Abstract
Recently, IL-12 emerged as a critical player in type 2 diabetes complications. We previously reported that ischemia-induced angiogenesis is compromised in type 2 diabetic mice. In this study, we determined that IL-12 disruption rescued angiogenesis and arteriogenesis in type 2 diabetic mice. To induce type 2 diabetes, wild-type (WT), p40IL-12−/− (p40−/−), and p35IL-12−/− (p35−/−) mice were fed a high-fat diet (HFD) for 12 weeks. Body weight, glucose test tolerance, and insulin test tolerance were assessed. After 12 weeks of an HFD, the femoral artery was ligated and blood flow recovery was measured every week for 4 weeks. WT, p40−/−, and p35−/− mice fed an HFD become obese after 12 weeks and exhibit glucose intolerance and insulin resistance. Blood flow recovery was fully restored in 2 to 3 weeks after femoral artery ligation in all groups of mice fed a normal diet. However, after 12 weeks of an HFD, blood flow recovery was compromised in WT mice, whereas it was fully recovered in p40−/− and p35−/− mice. The mechanism of blood flow recovery involves an increase in capillary/arteriole density, endothelial nitric oxide synthase/Akt/vascular endothelial growth factor receptor 2 signaling, and a reduction in oxidative stress and inflammation. The disruption of IL-12 promotes angiogenesis and increases blood flow recovery in obese type 2 diabetic mice by an endothelial nitric oxide synthase/Akt/vascular endothelial growth factor receptor 2/oxidative stress–inflammation–dependent mechanism.
Diabetes mellitus affects >347 million individuals worldwide, leading to >4.6 million deaths each year, and its prevalence substantially continues to increase globally.
Cardiovascular dysfunctions are the primary chronic complications, which cause tremendous morbidity, disability, and mortality in patients with type 2 diabetes. Approximately 80% of deaths in patients with diabetes are closely related to vascular complications. Diabetes affects the function and the structure of the small and large blood vessels.1 The development of new vessels in ischemic organs is blunted in diabetic patients, which contributes to delayed wound healing, increased risk of rejection of transplanted organs, exacerbated peripheral limb ischemia (foot ulceration and amputation), and even cardiac mortality.2, 3, 4 Moreover, the mechanisms underlying diabetes-impaired angiogenesis are not completely understood.
We previously reported that angiogenesis is blunted in type 2 diabetic mice under chronic ischemia in the hind limb,5, 6, 7 because of inflammation. Recently, inflammation has emerged as a central contributor to the progression of insulin resistance and the pathogenesis of type 2 diabetes and its vascular complications.8, 9, 10
IL-12 is a heterodimeric cytokine composed of two disulfide-linked protein subunits, p35 and p40, produced by dendritic cells, macrophages, and natural killer cells. IL-12 has been shown to increase in type 2 diabetes and has been involved in the pathogenesis of atherosclerosis, macrovascular complications, and diabetic retinopathy.11, 12, 13, 14 However, little is known about whether IL-12 plays a role in the process of ischemia-induced angiogenesis and arteriogenesis in type 2 diabetes.
Accordingly, using genetically deficient p40−/− and p35−/− mice, we examined whether IL-12 is involved in ischemia-induced neovascularization in the setting of type 2 diabetes. We used a well-established mouse model of neovascularization with unilateral hind-limb chronic ischemia and a high-fat diet (HFD) to induce type 2 diabetes.2, 15
Our aim was to determine the role and mechanism of IL-12 in ischemia-induced neovascularization in mice fed an HFD. We hypothesized that the disruption of IL-12 is an important mechanism that triggers angiogenesis, which protects tissues against chronic ischemia in type 2 diabetes.
Materials and Methods
Animal Model and Hind-Limb Ischemia Surgery
C57BL/6J males [wild type (WT)], p40−/−, and p35−/− on a C57BL/6J background were purchased from Jackson Laboratory (Bar Harbor, ME). To induce diabetes, mice were fed an HFD, 60% high fat [TD.06414 Adjusted Calories Diet 60/Fat; ENVIGO (Teklad, Madison, Wisconsin)] for 12 weeks.2, 15 After 12 weeks, hind-limb ischemia was performed.
Hind-limb ischemia was induced in all mice, as previously reported,5, 6, 7 by ligating the left common femoral artery proximal to the origin of the profunda femoris artery. Mice were anesthetized with 1% to 3% of isoflurane, and a skin incision was made at the left hind limb of the mice. The common femoral vein and nerve were dissected and separated from the femoral artery. The femoral artery was ligated, and then the incision was sutured. These studies were performed according to the principles of the NIH Guide for the Care and Use of Laboratory Animals16 and were approved by the Eastern Virginia Medical School (Norfolk, VA) Institutional Animal Care and Use Committee.
Body Weight and Fasting Blood Glucose
Body weight and fasting blood glucose level were measured once a week for 12 weeks.
Glucose Tolerance Test and Insulin Tolerance Test
The glucose tolerance test and insulin tolerance test were performed once a month for 3 months.
Glucose Tolerance Test
Mice were fasted overnight. Blood samples were obtained from the tail vein using an Ultra Touch glucometer (True test; Trividia Health Inc, Fort Lauderdale, FL). Mice then received an i.p. injection of 2 g/kg glucose. Then, tail vein lancing (using a small sterile needle) was used to draw blood (approximately 2 μL) to determine blood glucose levels. Blood glucose levels were measured with an Ultra Touch glucometer at 0, 10, 20, 30, 60, 90, and 120 minutes after glucose injection.
Insulin Tolerance Test
Mice were fasted for 6 hours and then injected with 1 U/kg i.p. of insulin. Then, blood glucose was measured at 15, 30, 45, and 60 minutes after insulin injection.
Laser Doppler Measurement of Hind-Limb Blood Flow
Each mouse was warmed to a core temperature of 37°C. Then, hind-limb blood flow measurements were performed over the region of interest before surgery, immediately after surgery, and serially over the 4-week period with laser Doppler perfusion imaging (Moor Instruments, Wilmington, DE).5, 6, 7
In Vivo Treatment with IL-12
One week before femoral ligation, p40−/− mice fed an HFD were given injections of recombinant murine IL-12 (i.p., 25 ng, three times per week) for a period of 4 weeks. IL-12 was purchased from Peprotech (Rocky Hill, NJ).
IL-12 and Migration of Vascular Smooth Muscle Cells
Vascular smooth muscle cells were cultured in the complete growth medium (Dulbecco's modified Eagle's medium) to confluence in a humidified incubator containing 5% CO2 at 37°C. Confluent cell monolayers were incubated for 24 hours with the starvation media (low-glucose Dulbecco's modified Eagle's medium without fetal bovine serum) before the experiments. Then, they were scraped with a 200-μL standard pipette tip to generate scratch wounds and washed three times with phosphate-buffered saline to remove any loosely attached cells. The wounded monolayers were then incubated for 24 hours after treatment with and without 50 ng of IL-12. Images of injured areas at 0 and 24 hours were taken at ×10 magnification.
Enzyme-Linked Immunosorbent Assay and Kits
All of the assays and kits were used according to the manufacturer's recommendations. IL-12, IL-10, IL-6, and monocyte chemoattractant protein (MCP)-1 enzyme-linked immunosorbent assay kits were purchased from BioLegend (San Diego, CA). Vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) were purchased from R&D Systems (Minneapolis, MN). The thiobarbituric acid reactive substances assay kit was purchased from Cayman Chemical (Ann Arbor, MI).
Western Blot Analysis
Hind-limb tissues were homogenized in T-PER Tissue Protein Extraction Reagent (Thermo Fisher Scientific, Waltham, MA) containing a cocktail of protease and phosphatase inhibitors. Equal amounts of protein were loaded into polyacrylamide-SDS gels (Bio-Rad Laboratories Inc., Des Plaines, IL) and transferred onto nitrocellulose membranes (Bio-Rad Laboratories Inc.). The blots were blocked with 5% bovine serum albumin for 1 hour and probed with primary antibodies for target proteins overnight at 4°C. Immunoblots were next probed with the fluorophore-labeled secondary antibodies (LI-COR Biosciences, Lincoln, NE) for 1 hour at room temperature. Final protein expression was detected using the Odyssey imaging system (LI-COR Biosciences) and quantified using ImageJ software version 1.51 (NIH, Bethesda, MD; http://imagej.nih.gov/ij). Antibodies for phosphorylated endothelial nitric oxide synthase (eNOS) (serine 1177), phosphorylated VEGF receptor (VEGFR) 2, total Akt, phosphorylated Akt, total STAT4, NADPH oxidase isoform (Nox)2, and Nox4 were purchased from Cell Signaling Technology (Danvers, MA). Antibodies for phosphorylated STAT4 (PY693) and eNOS/NOS type III were purchased from BD Biosciences (San Jose, CA). Secondary antibody conjugates were purchased from LI-COR Biosciences.
NADPH Oxidase Activity Assay
NADPH oxidase activity was performed as previously described.17 Superoxide anion levels generated by NADPH oxidase were measured in lysates of ischemic muscles using a lucigenin chemiluminescence assay. Briefly, lysates were prepared in a sucrose buffer containing 50 mmol/L KH2PO4, 1 mmol/L EGTA, and 150 mmol/L sucrose (pH 7.0), with protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN), in a tissue homogenizer on ice. Aliquots of the homogenates were used immediately. To start the assay, a volume of 100 μL of each lysate was used in a total volume of 1 mL phosphate-buffered saline buffer, preheated at 37°C, containing 5 μmol/L lucigenin and 100 μmol/L NADPH. Blank samples were prepared using 100 μL of sucrose buffer. Lucigenin activity was measured every 30 seconds for 10 minutes in a luminometer (20/20, single-tube luminometer; Turner BioSystems, Sunnyvale, CA) until enzymatic activity reached the plateau. Data are expressed as area under the curve of relative light units normalized to protein content (in μg protein).17
Immunohistochemistry
Immumohistochemistry for capillary/arteriole density and inflammation was performed as previously described.5, 18, 19 Immunofluorescence staining was performed on hind-limb muscles for α-smooth muscle actin, CD31, F4/80, p40, and p35 expression. Immunoperoxidase staining was also performed for p40, p35, and F4/80 expression.
Real-Time Quantitative PCR
Total RNA was isolated using RNAzol-RT reagent (Molecular Research Center, Cincinnati, OH) from ischemic hind-limb muscles from all groups. The RNA was then reverse transcribed using NEB M-MuLV Reverse Transcriptase (New England Biolabs, Ipswich, MA) and subjected to real-time quantitative PCR using the TaqMan and Bio-Rad CFX96 Real-Time PCR Detection System (Bio-Rad Laboratories Inc.). Real-time quantitative PCR was performed in triplicate using the following TaqMan assays (Thermo Fisher Scientific): Nox2 (Mm01287743_m1), Nox4 (Mm00479246_m1), tumor necrosis factor (TNF)-α (Mm00443258_m1), p40 (Mm00434174_m1), p35 (Mm.PT.5813818295), IL-10 (Mm.PT.58 13531087), interferon (IFN)-γ (Mm.PT.5841769240), arginase-1 (Mm.PT.588651372), Mannose receptor C type 1 (Mm.PT.5831835913), C-type lectin domain containing 10A (Clec10a; Mm.PT.5831835913), and chemokine (C-C motif) ligand (Mm.PT.5842151692). A two-step reaction protocol was used with an initial denaturation of 1 minute at 95°C, followed by 40 cycles of 95°C for 15 seconds, then 60°C for 45 seconds, using the Real-Time Thermal Cycler CFX96 Optics Module (Bio-Rad Laboratories Inc.). Final normalized gene expression was calculated using α-actin mRNA as an endogenous control.
Statistical Analysis
Results are expressed as means ± SEM. One- and two-way analysis of variance were used when appropriate. Comparisons between groups were performed with t-tests when the analysis of variance test was statistically significant. P < 0.05 was considered significant.
Results
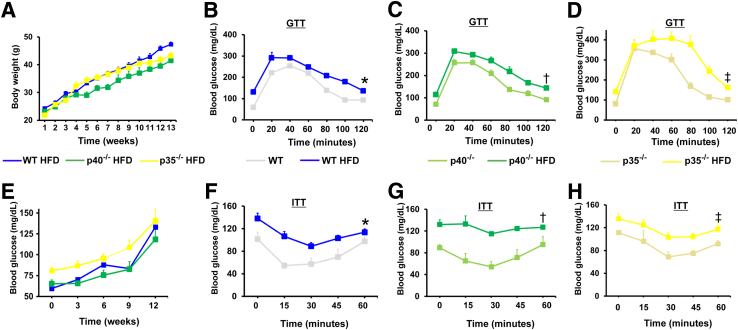
In WT, p40−/−, and p35−/− mice fed with an HFD for 12 weeks, a similar increase in the body weight and blood glucose levels (Figure 1, A and B) was observed. The glucose tolerance was similar between WT and p40−/− mice fed a chow diet (Figure 1, C and E). However, glucose tolerance for a chow diet was higher within the first 60 minutes in p35−/− mice compared with WT and p40−/− mice (Figure 1, C, E, and G). The insulin tolerance was similar in all mice fed a chow diet (Figure 1, D, F, and H). When these mice were fed an HFD for 12 weeks, the glucose test tolerance was compromised in WT and p40−/− mice but exacerbated in the p35−/− mice (Figure 1, C, E, and G). However, the insulin test tolerance was reduced in all groups of mice (Figure 1, D, F, and H). These results showed that HFD induced insulin resistance in WT, p40−/−, and p35−/− mice. Our data suggest that p35 could be involved in the etiology of obesity and type 2 diabetes induced by HFD feeding.
Figure 1.
Body weight and blood glucose levels during glucose tolerance tests (GTTs) and insulin tolerance tests (ITTs) of mice during a 12-week feeding of a high-fat diet (HFD) or a normal diet. A and B: Male wild-type (WT), p40−/−, and p35−/− mice were fed an HFD for 12 weeks; body weight (A) and fasted blood glucose (B) were assessed weekly. C–G: Glucose test tolerance performed on WT (C), p40−/− (E), and p35−/− (G) mice fed either an HFD or a normal diet. D–H: Insulin test tolerance performed in WT (D), p40−/− (F), and p35−/− (H) mice fed either a chow diet or an HFD. Blood glucose measurement during GTT experiments was performed in mice that fasted overnight, whereas the ITT procedure was performed in mice that fasted for 6 hour. Data are expressed as means ± SEM (A–H). n = 6 (A–H). ∗P < 0.05 versus WT fed a normal diet; †P < 0.05 versus p40−/− fed a normal diet; ‡P < 0.05 versus p35−/− fed a normal diet.
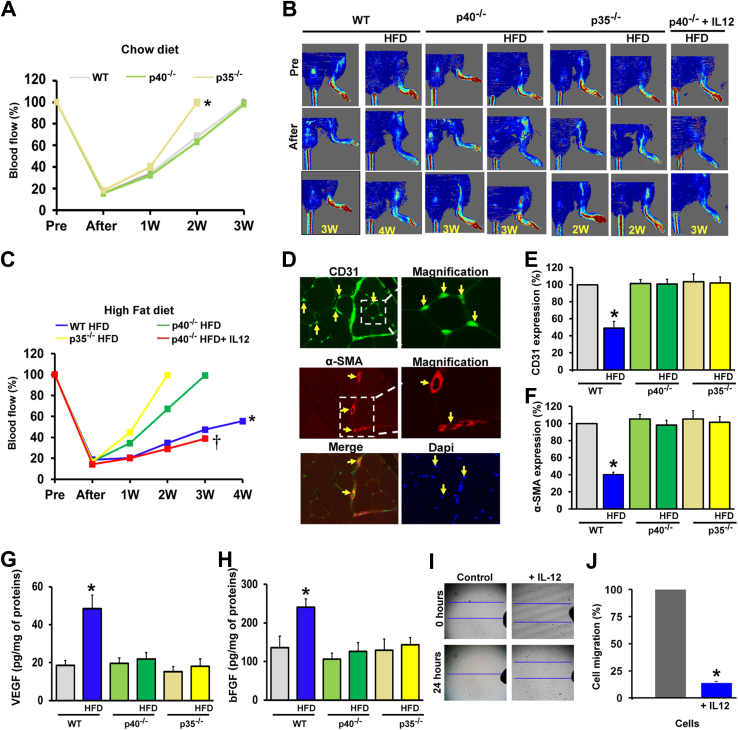
To determine the in vivo contribution of IL-12 on angiogenesis, femoral artery ligation was performed in WT, p40−/−, and p35−/− mice fed a chow diet and an HFD. Laser Doppler perfusion imaging was performed to measure blood flow before and immediately after femoral artery ligation and at weeks 1, 2, and 3, until reaching full recovery. After femoral artery ligation, blood flow decreased by 80% in the hind limb in all of the groups of mice (Figure 2, A and B). For a normal chow diet, total blood flow recovery was faster in p35−/− mice (2 weeks) compared with WT and p40−/− mice (3 weeks) (Figure 2A).
Figure 2.
Effect of IL-12 deficiency on ischemia-induced neovascularization in mice fed a normal diet or a high-fat diet (HFD). A and B: Quantitative data for blood flow recovery in wild-type (WT), p40−/−, and p35−/− mice fed a chow diet (A) or an HFD (B). C: Representative blood flow imaging measured with laser Doppler perfusion imaging (Moor Instruments) in the ischemic hind limb of WT, p40−/−, and p35−/− mice fed a chow diet or an HFD and p40−/− mice fed an HFD receiving exogenous IL-12 (25 ng, three times per week for 4 weeks) before surgery (Pre; femoral ligation), right after surgery, and once a week for 3 to 4 weeks (W). D: CD31 (capillaries; green), α-smooth muscle actin (α-SMA; arterioles; red) and DAPI (cell nuclei; blue) immunostaining of ischemic muscles in WT mice. The arrows show the specific staining. E and F: Cumulative data of CD31 and α-smooth muscle actin expression in the ischemic limb in WT, p40−/−, and p35−/− mice fed a chow diet or an HFD. G and H: Vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) levels measured by enzyme-linked immunosorbent assay in ischemic hind-limb muscle lysates from WT, p40−/−, and p35−/− mice fed a chow diet or an HFD. I and J: Effect of IL-12 on cell migration. Using the wound-healing assay, the wound was generated by a scratch on confluent vascular smooth muscle cells; the wound closure was monitored after scratch (0 hours) and at 24 hours and imaged with phase-contrast microscopy (Olympus, Lombard, IL). The migration assay was performed in three independent experiments. The P values were determined for control and IL-12–treated cells and P < 0.05 was considered statistically significant. Data are expressed as means ± SEM (E–H). n = 6 (C and E–H). ∗P < 0.05 versus all groups; †P < 0.05 versus p40−/− HFD group. Original magnification, ×10 (I).
Blood flow recovery was significantly compromised in WT mice fed an HFD compared with WT fed a chow diet. The recovery reached only 50% within 4 weeks after the femoral artery ligation (Figure 2B), whereas it reached 100% in p40−/− and p35−/− mice fed an HFD within 2 to 3 weeks (Figure 2B).
Figure 2C illustrates representative blood flow in all mice fed either a chow diet or an HFD before and after femoral artery ligation. These data indicate that the deletion of IL-12 protects the hind-limb muscle from chronic ischemia by promoting angiogenesis/arteriogenesis and, therefore, blood flow perfusion. To demonstrate that the stimulation of angiogenesis observed in mice lacking IL-12 and fed an HFD was because of the IL-12 deficiency, we tested the effect of administrating exogenous IL-12 (25 ng per mouse) to p40−/− mice fed an HFD 1 week before femoral artery ligation and 1, 2, and 3 weeks after. Blood flow recovery was impaired in p40−/− mice receiving IL-12 after femoral artery ligation compared with untreated p40−/− mice fed an HFD (Figure 2, B and C). Indeed, the administration of IL-12 to p40−/− mice blunted the promotion of angiogenesis. Together, these findings support the critical role of IL-12 in impaired angiogenesis in type 2 diabetes.
The blood flow data were supported by the blood vessel density measurement assessed by immunostaining in hind-limb muscle. The capillary density determined by CD31 (a specific marker for the endothelial cell) staining was significantly reduced in WT mice fed an HFD compared with all other groups of mice (Figure 2, D and E). Similar results were obtained with arteriole density assessed with α-smooth muscle actin staining (Figure 2, D and F). Moreover, ischemic limbs from p40−/− and p35−/− mice fed an HFD exhibit a higher capillary and arteriole density compared with WT mice fed an HFD (Figure 2, D–F).
VEGF and bFGF are factors known to play a major role in angiogenesis. The tissue level of VEGF and bFGF was measured in the ischemic hind limb of all mice. VEGF and bFGF levels were significantly increased in WT mice fed an HFD compared with WT, p40−/−, and p35−/− mice fed a chow diet (Figure 2, G and H). Interestingly, VEGF and bFGF levels were reduced in the ischemic limb of p40−/− and p35−/− mice fed an HFD (Figure 2, G and H).
To demonstrate the antiangiogenic effect of IL-12, we performed an in vitro cell injury and migration assay. We found that exogenous IL-12 significantly blunted the migration of smooth muscle cells (Figure 2, I and J). Together, these results established the following: the excess in IL-12 in type 2 diabetic mice blunts angiogenesis and arteriogenesis in response to chronic ischemia; and the disruption of IL-12 triggers the angiogenesis and arteriogenesis events in response to chronic ischemia.
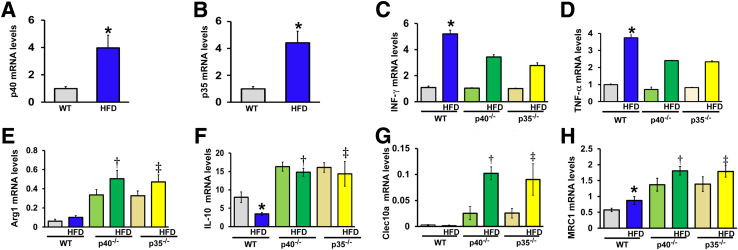
Figure 3, A and B, illustrate the increase in p35 and p40 mRNA levels in the hind-limb muscle from WT mice fed an HFD compared with WT mice fed a chow diet. These results indicate that IL-12 (p40 and p35) was up-regulated in type 2 diabetes.
Figure 3.
Effect of a high-fat diet (HFD) and IL-12 deficiency on cytokine expression in the ischemic hind limb. A and B: p40 and p35 relative mRNA levels, as assessed by real-time quantitative PCR in ischemic hind-limb muscle. C: Interferon (IFN)-γ relative mRNA level in wild-type (WT), p40−/−, and p35−/− mice fed a control diet or an HFD. D: Tumor necrosis factor (TNF)-α relative mRNA level in WT, p40−/−, and p35−/− mice fed a chow diet or an HFD. E–H: Relative mRNA levels for markers for M2 macrophages in WT, p40−/−, and p35−/− mice fed a chow diet or an HFD. Arginase-1 (Arg1; E), IL-10 (F), C-type lectin domain containing 10A (Clec10a; G), and Mannose receptor C-type 1 (MRC1; H) mRNA levels. All cytokine gene expression levels were normalized to β-actin. Data are expressed as means ± SEM. n = 5 to 6 (A–H). ∗P < 0.05 versus WT; †P < 0.05 versus WT HFD or WT; ‡P < 0.05 versus WT HFD.
It is well documented that macrophages are the key cell type in orchestrating the angiogenic and healing responses in ischemic tissues.20 On the basis of the activation route, macrophages can generally be divided into two major populations: M1 proinflammatory macrophages and M2 anti-inflammatory/proangiogenic macrophages. To determine the status of M1/M2 macrophages in our model of HFD-induced diabetes, we analyzed the expression of genes reflecting proinflammatory and anti-inflammatory macrophages in ischemic limbs of WT, p40, and p35 mice. INF-γ and TNF-α, which are downstream signaling to IL-12, were increased in WT mice fed an HFD and reduced in p40−/− and p35−/− mice fed an HFD (Figure 3, C and D), suggesting a role of IL-12 on proinflammatory cytokine regulation. Expressions of anti-inflammatory factors and markers for M2 macrophages, arginase-1, IL-10, Clec10a, and MRC1 were all increased in the ischemic limb of p40−/− and p35−/− mice fed either a chow diet or an HFD compared with WT mice fed an HFD or a chow diet (Figure 3, E–H).
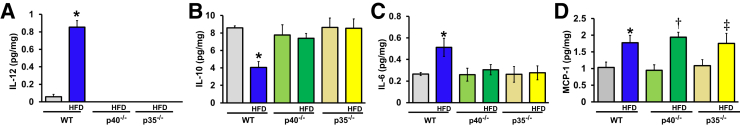
Moreover, we assessed the levels of IL-12, IL-10, IL-6, and MCP-1 cytokines at the protein levels by enzyme-linked immunosorbent assay. IL-12 was only detected in the ischemic limb of WT mice (Figure 4A). Consistent with previous studies,21, 22 our results confirmed that p40−/− and p35−/− mice were unable to make the bioactive IL-12 cytokine22 and also showed that an HFD induced an up-regulation of IL-12 in the ischemic limb of WT mice. The IL-10 cytokine was decreased in the ischemic limb of WT mice fed an HFD compared with WT mice fed a chow diet but prevented in p40−/− and p35−/− mice (Figure 4B). Moreover, an HFD induced an increase of the IL-6 level in the ischemic limb of WT mice; this was blunted in p40−/− and p35−/− mice (Figure 4C).
Figure 4.
Cytokine expression in ischemic hind limb. IL-12 (A), IL-10 (B), IL-6 (C), and monocyte chemoattractant protein (MCP)-1 (D) protein concentrations in ischemic limb of wild-type (WT), p40−/−, and p35−/− mice fed a chow diet or a high-fat diet (HFD) were quantified by enzyme-linked immunosorbent assay. Data are expressed as means ± SEM (A–D). n = 5 to 6 (A–D). ∗P < 0.05 versus WT; †P < 0.05 versus WT HFD or WT; ‡P < 0.05 versus WT HFD.
Interestingly, an HFD increased the level of MCP-1 in the ischemic limb of WT, p40−/−, and p35−/− mice. Unlike IL-6, MCP-1 level was not affected by p40 or p35 deletion (Figure 4D).
Together, our data showed that IL-12 deficiency up-regulated the expression of anti-inflammatory cytokines and reduced the expression of proinflammatory cytokines in the ischemic limb of type 2 diabetic mice.
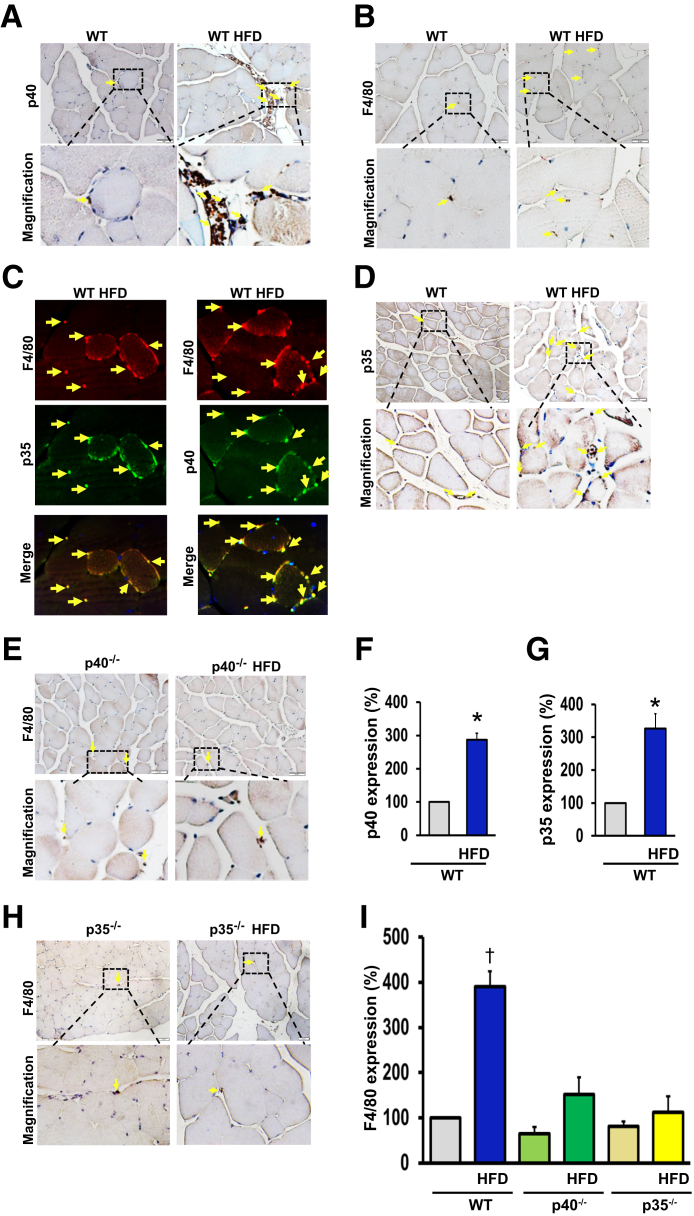
Using p40 and p35 antibodies, immunohistochemical analysis confirmed our real-time quantitative RT-PCR data showing the up-regulation of p40 and p35 mRNA levels in the ischemic limb of WT mice fed an HFD (Figure 5, A, D, F, and G).
Figure 5.
A and B: Immunohistochemistry images using p40 and p35 antibodies to detect p40 and p35 IL-12 in the hind-limb ischemic muscle of wild-type (WT) mice fed a chow diet or a high-fat diet (HFD). C and D: Quantification of immunohistochemistry staining for p40 and p35 of WT mice fed a chow diet or an HFD. E–G: Immunohistochemistry images using F4/80 antibody to detect macrophages in hind-limb muscle of WT (E), p40−/− (F), and p35−/− (G) mice fed a chow diet or an HFD. H: Quantification of immunohistochemistry images for F4/80 in hind-limb muscle of WT, p40−/−, and p35−/− mice fed a chow diet or an HFD. I: Double immunofluorescence for p40 (green), p35 (green), and the macrophage marker F4/80 (red) using specific antibodies, followed by fluorescent-labeled secondary antibodies, in hind-limb muscle of WT mice fed an HFD. Arrows show the specific staining for each antibody. All of the data shown are representative of six separate animals. Data are expressed as means ± SEM. ∗P < 0.05 versus WT; †P < 0.05 versus all groups. Original magnification, ×20 (A, B, D, E, and H); ×50 (C).
We also demonstrated that the number of F4/80-positive cells (a marker for macrophages) was significantly higher in WT mice fed an HFD mice compared with WT mice fed a chow diet (Figure 5, B and I). However, in the p40−/− or p35−/− mice, the number of macrophages was low and was not increased by an HFD (Figure 5, E, H, and I). Moreover, the double immunostaining between F4/80 (macrophage marker) and p35 or between F4/80 and p40 revealed that the source of IL-12 was mainly from the macrophages (Figure 5C).
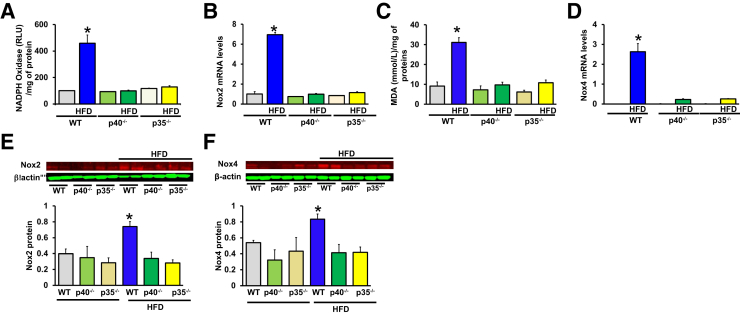
We previously reported that oxidative stress was increased in type 2 diabetic mice.19 In the present study, we found an increase of oxidative stress, as assessed by thiobarbituric acid reactive substances malonedialdehyde levels and NADPH oxidase activity, in ischemic hind limb muscle from WT mice fed an HFD compared with WT mice fed a chow diet; oxidative stress was blunted in p40−/− and p35−/− mice fed an HFD (Figure 6, A and B).
Figure 6.
Effect of p40 and p35 deficiency on oxidative stress in ischemic muscle limb. A: NADPH oxidase superoxide activity was measured by lucigenin chemiluminiscent assay in ischemic muscle limb homogenates from wild-type (WT), p40−/−, p35−/− mice fed a chow diet or a high-fat diet (HFD). B and E: NADPH oxidase NADPH oxidase (Nox)2 mRNA and protein levels in ischemic muscle lysates from WT, p40−/−, and p35−/− mice fed a chow diet or an HFD. C: Thiobarbituric acid reactive substances presented as concentration of malondialdehyde (MDA) in the ischemic limb of WT, p40−/−, and p35−/− mice fed a chow diet or an HFD. D and F: NADPH oxidase Nox4 mRNA and protein levels in ischemic muscles lysates from WT, p40−/−, and p35−/− mice fed a chow diet or an HFD. Data are expressed as means ± SEM (A–F). n = 6 (A–F). ∗P < 0.05 versus all groups. RLU, relative light units.
Recent evidence demonstrated that NADPH oxidases NOX2 and NOX4 were increased by an HFD.23 Accordingly, real-time quantitative RT-PCR and Western blot analysis showed that Nox2 and Nox4 levels were up-regulated in hind-limb muscles from WT mice fed an HFD but not in p40−/− and p35−/− mice fed an HFD (Figure 6, C–F). We could not detect Nox1 expression in ischemic limb muscle from all mice. These data indicate that IL-12 disruption promotes angiogenesis and arteriogenesis in response to chronic ischemia, more likely through the inhibition of oxidative stress via the down-regulation of Nox2 and Nox4 expression.
It is well documented that IL-12 acts via the STAT4 signaling pathway.24
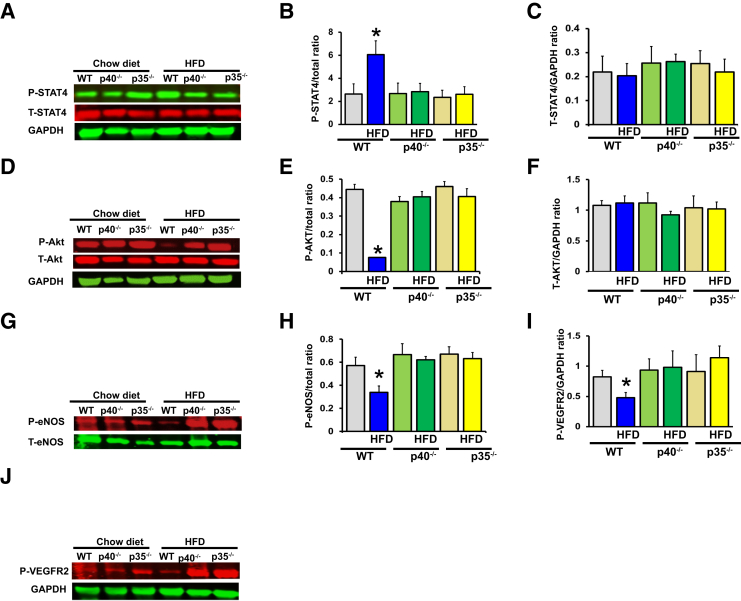
Signal transduction through the IL-12 receptor induces tyrosine phosphorylation of the Janus family kinases TYK2 and JAK2, which, in turn, phosphorylate STAT4. Our data show that phosphorylated STAT4 was increased in the hind-limb muscle from WT mice fed an HFD (Figure 7, A and B). However, the increase in phosphorylated STAT4 was blunted in p40−/− and p35−/− mice fed an HFD (Figure 7, A and B). We did not observe any changes in total STAT4 between all groups of mice (Figure 7, A and C). These data suggest that an HFD was able to activate IL-12 signaling via STAT4 phosphorylation and was prevented by p40 or p35 deletion.
Figure 7.
Effect of p40 and p35 deficiency on IL-12 signaling, survival pathway, and proangiogenic factors in ischemic muscle limb. Representative immunoblots and densitometric analysis of the expression of phosphorylated STAT4 (P-STAT4; PY693; A and B), total STAT4 (T-STAT4; A and C), phosphorylated Akt (P-Akt; serine 473; D and E), total Akt (T-Act; D and F), phosphorylated endothelial nitric oxide synthase (eNOS) (P-eNOS; serine 1177; G and H), total e-NOS (T-eNOS; G and H), and phosphorylated vascular endothelial growth factor receptor 2 (VEGFR2; tyrosine 1175; I and J). β-Actin was used as loading control. Densitometric measurements were performed to evaluate the fold increase in protein expression or activity. Data are expressed as means ± SEM. n = 6 (A–J). ∗P < 0.05 versus all groups. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
The angiogenic factors (phosphorylated eNOS, Akt, and VEGFR2) were significantly reduced in ischemic hind-limb muscle from WT mice fed an HFD (Figure 7, D–J) but normalized in p40−/− and p35−/− mice fed an HFD (Figure 7, D–J), suggesting that IL-12 promotes angiogenesis by regulating proangiogenic factors.
Discussion
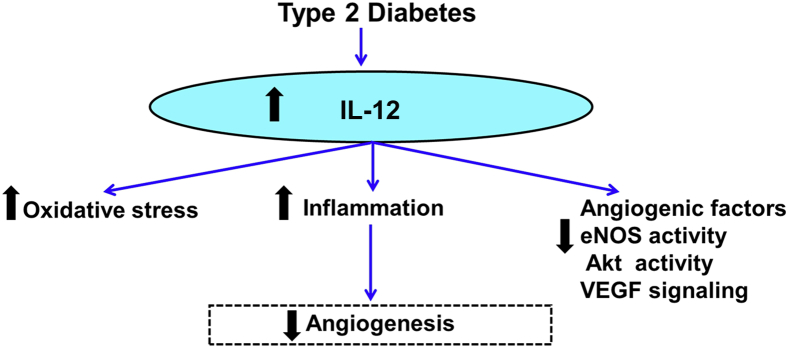
The present study provided evidence that the disruption of IL-12 promotes angiogenesis, which rescues tissue blood flow perfusion in obese type 2 diabetic mice. We also demonstrated that the deletion of IL-12 stimulates neovascularization events through a mechanism that involves a decrease in oxidative stress and an increase in the angiogenic factors (eNOS, Akt, and VEGFR2). The beneficial effects of lacking IL-12 were associated with reduced inflammation, increased M2 macrophages, and reduced oxidative stress in the ischemic limb of type 2 diabetic mice, suggesting that inflammation and oxidative stress account for the impaired ischemia-induced neovascularization in type 2 diabetes (Figure 8). These results are consistent with our previous finding indicating that angiogenesis in response to hind-limb ischemia was abrogated during diabetes.5, 6, 7 Despite a huge number of positive animal studies on angiogenesis, the clinical trials on therapeutic angiogenesis are not conclusive. With a disease as complex as diabetes, many factors are likely to be involved in angiogenesis defect.25 In the present study, we used a preclinical relevant murine model of type 2 diabetic obese mice induced by an HFD to study the in vivo contribution of IL-12 on neovascularization. The HFD mice model is a robust and efficient model for impaired glucose tolerance and progressive insulin resistance and type 2 diabetes.2, 15
Figure 8.
Summary diagram that illustrates potential mechanisms by which IL-12 impairs ischemia-induced angiogenesis in type 2 diabetes via oxidative stress, inflammation, and angiogenic factors. eNOS, endothelial nitric oxide synthase; VEGF, vascular endothelial growth factor.
In agreement with previous studies, we showed that p40−/− and p35−/− mice were unable to produce a biological active of IL-12,21, 22 the heterodimeric cytokine IL-12 (70 kDa) composed of p35 (35 kDa) and p40 (40 kDa) (Figure 4 A), and IL-12 production is enhanced in obese type 2 diabetic patients and animal models.11, 13, 14, 26 We found an increase of IL-12 in the hind-limb muscle of our diabetic mice (fed an HFD for 12 weeks) compared with nondiabetic mice (fed a chow diet). WT, p40IL-12−/−, and p35IL-12−/− mice fed an HFD for 12 weeks exhibit a gain in body weight, impaired glucose test tolerance, and insulin resistance. These data suggest that IL-12 is unlikely to be involved in the development and etiologies of obesity and type 2 diabetes. However, p35−/− mice fed an HFD appear to be more sensitive to glucose test tolerance than p40 or WT mice. Further investigations are needed to explore the possible link between p35 and insulin resistance and type 2 diabetes development. In fact, a recent study reported an association without cause-effect between lipid abnormalities and p40 levels in obese patients.27 Moreover, IL-12 secretion and production are enhanced under hyperglycemia and diabetes conditions, suggesting that augmented IL-12 level and release are the consequences of obesity and type 2 diabetes, rather than a cause.28
The role of IL-12 in angiogenesis and arteriogenesis is still unknown, especially in obesity associated with type 2 diabetes. This study is the first evidence to show the in vivo contribution of IL-12 in impaired neovascularization in the ischemic hind limb of type 2 diabetic mice. The disruption of IL-12 in type 2 diabetic mice was able to stimulate angiogenesis efficiently and rescue blood flow perfusion in mice with type 2 diabetes and insulin resistance.
For a chow diet, IL-12 disruption did not significantly affect the underlying mechanism of angiogenesis and arteriogenesis in our model of hind-limb ischemia. Nevertheless, IL-12 was reported to inhibit bFGF-induced neovascularization in a s.c. Matrigel.29 The difference could be related to the model used and the IL-12 expression levels in the hind-limb muscle. In fact, the level of IL-12 was low in the control limb muscle. Also, using the hind-limb ischemia model, a recent study showed that p35−/− mice fully recovered blood flow within 3 days after femoral artery ligation.30 In the present study, the blood flow recovery was complete 3 weeks after femoral artery ligation in WT, p40−/−, and p35−/− mice. The discrepancy between our results and those of Du and colleagues30 could be because of technical setting at the time of blood flow measurement. Laser Doppler perfusion measurement is sensitive to temperature changes, and a high temperature could lead to high values in blood flow perfusion assessment.
During the past decade, it became apparent that inflammation is a key feature of obesity and type 2 diabetes. In fact, obesity, insulin resistance, and type 2 diabetes are closely associated with chronic inflammation, characterized by abnormal cytokine production and activation of inflammatory signaling pathways.31, 32, 33, 34
Blood flow measurement and immunohistochemistry analyses indicate that blood flow recovery was strongly associated with an increase in capillary and arteriole density in the ischemic limb of p40−/− and p35−/− mice fed an HFD. These results highlight the protective role of IL-12 disruption in neovascularization in obese type 2 diabetic mice.
The mechanism by which IL-12 disruption protects the angiogenesis and arteriogenesis could be through the reduction of inflammation (reduction of macrophage infiltration, reduction of proinflammatory cytokines, and increase of anti-inflammatory cytokines). Macrophages have a remarkable plasticity to effectively respond to environmental changes. They can modify their phenotypes M1 or M2 to exert different functions. Both subtypes of macrophages are required during neovascularization; the release of chemofactors, such as MCP-1 by M1, plays an important role in arteriogenesis.35
In the present study, TNF-α, IFN-γ, and IL-6, potent inducers of inflammation, were highly up-regulated in ischemic limbs from WT mice fed an HFD compared with WT mice fed a chow diet; they were significantly reduced in p40−/− and p35−/− mice fed an HFD. MCP-1, another proinflammatory cytokine, was up-regulated in the ischemic limb of all mice fed an HFD and was not affected by the deletion of p40 or p35. The blockade of MCP-1 activity was able to impair blood flow recovery after hind-limb ischemia in control mice.36 Further studies are needed to explore the role of MCP-1 in ischemia-induced neovascularization in type 2 diabetes.
Macrophages, major sources of proinflammatory factors, were also increased by an HFD in WT mice and drastically inhibited in p40−/− and p35−/− mice.
In line with our results, a clear link was established between proinflammatory TNF-α, obesity, and inflammation.37 Moreover, TNF-α has been shown to be involved in ischemia-induced neovascularization38, 39 and could account for the beneficial effect of lacking IL-12 on ischemia-induced neovascularization in diabetes. Furthermore, IFN-γ could also mediate the inhibitory effect of IL-12 on angiogenesis observed in our diet-induced type 2 diabetic mice. The mRNA expression of IFN-γ was up-regulated in the ischemic limb of type 2 diabetic mice, whereas it was down-regulated in the mice lacking p40 or p35 compared with wild type. In fact, IFN-γ has been shown to be a mediator of angiogenesis inhibition by IL-12 using a Matrigel angiogenesis system.29, 40
IFN-γ biases M1 polarization,41, 42 which could explain the inhibitory effect of IL-12 on angiogenesis in type 2 diabetes. Further investigations are needed to determine the effect of the IL-12/IFN-γ/M1 macrophage polarization axis on angiogenesis and arteriogenesis in diabetes.
Indeed, M2 macrophages are known to express angiogenic factors, such as VEGF, insulin-like growth factor-1, and hepatocyte growth factor, and promote wound repair and neovascularization.43 Our results show that M2 macrophage markers, ARG1, IL-10, Clec10a, and MRC1, were all up-regulated in the ischemic limb of p40−/− and p35−/− mice compared with WT mice fed an HFD. These results suggest the potential involvement of M2 macrophages in ischemia-induced neovascularization in type 2 diabetic mice.
Recently, oxidative stress has emerged as a feature of obesity and is a major factor in the development of insulin resistance in obesity44; it has been shown to play a major role in diabetic vascular complications.19 Indeed, inhibiting oxidative stress in diabetic mice may account for the beneficial role of deleting IL-12 in ischemia-induced neovascularization in type 2 diabetes. The exact mechanism by which IL-12 regulates oxidative stress is yet to be determined. Lacking IL-12 protects type 2 diabetic mice against oxidative stress.
The induction of neovascularization is a consequence of a balance between proangiogenic and antiangiogenic factors. In line with previous studies,5, 7, 45 we demonstrated that the VEGF level was increased and eNOS and VEGF signaling (VEGFR2 phosphorylation) were reduced in the ischemic limb of dietary-induced type 2 diabetes. Indeed, an increase of soluble and membrane-bound VEGFR1 may limit the ability of VEGF to bind to VEGFR2, resulting in decreased angiogenesis.45
Moreover, bFGF was also increased in the ischemic limb of WT mice fed an HFD compared with WT mice fed a chow diet. It is well known that bFGF is a potent angiogenic factor that requires the activation of the VEGF system.46 Interestingly, the advanced glycation end product has been shown to be present in type 2 diabetes and associated with vascular complications.47 Moreover, bFGF has been shown to be modified by the advanced glycation end product in diabetes. The glycation of bFGF by advanced glycation end product in vitro and in vivo was able to reduce its angiogenic properties,48, 49 which, in turn, can inhibit VEGF-dependent signaling.
We also found that p40 and p35 deficiency enhanced eNOS and VEGF signaling. Moreover, both VEGF and bFGF levels were reduced in p40−/− and p35−/− mice fed an HFD. These results suggest that the eNOS pathway, VEGF expression, bFGF expression, and VEGF signaling are regulated by IL-12. Further studies are needed to delineate the mechanism linking IL-12 to eNOS, VEGF, and bFGF signaling.
In conclusion, we provided new insights into the essential role of IL-12 in ischemia-induced neovascularization. The disruption of IL-12 in obese type 2 diabetic mice promotes the induction of angiogenesis and arteriogenesis and, therefore, blood flow perfusion in response to chronic ischemia. The protective mechanism of IL-12 disruption in obesity and type 2 diabetes involved the following: i) the reduction in oxidative stress and IL-12 downstream signaling (STAT4 phosphorylation), ii) the increase in angiogenic factor activity (eNOS, Akt, and VEGFR2), iii) the reduction of inflammation and proinflammatory cytokines, and iv) the increase of anti-inflammatory cytokines and M2 macrophage markers (Figure 8).
Footnotes
Supported by an Eastern Virginia Medical School Research Emphasis grant (S.B.), NIH grant R01HL095566 (K.M.), and an Egyptian Cultural Bureau fellowship (M.A.).
Disclosures: None declared.
References
- 1.Fowler M.J. Microvascular and macrovascular complications of diabetes. Clin Diabetes. 2011;29:116–122. [Google Scholar]
- 2.Winzell M.S., Ahrén B. The high-fat diet–fed mouse a model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes. 2004;53:S215–S219. doi: 10.2337/diabetes.53.suppl_3.s215. [DOI] [PubMed] [Google Scholar]
- 3.Waltenberger J. New horizons in diabetes therapy: the angiogenesis paradox in diabetes: description of the problem and presentation of a unifying hypothesis. Immunol Endocr Metab Agents Med Chem. 2007;7:87–93. [Google Scholar]
- 4.Costa P.Z., Soares R. Neovascularization in diabetes and its complications: unraveling the angiogenic paradox. Life Sci. 2013;92:1037–1045. doi: 10.1016/j.lfs.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Amin A., Choi S.K., Galan M., Kassan M., Partyka M., Kadowitz P., Henrion D., Trebak M., Belmadani S., Matrougui K. Chronic inhibition of endoplasmic reticulum stress and inflammation prevents ischaemia-induced vascular pathology in type II diabetic mice. J Pathol. 2012;227:165–174. doi: 10.1002/path.3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi S.-K., Galán M., Partyka M., Trebak M., Belmadani S., Matrougui K. Chronic inhibition of epidermal growth factor receptor tyrosine kinase and extracellular signal-regulated kinases 1 and 2 (ERK1/2) augments vascular response to limb ischemia in type 2 diabetic mice. Am J Pathol. 2012;180:410–418. doi: 10.1016/j.ajpath.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amin A.H., Elmageed Z.Y.A., Nair D., Partyka M.I., Kadowitz P.J., Belmadani S., Matrougui K. Modified multipotent stromal cells with epidermal growth factor restore vasculogenesis and blood flow in ischemic hind-limb of type II diabetic mice. Lab Invest. 2010;90:985–996. doi: 10.1038/labinvest.2010.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donath M.Y., Shoelson S.E. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11:98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- 9.Fink L.N., Costford S.R., Lee Y.S., Jensen T.E., Bilan P.J., Oberbach A., Blüher M., Olefsky J.M., Sams A., Klip A. Pro-inflammatory macrophages increase in skeletal muscle of high fat-fed mice and correlate with metabolic risk markers in humans. Obesity. 2014;22:747–757. doi: 10.1002/oby.20615. [DOI] [PubMed] [Google Scholar]
- 10.Tataranni P.A., Ortega E. A burning question does an adipokine-induced activation of the immune system mediate the effect of overnutrition on type 2 diabetes? Diabetes. 2005;54:917–927. doi: 10.2337/diabetes.54.4.917. [DOI] [PubMed] [Google Scholar]
- 11.Winkler G., Salamon F., Salamon D., Speer G., Simon K., Cseh K. Elevated serum tumour necrosis factor-alpha levels can contribute to the insulin resistance in type II (non-insulin-dependent) diabetes and in obesity. Diabetologia. 1998;41:860–861. doi: 10.1007/s001250051000. [DOI] [PubMed] [Google Scholar]
- 12.Uyemura K., Demer L., Castle S., Jullien D., Berliner J., Gately M., Warrier R., Pham N., Fogelman A., Modlin R. Cross-regulatory roles of interleukin (IL)-12 and IL-10 in atherosclerosis. J Clin Invest. 1996;97:2130. doi: 10.1172/JCI118650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wegner M., Winiarska H., Bobkiewicz-Kozłowska T., Dworacka M. IL-12 serum levels in patients with type 2 diabetes treated with sulphonylureas. Cytokine. 2008;42:312–316. doi: 10.1016/j.cyto.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 14.Mishra M., Kumar H., Bajpai S., Singh R.K., Tripathi K. Level of serum IL-12 and its correlation with endothelial dysfunction, insulin resistance, proinflammatory cytokines and lipid profile in newly diagnosed type 2 diabetes. Diabetes Res Clin Pract. 2011;94:255–261. doi: 10.1016/j.diabres.2011.07.037. [DOI] [PubMed] [Google Scholar]
- 15.Hagberg C.E., Mehlem A., Falkevall A., Muhl L., Fam B.C., Ortsäter H., Scotney P., Nyqvist D., Samén E., Lu L. Targeting VEGF-B as a novel treatment for insulin resistance and type 2 diabetes. Nature. 2012;490:426–430. doi: 10.1038/nature11464. [DOI] [PubMed] [Google Scholar]
- 16.Committee for the Update of the Guide for the Care and Use of Laboratory Animals; National Research Council . ed 8. National Academies Press; Washington, DC: 2011. Guide for the Care and Use of Laboratory Animals. [Google Scholar]
- 17.Kassan M., Galan M., Partyka M., Trebak M., Matrougui K. Interleukin-10 released by CD4+ CD25+ natural regulatory T cells improves microvascular endothelial function through inhibition of NADPH oxidase activity in hypertensive mice. Arterioscler Thromb Vasc Biol. 2011;31:2534–2542. doi: 10.1161/ATVBAHA.111.233262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Belmadani S., Bernal J., Wei C.-C., Pallero M.A., Dell'Italia L., Murphy-Ullrich J.E., Berecek K.H. A thrombospondin-1 antagonist of transforming growth factor-β activation blocks cardiomyopathy in rats with diabetes and elevated angiotensin II. Am J Pathol. 2007;171:777–789. doi: 10.2353/ajpath.2007.070056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Belmadani S., Palen D.I., Gonzalez-Villalobos R.A., Boulares H.A., Matrougui K. Elevated epidermal growth factor receptor phosphorylation induces resistance artery dysfunction in diabetic db/db mice. Diabetes. 2008;57:1629–1637. doi: 10.2337/db07-0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koh T.J., DiPietro L.A. Inflammation and wound healing: the role of the macrophage. Expert Rev Mol Med. 2011;13:e23. doi: 10.1017/S1462399411001943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mattner F., Magram J., Ferrante J., Launois P., Di Padova K., Behin R., Gately M.K., Louis J.A., Alber G. Genetically resistant mice lacking interleukin-12 are susceptible to infection with Leishmania major and mount a polarized Th2 cell response. Eur J Immunol. 1996;26:1553–1559. doi: 10.1002/eji.1830260722. [DOI] [PubMed] [Google Scholar]
- 22.Magram J., Connaughton S.E., Warrier R.R., Carvajal D.M., Wu C.Y., Ferrante J., Stewart C., Sarmiento U., Faherty D.A., Gately M.K. IL-12-deficient mice are defective in IFN gamma production and type 1 cytokine responses. Immunity. 1996;4:471–481. doi: 10.1016/s1074-7613(00)80413-6. [DOI] [PubMed] [Google Scholar]
- 23.DiStasi M.R., Mund J.A., Bohlen H.G., Miller S.J., Ingram D.A., Dalsing M.C., Unthank J.L. Impaired compensation to femoral artery ligation in diet-induced obese mice is primarily mediated via suppression of collateral growth by Nox2 and p47phox. Am J Physiol Heart Circ Physiol. 2015;309:H1207–H1217. doi: 10.1152/ajpheart.00180.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Torpey N., Maher S.E., Bothwell A.L., Pober J.S. Interferon alpha but not interleukin 12 activates STAT4 signaling in human vascular endothelial cells. J Biol Chem. 2004;279:26789–26796. doi: 10.1074/jbc.M401517200. [DOI] [PubMed] [Google Scholar]
- 25.Simons M. Angiogenesis, arteriogenesis, and diabetes: paradigm reassessed? J Am Coll Cardiol. 2005;46:835–837. doi: 10.1016/j.jacc.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 26.Nam H., Ferguson B.S., Stephens J.M., Morrison R.F. Impact of obesity on IL-12 family gene expression in insulin responsive tissues. Biochim Biophys Acta. 2013;1832:11–19. doi: 10.1016/j.bbadis.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nikołajuk A., Karczewska-Kupczewska M., Straczkowski M. Relationship between serum IL-12 and p40 subunit concentrations and lipid parameters in overweight and obese women. Metab Syndr Relat Disord. 2015;13:336–342. doi: 10.1089/met.2014.0164. [DOI] [PubMed] [Google Scholar]
- 28.Chu C.-M., Kuo S.-F., Hua C.-C., Wu S.-Y., Chuang D.-Y., Wu H.-P. Glucose increases interleukin-12 gene expression and production in stimulated peripheral blood mononuclear cells of type 2 diabetes patients. Biomed J. 2014;37:293. doi: 10.4103/2319-4170.132887. [DOI] [PubMed] [Google Scholar]
- 29.Sgadari C., Angiolillo A., Tosato G. Inhibition of angiogenesis by interleukin-12 is mediated by the interferon-inducible protein 10. Blood. 1996;87:3877–3882. [PubMed] [Google Scholar]
- 30.Kan X., Wu Y., Ma Y., Zhang C., Li P., Wu L., Zhang S., Li Y., Du J. Deficiency of IL-12p35 improves cardiac repair after myocardial infarction by promoting angiogenesis. Cardiovasc Res. 2016;109:249–259. doi: 10.1093/cvr/cvv255. [DOI] [PubMed] [Google Scholar]
- 31.Wellen K.E., Hotamisligil G.S. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pillon N.J., Bilan P.J., Fink L.N., Klip A. Cross-talk between skeletal muscle and immune cells: muscle-derived mediators and metabolic implications. Am J Physiol Endocrinol Metab. 2013;304:E453–E465. doi: 10.1152/ajpendo.00553.2012. [DOI] [PubMed] [Google Scholar]
- 33.Herder C., Haastert B., Müller-Scholze S., Koenig W., Thorand B., Holle R., Wichmann H.-E., Scherbaum W.A., Martin S., Kolb H. Association of systemic chemokine concentrations with impaired glucose tolerance and type 2 diabetes results from the cooperative health research in the region of Augsburg survey S4 (KORA S4) Diabetes. 2005;54:S11–S17. doi: 10.2337/diabetes.54.suppl_2.s11. [DOI] [PubMed] [Google Scholar]
- 34.Van Greevenbroek M., Schalkwijk C., Stehouwer C. Obesity-associated low-grade inflammation in type 2 diabetes mellitus: causes and consequences. Neth J Med. 2013;71:174–187. [PubMed] [Google Scholar]
- 35.Shireman P.K. The chemokine system in arteriogenesis and hind limb ischemia. J Vasc Surg. 2007;45(Suppl A):A48–A56. doi: 10.1016/j.jvs.2007.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Niiyama H., Kai H., Yamamoto T., Shimada T., Sasaki K., Murohara T., Egashira K., Imaizumi T. Roles of endogenous monocyte chemoattractant protein-1 in ischemia-induced neovascularization. J Am Coll Cardiol. 2004;44:661–666. doi: 10.1016/j.jacc.2004.04.046. [DOI] [PubMed] [Google Scholar]
- 37.Hotamisligil G.S., Shargill N.S., Spiegelman B.M. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 38.Luo D., Luo Y., He Y., Zhang H., Zhang R., Li X., Dobrucki W.L., Sinusas A.J., Sessa W.C., Min W. Differential functions of tumor necrosis factor receptor 1 and 2 signaling in ischemia-mediated arteriogenesis and angiogenesis. Am J Pathol. 2006;169:1886–1898. doi: 10.2353/ajpath.2006.060603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leibovich S.J., Polverini P.J., Shepard H.M., Wiseman D.M., Shively V., Nuseir N. Macrophage-induced angiogenesis is mediated by tumour necrosis factor-α. Nature. 1987;329:630–632. doi: 10.1038/329630a0. [DOI] [PubMed] [Google Scholar]
- 40.Angiolillo A.L., Sgadari C., Tosato G. A role for the interferon-inducible protein 10 in inhibition of angiogenesis by interleukin-12. Ann N Y Acad Sci. 1996;795:158–167. doi: 10.1111/j.1749-6632.1996.tb52664.x. [DOI] [PubMed] [Google Scholar]
- 41.Mantovani A., Sica A., Sozzani S., Allavena P., Vecchi A., Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 42.Mantovani A., Sica A., Locati M. Macrophage polarization comes of age. Immunity. 2005;23:344–346. doi: 10.1016/j.immuni.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 43.Kodelja V., Muller C., Tenorio S., Schebesch C., Orfanos C.E., Goerdt S. Differences in angiogenic potential of classically vs alternatively activated macrophages. Immunobiology. 1997;197:478–493. doi: 10.1016/S0171-2985(97)80080-0. [DOI] [PubMed] [Google Scholar]
- 44.Furukawa S., Fujita T., Shimabukuro M., Iwaki M., Yamada Y., Nakajima Y., Nakayama O., Makishima M., Matsuda M., Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hazarika S., Dokun A.O., Li Y., Popel A.S., Kontos C.D., Annex B.H. Impaired angiogenesis after hindlimb ischemia in type 2 diabetes mellitus: differential regulation of vascular endothelial growth factor receptor 1 and soluble vascular endothelial growth factor receptor 1. Circ Res. 2007;101:948–956. doi: 10.1161/CIRCRESAHA.107.160630. [DOI] [PubMed] [Google Scholar]
- 46.Murakami M., Nguyen L.T., Zhuang Z.W., Moodie K.L., Carmeliet P., Stan R.V., Simons M. The FGF system has a key role in regulating vascular integrity. J Clin Invest. 2008;118:3355–3366. doi: 10.1172/JCI35298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Su J., Lucchesi P.A., Gonzalez-Villalobos R.A., Palen D.I., Rezk B.M., Suzuki Y., Boulares H.A., Matrougui K. Role of advanced glycation end products with oxidative stress in resistance artery dysfunction in type 2 diabetic mice. Arterioscler Thromb Vasc Biol. 2008;28:1432–1438. doi: 10.1161/ATVBAHA.108.167205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Facchiano F., Lentini A., Fogliano V., Mancarella S., Rossi C., Facchiano A., Capogrossi M.C. Sugar-induced modification of fibroblast growth factor 2 reduces its angiogenic activity in vivo. Am J Pathol. 2002;161:531–541. doi: 10.1016/S0002-9440(10)64209-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Duraisamy Y., Slevin M., Smith N., Bailey J., Zweit J., Smith C., Ahmed N., Gaffney J. Effect of glycation on basic fibroblast growth factor induced angiogenesis and activation of associated signal transduction pathways in vascular endothelial cells: possible relevance to wound healing in diabetes. Angiogenesis. 2001;4:277–288. doi: 10.1023/a:1016068917266. [DOI] [PubMed] [Google Scholar]