Chronic lymphocytic leukemia (CLL) and B-cell lymphomas represent a heterogeneous group of common hematologic malignancies arising as a consequence of dysregulated B-cell differentiation and expansion of an abnormal B-cell clone1. Due to an unmet clinical need in elderly patients with relapsed/refractory B-cell malignancies for whom currently there are no curative options2,3, we evaluated the clinical activity and safety of acalisib (GS-9820, 6-fluoro-3-phenyl-2-[(1S)-1-(9H-purin-6-ylamino) ethyl]-4(3H)-quinazolinone), a second-generation inhibitor of phosphoinositide 3-kinase delta (PI3Kδ).
In a human basophil activation assay, acalisib was most selective for PI3Kδ (IC50 12.7 nM), compared with other PI3K class I isoforms. Acalisib suppressed IgE receptor I PI3Kδ-mediated CD63 expression with an EC50 of 14 nM. Its high selectivity was confirmed by the fact that no binding to other kinases was observed at 10 µM acalisib concentration4.
We initiated a phase 1b, open-label, dose escalation and expansion study of acalisib monotherapy in adults with recurrent lymphoid malignancies, who had measurable lymphadenopathy and required therapy for their cancers (NCT01705847). Eligible patients were enrolled into escalating dose cohorts utilizing a 3 + 3 design at the following oral acalisib doses: 50, 100, 200, and 400 mg twice daily. The primary endpoint of the study was to evaluate the maximum tolerated dose (MTD) within the tested dose range. The secondary study objectives were to characterize the dose-limiting toxicities, efficacy (per standard response criteria5,6), and overall safety profile of acalisib (details in Supplemental Materials).
From 21 November 2012 to 30 April 2014, 39 patients were enrolled in four centers in the Netherlands. The final data cutoff presented herein is 17 August 2016, with a median (min, max) follow-up of 6 (0.03, 37.0) months. In total, 38 patients received ≥1 dose of acalisib—four, three and three patients in the acalisib 50, 100, and 200 mg twice-daily dose-finding cohorts, respectively, and 29 patients in the 400 mg twice-daily expansion cohort. Thirty-four (89.5%) patients discontinued prior to study closure, primarily due to progressive disease (12 (32%)) (Supplemental Table 1).
The majority of patients were male 26/38 (68%), with a median age (range) of 69 (48–81) years and a median (Q1, Q3) number of 3 (2, 4) prior therapies. Overall, 22 (57.9%) patients had CLL, 15 (39.5%) had non-Hodgkin’s lymphoma (NHL) and 1 (2.6%) had (Hodgkin’s lymphoma (HL); >50% had refractory disease and 34.2% had relapsed after the last therapy (Supplemental Table 2).
After a median treatment duration of 5.8 months, the overall response rate (ORR) (95% confidence interval (CI)) in all dose cohorts per independent review committee assessment was 42.1% (26.3, 59.2) with 16 partial responses (PRs) (Supplemental Table 3). Responses were observed across all dose cohorts (Supplemental Fig. 1). The ORR (95% CI) for the 29 patients who received 400 mg acalisib twice daily as their initial dose level was 41.4% (23.5, 61.1), with 12 PRs (Supplemental Table 3). When efficacy was analyzed by disease type, a higher ORR (95% CI) was noted in patients with CLL (53.3% (26.6, 78.7)), compared with NHL/HL patients (28.6% (8.4, 58.1)) (Supplemental Table 3).
The lymph node response (95% CI) was 12/14 (85.7% (57.2, 98.2)) in patients with CLL and 4/11 (36.4% (10.9, 69.2)) in patients with NHL/HL. Among patients with CLL, the best percent change from baseline in the sum of the products of the greatest perpendicular diameters exceeding 50% was observed in all dose cohorts, whereas patients with NHL responded only to the highest dose of acalisib (Fig. 1).
Fig. 1. Best percent change from baseline in the SPD per disease type and IRC assessmentsa.
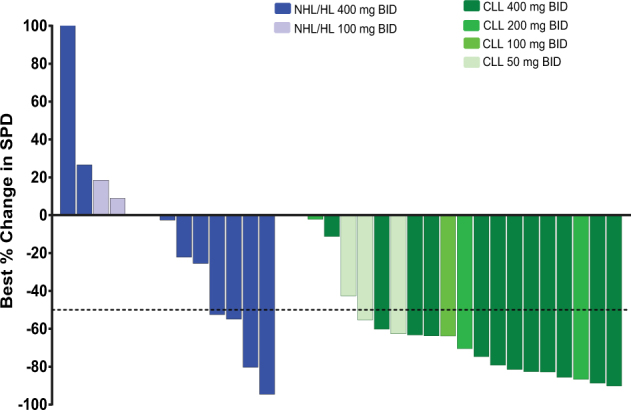
This analysis includes patients with both baseline and post-baseline SPD measurements. Baseline is defined as the last measurement before the first dose of GS-9820. aPatients were analyzed based on the initial dosing level they received. CLL chronic lymphocytic leukemia, NHL/HL non-Hodgkin’s and Hodgkin’s lymphoma, SPD sum of the products of the greatest perpendicular diameters
After a median (Q1, Q3) follow-up of 4.5 (2, 16) months, the median (95% CI) Kaplan–Meier estimate of PFS for the 29 patients initially treated with 400 mg acalisib twice daily was 8.2 (3.4, 16.6) months; 16.6 (3.4, NR) months for patients with CLL and 4.0 (1.6, 16.4) months for patients with NHL/HL (Supplemental Fig. 2).
The median (Q1, Q3 (range)) duration of exposure to acalisib was 5.8 (2, 17 (0–37)) months for all 38 treated patients, and 4.5 (2, 16 (0–30)) months for the 29 patients who received 400 mg twice daily as their initial treatment. Doses ranging from 50 to 400 mg twice daily were not associated with dose-limiting toxicities (DLTs) and the MTD dose has not been determined.
Adverse events (AEs, not exposure-adjusted) were reported in all treated patients (Supplemental Table 4–6) and included all AEs, laboratory abnormalities, and serious AEs. Treatment-emergent AEs (TEAEs) considered related to acalisib were reported by 30 (78.9%) patients, of whom 21 (55.3%) had grade ≥3 events. The most frequent TEAEs were diarrhea, rash, elevated liver transaminases, and infections (Table 1). Grade ≥3 infections (pneumonia, viral pneumonia, bronchiolitis, and Pneumocystis jirovecii pneumonia) were reported individually in four patients. Although at the time of the first interim analysis performed in July 2013 none of the patients had grade ≥3 elevations of hepatic transaminase levels, extended duration of treatment led to 10.5% and 7.9% of patients with grade ≥3 laboratory alanine aminotransferase (ALT) and aspartate aminotransferase (AST) elevations, respectively. After dose interruption, 7.9% of these events resolved to grade 1.
Table 1.
Adverse events related to acalisib in ≥5% of all patients, and all acalisib-related infections
| Adverse events, n (%) | 50 mg BID (N = 3) | 100 mg BID (N = 3) | 200 mg BID (N = 3) | 400 mg BID (N = 29) | Total (N = 38) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| All | Grade ≥3 | All | Grade ≥3 | All | Grade ≥3 | All | Grade ≥3 | All | Grade ≥3 | |
| AEs related to acalisib | 3 (100) | 1 (33.3) | 2 (66.7) | 1 (33.3) | 2 (66.7) | 1 (33.3) | 23 (79.3) | 18 (62.1) | 30 (78.9) | 21 (55.3) |
| Diarrhea | 2 (66.7) | 1 (33.3) | 0 | 0 | 1 (33.3) | 0 | 6 (20.7) | 3 (10.3) | 9 (23.7) | 4 (10.5) |
| Rash | 0 | 0 | 0 | 0 | 0 | 0 | 8 (27.6) | 4 (13.8) | 8 (21.1) | 4 (10.5) |
| Weight decreased | 1 (33.3) | 0 | 0 | 0 | 0 | 0 | 5 (17.2) | 1 (3.4) | 6 (15.8) | 1 (2.6) |
| Alanine aminotransferase increased | 0 | 0 | 1 (33.3) | 0 | 0 | 0 | 4 (13.8) | 1 (3.4) | 5 (13.2) | 1 (2.6) |
| Aspartate aminotransferase increased | 0 | 0 | 1 (33.3) | 0 | 0 | 0 | 4 (13.8) | 1 (3.4) | 5 (13.2) | 1 (2.6) |
| Dysgeusia | 0 | 0 | 0 | 0 | 0 | 0 | 5 (17.2) | 0 | 5 (13.2) | 0 |
| Cough | 0 | 0 | 0 | 0 | 0 | 0 | 4 (13.8) | 0 | 4 (10.5) | 0 |
| Pyrexia | 0 | 0 | 0 | 0 | 0 | 0 | 3 (10.3) | 0 | 3 (7.9) | 0 |
| Nausea | 0 | 0 | 0 | 0 | 0 | 0 | 3 (10.3) | 0 | 3 (7.9) | 0 |
| Neutrophil count decreased | 0 | 0 | 0 | 0 | 0 | 0 | 3 (10.3) | 3 (10.3) | 3 (7.9) | 3 (7.9) |
| Drug eruption | 0 | 0 | 0 | 0 | 0 | 0 | 2 (6.9) | 0 | 2 (5.3) | 0 |
| Blood alkaline phosphatase increased | 0 | 0 | 0 | 0 | 0 | 0 | 2 (6.9) | 0 | 2 (5.3) | 0 |
| Anemia | 0 | 0 | 0 | 0 | 0 | 0 | 2 (6.9) | 1 (3.4) | 2 (5.3) | 1 (2.6) |
| Hypokalemia | 0 | 0 | 0 | 0 | 0 | 0 | 2 (6.9) | 1 (3.4) | 2 (5.3) | 1 (2.6) |
| All acalisib-related infections and infestations, by preferred term | ||||||||||
| Pneumonia | 1 (33.3) | 0 | 0 | 0 | 0 | 0 | 1 (3.4) | 1 (3.4) | 2 (5.3) | 1 (2.6) |
| Lung infection | 0 | 0 | 0 | 0 | 0 | 0 | 1 (3.4) | 0 | 1 (2.6) | 0 |
| Bronchiolitis | 0 | 0 | 0 | 0 | 0 | 0 | 1 (3.4) | 1 (3.4) | 1 (2.6) | 1 (2.6) |
| Pneumonia viral | 0 | 0 | 0 | 0 | 0 | 0 | 1 (3.4) | 1 (3.4) | 1 (2.6) | 1 (2.6) |
| Mycobacterial infection | 0 | 0 | 0 | 0 | 0 | 0 | 1 (3.4) | 0 | 1 (2.6) | 0 |
| Varicella zoster virus infection | 0 | 0 | 0 | 0 | 0 | 0 | 1 (3.4) | 0 | 1 (2.6) | 0 |
| Parainfluenza virus infection | 0 | 0 | 0 | 0 | 0 | 0 | 1 (3.4) | 0 | 1 (2.6) | 0 |
| Pneumocystis jirovecii pneumonia | 0 | 0 | 0 | 0 | 0 | 0 | 1 (3.4) | 1 (3.4) | 1 (2.6) | 1 (2.6) |
| Upper respiratory tract infection | 0 | 0 | 0 | 0 | 0 | 0 | 1 (3.4) | 0 | 1 (2.6) | 0 |
| Urinary tract infection | 0 | 0 | 0 | 0 | 1 (33.3) | 1 (33.3) | 0 | 0 | 1 (2.6) | 1 (2.6) |
Patients who experienced multiple events within the same preferred term are counted once
BID twice daily
Acalisib-related AEs that led to permanent discontinuation from the study drug occurred in the acalisib 400 mg twice-daily cohort only, and included: grade 5 leukoencephalopathy, grade 2 exanthema, grade 2 allergic reaction (rash), grade 3 hemolysis, grade 3 hypersensitivity allergic reaction, and grade 3 ALT and AST elevations.
In the overall study population, nine (23.7%) patients had an AE that led to death—these included two patients with pneumonia, two with respiratory failure, and one patient each with dyspnea, general physical health deterioration, leukoencephalopathy, pulmonary sepsis, and tumor embolism. Among these nine patients, one patient was in the 50 mg acalisib twice-daily cohort, and the remaining eight patients were treated with 400 mg acalisib twice daily.
In conclusion, acalisib demonstrated clinical activity in the study population. The prominent TEAEs were due to a spectrum of mostly infectious and immune-mediated toxicities described for other PI3Kδ inhibitors (idelalisib, duvelisib)7,8, which may point to a class effect rather than a specific drug effect9–11.
Electronic supplementary material
Acknowledgements
We extend our thanks to the patients and their families and to all the participating study investigators and their staff. This study was sponsored by Gilead Sciences, Inc. Medical writing and editorial support was provided by Ewa Wandzioch, PhD, and Meryl Gersh, PhD, of AlphaBioCom, LLC, King of Prussia, PA, and was funded by Gilead Sciences, Inc.
Author contributions
A.P.K. and A.H.A. performed research, analyzed the data, supervised the study, and created the initial draft of the manuscript. M.S. collected/assisted in data collection, study submission, and study logistic. S.H.T. and M.E.D.C. treated patients in the trial. M.J.K. recruited and treated patients, and provided oversight for the study/study team. L.D. performed research and contributed to the data interpretation. R.L. performed research, supervised the study, provided patient support and guidance, and conducted the trial on a daily basis. Employees of Gilead Sciences, Inc., A.H.A., J.G., L.D., and Y.X., contributed to the study design, implementation, and data analyses. All authors critically reviewed and revised each draft of the manuscript and approved of the final submitted version. E.W. and M.G. provided medical writing and editorial support. All authors and writers had access to study data at all times when preparing this manuscript.
Conflict of interest
M.J.K., A.P.K., and M.E.D.C. have received honoraria for advisory boards and presentations from Gilead Sciences, Inc. J.K.D., M.S., and R.L. have nothing to disclose. A.H.A., J.G., L.D., and Y.X. are employees of Gilead Sciences, Inc. Medical writers E.W. and M.G. were sponsored by Gilead Sciences, Inc.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Information accompanies this paper at 10.1038/s41408-018-0055-x.
References
- 1.Shaffer AL, Rosenwald A, Staudt LM. Lymphoid malignancies: the dark side of B-cell differentiation. Nat. Rev. Immunol. 2002;2:920–932. doi: 10.1038/nri953. [DOI] [PubMed] [Google Scholar]
- 2.Eichhorst BF, et al. First-line therapy with fludarabine compared with chlorambucil does not result in a major benefit for elderly patients with advanced chronic lymphocytic leukemia. Blood. 2009;114:3382–3391. doi: 10.1182/blood-2009-02-206185. [DOI] [PubMed] [Google Scholar]
- 3.Tallarico M, et al. Toxicities and related outcomes of elderly patients (pts) (≥65 years) with hematologic malignancies in the contemporary era (Alliance A151611) Blood. 2016;128:536. [Google Scholar]
- 4.Shugg RP, et al. Effects of isoform-selective phosphatidylinositol 3-kinase inhibitors on osteoclasts: actions on cytoskeletal organization, survival, and resorption. J. Biol. Chem. 2013;288:35346–35357. doi: 10.1074/jbc.M113.507525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheson BD, et al. Revised response criteria for malignant lymphoma. J. Clin. Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 6.Hallek M, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446–5456. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coutre SE, et al. Management of adverse events associated with idelalisib treatment: expert panel opinion. Leuk. Lymphoma. 2015;56:2779–2786. doi: 10.3109/10428194.2015.1022770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flinn I, et al. A phase 1 evaluation of duvelisib (IPI-145), a PI3K-δ,γ inhibitor, in patients with relapsed/refractory iNHL. Blood. 2014;124:802. [Google Scholar]
- 9.Patnaik A, et al. First-in-human phase I study of copanlisib (BAY 80-6946), an intravenous pan-class I phosphatidylinositol 3-kinase inhibitor, in patients with advanced solid tumors and non-Hodgkin’s lymphomas. Ann. Oncol. 2016;27:1928–1940. doi: 10.1093/annonc/mdw282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lampson BL, et al. Idelalisib given front-line for treatment of chronic lymphocytic leukemia causes frequent immune-mediated hepatotoxicity. Blood. 2016;128:195–203. doi: 10.1182/blood-2016-03-707133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel, M. R. et al. Early clinical activity and pharmacodynamic effects of duvelisib, a PI3Kδγ inhibitor, in patients with treatment naïve CLL. J. Clin. Oncol.33, (2015) (Suppl; Abstr 7074).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


