Abstract
Aims
The drug burden index (DBI) is a dose‐related measure of anticholinergic and sedative drug exposure. This cross‐sectional study described DBI in older adults with intellectual disabilities (ID) and the most frequently reported therapeutic classes contributing to DBI and examined associations between higher DBI scores and potential adverse effects as well as physical function.
Methods
This study analysed data from Wave 2 (2013/2014) of the Intellectual Disability Supplement to the Irish Longitudinal Study on Ageing (IDS‐TILDA), a representative study on the ageing of people with ID in Ireland. Self‐ and objectively‐reported data were collected on medication use and physical health, including health conditions. The Barthel index was the physical function measure.
Results
The study examined 677 individuals with ID, of whom 644 (95.1%) reported taking medication and 78.6% (n = 532) were exposed to medication with anticholinergic and/or sedative activity. 54.2% (n = 367) were exposed to high DBI score (≥1). Adjusted multivariate regression analysis revealed no significant association between DBI score and daytime dozing, constipation or falls. After adjusting for confounders (sex, age, level of ID, comorbidities, behaviours that challenge, history of falls), DBI was associated with significantly higher dependence in the Barthel index (P = 0.002).
Conclusions
This is the first time DBI has been described in older adults with ID. Scores were much higher than those observed in the general population and higher scores were associated with higher dependence in Barthel index activities of daily living.
Keywords: ageing, drug utilization, pharmacoepidemiology
What is Already Known about this Subject
The drug burden index (DBI) is a tool that quantitatively evaluates the exposure of an individual to medications with anticholinergic and sedative activity.
Higher DBI scores have been associated with poorer physical and cognitive function in community‐dwelling older people without intellectual disabilities (ID).
What this Study Adds
We evaluated DBI in older adults with ID using data from a nationally representative study in Ireland.
The DBI of older people with ID is higher than that of the older general population, particularly the anticholinergic component, and this reflects the different pattern of multimorbidity of the ID population, in particular higher levels of mental health and neurological morbidities.
Higher DBI scores were significantly associated with having higher levels of dependence as measured by the Barthel index after adjusting for relevant confounders.
Introduction
People with intellectual disabilities (ID) may be exposed to high levels of polypharmacy, including medicines with sedative and anticholinergic effects 1, 2. People with ID become multimorbid with age and as a result may be exposed to a high burden of medications 3. This multimorbidity includes a high prevalence of mental health conditions (such as depression, schizophrenia, bipolar affective disorder and anxiety disorders), neurological disease (such as epilepsy and dementia) and gastrointestinal disease (such as gastroesophageal reflux disease and peptic ulcer disease) 4.
Medications with sedative and/or anticholinergic activity may have a significant negative impact on the health of older people. Sedative medications commonly produce adverse cognitive and psychomotor events, increased falls and fractures liability and daytime fatigue – effects that become clinically significant in those with additional risk factors for falls and cognitive impairment 5, 6, 7, 8, 9, 10. Use of medications with sedative effects has been associated with frailty and poorer performance in instrumental activities of daily living in older adults 11, 12.
Older adults in the general population can be particularly sensitive to medications with anticholinergic effects, and the classic adverse effects such as dry mouth, reduced gastric motility, blurred vision and sedation can be compounded in this population to produce difficulties in communication, constipation and falls 13, 14.
A high burden of medicines with anticholinergic properties has been reported in two‐thirds of older adults with ID, with high levels of anticholinergic cognitive burden (ACB) exposure in three in 10 older adults with ID 1, 2. In contrast, community‐dwelling older adults without ID have reported a total ACB exposure of 23% 13. Use of neuroleptic and antipsychotic medication in the ID population have been reported at between 21–50%, while sedative exposure has been reported at between 10–24% 2, 15, 16, 17, 18 and total exposure to central nervous system agents has been reported as high as 60%, compared to 10–25% in the population without ID 19. This burden in people with ID has been associated with various adverse effects, including chronic constipation and daytime drowsiness 1.
Several alternative scales are available to quantify anticholinergic and sedative load separately. The ACB scale categorizes medications as having absent (ACB Score 0), possible (ACB Score 1) or definite (ACB Score 2 or 3) anticholinergic properties 1. The Sedative Load model classifies medications as primary sedatives (Group 1), drugs with sedation as a prominent side effect (Group 2), drugs with sedation as a potential adverse effect (Group 3) and drugs with no known sedative effect (Group 4) 20. The drug burden index (DBI) is a tool that quantitatively evaluates the burden of both anticholinergic and sedative medications on an individual. The DBI offers a dose‐related measure of burden, unlike these other indices available, by considering the relationship between prescribed dose and the dose response curve. Scores from the relevant medications are added together to give a total DBI score for the individual 21, 22. Associations between DBI scores and objective function measures have been analysed to identify the effect of these types of medications on cognitive and physical performance in older adults. A higher DBI score has been associated with a number of negative outcomes: poor cognitive and physical performance; reduced gait speed and grip strength; poorer performance in instrumental activities of daily living; frailty; and falls 21, 22, 23, 24, 25, 26, 27, 28.
The DBI can be tailored to evaluate appropriate prescribing in several settings, and has been validated internationally 22, 29. The index includes a range of medications with sedative properties, offering a broader evaluation than previous research that only examined single classes of sedatives 11. A recent systematic review identified the DBI as the most suitable tool for use in the evaluation of anticholinergic burden in longitudinal studies of older adults 30.
Failure to identify the side effects of anticholinergics in older people in the general population occurs due to reduced health expectations and misinterpretation of side effects as age related illness 13, a difficulty that is further compounded in the population with ID due to their additional difficulties in communication and diagnostic overshadowing 31, 32. Even though people with ID have a high drug burden, there is a lack of research into specific measurement of drug burden of sedative and anticholinergic medicines and guidance for intervention in this population. Given evidence to date of high sedative and anticholinergic burden in older adults with ID, the aim of this study was to evaluate and describe the cumulative drug burden for older people with ID.
In Ireland, similar to the practice in other developed countries, current policy emphasizes deinstitutionalization of people with ID. This specifically aims to encourage movement from congregated settings (i.e. housing units of 10 or more people) into community housing alongside the general population. While community‐based models appear to achieve better outcomes for people with ID, loss of specialist medical services and greater use of general primary care practices may mean the medical needs of people with ID as they age are not fully addressed 2, 33.
The primary objectives of this study were:
To create an inventory of medications with clinically significant anticholinergic and sedative activity for use in the study setting;
To determine the characteristics in an older population with ID that are associated with the drug burden measured by the DBI;
To describe the drug burden in older adults with ID and the most frequently reported therapeutic classes contributing to total burden; and
To examine the association between drug burden and potential adverse effects (daytime dozing, chronic constipation and falls) and Barthel index activities of daily living.
Methods
Study population
The data for this study were drawn from Wave 2 (2013/2014) of the Intellectual Disability Supplement to the Irish Longitudinal Study on Ageing (IDS‐TILDA); a large scale, nationally representative longitudinal study that examines the ageing of persons with ID. This study has been described in detail elsewhere 33, 34. The National Intellectual Disability Database (NIDD) collates information for all people in the Republic of Ireland with an ID eligible for or receiving services and it provided the sampling frame for Wave 1 of the study. A total of 1800 personal identification numbers were randomly selected by staff at NIDD. An invitation pack was sent to each potential participant with a consent form. When an individual was unable to provide consent independently, a family member/guardian could sign a letter of agreement for their family member to participate. Participants lived independently/with family, in community group homes or in residential settings. A total of 753 individuals participated in Wave 1 of the study (2009/2010). Participants were aged 40 years or older to account for the reduced life expectancy and presentation of older age conditions at a younger age in people with ID 35. Approval for the study was granted by Faculty of Health Sciences Research Ethics Committee in Trinity College Dublin. In addition, local and/or regional ethical committee approval was granted from each service provider (n = 138).
All living Wave 1 participants (n = 719) were invited to participate in Wave 2 (2013/2014). The study population with available medication data was 677 (95.6%; Figure 1).
Figure 1.
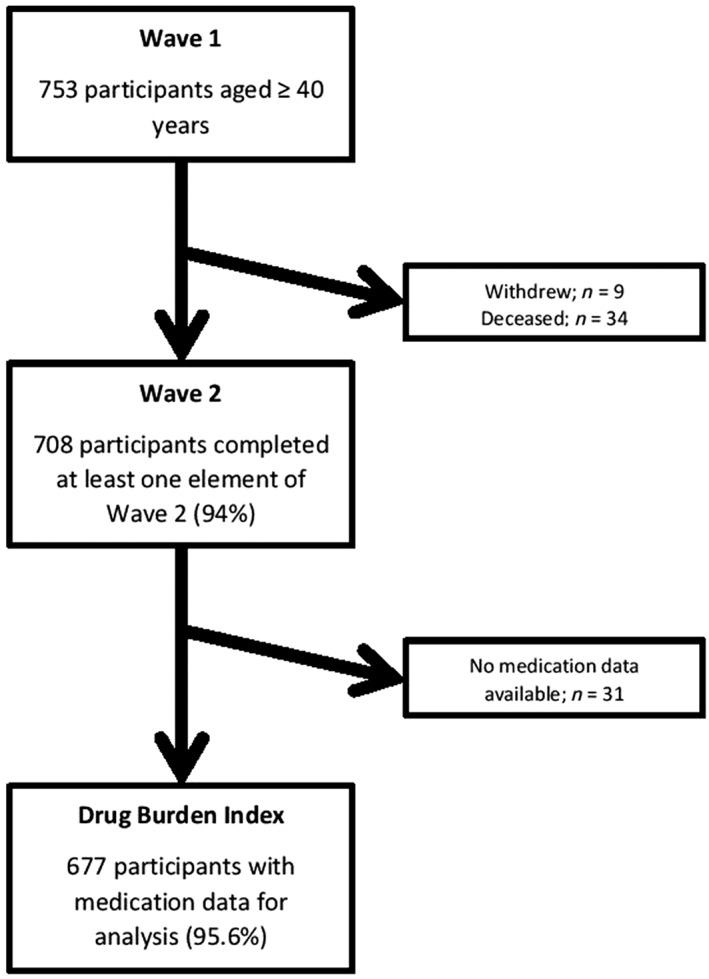
Flow chart of Intellectual Disability Supplement to the Irish Longitudinal Study on Ageing
Participants were invited to complete a preinterview questionnaire (PIQ) and these answers were confirmed in the face‐to‐face interview with a computer‐assisted personal interview. The PIQ contained a section on medication use. The design of this medication data section was improved from Wave 1 to Wave 2 of the study to collect more consistent, accurate information on dose and frequency data. To facilitate data capture further, the PIQ was sent to each participant/carer 1 week in advance of interview to allow time to access medical files if necessary. In the majority of cases (92.8%; n = 628), these data were recorded by proxy (key worker or family member known to participants for at least 6 months).
Field researchers came from a variety of backgrounds, but all had experience in working with people with ID. They were provided with three full training days in data collection and a further refresher day prior to beginning interviews. A pharmacist (M.O'D.) provided medication data capture training to all field researchers. Several interview styles were used: direct interview with the participant; assisted interview where a proxy assisted the participant; and interview where questions were answered by a proxy only, with or without the participant present.
In addition, a health assessment was included to obtain objective physical health measures, which included body mass index and Lunar Achilles GE quantitative ultrasound 33. The health assessments were conducted separate from the main interview by a registered nurse in intellectual disability who was a researcher in the IDS‐TILDA study. Each of the Wave 2 participants was invited to take part and to facilitate and support the individual's participation, adaptable and accessible materials, and methods were developed. This component has been described in detail elsewhere 33, 36, 37.
The STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) reporting guidelines for cross‐sectional studies were used 38, 39.
Medication exposure
Participants/proxies were asked: “Can you tell me what medications (including prescribed or over the counter) and supplements you take on a regular basis (like every day or every week)?”
The participant/proxy recorded their medication data by brand name/International Nonproprietary Name, dose, frequency, route of administration and date on which medicine was initiated in the PIQ.
Medications were categorized by Anatomical Therapeutic Chemical (ATC) code and verified by two pharmacists 40. Medicines recorded for indications which were not regular (for example: treatments for predental/medical procedures, prephlebotomy treatments, alternate month usage of antihistamines) were excluded from the calculations. As per previous studies, “as required” or “prn” medications were also excluded from DBI calculations because the contribution to the burden of anticholinergic and/or sedative activity from the intermittent use of these medications cannot be reliably estimated 21, 41, 42.
Medication inventory of sedative and anticholinergic medicines
Medications with clinically significant anticholinergic and/or sedative activity were identified by reference to relevant studies 1, 12, 20, 21, 25, 43 in addition to detailed examination of the licenced product information (Summary of Product Characteristics, SmPC) for the Republic of Ireland as available from the Health Products Regulatory Authority (HPRA) 44. The final list of 258 medications (117 anticholinergic, 141 sedative) was decided upon by consensus by three pharmacists (M.H., M.O. and J.O.; Figure 2).
Figure 2.
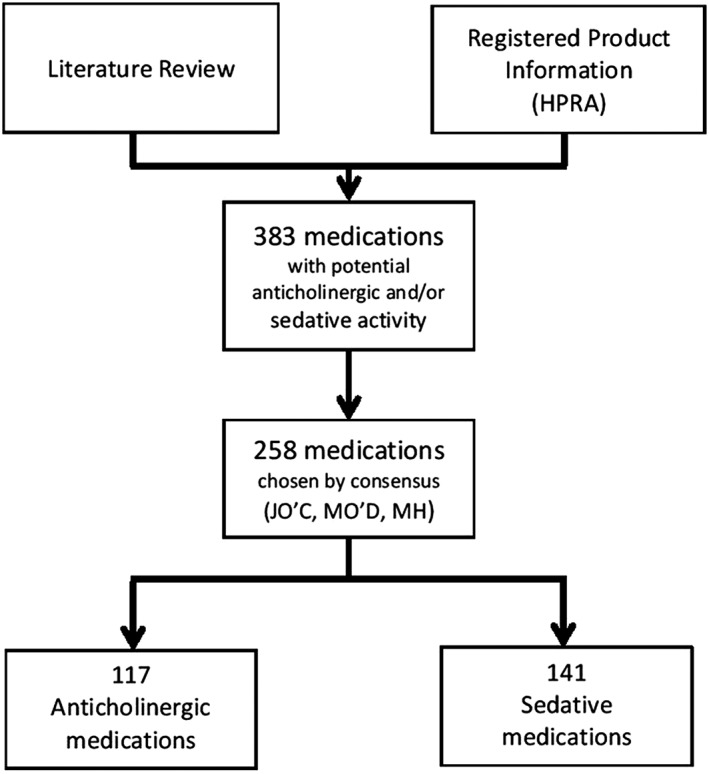
Development of medication inventory
Table 1 represents a summary of the search terms used to identify sedative potential during review of the SmPC. The list of search terms used was modelled on those used in the sedative load model: sedating, sedative, drowsiness, sleepiness, lassitude, exhaustion, tiresome, fatigability 20.
Table 1.
Sedative search terms
| Sedative search terms | Clinically significant adverse effect frequency |
|---|---|
|
• Fatigue
• Asthenia • Somnolence • Sedation • Drowsiness |
• Very common: occurring in >1/10 users • Common: occurring in >1/100 and <1/10 users |
This inventory of medications was categorized according to the nature of the burden. Medications with both anticholinergic and sedative effects were classified primarily as anticholinergic as per previous studies 21, 23, 45, 46.
Prochlorperazine was recoded from the ATC code N05AB04 (antipsychotics) to A04A (antiemetics and antinauseants) as the dosages reported by this population exclusively fell within the total daily dosage range used for treatment of Ménière's syndrome, nausea and vomiting (10–40 mg according to the Irish SmPC) as opposed to those indicated for psychotic episodes (75–100 mg).
Depot preparations administered less frequently than weekly (intramuscular flupentixol, fluphenazine, risperidone, zuclopenthixol) were also included due to their release profile.
Topical products were excluded for the purposes of this analysis due to insignificant systemic effects. The only exception was ophthalmological atropine, which was considered to have clinically significant systemic anticholinergic properties 47.
Inhaled anticholinergic preparations (ipratropium and tiotropium) were excluded due to low prevalence of users (n = 11; 1.6%) and high proportion of missing dose information for these preparations (n = 7; 64%).
Medicines for which the primary indication is acute seizure control (rectal diazepam, buccal midazolam) and migraine (sumatriptan), are used on an intermittent basis and were excluded as the DBI measures exposure to regularly used medications.
Calculation of DBI
The accepted standard approach to calculation of the DBI was used 21. Scores were calculated for each individual according to the formula:
where BAC indicates the anticholinergic burden and BS indicates the sedative burden 21. A model of pharmacological effect (E) was developed, which assumes that the anticholinergic and sedative burdens of individual drugs are additive linearly:
α is a proportionality constant, D is the daily dose and DR50 is the daily dose to achieve 50% of maximal contributory effect at steady state 22. As the DR50 of anticholinergic and sedative activity is not easily identifiable, the minimum daily dose (MDD) is used as a substitute. MDDs were selected based on the lowest effective daily dose listed in the Irish medicinal product authorization from the HPRA.
The final version of the DBI calculation is as follows:
where δ is the MDD.
DBI scores for participants were calculated as a continuous variable and then transformed into a categorical variable with three levels: DBI score 0 (no DBI exposure), DBI score 0 > 1 (low) and DBI score ≥ 1 (high). This reflects the categories used elsewhere in the literature 21, 23, 24, 25, 26, 27, 28, 42, 48, 49.
Missing dose information
When dose information was missing for a medication (n = 43), a median dosage figure was used. This is consistent with the strategy used in preceding DBI studies 21, 23, 24, 26. For one medication (diphenhydramine), no median dose was available, therefore the MDD was substituted.
Physical function measure
The Barthel index is a measure of assessing disability in those receiving rehabilitation for neuromuscular and musculoskeletal conditions and has become a reliable method of measuring function in older populations. It consists of an ordinal scale of 10 instrumental activities of daily living (range 0–20) 50, 51. It considers the level of dependence an individual has with regard to mobility, using stairs, dressing, bathing, grooming, feeding, transfer, toileting, and bladder and bowel continence. A modified form of Barthel index activities of daily living was created for this population (Table S1). Each participant was given a composite score between 0 and 20 based on their self‐/proxy‐report of difficulty experienced with each activity. Lower scores indicated poorer physical function. Barthel index scores were categorized as per Wade and Collin 52 classifications: total dependence (0–4), severe dependence (5–12), moderate dependence (13–18), mild dependence (19) and total independence (20).
Participants with two or more missing values (i.e. those who answered “don't know”, gave an unclear response or preferred not to answer) were excluded from the Barthel index evaluation (n = 42). This method of handling missing data was as per a previous study 53.
Covariates
Demographic characteristics: Covariates were sex, age range (44–49 years; 50–64 years; 65+ years), level of ID (mild; moderate; severe/profound), type of residence (independent, community group home, residential care) and behaviours that challenge (yes/no).
Level of ID is correlated by intelligence quotient scores as follows; mild (50–55 to approximately 70), moderate (35–40 to 50–55) and severe/profound (below 35–40), and correct classification was obtained from case notes for each participant 54. Those with unverified level of ID (n = 53) were excluded from regression analysis.
Community group homes are in a community setting with staff support for small groups of people with ID. Residential settings were defined as living arrangements where 10 or more people share a single living unit or where the living arrangements are campus based.
Behaviours that challenge were defined as any behaviour that: “(1) is a barrier to a person participating in and contributing to their community (including both active and passive behaviours); (2) undermines directly or indirectly a person's rights, dignity or quality of life; and (3) poses a risk to the health and safety of a person and those with whom they live and work” 55. Behaviours that challenge were measured by response to a question on verbal aggression, physical aggression, destructive behaviour, self‐injurious behaviour or other behaviours that challenge. A more detailed description of the definition of behaviours that challenge is available in Supplementary Information Description SD1. Behaviours that challenge were included as a covariate to adjust for potential confounding by indication due to evidence that already exists in the literature reporting the extensive use of psychotropic medication in the management of behaviours that challenge in the ID population 18, 56, 57, 58.
-
Potential adverse effects: Three potential adverse effects were selected for assessment due to previous associations identified in the general population in DBI studies and in ACB studies of older adults with ID 1, 27.
Daytime dozing: This was identified by asking participants and/or proxies “How likely are you to doze off and fall asleep during the day?” Where a participant answered “most of the time” or “sometimes”, they were considered to have daytime dozing (yes), while “rarely” and “never” indicated no daytime sleep problem (no) 59.
Chronic constipation: Participants/proxies were asked “have you ever had a doctor's diagnosis of chronic constipation?” to which they answered “yes”, “no” or “don't know”.
Fall in the previous 12 months: Participants/proxies were asked “in the past year have you had any fall including a slip or trip in which you lost your balance and landed on the floor or ground or lower level?” to which they answered “yes”, “no” or “don't know”.
Functional comorbidity index: A modified functional comorbidity index (FCI; Table S2) was developed for the population and provided a continuous score between 0 and 16 by summing the presence of a reported doctor's diagnosis (in response to the question “have you ever had a doctor's diagnosis of…”) for the following conditions: arthritis; osteoporosis/osteopenia (self/proxy report of doctor's diagnosis and/or objective evidence from quantitative ultrasound); asthma; lung disease; angina; congestive heart failure (or heart disease); myocardial infarction; neurological disease; stroke or transient ischaemic attack; diabetes mellitus type I or II; upper gastrointestinal disease (e.g. ulcer, hernia, reflux); depression (unipolar or bipolar); anxiety or panic disorder; visual impairment (e.g. cataracts, glaucoma, macular degeneration); hearing impairment; overweight/obese (data obtained from health assessment). Similar modified versions of the FCI have been used in a number of previous DBI studies adapted for the populations being studied 24, 27, 45, 46, 60. Participants with two or more missing conditions were excluded from the FCI score evaluation, reflecting the method used in a previous study 53. Table 4 (multivariate regression analysis) and Figure 3 (analysis of covariance for Barthel index) were adjusted for comorbidities using the FCI.
-
Polypharmacy: This was measured as a categorical variable. Definitions were as follows:
-
➢
Excessive polypharmacy: Concurrent use of 10 or more different drugs.
-
➢
Polypharmacy: Use of five to nine drugs.
-
➢
No polypharmacy: Use of four drugs or fewer (included those taking no medicines) 2.
-
➢
Statistical analyses
Analyses were performed using Microsoft Excel 2010 (Microsoft Corporation) and Statistical Package for Social Sciences (SPSS) version 21.0 (IBM Corporation). Statistical significance was set at P < 0.05.
Descriptive statistics (percentages and 95% confidence intervals) described the characteristics of the study populations. Medians and interquartile range (IQR) were reported as the data were not normally distributed. Univariate analysis was used to examine the associations between the three DBI levels (0, 0 > 1, ≥1; the dependent variable) and the demographic and clinical variables. For the categorical variables, χ2 tests for independence were used to test for significant associations between the three DBI levels.
Multinomial logistic regression was carried out to identify the relationship between DBI scores of 0 > 1 and ≥1 and potential adverse effects. The reference category was set as those with DBI score = 0. The model was adjusted for demographic variables – age, gender, level of ID, type of residence, behaviours that challenge, comorbidities (FCI) and number of non‐DBI medicines; potential adverse effects included were daytime dozing, chronic constipation and fall in the previous 12 months. Those with unverified level of ID (n = 53) were excluded from the regression analysis. Those living independently (n = 102) or in community group homes (n = 298) were combined as a single group (n = 400) due to small numbers in the subgroups.
To test for multicollinearity between the independent factors, two strategies were employed. Variance inflation factors (VIF) and Spearman's correlation coefficients of the independent variables were examined. A VIF cut‐off of >2 was employed 61. If the VIF for one of the variables is >2, there is collinearity associated with that variable. All VIFs were below the threshold of 2. Spearman's correlation coefficients were interpreted using Dancy and Reidy's categorisation 62. Here, correlations of ±1 are interpreted as a perfect correlation, values between ±0.7 to ±0.9 are interpreted as strong correlations, values in the range ± 0.4 to ±0.6 are categorised as moderate correlations, values between ±0.1 to ±0.3 are weak correlations and a value of 0 is zero correlation, implying that there is no correlation. All correlations fell below 0.4, indicating only weak correlations, and were thus not of concern.
Analysis of covariance (ANCOVA) was used to compare the adjusted mean of the continuous variable for the Barthel index between subjects exposed to three different levels of DBI ranges [means were adjusted for sex, age, level of ID, behaviours that challenge, comorbidities (FCI) and history of falls]. This reflected the practice used in a similar study of DBI and physical function measures 24.
Sample size calculation for the logistic regression was based on the guideline of Peduzzi et al. 63: for a minimum number of cases (n) needed for the study; n = 10 k/p, where p is the smallest of the proportions of negative or positive cases in the population and k is the number of covariates (independent variables) 63. For the multinomial logistic regression, there were 10 covariates and the proportion of negative cases (DBI 0) was 0.21, therefore a minimum sample size of n = 476 was needed, which our sample size (n = 484) exceeded.
Missing data
Missing data for the Barthel index and FCI analyses are described in Table S3. Higher rates of missing data were observed in those with mild level of ID (26.8%; n = 40), those living in independent settings (32.4%; n = 33) and those in the younger age group (44–49 years; 24.6%; n = 46) for the FCI. Fewer data were missing overall for Barthel index and the profile was slightly different, with more people with moderate ID (7.0%; n = 20), living in community group homes (7.7%; n = 23) and in the older age group (65+ years; 7.7%; n = 11) missing Barthel index scores.
Results
Of the 708 participants who partook in Wave 2, 677 (95.6%) had available medication data. Of the study population (n = 677), 56.1% (n = 380) were female and 44.1% (n = 298) lived in a community group home (Table 2). The median number of medications per participant was 6.00 (IQR 6.00). Of the 677 participants, 95.1% (n = 644) reported taking medicines. Overall, 78.6% (n = 532) of the population were exposed to medications with anticholinergic and/or sedative effects (DBI Score > 0) and the median number of DBI medications was 2.00 (IQR 3.00; Table 2). 51.3% (n = 347) of participants were exposed to anticholinergic medications only and 32.1% (n = 217) were exposed to sedative medications only. Median number of comorbidities (FCI) was 3.00 (IQR 2.00) and 41.2% (n = 264) of participants reported having neurological disease. With respect to potential adverse effects, 34.9% (n = 232) reported daytime dozing, 38.4% (n = 257) reported chronic constipation and 28.5% (n = 190) reported having a fall in the previous 12 months. 52.0% (n = 352) reported behaviours that challenge. Level of polypharmacy was high, with 62.2% (n = 421) reporting taking five or more medications. 21.4% (n = 145) of participants had no exposure to anticholinergic or sedative medications (DBI score = 0), while 24.4% (n = 165) had a DBI score 0 > 1 and 54.2% (n = 367) had a score of 1 or higher.
Table 2.
Baseline characteristics of the study population
| Characteristics |
Number of participants
% (95% CI) |
|---|---|
| Sex (n = 677) | |
| Male (n = 297) | 43.9 (40.16–47.64) |
| Female (n = 380) | 56.1 (52.36–59.84) |
| Age (n = 676) | |
| 44–49 years (n = 187) | 27.6 (24.23–30.97) |
| 50–64 years (n = 347) | 51.3 (47.53–55.07) |
| 65 years + (n = 142) | 21.0 (17.93–24.07) |
| Level of ID (n = 624) | |
| Mild (n = 149) | 23.9 (20.55–27.25) |
| Moderate (n = 287) | 44.0 (40.11–47.89) |
| Severe/profound (n = 188) | 30.1 (26.5–33.7) |
| Type of residence (n = 676) | |
| Independent (n = 102) | 15.0 (12.31–17.69) |
| Community group home (n = 298) | 44.1 (40.36–47.84) |
| Residential care (n = 276) | 40.8 (37.1–44.5) |
| Barthel index (n = 635) | |
| Total independence (n = 113) | 17.8 (14.9–21.0) |
| Mild dependence (n = 50) | 7.9 (5.9–10.2) |
| Moderate dependence (n = 274) | 43.1 (39.3–47.1) |
| Severe dependence (n = 112) | 17.6 (14.8–20.8) |
| Total dependence (n = 86) | 13.5 (11.0–16.5) |
| Median (IQR) FCI Score (n = 532) | 3.00 (2.00) |
| Neurological disease (n = 641) | 41.2 (37.3–45.1; n = 264) |
| Depression (n = 673) | 28.4 (25.0–32.0; n = 191) |
| Anxiety (n = 673) | 19.2 (16.3–22.3; n = 129) |
| Daytime dozing (n = 665) | 34.9 (31.3–38.6; n = 232) |
| Chronic constipation (n = 669) | 38.4 (34.7–42.2; n = 257) |
| Fall in previous 12 months (n = 667) | 28.5 (25.07–31.93; n = 190) |
| Behaviours that challenge (n = 677) | 52.0 (48.2–55.8; n = 352) |
| Polypharmacy (n = 677) | |
| No polypharmacy (n = 256) | 37.8 (34.1–41.6) |
| Polypharmacy (n = 258) | 38.1 (34.4–41.9) |
| Excessive polypharmacy (n = 163) | 24.1 (20.9–27.5) |
| Exposure to any medications (n = 677) | 95.1 (93.47–96.73; n = 644) |
| Median (IQR) no. of medications | 6.00 (6.00) |
| Median (IQR) no. of DBI medications | 2.00 (3.00) |
| Median (IQR) no. of non‐DBI medications | 4.00 (5.00) |
| Exposure to DBI medications | |
| Anticholinergic only (n = 347) | 51.3 (47.5–55.1) |
| Sedative only (n = 217) | 32.1 (28.6–35.6) |
| Total (anticholinergic and/or sedative; n = 532) | 78.6 (75.3–81.6) |
| DBI score | |
| 0 (n = 145) | 21.4 (18.31–24.49) |
| 0 > 1 (n = 165) | 24.4 (21.16–27.64) |
| ≥ 1 (n = 367) | 54.2 (50.45–57.95) |
| Median (IQR) DBI score | 1.10 (±1.73) |
| Median (IQR) DBA score | 1.00 (±1.13) |
| Median (IQR) DBS score | 0.69 (±0.79) |
| Range (min–max) | 0–5.47 |
CI, confidence interval; ID, intellectual disabilities; IQR, interquartile range; FCI, functional comorbidity index; DBI, drug burden index; DBA, anticholinergic component of drug burden index; DBS, sedative component of drug burden index.
Table 3 displays the exposure of participants to at least one member of the individual therapeutic drug classes in descending order and classified by anticholinergic or sedative status. Overall, 44% (n = 298) of participants were exposed to one or more antipsychotic medications with risperidone being most common (15.7%, n = 106). The most commonly reported sedative class was the ATC class N05C (hypnotics and sedatives), and the most commonly reported drug within this class was zopiclone, which 2.8% (n = 19) of the participants reported taking on a regular basis. Valproic acid was the most commonly reported drug overall, with 19.4% (n = 131) of participants exposed to this medication. Other therapeutic classes reported by <5% in decreasing prevalence included in Table S4.
Table 3.
Prevalence of exposure to drug classes with anticholinergic and sedative activity
| Therapeutic DBI drug class and most frequently reported medicine within each class | ATC code | % of population exposed, (95% CI; n) |
|---|---|---|
| Anticholinergic | ||
| Antipsychotics | N05A | 44.0 (40.26–47.74; n = 298) |
| Risperidone | N05AX08 | 15.7 (12.9–18.4; n = 106) |
| Antiepileptics | N03A | 42.4 (38.68–46.12; n = 287) |
| Valproic acid | N03AG01 | 19.4 (16.4–22.3; n = 131) |
| Antidepressants | N06A | 27.8 (24.43–31.17; n = 188) |
| Escitalopram | N06AB10 | 5.8 (4.0–7.5; n = 39) |
| Anticholinergic agents | N04A | 13.0 (10.47–15.52; n = 88) |
| Biperiden | N04AA02 | 8.7 (6.6–10.8; n = 59) |
| Anxiolytics | N05B | 11.5 (9.1–13.9; n = 78) |
| Diazepam | N05BA01 | 6.1 (4.3–7.9; n = 41) |
| Diuretics | C03(A, C, D) | 7.4 (5.43–9.37; n = 55) |
| Furosemide | C03CA01 | 5.0 (3.4–6.7; n = 34) |
| Antihistamines | R06A | 5.5 (3.78–7.22; n = 37) |
| Cetirizine | R06AE07 | 1.6 (0.7–2.6; n = 11) |
| Sedative | ||
| Hypnotics and sedatives | N05C | 7.1 (5.17–9.03; n = 48) |
| Zopiclone | N05CF01 | 2.8 (1.6–4.1; n = 19) |
| Antidementia drugs | N06D | 3.4 (2.03–4.77; n = 23) |
| Donepezil | N06DA02 | 2.2 (1.1–3.3; n = 15) |
| Drugs for benign prostate hypertrophy | G04C | 1.8 (0.8–2.8; n = 12) |
| Tamsulosin | G04CA02 | 1.8 (0.8–2.8; n = 12) |
Other therapeutic classes reported by <5% listed in Supporting Information Table S4.
Table S5 displays the univariate analysis of DBI score and specific population parameters.
Multivariate regression analysis of the relationship between DBI scores and potential adverse effects (Table 4), adjusted for sex, age, type of residence, level of ID, behaviours that challenge, comorbidities (FCI) and number of non‐DBI medicines, revealed that daytime dozing, chronic constipation and history of falls were not significantly associated with DBI score > 0 (P = 0.764 and 0.094; P = 0.486 and 0.102; P = 0.168 and 0.731, respectively).
Table 4.
Multivariate analysis of drug burden index scores and potential adverse effects (n = 484)a
| DBI 0 > 1 | DBI ≥ 1 | |||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Daytime dozing | ||||
| No | 1 (reference) | 1 (reference) | ||
| Yes | 1.108 (0.568;2.162) | 0.764 | 1.670 (0.916;3.044) | 0.094 |
| Chronic constipation | ||||
| No | 1 (reference) | 1 (reference) | ||
| Yes | 1.276 (0.643;2.532) | 0.486 | 1.679 (0.902;3.123) | 0.102 |
| Fall in the previous 12 months | ||||
| No | 1 (reference) | 1 (reference) | ||
| Yes | 0.611 (0.303;1.231) | 0.168 | 1.113 (0.605;2.046) | 0.731 |
Reference category: DBI 0. P < 0.05 is significant, all significant factors in bold. Cox and Snell r2 = 0.245, Nagelkerke r2 = 0.284. Data are adjusted odds ratio (OR) with 95% confidence interval (CI). Model adjusted for sex, age, level of intellectual disabilities, type of residence, behaviours that challenge, comorbidities (functional comorbidity index) and number of non‐drug burden index medicines.
Figure 3 displays adjusted means for Barthel index activities of daily living.
Figure 3.
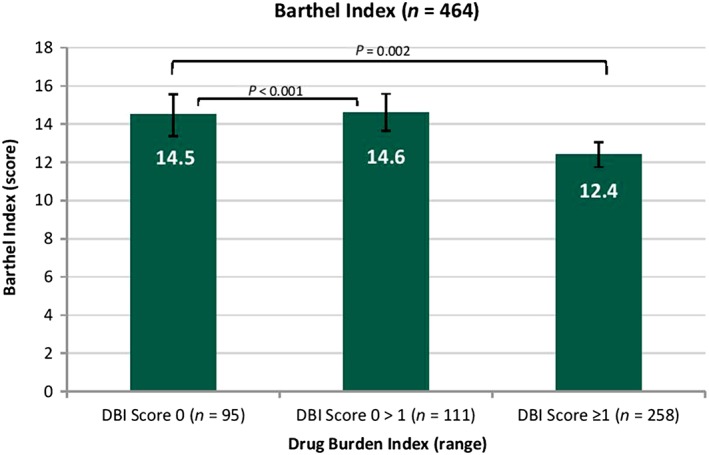
Analysis of covariance for the association of adjusted means of Barthel index with increasing drug burden index. Means are adjusted for sex, age, level of intellectual disability, comorbidities (functional comorbidity index), behaviours that challenge and history of falls. Drug burden index is grouped into three intervals (0, 0 > 1 and ≥1). Error bars show 95% confidence interval. Barthel index instrumental activities of daily living:\ lower score indicates worse function
Significantly lower scores in Barthel index activities of daily living were identified for those with a DBI score of ≥1 (P = 0.002; mean score 12.4, 95% CI 11.7–13.0) compared to those with DBI 0 (mean score 14.5, 95% CI 13.4–15.6) after adjusting for cofounders (gender, age, level of ID, comorbidities (FCI), behaviours that challenge and history of falls). There was also significant difference in performance for those with a DBI score of 0 > 1 (P < 0.001; mean score 14.6, 95% CI 13.6–15.6) compared to those with no DBI exposure.
Discussion
Principal findings
To our knowledge this is the first study to describe DBI in a representative population of older adults with an intellectual disability. Our findings reveal that in contrast to existing studies on older adults without ID, older people with ID had higher cumulative exposure to both sedative and anticholinergic medicines; over three‐quarters of the study population were exposed to at least one anticholinergic or sedative medication (DBI Score > 0; Table 2). This reflects the higher levels of multimorbidity in this population, in particular mental health and neurological morbidity 4. After adjusting for confounders, multivariate analysis identified that daytime dozing, chronic constipation and history of falls were not significantly associated with DBI score (P > 0.05; Table 4). Analysis of covariance identified higher levels of dependence in Barthel index activities of daily living as DBI scores increased (P < 0.05; Figure 3).
Comparison with other studies
The DBI scores were much higher in our study than in published studies of older adults in the general population (Table S6). Patterns of mutimorbidity in people with ID differ substantially from those in people without ID 3, 64. In particular, depression rates varied between 9–22% in older adults without ID in preceding DBI studies, while lifetime prevalence was reported at 28% in IDS‐TILDA participants. Patterns of neurological disease were also different. Epilepsy was the predominant neurological condition in IDS‐TILDA (36%) 33, levels of epilepsy are higher in people with ID compared to those in the general population 65. Other DBI studies most commonly reported dementia or cognitive impairment 24, 26, 27, 46, 48, 53, 60, 66 both of which are difficult to assess in people with ID (Table S6).
As a result, there is a noticeable contrast in exposure and contributing therapeutic classes observed in those with ID compared to older adults without ID. Antipsychotic and antiepileptic medicines are most prevalent in our cohort (44% and 42%, respectively, Table 3) and are frequently associated with anticholinergic effects 1, while only 1–10% of participants in previous DBI studies of older adults have reported exposure to antipsychotics and 2% reported exposure to antiepileptics. 24, 27. In the population without ID, sedative exposure was higher in two of the five studies, while anticholinergic exposure was substantially lower compared to the anticholinergic burden (51%) in this population (Table S6).
Prevalence of therapeutic drug classes
Antiepileptic medications (ATC N03A) accounted for over one‐quarter of the overall burden (Table 3), in contrast to studies of drug burden in the existing literature that do not report antiepileptic medication as a significant contributor to drug burden 21, 23, 24, 25, 26, 27, 28, 42, 48, 49. In addition to causing anticholinergic and sedative effects, antiepileptic medications also require careful monitoring and have the potential to introduce other clinical implications, including drug–drug interactions 67. Multiple antipsychotic use is prevalent in this population and anticholinergics such as biperiden are often co‐prescribed for movement disorders 1 and these add significantly to the anticholinergic burden along with antidepressants and anxiolytics (Table 3). The concurrent use of antipsychotics and anticholinergics requires caution 68 and has been associated with constipation and laxative use 1 in an earlier wave of this cohort. Hypnotics and sedatives were the dominant component of the sedative burden (7.1% of population exposed) while antidementia drugs were less prevalent (3.4%). However, with drugs from 38 therapeutic classes (Table 3 and Supporting Information Table S4) contributing to the DBI scores of the participants, the sources of the burden reflect the diverse range of drugs used in people with ID 64.
Potential adverse effects and drug burden
Although levels of daytime dozing were high (35%), it was not significantly associated with DBI score after adjusting for confounding factors (DBI 0 > 1, P = 0.764; DBI ≥ 1, P = 0.094; Table 4). The effect of medications on sleep in adults with ID not clear cut. It has been reported that the circadian sleep–wake rhythm in older adults with ID is less stable and more fragmented than older adults without ID. Higher age, dementia, depression and epilepsy have been associated with this disturbed sleep cycle, while no independent association was found with taking antiepileptic, antidepressant, antipsychotic or benzodiazepine medications 69. In contrast, a systematic review of sleep disturbance in ID identified a number of studies that found associations between medication use and sleep problems 70. These studies, however, examined early morning waking, broken sleep, snoring and nocturnal incontinence rather than daytime dozing. As sleep patterns are different in this population and a number of different factors may be influencing levels of daytime sedation, and, since the DBI combines sedative and anticholinergic measures, it is not surprising that a conclusive association was not found. Further investigation is required to examine associations between DBI, its sedative and anticholinergic components and other measures of sleep quality in this population.
After adjusting for confounders, we identified no association between chronic constipation and higher DBI scores (DBI 0 > 1, P = 0.486; DBI ≥ 1, P = 0.102; Table 4). However, it is noteworthy that almost of the ten reporting constipation were exposed to anticholinergic and/or sedative medication, and almost two‐thirds had a DBI score of ≥1 (Supporting Information Table S5). Overall, there is a high prevalence of constipation among the population with ID (38%). Although the cause of constipation is multifactorial, it is acknowledged that medications with anticholinergic action contribute to constipation in older people 71. It has been found that medications are strongly associated with the presence of constipation in older adults with ID, in particular antiepileptic medications and antipsychotic medications, due to slowing down the transit times of the large bowel as a result of their anticholinergic activity 1, 72. People reporting constipation generally also report lower health‐related quality of life 73, 74, 75, thus the impact of the anticholinergic medication component of the DBI should not be underestimated in this area.
History of falls was not significant after multivariate regression in our population of older adults with ID (DBI 0 > 1, P = 0.168; DBI ≥ 1, P = 0.731, Table 4), which is in contrast to the findings of DBI studies in older adults without ID 27, 49. It is possible that the susceptibility to falls from anticholinergic and sedative medications may be different in older adults with ID as the long‐term use of these medications in this population may result in the absence of the starting effect, which has been associated with falls in adults who commence these types of medications later in life 76. The susceptibility to anticholinergic and/or sedative effects may vary with age and with the cause of ID and pattern of multimorbidity in this cohort. In addition, seizure disorders have been identified as one of the major risk factors for falls in adults with ID 77, 78, thus antiepileptic medications, despite being anticholinergic and sedative in nature, may provide seizure control, which could affect the relationship between DBI and rate of falls differently to that observed in the general population. Individuals with higher dependency and/or multimorbidity may be monitored more closely for falls and risk of falls, or may be immobile due to factors such as poor health and level of disability.
It is worth noting that confidence intervals across all the categories were quite wide, indicating that there was still wide variation remaining after adjusting for confounding factors.
Factors associated with drug burden and physical function measures
Higher DBI scores were significantly associated with higher levels of dependency in Barthel index activities of daily living after adjusting for relevant confounders including level of ID in this study (P < 0.05, Figure 3). This is similar to the findings of a study of DBI and instrumental activities of daily living in older Australian men 24. Compared to older adults without ID, people with ID may have lower scores in the Barthel index, which in turn may further be affected by DBI. However, as this is a cross‐sectional study, it is not possible to establish causality, but with repeated assessments over several waves of the cohort, further analysis will be possible.
Impact of findings on practice
Difficulties in patient assessment, including, but not limited to, problems with communication, staff shortages and time constraints, can hinder care and leave individuals with ID vulnerable to prescribing that is not regularly reviewed, the prescribing cascade or initiation of inappropriate drugs 31. Devolving responsibility and fragmentation of care have been identified as barriers to deprescribing anticholinergic and sedative medications in older adults without ID 79. This is particularly relevant to older people with ID, due to the variety of medical conditions experienced. Higher rates of epilepsy, as outlined above, require the attention of specialist care, but unless there is adequate multidisciplinary involvement in review, deprescribing cannot take place, as general practitioners feel specialist prescribers must conduct the deprescribing and vice versa 79, 80.
People with ID in older age profile may experience different susceptibility to certain adverse effects of anticholinergic and sedative medications compared to the general population. A recent study in the UK found that people with ID are more likely to experience movement‐related side effects from antipsychotic medications 81. The effect of the drug burden itself may compromise individuals who have difficulties expressing their symptoms and whose expression of adverse drug effects may be limited. This may make it more challenging for carers and clinicians to assess and monitor these patients effectively. Therefore, the DBI is a valuable tool to review these medications regimens 82.
The association of drug burden with poor performance in Barthel index activities of daily living indicates the potential impact this burden has on the quality of life of older adults with intellectual disabilities. Further longitudinal examination of this burden is necessary. Evidence here should encourage greater attention to reducing polypharmacy, selection of alternative treatment options and finding means to systematically reduce sedative and anticholinergic drug burden.
Recognition of the impact of anticholinergic and sedative medications on physical and cognitive function, collaboration between patients, carers and healthcare professionals and reaction to deprescribing triggers have all been acknowledged as facilitators for optimising medication use in people without ID 79. Indeed, targeted deprescribing of drugs with anticholinergic and sedative effects is already underway in the older age population without ID, guided by the DBI 83.
While regular medication reviews are part of good case management, this is a population for whom review is often inhibited by difficulties in communication, high levels of morbidity and polypharmacy, and numerous specialist and nonspecialist prescribers. The DBI could be a screening tool to trigger medication review for older adults with ID. It can alert the prescriber to the existing status of the individual, make them mindful of the current burden, trigger a more frequent review of medications, allow for possible rationalisation of therapy, and inform further prescribing.
The potential of the DBI tool as a trigger for deprescribing, and an enabler for medication review of people with ID, who often cannot speak for themselves, should be investigated. Dissemination of the findings of this study, education of professionals, patients and carers in optimizing the use of anticholinergics and sedatives, encouraging the identification of adverse effects from these medicines, and recognizing the absence of symptoms can contribute to the optimizing of medication use in this population. Longitudinal follow‐up is required to establish fully the association of DBI with this population, as this study provides only baseline data, which may be further investigated in future waves of the study.
Strengths and limitations
Our study has five important strengths. First, the use of a large, nationally representative sample of older adults with ID selected at random in Ireland allowed sufficient power for multivariate analysis and is representative of the older population with ID in Ireland. Second, comprehensive medication data were recorded for the majority of Wave 2 participants (95.6%) and this medication data were cross‐checked by interviewers. While collection of medication data was carried out by nonpharmacists, the training provided by a pharmacist (M.O.) and design of the medication data section facilitated high quality data capture. Participants and/or their proxies received the medication data section in advance of the face‐to‐face interview, allowing time to consult medical files to capture this information accurately. Third, detailed assessment of health characteristics provided data on potential confounding factors for our analysis. Fourth, we used the DBI, which is a score that has been validated across a number of studies internationally and is a robust measure of anticholinergic and sedative drug effects. It also considers the dose each participant is exposed to, which is useful as adverse effects may often be dose‐related. A comprehensive approach was used to create the DBI inventory for use in an Irish population, and this list was both developed and approved by consensus of three pharmacists. Fifth, objective measures of physical performance were selected for examining physical function outcomes.
There are a few limitations: as this is a cross‐sectional observational study, we can only describe the associations between DBI scores, potential adverse effects and physical function outcomes. This correlation does not imply causality, particularly with respect to physical function. While it is not possible to establish the effect of DBI scores on functional decline at present, this study offers the scope for further longitudinal analysis of data from IDS‐TILDA by identifying the baseline levels of exposure and function in this population.
Although bias was removed where possible in our multivariate analysis by making adjustment for confounders, residual confounding factors may remain.
With respect to the multivariate regression and Barthel index measure of dependency, it should be noted that the numbers of participants with all information available is restricted to 70% of the overall population due to missing data of participants who were unable to complete these elements of the interview. Thus, interpretation of these data should be conservative as it may not be fully representative of the entire population. The highest rates of missing data were observed in those with mild/moderate level of ID and those living independently or in community group homes.
In conclusion, this study has highlighted extensive use of medications with both anticholinergic and sedative properties in older adults with intellectual disabilities. This is the first time a study has examined the combined anticholinergic and sedative exposure of a cohort of people with ID. In addition, this high burden has been shown to have an association with higher dependency in Barthel index activities of daily living. Use of the DBI as a tool for clinicians could help guide prescribing practice and multidisciplinary involvement would be essential for the development of optimal medicine regimens and improvement of health outcomes for older adults with ID.
Competing Interests
There are no competing interests to declare.
The authors would like to thank the people with ID who participated in this study, their families, carers, the services involved, the IDS‐TILDA Scientific Advisory Committee and the Intellectual Disability Consultative Groups for their support. They would like to acknowledge the contributions of Dr Rachael Carroll. The authors would also like to acknowledge funding for the IDS‐TILDA study from the Health Research Board and the Department of Health. The views expressed are those of the authors and are not necessarily those of the Department of Health, The Health Research Board or Trinity College Dublin.
This research was funded by the Department of Health in Ireland and managed by the Health Research Board. The lead author (J.O.) received funding for a PhD from the Trinity College Dublin Studentship, Dean's Fund and Non‐Foundation Scholarship. M.O. received funding for this study from the Dean's Fund Award, Faculty of Health Sciences, Trinity College Dublin. The funding bodies did not play a role in the study design or writing of the manuscript.
Contributors
J.O., E.B., N.M., C.O., C.D., P.M., M.M., M.C.H. and M.O. contributed to the overall conception and design of the study. J.O. and C.D. undertook the data extraction. J.O. and M.O. carried out the statistical analyses of the study; J.O. wrote the first draft of this manuscript. M.O., P.M., M.M., C.O., C.D., N.M., E.B. and M.C.H. revised the manuscript. All authors, external and internal, had full access to all of the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis. All authors contributed to the interpretation of results and drafting of this manuscript. All authors read and approved the final manuscript. J.O. and M.M. are the guarantors.
Supporting information
Supporting info item
Table S1 Modified Barthel index
Table S2 Modified functional comorbidity index
Table S3 Description of missing physical function scores
Table S4 Other therapeutic classes reported by <5% in decreasing prevalence
Table S5 Drug burden index binary table
Table S6 Review of existing drug burden index literature – observational studies
O'Connell, J. , Burke, É. , Mulryan, N. , O'Dwyer, C. , Donegan, C. , McCallion, P. , McCarron, M. , Henman, M. C. , and O'Dwyer, M. (2018) Drug burden index to define the burden of medicines in older adults with intellectual disabilities: An observational cross‐sectional study. Br J Clin Pharmacol, 84: 553–567. doi: 10.1111/bcp.13479.
References
- 1. O'Dwyer M, Maidment ID, Bennett K, Peklar J, Mulryan N, McCallion P, et al Association of anticholinergic burden with adverse effects in older people with intellectual disabilities: an observational cross‐sectional study. Br J Psychiatry 2016; 209: 1–7. [DOI] [PubMed] [Google Scholar]
- 2. O'Dwyer M, Peklar J, McCallion P, McCarron M, Henman M. Factors associated with polypharmacy and excessive polypharmacy in older people with intellectual disability differ from the general population: a cross‐sectional observational nationwide study. BMJ Open 2016; 6: e010505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Santosh PJ, Baird G. Psychopharmacotherapy in children and adults with intellectual disability. Lancet 1999; 354: 233–242. [DOI] [PubMed] [Google Scholar]
- 4. McCarron M, Swinburne J, Burke E, McGlinchey E, Carroll R, McCallion P. Patterns of multimorbidity in an older population of persons with an intellectual disability: results from the intellectual disability supplement to the Irish longitudinal study on aging (IDS‐TILDA). Res Dev Disabil 2013; 34: 521–527. [DOI] [PubMed] [Google Scholar]
- 5. Glass J, Lanctôt KL, Herrmann N, Sproule BA, Busto UE. Sedative hypnotics in older people with insomnia: meta‐analysis of risks and benefits. BMJ 2005; 331: 1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Weiner DK, Hanlon JT, Studenski SA. Effects of central nervous system polypharmacy on falls liability in community‐dwelling elderly. Gerontology 1998; 44: 217–221. [DOI] [PubMed] [Google Scholar]
- 7. Rubenstein LZ. Falls in older people: epidemiology, risk factors and strategies for prevention. Age Ageing 2006; 35 (S2): ii37–ii41. [DOI] [PubMed] [Google Scholar]
- 8. Johnell K, Fastbom J. Gender and use of hypnotics or sedatives in old age: a nationwide register‐based study. Int J Clin Pharmacol 2011; 33: 788–793. [DOI] [PubMed] [Google Scholar]
- 9. Levy HB. Non‐benzodiazepine hypnotics and older adults: what are we learning about zolpidem? Expert Rev Clin Pharmacol 2014; 7: 5–8. [DOI] [PubMed] [Google Scholar]
- 10. Vestergaard P, Prieto‐Alhambra D, Javaid MK, Cooper C. Fractures in users of antidepressants and anxiolytics and sedatives: effects of age and dose. Osteoporos Int 2013; 24: 671–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gnjidic D, Le Couteur DG, Hilmer SN, Cumming RG, Blyth FM, Naganathan V, et al Sedative load and functional outcomes in community‐dwelling older Australian men: the CHAMP study. Fundam Clin Pharmacol 2014; 28: 10–19. [DOI] [PubMed] [Google Scholar]
- 12. Peklar J, O'Halloran AM, Maidment ID, Henman MC, Kenny RA, Kos M. Sedative load and frailty among community‐dwelling population aged ≥65 years. J Am Med Dir Assoc 2015; 16: 282–289. [DOI] [PubMed] [Google Scholar]
- 13. Mintzer J, Burns A. Anticholinergic side‐effects of drugs in elderly people. J R Soc Med 2000; 93: 457–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Feinberg M. The problems of anticholinergic adverse effects in older patients. Drugs Aging 1993; 3: 335–348. [DOI] [PubMed] [Google Scholar]
- 15. Molyneux P, Emerson E, Caine A. Prescription of psychotropic medication to people with intellectual disabilities in primary health‐care settings. J Appl Res Intellect Disabil 1999; 12: 46–57. [Google Scholar]
- 16. de Kuijper G, Hoekstra P, Visser F, Scholte FA, Penning C, Evenhuis H. Use of antipsychotic drugs in individuals with intellectual disability (ID) in the Netherlands: prevalence and reasons for prescription. J Intellect Disabil Res 2010; 54: 659–667. [DOI] [PubMed] [Google Scholar]
- 17. Stolker JJ, Koedoot PJ, Heerdink ER, Leufkens HG, Nolen WA. Psychotropic drug use in intellectually disabled group‐home residents with behavioural problems. Pharmacopsychiatry 2002; 35: 19–23. [DOI] [PubMed] [Google Scholar]
- 18. Robertson J, Emerson E, Gregory N, Hatton C, Kessissoglou S, Hallam A. Receipt of psychotropic medication by people with intellectual disability in residential settings. J Intellect Disabil Res 2000; 44: 666–676. [DOI] [PubMed] [Google Scholar]
- 19. Chitty KM, Evans E, Torr JJ, Iacono T, Brodaty H, Sachdev P, et al Central nervous system medication use in older adults with intellectual disability: results from the successful ageing in intellectual disability study. Aust N Z J Psychiatry 2016; 50: 352–362. [DOI] [PubMed] [Google Scholar]
- 20. Linjakumpu T, Hartikainen S, Klaukka T, Koponen H, Kivelä SL, Isoaho R. A model to classify the sedative load of drugs. Int J Geriatr Psychiatry 2003; 18: 542–545. [DOI] [PubMed] [Google Scholar]
- 21. Hilmer SN, Mager DE, Simonsick EM, Cao Y, Ling SM, Windham G, et al A drug burden index to define the functional burden of medications in older people. Arch Intern Med 2007; 167: 781–787. [DOI] [PubMed] [Google Scholar]
- 22. Kouladjian L, Gnjidic D, Chen TF, Mangoni AA. Drug burden index in older adults: theoretical and practical issues. Clin Interv Aging 2014; 9: 1503–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hilmer SN, Mager DE, Simonsick EM, Ling SM, Windham BG, Harris TB, et al Drug burden index score and functional decline in older people. Am J Med 2009; 122: 1142–1149.e1‐2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gnjidic D, Cumming RG, Le Couteur DG, Handelsman DJ, Naganathan V, Abernethy DR, et al Drug burden index and physical function in older Australian men. Br J Clin Pharmacol 2009; 68: 97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gnjidic D, Hilmer SN, Blyth FM, Naganathan V, Cumming RG, Handelsman DJ, et al High‐risk prescribing and incidence of frailty among older community‐dwelling men. Clin Pharmacol Ther 2012; 91: 521–528. [DOI] [PubMed] [Google Scholar]
- 26. Gnjidic D, Le Couteur DG, Naganathan V, Cumming RG, Creasey H, Waite LM, et al Effects of drug burden index on cognitive function in older men. J Clin Psychopharmacol 2012; 32: 273–277. [DOI] [PubMed] [Google Scholar]
- 27. Wilson NM, Hilmer SN, March LM, Cameron ID, Lord SR, Seibel MJ, et al Associations between drug burden index and falls in older people in residential aged care. J Am Geriatr Soc 2011; 59: 875–880. [DOI] [PubMed] [Google Scholar]
- 28. Gnjidic D, Hilmer SN, Hartikainen S, Tolppanen A‐M, Taipale H, Koponen M, et al Impact of high risk drug use on hospitalization and mortality in older people with and without Alzheimer's disease: a National Population Cohort Study. PLoS ONE 2014; 9: e83224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Salahudeen MS, Hilmer SN, Nishtala PS. Comparison of anticholinergic risk scales and associations with adverse health outcomes in older people. J Am Geriatr Soc 2015; 63: 85–90. [DOI] [PubMed] [Google Scholar]
- 30. Cardwell K, Hughes CM, Ryan C. The association between anticholinergic medication burden and health related outcomes in the 'Oldest Old': a systematic review of the literature. Drugs Aging 2015; 32: 835–848. [DOI] [PubMed] [Google Scholar]
- 31. Krahn GL, Hammond L, Turner A. A cascade of disparities: health and health care access for people with intellectual disabilities. Dev Disabil Res Rev 2006; 12: 70–82. [DOI] [PubMed] [Google Scholar]
- 32. O'Dwyer M, Mestrovic A, Henman M. Pharmacists' medicines‐related interventions for people with intellectual disabilities: a narrative review. Int J Clin Pharmacol 2015; 37: 566–578. [DOI] [PubMed] [Google Scholar]
- 33. Burke E, McCarron M, McCallion P. Advancing years, different challenges: Wave 2 IDS‐TILDA findings of the ageing of people with intellectual disability. Dublin: School of Nursing and Midwifery, Trinity College, 2014; 1–178. [Google Scholar]
- 34. McCarron M, Swinburne J, Burke E, McGlinchey E, Mulryan N, Andrews V, et al Growing Older with an Intellectual Disability in Ireland 2011: First Results from the Intellectual Disability Supplement to the Irish Longitudinal Study on Ageing. Dublin: School of Nursing, Trinity College, 2011. [Google Scholar]
- 35. Coppus AMW. People with intellectual disability: what do we know about adulthood and life expectancy? Dev Disabil Res Rev 2013; 18: 6–16. [DOI] [PubMed] [Google Scholar]
- 36. Burke E. Bone health in older adults with intellectual disability. Trinity College: Dublin, 2016. [Google Scholar]
- 37. Burke E, Walsh J, McCallion P, McCarron M. One size does not fit all, making reasonable adjustment to facilitate the engagement of people with intellectual disability in objective health assessment. In: IASSIDD Congress, Melbourne, Australia, 2016.
- 38. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg 2014; 12: 1495–1499. [DOI] [PubMed] [Google Scholar]
- 39. Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, et al Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. Int J Surg 2014; 12: 1500–1524. [DOI] [PubMed] [Google Scholar]
- 40. WHO Collaborating Centre for Drug Statistics Methodology . Anatomical therapeutic chemical classification system [internet]. In: ATC/DDD Index, 2016. Available at http://www.whocc.no/atc_ddd_index/ (last accessed January–June 2016).
- 41. Nishtala PS, Hilmer SN, McLachlan AJ, Hannan PJ, Chen TF. Impact of residential medication management reviews on drug burden index in aged‐care homes: a retrospective analysis. Drugs Aging 2009; 26: 677–686. [DOI] [PubMed] [Google Scholar]
- 42. Castelino RL, Hilmer SN, Bajorek BV, Nishtala P, Chen TF. Drug burden index and potentially inappropriate medications in community‐dwelling older people: the impact of home medicines review. Drugs Aging 2010; 27: 135–148. [DOI] [PubMed] [Google Scholar]
- 43. Indiana University of Aging Research . The Anticolinergic cognitive burden scale (2012 update). In, 2012. Available at http://www.agingbraincare.org/uploads/products/ACB_scale_‐_legal_size.pdf (last accessed January–June 2016).
- 44. Health Products Regulatory Authority . Health products regulatory authority [internet]. In: Medicines, 2016. Available at http://www.hpra.ie (last accessed January–June 2016).
- 45. Gnjidic D, Le Couteur DG, Abernethy DR, Hilmer SN. Drug burden index and beers criteria: impact on functional outcomes in older people living in self‐care retirement villages. J Clin Pharmacol 2012; 52: 258–265. [DOI] [PubMed] [Google Scholar]
- 46. Gnjidic D, Bell JS, Hilmer SN, Lonnroos E, Sulkava R, Hartikainen S. Drug burden index associated with function in community‐dwelling older people in Finland: a cross‐sectional study. Ann Med 2012; 44: 458–467. [DOI] [PubMed] [Google Scholar]
- 47. Kaila T, Korte JM, Saari KM. Systemic bioavailability of ocularly applied 1% atropine eyedrops. Acta Ophthalmol Scand 1999; 77: 193–196. [DOI] [PubMed] [Google Scholar]
- 48. Best O, Gnjidic D, Hilmer SN, Naganathan V, McLachlan AJ. Investigating polypharmacy and drug burden index in hospitalised older people. Intern Med J 2013; 43: 912–918. [DOI] [PubMed] [Google Scholar]
- 49. Nishtala PS, Narayan SW, Wang T, Hilmer SN. Associations of drug burden index with falls, general practitioner visits, and mortality in older people. Pharmacoepidemiol Drug Saf 2014; 23: 753–758. [DOI] [PubMed] [Google Scholar]
- 50. Collin C, Wade DT, Davies S, Horne V. The Barthel ADL index: a reliability study. Int Disabil Stud 1988; 10: 61–63. [DOI] [PubMed] [Google Scholar]
- 51. Sainsbury A, Seebass G, Bansal A, Young JB. Reliability of the Barthel index when used with older people. Age Ageing 2005; 34: 228–232. [DOI] [PubMed] [Google Scholar]
- 52. Wade D, Collin C. The Barthel ADL index: a standard measure of physical disability? Int Disabil Stud 1988; 10: 64–67. [DOI] [PubMed] [Google Scholar]
- 53. Jamsen KM, Bell JS, Hilmer SN, Kirkpatrick CM, Ilomaki J, Le Couteur D, et al Effects of changes in number of medications and drug burden index exposure on transitions between frailty states and death: the concord health and ageing in men project cohort study. J Am Geriatr Soc 2016; 64: 89–95. [DOI] [PubMed] [Google Scholar]
- 54. American Psychiatric Association . Diagnostic and statistical manual of mental disorders, 5th Editon edn. Arlington, VA: American Psychiatric Publishing, 2013. [Google Scholar]
- 55. McVilly KR, Bristow S, Foreman P, Goddard L. Australian Society for the Study of Intellectual Disability. Positive behaviour support for people with intellectual disability : evidence‐based practice, promoting quality of life. Australian Society for the Study of Intellectual Disability: Putney, N.S.W, 2002. [Google Scholar]
- 56. Matson JL, Neal D. Psychotropic medication use for challenging behaviors in persons with intellectual disabilities: an overview. Res Dev Disabil 2009; 30: 572–586. [DOI] [PubMed] [Google Scholar]
- 57. McGillivray JA, McCabe MP. Pharmacological management of challenging behavior of individuals with intellectual disability. Res Dev Disabil 2004; 25: 523–537. [DOI] [PubMed] [Google Scholar]
- 58. McGillivray JA, McCabe MP. Emerging trends in the use of drugs to manage the challenging behaviour of people with intellectual disability. J Appl Res Intellect Disabil 2005; 19: 163–172. [Google Scholar]
- 59. Mulryan N, Reilly E, Carroll R, O'Dwyer M, Lawlor B, McCallion P, et al Sleep problems and associated health variables in an older Irish population with down syndrome: results from the intellectual disability supplement to the Irish longitudinal study on ageing (IDS‐TILDA). J Intellect Disabil Res 2016; 60: 711. [Google Scholar]
- 60. Lonnroos E, Gnjidic D, Hilmer SN, Bell JS, Kautiainen H, Sulkava R, et al Drug burden index and hospitalization among community‐dwelling older people. Drugs Aging 2012; 29: 395–404. [DOI] [PubMed] [Google Scholar]
- 61. Kutner MH, Nachtsheim C, Neter J. Applied linear regression models. McGraw‐Hill/Irwin: New York, 2004. [Google Scholar]
- 62. Dancy C, Reidy J. Statistics without maths for psychology. Pearson Education Limited: Harlow, 2004. [Google Scholar]
- 63. Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol 1996; 49: 1373–1379. [DOI] [PubMed] [Google Scholar]
- 64. Peklar J, Kos M, O'Dwyer M, McCarron M, McCallion P, Kenny RA, et al Medication and supplement use in older people with and without intellectual disability: an observational, cross‐sectional study. PLoS One 2017; 12: e0184390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. McCarron M, O'Dwyer M, Burke E, McGlinchey E, McCallion P. Epidemiology of epilepsy in older adults with an intellectual disability in Ireland: associations and service implications. Am J Intellect Dev Disabil 2014; 119: 253–260. [DOI] [PubMed] [Google Scholar]
- 66. Dauphinot V, Faure R, Bourguignon L, Goutelle S, Krolak‐Salmon P, Mouchoux C. Factors associated with changes in exposure to anticholinergic and sedative medications in elderly hospitalized patients: multicentre longitudinal study. Eur J Neurol 2016. [DOI] [PubMed] [Google Scholar]
- 67. Perucca E. The new generation of antiepileptic drugs: advantages and disadvantages. Br J Clin Pharmacol 1996; 42: 531–543. [DOI] [PubMed] [Google Scholar]
- 68. Taylor D, Paton C, Kapur S. The Maudsley Prescribing Guidelines in Psychiatry, 12th Editon edn. John Wiley & Sons: London, 2015. [Google Scholar]
- 69. Maaskant M, van de Wouw E, van Wijck R, Evenhuis HM, Echteld MA. Circadian sleep‐wake rhythm of older adults with intellectual disabilities. Res Dev Disabil 2013; 34: 1144–1151. [DOI] [PubMed] [Google Scholar]
- 70. van de Wouw E, Evenhuis HM, Echteld MA. Prevalence, associated factors and treatment of sleep problems in adults with intellectual disability: a systematic review. Res Dev Disabil 2012; 33: 1310–1332. [DOI] [PubMed] [Google Scholar]
- 71. Collamati A, Martone AM, Poscia A, Brandi V, Celi M, Marzetti E, et al Anticholinergic drugs and negative outcomes in the older population: from biological plausibility to clinical evidence. Aging Clin Exp Res 2016; 28: 25–35. [DOI] [PubMed] [Google Scholar]
- 72. McDermott A. A descriptive quantitative study examining the prevalence of constipation in older adults with intellectual disabilities in Ireland. Trinity College: Dublin, Ireland, 2016. [Google Scholar]
- 73. Irvine EJ, Ferrazzi S, Pare P, Thompson WG, Rance L. Health‐related quality of life in functional GI disorders: focus on constipation and resource utilization. Am J Gastroenterol 2002; 97: 1986–1993. [DOI] [PubMed] [Google Scholar]
- 74. Dennison C, Prasad M, Lloyd A, Bhattacharyya SK, Dhawan R, Coyne K. The health‐related quality of life and economic burden of constipation. Pharmacoeconomics 2012; 23: 467–476. [DOI] [PubMed] [Google Scholar]
- 75. Gliav A, Lindberg G. Quality of life in patients with different types of functional constipation. Scand J Gastroenterol 1997; 32: 1083–1089. [DOI] [PubMed] [Google Scholar]
- 76. Chiba Y, Shimada A, Yoshida F, Keino H, Hasegawa M, Ikari H, et al Risk of fall for individuals with intellectual disability. Am J Intellect Dev Disabil 2009; 114: 225–236. [DOI] [PubMed] [Google Scholar]
- 77. Hsieh K, Rimmer J, Heller T. Prevalence of falls and risk factors in adults with intellectual disability. Am J Intellect Dev Disabil 2012; 117: 442–454. [DOI] [PubMed] [Google Scholar]
- 78. Cox C, Clemson L, Stancliffe R, Durvasula S, Sherrington C. Incidence of and risk factors for falls among adults with an intellectual disability. J Intellect Disabil Res 2010; 54: 1045–1057. [DOI] [PubMed] [Google Scholar]
- 79. Kouladjian L, Gnjidic D, Reeve E, Chen TF, Hilmer SN. Health care practitioners' perspectives on deprescribing anticholinergic and sedative medications in older adults. Ann Pharmacother 2016; 50: 625–636. [DOI] [PubMed] [Google Scholar]
- 80. Jansen DE, Krol B, Groothoff JW, Post D. Towards improving medical Care for People with intellectual disability living in the community: possibilities of integrated care. J Appl Res Intellect Disabil 2005; 19: 214–218. [Google Scholar]
- 81. Sheehan R, Horsfall L, Strydom A, Osborn D, Walters K, Hassiotis A. Movement side effects of antipsychotic drugs in adults with and without intellectual disability: UK population‐based cohort study. BMJ Open 2017; 7: e017406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kouladjian L, Gnjidic D, Chen TF, Hilmer SN. Development, validation and evaluation of an electronic pharmacological tool: the drug burden index calculator(c). Res Social Adm Pharm 2016; 12: 865–875. [DOI] [PubMed] [Google Scholar]
- 83. Ailabouni N, Mangin D, Nishtala PS. Deprescribing anticholinergic and sedative medicines: protocol for a feasibility trial (DEFEAT‐polypharmacy) in residential aged care facilities. BMJ Open 2017; 7: e013800. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting info item
Table S1 Modified Barthel index
Table S2 Modified functional comorbidity index
Table S3 Description of missing physical function scores
Table S4 Other therapeutic classes reported by <5% in decreasing prevalence
Table S5 Drug burden index binary table
Table S6 Review of existing drug burden index literature – observational studies


