Abstract
Patients with later-life depression (LLD) show abnormal gray matter (GM) volume, white matter (WM) integrity and functional connectivity in the anterior cingulate cortex (ACC) and posterior superior temporal gyrus (pSTG), but it remains unclear whether these abnormalities persist over time. We examined whether structural and functional abnormalities in these two regions are present within the same subjects during depressed vs. remitted phases. Sixteen patients with LLD and 30 healthy subjects were studied over a period of 1.5 years. Brain images obtained with a 3-Tesla magnetic resonance imaging (MRI) system were analyzed by voxel-based morphometry of the GM volume, and diffusion tensor imaging (DTI) and resting-state functional MRI were used to assess ACC–pSTG connectivity. Patients with LLD in the depressed and remitted phases showed significantly smaller GM volume in the left ACC and left pSTG than healthy subjects. Both patients with LLD in the depressed and remitted phases had significantly higher diffusivities in the WM tract of the left ACC–pSTG than healthy subjects. Remitted patients with LLD showed lower functional ACC–pSTG connectivity compared to healthy subjects. No difference was found in the two regions between depressed and remitted patients in GM volume, structural or functional connectivity. Functional ACC–pSTG connectivity was positively correlated with lower global function during remission. Our preliminary data show that structural and functional abnormalities of the ACC and pSTG occur during LLD remission. Our findings tentatively reveal the brain pathophysiology involved in LLD and may aid in developing neuroanatomical biomarkers for this condition.
Keywords: cingulate cortex, connectivity, gray matter volume, late-life depression, magnetic resonance imaging, resting state functional magnetic resonance imaging, superior temporal gyrus, white matter integrity
Introduction
Accumulated evidence from structural neuroimaging studies suggests that patients with later-life depression (LLD) show abnormal gray matter (GM) and white matter (WM) brain structures. Patients with LLD have a significantly smaller GM volume in the anterior cingulate cortex (ACC), superior temporal gyrus (STG), middle temporal gyrus, inferior frontal, orbitofrontal cortex, insula, hippocampus, amygdala and putamen than healthy individuals (Andreescu et al., 2008; Sexton et al., 2013; Du et al., 2014; Smagula and Aizenstein, 2016). In terms of WM structures, patients with LLD showed abnormal connectivity in the cingulum, uncinate fasciculus (Charlton et al., 2015), temporal lobe (Charlton et al., 2015), superior longitudinal fasciculus, superior frontal gyrus, corpus callosum (Reppermund et al., 2014) and parahippocampal gyrus (Guo et al., 2014). In our previous study, patients with LLD showed smaller GM volumes in the ACC, posterior STG (pSTG), and orbitofrontal cortex and abnormal WM integrity of the uncinate fasciculus, in comparison to healthy subjects (Harada et al., 2016). However, it remains unclear whether abnormalities of GM volume and WM connectivity are progressive or consistent over time.
Longitudinal neuroimaging studies of individuals with LLD have begun to address this issue. Measured over a 2-year period, the GM volume in patients with LLD a showed greater reduction in left hippocampal GM volume than in non-depressed subjects (Steffens et al., 2011). Although there is some evidence of change in WM intensities in patients with LLD over the long-term (Chen et al., 2006; Teodorczuk et al., 2007; Firbank et al., 2012), we are aware of only one longitudinal study of structural WM connectivity in patients with LLD to date (Khalaf et al., 2015). That study found no significant differences in the fractional anisotropy of WM tracts between LLD remitters and non-remitters over a 12-week antidepressant-treatment period (Khalaf et al., 2015).
While there is evidence of abnormal functional connectivity in resting state functional magnetic resonance imaging (rs-fMRI) studies in patients with LLD, the results remain inconclusive. Patients with LLD showed decreased, increased and comparable functional connectivity in a variety of regions as compared to healthy subjects in cross-sectional (Liu et al., 2012; Sexton et al., 2012; Andreescu et al., 2013; Wang et al., 2015; Eyre et al., 2016) and longitudinal studies (Wu et al., 2011; Alexopoulos et al., 2012; Andreescu et al., 2013). Functional connectivity of regions in the default-mode network has also been well investigated in LLD (Wu et al., 2011; Alexopoulos et al., 2012; Liu et al., 2012; Sexton et al., 2012; Andreescu et al., 2013; Wang et al., 2015; Eyre et al., 2016). This network is thought to be an organized functional network of several brain regions that are active during the resting state and inhibited during the performance of active tasks (Raichle et al., 2001). It involves activity in the ACC, posterior cingulate cortex, precuneus, medial frontal cortex, inferior parietal and the pSTG nearby the temporoparietal junction area (Mars et al., 2012). The network also largely overlaps with the social cognitive network (Mars et al., 2012); for instance, the neural network of “Theory of Mind” involves the connectivity between the ACC and the pSTG/temporoparietal junction area (Abu-Akel and Shamay-Tsoory, 2011). We previously reported a reduced GM volume in the ACC and pSTG in patients with LLD (Harada et al., 2016). Thus, in the present study, we focused on these two regions.
The aim of the present study was to determine whether the GM volume of the ACC and pSTG, and structural and functional connectivity in the ACC–pSTG network, would be longitudinally altered during the depressed and remitted phases in patients with LLD. We hypothesized that, compared to healthy participants, patients with LLD would show a reduced GM volume in the ACC and pSTG, and reduced WM integrity and functional connectivity in the ACC–pSTG during the depressed phase. We also expected that these structural and functional abnormalities would recover after remission.
Materials and Methods
Participants
This study was carried out in accordance with the latest version of the Declaration of Helsinki. The Institutional Review Board of Yamaguchi University Hospital approved this study. Written informed consent was obtained from all participants after providing them with a complete description of the study.
We studied 46 participants, including 16 patients who met the criteria of the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision (American Psychiatric Association, 2000), for major depressive disorder, and 30 healthy subjects (Table 1). Patients with LLD and healthy subjects were not significantly different in terms of sex distribution, age, or years of education. Patients were recruited from Yamaguchi University Hospital and referred by clinics and hospitals in the area. Healthy participants were recruited by word-of-mouth or by advertising in the community. Patients were diagnosed and healthy subjects were screened based on clinical interviews and the Mini International Neuropsychiatric Interview (M.I.N.I., Japanese version 5.0.0; Otsubo et al., 2005). We confirmed the diagnoses in case conferences and laboratory meetings with distinguished clinical psychiatrists and trained research psychiatrists of our University.
Table 1.
Demographic and clinical characteristics of the participants.
| LLD (n = 16) | Healthy (n = 30) | ||
|---|---|---|---|
| Depressed | Remitted | ||
| Age (years) | 56 [53.3–65.5] | 58 [54–67] | 58 [54–63.5] |
| Sex (Female %) | 10 (62.5%) | 19 (63.3%) | |
| Education (years) | 13 [12–15.8] | 14 [12–16] | |
| MMSE | 28.5 [26.3–30] | 30 [28.8–30] | |
| Age of onset (years) | 51 [33.5–59.8] | - | |
| Duration (years) | 7.5 [2.8–22.8] | 9.3 [4.9–24.6] | - |
| Number of episode | 2 [2–2] | - | |
| SIGH-Da,b | 20 [19–22.8] | 2 [0.3–4.8] | 1 [0–1] |
| GAFa,b | 51 [51–60] | 89.5 [81.3–90] | 90 [90–95] |
| IMP-eq (mg) | 143.8 [81.3–253.1] | 200 [81.3–309.4] | - |
Data represent median [inter-quartile range]. LLD, later-life depression; MMSE, Mini-mental state examination; SIGH-D, Structured Interview Guide for the Hamilton Rating for Depression Scale; GAF, Global Assessment of Functioning; IMP-eq., Imipramine equivalent. aDepressed vs. Remitted; p < 0.05 by Wilcoxon signed ranks test. bDepressed vs. Healthy; p < 0.05 by Mann-Whitney U test.
Some of the subjects participated in our previous study (Harada et al., 2016). Patients with a history of or current substance abuse/dependence and other psychotic illnesses, as assessed by the M.I.N.I. were excluded from the study. Using interviews, blood tests and physical examinations, subjects with endocrinological disease, head trauma, neurological disease, a family history of hereditary neurological disorders, or other medical conditions (i.e., hypertension, diabetes, active liver disease, kidney problems, or respiratory problems) were excluded from the study. Two patients had comorbid panic disorder without agoraphobia. Fourteen patients were taking medication at the time of study participation. Seven patients were taking only antidepressants; five were taking antidepressants and antipsychotics; one was taking an antidepressant and a mood stabilizer; and one was taking an antidepressant, an antipsychotic, and a mood stabilizer. Of the remaining two patients, one was drug-naïve, and the other had been medication-free for at least 4 years. The medication load was assessed using imipramine-equivalent doses (Inada and Inagaki, 2015). The imipramine-equivalent dosage did not differ significantly between the depressed and remitted phase in patients with LLD. Healthy subjects were excluded if they had an immediate family member with a psychiatric disorder. Current mood states were evaluated using the Structured Interview Guide for the Hamilton Depression Rating Scale (SIGH-D; Williams, 1988). Any participant who was left-handed or ambidextrous was excluded from the analysis (Oldfield, 1971). The Global Assessment of Functioning scale (GAF, 2000) was used to assess social functioning. None of the participants were demented at the time of study participation, according to a Mini-Mental State Examination (MMSE; Folstein et al., 1975) and clinical interview. Patients with LLD underwent clinical evaluations and MRI examination during the depressed and remitted phases. Remission was defined using the DSM-IV criteria for full remission, with a specifier depressive episode, and with a score < 8 on the SIGH-D. The median [inter-quartile range] of duration from the depressed phase to recovery phase was 514.5 [145.2–877.3] days.
Magnetic Resonance Imaging
Brain images were collected on a Siemens 3-Tesla MRI system (Siemens Medical System, Skyra, Erlangen, Germany) using T1-weighted imaging, diffusion tensor imaging (DTI) with eigenvalues, and rs-fMRI. The T1-weighted parameters were as follows: field of view, 270 mm; view matrix, 256 × 256; repetition time, 2300 ms; echo time, 2.95 ms; flip angle, 9°; and slice thickness, 1.2 mm. The T2-weighted parameters were as follows: field of view, 240 mm; view matrix, 394 × 512; repetition time, 5000 ms; echo time, 87 ms; flip angle, 150°; and slice thickness, 6 mm. The DTI parameters were as follows: field of view, 235 mm; view matrix, 100 × 100; repetition time, 8700 ms; echo time, 88 ms; flip angle, 90°; and slice thickness, 2.4 mm. Two b values were used; one image was acquired at 0 s/mm2 (no diffusion weighting) and 29 noncoplanar images were acquired at 1000 s/mm2 (diffusion-weighted b value). The rs-fMRI parameters were as follows: field of view, 220 mm; view matrix, 64 × 64; repetition time, 2500 ms; echo time, 30 ms; flip angle, 80º, slice thickness, 4 mm. MR images were manually checked for quality before the analyses and abnormal findings were evaluated by radiologists who were blinded to the participants’ diagnoses.
Image Analysis
GM Volume
Image preprocessing was performed using VBM81 in the SPM8 software (Wellcome Department of Imaging Neuroscience, London, UK) running under Matlab R2015b (MathWorks, Natick, MA, USA). All original images were manually aligned on the anterior-posterior commissure line. T1-weighted images were segmented and imported into a format that could be used by the VBM8 algorithm. The segmentation procedure automatically removed non-brain tissues, including the scalp, skull and dural venous sinuses. The segmented images were normalized to Montreal Neurological Institute (MNI) space using the template assembled in VBM8, and were smoothed with an 8-mm Gaussian filter.
WM Connectivity
DTI scans were processed using the Functional MRI of the Brain Software Library (FSL version 5.1; Oxford, UK2). Eddy-current distortions and head displacements were adjusted through affine registration of the 31 diffusion volumes to the first b0 volume using FSL’s Linear Registration Tool. The b-vector table (i.e., gradient directions) for each participant was then aligned to the rotation parameters of this linear correction. Non-brain tissue was removed using FSL’s Brain Extraction Tool. Fractional anisotropy, as well as axial, radial, and mean diffusivities, were then estimated at each voxel of the brain by fitting a diffusion tensor model to the raw diffusion data using weighted least-squares in FSL’s Diffusion Toolbox.
The local (i.e., within-voxel) probability density functions of the principal diffusion direction were estimated using Markov Chain Monte Carlo sampling in FSL’s Bedpostx tool (Behrens et al., 2007). A spatial probability density function across voxels was then estimated based on these local probability density functions using FSL’s Probtrackx tool (Behrens et al., 2007), in which 5000 samples were taken for each input voxel with a 0.2-curvature threshold, 0.5-mm step length, and 2000 steps per sample.
A 5-mm sphere was created at the pSTG peak in the VBM finding (MNI: −46.5, −61.5, 18) in MNI 1-mm space. The ACC was defined using Freesurfer 5.3.0 (Fischl et al., 1999, 2002) on MNI 1-mm brain space. Probabilistic tractography was used to segment the WM structure between the pSTG sphere and the ACC. The mean of the axial, radial, and mean diffusivities, as well as the fractional anisotropy was calculated within the WM tract for each individual. Probabilistic tractography failed to segment the WM tract of nine healthy subjects and three patients with LLD in remission.
Functional Connectivity
Script libraries (fcon) from the 1000 Functional Connectomes Project3 (Biswal et al., 2010) were used for preprocessing and region of interest (ROI) analyses. Resting state images were first motion-corrected and spatially smoothed using a 6-mm full-width at half-maximum Gaussian kernel. The structural T1 images were individually registered to the MNI152 2-mm brain. Using this registration, 12 affine parameters were created between the rs-fMRI volume and MNI152 with a 2-mm space, such that a seed ROI could later be registered to each individual rs-fMRI space. The time series were band-pass filtered (between 0.005 Hz and 0.1 Hz), and each resting state volume was regressed by WM and cerebrospinal fluid signal fluctuations as well as the six motion parameters. For ROI connectivity analysis, ROIs were determined in the MNI152 2-mm space. The same pSTG and ACC ROIs from the WM analysis were registered to the MNI 2-mm space. Between these two ROIs, a Pearson’s product moment correlation coefficient was calculated for each subject and then transformed to the Z standard score.
Statistical Analysis
GM Volume
We analyzed the imaging data with SPM8 software, which implements a general linear model. We examined the VBM analysis of the whole brain and of an ROI. The left ACC and bilateral STG were defined as an ROI according to our previous study (Harada et al., 2016). SPM8 was used to assess the difference in GM volume between patients with LLD (depressed and remitted) and healthy subjects, using a two-sample t-test, with age, sex, years of education, and total brain volume as covariates. Comparison between depressed patients with LLD and remitted patients with LLD was performed using a paired t-test in SPM8. A voxel-wise t-test was performed with the threshold set at p < 0.05 corrected by family-wise error (pFWE) in SPM8. We anatomically identified the brain regions using automated anatomical labeling (Tzourio-Mazoyer et al., 2002) via the Wake Forest University Pick Atlas (version 3.044; Maldjian et al., 2004) and the Atlas of the Human Brain (Mai et al., 2008). All results are presented in MNI coordinates.
WM Connectivity
We compared the mean diffusivity, radial diffusivity, axial diffusivity and fractional anisotropy values of the left ACC–pSTG WM tract between depressed patients with LLD and healthy subjects and between remitted patients with LLD and healthy subjects, using a Mann-Whitney U test. We also compared these values of the WM tract between depressed and remitted patients with LLD using the Wilcoxon signed-rank test. SPSS Statistics version 20 for Windows (IBM, Chicago, IL, USA) was used for statistical analysis.
Functional Connectivity
To test functional connectivity changes and differences between groups, Z scores of functional connectivity of the ACC–pSTG tract were examined and compared between depressed patients with LLD and healthy subjects, and between remitted patients with LLD and healthy subjects, using the Mann-Whitney U test, and between depressed and remitted patients with LLD using Wilcoxon’s signed-rank test.
Multi-modal and Clinical Associations
Spearman’s rank correlation coefficients were tested to determine the association between clinical variables and regions in patients with LLD when statistical analysis showed significance between patients with LLD and healthy participants. The clinical variables included the duration of illness, the duration of recovery, the number of episodes, imipramine equivalent and the total SIGH-D and GAF scores. We also examined the correlation between the subscore of “feelings of guilt” in the SIGH-D and the results of MRI analyses, because this feeling represents the core feature of cognitive disturbance in depression and is associated with the ACC and pSTG for mood processing and with the default-mode network (Basile et al., 2011; Andrews-Hanna et al., 2014; Hamilton et al., 2015; details in “Discussion” section). Statistical significance was set at p < 0.05.
Results
GM Volume
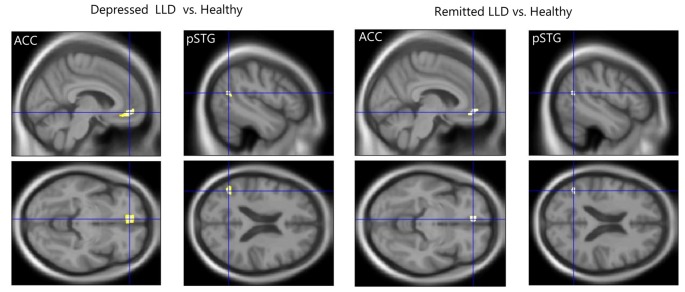
The whole brain analysis demonstrated that depressed patients with LLD showed smaller GM volumes in the left pSTG than healthy subjects (the coordinates of the voxel of maximum, x = −47, y = −61, z = 18, T = 6.55, k = 77, PFWE < 0.001). No other regional GM volume was significantly different in the whole brain analysis. There was no significant difference in the GM volume in the whole brain analysis between remitted patients with LLD and healthy subjects, or between patients with LLD in the depressed and remitted phases. For the ROI analysis, depressed patients with LLD had significantly smaller GM volumes in the left ACC (the coordinates of the voxel of maximum, x = −6, y = 45, z = −9, T = 5.25, k = 440, PFWE < 0.001) and left pSTG (x = −47, y = −61, z = 18, T = 6.55, k = 186, PFWE < 0.001) than healthy subjects. Remitted patients with LLD also showed significantly smaller GM volumes in the left ACC (x = −6, y = 44, z = −11, T = 4.05, k = 162, PFWE = 0.010) and left pSTG (x = −47, y = −61, z = 18, T = 5.40, k = 93, PFWE = 0.003) than healthy participants (Figure 1). There was no significant difference in the GM volume of the two regions between patients with LLD in the depressed and remitted phases.
Figure 1.
Reduced gray matter (GM) volume in patients with later-life depression (LLD). Patients with acute LLD showed significantly smaller GM volumes in the left anterior cingulate cortex (ACC) and posterior superior temporal gyrus (pSTG) than did healthy subjects. Patients with LLD in remission showed significantly smaller GM volumes in the left ACC and pSTG than healthy participants. The cross-hairs mark the coordinates of the voxel of maximal statistical significance.
WM Connectivity
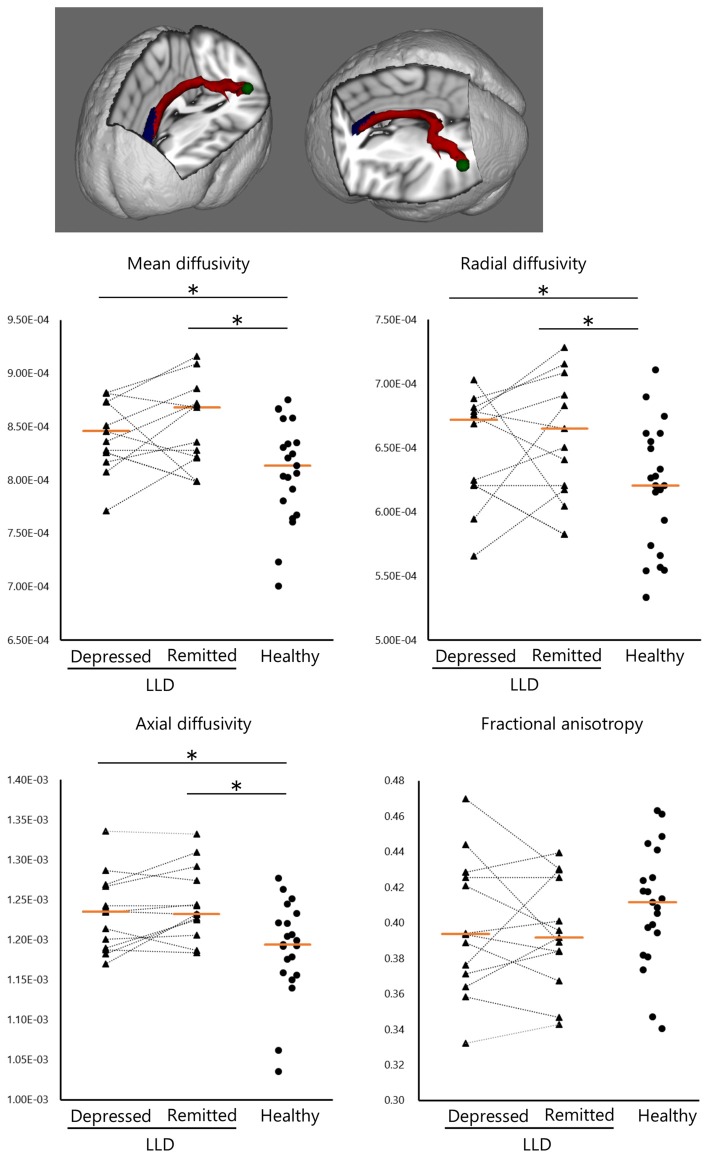
We found that depressed patients with LLD showed significantly higher mean diffusivity, radial diffusivity, and axial diffusivity, but not fractional anisotropy, in the WM tract of the left ACC–pSTG than healthy subjects (mean diffusivity, U = 251, P = 0.01; radial diffusivity, U = 239, P = 0.03; axial diffusivity, U = 242, P = 0.02; fractional anisotropy, U = 140, P = 0.40; Figure 2). Similarly, remitted patients with LLD had significantly higher scores on those parameters than healthy participants (mean diffusivity, U = 210, P < 0.01; radial diffusivity, U = 201, P = 0.02; axial diffusivity, U = 101, P < 0.01; fractional anisotropy, U = 101, P = 0.22). There was no statistically significant difference between depressed and remitted patients with LLD in any parameters of WM tracts (mean diffusivity, Z = −1.02, P = 0.31; radial diffusivity, Z = −0.47, P = 0.64; axial diffusivity, Z = −1.49, P = 0.14; fractional anisotropy, Z = −0.39, P = 0.70).
Figure 2.
Structural white matter (WM) connectivity between the left anterior cingulate and posterior superior temporal gyrus (ACC–pSTG). Patients with LLD (n = 13) in the depressed and remitted phases demonstrated significantly higher WM connectivity in the ACC–pSTG region than did healthy subjects (n = 21), in terms of mean, radial and axial diffusivity, but not in fractional anisotropy. Tangerine bars represent the median value of Z-score for each diffusivity. Y-axis represents z-score. *p < 0.05 by Mann-Whitney U test or Wilcoxon signed ranks test.
Functional Connectivity
Remitted patients with LLD showed lower functional connectivity in the left ACC–pSTG than did healthy subjects (U = 127, P < 0.01; Figure 3). The functional connectivity was not statistically significantly different between depressed and remitted patients with LLD or between depressed patients with LLD and healthy subjects.
Figure 3.
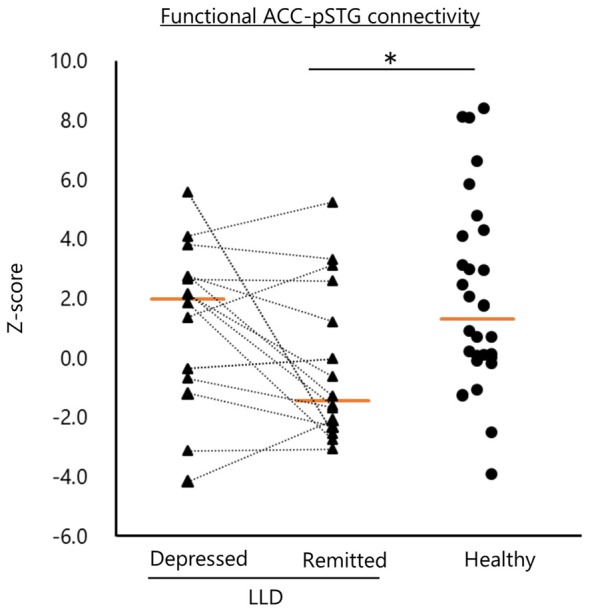
Functional connectivity of the left anterior cingulate and posterior superior temporal gyrus (ACC–pSTG). Patients with remitted LLD (n = 16) showed lower functional connectivity in the left ACC–pSTG than healthy subjects (n = 30). Tangerine bars represent the median value of Z-score for each group. *p < 0.05 by Mann-Whitney U test.
Multi-modal and Clinical Associations
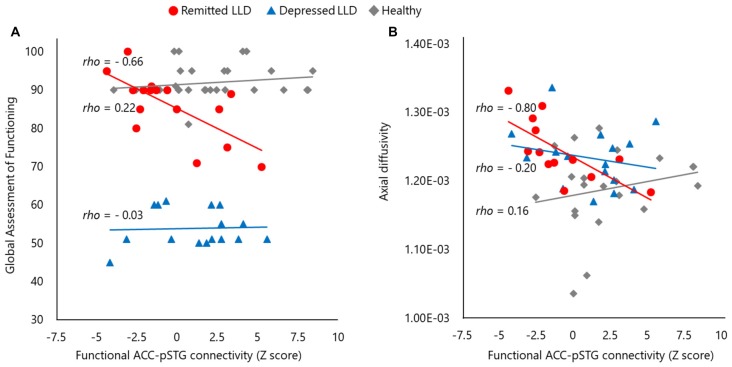
There was no significant correlation between clinical variables, including “feelings of guilt” in the SIGH-D, and the structural or functional MRI findings in depressed patients with LLD and healthy subjects. The functional connectivity of the ACC–pSTG was significantly and inversely correlated with the GAF score (rho = −0.66, p < 0.01) and the axial diffusivity of the ACC–pSTG WM tract (rho = −0.80, p < 0.01) in patients with LLD in the remitted phase, but not in patients with LLD in the depressed phase or healthy subjects (Figure 4). These two results remained statistically significant after Bonferroni correction. The other clinical variables, including medication load, were not significantly correlated with any result of structural or functional MRI in remitted patients with LLD.
Figure 4.
The scatter plots of between functional and structural connectivity and the global functioning. Functional connectivity of anterior cingulate and posterior superior temporal gyrus pSTG (ACC–pSTG) showed a significantly inverse correlation in the score of global assessment of functioning (A) and structural ACC–pSTG connectivity (B) in patients with LLD in the remitted phase (red circle), but not in patients with LLD in the depresses phase (blue triangle) or healthy participants (gray diamond).
To verify the significance of these results (Figure 4), we performed an exploration of the differences in the two correlations between the groups, using Fisher’s Z test. In terms of functional connectivity and GAF correlation, there was a significant difference between the remitted phase (Z = −0.80) and the depressed phase (Z = 0.03) in patients with LLD (Z = 2.12, p = 0.03) and between remitted patients and healthy subjects (Z = 0.21; Z = 3.03, p < 0.01), but not between depressed patients with LLD and healthy subjects (Z = 0.56, p = 0.58). In terms of functional connectivity and axial diffusivity correlation, there was a significant difference between the remitted phase (Z = −1.09) and depressed phase (Z = −0.21) in patients with LLD (Z = 2.25, p = 0.02), and between remitted patients and healthy subjects (Z = −0.12; Z = 2.65, p < 0.01), but not between depressed patients with LLD and healthy subjects (Z = 0.22, p = 0.83). These results suggested that the two observed statistically significant correlations were valid in patients with LLD in the remitted phase.
Discussion
Although this is a preliminary study, no other longitudinal investigation of structural and functional connectivity abnormalities in patients with LLD has been reported to date. Acutely depressed patients with LLD, relative to healthy subjects, had a smaller GM volume in the ACC and pSTG, higher WM connectivity, and comparable functional connectivity between the two regions. These GM and WM anomalies remained abnormal after remission in patients with LLD, while the functional connectivity declined in patients with LLD during remission relative to healthy subjects. Furthermore, ACC–pSTG functional connectivity was associated with structural connectivity of the ACC–pSTG and global functioning in the remitted phase. The present results suggest that: (1) structural abnormalities in the ACC and pSTG do not appear to be linked to mood state; (2) functional abnormality of the ACC–pSTG connection is associated with functional outcome; and (3) GM volume and structural and functional connectivity of ACC and pSTG are, to some extent, linked to each other. These tentative findings indicate that the ACC–pSTG is both structurally and functionally involved in the pathophysiology of LLD.
The ACC connects structurally and functionally with a variety of brain areas, including the lateral prefrontal cortex, orbitofrontal cortex, parietal cortex, amygdala, STG, nucleus accumbens, hypothalamus, insula, raphe nucleus and hippocampus (Morecraft and Van Hoesen, 1992; Devinsky et al., 1995; Margulies et al., 2007; Ikuta et al., 2017). The ACC plays a role in processing stimulation, error detection, prediction of the problem, incentive and modulation of emotional reaction (Bush et al., 2000; Gasquoine, 2013). Substantial neuroimaging studies have shown that the ACC is deeply involved in depression. One review of preclinical and clinical studies has suggested that a neural network that includes orbital and medial prefrontal cortex regions, such as the ACC, the limbic system, the STG, and the temporal lobe is associated with depression (Price and Drevets, 2010). Deep brain stimulation of the subgenual ACC was effective for biological treatment of patients with treatment-resistant depression (Mayberg et al., 2005).
However, the evidence for reduced GM volume of ACC in patients with LLD is inconsistent (Lavretsky et al., 2007; Yuan et al., 2008; Weber et al., 2010). A reduced GM volume of the ACC was observed during both depressed and remitted phases in the current as well as a prior study (Harada et al., 2016). The reasons for inconsistency across studies of LLD may be related to methodological differences, such as the mood state, the mean age of participants, the number of episodes, and the duration of illness. Further studies controlling for these background demographics are needed to obtain conclusive data regarding abnormal GM volumes in the ACC in patients with LLD.
The pSTG is a part of the temporoparietal junction area (Abu-Akel and Shamay-Tsoory, 2011). This area plays a crucial role in integrating information from both the external environment as well as from within the body, and in collecting and processing all of this information (Abu-Akel and Shamay-Tsoory, 2011). The area is also known to be a key player in cognitive and affective theory of mind (Saxe and Kanwisher, 2003; Corradi-Dell’Acqua et al., 2014), such as false belief (Apperly et al., 2004; Aichhorn et al., 2009). Structured studies have shown reduced GM volume and density of the STG in mood disorders (Nugent et al., 2006; Abe et al., 2010; Peng et al., 2011). Our results support these findings.
The present study demonstrated no difference in the GM volume in the ACC or pSTG in the same patients during depressed and remitted phases. To our knowledge, no previous longitudinal neuroimaging study has measured such regional GM volumes in patients with LLD. Steffens et al. (2011) have demonstrated that patients with LLD had a reduced hippocampal GM volume over a 2-year period, while non-depressed subjects did not, and the reduction of hippocampal GM volumes was associated with a decline in the MMSE for 2–2.5 years in patients with LLD. They also measured hippocampal GM volume using a manual tracing method and did not examine the GM volume of other regions, while we performed a whole brain VBM analysis. Their study did not show whether patients with LLD were remitted when the GM volume was measured for a second time. They determined the MMSE score at the baseline and at a second point, while we obtained the MMSE score in the remitted phase, because depressed patients often demonstrate psychomotor retardation, which may affect the MMSE score. Thus, it was unknown whether the patients showed cognitive decline from the baseline to the second assessment point. The sample size in total was 162 in their study, vs. the 46 participants in the current study. These methodological differences between the two studies make it difficult to interpret the differences in results. More longitudinal studies with large samples, controlling for background demographics, are required to obtain conclusive data on the abnormal GM volumes in patients with LLD.
In terms of the ACC–pSTG connectivity, the current study showed structural and functional abnormalities in patients with LLD, and these abnormalities were associated with clinical manifestations. The pSTG and ACC are anatomically and functionally connected in the monkey (Yukie and Shibata, 2009) and humans (Margulies et al., 2007). The two regions are involved in the default-mode network and social cognitive network (Margulies et al., 2007; Menon, 2011). Reviews of rs-fMRI studies of depression have shown abnormal functional connectivity between the two regions (Kaiser et al., 2015; Mulders et al., 2015). Although we are not aware of a study that directly examined functional connectivity of the ACC–pSTG in depression, our results contribute to such evidence.
The WM ACC–pSTG connectivity showed state-independent abnormality in patients with LLD: the axial, radial, and mean diffusivities of the ACC–pSTG tract were higher in patients with LLD. Fractional anisotropy of the ACC–pSTG did not show a significant difference between patients with LLD and healthy subjects. This lack of difference in fractional anisotropy is consistent with our finding that both axial and radial diffusivities are higher in the patients with LLD. Fractional anisotropy is an estimate of the superlativeness of axial diffusivity as compared to the secondary and tertiary diffusivities, radial diffusivity is the mean of the secondary and tertiary diffusivities, while mean diffusivity (also known as the apparent diffusion coefficient) is the mean of the axial, secondary, and tertiary diffusivities. The lack of difference in fractional anisotropy and the higher mean diffusivity in the LLD group is the result of the higher axial and radial diffusivities. Therefore, higher mean diffusivity is the main structural connectivity finding of the current study. Increased mean diffusivity has been repeatedly reported in multiple sclerosis, especially during the acute phase (Christiansen et al., 1993; Tievsky et al., 1999; Werring et al., 2000; Rosso et al., 2006). Our results may imply de-myelinated WM tissue in LLD.
We cautiously propose the following speculative model to describe the MRI results, based on the above discussion. Patients with LLD are structurally and functionally vulnerable in the ACC–pSTG during the asymptomatic state. This may involve maintaining a balance with low-grade ACC–pSTG activity. In an acutely depressed state, the functional ACC–pSTG connectivity would increase to compensate for the weak neural activity of the small GM volume in these two regions, in order to regulate negative ruminative mood and cognition. Subsequently, the functional ACC–pSTG connectivity during the depressed phase would be relatively overactive in these patients, although seeming to be in a normal range as compared to healthy subjects. Then, this hyper-functional connectivity would induce an imbalance between structure and function of the ACC–pSTG, leading to inappropriate mood and cognitive processing, and may result in symptomatic manifestation. However, the overactivation of STG in an rs-fMRI study was observed in depressive patients with a history of suicide attempt, as compared to those without such a history and healthy subjects (Cao et al., 2016). The study also showed that the overactivation was also associated with abnormal impulsivity in suicide attempters. The investigators stated a similar interpretation of the results, together with the finding of a small GM volume in the STG in suicide attempters in another study (Pan et al., 2015). However, this model is speculative, because the functional connectivity was not different between the depressed and remitted stages in patients with LLD, and no other functional or structural connectivity was investigated. To test such a model, substantial multi-modal neuroimaging studies for depression and depression-like animal model studies would be required to elucidate the neural mechanism of mood regulation and cognitive processing. A potential molecular mechanism of the structural and functional vulnerability in LLD may be the effect of inflammation on dysfunctional processing. Some evidence has suggested that structural abnormalities, e.g., vascular depression and hypoperfusion of the brain, were observed in patients with LLD (Alexopoulos et al., 1997; Matsuo et al., 2005); these abnormalities may trigger microglial activation and subsequent neuroinflammation, and this abnormal neuroinflammation processing may lead to demyelination and neurodegeneration (Popa-Wagner et al., 2014; Becker, 2016). To address this hypothesis, inflammatory biomarkers, including interleukin 6 and 8, interferon-alpha, IL-6, IL-8, IFN-alpha and TNF-alpha, together with measurement of structural and functional neuroimaging, would be necessary.
There are some limitations to this study: first, the sample size was small. Thus, statistical power would be somewhat limited, although we applied non-parametric statistical tests. Second, medication may have affected the MRI results, although the effect of medication was inconclusive in patients with mood disorders in meta-analytical and review studies (Koolschijn et al., 2009; Bora et al., 2012; Matsuo et al., 2012). Although the GM volume of the ACC and pSTG, and structural and functional connectivity of the two regions were not associated with medication load in the present study, we cannot exclude the possibility that medication could have masked the results. Third, as we conducted a seed-based approach in the ACC and pSTG, other regions relevant to mood and cognitive processing in LLD may have indirectly influenced the results related to the functional connectivity of these two regions. Lastly, we did not perform MRI re-scanning in healthy subjects; thus it cannot be excluded that structural and functional changes in certain regions may have occurred in healthy subjects over the observation period, which may alter the conclusions of the current study.
In conclusion, the findings of the present preliminary study provide tentative evidence that structural and functional abnormalities of the ACC and pSTG are observed during remission of LLD. Structural connectivity of the ACC–pSTG was independent of mood state and functional ACC–pSTG connectivity was associated with functional outcome. Our findings reveal some insights into brain pathophysiology and may aid in developing neuroanatomical biomarkers for LLD.
Author Contributions
KM conceived and designed the experiments. KH, MN, TW, MH, TM, HY and YW performed the experiments and collected the data. KH and TI analyzed MR data. KH, TI and KM discussed the results and wrote the article.
Conflict of Interest Statement
TI has received speaker’s honoraria from Eli Lilly, Daiichi Sankyo and Dainippon Sumitomo. KM received honoraria from GlaxoSmithKline, Mochida Pharmaceutical, and Meiji Seika Pharma and research donations from Otsuka Pharmaceutical. YW received research donations from MSD, GlaxoSmithKline, Eli Lilly and Company, Yoshitomiyakuhin, Shinogi, Pfizer, Janssen Pharma, Meiji Seika Pharma, FujiFilum RI Pharma, Takeda Pharmaceutical, Astellas, Dainippon Sumitomo Pharma and Otsuka Pharmaceutical. The other authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank all the participants in the study. We also thank Ms. Ayumi Kobayashi, Ms. Kumiko Hara, Ms. Kiyoe Yamauchi, and other staff members at Division of Neuropsychiatry, Department of Neuroscience, Yamaguchi University Graduate School of Medicine for their help in data collection.
Funding. This work was supported by Grants-in-Aid for “Integrated research on neuropsychiatric disorders” from the Strategic Research Program for Brain Sciences by the Ministry of Education, Culture, Sports, Science and Technology of Japan (SRPBS) and Japan Agency for Medical Research and Development (AMED), and Culture of Japan and Japan Society for the Promotion of Science (JSPS) KAKENHI grant numbers 15K09832. Image preprocessing was partly performed using the supercomputer cluster at the Mississippi Center for Supercomputing Research partly funded by the National Science Foundation (EPS- 0903787).
References
- Abe O., Yamasue H., Kasai K., Yamada H., Aoki S., Inoue H., et al. (2010). Voxel-based analyses of gray/white matter volume and diffusion tensor data in major depression. Psychiatry Res. 181, 64–70. 10.1016/j.pscychresns.2009.07.007 [DOI] [PubMed] [Google Scholar]
- Abu-Akel A., Shamay-Tsoory S. (2011). Neuroanatomical and neurochemical bases of theory of mind. Neuropsychologia 49, 2971–2984. 10.1016/j.neuropsychologia.2011.07.012 [DOI] [PubMed] [Google Scholar]
- Aichhorn M., Perner J., Weiss B., Kronbichler M., Staffen W., Ladurner G. (2009). Temporo-parietal junction activity in theory-of-mind tasks: falseness, beliefs, or attention. J. Cogn. Neurosci. 21, 1179–1192. 10.1162/jocn.2009.21082 [DOI] [PubMed] [Google Scholar]
- Alexopoulos G. S., Hoptman M. J., Kanellopoulos D., Murphy C. F., Lim K. O., Gunning F. M. (2012). Functional connectivity in the cognitive control network and the default mode network in late-life depression. J. Affect. Disord. 139, 56–65. 10.1016/j.jad.2011.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulos G. S., Meyers B. S., Young R. C., Campbell S., Silbersweig D., Charlson M. (1997). ‘Vascular depression’ hypothesis. Arch. Gen. Psychiatry 54, 915–922. 10.1001/archpsyc.1997.01830220033006 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (2000). Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR). Arlington, VA: American Psychiatric Publishing. [Google Scholar]
- Andreescu C., Butters M. A., Begley A., Rajji T., Wu M., Meltzer C. C., et al. (2008). Gray matter changes in late life depression—a structural MRI analysis. Neuropsychopharmacology 33, 2566–2572. 10.1038/sj.npp.1301655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreescu C., Tudorascu D. L., Butters M. A., Tamburo E., Patel M., Price J., et al. (2013). Resting state functional connectivity and treatment response in late-life depression. Psychiatry Res. 214, 313–321. 10.1016/j.pscychresns.2013.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna J. R., Smallwood J., Spreng R. N. (2014). The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Ann. N Y Acad. Sci. 1316, 29–52. 10.1111/nyas.12360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apperly I. A., Samson D., Chiavarino C., Humphreys G. W. (2004). Frontal and temporo-parietal lobe contributions to theory of mind: neuropsychological evidence from a false-belief task with reduced language and executive demands. J. Cogn. Neurosci. 16, 1773–1784. 10.1162/0898929042947928 [DOI] [PubMed] [Google Scholar]
- Basile B., Mancini F., Macaluso E., Caltagirone C., Frackowiak R. S., Bozzali M. (2011). Deontological and altruistic guilt: evidence for distinct neurobiological substrates. Hum. Brain Mapp. 32, 229–239. 10.1002/hbm.21009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker K. J. (2016). Inflammation and the silent sequelae of stroke. Neurotherapeutics 13, 801–810. 10.1007/s13311-016-0451-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens T. E., Berg H. J., Jbabdi S., Rushworth M. F., Woolrich M. W. (2007). Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? Neuroimage 34, 144–155. 10.1016/j.neuroimage.2006.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B. B., Mennes M., Zuo X.-N., Gohel S., Kelly C., Smith S. M., et al. (2010). Toward discovery science of human brain function. Proc. Natl. Acad. Sci. 107, 4734–4739. 10.1073/pnas.0911855107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora E., Fornito A., Pantelis C., Yücel M. (2012). Gray matter abnormalities in major depressive disorder: a meta-analysis of voxel based morphometry studies. J. Affect. Disord. 138, 9–18. 10.1016/j.jad.2011.03.049 [DOI] [PubMed] [Google Scholar]
- Bush G., Luu P., Posner M. I. (2000). Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn. Sci. 4, 215–222. 10.1016/s1364-6613(00)01483-2 [DOI] [PubMed] [Google Scholar]
- Cao J., Chen X., Chen J., Ai M., Gan Y., Wang W., et al. (2016). Resting-state functional MRI of abnormal baseline brain activity in young depressed patients with and without suicidal behavior. J. Affect. Disord. 205, 252–263. 10.1016/j.jad.2016.07.002 [DOI] [PubMed] [Google Scholar]
- Charlton R. A., Leow A., Gadelkarim J., Zhang A., Ajilore O., Yang S., et al. (2015). Brain connectivity in late-life depression and aging revealed by network analysis. Am. J. Geriatr. Psychiatry 23, 642–650. 10.1016/j.jagp.2014.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P. S., McQuoid D. R., Payne M. E., Steffens D. C. (2006). White matter and subcortical gray matter lesion volume changes and late-life depression outcome: a 4-year magnetic resonance imaging study. Int. Psychogeriatr. 18, 445–456. 10.1017/s1041610205002796 [DOI] [PubMed] [Google Scholar]
- Christiansen P., Gideon P., Thomsen C., Stubgaard M., Henriksen O., Larsson H. B. (1993). Increased water self-diffusion in chronic plaques and in apparently normal white matter in patients with multiple sclerosis. Acta Neurol. Scand. 87, 195–199. 10.1111/j.1600-0404.1993.tb04100.x [DOI] [PubMed] [Google Scholar]
- Corradi-Dell’Acqua C., Hofstetter C., Vuilleumier P. (2014). Cognitive and affective theory of mind share the same local patterns of activity in posterior temporal but not medial prefrontal cortex. Soc. Cogn. Affect. Neurosci. 9, 1175–1184. 10.1093/scan/nst097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinsky O., Morrell M. J., Vogt B. A. (1995). Contributions of anterior cingulate cortex to behaviour. Brain 118, 279–306. 10.1093/brain/118.1.279 [DOI] [PubMed] [Google Scholar]
- Du M., Liu J., Chen Z., Huang X., Li J., Kuang W., et al. (2014). Brain grey matter volume alterations in late-life depression. J. Psychiatry Neurosci. 39, 397–406. 10.1503/jpn.130275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyre H. A., Yang H., Leaver A. M., Van Dyk K., Siddarth P., Cyr N. S., et al. (2016). Altered resting-state functional connectivity in late-life depression: a cross-sectional study. J. Affect. Disord. 189, 126–133. 10.1016/j.jad.2015.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firbank M. J., Teodorczuk A., van der Flier W. M., Gouw A. A., Wallin A., Erkinjuntti T., et al. (2012). Relationship between progression of brain white matter changes and late-life depression: 3-year results from the LADIS study. Br. J. Psychiatry 201, 40–45. 10.1192/bjp.bp.111.098897 [DOI] [PubMed] [Google Scholar]
- Fischl B., Salat D. H., Busa E., Albert M., Dieterich M., Haselgrove C., et al. (2002). Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33, 341–355. 10.1016/S0896-6273(02)00569-X [DOI] [PubMed] [Google Scholar]
- Fischl B., Sereno M. I., Dale A. M. (1999). Cortical surface-based analysis: II. Inflation, flattening, and a surface-based coordinate system. Neuroimage 9, 195–207. 10.1006/nimg.1998.0396 [DOI] [PubMed] [Google Scholar]
- Folstein M. F., Folstein S. E., McHugh P. R. (1975). “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 12, 189–198. 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- Gasquoine P. G. (2013). Localization of function in anterior cingulate cortex: from psychosurgery to functional neuroimaging. Neurosci. Biobehav. Rev. 37, 340–348. 10.1016/j.neubiorev.2013.01.002 [DOI] [PubMed] [Google Scholar]
- Guo W., Liu F., Xun G., Hu M., Guo X., Xiao C., et al. (2014). Disrupted white matter integrity in first-episode, drug-naive, late-onset depression. J. Affect. Disord. 163, 70–75. 10.1016/j.jad.2014.03.044 [DOI] [PubMed] [Google Scholar]
- Hamilton J. P., Farmer M., Fogelman P., Gotlib I. H. (2015). Depressive rumination, the default-mode network and the dark matter of clinical neuroscience. Biol. Psychiatry 78, 224–230. 10.1016/j.biopsych.2015.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada K., Matsuo K., Nakashima M., Hobara T., Higuchi N., Higuchi F., et al. (2016). Disrupted orbitomedial prefrontal limbic network in individuals with later-life depression. J. Affect. Disord. 204, 112–119. 10.1016/j.jad.2016.06.031 [DOI] [PubMed] [Google Scholar]
- Ikuta T., Matsuo K., Harada K., Nakashima M., Hobara T., Higuchi N., et al. (2017). Disconnectivity between dorsal raphe nucleus and posterior cingulate cortex in later life depression. Front. Aging Neurosci. 9:236. 10.3389/fnagi.2017.00236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inada T., Inagaki A. (2015). Psychotropic dose equivalence in Japan. Psychiatry Clin. Neurosci. 69, 440–447. 10.1111/pcn.12275 [DOI] [PubMed] [Google Scholar]
- Kaiser R. H., Andrews-Hanna J. R., Wager T. D., Pizzagalli D. A. (2015). Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA Psychiatry 72, 603–611. 10.1001/jamapsychiatry.2015.0071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalaf A., Edelman K., Tudorascu D., Andreescu C., Reynolds C. F., Aizenstein H. (2015). White matter hyperintensity accumulation during treatment of late-life depression. Neuropsychopharmacology 40, 3027–3035. 10.1038/npp.2015.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolschijn P. C., van Haren N. E., Lensvelt-Mulders G. J., Hulshoff Pol H. E., Kahn R. S. (2009). Brain volume abnormalities in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Hum. Brain Mapp. 30, 3719–3735. 10.1002/hbm.20801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavretsky H., Ballmaier M., Pham D., Toga A., Kumar A. (2007). Neuroanatomical characteristics of geriatric apathy and depression: a magnetic resonance imaging study. Am. J. Geriatr. Psychiatry 15, 386–394. 10.1097/jgp.0b013e3180325a16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F., Hu M., Wang S., Guo W., Zhao J., Li J., et al. (2012). Abnormal regional spontaneous neural activity in first-episode, treatment-naive patients with late-life depression: a resting-state fMRI study. Prog. Neuropsychopharmacol. Biol. Psychiatry 39, 326–331. 10.1016/j.pnpbp.2012.07.004 [DOI] [PubMed] [Google Scholar]
- Mai J. K., Paxinos G., Voss T. (2008). Atlas of the Human Brain. New York, NY: Elsevier. [Google Scholar]
- Maldjian J. A., Laurienti P. J., Burdette J. H. (2004). Precentral gyrus discrepancy in electronic versions of the Talairach atlas. Neuroimage 21, 450–455. 10.1016/j.neuroimage.2003.09.032 [DOI] [PubMed] [Google Scholar]
- Margulies D. S., Kelly A. M., Uddin L. Q., Biswal B. B., Castellanos F. X., Milham M. P. (2007). Mapping the functional connectivity of anterior cingulate cortex. Neuroimage 37, 579–588. 10.1016/j.neuroimage.2007.05.019 [DOI] [PubMed] [Google Scholar]
- Mars R. B., Neubert F. X., Noonan M. P., Sallet J., Toni I., Rushworth M. F. (2012). On the relationship between the “default mode network” and the “social brain”. Front. Hum. Neurosci. 6:189. 10.3389/fnhum.2012.00189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo K., Onodera Y., Hamamoto T., Muraki K., Kato N., Kato T. (2005). Hypofrontality and microvascular dysregulation in remitted late-onset depression assessed by functional near-infrared spectroscopy. Neuroimage 26, 234–242. 10.1016/j.neuroimage.2005.01.024 [DOI] [PubMed] [Google Scholar]
- Matsuo K., Sanches M., Brambilla P., Soares J. C. (2012). “Structural brain abnormalities in bipolar disorder,” in The Bipolar Brain. Integrating Neuroimaging and Genetics, ed. Strakowski S. M. (New York, NY: Oxford University Press, Inc.), 17–52. [Google Scholar]
- Mayberg H. S., Lozano A. M., Voon V., McNeely H. E., Seminowicz D., Hamani C., et al. (2005). Deep brain stimulation for treatment-resistant depression. Neuron 45, 651–660. 10.1016/j.neuron.2005.02.014 [DOI] [PubMed] [Google Scholar]
- Menon V. (2011). Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn. Sci. 15, 483–506. 10.1016/j.tics.2011.08.003 [DOI] [PubMed] [Google Scholar]
- Morecraft R. J., Van Hoesen G. W. (1992). Cingulate input to the primary and supplementary motor cortices in the rhesus monkey: evidence for somatotopy in areas 24c and 23c. J. Comp. Neurol. 322, 471–489. 10.1002/cne.903220403 [DOI] [PubMed] [Google Scholar]
- Mulders P. C., van Eijndhoven P. F., Schene A. H., Beckmann C. F., Tendolkar I. (2015). Resting-state functional connectivity in major depressive disorder: a review. Neurosci. Biobehav. Rev. 56, 330–344. 10.1016/j.neubiorev.2015.07.014 [DOI] [PubMed] [Google Scholar]
- Nugent A. C., Milham M. P., Bain E. E., Mah L., Cannon D. M., Marrett S., et al. (2006). Cortical abnormalities in bipolar disorder investigated with MRI and voxel-based morphometry. Neuroimage 30, 485–497. 10.1016/j.neuroimage.2005.09.029 [DOI] [PubMed] [Google Scholar]
- Oldfield R. C. (1971). The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9, 97–113. 10.1016/0028-3932(71)90067-4 [DOI] [PubMed] [Google Scholar]
- Otsubo T., Tanaka K., Koda R., Shinoda J., Sano N., Tanaka S., et al. (2005). Reliability and validity of Japanese version of the mini-international neuropsychiatric interview. Psychiatry Clin. Neurosci. 59, 517–526. 10.1111/j.1440-1819.2005.01408.x [DOI] [PubMed] [Google Scholar]
- Pan L. A., Ramos L., Segreti A., Brent D. A., Phillips M. L. (2015). Right superior temporal gyrus volume in adolescents with a history of suicide attempt. Br. J. Psychiatry 206, 339–340. 10.1192/bjp.bp.114.151316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J., Liu J., Nie B., Li Y., Shan B., Wang G., et al. (2011). Cerebral and cerebellar gray matter reduction in first-episode patients with major depressive disorder: a voxel-based morphometry study. Eur. J. Radiol. 80, 395–399. 10.1016/j.ejrad.2010.04.006 [DOI] [PubMed] [Google Scholar]
- Popa-Wagner A., Buga A. M., Tica A. A., Albu C. V. (2014). Perfusion deficits, inflammation and aging precipitate depressive behaviour. Biogerontology 15, 439–448. 10.1007/s10522-014-9516-1 [DOI] [PubMed] [Google Scholar]
- Price J. L., Drevets W. C. (2010). Neurocircuitry of mood disorders. Neuropsychopharmacology 35, 192–216. 10.1038/npp.2009.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle M. E., MacLeod A. M., Snyder A. Z., Powers W. J., Gusnard D. A., Shulman G. L. (2001). A default mode of brain function. Proc. Natl. Acad. Sci. U S A 98, 676–682. 10.1073/pnas.98.2.676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppermund S., Zhuang L., Wen W., Slavin M. J., Trollor J. N., Brodaty H., et al. (2014). White matter integrity and late-life depression in community-dwelling individuals: diffusion tensor imaging study using tract-based spatial statistics. Br. J. Psychiatry 205, 315–320. 10.1192/bjp.bp.113.142109 [DOI] [PubMed] [Google Scholar]
- Rosso C., Remy P., Creange A., Brugieres P., Cesaro P., Hosseini H. (2006). Diffusion-weighted MR imaging characteristics of an acute strokelike form of multiple sclerosis. AJNR Am. J. Neuroradiol. 27, 1006–1008. [PMC free article] [PubMed] [Google Scholar]
- Saxe R., Kanwisher N. (2003). People thinking about thinking people. The role of the temporo-parietal junction in “theory of mind”. Neuroimage 19, 1835–1842. 10.1016/s1053-8119(03)00230-1 [DOI] [PubMed] [Google Scholar]
- Sexton C. E., Allan C. L., Le Masurier M., McDermott L. M., Kalu U. G., Herrmann L. L., et al. (2012). Magnetic resonance imaging in late-life depression: multimodal examination of network disruption. Arch. Gen. Psychiatry 69, 680–689. 10.1001/archgenpsychiatry.2011.1862 [DOI] [PubMed] [Google Scholar]
- Sexton C. E., Mackay C. E., Ebmeier K. P. (2013). A systematic review and meta-analysis of magnetic resonance imaging studies in late-life depression. Am. J. Geriatr. Psychiatry 21, 184–195. 10.1016/j.jagp.2012.10.019 [DOI] [PubMed] [Google Scholar]
- Smagula S. F., Aizenstein H. J. (2016). Brain structural connectivity in late-life major depressive disorder. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 1, 271–277. 10.1016/j.bpsc.2015.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens D. C., McQuoid D. R., Payne M. E., Potter G. G. (2011). Change in hippocampal volume on magnetic resonance imaging and cognitive decline among older depressed and nondepressed subjects in the neurocognitive outcomes of depression in the elderly study. Am. J. Geriatr. Psychiatry 19, 4–12. 10.1097/JGP.0b013e3181d6c245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teodorczuk A., O’Brien J. T., Firbank M. J., Pantoni L., Poggesi A., Erkinjuntti T., et al. (2007). White matter changes and late-life depressive symptoms: longitudinal study. Br. J. Psychiatry 191, 212–217. 10.1192/bjp.bp.107.036756 [DOI] [PubMed] [Google Scholar]
- Tievsky A. L., Ptak T., Farkas J. (1999). Investigation of apparent diffusion coefficient and diffusion tensor anisotrophy in acute and chronic multiple sclerosis lesions. AJNR Am. J. Neuroradiol. 20, 1491–1499. [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., et al. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15, 273–289. 10.1006/nimg.2001.0978 [DOI] [PubMed] [Google Scholar]
- Wang Z., Yuan Y., Bai F., Shu H., You J., Li L., et al. (2015). Altered functional connectivity networks of hippocampal subregions in remitted late-onset depression: a longitudinal resting-state study. Neurosci. Bull. 31, 13–21. 10.1007/s12264-014-1489-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Giannakopoulos P., Delaloye C., de Bilbao F., Moy G., Moussa A., et al. (2010). Volumetric MRI changes, cognition and personality traits in old age depression. J. Affect. Disord. 124, 275–282. 10.1016/j.jad.2009.11.016 [DOI] [PubMed] [Google Scholar]
- Werring D. J., Brassat D., Droogan A. G., Clark C. A., Symms M. R., Barker G. J., et al. (2000). The pathogenesis of lesions and normal-appearing white matter changes in multiple sclerosis: a serial diffusion MRI study. Brain 123, 1667–1676. 10.1093/brain/123.8.1667 [DOI] [PubMed] [Google Scholar]
- Williams J. B. (1988). A structured interview guide for the Hamilton Depression Rating scale. Arch. Gen. Psychiatry 45, 742–747. 10.1001/archpsyc.1988.01800320058007 [DOI] [PubMed] [Google Scholar]
- Wu M., Andreescu C., Butters M. A., Tamburo R., Reynolds C. F., III., Aizenstein H. (2011). Default-mode network connectivity and white matter burden in late-life depression. Psychiatry Res. 194, 39–46. 10.1016/j.pscychresns.2011.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y., Zhu W., Zhang Z., Bai F., Yu H., Shi Y., et al. (2008). Regional gray matter changes are associated with cognitive deficits in remitted geriatric depression: an optimized voxel-based morphometry study. Biol. Psychiatry 64, 541–544. 10.1016/j.biopsych.2008.04.032 [DOI] [PubMed] [Google Scholar]
- Yukie M., Shibata H. (2009). “Temporocingulate interactions in the monkey,” in Cingulate Neurobiology and Disease, ed. Vogt B. A. (New York, NY: Oxford University Press Inc.), 95–112. [Google Scholar]