Abstract
Aims
To determine the incidence of drug‐related deaths (DRD) in a university hospital in 2015, to describe their characteristics, and to discover risk factors of DRD.
Methods
An analytic and retrospective cohort study. Patients with a death diagnosed predefined from a list of medical conditions potentially caused by drugs were the selected cases for further review. Causality assessment was evaluated by a local drug safety committee.
Results
Out of 1135 inpatient deaths, 73 DRD were included (six were hospital‐acquired). The incidence of DRD of all hospital admissions was 0.34%, and the incidence of all deaths cases was 7%. Drugs were the cause of death in 38 patients (52%) and a contributive role in 35 (48%). The median age of DRD patients was 72 years (range 19–94) and 72.6% were men. The median hospital stay, Charlson score and number of drugs were 5 days, 2 points and seven drugs respectively. The most frequent DRD were cerebral haemorrhages and infections in drug‐immunosuppressed patients (32, 43.8%, each group). The most frequently involved drugs were antineoplastics and glucocorticosteroids (40% and 18%), and antithrombotics (33%); drug–drug interactions were present in 44% DRD. Sex, age and number of drugs were risk factors of DRD.
Conclusions
Adverse drug reactions were a significant cause of death in hospitalized patients, mainly haemorrhages and infections precipitated by drug–drug interactions. Risk factors for DRD were sex, age and number of drugs. Preventable DRD and measures to avoid them should be accurately assessed in further studies.
Keywords: adverse drug reaction, epidemiology, hospital mortality, incidence
What is Already Known about this Subject
Fatal adverse drug reactions represent a relevant cause of death in hospitals.
There are few studies focused on assessing the incidence of drug‐related deaths (DRD).
The incidence rates of DRD show a wide variability.
What this Study Adds
Additive or synergistic effects were implicated in almost half of DRD.
Almost half of the DRD cases were preventable according to Schumock and Thornton criteria.
Risk factors of DRD were sex, age, and number of drugs.
Incidence data of DRD in tertiary hospitals can vary according to the methods used to select cases and for drug causality assessment; however, risk factors of DRD were similar among different studies.
Introduction
Medicines are not always effective in improving clinical outcomes for all patients treated. Although most drugs generally have a good safety profile, some patients present with an adverse drug reaction (ADR). ADRs are an important cause of morbidity that occur in about 10% in ambulatory care setting, in 10–20% of hospital inpatients, and accounts for 5% of all hospital admissions 1, 2. ADR increases both the length of hospital stay and costs. Moreover, ADR is also a relevant cause of mortality. Among iatrogenic causes, which represented the third leading cause of death in the USA in 2000, where ADRs caused around 106 000 deaths in a year 3. Lazarou et al. estimated fatal ADR to be between the fourth and sixth leading cause of death in the USA 4.
There are many studies and meta‐analyses assessing the incidence of ADR in hospitalized patients, as well as assessing ADR incidence of patients attended in the emergency rooms 4, 5. However, data on fatal ADR occurrence rate is often a secondary outcome and, therefore, less reported. To our knowledge, there are only four published studies designed to assess the incidence of drug‐related death (DRD) in a tertiary hospital, with long periods of study; and only two of them assessed the risk factors 6, 7. Their DRD incidences show a widely variability, ranging from 0.02 to 0.95% for incidences of hospital admissions, and from 3% to >18% for incidences of patient deaths in hospital 6, 7, 8, 9.
The main objectives of this study were to determine the DRD incidence in a single tertiary care hospital in 2015, and to determine the risk factors for DRD. Other secondary objectives of the study were to discover which drugs were involved, to describe the characteristics of ADR, to assess drug–drug interactions, and to identify preventable DRD.
Materials and methods
We followed the STROBE Statement to report the study sections and their content 10.
Study design
This is an analytic and retrospective cohort study performed in the Germans Trias i Pujol Hospital, which is a tertiary care hospital with 511 beds for a population of about 850 000 people living in the Barcelonès Nord I Maresme area of Barcelona, in Catalonia, Spain. The study was approved by the Research Ethics Committee of the Germans Trias i Pujol Hospital in February 2016, and was registered in the ClinicalTrials.gov (identifier: NCT02838212; https://clinicaltrials.gov/ct2/show/NCT02838212). All included cases of fatal ADR were reported to the Spanish Pharmacovigilance System.
Participants and selection criteria
From a list of patients who died in the hospital throughout 2015, only the patients from whom we had a death diagnosis were selected for this study. Therefore, patients who died in the emergency room were not included as the diagnosis was not accessible.
Patients were selected as potential cases for further review if their death diagnosis was in a predefined list of diseases and syndromes. This list included medical conditions potentially caused by drugs (Table 1). When a specific drug was mentioned in the diagnosis, that case was also selected. The selection of the cases was done by A.L.A. and reviewed by E.M.
All potential cases were assessed to determine whether death was related to drugs; and any drug‐related case was included and considered an actual DRD. Therefore, DRD cases were dead hospitalized patients in 2015 with one or more drugs related to death. Patients whose death was not related to a drug were excluded and considered non‐DRD cases. The definition of ADR used was “a response to a medicinal product which is noxious and unintended”, from the last Directive of the European Parliament and of the Council (2010/84/EU) 11.
Table 1.
Frequency of selected and included patients according to death diagnosis
| Medical condition | Selected patients | Included patients |
|---|---|---|
| Acute renal failure | 11 | 1 |
| Agranulocytosis, leukopenia, neutropenia | 4 | 2 |
| Allergic reaction, anaphylactic shock, angioedema | 0 | 0 |
| Aplastic anaemia, pancytopenia, | 0 | 0 |
| Aseptic meningitis | 0 | 0 |
| Arrhythmia, atrioventricular block, syncope, torsade de pointes | 10 | 2 |
| Confusional syndrome or delirium | 1 | 1 |
| Cushing syndrome | 0 | 0 |
| Drug intoxication, suicide attempt | 1 | 1 |
| Encephalopathy | 0 | 0 |
| Erythema nodosum | 0 | 0 |
| Gastric or duodenal ulcer | 5 | 1 |
| Guillain–Barré syndrome | 0 | 0 |
| Haemolytic anaemia | 1 | 0 |
| Haemorrhage, haematoma, gastrointestinal bleeding upper/lower | 82 | 34 |
| Hepatitis | 1 | 0 |
| Hyperkalaemia, hypokalaemia, hyponatraemia | 1 | 0 |
| Infections, sepsis | 140 | 30 |
| Metabolic acidosis | 0 | 0 |
| Multiforme erythema, Stevens–Johnson syndrome, toxic epidermal necrolisis | 0 | 0 |
| Myopathy | 0 | 0 |
| Neuroleptic malignant syndrome | 0 | 0 |
| Pancreatitis | 10 | 0 |
| Parkinsonism | 0 | 0 |
| Pneumonitis | 2 | 1 |
| Pulmonary fibrosis | 5 | 0 |
| Pulmonary thromboembolism | 0 | 0 |
| Rhabdomyolysis | 0 | 0 |
| Syndrome of the inadequate secretion of antidiuretic hormone | 0 | 0 |
| Systemic lupus erythematosus | 0 | 0 |
| Thrombocytopenia | 0 | 0 |
| Vasculitis | 0 | 0 |
| Other (including drug in the diagnosis) | 9 | 0 |
| Total patients | 284 | 73 |
Outcomes
Primary outcome
DRD occurrence rate or incidence of all hospital admissions in 2015. This was calculated with the number of dead patients in our hospital throughout 2015 whose cause of death was related to a drug as numerator (DRD cases) and the number of patients admitted to the hospital in the same period as denominator.
Secondary outcomes
DRD occurrence rate of inpatients deaths in 2015. Inpatient deaths refer to patients dying during hospitalization. This was calculated with the number of patients who died in hospital in 2015 in whom the cause of death was related to a drug as a numerator (DRD cases) and the number of patients with an available diagnosis of death in the same period as a denominator.
Characteristics of DRD and involved drugs: (i) number of DRD cases with the involved drug starting the week before; (ii) number of DRD cases with drug–drug interactions between involved drugs; (iii) number of hospital‐acquired DRD cases, where ADR started during the hospitalization period; (iv) number of DRD with polymedication; (v) number of DRD cases with autopsy; (vi) number of DRD cases in which the suspected drug had a contributive or causal role; and (vii) number of preventable DRD cases assessed by Schumock and Thornton criteria 12.
Causality of DRD assessed by the number of DRD cases with certain, probable or possible categories applying World Health Organization‐Uppsala Monitoring Centre (WHO‐UMC) criteria 13, and the Naranjo algorithm 14.
Risk factors for DRD or independent associations (sex, age, number of drugs, and comorbidity).
Data sources
The list of dead patients from 2015 and the number of admissions were obtained from the database of the hospital information system. Diagnoses of death were done by the physicians who attended the patient; and were categorized according to the ICD‐9‐MC diagnosis coding (International Classification of Diseases: Ninth Revision–Clinical Modification). Death certificates were not used in this study.
To assess the selected cases, drugs, comorbidities, haematological, biochemical and radiological tests were extracted from the hospital medical charts by A.L.A., Y.S. or E.M. To minimize information bias, related information on drugs was also extracted from the primary care medical registry. However, when the data on the indication of drug use were missing, their attribution was decided according to the pathological history of patients. When we encountered discrepancies in medication information, data were discussed with another rater to reach a consensus. Charlson score, length of hospital stay, sex and age at death were obtained for inpatients from the hospital electronic database information system.
Variables
For DRD cases, the Anatomical Therapeutic Chemical (ATC) classification system was used to classify involved medication (http://www.whocc.no/atc_ddd_index/). The number of involved drugs, duration and indication, doses and route of administration, as well as the characteristics of ADR were extracted. Polymedication was defined when patient received at least 10 drugs. The duration of the related drug was classified as: ‘acute’ when it was started within the week before the onset of the ADR, ‘subacute’ when it was started between 1 week and 6 months prior to ADR, and ‘chronic’ when it was started more than 6 months before the ADR. In cases where two or more drugs with different starting times were involved in the DRD, the most recent was collected, because it was more likely that the drug–drug interaction appeared when the most recent drug was introduced. Comorbidity was measured using Charlson comorbidity index 15. When the hospitalization length was <24 h, it was counted as 0 days.
ADR causality assessment
Potential cases of DRD were presented in the Drug Safety Committee of the Hospital to assess causality attribution. This Committee is responsible for assessing all the ADR reported in the Pharmacovigilance Program of the hospital. The probability of a fatal ADR was classified into one of the six categories of the WHO‐UMC's causality classification (certain, probable/likely, possible, unlikely, conditional/unclassified and unassessable/unclassifiable) 13. We also used the Naranjo algorithm to score the causal probability 14, using a web tool (http://pmidcalc.org/7249508). ADR were classified as definite (9–12 points), probable (5–8 points), possible (1–4 points) or doubtful (0 points). Two evaluators (A.L.A. and E.M.) assessed both causality methods for each DRD case to increase validity to the study. Consensus was reached when discrepancies between scores were present. All involved drugs in a fatal ADR were classified as causing death when the ADR was directly produced by the drug; or contributing to death when drug with another factor concomitantly precipitated the ADR. In addition, autopsy of the patient was recorded in order to confirm the cause of the DRD.
Preventability of ADR
The Schumock and Thornton criteria checklist was applied to identify preventable DRD 12. The seven criteria assessed mainly the appropriateness of drug according to the patient's condition and to pharmacokinetic characteristics of the drug, drug monitoring, and drug–drug interactions. A DRD was considered to be preventable when it met at least one of the following criteria: (i) the drug was not appropriate for the patient's condition; (ii) the dose, frequency and route of administration were inappropriate for the patient's age, weight or disease state; (iii) therapeutic drug monitoring or other necessary laboratory test was not performed; (iv) the patient had a history of allergy or previous reaction to the administered drug; (v) a documented drug interaction was involved in the ADR; (vi) a serum concentration above the therapeutic range was documented; and (vii) noncompliance was involved in the ADR.
Statistical analysis
Summary statistics are presented as percentages in the case of categorical variables and as median (range) in the case of continuous variables. Characteristics of the DRD cases and non‐DRD cases were compared with the Pearson chi‐square or the Fisher exact test, as appropriate for categorical variables, as well as Wilcoxon rank‐sum test for continuous variables. Missing data were not included in the analysis.
Any independent association between patients' characteristics and DRD was assessed by using a logistic regression. DRD was the dependent variable. The variables included in the logistic regression model were age, sex, number of drugs and Charlson comorbidity index score.
For all analyses and comparisons, a two‐sided P value <0.05 was used to determine statistical significance. All statistics were performed using the SPSS software package for Windows, version 19.0 (SPSS Inc, Chicago, IL, USA) and R package v.3.3.1 (21–06‐2016; R CoreTeam. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2016. https://www.r‐project.org/).
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 16, and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 17.
Results
In 2015, there were 21 483 admissions and 1135 inpatient deaths (18.9%, 1135/21 483) in Germans Trias I Pujol Hospital. The diagnosis of death was available in 1036 patients (91.3%, 1036/1135). A total of 281 patients (24.7%, 281/1135) were selected as potential DRD cases and reviewed. After drug causality assessment, 73 patients were DRD cases and 208 were non‐DRD (Figure 1). DRD occurence rate of all hospital admissions was 0.34% (73/21 483), and the rate of inpatient deaths cases was 7.05% (73/1036).
Figure 1.
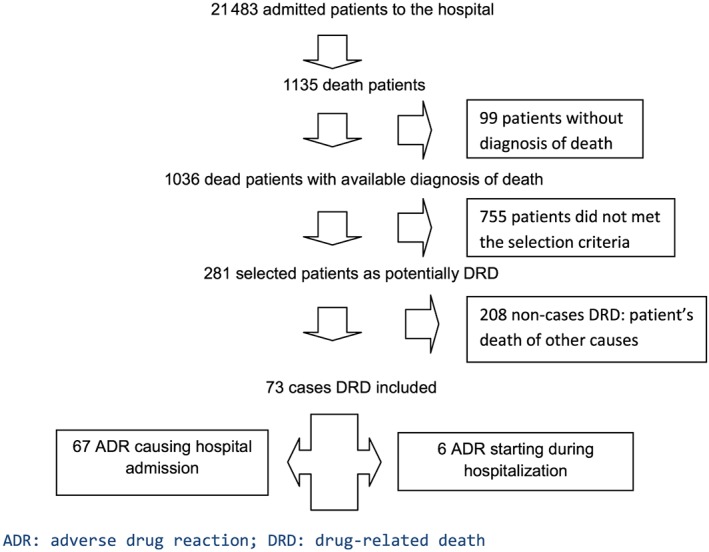
Flow chart of patient selection
For DRD cases, the median age of patients was 72 years (range 19–94), of whom 72.6% were men. Median Charlson score was 2 points (range 0–8), median number of drugs during ADR episodes per patient was seven (range 2–14) and median hospital stay was 5 days (range 0–57).
Characteristics of fatal ADR
ADR was the cause of hospital admission in 67 cases (91.8%, 67/73), and the ADR started during hospitalization in six patients (8.2%, 6/73; Figure 1).
The most frequent DRD were haemorrhage (34 cases, 46.6%) followed by sepsis or infections in drug‐immunosuppressed patients (32, 43.8%). The remaining types of DRD are detailed in Table 2.
Table 2.
Frequency and types of drug‐related deaths (DRD)
| Type of DRD | n | % |
|---|---|---|
| Haemorrhages | 34 | 46.5 |
| ‐ Cerebral | 32 | 43.8 |
| ‐ Other locations | 2 | 2.7 |
| ‐ (gastrointestinal, retroperitoneal haematoma) | ||
| Infections in immunosuppressed patients | 32 | 43.8 |
| ‐ Lung infection | 18 | 24.6 |
| ‐ Sepsis | 12 | 16.4 |
| ‐ Fever in neutropenic patients | 2 | 2.7 |
| Atrioventricular block | 2 | 2.7 |
| Acute renal failure | 1 | 1.4 |
| Acute confusional syndrome | 1 | 1.4 |
| Interstitial pneumonitis | 1 | 1.4 |
| Duodenal ulcer with haemorrhage | 1 | 1.4 |
| Benzodiazepine intoxication | 1 | 1.4 |
| Total | 73 | 100 |
Characteristics of drugs related to ADR
In 41 (56.2%, 41/73) DRD cases, there was only one drug involved, with two drugs in 23 (31.5%, 23/73), three drugs in eight (11%, 8/73), and four different related drugs in one case (1.4%, 1/73). In 32 (43.8%, 32/73) DRD cases, a drug–drug interaction was present; all were pharmacodynamic and synergistic interactions. The number of DRD patients with polymedication was 32 (43.8%, 32/73).
In total, there were 116 involved drugs for 73 DRD cases. Forty‐six drugs (39.6%, 46/116) were classified in ATC category L, 38 (32.8%, 38/116) in category B and 21 (18.1%, 21/116) in category H (Table 3). The most commonly involved drugs were acetylsalicylic acid (in 20 DRD cases, 17.2%), prednisone (in 15, 12.9%) and acenocoumarol (in 11, 9.5%; Table 4). Seventy‐three drugs (62.9%, 73/116) were concomitantly administered with other drugs.
Table 3.
The Anatomical Therapeutic Chemical (ATC) classification system of involved drugs
| ATC category | Therapeutic Area | n | % |
| A | Alimentary tract and metabolism | 0 | 0 |
| B | Blood and blood forming organs | 38 | 32.8 |
| C | Cardiovascular system | 5 | 4.3 |
| D | Dermatologicals | 0 | 0 |
| G | Genito‐urinary system and sex hormones | 0 | 0 |
| H | Systemic hormonal preparations, excluding sex‐hormones and insulins | 21 | 18.1 |
| J | Anti‐infectives for systemic use | 0 | 0 |
| L | Antineoplastic and immunomodulating agents | 46 | 39.6 |
| M | Musculoskeletal system | 1 | 0.9 |
| N | Nervous system | 5 | 4.3 |
| P | Antiparasitic products, insecticides and repellents | 0 | 0 |
| R | Respiratory system | 0 | 0 |
| S | Sensory organs | 0 | 0 |
| V | Various | 0 | 0 |
| Total | 116 | 100 |
Table 4.
Drugs involved in drug‐related death cases
| Drug | n | % | Drug–drug interaction |
|---|---|---|---|
| Acetylsalicylic acid | 20 | 17.2% | 4 (20%) |
| Prednisone | 15 | 12.9% | 13 (86.7%) |
| Acenocoumarol | 11 | 9.5% | 1 (9.1%) |
| Dexamethasone | 6 | 5.2% | 5 (83.3%) |
| Ciclosporin | 5 | 4.3% | 5 (100%) |
| Clopidogrel | 4 | 3.4% | 3 (75%) |
| 5‐Fluorouracil | 3 | 2.6% | 2 (100%) |
| Mycophenolic acid | 3 | 2.6% | 3 (100%) |
| Methotrexate | 3 | 2.6% | 2 (66.7%) |
| Enoxaparin | 2 | 1.7% | 0 (0%) |
| Pentoxifylline | 2 | 1.7% | 2 (100%) |
| Bevacizumab | 2 | 1.7% | 2 (100%) |
| Pemetrexed | 2 | 1.7% | 2 (100%) |
| Paclitaxel | 2 | 1.7% | 1 (50%) |
| Tacrolimus | 2 | 1.7% | 2 (100%) |
| Topotecan | 2 | 1.7% | 2 (100%) |
| Amiodarone, azacitidine, azathioprine, bendamustine, blinatumomab, carboplatin, carmustine, ceritinib, cetuximab, cyclophosphamide, cytarabine, dexketoprofen, docetaxel, doxorubicin, etoposide, everolimus, fentanyl, furosemide, gemcitabine, ivabradine, leflunomide, lorazepam, melphalan, mitomycin, nilotinib, nivolumab, oxaliplatin, paracetamol, rituximab, ruxolitinib, sertraline, venlafaxine | 1 each (32) | 0.9% each | 0 |
| Total | 116 | 100 | 48 drugs (41%) |
The medical indications for the drugs were atrial fibrillation and solid tumours (15 DRD cases in each indication, 20.5%, 15/73; Table 5).
Table 5.
Indications of drugs involved to drug‐related death cases
| Medical indications | n | % | Detailed medical indications |
|---|---|---|---|
| Atrial fibrillation | 15 | 20.5% | Chronic atrial fibrillation (14), paroxistic atrial fibrillation (1) |
| Solid tumour | 15 | 20.5% | Lung (6), colon and rectum (3), base of tongue (2), breast (2), prostate (1), pancreas (1) |
| Transplant | 8 | 11% | Bone marrow transplant (5), kidney transplant (3) |
| Hematologic disease | 7 | 9.6% | Leukaemia (3) myelodysplastic syndrome (2), lymphoma (1), multiple myeloma (1) |
| Coronary heart disease | 6 | 8.2% | ‐ |
| Stroke | 5 | 6.8% | Transitory ischemic stroke (3), stroke (2) |
| Unknown | 5 | 6.8% | Probably primary cardiovascular prophylaxis (5) |
| Peripheral artery disease | 4 | 5.6% | ‐ |
| Pain | 2 | 2.8% | Chronic pain (1), postoperative pain (1) |
| Other | 6 | 8.2% | Rheumatoid arthritis (1), vasculitis (1), lung fibrosis (1), valvular prosthesis (1), suicide attempt (1), motor neuron disease (1) |
| Total | 73 | 100 | ‐ |
The related drug was started within the week before the ADR in eight patients (11%, 8/73), the duration of treatment was subacute in 27 patients (37%, 27/73) and chronic in 36 (49.3%, 36/73); the beginning of the drug was unknown in two DRD cases (2.7%, 2/73; Table 6).
Table 6.
Characteristics of patients and drug‐related deaths (DRD)
| Haemorrhages DRD | Infections DRD | All DRD | |
|---|---|---|---|
| Characteristics of patients with DRD | |||
| Age (median, range) | 77.5 (57–92) | 67.5 (19–82) | 72 (19–94) |
| Sex: women, n (%) | 8 (23.5%) | 9 (28.1%) | 20 (27.4%) |
| Number of drugs (median – range) | 7 (2–14) | 6.5 (4–10) | 7 (2–14) |
| Polymedication, n (%) | 11 (32.3%) | 13 (40.6) | 32 (43.8%) |
| Charlson score (median ‐ range) | 1 (0–8) | 3 (0–8) | 2 (0–8) |
| Days in hospital (median ‐ range) | 5 (0–57) | 5 (0–56) | 5 (0–57) |
| Characteristics of ADR | |||
| ADR was the cause of hospital admission, n ( %) | 33 (97.1%) | 28 (87.5%) | 67 (91.8%) |
| Drug–drug interaction, n (%) | 5 (14.7%) | 25 (78.1%) | 32 (43.8%) |
| Number of involved drugs (median – range) | 1 (1–3) | 2 (1–4) | 1 (1–4) |
| Time from the start of involved drugs, n (%) | a | a | |
| ≤ 1 week | 2 (6.2%) | 3 (9.4%) | 8 (11%) |
| >1 week–6 months | 1 (3.1%) | 25 (78.1%) | 27 (38%) |
| >6 months | 29 (90.7%) | 4 (12.5%) | 36 (51%) |
| Drugs were the cause of death, n (%) | 2 (5.9%) | 31 (96.9%) | 38 (52.1%) |
| Drugs contributed to death, n (%) | 32 (94.1%) | 1 (3.1%) | 35 (47.9%) |
| Total | 34 | 32 | 73 |
data not available in two patients
Drugs were the cause of death in 38 DRD cases (52.1%, 38/73) and contributed to death in 35 patients (47.9%, 35/73). The main contributive causes for intracranial haemorrhages were falls with head injuries (13/73, 40.6%) and hypertension (15/73, 46.9%).
No medication errors on type of drug, dosing or route of administration were identified. One patient took an overdose of benzodiazepines causing DRD by intoxication.
Hospital‐acquired fatal ADR
In six patients (8.2%, 6/73), ADR started during the hospitalization. Having previously received cytotoxic and/or immunosuppressant agents for haematological malignancies, four patients presented respiratory infection or sepsis. One patient, admitted to another hospital for appendicitis and treated with intravenous dexketoprofen for pain, was transferred to our hospital 3 days after for haemorrhagic shock due to an active bleeding of duodenal ulcer that persisted despite multiple endoscopic sclerosing treatments. Another patient, hospitalized at another centre for respiratory tract infection and treated with enoxaparin for atrial fibrillation, was transferred to our hospital a week later for an extensive retroperitoneal haematoma after a fall. The patient presented with haemorrhagic shock due to an active bleeding of the uterine artery.
The incidence of hospital‐acquired fatal ADR calculated from all admissions to the hospital was 0.03% (6/21 483). The incidence of hospital‐acquired fatal ADR was 0.58% of dead patients (6/1036).
ADR causality assessment
All deaths were classified as probably or possibly related to drugs, regardless of the assessment method used. When applying WHO‐UMC classification, 37 (50.7%, 37/73) ADR were possible and 36 (49.3%, 36/73) probable related to drugs. Median Naranjo score of DRD cases was 4 (range 3–7). ADRs were classified into two categories: possible (scores range 2–4; 46/73 ADR, 63%) and probable (scores range 5–8; 27/73 ADR, 37%).
No autopsies were performed, although permission was requested in three cases, but their families denied. In another patient, the local police requested a forensic autopsy.
Preventability of ADR
DRD were potentially preventable in 34 cases (46.6%, 34/73) according to Schumock and Thornton criteria. The most frequent criterion we found was that a documented drug–drug interaction was involved in the ADR (32 cases, 94.1%). Three patients met two different criteria (4,1%, 3/73; Table 7).
Table 7.
Frequency of drug‐related deaths (DRD) cases meeting the Schumock and Thornton criteria
| Schumock and Thornton criteria | Number of DRD cases meeting the criteria |
|---|---|
| (1) the drug was not appropriate for the patient's condition | 4 |
| (2) the dose, frequency and route of administration were inappropriate for the patient's age, weight or disease state | 1a |
| (3) therapeutic drug monitoring or other necessary laboratory test was not performed | 0 |
| (4) the patient had a history of allergy or previous reaction to the administered drug | 0 |
| (5) a documented drug interaction was involved in the ADR | 32 |
| (6) a serum concentration above the therapeutic range was documented | 0 |
| (7) noncompliance was involved in the ADR | 0 |
duration of treatment was not appropriate
Risk factors of DRD
Univariate analysis showed statistically significant differences in all assessed variables: sex, age, number of drugs and Charlson comorbidity score (Table 8). Multivariate logistic regression analysis to identify independent associations with DRD showed statistically significant differences in sex, age and number of drugs, but not in Charlson comorbidity score (Table 9).
Table 8.
Comparison between drug‐related deaths (DRD) cases and deaths for other causes (non‐DRD cases)
| DRD Cases (n = 73) | Non‐DRD cases (n = 208) | OR (95% CI) | P value | |
|---|---|---|---|---|
| Age (years), median (range) | 72 (19–94) | 76.5 (0–98) | 0.98 (0.96–1.00) | 0.0088 |
| Sex, female, n (%) | 20 (27.4) | 97 (46.6) | 0.43 (0.24–0.76) | 0.0041 |
| Charlson comorbidity score, median (range) | 2 (0–8) | 1 (0–12) | 1.13 (1.01–1.26) | 0.014 |
| Number of drugs, median (range; at the admission time) | 8 (2–21) | 7 (0–20)a | 1.09 (1.03–1.16) | 0.0023 |
| Polymedication, n (%) | 32 (43.8%) | 64 (31.7%) | 1.68 (0.97–2.91) | 0.062 |
| Days in hospital, median (range) | 5 (0–57) | 7 (0–114) | 0.98 (0.96–1.01) | 0.172 |
missing data in six patients
Table 9.
Multivariate analysis comparing drug‐related deaths to deaths for other causes
| Multivariate analysis | |||
|---|---|---|---|
| OR | 95% CI | P value | |
| Age | 0.98 | 0.96–0.99 | 0.0197 |
| Sex (reference variable: women) | 0.47 | 0.25–0.84 | 0.0131 |
| Total number of drugs | 1.10 | 1.03–1.17 | 0.0047 |
| Charlson comorbidity score | 1.04 | 0.92–1.18 | 0.4795 |
In the univariate analysis, the Charlson comorbidity score effect was statistically significant. However, when adjusting by age, this effect was not statistically significant (P = 0.4795). DRD patients were more often men, younger and had received more drugs than those who died for other causes (Table 9).
Posthoc analysis: characteristics of DRD cases
Since ADRs could be grouped into two large groups, haemorrhages and infections, we proceeded to detail and compare the characteristics of each group (Table 6). Statistically significant differences were found in the following variables: age [odds ratio (OR) 1.09; 95% confidence interval (CI) 1.04–1.16], drug–drug interactions (OR 18.12; 95% CI 5.35–69.13), number of drugs (OR 0.10; 95% CI 0.03–0.28), and Charlson score (OR 1.58; 95% CI 1.23–2.13).
Discussion
In our results, DRD occurrence rate of all hospital admissions was 0.34%, and the occurrence rate of all inpatient deaths was 7%.
When we compared these results to data of available ADR meta‐analysis or reviews, our incidences were similar to those described in a recent review of 47 European observational studies, where the mean rate of fatal ADRs was 0.14% of all admissions 18; and very similar to those reported in a meta‐analysis of 39 studies about ADR in hospitalized patients in the USA with a rate of fatal ADR of 0.32% of all hospital admission. Moreover, the authors of the latter review have suggested a rate of fatal ADR of 4.6% of deaths from all causes 4. The retrospective design and the methods used in the present study differ from those studies included in the reviews.
Our results were also compared to available four studies with similar methodological design which were conducted in Finland and Spain 6, 7, 8, 9. The occurrence rate of DRD of all hospital admissions in our study was 10 times higher than the Finnish studies (Spanish data not reported) 4, 6. By contrast, the occurrence rate of all inpatient deaths of the present study was much smaller than the mentioned Spanish study (with rates twice higher) 7. The most important common characteristics between these four studies and our study are that they all were conducted in the last 15 years, in a single European centre, have a retrospective design, the study period was about a year, and the incidence of DRD was the main objective of the study. However, there are also important methodological differences, mainly the method used for selecting patients and the ADR definition used, which could explain the differences in the occurrence rates. As to the ADRs used in the studies, although there are many different definitions of ADRs 19, the most commonly used was the WHO definition 20. We chose the definition proposed in the last European pharmacovigilance legislation (Directive of the European Parliament and of the Council 2010/84/EU), which widened the definition of ADR (including, for example, doses and indications not authorized for the regulatory agencies) 11.
Finally, the results of our study differed from other studies with heterogeneous designs such as studies conducted in a specific hospital department, or with various hospitals participating, and those performed for longer or shorter periods of time 21, 22.
The incidence of hospital‐acquired fatal ADR of all admitted patients in our study was 10‐fold higher than a previous similar study, but 10‐fold lower than in another study which assessed specifically ADR in hospital inpatients 8, 23.
The most common DRD cases were haemorrhages and infections, which supports the findings in a population based study and in other previous studies of hospitalized patients 8, 9, 24, 25. Therefore, the most frequently involved drugs were antithrombotic agents and antineoplastics and/or glucocorticosteroids, in line with previous studies 8, 9, 25, 26, 27. Drug–drug interactions were linked to almost half of DRD cases, similar to another study 22.
ADR causality assessment
We used Naranjo algorithm and WHO‐UMC criteria for individual causality assessment in each DRD to improve the accuracy of our assessment. However, although both causality systems are the most frequently used worldwide, limitations are present when assessing dead patients, such as the criteria of drug dechallenge and drug rechallenge, which are not applicable in these cases.
In some cases with drug monitoring or measurements of biomarkers missing, with a fulminant death, or with a very rare ADR, the cause of death cannot be completely clear. If an ADR produces an anatomopathological lesion, the autopsy findings could be useful to determine or to verify the cause of death 28. In the present study, in only three patients were autopsies requested to perform further anatomopathological examinations, which were denied by their families. This low number of requested autopsies could be explained because all the ADRs leading to death were related to the mechanism of action of the involved drug/s (type A reaction) 29, and could have been predicted and even expected, leading to a low degree of uncertainty about the cause of death.
Preventability of ADR
No errors on drug, dosing or route of administration were identified, probably due to computerized prescribing systems implemented in hospitals. The number of DRD potentially preventable according to the Schumock and Thornton criteria was similar to previous studies 20. The most frequent Schumock and Thornton criterion met was that a documented drug–drug interaction was involved in the ADR. A pharmacodynamic drug–drug interaction was implicated in 44% of DRD cases. Previous studies highlighted that drug–drug interactions are a real problem in clinical practice 30. However, several diseases, such as cancer, pain or organ transplantation, require combined therapies to increase the effectiveness of drugs due to a beneficial synergic pharmacodynamic drug–drug interaction 31, although this can also lead to harmful interactions. All these drug–drug interactions are therefore well known and documented.
Preventability of DRD needs further analysis to propose effective intervention strategies to diminish the number of DRDs. Improved awareness, prevention and treatment of ADR could reduce the occurrence rate of ADR and fatal ADR.
Risk factors for ADR‐related deaths
In our study, male gender, younger elderly patients and higher number of drugs were significantly higher among DRD cases than non‐DRD cases. This surprised us because, in general, ADRs are more frequent in patients who are women, elderly and with high comorbidity 22, 32. Higher comorbidity has been found as a risk factor of presenting fatal ADR in several studies, but the methods used for their assessment were different between the studies; in two of them, comorbidity was measured by counting the diseases o patients 7, 23 and in one, a modified Charlson score was used 20. In the present study, the Charlson score was calculated taking into account comorbidities at admission to hospital and also during the hospitalization stay; which could explain differences between those results. By contrast, age and sex were not independent risk factors of DRD in other studies 7, 21. The number of medications, is a common risk factor of ADR previously described in other studies 6, 7, 23. This study lacked power to detect differences in polymedication between both groups.
Strengths and limitations
By the nature of the design, the main limitation of this study is that it is a single‐centre and retrospective study. There is likely to be a variation between different hospitals because of the differences in characteristics of the patients attending, as well as available medical specialties. This is why our results reflect DRD only in a tertiary hospital and not in the general population. Prospective studies are designed with specific data collection methods, and therefore may be more complete than retrospective studies. One disadvantage of a prospective cohort study is the long follow‐up period required to wait for events or diseases to occur 33; and taking into account that the studied event in this case was death, greatly complicates the follow‐up. Obtaining relevant clinical information of high quality on patients is difficult and, hence the number of DRD might have been underestimated. Moreover, the information about the use of over the counter drugs and herbal remedies is usually lacking in case records.
Our methods for the selection of deaths cases were designed to increase the efficiency of the study, that is, to identify the highest number of fatal ADRs using the minimum means in time and effort, despite their limitations. Selection was based on a pre‐defined list of diseases and syndromes potentially caused by drugs, which has its own limitations by restricting cases; leading clearly to an underestimation occurrences rates of death. Similar list has previously been used to identify ADR from admission diagnoses, considering low the number of nonidentified cases 34. However, to our knowledge, identifying fatal ADR from a diagnosis of death has not been used previously, although we believe that this could also be acceptable. In addition, the diagnosis of death obtained from the hospital database, from which the patients were selected, is also a limitation of the study. Moreover, this diagnosis was missing in almost 10% of patients. Therefore, in line with these limitations, it is extremely important that diagnosis or other medical features should be coded to analyse such data in further studies. If physicians are not aware of this, the problem will not be solved. However, more accurate diagnoses are expected to be included in death certificates, such as immediate and underlying cause, together with the cause of death. Diagnosis of a patient's death might be different from that of their death certificate; nevertheless, death certificates were not used in this study.
The main strengths of this study are the assessed sample size and the duration of the study, which were representative of all hospitalized patients in our setting, the definition of ADR used, currently in force in the regulatory framework of the European Union and the assessment of causality by a Safety Committee with expertise on ADR, as well as both causality methods used (WHO‐UMC and Naranjo algorithm) by two raters.
Our findings suggest that drugs are an important cause of death in hospitalized patients. Drugs are directly or indirectly responsible in at least one of 15 dead patients in our hospital, resulting in a significant health burden. Haemorrhages and infections were seen in a majority of DRD; and antithrombotic agents and antineoplastics combined with glucocorticosteroids were implicated in most of these events. Drug–drug interactions were involved in almost half of DRD. Risk factors for DRD were sex, age and number of drugs. Preventable DRD should be accurately assessed in further studies and preventive measures should be implemented in clinical practice.
Competing Interests
E.M., A.L.A., Y.S., J.R., and M.F. declare that they have no conflict of interest. This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
The study was approved by the Research Ethics Committee of the Germans Trias i Pujol Hospital in February 2016.
Formal consent is not required for this type of study.
Contributors
A.L.A. contributed to selection of cases, data acquisition and causality assessment of the included cases, interpretation of the data, and writing the manuscript. Y.S. contributed to data acquisition and causality assessment of the included cases, interpretation of the data, and writing the manuscript. J.R. contributed to statistical analysis and interpretation of the data. E.M. conceived the idea, contributed to study design, review selected cases, data acquisition and causality assessment of the included cases, analysis and interpretation of the data, and writing the first draft of manuscript. M.F. contributed to causality assessment of the included cases, interpretation of the data, and writing the manuscript. All the authors contributed to the final draft and approved its content.
Montané, E. , Arellano, A. L. , Sanz, Y. , Roca, J. , and Farré, M. (2018) Drug‐related deaths in hospital inpatients: A retrospective cohort study. Br J Clin Pharmacol, 84: 542–552. doi: 10.1111/bcp.13471.
References
- 1. Taché SV, Sönnichsen A, Ashcroft DM. Prevalence of adverse drug events in ambulatory care: a systematic review. Ann Pharmacother 2011; 45: 977–989. [DOI] [PubMed] [Google Scholar]
- 2. Pirmohamed M, Breckenridge AM, Kitteringham NR, Park BK. Adverse drug reactions. BMJ 1998; 316: 1295–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Starfield B. Is US health really the best in the world? JAMA 2000; 284: 483–485. [DOI] [PubMed] [Google Scholar]
- 4. Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients. A meta‐analysis of prospective studies. JAMA 1998; 279: 1200–1205. [DOI] [PubMed] [Google Scholar]
- 5. Kongkaew C, Noyce PR, Ashcroft DM. Hospital admissions associated with adverse drug reactions: a systematic review of prospective observational studies. Ann Pharmacother 2008; 42: 1017–1025. [DOI] [PubMed] [Google Scholar]
- 6. Pardo Cabello AJ, González Contreras LG, Manzano Gamero MV, Gómez Jiménez FJ, Puche Cañas E. Prevalence of fatal adverse drug reactions in hospitalized patients. Int J Clin Pharmacol Ther 2009; 47: 596–602. [DOI] [PubMed] [Google Scholar]
- 7. Pardo Cabello AJ, Del Pozo Gavilán E, Gómez Jiménez FJ, Mota Rodríguez C, Luna Del Castillo Jde D, Puche Cañas E. Drug‐related mortality among inpatients: a retrospective observational study. Eur J Clin Pharmacol 2016; 72: 731–736. [DOI] [PubMed] [Google Scholar]
- 8. Lapatto‐Reinilouto O, Patinen L, Niemi M, Backman JT, Neuvonen PJ. Drug‐related inadvertent deaths in a university hospital – a declining trend. Basic Clin Pharmacol Toxicol 2015; 117: 421–426. [DOI] [PubMed] [Google Scholar]
- 9. Juntti‐Patinen L, Neuvonen PJ. Drug‐related deaths in a university central hospital. Eur J Clin Pharmacol 2002; 58: 479–482. [DOI] [PubMed] [Google Scholar]
- 10. Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, et al Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. PLoS Med 2007; 4: e297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Commission directive 2010/84/EU of the European Parliament and the council of 15 December 2010 amending as regards pharmacovigilance, directive 2001/83/EC on the community code relating to medicinal products for human use. Official Journal of the European Union 31.12.2010: L348/74‐L348/99. Available at http://ec.europa.eu/health/files/eudralex/vol‐1/dir_2010_84/dir_2010_84_en.pdf (last accessed 23 September 2017).
- 12. Schumock GT, Thornton JP. Focusing on the preventability of adverse drug reactions. Hosp Pharm 1992; 27: 538. [PubMed] [Google Scholar]
- 13. World Health Organization . The use of the WHO‐UMC system for standardized case causality assessment. Uppsala: The Uppsala Monitoring Centre; Available at http://who‐umc.org/Graphics/24734.pdf (last accessed 23 September 2017). [Google Scholar]
- 14. Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981; 30: 239–245. [DOI] [PubMed] [Google Scholar]
- 15. Charlson ME, Pompei P, Ales KL, Mackenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40: 373–383. [DOI] [PubMed] [Google Scholar]
- 16. Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SPH, et al The IUPHAR/BPS guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 2016; 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Alexander SPH, Kelly E, Marrion NV, Peters JA, Faccenda E, Harding SD, et al The Concise Guide to PHARMACOLOGY 2017/18: Overview. Br J Pharmacol 2017; 174: S1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bouvy JC, De Bruin ML, Koopmanschap MA. Epidemiology of adverse drug reactions in Europe: a review of recent observational studies. Drug Saf 2015; 38: 437–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet 2000; 356: 1255–1259. [DOI] [PubMed] [Google Scholar]
- 20. World health organization technical report series (1972, no 498). International Drug Monitoring. The Role of the National Centres. Report of a WHO Meeting. Geneva, Switzerland. Available at http://apps.who.int/iris/bitstream/10665/40968/1/WHO_TRS_498.pdf (last accessed 23 September 2017). [PubMed]
- 21. Mouton JP, Mehta U, Parrish AG, Wilson DP, Stewart A, Njuguna CW, et al Mortality from adverse drug reactions in adult medical inpatients at four hospitals in South Africa: a cross‐sectional survey. Br J Clin Pharmacol 2015; 80: 818–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ebbesen J, Buajordet I, Erikssen J, Brørs O, Hilberg T, Svaar H, et al Drug‐related deaths in a department of internal medicine. Arch Intern Med 2001; 161: 2317–2323. [DOI] [PubMed] [Google Scholar]
- 23. Davies EC, Green CF, Taylor S, Williamson PR, Mottram DR, Pirmohamed M. Adverse drug reactions in hospital in‐patients: a prospective analysis of 3695 patient‐episodes. PLoS One 2009; 4: e4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Buajordet I, Ebbesen J, Erikssen J, Brørs O, Hilberg T. Fatal adverse drug events: the paradox of drug treatment. J Intern Med 2001; 250: 327–341. [DOI] [PubMed] [Google Scholar]
- 25. Wester K, Jonnson AK, Sigset O, Druid H, Hagg S. Incidence of fatal adverse drug reactions: a population based study. Br J Clin Pharmacol 2008; 65: 573–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Porter J, Jick H. Drug‐related deaths among medical inpatients. JAMA 1977; 237: 879–881. [PubMed] [Google Scholar]
- 27. Caranasos GJ, May FE, Stewart RB, Cluff LE. Drug‐associated deaths of medical inpatients. Arch Intern Med 1976; 136: 872–875. [PubMed] [Google Scholar]
- 28. Chopra N, de Leon J. Clozapine‐induced myocarditis may be associated with rapid titration: a case report verified with autopsy. Int J Psychiatry Med 2016; 51: 104–115. [DOI] [PubMed] [Google Scholar]
- 29. Rawlins MD, Thompson JW. Mechanisms of adverse drug reactions In: Textbook of adverse drug reactions, ed Davies DM. Oxford: Oxford University Press, 1991; 18–45. [Google Scholar]
- 30. Leone R, Magro L, Moretti U, Cutroneo P, Moschini M, Motola D, et al Identifying adverse drug reactions associated with drug‐drug interactions: data mining of a spontaneous reporting database in Italy. Drug Saf 2010; 33: 667–675. [DOI] [PubMed] [Google Scholar]
- 31. van Leeuwen RW, Brundel DH, Neef C, van Gelder T, Mathijssen RH, Burger DM, et al Prevalence of potential drug‐drug interactions in cancer patients treated with oral anticancer drugs. Br J Cancer 2013; 108: 1071–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hofer‐Dueckelmann C, Prinz E, Beindl W, Szymanski J, Fellhofer G, Pichler M, et al Adverse drug reactions (ADRs) associated with hospital admissions ‐ elderly female patients are at highest risk. Int J Clin Pharmacol Ther 2011; 49: 577–586. [DOI] [PubMed] [Google Scholar]
- 33. Song JW, Chung KC. Observational studies: cohort and case‐control studies. Plast Reconstr Surg 2010; 126: 2234–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pedrós C, Quintana B, Rebolledo M, Porta N, Vallano A, Arnau JM. Prevalence, risk factors and main features of adverse drug reactions leading to hospital admission. Eur J Clin Pharmacol 2014; 70: 361–367. [DOI] [PubMed] [Google Scholar]


