Abstract
Classic Ehlers-Danlos syndrome (cEDS) is characterized by fragile, hyperextensible skin and hypermobile joints. cEDS can be caused by heterozygosity for missense mutations in genes COL5A2 and COL5A1, which encode the α2(V) and α1(V) chains, respectively, of collagen V, and is most often caused by COL5A1 null alleles. However, COL5A2 null alleles have yet to be associated with cEDS or other human pathologies. We previously showed that mice homozygous null for the α2(V) gene Col5a2 are early embryonic lethal, whereas haploinsufficiency caused aberrancies of adult skin, but not a frank cEDS-like phenotype, as skin hyperextensibility at low strain and dermal cauliflower-contoured collagen fibril aggregates, two cEDS hallmarks, were absent. Herein, we show that ubiquitous postnatal Col5a2 knockdown results in pathognomonic dermal cauliflower-contoured collagen fibril aggregates, but absence of skin hyperextensibility, demonstrating these cEDS hallmarks to arise separately from loss of collagen V roles in control of collagen fibril growth and nucleation events, respectively. Col5a2 knockdown also led to loss of dermal white adipose tissue (WAT) and markedly decreased abdominal WAT that was characterized by miniadipocytes and increased collagen deposition, suggesting α2(V) to be important to WAT development/maintenance. More important, Col5a2 haploinsufficiency markedly increased the incidence and severity of abdominal aortic aneurysms, and caused aortic arch ruptures and dissections, indicating that α2(V) chain deficits may play roles in these pathologies in humans.
Type V collagen [col(V)] is a quantitatively minor fibril-forming collagen broadly expressed in vertebrate tissues as an α1(V)2α2(V) heterotrimer, comprising one α2(V) and two α1(V) chains.1 In addition, α1(V)3 homotrimers and α1(V)α2(V)α3(V) heterotrimeric forms of col(V) also have been reported in tissues, although the significance and functions of such forms are only beginning to be explored.2, 3, 4, 5, 6 Early evidence suggested a pericellular or basement membrane–associated distribution for col(V) forms.7, 8 However, subsequent studies have demonstrated that α1(V)2α2(V) heterotrimers are incorporated into the growing fibrils of the much more abundant fibrillar collagen I fibrils, and play roles in regulating the nucleation, size, and shapes of the resulting collagen I/col(V) heterotypic fibrils.9, 10, 11
Human patients with the heritable connective tissue disorder classic Ehlers-Danlos syndrome (cEDS) present with skin hyperextensibility, joint hypermobility, atrophic scarring, and easy bruising.12 Most cEDS cases are due to heterozygosity for null alleles for the α1(V) gene COL5A1.12, 13 Similarly, a null allele in the mouse α1(V) gene Col5a1 causes a cEDS-like phenotype in mice.14, 15 In addition, a number of human cEDS cases have now been shown to be due to heterozygosity for missense mutations in COL5A1, or in the α2(V) gene COL5A2.12 Interestingly, however, no human cEDS case has yet been associated with heterozygosity for a COL5A2 null allele, leading to the suggestion that haploinsufficiency for the α2(V) chain may not lead to cEDS or, perhaps, to any clinically abnormal phenotype.12
To determine in vivo effects of reduced or absent α2(V) chains, we recently generated and characterized mice with constitutively null Col5a2 alleles.16 Homozygosity for the null Col5a2 allele yielded embryonic lethality at approximately 12 days after conception,16 approximately 2 days later than the embryonic lethality previously described for homozygous null Col5a1−/− mice,11 although embryos of both genotypes had similar cardiovascular signs, suggesting deficits in early cardiovascular integrity. However, although Col5a1−/− embryos lacked identifiable mesenchymal collagen fibrils,11 Col5a2−/− embryos had identifiable mesenchymal collagen fibrils, albeit with abnormally large diameters and abnormal configurations.16 Differences in collagen fibrils and survival time between the two types of homozygous null embryos suggested that aberrant α1(V)3 homotrimers, which are formed by α1(V) chains in the absence of α2(V) chains,17, 18 can functionally compensate, in part, for loss of α1(V)2α2(V) heterotrimers.
Unlike heterozygous Col5a1+/− adult mice, which exhibit hyperextensible skin at both high and low stress, and on electron microscopic examination display large-diameter dermal aggregates of collagen fibrils with cauliflower-like contours,14 cardinal features of cEDS,12 adult heterozygous Col5a2+/− mice exhibited hyperextensible skin at high, but not low, stress and dermal collagen fibrils with relatively mild abnormalities in contour and diameter.16 Together, these data suggested that human COL5A2+/− heterozygotes, although likely more susceptible to skin tearing/wounding than normal, might not present with frank cEDS. This conclusion, together with the finding that aortas of Col5a2+/− heterozygous mice have reduced tensile strength and increased elasticity, raised the question of the clinical ramifications of the COL5A2+/− genotype in human populations.16
In the current report, we further elucidated the roles of α2(V) collagen chain in adult tissues and pathology, in part by using a conditional-null mouse model with floxed Col5a2 alleles that avoids embryonic lethality and allows study of the effects of postnatal Col5a2 ablation. Data presented herein show the α2(V) chain to play important roles in growth and that sufficiently low α2(V) chain levels yield skin that, like that of cEDS patients,19 has delayed and aberrant wound healing, has greatly reduced breaking strength/tensile strength, and contains the large cauliflower-like collagen fibril aggregates pathognomic of cEDS.16 However, unlike skin of cEDS patients, this skin lacks hyperextensibility and had almost total absence of dermal white adipose tissue (WAT). The latter, along with abdominal WAT depots of reduced size and fibrotic appearance, and in which adipocytes had a miniadipocyte appearance, suggests a role for α2(V) in WAT development and/or maintenance. Key observations include the finding that α2(V) chain haploinsufficiency yields markedly increased incidence, diameters, and pathology of abdominal aortic aneurysms (AAAs), and high susceptibility to fatal aortic arch ruptures and dissections in an angiotensin II–induced aneurysm mouse model. Thus, deficits in α2(V) chain levels/function may play roles in these pathologies in humans.
Materials and Methods
Col5a2-Floxed Mice
The generation of Col5a2fl/fl mice has been described previously.16
Induced Deletion of Col5a2
C57BL/6 Col5a2fl/fl mice were mated with transgenic Cre-ERT2 mice in which Cre recombinase expression was driven by the ubiquitin C (Ubc) promoter (JAX strain 008085),20 to generate Ubc Cre-ERT2; Col5a2fl/fl mice. Excision of Col5a2 sequences was then induced with tamoxifen, administered into the peritoneum (i.p.) of recently weaned 4-week-old male mice (0.5 μmol/L per gram body weight), weighing approximately 13 to 15 g, for a total of 10 days (two blocks of 5 consecutive days separated by 2 days without treatment). Littermates without the Ubc Cre-ERT2 transgene were used as controls and were similarly treated with tamoxifen. Efficacy of tamoxifen-induced excision of Col5a2 was assayed via PCR analysis of genomic DNA from ear samples digested in DirectPCR Lysis Reagent (Viagen Biotech Inc., Los Angeles, CA). Oligonucleotide forward (5′-GGTGATGGATGCTGACTTTG-3′/5′-AGCTTCTGTGCGTGCCCTGG-3′) and reverse (5′-GGAGGGGAGGATAAAGAGCA-3′) primers were used to detect the presence of floxed or excised alleles, represented by approximately 460- and approximately 510-bp bands, respectively, on resolution on 2.5% agarose gels.
Col5a2fl/fl mice also were mated with transgenic Cre-ERT2 mice, in which Cre expression is driven by the myosin heavy chain 11 promoter (JAX strain 019079),21 specific to smooth muscle cells, to generate Myh11 Cre-ERT2; Col5a2fl/fl mice. Male mice, all of which must contain the Y chromosome–linked Myh11 Cre-ERT2 transgene, were injected with either tamoxifen or vehicle (98% corn oil and 2% ethanol) using the same dosage and administration schedule as described above for Ubc-Cre; Col5a2fl/fl mice.
All mice were housed and treated in accordance with NIH's Guide for the Care and Use of Laboratory Animals,22 using protocols approved by the Research Animal Resources Center of the University of Wisconsin–Madison.
Splinted Wound Healing
Splinted wound healing was performed on 8- to 10-week-old mice, as described previously.23 Briefly, all wounding procedures were performed under isoflurane anesthesia. The backs of mice were shaved and sterilized. Two full-thickness wounds were generated on the midback using a 5-mm biopsy tool (Integra Miltex, York, PA). Circular, silicon splints, with a 6-mm inner diameter, were adhered around the edges of the wound using cyanoacrylate glue (Elmer's Products, Atlanta, GA) and fastened in place with four interrupted sutures. Wounds were covered by protective dressings for the duration of the experiment. Wound closure was analyzed using calipers by measuring two diameters per wound and using the average to estimate wound area. After sacrifice, the wounded tissue was carefully dissected for further analysis.
Dermal Fibroblast Culturing and Immunoblotting
Full-thickness skin was excised from the axillary region of 15-week-old adult mice. The tissue was then minced using a pair of scalpels and was digested overnight in 0.5 mg/mL type II collagenase (Worthington Biochemical Corporation, Lakewood, NJ) in Dulbecco's modified Eagle's medium supplemented with 20% fetal bovine serum, in a 5% CO2 atmosphere at 37°C. The digest was pelleted at 1500 × g for 10 minutes, the supernatant was removed, and the pellet was subsequently resuspended and plated in Dulbecco's modified Eagle's medium and 20% fetal bovine serum growth medium.
For immunoblotting, cells were washed twice with phosphate-buffered saline to remove residual growth medium before being serum starved in Dulbecco's modified Eagle's medium supplemented with 75 μg/mL ascorbic acid and 40 μg/mL soybean trypsin inhibitor (Sigma, St. Louis, MO). The following day, conditioned media were collected and concentrated by centrifugation filters (Millipore, Billerica, MA). Samples were boiled in Laemmli sample buffer and 2.5% β-mercaptoethanol before loading onto a 6% acrylamide gel and SDS-PAGE. Primary antibodies specific for the mouse α1(V) proline/arginine-rich protein and variable domains or the α2(V) cysteine-rich domain were used, as previously described.16 Anti–procollagen C-proteinase enhancer 1 antibody (Sigma) was used at a 1:1000 dilution. Goat anti-rabbit horseradish peroxidase–conjugated secondary antibody (Bio-Rad Laboratories Inc., Hercules, CA) was used at a 1:5000 dilution. Detection was with chemiluminescent substrate (Thermo Scientific, Waltham, MA).
Histologic Analyses
Hair was removed from subscapular skin samples, and WAT depots were isolated; samples were then fixed overnight in 10% formalin, followed by embedding in paraffin, and dividing into sections (5 μm thick). For hematoxylin and eosin staining, sections were fixed for 5 minutes in 10% formalin, then rinsed with water, and stained in modified Harris hematoxylin solution (Newcomer Supply, Inc., Middleton, WI) for 4 minutes. Samples were dehydrated in 1% acid alcohol, and the nuclei were blued in 1% ammonia water for 30 seconds. Cytoplasmic staining was achieved by immersing the slides in 1% alcoholic eosin Y solution (Newcomer Supply, Inc.). Slides were then dehydrated through 95% and 100% ethanol, cleared in xylene, and mounted with Cytoseal 60 (Thermo Fisher).
For trichrome staining, sections were fixed for 5 minutes in 10% formalin and placed in Bouin's solution for 1 hour at 60°C. Slides were washed until tissue sections were clear. Tissue nuclei were stained with working Wiegert's Iron Hematoxylin solution (Newcomer Supply, Inc.) for 10 minutes. Slides were then stained for collagen with Gomori's one-step trichrome solution (Newcomer Supply, Inc.) for 20 minutes. Last, slides were rinsed with 1% glacial acetic acid to enhance contrast, dehydrated through 95% and 100% ethanol, cleared in xylene, and mounted with Cytoseal 60. Bright-field images were captured using a Zeiss Axiophot 2 microscope (Zeiss, Thornwood, NY) with an attached charge-coupled device camera.
Transmission Electron Microscopy
Mouse subscapular skin was fixed in 4% paraformaldehyde and 2.5% glutaraldehyde in 0.1 mol/L sodium cacodylate buffer, pH 7.4, with 8.0 mmol/L CaCl2 for 2 to 4 hours and post-fixed with 1% osmium tetroxide. Further sample preparation and imaging was as previously described.16
Biomechanical Analysis of Skin
Tensiometric properties of moist skin strips (20 × 30 mm, trimmed to generate a 5-mm waist that provided a uniform breaking pattern) from the dorsal midline were evaluated using an Instron Tensiometer (model 5542; Instron, Canton, MA). Mechanical properties (breaking energy and Young's modulus) were calculated with Bluehill software version 2.32 (Instron, Norwood, MA).
Elastase and Angiotensin II Infusion Models for Aortic Aneurysms
For the elastase model, 12- to 15-week-old adult mice were anesthetized and underwent sterile preparation. A laparotomy was performed to expose the abdominal aorta. The aorta was temporarily ligated at two ends, and an aortotomy was performed between the ligations. Through the aortotomy, the aorta was perfused with 0.414 U/mL type I porcine pancreatic elastase (Sigma) in saline for 5 minutes at a pressure of 100 mmHg. After perfusion, the aortotomy was closed and the ligations were removed. Preperfusion measurements of aorta diameters were recorded using digital calipers, and measurements were taken again on sacrifice 2 weeks after surgery. The percentage change in diameter was calculated by comparing these two values.
For the angiotensin II (Ang II) infusion model, constitutively heterozygous null Col5a2+/− mice16 were crossed with Ldlr−/− mice (Jackson Laboratory, Bar Harbor, ME), and Col5a2+/−;Ldlr−/− and Col5a2+/+;Ldlr−/− control male mice at the age of 6 to 8 months were subjected to a 28-day infusion of Ang II. Alzet model 2004 osmotic minipumps (Durect Corp., Cupertino, CA), implanted s.c., were used to deliver Ang II (1000 ng/kg per minute; Sigma-Aldrich, St. Louis, MO) or vehicle (normal saline). Mice were anesthetized with isoflurane and administered buprenorphine (0.05 to 0.10 mg/kg) via i.p. injection after the procedure. At the completion of Ang II infusion, mice were perfused with normal saline, followed by 4% paraformaldehyde, at 100 mmHg for 5 minutes. Periadventitial tissues were carefully removed from the aortic wall, and a digital caliper (Absolute Digimatic; Mitutoyo, Aurora, IL) was used to measure maximal external diameters of suprarenal abdominal aortas. For this study, AAA was defined as ≥50% enlargement of maximal abdominal aorta diameter. Characterization of the severity of AAA formation was as previously described24: type I, dilated lumen in the suprarenal aorta with no thrombus; type II, remodeled tissue in the suprarenal region that frequently contains thrombus; type III, a pronounced bulbous form of type II that contains thrombus; and type IV, multiple aneurysms containing thrombus, some overlapping, in the suprarenal area of the aorta. Necropsy was performed as soon as possible on animals that expired before completion of the study. These animals were not included in the histologic or AAA quantification analyses, but only in the mortality data.
Statistical Analysis
Data are expressed as means ± SEM. To compare incidence of AAA formation between the two groups, χ2 test and two-tailed Fisher's exact probability test were used. A two-tailed t-test for normally distributed data and a Mann-Whitney U nonparametric test for skewed data that deviate from normality were used to compare two conditions. Differences with P < 0.05 were considered significant.
Results
Validation of Col5a2 Conditional Knockout
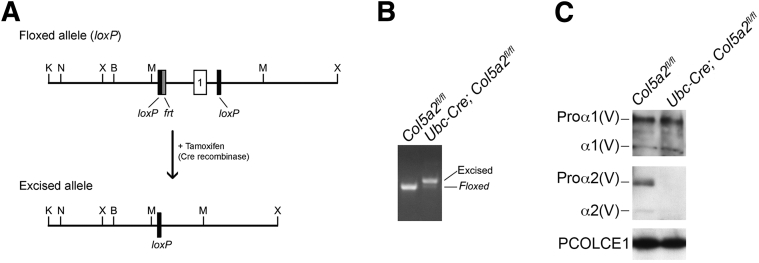
To determine the effects of ablating Col5a2 function in juvenile and adult mice, the embryonic demise associated with constitutive loss of Col5a2 function16 was avoided via conditional Col5a2 knockdown. Toward this end, mice were generated in which exon 1 and adjoining promoter and enhancer regions were flanked with flox (loxP) sites. Crossing with transgenic mice in which Cre expression is driven by the ubiquitin C promoter generated Ubc-Cre; Col5a2fl/fl mice, in which Cre activation by administration of tamoxifen excises Col5a2 sequences flanked by the loxP sites (Figure 1A). Genomic DNA extracted from ear punch samples after tamoxifen administration showed an upward mobility shift of approximately 50 bp from Ubc-Cre; Col5a2fl/fl DNA, indicating genomic excision by Cre, unlike Col5a2fl/fl littermate controls, which were also administered tamoxifen, but lacked the Ubc-Cre transgene (Figure 1B). Moreover, neither secreted pro-α2(V) chains nor mature α2(V) chains were detected in cultures of dermal fibroblasts isolated from 15-week-old tamoxifen-treated adult Ubc-Cre; Col5a2fl/fl mice (Figure 1C).
Figure 1.
Tamoxifen-induced Col5a2 ablation. A: Schematic shows placement of loxP and frt sequences, represented in black and gray, respectively. LoxP sequences flank exon 1 (open box) and adjoining promoter and enhancer sequences. On tamoxifen treatment, site-specific excision by induced Cre recombinase deletes LoxP-flanked sequences to produce the excised (ablated) allele. B: PCR genotyping of genomic DNA from Col5a2fl/fl and Ubc-Cre; Col5a2fl/fl mice treated with tamoxifen shows genomic excision of exon 1 only in mice (Ubc-Cre; Col5a2fl/fl) harboring the Ubc-Cre transgene. C: Immunoblotting of conditioned media from primary dermal fibroblasts isolated from Ubc-Cre; Col5a2fl/fl mice shows a striking deficit in α2(V) chain secretion, compared with Col5a2fl/fl fibroblasts. Both types of fibroblasts are seen to secrete similar levels of α1(V) chains. Procollagen C-proteinase enhancer 1 (PCOLCE1) was used as a loading control. B, BamH1; K, KpnI; M, MfeI; N, NcoI; X, XbaI.
Ubc-Cre; Col5a2fl/fl Mice Are Runted and Have Abnormalities of Skin and WAT
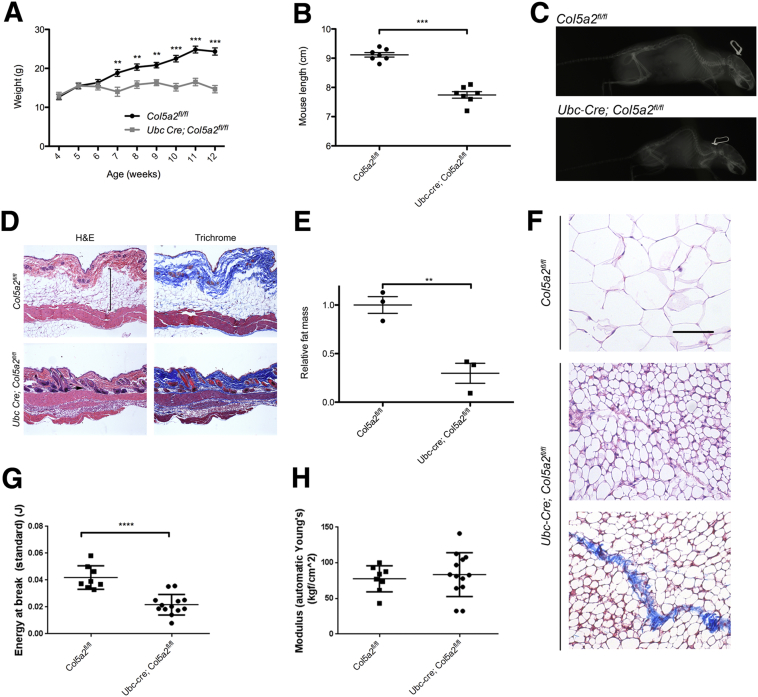
Ubc-Cre; Col5a2fl/fl mice failed to gain weight subsequent to tamoxifen treatment and had significantly decreased weight, compared with tamoxifen-treated Col5a2fl/fl controls, as early as 7 weeks of age (approximately 2 weeks after the final tamoxifen treatment). Weight differences further increased until weights of Ubc-Cre; Col5a2fl/fl mice were only approximately 60% those of Col5a2fl/fl controls at adulthood (Figure 2A). Ubc-Cre; Col5a2fl/fl mice were also of shorter length than Col5a2fl/fl controls (Figure 2B) and exhibited severe kypholordosis (Figure 2C).
Figure 2.
Morphologic appearance and features of skin and white adipose tissue (WAT) depots of Col5a2 adult mutant mice. A–C: Conditional loss of Col5a2 function results in markedly reduced mass (A) and length (B), and severe kypholordosis (C). The white outlined object above the head of each mouse in C is the ear tag. D: Hematoxylin and eosin (H&E) and Masson's trichrome staining of subscapular skin samples. Vertical bracket and arrow denote dermal WAT in Ubc-Cre; Col5a2fl/fl and Col5a2fl/fl littermate control skin, respectively. E: Masses of inguinal fat depots, normalized to the body weights of the mice from which they were recovered, were compared for Col5a2fl/fl and Ubc-Cre; Col5a2fl/fl mice. F: Representative H&E-stained sections are shown of Col5a2fl/fl and Ubc-Cre; Col5a2fl/fl inguinal fat depots. Masson's trichrome staining of a section of Ubc-Cre; Col5a2fl/fl inguinal fat depot is shown, evidencing a characteristic collagenous seam. Although only data for inguinal fat depots are shown in E and F, results were similar for gonadal fat depots. G and H: Biomechanical measurement shows Ubc-Cre; Col5a2fl/fl skin to have markedly decreased breaking strength (P < 0.0001; G), but no significant difference (P < 0.638; H) in extensibility (Young's modulus), compared with control skin. Data are presented as means ± SEM (A, B, E, G, and H). ∗∗P < 0.01, ∗∗∗P < 0.001, and ∗∗∗∗P < 0.0001. Scale bar = 50 μm (F).
Skin abnormalities are a hallmark of cEDS. We, therefore, subjected subscapular skin samples of Ubc-Cre; Col5a2fl/fl and control mice to histologic analysis. Hematoxylin and eosin staining showed Ubc-Cre; Col5a2fl/fl dermis to be markedly thinner, and seemingly more compact than control skin (Figure 2D). Masson's trichrome staining of serial sections confirmed a thinner, more compact, dermis and also suggested Ubc-Cre; Col5a2fl/fl dermis to be more collagen dense than control dermis (Figure 2D). Ubc-Cre; Col5a2fl/fl skin also apparently lacked dermal WAT, with an absence of morphologically identifiable adipocytes (Figure 2D). Consistent with these histologic results, on handling of mice, a thinning of the skin was readily apparent, and most evident while grasping mice by the scruff of the neck or other areas of dorsal skin.
To determine whether loss of WAT was limited to Ubc-Cre; Col5a2fl/fl skin, inguinal and gonadal fat depots were isolated from Ubc-Cre; Col5a2fl/fl and Col5a2fl/fl control mice and their weights were compared. Although Ubc-Cre; Col5a2fl/fl mice are approximately 60% the weight and approximately 60% the length of Col5a2fl/fl controls (Figure 2, A and B), Ubc-Cre; Col5a2fl/fl inguinal and gonadal fat depots were only 18% and 15% the mass of the corresponding Col5a2fl/fl depots, respectively; and were approximately 30% and approximately 25%, respectively, when normalized to the body weights of the mice from which they were recovered (Figure 2E). As Ubc-Cre; Col5a2fl/fl mice had food intake approximately 60% that of Col5a2fl/fl mice (data not shown), and thus proportional to their reduced size, the aggregate data from the much reduced WAT of skin and abdominal depots suggest that the α2(V) chain plays a role in WAT development and/or maintenance. Consistent with this possibility, the adipocytes in Ubc-Cre; Col5a2fl/fl inguinal and gonadal WAT depots were miniadipocytes, of an abnormally small size (Figure 2F). Ubc-Cre; Col5a2fl/fl inguinal and gonadal WAT depots were also abnormal in having seams of dense collagenous matrix not found in control inguinal or gonadal WAT depots (Figure 2F). These fibrotic seams in abdominal WAT depots were reminiscent of the dense collagenous matrix in Ubc-Cre; Col5a2fl/fl skin (Figure 2D), and are evidence of an abnormal collagenous matrix in both tissues.
Ubc-Cre; Col5a2fl/fl Skin Is Markedly Fragile, But Not Hyperextensible
To determine whether these differences translated into differences in mechanical strength, skin samples were examined for tensile strength, the amount of force required for the skin to stretch and break. Mechanical testing, and analysis that included correction for the thinner dorsal skin of Ubc-Cre; Col5a2fl/fl skin specimens, showed the latter to have markedly reduced breaking energy (toughness), with the breaking energy of Ubc-Cre; Col5a2fl/fl skin reduced by 49% compared with control skin (P < 0.0001) (Figure 2G). Surprisingly, however, the elastic modulus of Ubc-Cre; Col5a2fl/fl skin showed no significant difference with that of Col5a2fl/fl littermate controls (Figure 2H), indicating lack of the hyperextensibility characteristic of cEDS,12 and of the Col5a1+/− cEDS mouse model,14 and even of constitutively Col5a2+/− heterozygous null adult mice.16
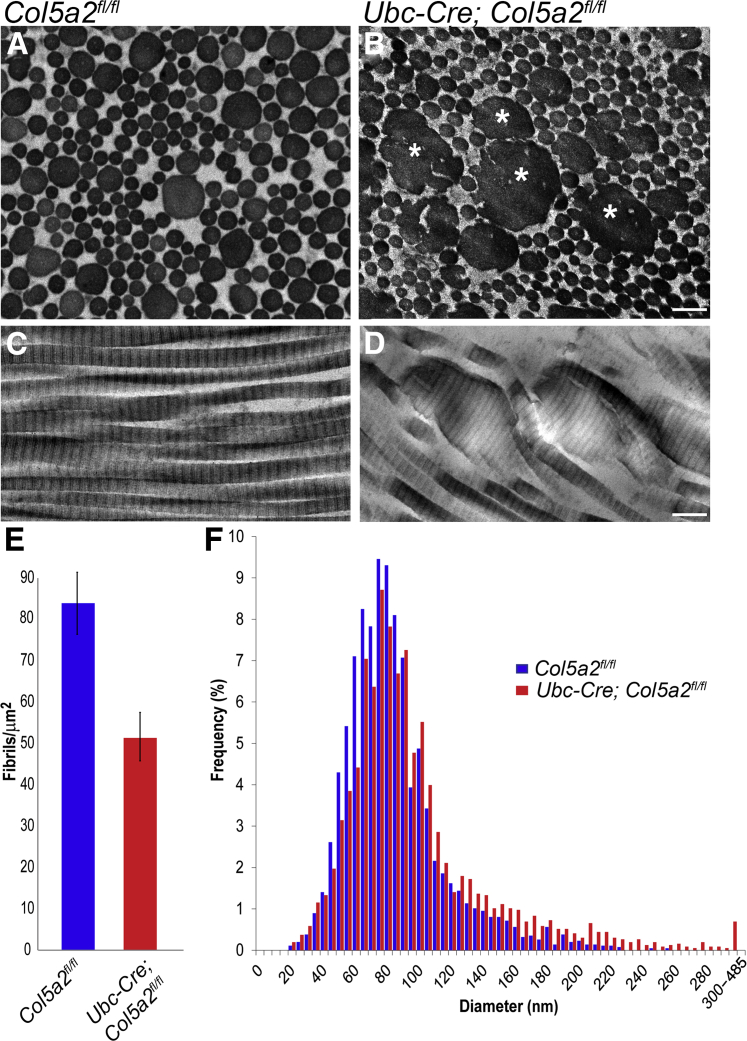
Ultrastructural Analysis Reveals Ubc-Cre; Col5a2fl/fl Mice to Have cEDS-Like Dermal Collagen Fibrillar Aggregates
cEDS is characterized, in part, by large-diameter aggregates of collagen fibrils with cross-sectional cauliflower-like contours.12 However, as null COL5A2 alleles have yet to be identified in cEDS patients,12 and as we previously found Col5a2+/− mice to have dermal collagen fibrils with relatively mild abnormalities in contour and diameter,16 we sought to determine the extent to which Ubc-Cre; Col5a2fl/lf mice would have changes in dermal collagen fibril size and geometry. To this end, transmission electron microscopy was used to examine skin ultrastructure. Ubc-Cre; Col5a2fl/fl and Col5a2fl/fl mice were both injected with tamoxifen and sacrificed at 15 weeks of age, at which time subscapular skin was collected. Analysis using transmission electron microscopy demonstrated that Ubc-Cre; Col5a2fl/fl skin had readily apparent large-diameter collagen fibril aggregates with cross-sectional cauliflower-like contours (Figure 3B), highly similar to the similar fibril aggregates that are a cardinal feature in the skin of cEDS patients.16 This feature was absent from control Col5a2fl/fl skin (Figure 3A). Examination of longitudinal collagen fibril profiles further emphasized the overall fibril disorganization and larger fibril diameters of Ubc-Cre; Col5a2fl/fl skin (Figure 3, C and D). Quantitative analyses of fibril cross sections also showed Ubc-Cre; Col5a2fl/fl skin to have a lower density of fibrils, compared to Col5a2fl/fl controls (P = 0.027) (Figure 3E), likely due, at least in part, to the presence of fibril aggregates and larger-diameter fibril diameters, such that there are fewer fibrils per unit space. Indeed, quantitative examination showed Ubc-Cre; Col5a2fl/fl skin to have a rightward shift in the distribution of collagen fibril diameters, with an extended right tail of atypically large-diameter sizes, ranging from 240 to 480 nm. The data thus indicate both an overall increase in collagen fibril diameters as well as a subpopulation of fibril aggregates of abnormally large diameters (Figure 3F).
Figure 3.
Transmission electron microscopic analysis of skin from 15-week-old Col5a2fl/fl and Ubc-Cre; Col5a2fl/fl mice. Ubc-Cre; Col5a2fl/fl mice (B) have large aggregates of collagen fibrils, known as collagen cauliflowers12 (asterisks), with cross-sectional cauliflower-like profiles not seen in Col5a2fl/fl controls (A). This alteration in fibril structure is highlighted in Ubc-Cre; Col5a2fl/fl (D) and Col5a2fl/fl (C) longitudinal sections. Mutant mice (red) also have decreased fibril density (E) and a corresponding distribution skewed toward larger fibril sizes (F) compared with controls (blue). Data are presented as means ± SEM (E). Scale bar = 200 nm (A–D).
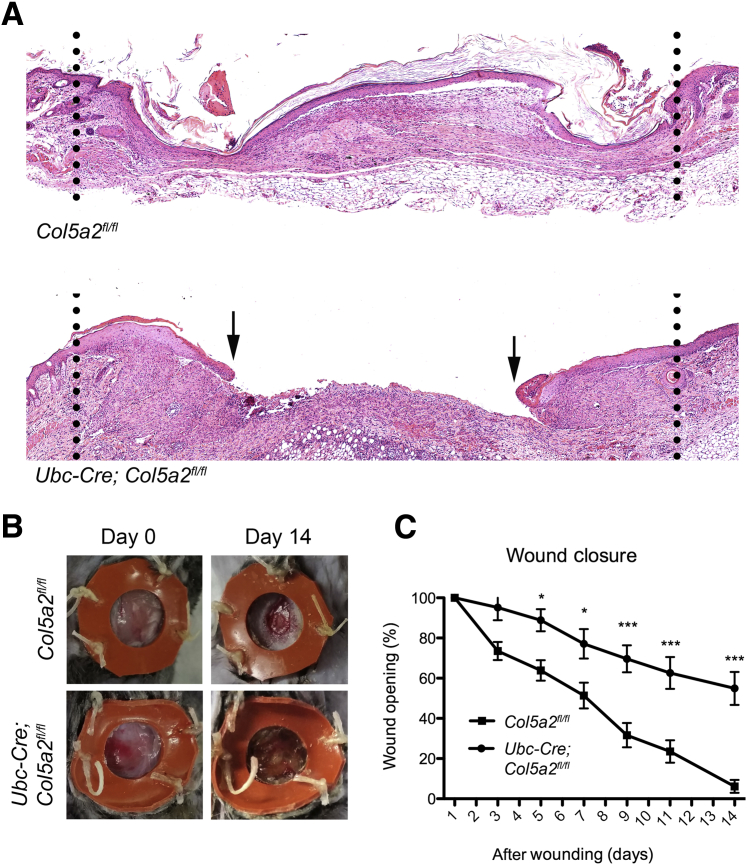
Ubc-Cre; Col5a2fl/fl Mice Have Impaired Wound Healing
Because cEDS patients have delayed and aberrant wound healing,19 we used a wound-healing assay to determine whether Ubc-Cre; Col5a2fl/fl mice might also have deficits in wound healing. Toward this end, 5-mm-diameter full skin thickness punch biopsy specimens were excised from Ubc-Cre; Col5a2fl/fl and Col5a2fl/fl control littermate mice. Wounds were sutured open to prevent contraction and to allow healing primarily by reepithelialization, extracellular matrix (ECM) deposition, and granulation tissue formation, the predominant mechanisms of wound healing in humans. Wounds were measured every other day for 2 weeks until the mice were sacrificed. Examination of wounds at 14 days revealed significantly delayed healing of Ubc-Cre; Col5a2fl/fl wounds, for which healing was always incomplete, as evidenced by readily apparent nonconjoined wound edges (Figure 4A), whereas wounds of Col5a2fl/fl controls reproducibly showed complete or nearly complete healing (Figure 4A). By 14 days after wounding, Ubc-Cre; Col5a2fl/fl wounds remained approximately 60% open, whereas Col5a2fl/fl wounds were essentially closed (Figure 4, B and C).
Figure 4.
Delayed wound healing because of Col5a2 ablation. A: Representative images are shown of hematoxylin and eosin–stained histologic sections of Col5a2fl/fl control and Ubc-Cre; Col5a2fl/fl wounds at 14 days after wounding. Dotted lines denote wound margins; arrows denote edge of migrating epithelial tongues in a Ubc-Cre; Col5a2fl/fl wound, whereas a Col5a2fl/fl control wound is completely reepithelialized. B: Representative images at wounding and 14 days after injury. C: Quantification of open wound areas shows wound closure to occur significantly more slowly in Ubc-Cre; Col5a2fl/fl than in Col5a2fl/fl control wounds. Data are expressed as means ± SEM (C). n = 9 (C, Ubc-Cre; Col5a2fl/fl wounds); n = 10 (C, Col5a2fl/fl wounds). ∗P < 0.05, ∗∗∗P < 0.001.
Col5a2 Ablation Predisposes to Increased Abdominal Aortic Aneurysm Diameters and to Aortic Arch Dissection and Rupture in Mouse Aortic Aneurysm Models
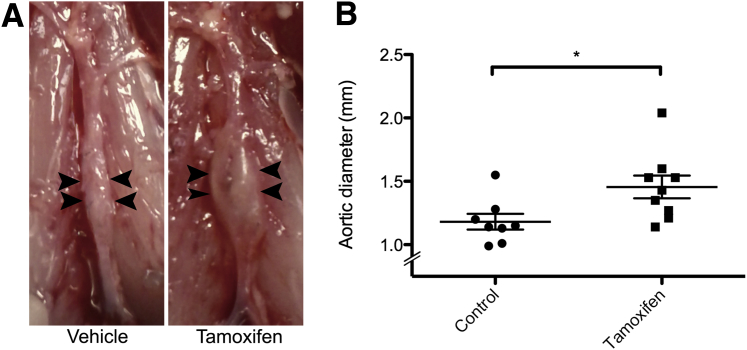
It has been reported that patients with cEDS have a higher incidence of dilated aortic roots.25 Therefore, the tensile strength of aortas of heterozygous Col5a1+/−14 and Col5a2+/−16 mice were previously examined ex vivo and were shown to have decreased aortic tensile strength and stiffness. Thus, we previously suggested that deficits in col(V) content might predispose to aortic aneurysms.16 To test this, however, Ubc-Cre; Col5a2fl/fl mice, which had relatively small body mass (Figure 2, A and B), also had large decreases in the sizes of their aortas, which were, therefore, not appropriate for comparison to controls in aorta induction experiments. It was also anticipated that Ubc-Cre; Col5a2fl/fl mice might have poor survivability in such studies, because of a general failure to thrive. Thus, instead, Col5a2fl/fl mice were crossed with transgenic Myh11-CreERT2 mice21 to produce Myh11-Cre; Col5a2fl/fl mice, in which tamoxifen induction of Cre activity induces Col5a2 excision only in gastrointestinal smooth muscle cells (SMCs) and in vascular SMCs,26 with the latter a major source of secreted collagens in vessel walls. The Myh11-Cre transgene is Y linked, and all male littermates in these crosses contain the Myh11-Cre transgene. Some of these mice were treated with tamoxifen, for Col5a2 excision, whereas control littermates, with the same genotype, were treated with vehicle. Subsequent to tamoxifen/vehicle treatment, there were no differences in weight, nor were other gross external differences noted between the two groups of mice (data not shown). In a model in which elastase perfusion of the abdominal aorta induces AAAs,27 AAAs were induced in both groups of mice approximately 8 weeks after tamoxifen/vehicle treatment. Interestingly, tamoxifen-treated mice had larger aneurysms than the vehicle-treated mice (158.8% change ± 16.66% versus 110.0% change ± 10.75%; P < 0.033), indicating that Col5a2 ablation in aortic SMCs was sufficient to render the aorta more prone to dilation (Figure 5, A and B).
Figure 5.
Col5a2 knockdown in vascular SMCs increases the diameters of elastase-induced AAAs. A: Image of representative AAAs, 2 weeks after elastase treatment, of Myh11-Cre; Col5a2fl/fl mice treated with tamoxifen or with vehicle. Arrowheads indicate borders of aortic dilation. B: Quantification of AAA maximal external infrarenal aortic diameters. n = 9 (A, tamoxifen treatment); n = 8 (A, vehicle treatment). ∗P < 0.05.
The fact that SMCs represent only a fraction of cells of which the aortic wall is composed complicated our ability to ascertain the efficiency with which Col5a2 sequences were excised using the Myh11-Cre transgene (described above). In addition, although Myh11-Cre should excise Col5a2 sequences from mature SMCs, it would not excise Col5a2 sequences from progenitor/stem cells involved in SMC turnover and replacement, as such SMC-lineage cells do not yet express Myh11.28 Thus, SMCs from which Col5a2 had been excised could be replaced by progenitors that retained ability to express Col5a2. For these reasons, we thought that the Myh11-Cre; Col5a2fl/fl studies described above might underrepresent Col5a2 roles in aortic aneurysm pathogenesis. Thus, we sought to bolster such experiments by examining aortic aneurysm formation in Col5a2+/− heterozygotes, in which all aortic cells would be Col5a2 haploinsufficient. For this study, Col5a2+/− mice were crossed onto an Ldlr−/− background, for use in the model system in which Ang II is infused into dyslipidemic mice to induce AAAs with various pathological features of human AAAs.29, 30
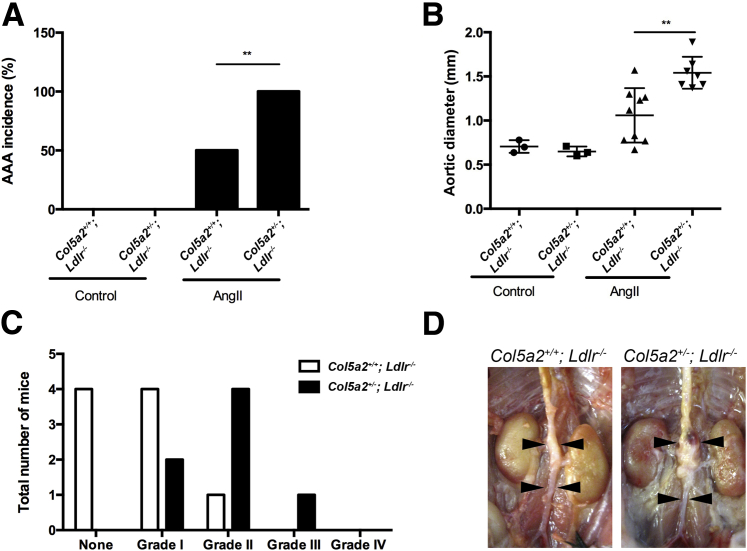
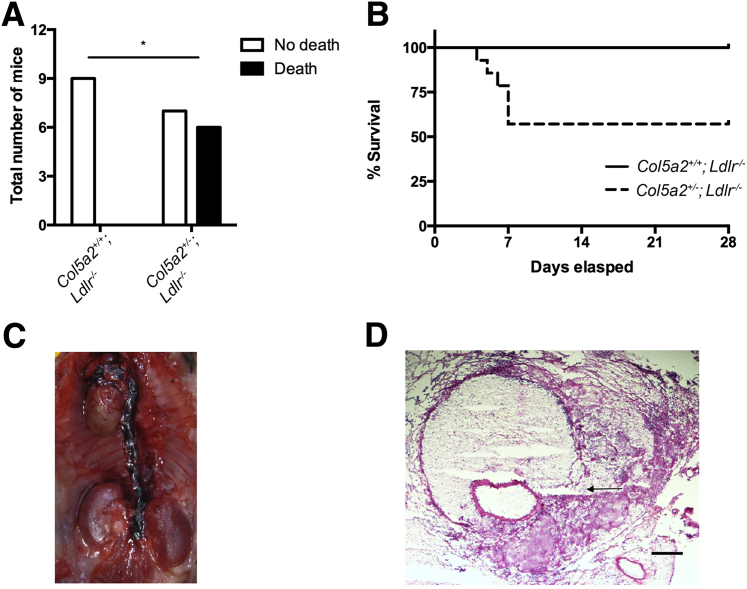
In the Ang II infusion model, Col5a2+/− mice had marked increases in the incidence (Figure 6A), diameters (Figure 6B), and severity of vascular pathology (Figure 6C) of induced AAAs, compared with Col5a2+/+ controls. Representative images of Col5a2+/− and Col5a2+/+ Ang II–induced AAAs are shown in Figure 6D. Surprisingly, in addition to effects of Ang II infusion on AAA size, 6 of the 13 Col5a2+/− mice subjected to Ang II infusion died within the first 7 days of the start of Ang II treatment (Figure 7, A and B) of aortic arch rupture and dissection (Figure 7, C and D).
Figure 6.
Col5a2 deficiency also exacerbates Ang II–induced AAA formation in mice. A: AAA incidence. B: Quantification of AAA maximal external suprarenal aortic diameter on day 28. An AAA is defined as percentage increase in aortic diameter of ≥50% (red dashed line). C: AAA classification in Col5a2+/+ versus Col5a2+/− mice after 28 days of Ang II infusion. D: Representative images of aortas. Arrowheads demarcate the area of the abdominal aorta treated with elastase. For comparing AAA incidence, Fisher's exact probability test was used. For comparison of aortic diameter differences, Mann-Whitney U test was used. n = 3 (A, both genotypes of saline controls); n = 9 (A and C, Col5a2+/+ Ang II group); n = 7 (A and C, Col5a2+/− Ang II group). ∗∗P < 0.01.
Figure 7.
Col5a2 deficiency predisposes mice to aortic dissection and ruptures after Ang II infusion. A: Mortality due to aortic dissection and rupture within the first 7 days of Ang II treatment. Fisher's exact probability test was used to compare Col5a2+/+ versus Col5a2+/−. Of the deaths, 50% were because of Debakey type I dissection, 16% were because of type II dissection, and 33% were because of type III dissection. B: Kaplan-Meier survival curve (P = 0.03, log-rank test). C: Representative image of dissection. D: Hematoxylin and eosin–stained section of thoracic dissection and rupture (arrow). n = 9 (A, Col5a2+/+); n = 13 (A, Col5a2+/−); n = 3 (A, type I dissection); n = 1 (A, type II dissection); n = 2 (A, type III dissection). ∗P < 0.05. Scale bar = 200 μm (D).
Discussion
The known molecular bases of cEDS include heterozygosity for COL5A1 null alleles or for missense COL5A2 mutations.12 However, there have been no described cEDS patients with COL5A2 null alleles. In a previous study of the phenotypic consequences of null alleles for α2(V)-encoding genes, we demonstrated16 that mice heterozygous for Col5a2 constitutively null alleles had cEDS-related skin features that included increased extensibility and decreased tensile strength at high stress. However, a frank cEDS phenotype was lacking, as skin lacked hyperextensibility at low stress and large-diameter cauliflower-shaped aggregates of collagen fibrils, both of which are pathognomonic for cEDS. We were interested in whether reducing Col5a2 expression to levels lower than those found in haploinsufficiency might produce a phenotype more typical of cEDS. Because complete loss of Col5a2 function in mice is embryonic lethal,16 the present study used the first described conditional knockdown for Col5a2. Ubiquitous ablation at weaning allowed assessment of α2(V) chain roles in late perinatal development, in growth/maturation and in mature tissues.
Initial gross histologic examination of Ubc-Cre; Col5a2fl/fl (Col5a2 knockdown) skin showed it to be extremely thin, with a compact, collagen-dense dermis in which dermal WAT was reduced to levels that were not readily detectable. This finding, along with our finding that abdominal fat depots were also much reduced in the Col5a2 knockdown mice, even when normalized to body weight, suggests an important role for the α2(V) chain in WAT development and/or maintenance, rather than a skin-specific effect [although the effect of α2(V) loss was most dramatic in skin].
Interestingly, the extreme thinness of Col5α2 knockdown dermis and lack of dermal WAT are not characteristic of cEDS. In contrast, transmission electron microscopy analysis showed Col5a2 knockdown skin to have the large-diameter cauliflower-contoured collagen fibril aggregates pathognomonic of cEDS. Thus, precipitous postnatal loss of α2(V) chain expression produced a phenotype overlapping, but not identical with, cEDS.
Col5a2 knockdown skin had a 49% loss of breaking energy (toughness), compared with controls, similar to the extent of reduction of tensile strength in Col5a1+/− skin in a cEDS mouse model.14 However, Col5a2 knockdown skin differs from cEDS skin, and even from the skin of Col5a2+/− heterozygotes,16 in lacking hyperextensibility, even under high stress. Thus, the presence of large cauliflower-shaped collagen fibril aggregates, but lack of hyperextensibility, in Col5α2 knockdown skin unlinks these two cardinal cEDS features, and shows that the cEDS skin hyperextensibility does not rely on the presence of large cauliflower-shaped collagen fibril aggregates. We speculate that, although the cauliflower-shaped collagen fibril aggregates form in Col5α2 knockdown skin because of α2(V) levels that, on tamoxifen-induced Col5a2 ablation, are lower than those found in haploinsufficiency, absence of hyperextensibility in Col5a2 knockdown skin derives from the persistence of some level of normal ECM deposited before tamoxifen treatment.
A seed-and-feed process has been proposed for the formation of fibrillar collagenous ECM,31 in at least some tissues. The seed phase of the process involves the extrusion of uniformly small-diameter collagen fibrils by stromal cells to form the fibrillar collagenous architecture of tissues in early prenatal development, whereas the feed phase occurs later in development and in homeostasis, and involves growth of collagen fibrils via fusion of small-diameter fibrils and accretion of collagen monomers onto fibril surfaces. Col(V) appears to play an important nucleation role in the seed phase of collagen fibrillogenesis, as collagen fibrils do not form in early embryonic tissues in the absence of col(V).11 Our current findings, in which collagen cauliflower-shaped aggregates are caused by postnatal Col5a2 knockdown, indicate that aberrantly low levels of normal α1(V)2α2(V) heterotrimers during the feed phase are sufficient for formation of such aggregates. In contrast, the previous observation that constitutive Col5a2 haploinsufficiency results in hyperextensibility,16 together with the present observation that even lower levels of postnatal Col5a2 expression do not, suggests that insufficient α1(V)2α2(V) levels during the seed phase of fibrillar collagenous ECM may be primarily responsible for the skin hyperextensibility of cEDS.
As noted above, the extreme thinness of Ubc-Cre; Col5α2fl/fl knockdown skin, deriving in large part from almost complete lack of dermal WAT, is not reminiscent of cEDS in humans, nor has it been reported for the Col5a1+/− cEDS mouse model.14 This difference in phenotypes may lie in the fact that decreased α1(V) chain levels result in only normal col(V), albeit at decreased levels, whereas decreased α2(V) chain levels result not only in lower levels of col(V), but also in the generation of an aberrant form of col(V). This is because, in the absence of sufficient α1(V) levels, excess α2(V) chains are unstable and are degraded, such that only normal α1(V)2α2(V) heterotrimers are deposited in tissues; however, in the absence of sufficient α2(V) chain levels, α1(V) chains can form stable α1(V)3 homotrimers.17, 18, 32 The result is that tissues in which α2(V) chains are at low levels would be affected not only by reduced deposition of α1(V)2α2(V) heterotrimers, but also by deposition of aberrant levels of α1(V)3 homotrimers. Thus, we speculate that aberrant ECM with lowered α1(V)2α2(V) content and elevated α1(V)3 content presents a microenvironment not conducive to adipogenesis and/or adipocyte maintenance. That the α2(V) chain may well play key roles in microenvironments that inform adipogenesis and/or adipocyte maintenance is supported by a study in which mice expressing a certain type of α2(V) chain with truncated N-terminal non-triple helical sequences had dermal WAT up to sixfold thicker than wild type.33 Consistent with possible key roles for col(V) in WAT biology is the finding that adiponectin overexpression leads to reduced expression of col(V) genes, accompanying a suppression of fat accumulation,34 and our previous finding that knockout of the gene for the α3(V) chain of col(V) leads to subtle reductions in the thickness of dermal WAT and substantial changes to WAT function.6 Interestingly, the appearance of abnormally small miniadipocytes in the reduced mass of Col5a2 knockdown abdominal WAT depots is reminiscent of miniadipocytes reported in knockout mouse models in which ECM is rendered relatively rigid and not easily remodeled by extracellular proteases.35 Such ECM is thought to impair the hypertrophic response necessary to complete adipocytic maturation,35 suggesting that the Col5a2 knockdown ECM found in WAT may be similarly abnormally rigid and resistant to remodeling.
We demonstrate Ubc-Cre; Col5a2fl/fl mice, like cEDS patients19 and Col5a1+/− mice,15 to have deficits in wound healing. However, we speculate that, in the case of the Col5a2 knockdown mice, the paucity of dermal WAT may to some degree contribute to the severity in the deficits in wound healing, as both adipose-derived stem cells and mature adipocytes have been suggested to be important to the reepithelialization, revascularization, and fibroblast repopulation necessary to skin repair.36
As cEDS patients have a higher incidence of dilated aortic roots than normal25 and mice haploinsufficient for Col5a114 or Col5a216 have aortas with diminished tensile strength, we sought to determine the extent to which lowered levels of α2(V) chains might affect the pathogenesis of aortic aneurysms. On treatment with tamoxifen, conditional Col5a2 knockdown in aortic smooth muscle cells was sufficient to increase the diameters of elastase-induced AAAs. Moreover, as tamoxifen has been shown to be protective against elastase-induced aneurysms in rodent models, in which it reduced aneurysm sizes by approximately 50%,37 the effect of deficits in α2(V) levels on aneurysm progression may well have been underestimated in our study. However, although it seems unlikely, we cannot formally dismiss the possibility that tamoxifen may have had differential effects on Ubc-Cre; Col5a2fl/fl and Col5a2fl/fl control mice.
In a follow-up study of Col5a2+/− heterozygotes, in which Ang II infusion induced AAAs with pathological features similar to those of human AAAs,29, 30 not only did Col5a2+/− mice have marked increases in the incidence, diameter, and severity of AAAs, compared with controls, but more than half died of aortic arch dissection and rupture. These data are exciting, in that they suggest that COL5A2 dysfunction in the general human population may predispose to aortic dilations, dissection, and rupture. That deficits in col(V) function can underlie loss of arterial integrity in humans is supported by recent reports of predisposition to rupture and dissection in the major arteries of a few cEDS patients with certain COL5A1 missense mutations,38 the occurrence of an aortic aneurysm in a cEDS patient with a COL5A1 splice site mutation,39 and COL5A1 or COL5A2 gene variants of unknown significance in two nonsyndromic cases of aortic root aneurysms.39
In sum, a previous study of Col5a2+/− mice16 together with the present study of conditional Col5a2 knockdown provide evidence indicating that insufficiency for α2(V) chains is unlikely to produce a frank cEDS-like phenotype, thus helping explain the absence of cEDS patients with COL5A2 null alleles.12 Moreover, the lack of an obvious phenotype in the absence of stress in Col5a2+/− mice suggests that COL5A2+/− individuals probably exist in the general human population, but would be unlikely, on routine clinical examination, to be diagnosed not only with cEDS, but perhaps with other clinical abnormalities as well. However, our findings that insufficiency for α2(V) chains can exacerbate AAA formation and predispose to dissecting and ruptured aneurysms of the aortic arch suggest COL5A2 as an important and novel candidate marker/target for the identification and treatment of nonsyndromic individuals prone to aortic aneurysm and dissection.
Footnotes
Supported by the Department of Veterans Affairs (J.M.D.), the University of Wisconsin School of Medicine and Public Health (D.S.G.), and NIH grants AR044745 (D.E.B.), R01HL088447 (B.L.), and AR047746 and AI084853 (D.S.G.).
Disclosures: None declared.
References
- 1.Fichard A., Kleman J.P., Ruggiero F. Another look at collagen V and XI molecules. Matrix Biol. 1995;14:515–531. doi: 10.1016/s0945-053x(05)80001-0. [DOI] [PubMed] [Google Scholar]
- 2.Niyibizi C., Fietzek P.P., van der Rest M. Human placenta type V collagens: evidence for the existence of an alpha 1(V) alpha 2(V) alpha 3(V) collagen molecule. J Biol Chem. 1984;259:14170–14174. [PubMed] [Google Scholar]
- 3.Rhodes R.K., Miller E.J. Physicochemical characterization and molecular organization of the collagen A and B chains. Biochemistry. 1978;17:3442–3448. doi: 10.1021/bi00610a003. [DOI] [PubMed] [Google Scholar]
- 4.Kumamoto C.A., Fessler J.H. Biosynthesis of A, B procollagen. Proc Natl Acad Sci U S A. 1980;77:6434–6438. doi: 10.1073/pnas.77.11.6434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dart M.L., Jankowska-Gan E., Huang G., Roenneburg D.A., Keller M.R., Torrealba J.R., Rhoads A., Kim B., Bobadilla J.L., Haynes L.D., Wilkes D.S., Burlingham W.J., Greenspan D.S. Interleukin-17-dependent autoimmunity to collagen type V in atherosclerosis. Circ Res. 2010;107:1106–1116. doi: 10.1161/CIRCRESAHA.110.221069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang G., Ge G., Wang D., Gopalakrishnan B., Butz D.H., Colman R.J., Nagy A., Greenspan D.S. Alpha3(V) collagen is critical for glucose homeostasis in mice due to effects in pancreatic islets and peripheral tissues. J Clin Invest. 2011;121:769–783. doi: 10.1172/JCI45096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Modesti A., Kalebic T., Scarpa S., Togo S., Grotendorst G., Liotta L.A., Triche T.J. Type V collagen in human amnion is a 12 nm fibrillar component of the pericellular interstitium. Eur J Cell Biol. 1984;35:246–255. [PubMed] [Google Scholar]
- 8.Gordon M.K., Foley J.W., Birk D.E., Fitch J.M., Linsenmayer T.F. Type V collagen and Bowman's membrane: quantitation of mRNA in corneal epithelium and stroma. J Biol Chem. 1994;269:24959–24966. [PubMed] [Google Scholar]
- 9.Birk D.E., Fitch J.M., Babiarz J.P., Linsenmayer T.F. Collagen type I and type V are present in the same fibril in the avian corneal stroma. J Cell Biol. 1988;106:999–1008. doi: 10.1083/jcb.106.3.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Birk D.E., Fitch J.M., Babiarz J.P., Doane K.J., Linsenmayer T.F. Collagen fibrillogenesis in vitro: interaction of types I and V collagen regulates fibril diameter. J Cell Sci. 1990;95(Pt 4):649–657. doi: 10.1242/jcs.95.4.649. [DOI] [PubMed] [Google Scholar]
- 11.Wenstrup R.J., Florer J.B., Brunskill E.W., Bell S.M., Chervoneva I., Birk D.E. Type V collagen controls the initiation of collagen fibril assembly. J Biol Chem. 2004;279:53331–53337. doi: 10.1074/jbc.M409622200. [DOI] [PubMed] [Google Scholar]
- 12.Symoens S., Syx D., Malfait F., Callewaert B., De Backer J., Vanakker O., Coucke P., De Paepe A. Comprehensive molecular analysis demonstrates type V collagen mutations in over 90% of patients with classic EDS and allows to refine diagnostic criteria. Hum Mutat. 2012;33:1485–1493. doi: 10.1002/humu.22137. [DOI] [PubMed] [Google Scholar]
- 13.Toriello H.V., Glover T.W., Takahara K., Byers P.H., Miller D.E., Higgins J.V., Greenspan D.S. A translocation interrupts the COL5A1 gene in a patient with Ehlers-Danlos syndrome and hypomelanosis of Ito. Nat Genet. 1996;13:361–365. doi: 10.1038/ng0796-361. [DOI] [PubMed] [Google Scholar]
- 14.Wenstrup R.J., Florer J.B., Davidson J.M., Phillips C.L., Pfeiffer B.J., Menezes D.W., Chervoneva I., Birk D.E. Murine model of the Ehlers-Danlos syndrome: col5a1 haploinsufficiency disrupts collagen fibril assembly at multiple stages. J Biol Chem. 2006;281:12888–12895. doi: 10.1074/jbc.M511528200. [DOI] [PubMed] [Google Scholar]
- 15.DeNigris J., Yao Q., Birk E.K., Birk D.E. Altered dermal fibroblast behavior in a collagen V haploinsufficient murine model of classic Ehlers-Danlos syndrome. Connect Tissue Res. 2016;57:1–9. doi: 10.3109/03008207.2015.1081901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park A.C., Phillips C.L., Pfeiffer F.M., Roenneburg D.A., Kernien J.F., Adams S.M., Davidson J.M., Birk D.E., Greenspan D.S. Homozygosity and heterozygosity for null Col5a2 alleles produce embryonic lethality and a novel classic Ehlers-Danlos syndrome-related phenotype. Am J Pathol. 2015;185:2000–2011. doi: 10.1016/j.ajpath.2015.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bentz H., Bachinger H.P., Glanville R., Kuhn K. Physical evidence for the assembly of A and B chains of human placental collagen in a single triple helix. Eur J Biochem. 1978;92:563–567. doi: 10.1111/j.1432-1033.1978.tb12778.x. [DOI] [PubMed] [Google Scholar]
- 18.Haralson M.A., Mitchell W.M., Rhodes R.K., Kresina T.F., Gay R., Miller E.J. Chinese hamster lung cells synthesize and confine to the cellular domain a collagen composed solely of B chains. Proc Natl Acad Sci U S A. 1980;77:5206–5210. doi: 10.1073/pnas.77.9.5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malfait F., Wenstrup R.J., De Paepe A. Clinical and genetic aspects of Ehlers-Danlos syndrome, classic type. Genet Med. 2010;12:597–605. doi: 10.1097/GIM.0b013e3181eed412. [DOI] [PubMed] [Google Scholar]
- 20.Ruzankina Y., Pinzon-Guzman C., Asare A., Ong T., Pontano L., Cotsarelis G., Zediak V.P., Velez M., Bhandoola A., Brown E.J. Deletion of the developmentally essential gene ATR in adult mice leads to age-related phenotypes and stem cell loss. Cell Stem Cell. 2007;1:113–126. doi: 10.1016/j.stem.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wirth A., Benyo Z., Lukasova M., Leutgeb B., Wettschureck N., Gorbey S., Orsy P., Horvath B., Maser-Gluth C., Greiner E., Lemmer B., Schutz G., Gutkind J.S., Offermanns S. G12-G13-LARG-mediated signaling in vascular smooth muscle is required for salt-induced hypertension. Nat Med. 2008;14:64–68. doi: 10.1038/nm1666. [DOI] [PubMed] [Google Scholar]
- 22.Committee for the Update of the Guide for the Care and Use of Laboratory Animals; National Research Council: Guide for the Care and Use of Laboratory Animals: Eighth Edition. Washington, DC, National Academies Press, 2011.
- 23.Dunn L., Prosser H.C., Tan J.T., Vanags L.Z., Ng M.K., Bursill C.A. Murine model of wound healing. J Vis Exp. 2013;75:e50265. doi: 10.3791/50265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daugherty A., Manning M.W., Cassis L.A. Antagonism of AT2 receptors augments angiotensin II-induced abdominal aortic aneurysms and atherosclerosis. Br J Pharmacol. 2001;134:865–870. doi: 10.1038/sj.bjp.0704331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wenstrup R.J., Meyer R.A., Lyle J.S., Hoechstetter L., Rose P.S., Levy H.P., Francomano C.A. Prevalence of aortic root dilation in the Ehlers-Danlos syndrome. Genet Med. 2002;4:112–117. doi: 10.1097/00125817-200205000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Thompson R.W., Liao S., Curci J.A. Vascular smooth muscle cell apoptosis in abdominal aortic aneurysms. Coron Artery Dis. 1997;8:623–631. doi: 10.1097/00019501-199710000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Tsui J.C. Experimental models of abdominal aortic aneurysms. Open Cardiovasc Med J. 2010;4:221–230. doi: 10.2174/1874192401004010221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Psaltis P.J., Simari R.D. Vascular wall progenitor cells in health and disease. Circ Res. 2015;116:1392–1412. doi: 10.1161/CIRCRESAHA.116.305368. [DOI] [PubMed] [Google Scholar]
- 29.Rush C., Nyara M., Moxon J.V., Trollope A., Cullen B., Golledge J. Whole genome expression analysis within the angiotensin II-apolipoprotein E deficient mouse model of abdominal aortic aneurysm. BMC Genomics. 2009;10:298. doi: 10.1186/1471-2164-10-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rateri D.L., Howatt D.A., Moorleghen J.J., Charnigo R., Cassis L.A., Daugherty A. Prolonged infusion of angiotensin II in apoE(-/-) mice promotes macrophage recruitment with continued expansion of abdominal aortic aneurysm. Am J Pathol. 2011;179:1542–1548. doi: 10.1016/j.ajpath.2011.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Canty E.G., Lu Y., Meadows R.S., Shaw M.K., Holmes D.F., Kadler K.E. Coalignment of plasma membrane channels and protrusions (fibripositors) specifies the parallelism of tendon. J Cell Biol. 2004;165:553–563. doi: 10.1083/jcb.200312071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Imamura Y., Steiglitz B.M., Greenspan D.S. Bone morphogenetic protein-1 processes the NH2-terminal propeptide, and a furin-like proprotein convertase processes the COOH-terminal propeptide of pro-alpha1(V) collagen. J Biol Chem. 1998;273:27511–27517. doi: 10.1074/jbc.273.42.27511. [DOI] [PubMed] [Google Scholar]
- 33.Andrikopoulos K., Liu X., Keene D.R., Jaenisch R., Ramirez F. Targeted mutation in the col5a2 gene reveals a regulatory role for type V collagen during matrix assembly. Nat Genet. 1995;9:31–36. doi: 10.1038/ng0195-31. [DOI] [PubMed] [Google Scholar]
- 34.Khan T., Muise E.S., Iyengar P., Wang Z.V., Chandalia M., Abate N., Zhang B.B., Bonaldo P., Chua S., Scherer P.E. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol Cell Biol. 2009;29:1575–1591. doi: 10.1128/MCB.01300-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang G., Greenspan D.S. ECM roles in the function of metabolic tissues. Trends Endocrinol Metab. 2012;23:16–22. doi: 10.1016/j.tem.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shook B., Rivera Gonzalez G., Ebmeier S., Grisotti G., Zwick R., Horsley V. The role of adipocytes in tissue regeneration and stem cell niches. Annu Rev Cell Dev Biol. 2016;32:609–631. doi: 10.1146/annurev-cellbio-111315-125426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grigoryants V., Hannawa K.K., Pearce C.G., Sinha I., Roelofs K.J., Ailawadi G., Deatrick K.B., Woodrum D.T., Cho B.S., Henke P.K., Stanley J.C., Eagleton M.J., Upchurch G.R. Tamoxifen up-regulates catalase production, inhibits vessel wall neutrophil infiltration, and attenuates development of experimental abdominal aortic aneurysms. J Vasc Surg. 2005;41:108–114. doi: 10.1016/j.jvs.2004.09.033. [DOI] [PubMed] [Google Scholar]
- 38.Monroe G.R., Harakalova M., van der Crabben S.N., Majoor-Krakauer D., Bertoli-Avella A.M., Moll F.L., Oranen B.I., Dooijes D., Vink A., Knoers N.V., Maugeri A., Pals G., Nijman I.J., van Haaften G., Baas A.F. Familial Ehlers-Danlos syndrome with lethal arterial events caused by a mutation in COL5A1. Am J Med Genet A. 2015;167:1196–1203. doi: 10.1002/ajmg.a.36997. [DOI] [PubMed] [Google Scholar]
- 39.Ziganshin B.A., Bailey A.E., Coons C., Dykas D., Charilaou P., Tanriverdi L.H., Liu L., Tranquilli M., Bale A.E., Elefteriades J.A. Routine genetic testing for thoracic aortic aneurysm and dissection in a clinical setting. Ann Thorac Surg. 2015;100:1604–1611. doi: 10.1016/j.athoracsur.2015.04.106. [DOI] [PubMed] [Google Scholar]