Abstract
Organophosphate-based compounds (OPs) represent a significant threat to warfighters (nerve agents) and civilian populations (pesticides). There is a pressing need to develop in vitro brain models that correlate to the in vivo brain to rapidly study OPs for neurotoxicity. Here we report on a microfluidic-based three-dimensional, four-cell tissue construct consisting of 1) a blood-brain barrier that has dynamic flow and membrane-free culture of the endothelial layer, and 2) an extracellular matrix (ECM)-embedded tissue construct with neuroblastoma, microglia, and astrocytes. We demonstrated this platform’s utility by measuring OP effects on barrier integrity, acetylcholinesterase (AChE) inhibition, viability and residual OP concentration with four model OPs. The results show that the OPs penetrate the blood brain barrier (BBB) and rapidly inhibit AChE activity, and that in vitro toxicity was correlated with available in vivo data. This paper demonstrates the potential utility of a membrane-free tetra-cultured brain on chip that can be scaled to high throughput as a cost-effective alternative method to animal testing.
Introduction
Organophosphate-based compounds (OPs) pose an acutely toxic threat to both warfighters and civilian populations, creating an urgent need to develop better protective measures and therapeutic interventions1. OP poisoning causes the inhibition of acetylcholinesterase (AChE)1–5, leading to the accumulation of acetylcholine (ACh) in synapses and neuromuscular junctions and thus hyper-cholinergic activity that results in excitotoxicity, seizures, and brain damage6–8. Recent studies demonstrated that OPs generated oxidative stress and lipid peroxidation in different cell types and elevated the production of reactive oxygen species (ROS) and DNA damage9,10. Mitochondrial-mediated and caspase-regulated apoptosis are associated with different signaling mechanisms including the mitogen-activated protein kinase (MAPK) pathways and activation of proapoptotic markers-Bax, and caspases-9/311,12. The blood brain barrier (BBB) provides the first line of defense for the central nervous system, and determines the rate at which OPs will reach the brain. The BBB is a selective diffusion barrier formed by specialized endothelial cells lining cerebral microvessels. These cells are characterized by high electrical resistance, epithelial-like tight junctions, and enriched expression of transport proteins that facilitate update of essential nutrients and efflux of xenobiotics13. The BBB interacts with other cellular components of the central nervous system (CNS), such as astrocytes, pericytes, and neuronal processes. However, many OPs readily cross the BBB, resulting in lethal CNS disruption14. Thus, understanding OP penetration of the BBB is the first critical piece of information required to predict acute neurotoxicity. This has been studied recently with the development of animal models of acute OP neurotoxicity15–18. However, relying exclusively on animal models severely hampers the speed with which new drug targets and candidate therapeutics can be identified and qualified, in addition to making development more expensive.
There is a clear need to develop more realistic in vitro brain models that simulate brain activities, mechanical environment, and complex physiological responses. Recent efforts to develop better in vitro brain models include brain organoids, mini-brain, microfluidic-based brain on a chip, tissue chip, and iPSC-derived microphysiological systems19–21. Recent studies account for differentiation to certain cellular subtypes of the human brain, including a myriad of neuronal subtypes, glial cells and endothelium. These in vitro systems may also be used to mimic diseases such as Alzheimer’s and Parkinson’s diseases22.
This paper reports on development of a perfusion-based3D brain culture platform that mimics the neurovascular environment. We constructed an in vitro brain model consisting of two membrane-free compartments: 1) an endothelial cell-lined vascular compartment, and 2) an extracellular matrix-embedded brain tissue compartment (neuroblastoma, astrocytes, and microglia). This plate-based microfluidic platform allows for potentially automated high throughput and high-content imaging with relatively fast readouts. We evaluated four OPs for concentration-dependent effects on: 1) overall cell viability/toxicity within the construct, 2) penetration of OPs across the model BBB, 3) inhibition of AChE activity in target cells following exposure, and 4) residual OP in endothelial vascular compartment.
Methods
Chemicals and reagents
Dimethyl methylphosphonate (DMMP, 97%), diethyl methylphophate (DEMP, 97%), diethyl cyanophosphonate (DECP, 90%), and diethyl chlorophosphate (DCP, 97%), (Scheme S1) were purchased from Sigma (St. Louis, MO, USA). Dulbecco’s modified eagle’s medium (DMEM) supplemented with antibiotics for cell culture was purchased from ATCC (Manassas, VA). Fetal bovine serum (FBS) was purchased from ATLANTA biologicals (Flowery Branch, GA). All other reagents were purchased from Sigma and Fisher Scientific unless otherwise noted.
Cell culture
Complete growth media for all cells (including co-cultures) consisted of DMEM supplemented with 10% FBS and antibiotics. Cryo-preserved bEnd.3 (immortalized murine brain endothelial cells), N2a (immortalized murine brain neuroblastoma), and C8-D1A (immortalized murine astrocytes), were purchased from ATCC. BV-2 (immortalized murine microglia) was obtained from Dr. G. Jean Harry (National Institute of Environmental Health Sciences, Research Triangle Park, NC). bEnd.3, C8-D1A, and BV-2 were maintained as previously described23. N2a cells were differentiated prior to incorporation in co-cultures by replacing complete medium with 0.2% FBS in DMEM for one day after passage. The cells were maintained in medium for 2–3 days followed by replacement with FBS-free DMEM one day prior to co-culture.
3D brain tetra-culture
The microfluidic chip (OrganoPlate, MIMETAS, the Netherlands) was used for tetra-culture and 3D blood-brain barrier construction. N2a, C8-D1A, and BV-2 cells were harvested and counted in complete growth media. The counted cells for brain compartment were spun down and the tube with the cell pellet was kept cool. The pellet was suspended in the extracellular matrix (ECM); one part 10 × phosphate-buffered saline (PBS) was added to nine parts concentrated rat tail collagen I solution (Corning, Collagen I Rat Tail, 10.21 mg/ml) diluted to 4 mg/ml with DMEM (without supplement), and the pH was adjusted to 7.0–7.4 with 20 mg/ml NaOH. Cells of 2 μl was added to either each gel inlet (brain lane) well of 2-lane. The plate was placed in an incubator (37 °C, 5% CO2) for 45 min to allow the gels to polymerize. Harvested bEnd.3 were counted and seeded at 2 μl of 1 × 107 cells/ml into the inlet wells (tunable perfusion lane) of the plate after gelling (brain lane). The plate was put on the side (angle of 75 degrees) for 1 h to allow the bEnd.3 cells to sediment against the gel. After addition of 50 μl medium to the outlet well, the tetra-cells were incubated again on the side (angle of 75 degrees) for 4 h to complete cell attachment. Additional medium (50 μl) was loaded into the inlet well and the plate was placed on an interval rocker (MIMETAS, The Netherlands), allowing bi-directional flow for perfusion. Medium was refreshed every 2 days.
Fluorescence microscopy
Cells (in 2-lane microfluidic channel) were fixed in 4% paraformaldehyde (PFA) in PBS (phosphate-buffered saline) for 15 min, washed twice with PBS for 5 min, and permeabilized with Triton X-100 (0.1% in PBS) for 5 min. Cells were blocked for 45 min at room temperature with 10% normal donor horse serum in PBS. Subsequently, cells were incubated with primary antibodies for 90 min, washed three times, incubated with secondary antibodies for 60 min and washed three times with PBS. Cells were counterstained with Hoechst (Invitrogen H3570). The following antibodies were used for immunohistochemistry: Claudin-5 (1:50, Invitrogen, 341600), Anti-MAP2 (1:100, Invitrogen PA110005), Goat anti-Rabbit AlexaFluor 568 (1:50, Invitrogen A11036), Goat anti-Chicken AlexaFluor 647 (1:100, Life Tech, A21449). Image data was acquired and processed using two-photon confocal microscope (ZEISS Multiphoton LSM 710) and ZEN software.
Cell viability
The cell viability was performed by using Hoechst to count all cells (blue), and Ethidium Homodimer (EthD-1, the “dead” agent) to stain the dead cells (red). The cells were washed two times with PBS buffer and incubated for 30 min with concentration of Hoechest (1:2000) and 4 μM EthD-1 in D-PBS. We took fluorescent images, automatically counted cells in image-Pro Plus (Media Cybernetics, Rockville, MD). % dead cells were calculated: % dead cells = (number of red foci/number of blue foci) × 100%23.
OP exposure and residual measurement
Following 5 days of 3D tetra-culture, the medium was removed. Four different OPs (DMMP, DEMP, DECP, and DCP) were dissolved in medium. OPs at concentrations of 10−1 to 10−7 M were added to the each inlet well of the neurovascular endothelial lane (blood lane). After 24 h, residual concentration of OPs in the blood lane was determined by LC-MS/MS, triple quadrupole mass spectrometer coupled with Shimadzu Prominence HPLC (API 3200, AB Sciex).
Acetylcholinesterase (AChE) assay
After four different OPs exposure for 24 h, media in brain on chip were taken and stored at −20 °C until AChE assay testing. AChE activities were measured according to the manufacturer’s instruction (Molecular Probes™ Amplex™ Acetylcholine/Acetlycholinesterase Assay Kit), their activity was measured in 200 µl total volume, using a CLARIOstar microplate reader at 25 °C (BMG LABTECH).
Results
Dynamic 3D tetra-culture platform
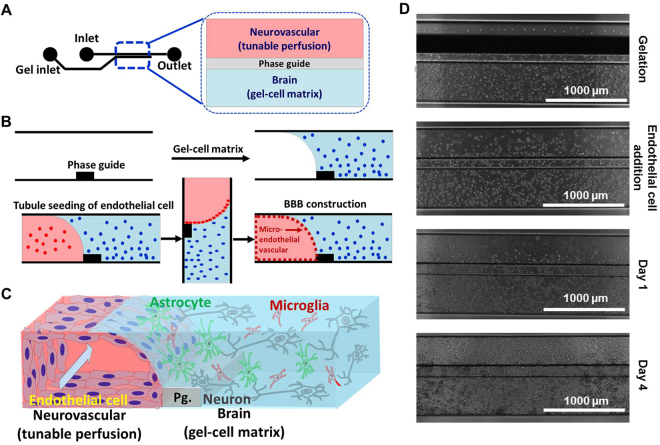
Figure 1 shows the process used to construct the brain on chip. We first loaded collagen-embedded neuroblastoma, astrocyte, and microglia (gel-cell matrix) into the brain lane using capillary pressure barriers called phase guides which enable separation of gel and fluid phases and form a membrane-free substrate for endothelial cell attachment22,24 and allowed the gels to polymerize (Fig. 1B). Endothelial cells were seeded against the gel-cell matrix of brain lane. Following stable attachment on the gel-cell matrix, endothelial cells formed a confluent monolayer, followed by formation of a tube-like structure (Fig. 1C). The plate was kept on a perfusion rocker which provided shear stress and fluidic flow. Figure 1C shows final brain tissue construct in which this scheme was successfully implemented for OPs screening. The four different cells grew well in two different compartments while forming a 3D structure (Fig. 1D).
Figure 1.
3D-brain construction for high content OPs toxicity screening; (A) structure and format application of in vitro cultureware, OrganoPlate consisting of 384 well microliter plate with 2 lanes, (B) experimental procedure for constructing 3D-neurovascular/brain compartment, (C) scheme of 3D tetra-culture for brain on chip, and (D) time lapsed images for 3D tetra-culture.
Integration of brain on chip construct
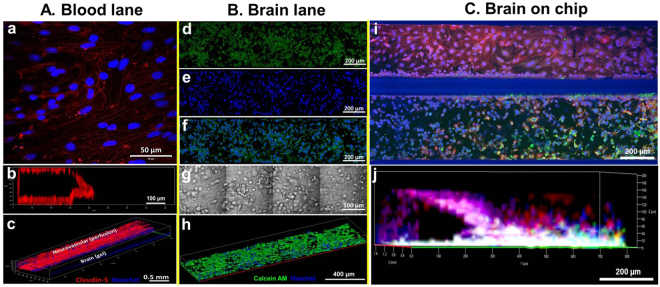
We optimized brain on chip construction by culturing two compartment lanes separately. We first injected collagen matrix containing neuroblastoma and glia into brain compartment and then seeded endothelial cells, 1.0 × 107 cells/ml against to collagen matrix (angle of 75 degrees) in the incubator for 4–6 hours. Following stable attachment of the endothelial cell on the collagen matrix, we placed brain on chip on the programmable rocker which can provide perfusion flow with an average medium flow of 1.5 μL h−1 25. As shown in Fig. 2A, endothelial cells formed a confluent monolayer (Fig. 2a), followed by formation of a tube like structure (Fig. 2b,c). Confocal images show neurovascular construct such as tight junction formation (claudin-5 red and nucleus blue).
Figure 2.
Construction confocal imaging of blood lane and brain lane; (A) blood lane only; (a) 2D stained image of tight junction (claudin-5, red) and nucleus (Hoechst, blue) for neurovascular, (b) cross-sectional 3D image of neurovascular, (c) 3D image of neurovascular, (B) brain lane only; (d) live (Calcein AM, green), (e) nucleus (Hoechst, blue), (f) merged image of live and nucleus images, (g) bright field image, (h) 3D image of merged construct of gel-3 different type cells matrix, (C) Brain on chip by the tetra-culture (claudin-5, red; calcein AM, green, and Hoechst, blue); (i) 2D stained image for blood and brain lanes, (j) 3D images of brain construct with endothelial, neuroblastoma, astrocyte, and microglia cells.
The brain compartment lane was also optimized by balancing cell density, 2.5 × 104 total cells in collagen gel, gelation time, medium compatibility, and ratio between different cells, astrocyte, neuroblastoma and microglia was 40%: 40%: 20%. Figure 2B shows stained images of brain construct of astrocyte, neuroblastoma and microglia with Calcein AM (green) and Hoechst (blue) after culturing for 5 days. In order to make sure of neuroblastoma differentiation, we cultured neuroblastoma only in collagen matrix in brain compartment lane in serum free medium for one day and we are able to observe neurite growths (Fig. S1). In brain lane (Fig. 2B), the result of three different cells growth can make sure well with cell’s shapes from bright field image (Fig. 2g).
Figure 2C shows confocal images of final brain on chip construct consisting of blood compartment lane (neurovascular with endothelial cell, b.End3 cells) and brain compartment lane (differentiated neuroblastoma N2a, astrocyte C8D1A, microglia BV2) unit. Claudin-5 (red) was stained for endothelial cells, live (green) and nucleus (blue). Figure 2j show confocal image of 3D constructed neurovascular structure with brain. Non-specific binding of claudin-5 (red) in the gel-cell matrix was observed (Fig. 2i and Fig. S2). We tested reproducibility of brain on chip platform. We took one column of 96-well plate of brain on chip and constructed 16 brains. Each brain on chip has cells ranging from 350 to 400 at certain layer in 3D brain construction (Fig. S3).
OP effects on AChE activity, viability, and residual Ops
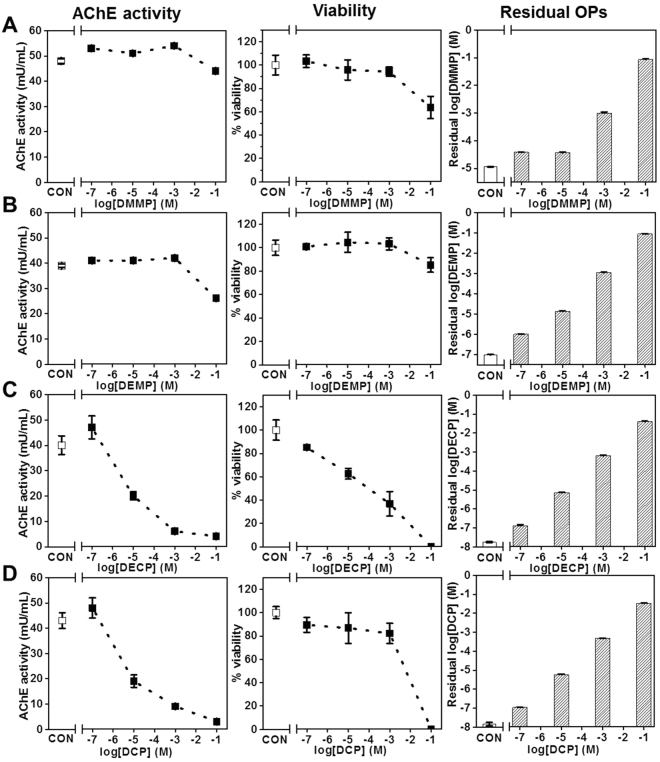
Four OPs were assessed for effects on AChE activity, cell viability, and residual OP in the blood lane using 3D dynamic tetra-culture model. To minimize cross-over of OPs, we added OPs from low concentration to high concentration with 3 replicates (Fig. S3). High-content image analyses were performed for viability and medium samples in blood lane were harvested for AChE analysis and residual OP measurement. These results were compared with control (no OP exposure) across all experiment (Fig. 3). In this model, baselines of control AChE expression/activity and viability were very close across all experiments. We applied concentrations of four OPs ranged from 10−7 M to 10−1 M for 24 hours. Figure 3 shows the measurement of AChE activity (left column), viability (center column), and residual OPs (right column). Based on AChE inhibition and viability, the results show that DMMP and DEMP (Fig. 3A and Fig. 3B) only have toxic effects on tetra-cultured brain at high concentrations (>10−3 M). The results for DECP and DCP show more potent toxic effect on brain tetra-cultured model in terms of AChE inhibition and viability. Concentration-dependent decreases in AChE were observed with DECP and DCP, with estimated IC50’s on the order of 10−5 M The residual concentrations of all OPs in the brain compartment correspond to the applied concentrations indicating high permeability across the BBB and metabolic stability within the time frame of the experiments.
Figure 3.
OP exposure results with optimized 3D dynamic tetra-culture brain on chip model. Controls (no OP) are presented on the left side of the broken x axis (labeled CON). AChE activity, viability, and LC-MS/MS data are mean ± S.D., n = 3 technical replicates.
Comparison to in vivo data
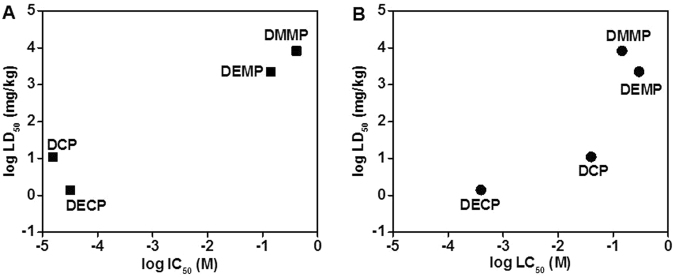
The compounds tested in this study are mainly used as chemical surrogates for nerve agents, and thus there are relatively few biological data sets available26–29. Estimated IC50 (AChE activity) and LC50 (viability) from Fig. 3 were summarized with LD50 in vivo for the OPs used in this study (Table 1). Plots between the known in vivo data and IC50 or LC50 showed reasonably good correlations (Fig. 4). From in vitro brain on chip model and in vivo data in literature, DECP and DCP shows more neurotoxicity than other OPs.
Table 1.
| Organophosphates | AChE activity (IC50, M) | Toxicity (LC50, M) | In vivo (LD50, mg/kg) |
|---|---|---|---|
| Dimethyl methylphosphonate (DMMP) | 4.1 × 10−1 | 1.5 × 10−1 | 8210 |
| Diethyl methylphosphonate (DEMP) | 1.4 × 10−1 | 3.0 × 10−1 | 2240 |
| Diethyl cyanophosphonate (DECP) | 3.2 × 10−5 | 4.0 × 10−4 | 1.4 |
| Diethyl chlorophosphate (DCP) | 1.6 × 10−5 | 4.0 × 10−2 | 11 |
Figure 4.
In vitro to in vivo toxicity correlation based on the log estimated IC50 (A, for AChE activity), LC50 (B, for in vitro viability), and LD50 for DMMP, DEMP, DECP, and DCP: (A) log LD50 vs. log IC50, and (B) log LD50 vs. log LC50.
Discussion
This platform provides an in vitro model of the BBB (endothelial cells and astrocytes) together with cells that mediate brain injury responses (microglia) and surrogates for the primary targets of OP toxicity (neurons) in 3D conditions with dynamic flow and membrane-free co-culture in a 96-well plate format. This represents a unique combination of features enhancing both physiological relevance and potential for throughput not previously achieved with in vitro models of the BBB. To demonstrate the utility of this model, we evaluated four OPs for concentration-dependent effects on: 1) overall cell viability/toxicity within the construct, 2) barrier integrity (Fig. S4A), 3) penetration of OP across the model BBB (Fig. S4B), and 4) inhibition of acetylcholinesterase (AChE) activity following exposure.
In this platform, the endothelial layer forms a 3D neurovascular structure against the membrane-free ECM–cells construct (200 μm height), which allows perfusion of nutrition and oxygen to ECM-cells construct and direct cell-cell contact between the parenchymal cells and the endothelium. Even though b.End3 endothelial cells do not form high-resistance tight junctions24, we observed significant changes from electrical resistance as well as FITC-Dextran diffusion experiment (Fig. 4S). An advantage of this platform is that the presence of an endothelial barrier creates a more physiologically relevant exposure route than monocultures (or co-cultures) of brain cells being exposed directly to chemicals. While we found that the OPs tested cross the model BBB and rapidly affect AChE activity of neuronal/glial co-cultures, often independent of cell toxicity, the endothelium may serve to reduce the toxicity of these substances by metabolic effects, even if it doesn’t impede their passage into brain tissue. Moreover, the barrier properties of the endothelium play a larger role in determining the neurotoxicity of other chemicals that do not so readily cross the BBB. The exact mechanism of cell death caused by OPs in these constructs is still unknown; however, in most of these experiments cell viability correlated well with reduced AChE activity (Fig. 3). The one exception was DCP (Fig. 3D) where large reductions in AChE activity occurred at lower concentrations than significant reductions in viability, suggesting involvement of additional mechanisms of toxicity such as mitochondrial-mediated and caspase-regulated apoptosis9–12.
It is critical to estimate an effective concentration in brain for a given blood concentration for in vitro to in vivo extrapolation. While physiologically based pharmacokinetic (PBPK) modeling can serve to predict effective concentrations, which eventually predict toxicity based on in vivo data, obviously such models really depend on having a priori in vivo data. This tetra-cultured brain culture platform has the potential to improve the correlations between in vivo data and the in vitro model, by providing a more functionally relevant exposure route towards more accurately predicting in vivo toxicity.
Electronic supplementary material
Acknowledgements
This work was supported by U.S. Department of Defense contact # D01 W911SR-14–2–0001–0010 awarded through the Minority-Supporting Institutions STEM Research and Development Consortium (MSRDC) at North Carolina A & T State University. Major Research Instrument grant by National Science Foundation (Award no. 1229392) for 2-photon confocal microscope was supported for this study. We would like to thank Dr. Sonia Grego at RTI International, (Research Triangle Park, NC, USA) and Dr. Remko van Vught at MIMETAS.
Author Contributions
Youngmi Koo, who is the first author in this manuscript, planned this study, conducted all the experiments, analyzed all the data and prepared manuscript. Brian T. Hawkins advised on the manuscript. Yeoheung Yun is the corresponding author in this manuscript and guided the research.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-20876-2.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sidell FR, Borak J. Chemical warfare agents: II. nerve agents. Annals of Emergency Medicine. 1992;21:865–871. doi: 10.1016/S0196-0644(05)81036-4. [DOI] [PubMed] [Google Scholar]
- 2.Bajgar J. Complex view on poisoning with never agents and organophosphates. Acta Medica (Hradec Králové) 2005;48:3–21. [PubMed] [Google Scholar]
- 3.Bajgar J, et al. Biochemical and behavioral effects of soman vapors in low concentrations. Inhalation Toxicology. 2004;16:497–507. doi: 10.1080/08958370490442430. [DOI] [PubMed] [Google Scholar]
- 4.Karami-Mohajeri S, Abdollahi M. Toxic influence of organophosphate, carbamate, and organochlorine pesticides on cellular metabolism of lipids, proteins, and carbohydrates: a systematic review. Hum Exp Toxicol. 2010;30:1119–40. doi: 10.1177/0960327110388959. [DOI] [PubMed] [Google Scholar]
- 5.Kaur S, Singh S, Chahal KS, Prakash A. Potential pharmacological strategies for the improved treatment of organophosphate-induced neurotoxicity. Canadian Journal of Physiology and Pharmacology. 2014;92:893–911. doi: 10.1139/cjpp-2014-0113. [DOI] [PubMed] [Google Scholar]
- 6.Slotkin TA. Does early-life exposure to organophosphate insecticides lead to prediabetes and obesity? Reproductive Toxicology. 2011;31:297–301. doi: 10.1016/j.reprotox.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Slotkin TA, Levin ED, Seidler FJ. Comparative developmental neurotoxicity of organophosphate insecticides: effects on brain development are separable from systemic toxicity. Environmental Health Perspectives. 2006;114:746–751. doi: 10.1289/ehp.8828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang J, et al. Transendothelial permeability of chlorpyrifos in RBE4 monolayers is modulated by astrocyte-conditioned medium. Molecular Brain Research. 2001;97:43–50. doi: 10.1016/S0169-328X(01)00296-0. [DOI] [PubMed] [Google Scholar]
- 9.Carlson K, Jortner BS, Ehrich M. Organophosphorus compound-induced apoptosis in SH-SY5Y human neuroblastoma cells. Toxicology and Applied Pharmacology. 2000;168:102–113. doi: 10.1006/taap.2000.8997. [DOI] [PubMed] [Google Scholar]
- 10.Park JH, Ko J, Hwang J, Koh HC. Dynamin-related protein 1 mediates mitochondria-dependent apoptosis in chlorpyrifos-treated SH-SY5Y cells. NeuroToxicology. 2015;51:145–157. doi: 10.1016/j.neuro.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 11.Kashyap MP, et al. Pkb/Akt1 mediates Wnt/GSK3β/β-Catenin signaling-induced apoptosis in human cord blood stem cells exposed to organophosphate pesticide monocrotophos. Stem Cells and Development. 2013;22:224–238. doi: 10.1089/scd.2012.0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kashyap MP, et al. Caspase cascade regulated mitochondria mediated apoptosis in monocrotophos exposed PC12 cells. Chemical Research in Toxicology. 2010;23:1663–1672. doi: 10.1021/tx100234m. [DOI] [PubMed] [Google Scholar]
- 13.Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 14.Amos ML, Smith ME. Effect of organophosphate administration on the expression of pro-opiomelanocortin-derived peptides in motoneurones. Neurotoxicology. 1998;19:989–997. [PubMed] [Google Scholar]
- 15.Faria M, et al. Zebrafish Models for Human Acute Organophosphorus Poisoning. Scientific Reports. 2015;5:1–15. doi: 10.1038/srep15591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bird SB, et al. Pharmacotherapy to protect the neuromuscular junction after acute organophosphorus pesticide poisoning. Annals of the New York Academy of Sciences. 2016;1374:86–93. doi: 10.1111/nyas.13111. [DOI] [PubMed] [Google Scholar]
- 17.Hernandez CM, et al. Repeated exposure to chlorpyrifos leads to prolonged impairments of axonal transport in the living rodent brain. NeuroToxicology. 2015;47:17–26. doi: 10.1016/j.neuro.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Voorhees JR, Rohlman DS, Lein PJ, Pieper AA. Neurotoxicity in preclinical models of occupational exposure to organophosphorus compounds. Frontiers in Neuroscience. 2016;10:590. doi: 10.3389/fnins.2016.00590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown JA, et al. Recreating blood-brain barrier physiology and structure on chip: A novel neurovascular microfluidic bioreactor. Biomicrofluidics. 2015;9:054124. doi: 10.1063/1.4934713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cho H, et al. Three-dimensional blood-brain barrier model for in vitro Studies of Neurovascular Pathology. Scientific Reports. 2015;5:15222. doi: 10.1038/srep15222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herland A, et al. Distinct contributions of astrocytes and pericytes to neuroinflammation identified in a 3D human blood-brain barrier on a chip. PLoS ONE. 2016;11:0150360. doi: 10.1371/journal.pone.0150360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yi Y, Park J, Lim J, Lee CJ, Lee S-H. Central nervous system and its disease models on a chip. Trends in Biotechnology. 2015;33:762–776. doi: 10.1016/j.tibtech.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 23.Hawkins BT, Hu T, Dougherty ER, Grego S. Modeling neuroinflammatory effects after chemical exposures in a scalable, three-dimensional cell culture system. Applied in vitro toxicology. 2016;2:223–234. doi: 10.1089/aivt.2016.0018. [DOI] [Google Scholar]
- 24.Rahman NA, et al. Immortalized endothelial cell lines for in vitro blood–brain barrier models: A systematic review. Brain Research. 2016;1642:532–545. doi: 10.1016/j.brainres.2016.04.024. [DOI] [PubMed] [Google Scholar]
- 25.Trietsch SJ, Israels GD, Joore J, Hankemeier T, Vulto P. Microfluidic titer plate for stratified 3D cell culture. Lab on a Chip. 2013;13:3548–3554. doi: 10.1039/c3lc50210d. [DOI] [PubMed] [Google Scholar]
- 26.Dimethyl methylphosphonate. U.S. National Library of Medicine Toxicology Data Network, https://chem.nlm.nih.gov/chemidplus/rn/756-79-6.
- 27.Diethyl methylphosphonate. U.S. National Library of Medicine Toxicology Data Network, https://chem.nlm.nih.gov/chemidplus/rn/683-08-9.
- 28.Diethyl cyanophosphate. U.S. National Library of Medicine Toxicology Data Network, https://chem.nlm.nih.gov/chemidplus/rn/2942-58-7.
- 29.Diethyl chlorophosphate. U.S. National Library of Medicine Toxicology Data Network, https://chem.nlm.nih.gov/chemidplus/rn/814-49-3.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.