The 2016 iteration of the World Health Organization (WHO) classification of myeloid neoplasms includes myelodysplastic syndrome with ring sideroblasts (MDS-RS) as a unique sub-type of MDS. Presence of dysplasia in single or multiple cell lineages with at least 15% ring sideroblasts (RS), or as few as 5% RS in the presence of SF3B1 mutations, further subcategorizes “MDS-RS” into: MDS-RS with single lineage dysplasia (SLD) and MDS-RS with multi-lineage dysplasia (MLD)1. In the prior 2008 WHO classification, MDS-RS-SLD was termed as refractory anemia with ringed sideroblasts and MDS-RS-MLD was included under refractory cytopenia with multi-lineage dysplasia, a category that also incorporated MDS patients with MLD without RS2. These changes in classification were prompted on the basis of morphological distinctions and their impact on prognosis. In addition, the strong phenotypic correlation of SF3B1 mutations with bone marrow (BM) RS3 and the prognostic irrelevance of BM RS percentage4, resulted in the inclusion of these mutations in the classification schema. The advent of next generation sequencing has established the molecular landscape in MDS, with frequent gene mutations including SF3B1 (20–30%), TET2 (~20%), ASXL1 (~14–15%), and RUNX1 (~8–9%)5–7. We pursued this study to assess the impact of degree of bone marrow dysplasia, in the context of gene mutations in patients with WHO defined MDS-RS.
Successive cases of MDS-RS, meeting the 2016 WHO criteria were identified from our institutional database from years 1994 to 2015. All patients had BM biopsies & cytogenetics at diagnosis. BM slides including Prussian blue stains for RS were re-reviewed to ensure compliance with the WHO criteria. Targeted exome sequencing for the following genes; TET2, ASXL1, DNMT3A, IDH1, IDH2, TP53, SRSF2, SF3B1, SH2B3, NPM1, FLT3, U2AF1, ZRSR2, JAK2, CSF3R, MPL, MFSD11, CEBPA, SETBP1, ZRSR2, RUNX1, IKZF1, CALR, KRAS, NRAS, CBL, PTPN11, STAG2, BCOR, and GATA2, was performed on diagnostic BM specimens from 64 patients by previously described methods8. Detailed clinical, laboratory and treatment data was collected. Mann–Whitney and Fisher exact test was used to assess differences among numerical and categorical variables between the SLD and MLD groups respectively. Multivariate analysis was performed using the Cox Proportional Hazards model. Statistics were performed via JMP Pro software (version 10).
Seventy six patients with MDS-RS met the study criteria, median age 73 years (range: 44–88), 50 (66%) males. Of these 57 (75%) were categorized as MDS-RS-SLD and 19 (25%) as MDS-RS-MLD. Six (8%) patients had an abnormal karyotype, which were further stratified into R-IPSS one (1.32%) intermediate, four (5.26%) poor, and one (1.32%) very poor cytogenetic risk groups. Overall R-IPSS stratification included 2 (2%) very low, 40 (53%) low, 22 (29%) intermediate, 9 (12%) high, and 3 (4%) very high-risk categories respectively. Targeted exome sequencing was available in 64 cases and demonstrated the following mutational frequencies; SF3B1 77%, ASXL1 16%, DNMT3A 13%, TET2 6%, TP53 5%, IDH1 3, and 2% each for SRSF2, PTPN11, ZRSR2, CSF3R, and U2AF1 (see Table 1).
Table 1.
Table displaying distribution of variables between MDS-RS, MDS-RS-SLD, and MDS-RS-MLD
| Variable; median value (range or %) | MDS-RS (n = 76) | SLD (n = 57) | MLD (n = 19) | P-value |
|---|---|---|---|---|
| Age (years) | 73 (44–88) | 73 (47–88) | 72 (44–81) | 0.22 |
| No. of males | 50 (66) | 36 (63) | 14 (74) | 0.4 |
| Hb; gm/dl | 9.3 (5.8–14.4) | 9.3 (5.8–13.4) | 10 (7.8–14.4) | 0.17 |
| WBC count x 109 per liter | 5.1 (1.2–17.6) | 5.7 (2.2–17.6) | 4.4 (1.2–9.4) | 0.02 * |
| ANC x 109 per liter | 3.06 (0.3–12.5) | 3.2 (0.6–12.5) | 2.13 (0.3–6.3) | 0.02 * |
| Platelet count x 109 per liter | 237 (7–819) | 255 (7–819) | 202 (27–438) | 0.05 * |
| BM ringed sideroblasts | 35 (10–80) | 50 (10–80) | 20 (15–70) | 0.004 * |
| BM blasts | 1(0–4) | 1(0–4) | 1(0–3) | 0.6 |
| Cytogenetics | ||||
| Abnormal karyotype (R-IPSS intermediate, poor and very poor cytogenetic risk groups) | 6 (8) | 4 (8) | 2 (11) | 0.5 |
| IPSS cytogenetic risk groups | ||||
| Good | 69 (91) | 52 (91) | 17 (11) | 0.5 |
| Intermediate | 2 (3) | 2 (4) | 0(0) | |
| Poor | 5 (6) | 3 (5) | 2 (11) | |
| R-IPSS cytogenetic risk groups | ||||
| Very good | 2 (3) | 1 (2) | 1 (5) | 0.6 |
| Good | 67 (88) | 51 (89) | 16 (84) | |
| Intermediate | 2 (3) | 2 (4) | 0(0) | |
| Poor | 4 (5) | 2 (4) | 2 (11) | |
| Very poor | 1 (1) | 1 (2) | 0(0) | |
| Genomic abnormalities | ||||
| SF3B1 | 49 (77) | 41 (82) | 8 (57) | 0.06 |
| ASXL1 | 10 (16) | 6 (12) | 4 (29) | 0.15 |
| DNMT3A | 8 (13) | 8 (16) | 0(0) | 0.04 * |
| TET2 | 4 (6) | 4 (8) | 0(0) | 0.2 |
| TP53 | 3 (5) | 3 (6) | 0(0) | 0.2 |
| IDH1 | 2 (3) | 2 (4) | 0(0) | 0.3 |
| SRSF2 | 1 (2) | 1 (2) | 0(0) | – |
| Others (PTPN11, ZRSR2, CSF3R, U2AF1) | 4 (6) | 4 (8) | 0(0) | – |
| Treatment | ||||
| HMA treatment | 5 (7) | 2 (4) | 3 (16) | 0.2 |
| Immunomodulatory treatment (Lenalidomide) | 1 (1) | 1 (2) | 0(0) | 0.83 |
| Allogeneic HSCT | 2 (3) | 1 (2) | 1 (5) | 0.16 |
| Outcomes | ||||
| Leukemic transformation | 2 (3) | 1 (2) | 1 (5) | 0.34 |
| Overall survival; median months (range) | 46 (0–190) | 47 (0–190) | 44 (6–123) | 0.2 |
* Signify statistically significant values
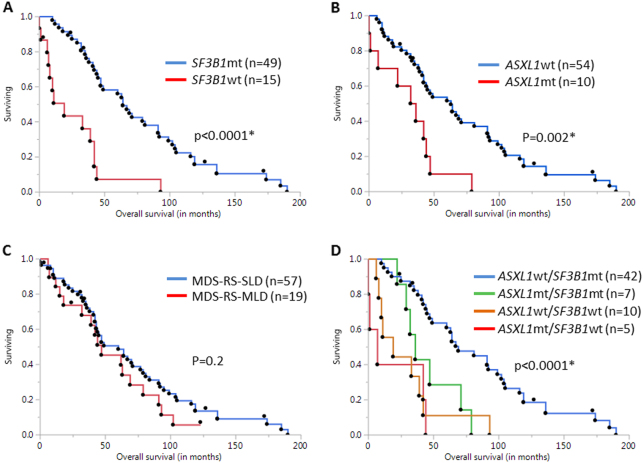
In comparison to MDS-RS-MLD, patients with MDS-RS-SLD had a higher frequency of SF3B1 (82 vs 57%, p = 0.06) and DNMT3A (16 vs 0%, p = 0.04) mutations, however the frequency of ASXL1 (12 vs 29%, p = 0.15) mutations was lower. At a median follow-up of 33 months, 68 (89%) deaths and 2 (3%) leukemic transformations were documented. Median survival (OS) of the entire cohort was 46 months (Range: 0–190 months). Survival for the MDS-RS-SLD group was 47 months, while that for MDS-RS-MLD was 44 months [Hazard ratio (HR) 1.4, p = 0.2]. On a univariate survival analysis that included age, sex, hemoglobin, white blood cell count with individual differential counts, platelet count, BM morphology (SLD versus MLD), peripheral blood blasts, cytogenetics and the aforementioned gene mutations, lack of SF3B1 mutations (HR 4.35, 95% CI 2.2–8.3, p < 0.0001, Fig. 1a) and presence of ASXL1 mutation (HR 3.13, 95% CI 1.4-6.3, p = 0.006, Fig. 1b) adversely impacted OS. Interestingly, multilineage dysplasia did not show a statistically significant correlation with outcomes (HR 1.4, 95% CI 0.8–2.5, p = 0.2) (Fig. 1c). In a multivariate analysis that included ASXL1 and SF3B1 mutations as variables, both the presence of ASXL1 mutations [HR of 2.3 (95% CI 1.0–4.7), p = 0.05] and the absence of SF3B1 mutations [HR 3.7 (95% CI 1.8–7.2), p = 0.0006] retained an independent and negative prognostic impact. In addition, both these mutations retained their prognostic impact when analyzed in the context of R-IPSS risk categories. Further, we assessed the combined effect of presence (mutated or mt) or absence (wild-type or wt) of ASXL1 and SF3B1 mutations on outcomes. Median OS was longest in the ASXL1wt/SF3B1mt (n = 42, 69 months) sub-group of patients in comparison to others (p < 0.0001), suggesting that the presence of both these mutations need to be assessed at diagnosis for an accurate prognostication (Fig. 1d). Patient numbers were limited for individual comparison with other sub-groups.
Fig. 1. Kaplan-Meir survival analysis for MDS-RS patients based on somatic mutational analysis.
a shows Kaplan-Meir survival curves for SF3B1 mutated (mt) and wild-type (wt) patients. Log-rank test was used to assess difference between groups. Median OS is significantly higher in the SF3B1 mt patients (64 versus 19 months, p < 0.0001*). b shows a similar analysis, depending on ASXL1 mutational status. Median OS in ASXL1 wt patients is much higher than ASXL1 mt patients (64 versus 39 months, p = 0.002*). c shows Kaplan–Meir survival curves among the two sub-groups based on bone marrow morphology (MDS-RS-SLD and MDS-RS-MLD). No significant survival difference was found between the two groups (p = 0.2). d shows Kaplan-Meir survival curves for four sub-groups, based on presence or absence of both SF3B1 and ASXL1 mutations. Median OS was highest in ASXL1 wt/SF3B1 mt (n = 42, 69 months) sub-group (p < 0.0001*), followed by ASXL1 mt/SF3B1 mt (n = 7, 39 months), ASXL1 wt/SF3B1 wt (n = 10, 19 months) and ASXL1 mt/SF3B1 wt (n = 5, 7 months) sub-groups, respectively
Incorporation of genomic alterations into existing WHO morphological classifications is critical for better classifying these disorders and to refine existing prognostic strategies9. Our study validates the prognostic impact of gene mutations in MDS-RS and also assesses their relevance in the context of existing morphological distinctions.
Mutations in SF3B1, a gene regulating pre-mRNA splicing, are known to have favorable outcomes in MDS-RS3,10 and our study has confirmed this observation. In contrast, ASXL1 mutations impact chromatin regulation by impairing activity of polycomb repressive complex 2 (PRC2), and are associated with adverse outcomes in myeloid neoplasms such as MDS, chronic myelomonocytic leukemia and myelofibrosis8,11,12. To our knowledge, ours is the first study to assess the prognostic impact of ASXL1 mutations in an independent large cohort of WHO-defined MDS-RS patients and has demonstrated an adverse impact on survival. In particular, the subgroup of MDS-RS patients with mutated SF3B1 and wild-type ASXL1 has the best prognosis. In conclusion, molecular abnormalities involving relevant genes take precedence in terms of prognostication within morphologically defined subsets of MDS and in fact, may be more relevant than existing morphological prognosticators such as degree of bone marrow dysplasia. Collaborative prospective efforts with larger number of patients are needed to validate these findings.
Acknowledgements
Current publication is supported in part by grants from the “The Gerstner Family Career Development Award” and the Mayo Clinic Center for Individualized Medicine, Mayo Clinic, Rochester, MN, USA”. This publication was supported by CTSA Grant Number KL2 TR000136 from the National Center for Advancing Translational Science (NCATS). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Arber DA, et al. The2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–2405. doi: 10.1182/blood-2016-03-643544. [DOI] [PubMed] [Google Scholar]
- 2.Vardiman JW, et al. The2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114:937–951. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 3.Patnaik MM, et al. SF3B1 mutations are prevalent in myelodysplastic syndromes with ring sideroblasts but do not hold independent prognostic value. Blood. 2012;119:569–572. doi: 10.1182/blood-2011-09-377994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patnaik MM, et al. Prognostic irrelevance of ring sideroblast percentage in World Health Organization-defined myelodysplastic syndromes without excess blasts. Blood. 2012;119:5674–5677. doi: 10.1182/blood-2012-03-415356. [DOI] [PubMed] [Google Scholar]
- 5.Bejar R, et al. Clinical effect of point mutations in myelodysplastic syndromes. N. Engl. J. Med. 2011;364:2496–2506. doi: 10.1056/NEJMoa1013343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Papaemmanuil E, et al. Somatic SF3B1 mutation in myelodysplasia with ring sideroblasts. N. Engl. J. Med. 2011;365:1384–1395. doi: 10.1056/NEJMoa1103283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patnaik MM, Tefferi A. Refractory anemia with ring sideroblasts (RARS) and RARS with thrombocytosis (RARS-T): 2017 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 2017;92:297–310. doi: 10.1002/ajh.24637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patnaik MM, et al. Prognostic interaction between ASXL1 and TET2 mutations in chronic myelomonocytic leukemia. Blood. Cancer J. 2016;6:e385. doi: 10.1038/bcj.2015.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tefferi A, et al. Targeted next-generation sequencing in myelodysplastic syndromes and prognostic interaction between mutations and IPSS-R. Am. J. Hematol. 2017;92:1311–1317. doi: 10.1002/ajh.24901. [DOI] [PubMed] [Google Scholar]
- 10.Malcovati L, et al. SF3B1 mutation identifies a distinct subset of myelodysplastic syndrome with ring sideroblasts. Blood. 2015;126:233–241. doi: 10.1182/blood-2015-03-633537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bejar R, et al. Validation of a prognostic model and the impact of mutations in patients with lower-risk myelodysplastic syndromes. J. Clin. Oncol. 2012;30:3376–3382. doi: 10.1200/JCO.2011.40.7379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thol F, et al. Prognostic significance of ASXL1 mutations in patients with myelodysplastic syndromes. J. Clin. Oncol. 2011;29:2499–2506. doi: 10.1200/JCO.2010.33.4938. [DOI] [PubMed] [Google Scholar]