metformin study from European Teratology Information Services (TIS) 1993–2015
| Metformin group | Reference group | Odds ratio (OR) and 95% confidence interval (95 CI) | ||||||
|---|---|---|---|---|---|---|---|---|
| Overall | Indication diabetes | Indication other | Overall | ORcrude (95 CI) | ORadj c (95 CI) | ORcrude (95 CI) | ORcrude (95 CI) | |
| Birth defects | n = 392a, n (%) | n = 219, n (%) | n = 173, n (%) | n = 431b, n (%) | Metformin overall vs. reference | Metformin with diabetes vs. reference | Metformin without diabetes vs. reference | |
| All birth defects without chromosomal or genetic origin | 32 (8.1) | 24 (11.0) | 8 (4.6) | 18 (4.2) | 2.04 (1.14–3.77) | 1.86 (0.95–3.71) | 2.84 (1.50–5.39) | 1.11 (0.45–2.53) |
| Major birth defects | 20 (5.1) | 17 (7.8) | 3 (1.7) | 9 (2.1) | 2.52 (1.17–5.89) | 1.70 (0.70–4.38) | 3.95 (1.77–9.41) | 0.83 (0.18–2.81) |
| Minor birth defects | 12 (3.1) | 7 (3.2) | 5 (2.9) | 9 (2.1) | 1.48 (0.62–3.66) | 1.92 (0.72–5.21) | 1.55 (0.55–4.21) | 1.40 (0.42–4.10) |
| Cardiac birth defects | 9d (2.3) | 6 (2.7) | 3 (1.7) | 5e (1.2) | 2.00 (0.67–6.03) | 1.53 (0.43–5.72) | 2.40 (0.72–8.41) | 1.50 (0.31–6.20) |
Restricted to pregnancies with information on the presence or absence of a birth defect: 28 birth defects (16 major and 12 minor) identified among 378 live born infants and 4 defects (4 major and 0 minor) among 14 pregnancy losses with histopathological exam (16%; 14/79)
Restricted to pregnancies with information on the presence or absence of a birth defect; 16 birth defects (7 major and 9 minor) among 421 live born and 2 defects (2 major and 0 minor) among 10 pregnancy losses with histopathological exam (17%; 10/58)
Adjusted for maternal age (continuous), BMI categories (≤25; 26–30, >31, missing), maternal hypertension (yes/no), use of >1 medication (yes/no), centre 8 (yes/no)
Contains two minor birth defects (1 patent or persistent foramen ovale; 1 interventricular septal hypertrophy)
Contains two minor birth defects (1 patent or persistent foramen ovale; 1 patent ductus arteriosus)
The risk of MBD without chromosomal or genetic origin was higher in the group exposed to metformin during the first trimester (5%; 20/392) than in the matched reference group (2%; 9/431). Similar trends were observed for minor BD (3% vs. 2%) and cardiac BD (2% vs. 1%). The risk of BD with chromosomal or genetic origin was similar in both groups (4/392 vs. 5/431). After adjustment for maternal age, BMI, maternal hypertension, use of >1 medication and centre, the OR for MBD was 1.70 (95% CI 0.70–4.38) and for cardiac BD specifically was 1.53 (95% CI 0.43–5.72). Further adjustment for indication yielded an OR for MBD of 0.80 (95% CI 0.19–2.77). However, as very few unexposed women had the indications to explore the impact of pre‐gestational diabetes in the association, we stratified the exposed group by indication.
As shown in Figure 1, the risk of MBD varied based on the presence of pre‐gestational diabetes, as well as diabetes severity criteria. The risk of MBD in patients exposed to metformin without pre‐gestational diabetes was 2% (3/173) and with a diagnosis of pre‐gestational diabetes it was 8% (17/219). Within the latter, the risk of MBD in patients having at least one severity criterion for diabetes was 10% (14/140). For this analysis, since adjustment for other covariates did not change the results, we present only the crude ORs. Compared to the unexposed reference, the OR for metformin without diabetes was 0.83 (95% CI 0.18–2.81) and for metformin with diabetes was 3.95 (95% CI 1.77–9.41). Details on infants with MBD in the metformin and reference group are provided in Table S1 (supplemental material).
Figure 1.
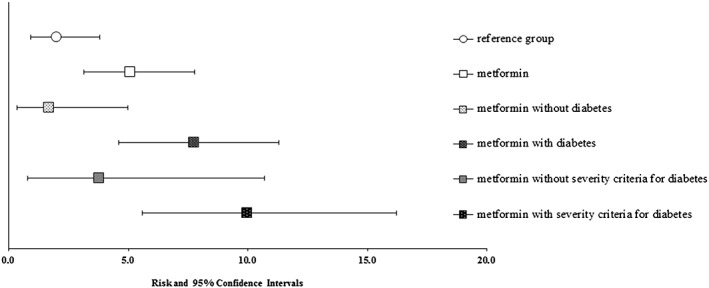
Risk of major birth defects stratified by exposure to metformin, indication and severity of diabetes
Spontaneous abortion and stillbirth
The mean gestational age at enrolment in the pregnancies included in this analysis (i.e. early enrolees) was 9 weeks for the metformin group and 8 weeks for the reference group. The cumulative risk of pregnancy losses was 10.8% in the reference group and 21% in women exposed to metformin early in pregnancy. The crude HR was 1.92 (95% CI 1.17–3.15) and the adjusted HR was 1.57 (95% CI 0.90–2.74). Within the exposed group, the risk of pregnancy loss was 24% among women with pre‐gestational diabetes and 17% when metformin was used for other indications (Figure 2). Compared to the reference, the crude HR was 2.51 (95% CI 1.44–4.36) for women with pre‐gestational diabetes and 1.38 (95% CI 0.74–2.59) for other indications.
Figure 2.
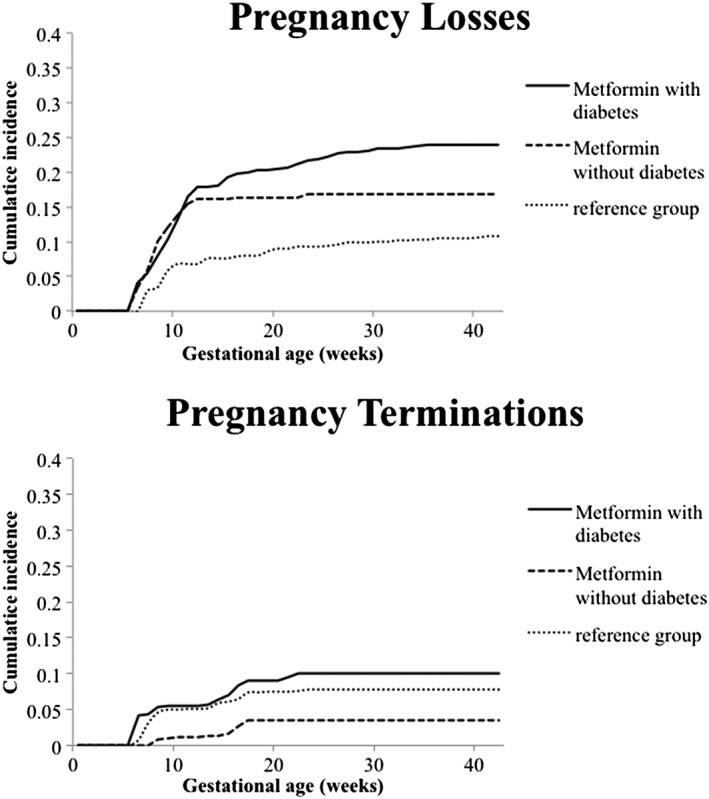
Cumulative incidence of pregnancy losses and terminations stratified by exposure to metformin and indication
Other outcomes
Details for other pregnancy and neonatal outcomes are presented in Table 3. The risk of ETOP was lower in the group exposed to metformin during the first trimester (2%; 9/458) than in the matched reference group (4%; 20/471). Inverse trends were observed for MTOP (3%; 12/458 vs. 1%; 6/471). The risk of preterm birth was higher in the metformin group (19%; 71/379 vs. 6%; 25/421 with ORcrude 3.63; 95% CI 2.27–5.97). After adjustment for maternal age, BMI, maternal hypertension, use of >1 medication and centre, the OR for preterm was ORadj 3.19 (95% CI 1.90–5.43). Within the exposed to metformin group, the risk of preterm was 25% (51/208) among women with pre‐gestational diabetes and 12% (20/170) when metformin was used for other indications. Compared to the unexposed reference, the ORcrude for metformin with diabetes was 5.28 (95% CI 2.91–9.80) and for non‐diabetic indications was 1.78 (95% CI 0.88–3.53). The assisted delivery risk was higher in the metformin group (64%; 235/379 vs. 38% 152/21 with ORcrude 2.92; 95% CI 2.18–3.92) and the association stayed significant after adjustment (ORadj 2.60; 95% CI 1.87–3.63). Within the exposed to metformin group, the risk of assisted delivery was 58.7% (122/208) among women with pre‐gestational diabetes and 65.3% (111/170) when metformin was used for other indications. Compared to the unexposed reference, the ORcrude for metformin with diabetes was 2.17 (95% CI 1.44–3.26) and for non‐diabetic indications it was 2.77 (95% CI 1.81–4.28).
Table 3.
Pregnancy outcomes and neonatal characteristics. Metformin study from European Teratology Information Services (TIS) 1993–2015
| Characteristic | Metformin group (n = 458) | Reference group (n = 479) |
|---|---|---|
| SAB, n | 49 | 29 |
| ETOP, n (%) | 9 (2) | 20 (4) |
| MTOP, n (%) | 12 (3) | 3 (<1) |
| Stillbirths, n (%) | 9 (2) | 6 (1) |
| Live births, n (%) | 379 (83) | 421 (88) |
| Among | Metformin live birth (n = 379) | Reference live birth (n = 421) |
|---|---|---|
| Gestational wk at birth | ||
| Missing, n (%) | 7 (2) | 7 (2) |
| Median (min‐max) [wk] | 38 (25–42) | 39 (27–42) |
| Preterm (<37 wk), n (%) | 71 (19) | 25 (6) |
| Sex, n (%) | ||
| Missing | 12 (3) | 13 (3) |
| Female | 190 (50) | 232 (56) |
| Birth weight a | ||
| Missing, n (%) | 16 (4) | 15 (4) |
| Median (min‐max) [g] | 3300 (740–5130) | 3300 (540–4785) |
| LBW, n (%) | 50 (13) | 30 (8) |
| Macrosomia, n (%) | 14 (4) | 2 (<1) |
| Delivery mode, n (%) | ||
| Missing | 15 (4) | 16 (4) |
| Vaginal deliveries | 131 (35) | 251 (62) |
| Assisted deliveries | 235 (62) | 152 (38) |
SAB, spontaneous abortion; ETOP, elective termination of pregnancy; MTOP, medical termination of pregnancy; LBW, low birth weight.
Not restricted to full term
When the metformin group was split into patients with a first trimester exposure only (discontinuers) and those with first trimester exposure and at least some second and third trimester exposure (continuers), and restricted to patients with available information for the assessed outcomes, the risk of preterm birth was higher in the continuers group (32%; 33/102 vs. 15%; 36/241 with ORcrude 2.70; 95% CI 1.58–4.64). After adjustment for maternal age, pre‐gestational diabetes, BMI, maternal hypertension, use of >1 medication and centre, the OR for preterm was 2.41 (95% CI 1.35–4.29). The risk of macrosomia in the continuers group was 5% (5/102) vs. 3% (8/241) with ORcrude 1.52 (95% CI 0.45–4.68). After adjustment, the OR for macrosomia was 1.02 (95% CI 0.28–3.42). The risk of assisted delivery in the continuers group was 72% (5/102) vs. 61% (146/241) with ORcrude (1.55; 95% CI 0.95–2.57). After adjustment, the OR for assisted delivery remained the same (ORadj 1.50; 95% CI 0.90–2.52). Detailed information is presented in Table S2 (supplemental material).
Discussion
This study prospectively evaluated 458 pregnancies exposed to metformin during pregnancy and revealed an increased risk for major birth defects and pregnancy losses in women exposed to metformin during specific periods of susceptibility compared to an unexposed population. However, the restriction of this association to women on metformin for their pre‐gestational diabetes suggests a significant confounding role of this indication.
In our study, reflecting the general population, a large proportion of the patients in the metformin group had a diagnosis of diabetes before pregnancy (63%) compared to those unexposed (<1%). Moreover, unsurprisingly, the frequency of other associated risk factors (e.g., obesity, hypertension and hyperlipidaemia) was also increased in the metformin group 24. These differences affect the risk of adverse pregnancy outcomes and therefore the comparability of the groups. Since adequate adjustment for indication was not possible, we based our assessment of confounding by pre‐gestational diabetes in the informal comparison with pregnancies exposed to metformin for other indications. As PCOS and some of the indications described in the other indications group have also been associated with insulin resistance, confounding by glucose management was probably not completely controlled. Thus, future studies should compare metformin exposed pregnancies with women with the same indication and treated with alternative therapies, for example those on insulin.
These results are in accordance with previous studies 13, 14, 25, 26, 27. In women with poorly controlled pre‐gestational diabetes, the risk of MBD has been shown to be 5–10% in live births 28. The risks in our cohort were similar, with 8% of MBD in pregnancies with pre‐gestational diabetes; increasing to 10.0% when the mother had at least one severity criterion for diabetes. However, the risk of MBD was similar in the reference group of patients not exposed to metformin (2.1%) and in those exposed to metformin for indications other than pre‐gestational diabetes (1.7%). Our findings suggest that the observed association between metformin and MBD could be explained by confounding by the underlying diabetes.
Whether diabetes increases a woman's risk of having a spontaneous abortion has been under debate for a long time 29. Overall studies suggest that poor metabolic control may be at increased risk of spontaneous abortion 25, 29, 30, 31, 32, 33, 34. Similarly, in our study the risk of pregnancy losses was higher (21%) in the group exposed to metformin with a diagnosis of pre‐gestational diabetes than in the metformin exposed group without pre‐gestational diabetes (17%). The higher risk estimate observed in the metformin exposed without pre‐gestational diabetes group, when compared to the reference group (10.8%), can probably be explained by the higher prevalence of PCOS reported in this group, a condition associated with higher spontaneous abortion rates 35, as well as the other diagnostics associated with insulin resistance.
The elevated risk for minor BD and cardiac BD in women on metformin is also likely explained by other baseline characteristics including obesity and hypertension 36, 37. The association between metformin and cardiac defects attenuated after adjustment for these risk factors. As minor BD are largely underreported to TISes, the increased risk could also be partially explained by a differential identification through closer postnatal monitoring of women with diabetes in the metformin group.
Besides the increased risk of MBD and SAB, diabetes in pregnancy is associated with other risks to the woman and to the developing fetus 5. Consistent with previous findings, we observed an increased risk of preterm birth and assisted deliveries in patients with at least some metformin use in the second and third trimester. The association attenuated for preterm after adjustment or when restricting to women with pre‐gestational diabetes diagnosis, but not for assisted deliveries. Larger studies have shown an increased risk of caesarean section with maternal pre‐existing diabetes and obesity of similar magnitude 38. In our study, adjusting for these covariates has not attenuated the association, suggesting that other factors are involved. Furthermore, interpretation of the results should be done cautiously as the sample size was small after restriction.
We observed an elevated proportion of women discontinuing the treatment during the first trimester (73%). This finding can partly be explained by the fact that metformin is often discontinued once the pregnancy is started when used for PCOS (12% of the patients were using metformin for PCOS) or because of a lack of adequate glucose control. The large proportion of discontinuing patients observed probably also reflects the concern regarding safety and supports the need for making these results available to women and prescribers.
The strengths and limitations of prospective observational pregnancy cohorts studies based on TIS data have been discussed in detail previously 16. Although this is the largest study published to date, the sample size is still too small and follow‐up duration too short to reach a final assessment on the safety and risks associated with the use of metformin in pregnancy. For example, it is important to note that the sample size was sufficient to detect only a fourfold increase in the risk of cardiac BD, given a baseline risk in the general population of 1% with a power of 80% and Type I error of 0.05. The main limitation of this study is the absence of a reference group composed of women treated for similar conditions. A reference group with patients taking insulin was unfortunately not available in the TIS cohort as this treatment is considered safe for use in pregnancy, thus only a few patients or their healthcare provider seek safety information on insulin. Although we cannot completely rule out any bias, the prospective approach of our study with similar procedures of data ascertainment across cohorts makes a substantial bias of exposure or outcome misclassification less likely. Finally, findings from this study might not be generalizable to other populations as the biological relations studied could be affected by characteristics of the studied population (i.e. medium‐ and high‐level educated women tend to be overrepresented among TIS enquirers 39) that differ from the general population. It remains, however, an important population to study in its own right.
In conclusion, pregnant women with pre‐gestational diabetes on metformin are at an increased risk for multiple adverse pregnancy outcomes. However, this risk appears to be due to the underlying diabetes, with no indication of a teratogenic or abortifacient effect of metformin itself. Future studies comparing metformin exposed pregnancies with women with the same indication and treated with alternative therapies, e.g., on insulin are warranted.
Competing Interests
There are no competing interests to declare.
We thank the European Network of Teratology Information Services (ENTIS) and its members, as well as the participating French regional pharmacovigilance centres that made this study possible.
The contribution of Alice Panchaud was supported by a grant from the Swiss National Science Foundation (SNSF no. P3SMP3—158808/1). The funding bodies had no role to play in study design, data collection and analysis, decision to publish and preparation of the manuscript. The corresponding author had full access to the data and made the final decision to submit for publication.
Contributors
A.Panchaud: study conception and design; acquisition of data; analysis and interpretation of data; drafting and revising the manuscript for intellectual content. V.R., D.B., E.A. and S.H.‐D.: analysis and interpretation of data, drafting and revising the manuscript for intellectual content. U.W., L.E.R., T.B. and C.C.: study conception and design; acquisition of data; drafting and revising the manuscript for intellectual content. T.V., N.B., M.DeS., A.Pistelli, A.D., F.B.‐S., M.C., H.D., A.Passier, Y.C.K., M.K.D., E.M., J.E. and G.K.: acquisition of data; drafting and revising the manuscript for intellectual content. All authors approved the final version and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Supporting information
Table S1 Details of birth defects. Metformin study from European Teratology Information Services (TIS) 1993 to 2015
Table S2 Pregnancy outcomes presented by metformin discontinuers and continuers. Metformin study from European Teratology Information Services (TIS) 1993 to 2015
Panchaud, A. , Rousson, V. , Vial, T. , Bernard, N. , Baud, D. , Amar, E. , De Santis, M. , Pistelli, A. , Dautriche, A. , Beau‐Salinas, F. , Cassina, M. , Dunstan, H. , Passier, A. , Kaplan, Y. C. , Duman, M. K. , Maňáková, E. , Eleftheriou, G. , Klinger, G. , Winterfeld, U. , Rothuizen, L. E. , Buclin, T. , Csajka, C. , and Hernandez‐Diaz, S. (2018) Pregnancy outcomes in women on metformin for diabetes or other indications among those seeking teratology information services. Br J Clin Pharmacol, 84: 568–578. doi: 10.1111/bcp.13481.
References
- 1. Foretz M, Guigas B, Bertrand L, Pollak M, Viollet B. Metformin: from mechanisms of action to therapies. Cell Metab 2014; 20: 953–966. [DOI] [PubMed] [Google Scholar]
- 2. Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al Management of hyperglycemia in type 2 diabetes: a patient‐centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012; 35: 1364–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nicholson W, Bolen S, Witkop CT, Neale D, Wilson L, Bass E. Benefits and risks of oral diabetes agents compared with insulin in women with gestational diabetes: a systematic review. Obstet Gynecol 2009; 113: 193–205. [DOI] [PubMed] [Google Scholar]
- 4. Zhao LP, Sheng XY, Zhou S, Yang T, Ma LY, Zhou Y, et al Metformin versus insulin for gestational diabetes mellitus: a meta‐analysis. Br J Clin Pharmacol 2015; 80: 1224–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. National Institute for Health and Care Excellence (NICE) . Diabetes in Pregnancy: Management of Diabetes and its Complications from Preconception to the Postnatal Period. London: NICE, 2015. [PubMed] [Google Scholar]
- 6. Rowan JA, Hague WM, Gao W, Battin MR, Moore MP, MiG Trial Investigators . Metformin versus insulin for the treatment of gestational diabetes. N Engl J Med 2008; 358: 2003–2015. [DOI] [PubMed] [Google Scholar]
- 7. Li G, Zhao S, Cui S, Li L, Xu Y, Li Y. Effect comparison of metformin with insulin treatment for gestational diabetes: a meta‐analysis based on RCTs. Arch Gynecol Obstet 2015; 292: 111–120. [DOI] [PubMed] [Google Scholar]
- 8. Beyuo T, Obed SA, Adjepong‐Yamoah KK, Bugyei KA, Oppong SA, Marfoh K. Metformin versus insulin in the management of pre‐gestational diabetes mellitus in pregnancy and gestational diabetes mellitus at the Korle Bu Teaching Hospital: a randomized clinical trial. PloS One 2015; 10: e0125712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ainuddin JA, Karim N, Zaheer S, Ali SS, Hasan AA. Metformin treatment in type 2 diabetes in pregnancy: an active controlled, parallel‐group, randomized, open label study in patients with type 2 diabetes in pregnancy. J Diabetes Res 2015; 2015: 325851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tertti K, Laine K, Ekblad U, Rinne V, Ronnemaa T. The degree of fetal metformin exposure does not influence fetal outcome in gestational diabetes mellitus. Acta Diabetol 2014; 51: 731–738. [DOI] [PubMed] [Google Scholar]
- 11. Tuchmann‐Duplessis H, Mercier‐Parot L. Repercussions of a hypoglycemic drug, N‐N‐dimethylbiguanide HCl, on gestation and fetal development in rats. C R Hebd Seances Acad Sci 1961; 253: 321–323. [PubMed] [Google Scholar]
- 12. Denno KM, Sadler TW. Effects of the biguanide class of oral hypoglycemic agents on mouse embryogenesis. Teratology 1994; 49: 260–266. [DOI] [PubMed] [Google Scholar]
- 13. Gilbert C, Valois M, Koren G. Pregnancy outcome after first‐trimester exposure to metformin: a meta‐analysis. Fertil Steril 2006; 86: 658–663. [DOI] [PubMed] [Google Scholar]
- 14. Cassina M, Dona M, Di Gianantonio E, Litta P, Clementi M. First‐trimester exposure to metformin and risk of birth defects: a systematic review and meta‐analysis. Hum Reprod Update 2014; 20: 656–669. [DOI] [PubMed] [Google Scholar]
- 15. Schaefer C, Hannemann D, Meister R. Post‐marketing surveillance system for drugs in pregnancy – 15 years experience of ENTIS. Reprod Toxicol 2005; 20: 331–343. [DOI] [PubMed] [Google Scholar]
- 16. Schaefer C, Ornoy A, Clementi M, Meister R, Weber‐Schoendorfer C. Using observational cohort data for studying drug effects on pregnancy outcome – methodological considerations. Reprod Toxicol 2008; 26: 36–41. [DOI] [PubMed] [Google Scholar]
- 17. Panchaud A, Csajka C, Merlob P, Schaefer C, Berlin M, De Santis M, et al Pregnancy outcome following exposure to topical retinoids: a multicenter prospective study. J Clin Pharmacol 2012; 52: 1844–1851. [DOI] [PubMed] [Google Scholar]
- 18. Winterfeld U, Klinger G, Panchaud A, Stephens S, Arnon J, Malm H, et al Pregnancy outcome following maternal exposure to mirtazapine: a multicenter, prospective study. J Clin Psychopharmacol 2015; 35: 250–259. [DOI] [PubMed] [Google Scholar]
- 19. Klieger‐Grossmann C, Weitzner B, Panchaud A, Pistelli A, Einarson T, Koren G, et al Pregnancy outcomes following use of escitalopram: a prospective comparative cohort study. J Clin Pharmacol 2012; 52: 766–770. [DOI] [PubMed] [Google Scholar]
- 20. Merks JH, van Karnebeek CD, Caron HN, Hennekam RC. Phenotypic abnormalities: terminology and classification. Am J Med Genet A 2003; 123A: 211–230. [DOI] [PubMed] [Google Scholar]
- 21. Rasmussen SA, Olney RS, Holmes LB, Lin AE, Keppler‐Noreuil KM, Moore CA, et al Guidelines for case classification for the National Birth Defects Prevention Study. Birth Defects Res A Clin Mol Teratol 2003; 67: 193–201. [DOI] [PubMed] [Google Scholar]
- 22. Meister R, Schaefer C. Statistical methods for estimating the probability of spontaneous abortion in observational studies – analyzing pregnancies exposed to coumarin derivatives. Reprod Toxicol 2008; 26: 31–35. [DOI] [PubMed] [Google Scholar]
- 23. Latouche A, Allignol A, Beyersmann J, Labopin M, Fine JP. A competing risks analysis should report results on all cause‐specific hazards and cumulative incidence functions. J Clin Epidemiol 2013; 66: 648–653. [DOI] [PubMed] [Google Scholar]
- 24. Scheen AJ, Van Gaal LF. Combating the dual burden: therapeutic targeting of common pathways in obesity and type 2 diabetes. Lancet Diabetes Endocrinol 2014; 2: 911–922. [DOI] [PubMed] [Google Scholar]
- 25. Dunne F, Brydon P, Smith K, Gee H. Pregnancy in women with type 2 diabetes: 12 years outcome data 1990–2002. Diabet Med 2003; 20: 734–738. [DOI] [PubMed] [Google Scholar]
- 26. Casson IF, Clarke CA, Howard CV, McKendrick O, Pennycook S, Pharoah PO, et al Outcomes of pregnancy in insulin dependent diabetic women: results of a five year population cohort study. BMJ 1997; 315: 275–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Towner D, Kjos SL, Leung B, Montoro MM, Xiang A, Mestman JH, et al Congenital malformations in pregnancies complicated by NIDDM. Diabetes Care 1995; 18: 1446–1451. [DOI] [PubMed] [Google Scholar]
- 28. Reece EA. Diabetes‐induced birth defects: What do we know? What can we do? Curr Diab Rep 2012; 12: 24–32. [DOI] [PubMed] [Google Scholar]
- 29. Mills JL, Simpson JL, Driscoll SG, Jovanovic‐Peterson L, Van Allen M, Aarons JH, et al Incidence of spontaneous abortion among normal women and insulin‐dependent diabetic women whose pregnancies were identified within 21 days of conception. N Engl J Med 1988; 319: 1617–1623. [DOI] [PubMed] [Google Scholar]
- 30. Miodovnik M, Lavin JP, Knowles HC, Holroyde J, Stys SJ. Spontaneous abortion among insulin‐dependent diabetic women. Am J Obstet Gynecol 1984; 150: 372–376. [DOI] [PubMed] [Google Scholar]
- 31. Miodovnik M, Skillman C, Holroyde JC, Butler JB, Wendel JS, Siddiqi TA. Elevated maternal glycohemoglobin in early pregnancy and spontaneous abortion among insulin‐dependent diabetic women. Am J Obstet Gynecol 1985; 153: 439–442. [DOI] [PubMed] [Google Scholar]
- 32. Miodovnik M, Mimouni F, Tsang RC, Ammar E, Kaplan L, Siddiqi TA. Glycemic control and spontaneous abortion in insulin‐dependent diabetic women. Obstet Gynecol 1986; 68: 366–369. [DOI] [PubMed] [Google Scholar]
- 33. Sutherland HW, Pritchard CW. Increased incidence of spontaneous abortion in pregnancies complicated by maternal diabetes mellitus. Am J Obstet Gynecol 1987; 156: 135–138. [DOI] [PubMed] [Google Scholar]
- 34. Wright AD, Nicholson HO, Pollock A, Taylor KG, Betts S. Spontaneous abortion and diabetes mellitus. Postgrad Med J 1983; 59: 295–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Palomba S, Falbo A, Orio F Jr, Zullo F. Effect of preconceptional metformin on abortion risk in polycystic ovary syndrome: a systematic review and meta‐analysis of randomized controlled trials. Fertil Steril 2009; 92: 1646–1658. [DOI] [PubMed] [Google Scholar]
- 36. van Gelder MM, Van Bennekom CM, Louik C, Werler MM, Roeleveld N, Mitchell AA. Maternal hypertensive disorders, antihypertensive medication use, and the risk of birth defects: a case‐control study. BJOG 2015; 122: 1002–1009. [DOI] [PubMed] [Google Scholar]
- 37. Stothard KJ, Tennant PW, Bell R, Rankin J. Maternal overweight and obesity and the risk of congenital anomalies: a systematic review and meta‐analysis. JAMA 2009; 301: 636–650. [DOI] [PubMed] [Google Scholar]
- 38. Ehrenberg HM, Durnwald CP, Catalano P, Mercer BM. The influence of obesity and diabetes on the risk of cesarean delivery. Am J Obstet Gynecol 2004; 191: 969–974. [DOI] [PubMed] [Google Scholar]
- 39. Beck E, Lechner A, Schaefer C. Who seeks Teratology Information Service's advice? Assessing the risk of selection bias in observational cohort studies on drug risks in pregnancy. Reprod Toxicol 2017; 67: 79–84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Details of birth defects. Metformin study from European Teratology Information Services (TIS) 1993 to 2015
Table S2 Pregnancy outcomes presented by metformin discontinuers and continuers. Metformin study from European Teratology Information Services (TIS) 1993 to 2015


