Abstract
The muscular dystrophies are genetically diverse. Shared pathological features among muscular dystrophies include breakdown, or loss of muscle, and accompanying fibrotic replacement. Novel strategies are needed to enhance muscle repair and function and to slow this pathological remodeling. Glucocorticoid steroids, like prednisone, are known to delay loss of ambulation in patients with Duchenne muscular dystrophy but are accompanied by prominent adverse effects. However, less is known about the effects of steroid administration in other types of muscular dystrophies, including limb-girdle muscular dystrophies (LGMDs). LGMD 2B is caused by loss of dysferlin, a membrane repair protein, and LGMD 2C is caused by loss of the dystrophin-associated protein, γ-sarcoglycan. Herein, we assessed the efficacy of steroid dosing on sarcolemmal repair, muscle function, histopathology, and the regenerative capacity of primary muscle cells. We found that in murine models of LGMD 2B and 2C, daily prednisone dosing reduced muscle damage and fibroinflammatory infiltration. However, daily prednisone dosing also correlated with increased muscle adipogenesis and atrophic remodeling. Conversely, intermittent dosing of prednisone, provided once weekly, enhanced muscle repair and did not induce atrophy or adipogenesis, and was associated with improved muscle function. These data indicate that dosing frequency of glucocorticoid steroids affects muscle remodeling in non-Duchenne muscular dystrophies, suggesting a positive outcome associated with intermittent steroid dosing in LGMD 2B and 2C muscle.
The muscular dystrophies cause progressive impairment of muscle architecture and functionality.1 The genetic etiology of these disorders is highly heterogeneous, and different mutations elicit different forms of muscular dystrophy with variable pathophysiologic outcomes.2 Duchenne muscular dystrophy (DMD) is an X-linked disease caused by genetic loss of functional dystrophin, a protein that anchors the intracellular contents to the myofiber membrane.3 The limb-girdle muscular dystrophies (LGMDs) are themselves genetically and clinically heterogeneous, mainly affecting hip and shoulder muscles.4 LGMD 2B, caused by loss-of-function mutations in the gene encoding dysferlin, is considered a defect of membrane repair and trafficking.5, 6, 7 Dysferlin is a membrane-associated protein, composed of multiple C2 domains, some of which bind phospholipids in the presence of Ca2+.8 LGMD 2C arises from disruption of γ-sarcoglycan, a dystrophin-associated protein, and is accompanied by a similar sarcolemmal fragility, as seen in dystrophin-deficient muscle.9, 10 LGMD 2B and 2C are effectively modeled in Dysferlin-null (Dysf-null) and Sgcg-null mice, respectively, recapitulating many of the same histopathological features seen in human LGMD 2B and 2C.5, 10
Synthetic glucocorticoid (GC) steroids, such as prednisone and deflazacort, are currently the only standard of clinical care for DMD patients.11 GC steroids differ from anabolic steroids, and in DMD, GC steroids are thought to act through reduction of inflammation, although the mechanisms are not fully known and likely extend beyond this one role.12 Although GC steroids are commonly used in DMD patients, long-term steroid dosing associates with many adverse effects, including obesity and stunted growth. This had led to attempts to reengineer GC steroids to avoid some of these unwanted effects.13
At present, GC steroid treatments are not recommended for clinical management of LGMDs. The ultrarare nature of LGMDs and their long time-course makes placebo-controlled trials difficult because assembly of sufficiently sized cohorts for study is difficult. One small double-blind, placebo-controlled trial was reported for LGMD 2B.14 Dysferlin-mutant LGMD 2B patients were treated with 1 mg/kg daily deflazacort for 1 month, and with 1 mg/kg every second day for the subsequent 5 months. This treatment was associated with a decline in muscle strength, which reversed after cessation of the steroid regimen.14 Little is known about the effect of GC steroids in other forms of LGMD. Intriguingly, beneficial effects with low-dose prednisone dosing (0.35 mg/kg daily) were reported in a case report of two patients with LGMD 2I due to FKRP mutations, and prednisolone treatment in a mouse model of LGMD 2I also showed some benefit.15, 16
A growing body of literature hints at a dual outcome of glucocorticoid steroids in muscle. Many studies report beneficial effects, and many others show that long-term steroid dosing leads to muscle atrophy, suggesting that outcome is profoundly influenced by disease context and drug dosing.17, 18, 19, 20, 21, 22 Recently, we reported that the overall outcome of GC steroid dosing in injured and dystrophic muscles is significantly altered by the frequency of drug dosing.23 We compared the molecular effects of once weekly to daily dosing of prednisone and deflazacort, and we showed that both regimens comparably enhanced myofiber repair in injured normal muscle and in dystrophin-deficient muscles. However, daily GC steroid dosing elicited significant muscle atrophy and muscle functional impairment. In contrast, weekly steroid dosing did not induce muscle mass loss and instead correlated with functional improvement in both voluntary and respiratory muscles.23 These promising results from weekly GC steroid dosing prompted investigation in mouse models of other forms of muscular dystrophy.
Herein, we compared the effects of weekly versus daily dosing of GC steroids in mouse models of LGMD 2B (Dysf-null) and LGMD 2C (Sgcg-null). We combined molecular and histological assays with functional assessment of muscle and determined the effect of GC steroids on cells isolated from treated muscles. Similar to what we found for dystrophin-deficient muscle, we found that both weekly and daily steroid dosing in Dysf-null and Sgcg-null mice correlated with enhanced myofiber membrane repair after microinjury in vitro, and this was accompanied by reduced immune and fibrotic muscle infiltrates in vivo. However, daily steroid dosing induced muscle atrophy and functional decline, whereas weekly dosing correlated with enhanced muscle performance and regeneration. Furthermore, unlike the daily GC steroid dosing, weekly GC steroid dosing associated with increased adipogenic remodeling of Dysf-null muscle. These results suggest beneficial effects of intermittent steroid regimens translatable to muscles affected by non-DMD muscular dystrophy, such as LGMD 2B and LGMD 2C.
Materials and Methods
Animals
Mice were housed in a specific pathogen-free facility in accordance with Northwestern University's Institutional Animal Care and Use Committee regulations. Mice were fed ad libitum and maintained on a 12-hour light/dark cycle. Wild-type (WT), Dysf-null mice from the 129T2/SvEmsJ background were used for studies involving steroid use in the absence of dysferlin.24, 25 Sgcg-null mice from the DBA/2J background were used for steroid studies in the absence of γ-sarcoglycan.26 Both female and male mice were included in study groups for reported experiments; specific sex distribution for experimental groups is reported in each figure legend. Cohort size (n = 5 mice per group) was determined on the basis of power analysis (G*Power v3.1, University of Dusseldorf, Dusseldorf, Germany; power, 0.80; α, 0.05) using data distribution values from previous studies in mdx mice and preliminary results in Dysf-null and Sgcg-null mice.23
Plasmids
A plasmid encoding human annexin A1 with a carboxy-terminal green fluorescent protein (GFP) was obtained from Origene (Rockville, MD; catalog number RG201569). GFP-tagged annexin A6 was described previously.27, 28
Electroporation, Myofiber Isolation, and Laser Injury
Flexor digitorum brevis fibers were electroporated in vivo, as previously described,29 with modifications as described.28, 30 Briefly, the hind limb footpad was injected with 10 μL of hyaluronidase (8 units) (catalog number H4272; Sigma, St. Louis, MO). After 2 hours, 20 μL of 2 μg/μL endotoxin free plasmid was injected into the footpad. Electroporation was conducted with the following specifications: 20 pulses, 20 milliseconds in duration for each, at 1 Hz, at 100 V/cm. Animals were allowed to recover for 7 days after electroporation to avoid injured fibers and to allow plasmid expression.31 Individual flexor digitorum brevis myofibers were then isolated from explanted muscles.30
Myofibers were dissected and laser injured, as described.27, 28, 30 Briefly, myofibers were dissociated in phosphate-buffered saline (PBS) supplemented with 0.2% bovine serum albumin and 4 mg/mL collagenase type II (catalog number 17101; Life Technologies, Grand Island, NY) at 37°C in 10% CO2. Muscle was triturated at 60 and 120 minutes after digestion onset. Fibers were then placed on MatTek confocal microscopy dishes (catalog number P35G-1.5-14-C; MatTek, Ashland, MA) in Ringer’s solution. After 30 minutes, myofibers were prepared for imaging, adding FM 4-64 dye (T-13320; Molecular Probes, Grand Island, NY) to a final concentration of 2.5 μm.
Laser injury was performed at room temperature using a Nikon A1R laser scanning confocal (Nikon, Tokyo, Japan) equipped with GaSP detectors through a 60× Apo λ 1.4 numerical aperture objective driven by Nikon Elements AR software version 4.5 (Nikon). A single pixel set as 120 nm (0.0144 μm2) was ablated using the 405-nm laser at 100% power for up to 5 seconds. Images were acquired as follows: one image just before damage (0 seconds), one image right after laser injury (bleach point), 10 images every 2 seconds after injury, and then one image every 10 seconds for up to 240 seconds after injury.
Z-stack projections were acquired from up to 35 consecutive images with a 150-nm interval between each step, using the Z-stack rendering built-in tool in NIS-elements AR version 4.5 (Nikon) or ImageJ version 1.0 (NIH, Bethesda, MD; http://imagej.nih.gov/ij). Fluorescence recovery after photobleaching was performed using a GFP-tagged annexin transgene on image sequences. Fluorescence recovery after photobleaching measurements were calculated on individual myofibers by ImageJ. Relative fluorescence in an 85-μm2 region encompassing the lesion area was calculated from images acquired as described above in myofiber isolation and laser damage, normalizing each frame to the preinjury intensity. This method allows comparisons of all strains and reduces variability introduced by differences in dye uptake or binding at time 0. GraphPad Prism software version 7.0a (Prism Software, La Jolla, CA) was used to calculate averages, and values were normalized to the prebleach intensity (F/F0). Time to cap formation was quantified as the postinjury time before the initial detection of nascent cap assembly at the injury site (generally approximately 0.5 μm in diameter perpendicular to the myofiber axis). All measurements were from n = 5 mice per group, n ≥ 10 myofibers per mouse. Statistical analysis used GraphPad Prism and a two-way analysis of variance; error bars represent the SEM.
Quality control for myofibers selected for laser ablation relied on the following parameters. Only myofibers adherent to the MatTek dish from end to end and not contracting during imaging were used. Imaged fibers were required to have intact sarcomeres and unruptured sarcolemma. The region of the myofiber selected for laser injury was required to be linear, without visible deformation or peripheral nuclei.
Analyses were conducted blinded to treatment group (M.Q.).
Drug Treatments
Prednisone (catalog number P6254; Sigma-Aldrich, St. Louis, MO) was resuspended in dimethyl sulfoxide (catalog number D2650; Sigma-Aldrich) to stock concentrations of 5 mg/mL. Dosing was on the basis of pretreatment weights (1 mg/kg body weight32) in 30 μL total PBS volume per injection. Vehicle treatment consisted of 7×/week of vehicle; weekly regimen, 1×/week prednisone dose, then 6× vehicle; daily regimen, 7×/week prednisone dose. Mice were injected daily via i.p. injection at 7 am. On injection days, stock solutions stored at −20°C were diluted into sterile Eppendorf tubes containing sterile PBS (catalog number 14190; Life Technologies). Sterile BD Micro-Fine IV Insulin Syringes (catalog number 14-829-1A; Fisher Scientific, Waltham, MA) were used to inject the i.p. cavity of nonsedated animals. Mice were weighed three times per week during the course of 4 weeks at 7 am. Body mass was expressed as fold change from day 0.
Grip Strength, Treadmill, Muscle Mechanics, Blood Drawing, and Plethysmography
Fore limb grip strength was measured using a meter (catalog number 1027SM; Columbus Instruments, Columbus, OH) blinded to treatment group (M.Q.). Mice performed 10 pulls, with 5 seconds rest on a flat surface between pulls. Mice were monitored for run to exhaustion on an automated treadmill (catalog number Exer3/6 without electrical stimulation grills; Columbus Instruments) with a 15-degree incline with a start speed at 1 m/minute with 1 m/minute2 acceleration. Exhaustion was defined when the animal stopped for >15 seconds on the rest pad in the absence of electrical stimuli. Immediately before sacrifice, in situ tetanic force from tibialis anterior muscles was measured using a Whole Mouse Test System (catalog number 1300A; Aurora Scientific, Aurora, ON, Canada) with a 1N dual-action lever arm force transducer (300C-LR; Aurora Scientific) in anesthetized animals (3% isoflurane in 100% O2). Parameters for tetanic isometric contractions were as follows: initial delay, 0.1 seconds; frequency, 200 Hz; pulse width, 0.5 milliseconds; duration, 0.5 seconds; and stimulation, 100 mA.33 Length was adjusted to a fixed baseline of 30 mN resting tension for all muscles/conditions. Fatigue analysis was conducted by reiterating the tetanic contraction every 10 seconds until muscle exhaustion (50 cycles). Time of contraction was assessed as time to maximum tetanic value within the 0.0- to 0.5-second range of each contraction cycle, whereas time of relaxation was assessed as time to 90% minimum tetanic value within the 0.5- to 0.8-second range.
Respiratory function was monitored by means of unanesthetized whole-body plethysmography using a Buxco Finepointe 4-site apparatus (Data Sciences International, St. Paul, MN). Individual mice were placed in a calibrated cylindrical chamber at room temperature. Each mouse was allowed to acclimate to the plethysmography chamber for 120 minutes before recording was initiated. Measurements were conducted on asleep mice, excluding values with rejection index >0 and frequency >250 beats per minute. Data were recorded for a total of 15 minutes broken into three consecutive 5-minute periods.
All physiological studies were conducted blinded to treatment group (M.Q.).
Histology, IF Microscopy, and Antibodies
Splenocytes were collected from minced, freshly isolated spleen by filtering the gross cell suspension with 100- and 40-μm cell strainers. Excised muscles were placed in 10% formaldehyde (catalog number 245-684; Fisher Scientific, Waltham, MA) for histological processing, immediately frozen in liquid nitrogen, and placed in precooled Nalgene cryovials. Then, they were stored at −80°C for molecular analyses or embedded in tissue freezing medium (catalog number TFM-5; Triangle Biomedical Sciences, Durham, NC) for immunofluorescence (IF) analyses. Sections (7 μm thick) from the center of paraffin-embedded muscles were stained with hematoxylin and eosin (catalog numbers 12013B and 1070C; Newcomer Supply, Middleton, WI) and Masson trichrome (catalog number HT-15; Sigma-Aldrich). Myofiber cross-sectional area (CSA) quantitation was conducted on 400 myofibers per diaphragm muscle per mouse (2000 myofibers per group) and 1200 myofibers per gastrocnemius muscle per mouse (7200 myofibers per WT group; 6000 myofibers per group). Diaphragm thickness was determined on 30 serial diaphragm sections per mouse (150 measurements per group). Sections (10 μm thick) from the center of frozen-embedded muscles were collected on the cryostat (chamber, −20°C; sample, −15°C; catalog number CM1950; Leica, Wetzlar, Germany) for immunostaining. At least 30 nonconsecutive sections were analyzed per muscle per condition.
IF staining was performed with the following conditions: 4% paraformaldehyde fixation (10 minutes, room temperature); permeabilization with 0.2% Triton (catalog number X-100; Sigma-Aldrich), 1% bovine serum albumin (catalog number A7906; Sigma-Aldrich), and PBS (30 minutes, room temperature); and blocking in 1% bovine serum albumin and 10% fetal bovine serum and PBS (30 minutes, room temperature). For macrophage detection, sections were incubated with anti-F4/80 conjugated to AlexaFluor488 (catalog number ab6640; Abcam, Cambridge, MA) at a dilution of 1:100 overnight at 4°C; nuclei were counterstained with 0.5 μg/mL Hoechst PBS (45 minutes, room temperature). For fiber typing, sections were incubated with primary antibodies BA-F8 (1:10), SC-71 (1:30), and BF-F3 (1:10; all from Developmental Studies Hybridoma Bank, Iowa City, IA) overnight at 4°C. Then, sections were incubated with secondary antibodies AlexaFluor350 anti-IgG2b, AlexaFluor488 anti-IgG1, and AlexaFluor594 anti-IgM (catalog numbers A21140, A21121, and 1010111, respectively; Life Technologies). Type 1 fibers stained blue, type 2A fibers stained green, and type 2B fibers stained red.34
Imaging was performed using a Zeiss Axio Observer A1 microscope (Zeiss, Oberkochen, Germany), using 10× and 20× objectives. Gryphax software version 1.0.6.598 (Jenoptik, Jena, Germany) was used for bright-field images, whereas ZEN 2 software version 2011 (Zeiss, Jena, Germany) was used for IF images. Area quantitation, length measurement, and cell counting were performed using ImageJ. Analyses were conducted blinded to treatment group (M.Q.).
Hydroxyproline Quantification
Frozen quadriceps muscles (100-mg portions) were used to dose hydroxyproline content, as previously described.35 Results were reported as mmol (hydroxyproline)/mg (tissue).
Serum Collection and CK Analysis
Serum was collected and processed, as described, from animals after at least 24 hours after performance tests.8 Serum creatine kinase (CK) was analyzed in triplicate for each mouse using the EnzyChrom Creatine Kinase Assay (catalog number ECPK-100; BioAssay Systems, Hayward, CA), following manufacturer's instructions. A Synergy HTX multimode plate reader (BioTek, Winooski, VT) was used to collect results, expressed as U/mL.
Real-Time Quantitative RT-PCR
Total RNA was extracted with Trizol (catalog number 15596018; Life Technologies) from 30 mg tissue, as per manufacturer's instructions. RNA (2 μg) was reverse transcribed with the qScript cDNA kit (catalog number 95048; Quanta Biosciences, Beverly, MA), following the kit's instructions. cDNA was diluted 1:7, and 2 μL was used per 10 μL real-time quantitative PCR (qPCR). Each qPCR contained 100 nmol/L primers and 5 μL iTaq SYBR Green Mix (catalog number 1725124; Bio-Rad, Hercules, CA). The list of primers and sequences is provided in Table 1. The CFX96 RealTime System (Bio-Rad) was used to run the qPCR (95°C, 15 seconds; 59°C, 60 seconds; 40 cycles) and quantitate fluorescence. Relative fold change among biological groups was calculated using Pgk as an internal normalizer.
Table 1.
List of Primers for Real-Time Quantitative PCR
| Gene | Primer |
|---|---|
| Anxa1 | F: 5′-GGAGAAAGGGGACAGACGTG-3′ |
| R: 5′-TGGCACACTTCACGATGGTT-3′ | |
| Anxa6 | F: 5′-TGGCTGCTGAGATCTTGGAAA-3′ |
| R: 5′-CGTCCTTGACATCCCCAGAC-3′ | |
| Fbxo32 | F: 5′-TTGGATGAGAAAAGCGGCAG-3′ |
| R: 5′-TACAGTATCCATGGCGCTCC-3′ | |
| Foxo1 | R: 5′-TTCCCAATGGCACAGTCCTT-3′ |
| Gzmb | F: 5′-ACAAAGGCAGGGGAGATCAT-3′ |
| R: 5′-CGAATAAGGAAGCCCCCACA-3′ | |
| Ifng | F: 5′-CGGCACAGTCATTGAAAGCC-3′ |
| R: 5′-TGTCACCATCCTTTTGCCAGT-3′ | |
| Klf15 | F: 5′-AAATGCACTTTCCCAGGCTG-3′ |
| R: 5′-CGGTGCCTTGACAACTCATC-3′ | |
| Mef2C | F: 5′-CATAACATGCCGCCATCTGC-3′ |
| R: 5′-ATCTCGAAGGGGTGGTGGTA-3′ | |
| Pgk | F: 5′-CAAAATGTCGCTTTCCAACAAG-3′ |
| R: 5′-AACGTTGAAGTCCACCCTCATC-3′ | |
| Pparg | F: 5′-CGGGCTGAGAAGTCACGTT-3′ |
| R: 5′-TGTGTCAACCATGGTAATTTCAGT-3′ | |
| Trim63 | F: 5′-AACCTGGAGAAGCAGCTGAT-3′ |
| R: 5′-AGACATGGACACTGAGCCAC-3′ |
F, forward; R, reverse.
Primary Cell Studies
Isolation of primary myoblasts and fibroadipogenic progenitors (FAPs) was conducted adapting previously reported conditions.36, 37, 38 Briefly, tibialis anterior muscles were explanted, minced, and digested in 5 mL/muscle Dulbecco’s modified Eagle’s medium supplemented with 0.06 collagenase type II (catalog numbers 11995 and 17101; Thermo Fisher Scientific, Waltham, MA) at 37°C for 40 minutes while shaking. Cells were filtered through 100- and 40-μm strainers, plated on 10-cm plastic dishes coated with collagen (1% solution in 20% acetic acid; catalog number C9791; Sigma; one muscle preparation per dish), and incubated at 37°C, 5% CO2 for 7 to 10 days to collect at least 105 cells for sorting. Myoblasts were sorted as the CD56+/CD140a− fraction, whereas FAPs were sorted as the CD56−/CD140a+ fraction with an FACSAria SORP 4-laser (Beckton Dickinson, Franklin Lakes, NJ), using allophycocyanin-conjugated anti-CD56 and phosphatidylethanolamine-conjugated anti-CD140a antibodies [catalog number FAB7820A (R&D Systems, Minneapolis, MN) and catalog number 11-1401-80 (Thermo Fisher Scientific), respectively; both used at 2 μL/105 cells/200 μL PBS volume]. Growth medium for myoblasts was Dulbecco’s modified Eagle’s medium supplemented with 20% fetal bovine serum [catalog number 2442 (lot number 14E332; Sigma], 1% penicillin/streptomycin (catalog number 15070; Thermo Fisher Scientific), 5 ng/mL basic fibroblast growth factor (catalog number 130-093-842; Miltenyi Biotec, Bergisch Gladbach, Germany), and 1% chicken embryo extract (catalog number NC9880840; Thermo Fisher Scientific). Medium for FAPs was Dulbecco’s modified Eagle’s medium supplemented with 20% fetal bovine serum, 1% penicillin/streptomycin, 1% non-essential amino acids, and 1% insulin-transferrin–selenium supplements (catalog numbers 11140050 and 41400045; Thermo Fisher Scientific). Cells were gated on the basis of isotype-stained or bare cell pools, and sorted through a 100-μm nozzle. Both primary populations were sorted in parallel from each mouse of each study group, and plated at 5000 cells/cm2 (eg, approximately 104 cells per multiwell 24 well). Plated cells were then directly used for analyses without further passaging. Spontaneous adipocyte conversion was assayed via Oil Red O staining on proliferating cells on 90% confluence.39 Unbiased myogenic differentiation was induced with 10-day incubation with Dulbecco’s modified Eagle’s medium supplemented with 1% penicillin/streptomycin and 2% horse serum (catalog number 16050-122; Thermo Fisher Scientific). Myoblast differentiation was analyzed by immunostaining myotubes for myosin heavy chain (clone MF20, used at 1:10 dilution; Developmental Studies Hybridoma Bank). Quantitative analyses included triplicates of each sorted cell population per mouse per group. Relative quantitative analyses per field were conducted on ≥10 fields randomly imaged across each replicate. Analyses were conducted blinded to treatment group (M.Q.).
Statistical Analysis
Statistical analyses were performed using GraphPad Prism software. Most comparisons relied on analysis of variance with Bonferroni multicomparison appropriate for the variables tested [one-way analysis of variance for one variable, two-way analysis of variance for two variables (typically time and treatment)]. When comparing two groups, a two-tailed t-test with Welch's correction (unequal variances) was used. For analysis of variance and t-test analyses, P < 0.05 was considered significant. Data were presented as single values (dot plots, histograms) when the number of data points was <10. In analyses pooling larger data point sets per group, Tukey distribution bars were used to emphasize data range distribution and histograms with error bars were used to emphasize shifts in average values. Analyses pooling data points over time were presented as marked line plots. Dot plots, histograms, and marked line plots depict means ± SEM. Box plots depict the Tukey distribution of the data pool: interquartile distribution; lower whisker, 25th percentile minus 1.5 times the interquartile range; upper whisker, 75th percentile plus 1.5 times the interquartile range.
Results
Glucocorticoid Steroids Enhance Sarcolemmal Repair in Dysf-Null Myofibers
It was recently found that a single pulse of GC steroids accelerated repair of the myofiber plasma membrane, the sarcolemma, in WT mice.23 This effect correlated with the up-regulation of Anxa1 and Anxa6, which encode annexin A1 and annexin A6, respectively. These annexins are components of the repair complex that forms at the site of sarcolemmal injury in isolated myofibers.28 Anxa6 is a modifier of muscular dystrophy.8, 27 Because of dysferlin's role in mediating membrane resealing,40 we queried whether GC steroids would elicit a similar effect on sarcolemmal repair in myofibers from Dysf-null mice, a genetic model of LGMD 2B.5, 41
Sarcolemmal repair was imaged in real time in live myofibers, as previously described,30 comparing myofibers from strain-matched Dysf-null and WT 9-month–old animals. After laser injury, the extent of sarcolemmal damage was quantified over time (240 seconds) and at end point (300 seconds), monitoring the accumulation rate of the lipophilic FM4-64 dye at the site of injury. Repair cap formation was monitored through expression from a GFP-tagged annexin A1 (GFP-ANXA1) construct, which had been electroporated into muscle in vivo 7 days before analysis. Images for FM6-64, which monitors the extent of sarcolemmal damage, and ANXA1-GFP, which measures the repair complex, were acquired simultaneously. To analyze the effects of a glucocorticoid steroid pulse, Dysf-null mice (n = 5 mice per group) were treated with a single i.p. injection of 1 mg/kg prednisone, 1 mg/kg deflazacort, or vehicle 24 hours before myofiber analysis, following the reported conditions of dosing and timing.23
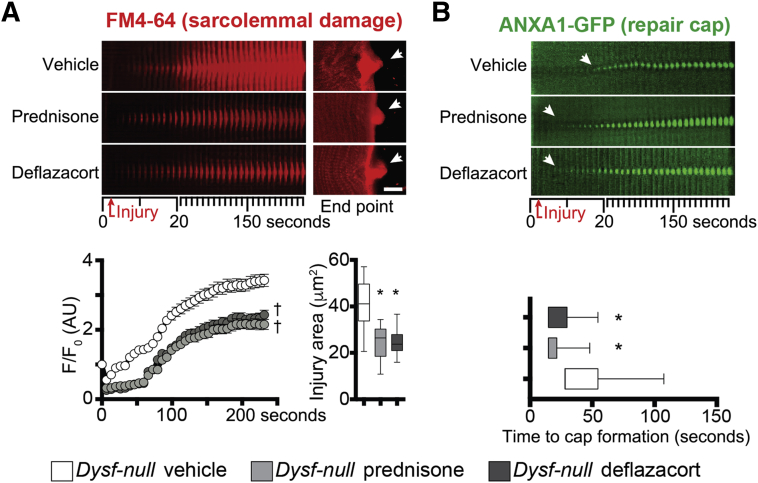
Steroid-treated Dysf-null myofibers had reduced accumulation of FM4-64 dye over time and at end point on sarcolemmal injury, as compared to vehicle-treated myofibers, consistent with a reduction in the extent of sarcolemmal damage under equal injury conditions (Figure 1A). Moreover, steroid-treated Dysf-null myofibers displayed a faster onset of repair cap formation, visualized by ANXA1-GFP accumulation at the injury site, resulting in a moderately increased cap size at end point (Figure 1B). Prednisone and deflazacort correlated with comparable trends in both parameters. Thus, a pulse of glucocorticoid steroids promoted sarcolemmal repair in isolated Dysf-null myofibers.
Figure 1.
Prednisone-accelerated sarcolemmal repair and repair cap formation in Dysf-null myofibers. A: A single dose of glucocorticoid steroids, either prednisone or deflazacort, decreased the extent of sarcolemmal injury in live myofibers, as quantitated by FM4-64 accumulation over time and injury area at analysis end point (arrows). Top left panels: Time-dependent real-time image stacks of injury site (10-μm-wide area). Top right panels: Z-stack rendering of FM4-64 accumulation at injury site at 300 seconds after injury. Bottom panels: Quantitative data are plotted. B: The single steroid dose associated with faster onset of the sarcolemmal repair cap, as monitored in real-time by green fluorescent protein (GFP)–labeled annexin A1 (ANXA1-GFP) accumulation at injury site (arrows). Top panels: Time-dependent image stacks (10-μm-wide area). Bottom panel: Quantitation of time (after injury) to cap onset is shown. Data are expressed as means ± SEM (marked line plots) or Tukey distribution (box plots). n = 5 mice (50 myofibers) per group (3 males and 2 females per group). ∗P < 0.05 versus vehicle (one-way analysis of variance test with Bonferroni multiple comparison); †P < 0.05 versus vehicle (two-way analysis of variance test with Bonferroni multiple comparison). Scale bar = 5 μm (A, right top panels). AU, arbitrary unit.
Weekly and Daily Steroid Regimens Ameliorate Sarcolemmal Repair in Dysf-Null Myofibers
Intermittent prednisone administration has been assessed in humans and mice with DMD, where it has been suggested to have comparable benefit to daily dosing with fewer adverse effects.42, 43, 44 Weekly and daily GC dosing with either prednisone or deflazacort associated with comparable amelioration of sarcolemmal repair in dystrophin-deficient mouse myofibers.23 In light of enhanced sarcolemmal repair seen after a single dose of GC steroids, we assessed weekly and daily GC steroid dosing in Dysf-null mice. Dysf-null mice (n = 5 mice per group, 9 months of age) were i.p. injected with 1 mg/kg prednisone either daily (seven doses/week) or weekly (one dose/week, vehicle for the remaining six doses), or with daily vehicle doses as control. Duration of treatment was 4 weeks, similar to what was assessed in dystrophin-deficient mice.23
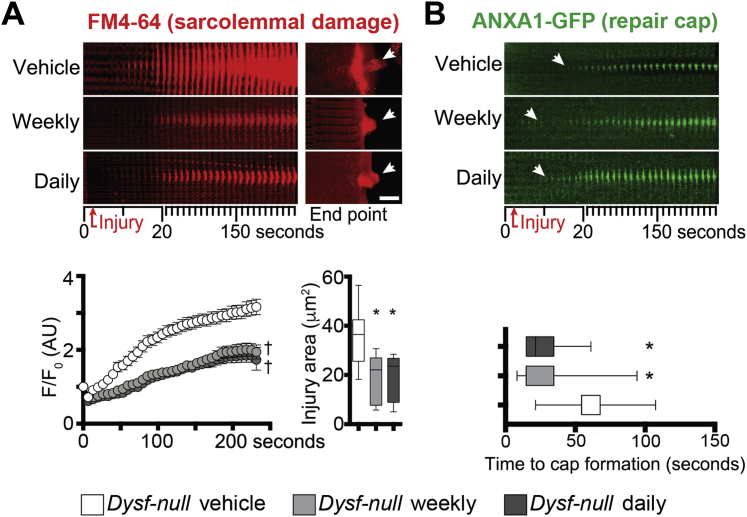
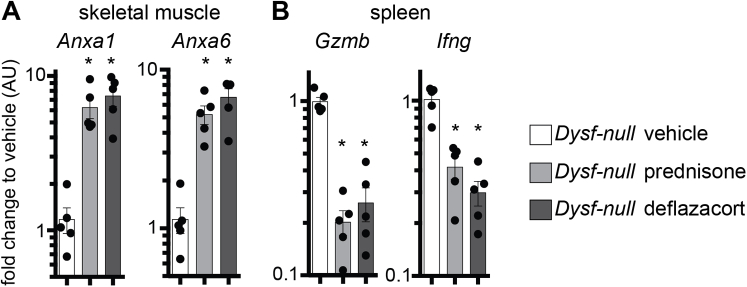
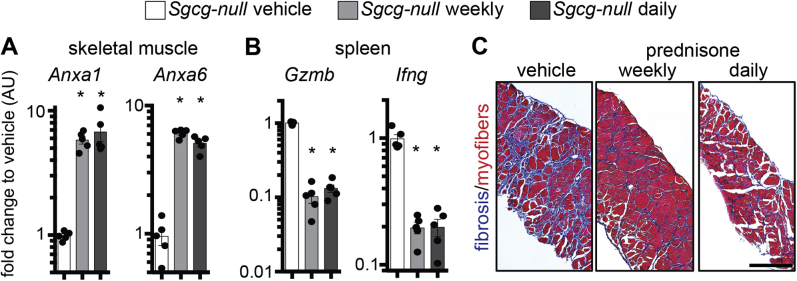
At treatment end point, myofibers from the flexor digitorum brevis muscles of control and treated animals were isolated and tested for sarcolemmal repair. Both prednisone regimens (weekly and daily) correlated with reduced extent of sarcolemmal damage when compared to vehicle-treated mice, as quantitated by FM4-64 accumulation on sarcolemmal injury (Figure 2A). Consistent with acute dosing of prednisone, 4 weeks of treatment was associated with faster onset of repair cap formation than in control myofibers, as visualized by ANXA1-GFP (Figure 2B). Weekly and daily steroid dosing elicited comparable trends. We next assayed quadriceps muscles from treated Dysf-null mice cohorts for Anxa1 and Anxa6 gene expression levels through qPCR. Four weeks after treatment onset, weekly and daily prednisone regimens resulted in comparable up-regulation of Anxa1 and Anxa6 in muscle, as compared to vehicle-treated muscle (Supplemental Figure S1A). Steroid uptake was confirmed by down-regulation of gene markers of immune cell activation (Gzmb and Ifng) in primary splenocytes of prednisone-treated mice, as compared to vehicle-treated control animals (Supplemental Figure S1B).
Figure 2.
Weekly and daily prednisone regimens enhance sarcolemmal repair in Dysf-null myofibers. A:Dysf-null mice were treated for 4 weeks with either weekly or daily prednisone. Both prednisone regimens resulted in improved sarcolemmal repair after laser-induced injury, as shown by diminished accumulation of FM4-64 over time (10-μm-wide area) and decreased sarcolemmal injury area at end point (arrows). B: Both steroid regimens correlated with faster onset of the annexin A1 (ANXA1) repair cap, as monitored through ANXA1–green fluorescent protein (GFP) accumulation at injury site over time (10-μm-wide area). Data are expressed as means ± SEM (marked line plots) or Tukey distribution (box plots). n = 5 mice (50 myofibers) per group (3 males and 2 females in vehicle and daily groups and 2 males and 3 females in weekly group). ∗P < 0.05 versus vehicle (one-way analysis of variance test with Bonferroni multiple comparison); †P < 0.05 versus vehicle (two-way analysis of variance test with Bonferroni multiple comparison). Scale bar = 5 μm (A). AU, arbitrary unit.
Thus, similar to what was observed for dystrophin-deficient muscle,23 both weekly and daily GC steroids correlated with annexin gene up-regulation, faster repair cap formation, and enhanced sarcolemmal repair in Dysf-null myofibers after 4 weeks of treatment.
Steroids Decrease Muscle Damage and Fibrosis in Dysf-Null Mice
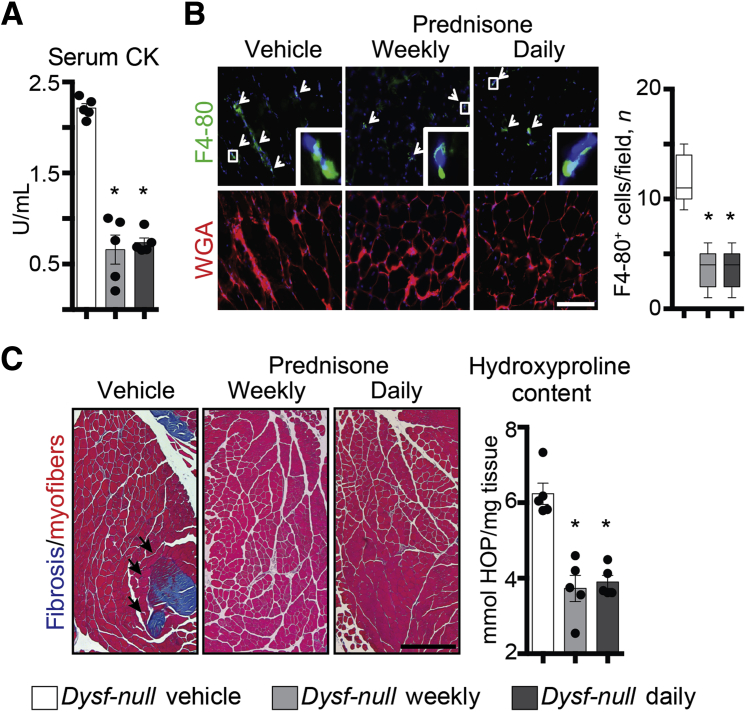
To gain insight into the overall effects of weekly and daily steroid regimens in muscle, muscle damage, the extent of immune infiltration, and the degree of fibrosis in Dysf-null mice at the end of the 4-week-long treatment were analyzed. Serum CK levels were comparably reduced after both prednisone regimens, as compared to vehicle-based treatment (Figure 3A). The extent of immune infiltration was quantitated as amount of F4/80+ macrophages in quadriceps muscle sections. Infiltrating macrophages, as quantified via IF microscopy of muscle sections, appeared comparably decreased after prednisone (Figure 3B). Fibrosis was visualized by Masson trichromic staining in gastrocnemius muscles, and measured as hydroxyproline concentration in quadriceps muscle tissue. Both fibrotic infiltrates and hydroxyproline content were reduced after steroid dosing, with comparable effects from both weekly and daily dosing (Figure 3C). Thus, consistent with findings related to sarcolemmal repair of isolated myofibers, these data indicate that both weekly and daily steroid regimens comparably increase muscle repair, thereby decreasing muscle damage and immune/fibrotic infiltration.
Figure 3.
Weekly and daily steroid regimens reduce muscle damage and fibrosis in Dysf-null mice. A: The same cohort of Dysf-null mice was assessed after 4 weeks of either weekly or daily prednisone. Both steroid regimens comparably decreased serum creatine kinase (CK) levels after a 4-week treatment, as compared to vehicle treatment. B: Immunostaining of quadriceps muscles revealed a decrease in infiltrating macrophages (F4/80+ cells; arrowheads and representative insets) in all steroid-treated mice, as compared to vehicle-treated animals. C: Fibrosis was comparably decreased in both prednisone regimens in hind limb skeletal muscles. Left panels: Masson trichrome staining of gastrocnemius muscle sections (arrows, fibrotic accumulation). Right panel: Quantitation of hydroxyproline content in quadriceps muscle tissue. Data are expressed as means ± SEM and single mouse values (histograms) or Tukey distribution (box plots). n = 5 mice per group (3 males and 2 females in vehicle and daily groups and 2 males and 3 females in weekly group). ∗P < 0.05 versus vehicle (one-way analysis of variance test with Bonferroni multiple comparison). Scale bars: 100 μm (B); 200 μm (C, left panels). HOP, hydroxyproline; WGA, wheat germ agglutinin.
Steroid Regimens Exert Divergent Effects on Dysf-Null Muscle Mass and Functionality
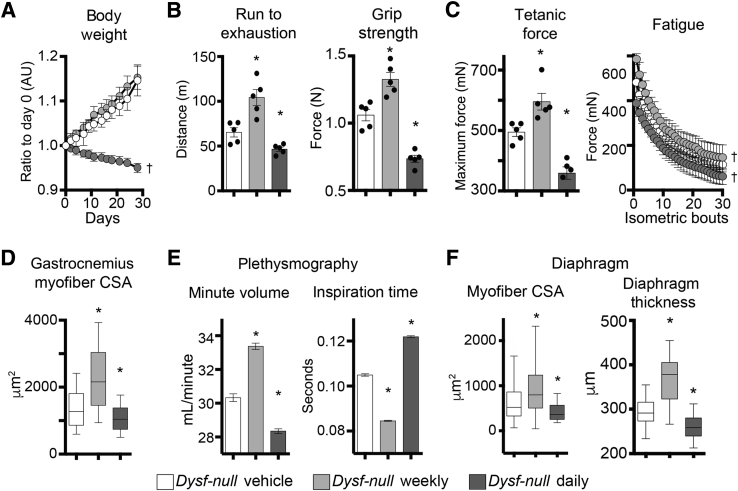
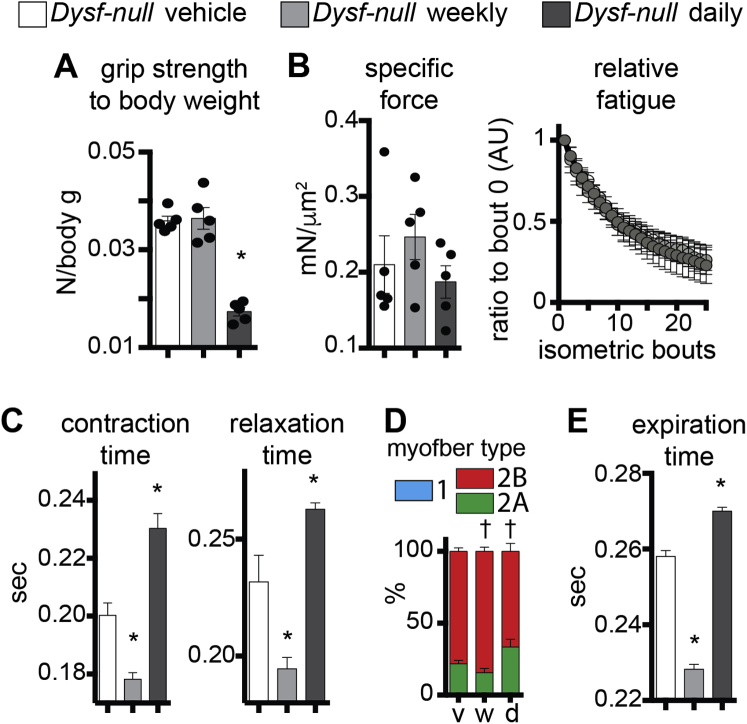
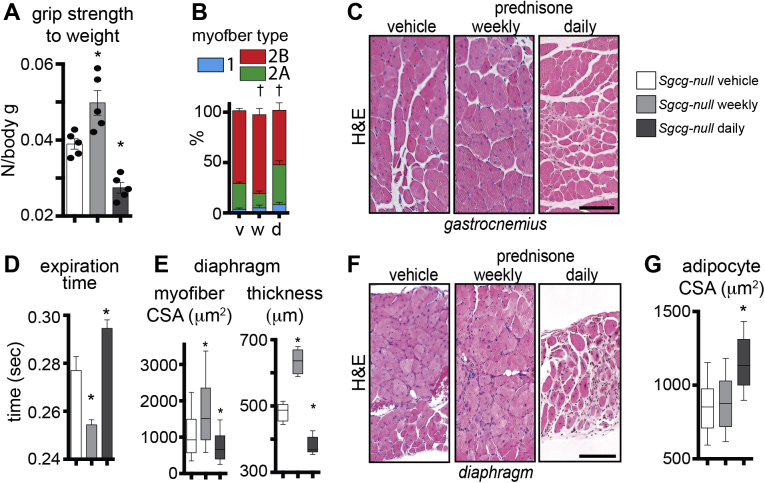
To investigate the effects of weekly and daily prednisone regimens in treated Dysf-null mice, we monitored mass and function of voluntary and respiratory muscles via an extensive panel of performance tests, and physiological and histological analyses. Analysis of body mass over time, measured as weight ratio to starting weight, identified that the daily prednisone regimen associated with gradual mass loss, whereas weekly dosing did not induce significant shifts from the weight trend of vehicle-treated animals (Figure 4A). Muscle performance was tested at treatment end point via run-to-exhaustion test on a treadmill and bilateral grip strength. Both performance tests showed increased performance after weekly dosing, and decreased performance after daily dosing, as compared to control animals (Figure 4B). Intriguingly, even when normalized to body mass, grip strength was significantly decreased after daily prednisone, suggesting a significant impact on muscle weakness in the fore limbs of treated mice (Supplemental Figure S2A).
Figure 4.
Weekly and daily prednisone regimens induce divergent effects on Dysf-null muscle function. A: Unlike weekly dosing, daily prednisone dosing reduced relative body mass over time. B: Weekly and daily prednisone regimens produced opposite effects on muscle performance, as measured by run-to-exhaustion treadmill and grip strength tests. Weekly dosing improved performance, whereas daily dosing decreased performance. C: Left panel: Weekly prednisone associated with increased maximum tetanic force of whole tibialis anterior muscle, as compared to vehicle-treated animals. Right panel: The increase in force remained stable for 30 consecutive isometric contractions. Daily prednisone dosing induced opposite effects. D: Weekly prednisone dosing induced hypertrophic remodeling, whereas daily prednisone dosing induced atrophic remodeling, of skeletal muscle, as quantitated by cross-sectional area (CSA) of gastrocnemius myofibers. E: Whole-body plethysmography showed that weekly prednisone treatment induced improved respiratory capacity, increasing minute volume and decreasing average inspiration time. Daily prednisone dosing yielded the opposite effects. F: Myofiber CSA and transverse thickness of diaphragm muscles followed trends observed in hind limb muscles (ie, increased after weekly prednisone and decreased after daily prednisone, as compared to vehicle-treated control animals). Data are expressed as means ± SEM (marked line plots and histograms), single mouse values (histograms), or Tukey distribution (box plots). n = 5 mice per group (3 males and 2 females in vehicle and daily groups and 2 males and 3 females in weekly group). ∗P < 0.05 versus vehicle (one-way analysis of variance test with Bonferroni multiple comparison); †P < 0.05 versus vehicle (two-way analysis of variance test with Bonferroni multiple comparison). AU, arbitrary unit.
Ex vivo muscle physiology was analyzed via tetanic force measurement in whole tibialis anterior muscles, on stimulation by isometric contractions. The weekly regimen correlated with increased maximum tetanic force, and the increase in force was stable for 30 consecutive contraction bouts, as compared to vehicle-treated muscles. Daily prednisone dosing exerted opposite effects (Figure 4C). However, specific force and relative fatigue analyses did not show significant changes among study cohorts, consistent with a proportional relationship between mass and strength (Supplemental Figure S2B). Contraction and relaxation times, related to tetanic contractions, were decreased after weekly dosing, and increased after the daily steroid regimen (Supplemental Figure S2C). Consistent with force analyses, histological analysis of hind limb muscles showed that weekly prednisone correlated with increased CSA of myofibers, whereas daily steroid dosing elicited a decline in myofiber mass when compared to vehicle-treated muscles (Figure 4D). Moreover, both prednisone regimens elicited modest, but significant, changes in fiber type composition in muscle, as assessed by myosin isoform immunostaining.34 Weekly prednisone dosing associated with a slight increase in relative abundance of type 2B fast, glycolytic myofibers, whereas the daily regimen associated with an increase in type 2A fast, oxidative myofibers (Supplemental Figure S2D).
To monitor the diaphragm muscle function, we performed whole-body plethysmography in treated and control Dysf-null mice at treatment end point. Weekly steroid dosing correlated with increased respiratory capacity, measured as minute volume, and with decreased average time for inspiration and expiration. Daily prednisone regimen induced opposite effects as compared to control animals (Figure 4E and Supplemental Figure S2E). Analogous to hind limb muscles, histological analysis of diaphragm muscles revealed that myofiber CSA and transverse thickness were increased after weekly prednisone, albeit variably, whereas daily dosing correlated with lower values of both parameters than control muscles (Figure 4F). Taken together, these assessments indicate that daily prednisone dosing correlated with muscle weakness and loss of muscle mass in dysferlin-deficient mice. Conversely, intermittent prednisone administration did not promote muscle mass and correlated with improved performance and strength of both voluntary and respiratory muscles in Dysf-null mice.
Weekly and Daily Prednisone Regimens Elicit Opposite Effects on Atrophy, Myogenesis, and Adipogenesis in Dysf-Null Muscle
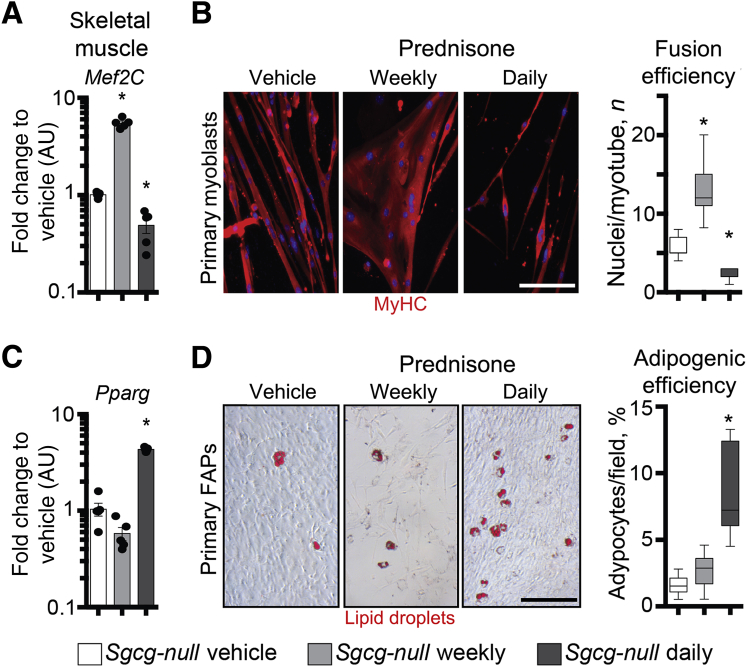
Recently, the transcriptional regulator Krüppel-like factor 15 has been proposed as a mediator of proergogenic remodeling by glucocorticoid steroids in dystrophic muscle.20 In WT and dystrophin-deficient mice, daily and weekly steroid regimens exert opposite effects on expression levels of Klf15 and muscle atrophy regulators.23 We, therefore, interrogated treated and control Dysf-null muscle for transcriptional trends of key candidate genes through qPCR. Klf15 was up-regulated after weekly dosing, and down-regulated after daily dosing, of prednisone, as compared to vehicle controls (Figure 5A). Conversely, Fbxo32 and Trim63, encoding for the atrophy agonists ATROGIN1 and MURF1, respectively, showed opposite trends. In addition, opposite to daily dosing, a weekly steroid regimen correlated with up-regulation of Mef2C, a key transcriptional factor implicated in muscle hypertrophy and regeneration.45, 46
Figure 5.
Weekly prednisone dosing promotes myogenic capacity, whereas daily dosing promotes adipogenesis, in Dysf-null mice. A: Real-time quantitative PCR of quadriceps muscle tissue showed transcriptional trends correlating with divergent ergogenic effects of weekly versus daily prednisone in Dysf-null muscle, including divergent effects on the myogenic factor Mef2C. B: Immunostaining of differentiated primary myoblasts (CD56+/CD140a− fraction, passage 0) showed that, after weekly prednisone, myoblasts increased fusion efficiency in multinucleated myotubes. Daily prednisone induced the opposite effects. C: The adipogenic factor Pparg was up-regulated in muscle after daily prednisone (left panels), and the extent of fatty infiltrates in quadriceps muscle was dramatically increased after daily prednisone dosing (right panel), as shown by Oil Red O staining. D: Daily prednisone dosing induced a dramatic increase in spontaneous adipocyte production by proliferating primary fibroadipogenic progenitors (FAPs; CD56−/CD140a+ fraction, passage 0), as demonstrated by Oil Red O staining of lipid-storing cells (left panels show representative images; right panel, quantitationData are expressed as means ± SEM, single mouse values (histograms), or Tukey distribution (box plots). n = 5 mice per group (3 males and 2 females in vehicle and daily groups and 2 males and 3 females in weekly group). ∗P < 0.05 versus vehicle (one-way analysis of variance test with Bonferroni multiple comparison). Scale bars: 100 μm (B and D); 200 μm (C). AU, arbitrary unit; MyHC, myosin heavy chain.
Because of the results with Mef2C, we interrogated the myogenic capability of resident myoblasts after vehicle or prednisone regimens. Primary myoblasts were sorted as the CD56+/CD140a− subfraction37, 38 of the mononuclear cell fraction from tibialis anterior muscles at treatment end point, and differentiated under serum starvation without serial passaging. When isolated from weekly GC steroid-treated muscles, primary myoblasts showed an increased rate of spontaneous fusion into myotubes in vitro, whereas myoblasts from daily GC steroid-treated muscles showed a decreased fusion efficiency as compared to cells from control animals (Figure 5B).
One of the adverse effects of chronic glucocorticoid steroid administration is obesity.47 This is especially relevant in Dysf-null muscle, where adipogenic replacement of myofibers is a prominent pathophysiologic feature during later stages of disease.25, 48, 49 To evaluate the effect of GC steroids on adipogenic replacement of muscle, we analyzed the expression levels of Pparg, a regulator of adipogenic potential, and the extent of lipid deposition in quadriceps muscle tissue. Pparg expression levels were up-regulated in muscle of daily, but not weekly, treated mice, as compared to control animals. Moreover, daily prednisone GC exposure associated with a sharp increase in the extent of lipid deposition in muscle, as revealed by Oil Red O staining of muscle sections (Figure 5C). We next tested the adipogenic capacity of resident FAPs, which are muscle interstitial cells with adipogenic potential.37 We isolated primary FAPs as the CD56−/CD140a+ subfraction of previously mentioned tibialis anterior mononuclear preparations, and assessed rate of spontaneous conversion into lipid-storing cells on proliferation without serial passaging. Daily GC prednisone was associated with increased adipogenic conversion of primary FAPs, as compared to weekly prednisone or vehicle control regimens (Figure 5D). More important, histological analysis of the ventral fat pad in treated and control mice revealed that daily steroid dosing also correlated with adipocyte hypertrophy in fat tissue (Supplemental Figure S3), a marker of propensity for obesity.50 Thus, daily GC steroid dosing correlated with increased lipid deposits in muscle, higher adipogenic conversion of resident FAPs, and increased adipocyte size in the fat tissue of Dysf-null mice. Daily GC prednisone dosing was able to promote muscle performance in the absence of enhanced adipogenic conversion in muscle and fat tissue adipocyte hypertrophy.
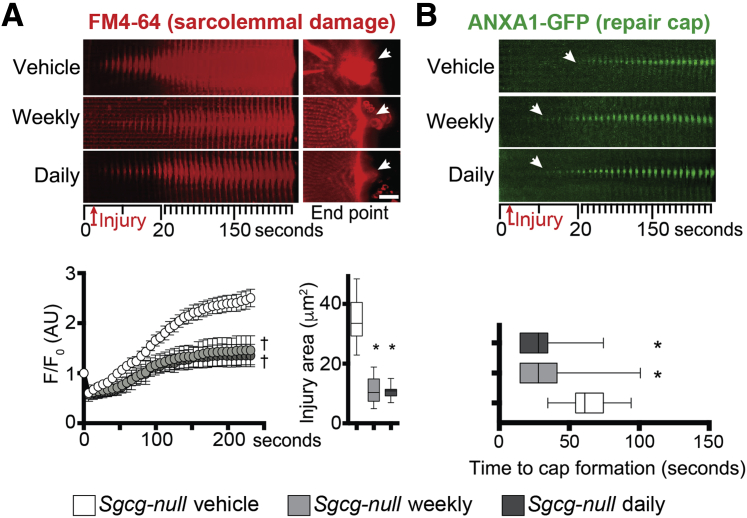
Prednisone Improves Sarcolemmal Repair in Sgcg-Null Mice
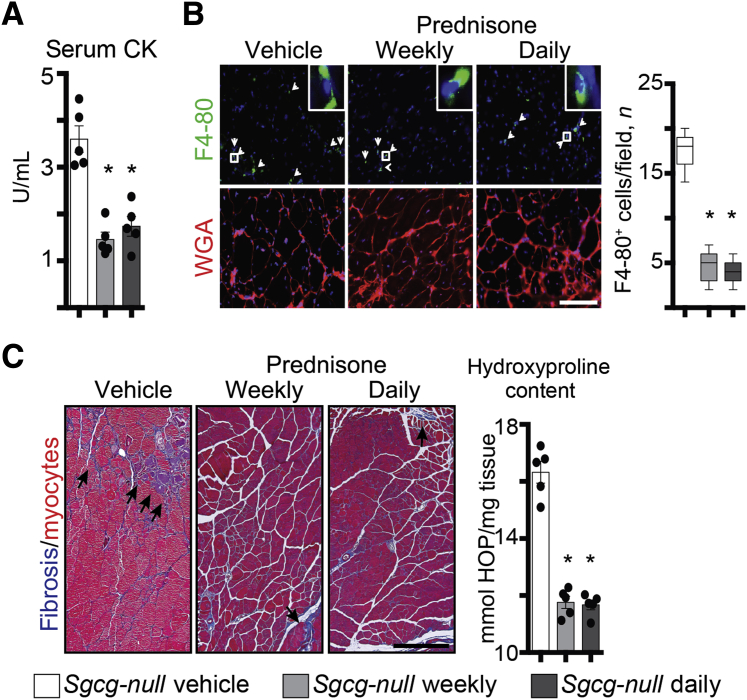
We similarly tested the effect of prednisone on muscle repair on myofibers isolated from Sgcg-null mice, a genetic model of LGMD type 2C. Sgcg-null mice received a 4-week-long treatment with vehicle, weekly prednisone, or daily prednisone (n = 5 mice per group), following the same conditions used for Dysf-null groups. At treatment end point, we analyzed the levels of sarcolemmal injury and overall muscle damage across groups. The extent of sarcolemmal damage after laser-driven injury in isolated myofibers was significantly smaller after both steroid regimens compared to vehicle injections, as quantitated through FM4-64 dye accumulation (Figure 6A). The onset of the annexin repair cap assembly was faster in myofibers from prednisone-treated mice than from vehicle-treated mice, as visualized by ANXA1-GFP (Figure 6B). Expression levels of Anxa1 and Anxa6 were comparably up-regulated in the quadriceps muscles of steroid-treated mice, as compared to vehicle-treated animals (Supplemental Figure S4A). Moreover, steroid uptake was confirmed by Gzmb and Ifng down-regulation in primary splenocytes (Supplemental Figure S4B). Overall muscle damage was reduced in both prednisone-treated groups when compared to vehicle-treated mice, as quantitated by serum CK levels (Figure 7A). Moreover, both prednisone regimens correlated with reduction in infiltrating F4/80+ macrophages in quadriceps muscles and reduction of fibrosis in hind limb and diaphragm muscles as compared to vehicle-treated controls (Figure 7B and C, and Supplemental Figure S4C). Thus, as with Dysf-null mice, both weekly and daily dosing of GC steroids correlated with improved sarcolemmal repair and reduced levels of muscle damage and immune/fibrotic infiltration in Sgcg-null muscle.
Figure 6.
Prednisone treatment of Sgcg-null mice enhances sarcolemmal repair and repair cap formation after injury. A: Laser injury was performed on Sgcg-null myofibers after 4 weeks of either weekly or daily prednisone treatment. Both weekly and daily prednisone reduced the extent of sarcolemmal damage after laser-induced injury, as shown by decreased FM4-64 accumulation over time (10-μm-wide area) and at end point (arrows). B: Both weekly and daily prednisone regimens correlated with faster onset (arrows) of accumulation of annexin A1–green fluorescent protein (ANXA1-GFP) in repair caps on the site of sarcolemmal injury (10-μm-wide area). Data are epressed as means ± SEM (marked line plots) or Tukey distribution (box plots). n = 5 mice (50 myofibers) per group (3 males and 2 females per group). ∗P < 0.05 versus vehicle (one-way analysis of variance test with Bonferroni multiple comparison); †P < 0.05 versus vehicle (two-way analysis of variance test with Bonferroni multiple comparison). Scale bar, 5 μm (A). AU, arbitrary unit.
Figure 7.
Comparable improvement in histopathological findings by weekly and daily prednisone in Sgcg-null mice. A: Weekly and daily prednisone similarly decreased serum creatine kinase (CK) levels after a 4-week treatment, as compared to vehicle treatment. B: Immunostaining of quadriceps muscles revealed a decrease in infiltrating macrophages (F4/80+ cells; arrowheads and representative insets) in all prednisone-treated mice, as compared to vehicle-treated animals. C: Fibrosis (arrows) was comparably decreased in hind limb skeletal muscles by both prednisone regimens. Left panels: Masson trichrome staining of gastrocnemius muscle sections. Right panel: Quantitation of hydroxyproline content in quadriceps muscle tissue. Data are expressed as means ± SEM, single mouse values (histograms), or Tukey distribution (box plots). n = 5 mice per group (3 males and 2 females per group). ∗P < 0.05 versus vehicle (one-way analysis of variance test with Bonferroni multiple comparison). Scale bars: 100 μm (B); 200 μm (C, left panels). HOP, hydroxyproline; WGA, wheat germ agglutinin.
Divergent Remodeling and Functional Effects from Weekly versus Daily Steroids in Sgcg-Null Muscles
Body mass declined over time in Sgcg-null mice with the daily prednisone regimen, whereas body mass slightly increased over time under vehicle or weekly steroid regimens (Figure 8A). At treatment end point, weekly prednisone was associated with better performance on the treadmill and grip strength assays, whereas daily prednisone induced opposite effects, as compared to vehicle-treated mice (Figure 8B). When normalized to body mass, grip strength was increased in weekly prednisone-treated mice and reduced in daily prednisone-treated mice (Supplemental Figure S5A). Stimulation of whole tibialis anterior muscles with isometric contractions showed that weekly prednisone correlated with increased maximum tetanic force, and this increase was maintained for up to 10 consecutive bouts, as compared to the vehicle regimen. Daily prednisone correlated with opposite effects (Figure 8C). Prednisone regimens associated with mildly divergent effects on myofiber type distribution in quadriceps muscle; weekly prednisone increased the type 2B ratio, whereas daily prednisone increased type 2A as compared to vehicle regimen (Supplemental Figure S5B). Myofiber CSA was increased after weekly dosing and decreased after daily dosing in gastrocnemius muscle (Figure 8D and Supplemental Figure S5C).
Figure 8.
Weekly prednisone improves muscle function in Sgcg-null mice, whereas daily prednisone induces atrophy. A: Unlike weekly dosing, daily prednisone reduced relative body weight over time. B: Weekly prednisone improved run to exhaustion and grip strength, whereas daily prednisone resulted in a reduction in both treadmill and grip strength performance, in Sgcg-null mice. C: Left panel: Weekly prednisone associated with increased tetanic force of whole tibialis anterior muscle, as compared to vehicle-treated animals. Right panel: The increase in force remained stable for 10 consecutive isometric contractions. Daily prednisone dosing induced opposite effects. D: Weekly prednisone induced hypertrophic remodeling, whereas daily dosing induced atrophic remodeling, of skeletal muscle, as quantitated by cross-sectional area (CSA) of gastrocnemius myofibers. E: Whole-body plethysmography showed that weekly prednisone treatment induced improved respiratory capacity, increasing minute volume and decreasing average inspiration time. Daily prednisone dosing induced opposite effects. F: Real-time quantitative PCR of quadriceps muscle tissue showed transcriptional trends correlating with divergent ergogenic effects of steroid regimens in muscle, including the ergogenic factor Klf15, which was up-regulated after weekly dosing, and the atrophic factors Fbxo32 and Trim63, which were up-regulated after daily dosing. Data are expressed as means ± SEM (marked line plots and histograms), single mouse values (histograms), or Tukey distribution (box plots). n = 5 mice per group (3 males and 2 females per group). ∗P < 0.05 versus vehicle (one-way analysis of variance test with Bonferroni multiple comparison); †P < 0.05 versus vehicle (two-way analysis of variance test with Bonferroni multiple comparison). AU, arbitrary unit.
Whole-body plethysmography and histology were used to monitor diaphragm function at treatment end point. As in Dysf-null animals, respiratory function was improved in Sgcg-null mice after weekly prednisone and was worsened after daily prednisone, as quantitated by minute volume and inspiration/expiration time (Figure 8E and Supplemental Figure S5D). Sgcg-null diaphragm muscle showed increased myofiber CSA and thickness after the weekly regimen, and opposite trends were quantitated after the daily steroid regimen (Supplemental Figure S5E and F). Furthermore, the quadriceps muscle from Sgcg-null mice demonstrated the same trends for gene expression associated with proergogenic or proatrophic remodeling. Weekly prednisone was linked to up-regulation of Klf15 and daily prednisone to reduced Klf15, as compared to the vehicle regimen. Fbxo32 and Trim63 expression levels were up-regulated after daily prednisone dosing, and down-regulated after weekly dosing (Figure 8F). Taken together, these data indicate that intermittent treatment of Sgcg-null mice with prednisone correlated with increased functionality without loss of muscle mass, whereas daily steroid dosing associated with atrophic muscle remodeling and loss of muscle strength and performance.
Steroid Regimens Induce Divergent Effects on Myogenic and Adipogenic Capacities in Sgcg-Null Muscle
Expression levels of promyogenic factor Mef2C were up-regulated in muscle after weekly prednisone and down-regulated after daily prednisone (Figure 9A). Accordingly, the weekly prednisone regimen correlated with increased fusion efficiency of primary myoblasts (CD56+/CD140a−) into myotubes under serum starvation, as compared to myoblasts isolated from vehicle-treated mice. Daily prednisone dosing correlated with decreased efficiency (Figure 9B). Conversely, the daily prednisone regimen produced up-regulation of proadipogenic Pparg expression in muscle tissue, and a mild increase in spontaneous conversion of primary muscle FAPs (CD56+/CD140a−) into lipid-storing cells (Figure 9C and D). Furthermore, daily prednisone associated with increased adipocyte hypertrophy in the ventral fat pad (Supplemental Figure S5G). Thus, steroid regimens correlated with divergent effects on myogenic and adipogenic capacities in Sgcg-null muscle. Although intermittent dosing correlated with increased myogenesis, daily dosing associated with adipocyte hypertrophy and higher adipogenic propensity of muscle FAPs.
Figure 9.
Weekly versus daily prednisone induces divergent effects on myogenic and adipogenic capacities in Sgcg-null muscles. A: Real-time quantitative PCR on quadriceps muscle tissue showed up-regulation of the myogenic factor Mef2C after weekly prednisone, and its down-regulation after daily prednisone. B: Immunostaining of differentiated primary myoblasts (passage 0) showed that, after weekly prednisone, myoblasts increased fusion efficiency in multinucleated myotubes. Daily prednisone induced the opposite effects. C: The adipogenic factor Pparg was up-regulated in muscle after daily prednisone dosing. D: Daily prednisone dosing also associated with an increase in spontaneous adipocyte production by proliferating primary fibroadipogenic progenitors (FAPs), as demonstrated by Oil Red O staining of lipid-storing cells (left panels show representative images; right panel, quantitation). Data are expressed as means ± SEM and single mouse values (histograms) or Tukey distribution (box plots). n = 5 mice per group (3 males and 2 females per group). ∗P < 0.05 versus vehicle (one-way analysis of variance test with Bonferroni multiple comparison). Scale bar = 100 μm (B and D, left panels). AU, arbitrary unit.
Discussion
Intermittent Prednisone Dosing Improves Muscle Performance in LGMD Mouse Models
To date, evaluation of glucocorticoid steroid regimens in the highly heterogeneous field of LGMD pathology mainly consists of scattered observations from clinical cases.51, 52 A deflazacort-based treatment trial of LGMD 2B patients, with limited cohort size, suggested detrimental effects of chronic daily dosing of glucocorticoid steroids on muscle strength.14 Our data show that both weekly and daily prednisone regimens improved muscle repair after laser injury, and this correlated with a reduction in serum CK in vivo. However, weekly versus daily prednisone dosing produced divergent responses with respect to muscle performance and atrophy. Daily prednisone treatment elicited a significant atrophic response, accompanied by an increase in the genes encoding atrophic factors like Fbxo32 and Trim63. In contrast, weekly prednisone treatment had the same beneficial effect on promoting sarcolemmal repair but without the atrophic effects. Weekly prednisone treatment was associated with enhanced muscle performance in vivo and in isolated muscles measured ex vivo. These findings are similar to recent observations made in mdx mice, a model for DMD.23 There are shared pathological mechanisms between dystrophin deficiency and γ-sarcoglycan loss, as both are components of the dystrophin complex, a complex critical for maintaining muscle membrane stability. Thus, the similar outcome with prednisone treatment could be anticipated in γ-sarcoglycan–deficient animals. However, the findings in dysferlin-deficient mice, a model for LGMD 2B, were unanticipated, as this defect is not specifically associated with impaired sarcolemmal fragility. These data support that enhancing muscle repair and muscle performance, through intermittent steroid dosing, may be broadly useful.
With respect to the translational relevance of the experimental weekly dosing of 1 mg/kg per dose, the question of whether daily dosing with smaller steroid amounts (ie, approximately 0.15 mg/kg per dose) can elicit similar improvement is still open. This question will likely be influenced by the different genetic contexts of dystrophic pathology. Indeed, daily dosing of approximately 0.3 mg/kg prednisone was reported as clinically beneficial in a case report of two FKRP-related LGMD 2I patients.15 However, a meta-analysis of DMD patients and steroid use suggested that 0.75 mg/kg was not as effective as higher dosing.53 Considering the literature and the findings in this report, it might be speculated that daily exposure increases the chance of atrophic remodeling, whereas the precise dose and pathological context influence timing and severity of remodeling. Conversely, pulsatile weekly dosing appears to reduce the pharmacological burden and adverse effects, while enhancing muscle repair and performance, as suggested by several studies.42, 43, 44, 53 However, detailed comparative studies of diverse dosing modalities in experimental animal models and human subjects are still needed.
Dysferlin deficiency disrupts the endogenous process of sarcolemmal resealing, whereas γ-sarcoglycan deficiency impairs the physical anchoring of sarcomeres to the membrane, thereby undermining sarcolemmal stability on muscle contraction.8, 10 Both models showed up-regulation of Anxa1 and Anxa6 after prednisone treatment. This same finding was seen in mdx mice, and along with Anxa1 and Anxa6, additional genes encoding components of the repair complex were increased.23 We found that the promoters of both Anxa1 and Anxa6 were engaged by the glucorcorticoid receptor,23 and it can be reasonably expected that this same mechanism occurs in most muscles, whether dystrophic or normal muscles after injury.
In addition to transcriptional changes in repair genes, steroid-associated improvement in sarcolemmal repair may also derive from plasma membrane remodeling that inherently alters susceptibility to injury. Glucocorticoid steroids can influence both composition and properties of outer membranes. In guinea pig cardiomyocytes, dexamethasone reduced membrane destabilization after calcium overload via inhibition of phospholipase A2.54 In rabbit cardiac endothelial cells, dexamethasone increased membrane fluidity by regulating eicosanoid production.55 However, targeted studies are required to quantitatively dissect how much repair gene levels and membrane properties contribute to prednisone-induced changes in sarcolemmal injury and repair.
Daily Prednisone Promoted Marked Adipogenesis in Dysferlin-Deficient Muscle
Pharmacological treatment strategies for dystrophic muscle often lack a precise understanding of the key cellular mediators of drug-related effects on the whole muscle tissue.56, 57 Herein, we described regimen-associated effects on resident myoblasts and FAPs of both Dysf-null and Sgcg-null muscles, although the finding was more dramatic in dysferlin-mutant muscle. In accordance with adipocyte hypertrophy trends in the ventral fat pad, the spontaneous adipogenic transformation of resident muscle FAPs was increased after daily, but not weekly, dosing of prednisone. However, FAPs from Dysf-null muscles appeared intrinsically more prone to convert into lipid-storing cells than those from Sgcg-null muscles, although possible background-related effects should be considered. This aspect is intriguing and warrants more in-depth investigation, considering the recent findings indicating a role for another annexin gene, Anxa2, in regulating the characteristic increase in fatty infiltrates observed in Dysf-null murine muscles.48
Taken together, our findings showed that daily steroid dosing, albeit beneficial to sarcolemmal repair and muscle damage, ultimately results in atrophy and weakness of Dysf-null and Sgcg-null muscles, consistent with findings in dystrophin-deficient muscles.20, 32 Conversely, intermittent steroid dosing improved repair without promoting atrophy or adipogenesis, ultimately resulting in improved muscle mass, strength, and performance. Regimen-specific divergent effects on muscle performance correlated with opposite transcriptional regulation of Klf15 versus atrophy-related genes, and the relevance of these molecular signatures for LGMD muscle supports that increases in Klf15 levels are as beneficial for LGMD muscle as they are for mdx muscle.20 This is particularly compelling for LGMD 2B, where prohypertrophic remodeling is not straightforward. When challenged with genetic gain of follistatin expression, a prohypertrophic manipulation, Dysf-null muscles showed exacerbation of muscle degeneration.58 More refined studies, especially with treatment duration >4 weeks, are required to shed light on the interplays between intermittent steroid dosing and muscle mass remodeling pathways. However, these data support further investigation into mechanisms and biomarkers of these divergent steroid effects so that these preclinical findings can be translated into humans with muscle disease.
Acknowledgments
We thank the Center for Advanced Microscopy and Dr. Constadina Arvanitis (Northwestern University) for outstanding support.
Footnotes
Supported by NIH grants U54 AR052646, RO1 NS047726, and R01 HL61322, and the Parent Project Muscular Dystrophy. M.Q. is supported by Muscular Dystrophy Association Development grant 479350 with cofunding from the American Association of Neuromuscular and Electrodiagnostic Medicine Foundation. Cell sorting was conducted at the Northwestern University Flow Cytometry Core Facility, supported by Cancer Center Support grant/National Cancer Institute grant CA060553.
Disclosures: None declared.
Supplemental material for this article can be found at http://dx.doi.org/10.1016/j.ajpath.2017.07.017.
Supplemental Data
Supplemental Figure S1.
Prednisone treatment associated with up-regulation of annexin gene expression in Dysf-null muscles. A: Weekly and daily prednisone treatment induced a comparable increase in Anxa1 and Anxa6 expression levels in quadriceps muscles of Dysf-null mice. B: Steroid uptake was confirmed by down-regulation of Gzmb and Ifng, markers of immune cell activation, in primary splenocytes of steroid-treated mice. Data are expressed as means ± SEM and single mouse values (histograms). n = 5 mice (50 myofibers) per group (3 males and 2 females in vehicle and daily groups and 2 males and 3 females in weekly group). ∗P < 0.05 versus vehicle (one-way analysis of variance test with Bonferroni multiple comparison). AU, arbitrary unit.
Supplemental Figure S2.
Weekly and daily prednisone-induced divergent functional effects on Dysf-null muscle. A: Daily prednisone dosing reduced grip strength normalized to body mass, suggesting a significant impact on intrinsic muscle weakness. B: Specific force and relative fatigue of tibialis anterior muscles did not significantly change across prednisone regimen types. C: Times of tibialis anterior muscle contraction and relaxation were reduced after weekly prednisone, and increased after daily prednisone. D: Weekly prednisone correlated with expansion of type 2B fast, glycolytic fibers and reduction of type 2A fast, oxidative fibers, as quantitated by immunostaining of quadriceps muscle sections. Daily prednisone had the opposite effects. Type 1 myofibers (blue bars) were found in negligible amounts in all conditions. E: Prednisone dosing schemes associated with the opposite effects on average expiration time, as quantitated through whole-body plethysmography, paralleling the effects on inspiration time. Data are expressed as means ± SEM (histograms). n = 5 mice per group (3 males and 2 females in vehicle and daily groups and 2 males and 3 females in weekly group). ∗P < 0.05 versus vehicle (one-way analysis of variance test with Bonferroni multiple comparison); †P < 0.05 versus vehicle (two-way analysis of variance test with Bonferroni multiple comparison). AU, arbitrary unit.
Supplemental Figure S3.
Daily prednisone dosing produced adipocyte hypertrophy. Left panels: Representative images of ventral fat pad histology. Right panel: Quantitation of adipocyte cross-sectional area (CSA), showing significantly increased CSA values after daily prednisone dosing. Data are expressed as box plots (Tukey distribution). n = 5 mice per group (3 males and 2 females in vehicle and daily groups and 2 males and 3 females in weekly group). ∗P < 0.05 versus vehicle (one-way analysis of variance test with Bonferroni multiple comparison).
Supplemental Figure S4.
Prednisone increased annexin gene expression and improved diaphragm histopathology in Sgcg-null muscles. A: Weekly and daily steroid regimens induced comparable increases in Anxa1 and Anxa6 expression levels in quadriceps muscles of Sgcg-null mice. B: Steroid uptake was confirmed by down-regulation of Gzmb and Ifng, markers of immune cell activation, in splenocytes of steroid-treated mice. C: Fibrosis of dystrophic diaphragm muscle was comparably reduced after both steroid regimens, as shown by Masson trichrome staining. Data are expressed as means ± SEM and single mouse values (histograms). n = 5 mice per group (3 males and 2 females in vehicle and daily groups and 2 males and 3 females in weekly group). ∗P < 0.05 versus vehicle (one-way analysis of variance test with Bonferroni multiple comparison). Scale bar = 100 μm (C). AU, arbitrary unit.
Supplemental Figure S5.
Weekly prednisone induced hypertrophy, and daily prednisone induced atrophy, of Sgcg-null muscles. A: Weekly prednisone injection increased the grip strength, as normalized to body weight, as compared to vehicle treatment. Daily prednisone dosing had the opposite effects. B: Weekly prednisone correlated with expansion of type 2B fast, glycolytic and reduction of type 2A fast, oxidative myofibers, as quantitated by immunostaining of quadriceps muscle sections. Daily dosing had the opposite effects. C: Histological analysis of gastrocnemius muscle sections showed hypertrophic remodeling of myofibers after weekly prednisone dosing, and atrophic remodeling after the daily regimen. D: Weekly steroid regimen decreased expiration time, whereas daily dosing correlated with increased time, as quantitated through whole-body plethysmography. E and F: Divergent effects on muscle remodeling were evidenced in diaphragm muscles, as shown by myofiber cross-sectional area (CSA) values, transverse thickness, and representative histology images. G: Daily steroid dosing correlated with adipocyte hypertrophy, as quantitated through histology analysis of ventral fat pad sections. Data are expressed as means ± SEM, single mouse values (histograms), or box plots (Tukey distribution). n = 5 mice per group (3 males and 2 females per group). ∗P < 0.05 versus vehicle (one-way analysis of variance test with Bonferroni multiple comparison); †P < 0.05 versus vehicle (two-way analysis of variance test with Bonferroni multiple comparison). Scale bars = 100 μm (C and F). H&E, hematoxylin and eosin.
References
- 1.Emery A.E. The muscular dystrophies. Lancet. 2002;359:687–695. doi: 10.1016/S0140-6736(02)07815-7. [DOI] [PubMed] [Google Scholar]
- 2.Aartsma-Rus A., Ginjaar I.B., Bushby K. The importance of genetic diagnosis for Duchenne muscular dystrophy. J Med Genet. 2016;53:145–151. doi: 10.1136/jmedgenet-2015-103387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guiraud S., Aartsma-Rus A., Vieira N.M., Davies K.E., van Ommen G.J., Kunkel L.M. The pathogenesis and therapy of muscular dystrophies. Annu Rev Genomics Hum Genet. 2015;16:281–308. doi: 10.1146/annurev-genom-090314-025003. [DOI] [PubMed] [Google Scholar]
- 4.Thompson R., Straub V. Limb-girdle muscular dystrophies: international collaborations for translational research. Nat Rev Neurol. 2016;12:294–309. doi: 10.1038/nrneurol.2016.35. [DOI] [PubMed] [Google Scholar]
- 5.Bittner R.E., Anderson L.V., Burkhardt E., Bashir R., Vafiadaki E., Ivanova S., Raffelsberger T., Maerk I., Hoger H., Jung M., Karbasiyan M., Storch M., Lassmann H., Moss J.A., Davison K., Harrison R., Bushby K.M., Reis A. Dysferlin deletion in SJL mice (SJL-Dysf) defines a natural model for limb girdle muscular dystrophy 2B. Nat Genet. 1999;23:141–142. doi: 10.1038/13770. [DOI] [PubMed] [Google Scholar]
- 6.Kerr J.P., Ward C.W., Bloch R.J. Dysferlin at transverse tubules regulates Ca(2+) homeostasis in skeletal muscle. Front Physiol. 2014;5:89. doi: 10.3389/fphys.2014.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lek A., Evesson F.J., Sutton R.B., North K.N., Cooper S.T. Ferlins: regulators of vesicle fusion for auditory neurotransmission, receptor trafficking and membrane repair. Traffic. 2012;13:185–194. doi: 10.1111/j.1600-0854.2011.01267.x. [DOI] [PubMed] [Google Scholar]
- 8.Demonbreun A.R., Allen M.V., Warner J.L., Barefield D.Y., Krishnan S., Swanson K.E., Earley J.U., McNally E.M. Enhanced muscular dystrophy from loss of dysferlin is accompanied by impaired annexin A6 translocation after sarcolemmal disruption. Am J Pathol. 2016;186:1610–1622. doi: 10.1016/j.ajpath.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noguchi S., McNally E.M., Ben Othmane K., Hagiwara Y., Mizuno Y., Yoshida M., Yamamoto H., Bonnemann C.G., Gussoni E., Denton P.H., Kyriakides T., Middleton L., Hentati F., Ben Hamida M., Nonaka I., Vance J.M., Kunkel L.M., Ozawa E. Mutations in the dystrophin-associated protein gamma-sarcoglycan in chromosome 13 muscular dystrophy. Science. 1995;270:819–822. doi: 10.1126/science.270.5237.819. [DOI] [PubMed] [Google Scholar]
- 10.Hack A.A., Ly C.T., Jiang F., Clendenin C.J., Sigrist K.S., Wollmann R.L., McNally E.M. Gamma-sarcoglycan deficiency leads to muscle membrane defects and apoptosis independent of dystrophin. J Cell Biol. 1998;142:1279–1287. doi: 10.1083/jcb.142.5.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gloss D., Moxley R.T., 3rd, Ashwal S., Oskoui M. Practice guideline update summary: corticosteroid treatment of Duchenne muscular dystrophy: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2016;86:465–472. doi: 10.1212/WNL.0000000000002337. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Bello L., Gordish-Dressman H., Morgenroth L.P., Henricson E.K., Duong T., Hoffman E.P., Cnaan A., McDonald C.M., CINRG Investigators Prednisone/prednisolone and deflazacort regimens in the CINRG Duchenne Natural History Study. Neurology. 2015;85:1048–1055. doi: 10.1212/WNL.0000000000001950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heier C.R., Damsker J.M., Yu Q., Dillingham B.C., Huynh T., Van der Meulen J.H., Sali A., Miller B.K., Phadke A., Scheffer L., Quinn J., Tatem K., Jordan S., Dadgar S., Rodriguez O.C., Albanese C., Calhoun M., Gordish-Dressman H., Jaiswal J.K., Connor E.M., McCall J.M., Hoffman E.P., Reeves E.K., Nagaraju K. VBP15, a novel anti-inflammatory and membrane-stabilizer, improves muscular dystrophy without side effects. EMBO Mol Med. 2013;5:1569–1585. doi: 10.1002/emmm.201302621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walter M.C., Reilich P., Thiele S., Schessl J., Schreiber H., Reiners K., Kress W., Muller-Reible C., Vorgerd M., Urban P., Schrank B., Deschauer M., Schlotter-Weigel B., Kohnen R., Lochmuller H. Treatment of dysferlinopathy with deflazacort: a double-blind, placebo-controlled clinical trial. Orphanet J Rare Dis. 2013;8:26. doi: 10.1186/1750-1172-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Darin N., Kroksmark A.K., Ahlander A.C., Moslemi A.R., Oldfors A., Tulinius M. Inflammation and response to steroid treatment in limb-girdle muscular dystrophy 2I. Eur J Paediatr Neurol. 2007;11:353–357. doi: 10.1016/j.ejpn.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 16.Wu B., Shah S.N., Lu P., Richardson S.M., Bollinger L.E., Blaeser A., Madden K.L., Sun Y., Luckie T.M., Cox M.D., Sparks S., Harper A.D., Lu Q.L. Glucocorticoid steroid and alendronate treatment alleviates dystrophic phenotype with enhanced functional glycosylation of alpha-dystroglycan in mouse model of limb-girdle muscular dystrophy with FKRPP448L mutation. Am J Pathol. 2016;186:1635–1648. doi: 10.1016/j.ajpath.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 17.Anderson J.E., McIntosh L.M., Poettcker R. Deflazacort but not prednisone improves both muscle repair and fiber growth in diaphragm and limb muscle in vivo in the mdx dystrophic mouse. Muscle Nerve. 1996;19:1576–1585. doi: 10.1002/(SICI)1097-4598(199612)19:12<1576::AID-MUS7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 18.Balaban B., Matthews D.J., Clayton G.H., Carry T. Corticosteroid treatment and functional improvement in Duchenne muscular dystrophy: long-term effect. Am J Phys Med Rehabil. 2005;84:843–850. doi: 10.1097/01.phm.0000184156.98671.d0. [DOI] [PubMed] [Google Scholar]
- 19.Ma K., Mallidis C., Bhasin S., Mahabadi V., Artaza J., Gonzalez-Cadavid N., Arias J., Salehian B. Glucocorticoid-induced skeletal muscle atrophy is associated with upregulation of myostatin gene expression. Am J Physiol Endocrinol Metab. 2003;285:E363–E371. doi: 10.1152/ajpendo.00487.2002. [DOI] [PubMed] [Google Scholar]
- 20.Morrison-Nozik A., Anand P., Zhu H., Duan Q., Sabeh M., Prosdocimo D.A., Lemieux M.E., Nordsborg N., Russell A.P., MacRae C.A., Gerber A.N., Jain M.K., Haldar S.M. Glucocorticoids enhance muscle endurance and ameliorate Duchenne muscular dystrophy through a defined metabolic program. Proc Natl Acad Sci U S A. 2015;112:E6780–E6789. doi: 10.1073/pnas.1512968112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sato A.Y., Richardson D., Cregor M., Davis H.M., Au E.D., McAndrews K., Zimmers T.A., Organ J.M., Peacock M., Plotkin L.I., Bellido T. Glucocorticoids induce bone and muscle atrophy by tissue-specific mechanisms upstream of E3 ubiquitin ligases. Endocrinology. 2017;158:664–677. doi: 10.1210/en.2016-1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schakman O., Gilson H., Kalista S., Thissen J.P. Mechanisms of muscle atrophy induced by glucocorticoids. Horm Res. 2009;72(Suppl 1):36–41. doi: 10.1159/000229762. [DOI] [PubMed] [Google Scholar]
- 23.Quattrocelli M., Barefield D.Y., Warner J.L., Vo A.H., Hadhazy M., Earley J.U., Demonbreun A.R., McNally E.M. Intermittent glucocorticoid steroid dosing enhances muscle repair without eliciting muscle atrophy. J Clin Invest. 2017;127:2418–2432. doi: 10.1172/JCI91445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Demonbreun A.R., Fahrenbach J.P., Deveaux K., Earley J.U., Pytel P., McNally E.M. Impaired muscle growth and response to insulin-like growth factor 1 in dysferlin-mediated muscular dystrophy. Hum Mol Genet. 2011;20:779–789. doi: 10.1093/hmg/ddq522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Demonbreun A.R., Rossi A.E., Alvarez M.G., Swanson K.E., Deveaux H.K., Earley J.U., Hadhazy M., Vohra R., Walter G.A., Pytel P., McNally E.M. Dysferlin and myoferlin regulate transverse tubule formation and glycerol sensitivity. Am J Pathol. 2014;184:248–259. doi: 10.1016/j.ajpath.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heydemann A., Huber J.M., Demonbreun A., Hadhazy M., McNally E.M. Genetic background influences muscular dystrophy. Neuromuscul Disord. 2005;15:601–609. doi: 10.1016/j.nmd.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 27.Swaggart K.A., Demonbreun A.R., Vo A.H., Swanson K.E., Kim E.Y., Fahrenbach J.P., Holley-Cuthrell J., Eskin A., Chen Z., Squire K., Heydemann A., Palmer A.A., Nelson S.F., McNally E.M. Annexin A6 modifies muscular dystrophy by mediating sarcolemmal repair. Proc Natl Acad Sci U S A. 2014;111:6004–6009. doi: 10.1073/pnas.1324242111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Demonbreun A.R., Quattrocelli M., Barefield D.Y., Allen M.V., Swanson K.E., McNally E.M. An actin-dependent annexin complex mediates plasma membrane repair in muscle. J Cell Biol. 2016;213:705–718. doi: 10.1083/jcb.201512022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DiFranco M., Quinonez M., Capote J., Vergara J. DNA transfection of mammalian skeletal muscles using in vivo electroporation. J Vis Exp. 2009;32:1520. doi: 10.3791/1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Demonbreun A.R., McNally E.M. DNA electroporation, isolation and imaging of myofibers. J Vis Exp. 2015;106:e53551. doi: 10.3791/53551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kerr J.P., Ziman A.P., Mueller A.L., Muriel J.M., Kleinhans-Welte E., Gumerson J.D., Vogel S.S., Ward C.W., Roche J.A., Bloch R.J. Dysferlin stabilizes stress-induced Ca2+ signaling in the transverse tubule membrane. Proc Natl Acad Sci U S A. 2013;110:20831–20836. doi: 10.1073/pnas.1307960110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sali A., Guerron A.D., Gordish-Dressman H., Spurney C.F., Iantorno M., Hoffman E.P., Nagaraju K. Glucocorticoid-treated mice are an inappropriate positive control for long-term preclinical studies in the mdx mouse. PLoS One. 2012;7:e34204. doi: 10.1371/journal.pone.0034204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quattrocelli M., Swinnen M., Giacomazzi G., Camps J., Barthelemy I., Ceccarelli G., Caluwe E., Grosemans H., Thorrez L., Pelizzo G., Muijtjens M., Verfaillie C.M., Blot S., Janssens S., Sampaolesi M. Mesodermal iPSC-derived progenitor cells functionally regenerate cardiac and skeletal muscle. J Clin Invest. 2015;125:4463–4482. doi: 10.1172/JCI82735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bloemberg D., Quadrilatero J. Rapid determination of myosin heavy chain expression in rat, mouse, and human skeletal muscle using multicolor immunofluorescence analysis. PLoS One. 2012;7:e35273. doi: 10.1371/journal.pone.0035273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heydemann A., Ceco E., Lim J.E., Hadhazy M., Ryder P., Moran J.L., Beier D.R., Palmer A.A., McNally E.M. Latent TGF-beta-binding protein 4 modifies muscular dystrophy in mice. J Clin Invest. 2009;119:3703–3712. doi: 10.1172/JCI39845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agley C.C., Rowlerson A.M., Velloso C.P., Lazarus N.L., Harridge S.D. Isolation and quantitative immunocytochemical characterization of primary myogenic cells and fibroblasts from human skeletal muscle. J Vis Exp. 2015;95:52049. doi: 10.3791/52049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arrighi N., Moratal C., Clement N., Giorgetti-Peraldi S., Peraldi P., Loubat A., Kurzenne J.Y., Dani C., Chopard A., Dechesne C.A. Characterization of adipocytes derived from fibro/adipogenic progenitors resident in human skeletal muscle. Cell Death Dis. 2015;6:e1733. doi: 10.1038/cddis.2015.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Uezumi A., Fukada S., Yamamoto N., Ikemoto-Uezumi M., Nakatani M., Morita M., Yamaguchi A., Yamada H., Nishino I., Hamada Y., Tsuchida K. Identification and characterization of PDGFRalpha+ mesenchymal progenitors in human skeletal muscle. Cell Death Dis. 2014;5:e1186. doi: 10.1038/cddis.2014.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fink T., Zachar V. Adipogenic differentiation of human mesenchymal stem cells. Methods Mol Biol. 2011;698:243–251. doi: 10.1007/978-1-60761-999-4_19. [DOI] [PubMed] [Google Scholar]
- 40.Bansal D., Miyake K., Vogel S.S., Groh S., Chen C.C., Williamson R., McNeil P.L., Campbell K.P. Defective membrane repair in dysferlin-deficient muscular dystrophy. Nature. 2003;423:168–172. doi: 10.1038/nature01573. [DOI] [PubMed] [Google Scholar]
- 41.Liu J., Aoki M., Illa I., Wu C., Fardeau M., Angelini C., Serrano C., Urtizberea J.A., Hentati F., Hamida M.B., Bohlega S., Culper E.J., Amato A.A., Bossie K., Oeltjen J., Bejaoui K., McKenna-Yasek D., Hosler B.A., Schurr E., Arahata K., de Jong P.J., Brown R.H., Jr. Dysferlin, a novel skeletal muscle gene, is mutated in Miyoshi myopathy and limb girdle muscular dystrophy. Nat Genet. 1998;20:31–36. doi: 10.1038/1682. [DOI] [PubMed] [Google Scholar]
- 42.Connolly A.M., Schierbecker J., Renna R., Florence J. High dose weekly oral prednisone improves strength in boys with Duchenne muscular dystrophy. Neuromuscul Disord. 2002;12:917–925. doi: 10.1016/s0960-8966(02)00180-3. [DOI] [PubMed] [Google Scholar]
- 43.Keeling R.M., Golumbek P.T., Streif E.M., Connolly A.M. Weekly oral prednisolone improves survival and strength in male mdx mice. Muscle Nerve. 2007;35:43–48. doi: 10.1002/mus.20646. [DOI] [PubMed] [Google Scholar]
- 44.Escolar D.M., Hache L.P., Clemens P.R., Cnaan A., McDonald C.M., Viswanathan V., Kornberg A.J., Bertorini T.E., Nevo Y., Lotze T., Pestronk A., Ryan M.M., Monasterio E., Day J.W., Zimmerman A., Arrieta A., Henricson E., Mayhew J., Florence J., Hu F., Connolly A.M. Randomized, blinded trial of weekend vs daily prednisone in Duchenne muscular dystrophy. Neurology. 2011;77:444–452. doi: 10.1212/WNL.0b013e318227b164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moretti I., Ciciliot S., Dyar K.A., Abraham R., Murgia M., Agatea L., Akimoto T., Bicciato S., Forcato M., Pierre P., Uhlenhaut N.H., Rigby P.W., Carvajal J.J., Blaauw B., Calabria E., Schiaffino S. MRF4 negatively regulates adult skeletal muscle growth by repressing MEF2 activity. Nat Commun. 2016;7:12397. doi: 10.1038/ncomms12397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang D.Z., Valdez M.R., McAnally J., Richardson J., Olson E.N. The Mef2c gene is a direct transcriptional target of myogenic bHLH and MEF2 proteins during skeletal muscle development. Development. 2001;128:4623–4633. doi: 10.1242/dev.128.22.4623. [DOI] [PubMed] [Google Scholar]
- 47.Davidson Z.E., Ryan M.M., Kornberg A.J., Sinclair K., Cairns A., Walker K.Z., Truby H. Observations of body mass index in Duchenne muscular dystrophy: a longitudinal study. Eur J Clin Nutr. 2014;68:892–897. doi: 10.1038/ejcn.2014.93. [DOI] [PubMed] [Google Scholar]
- 48.Defour A., Medikayala S., Van der Meulen J.H., Hogarth M.W., Holdreith N., Malatras A., Duddy W., Boehler J., Nagaraju K., Jaiswal J.K. Annexin A2 links poor myofiber repair with inflammation and adipogenic replacement of the injured muscle. Hum Mol Genet. 2017;26:1979–1991. doi: 10.1093/hmg/ddx065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grounds M.D., Terrill J.R., Radley-Crabb H.G., Robertson T., Papadimitriou J., Spuler S., Shavlakadze T. Lipid accumulation in dysferlin-deficient muscles. Am J Pathol. 2014;184:1668–1676. doi: 10.1016/j.ajpath.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 50.Muir L.A., Neeley C.K., Meyer K.A., Baker N.A., Brosius A.M., Washabaugh A.R., Varban O.A., Finks J.F., Zamarron B.F., Flesher C.G., Chang J.S., DelProposto J.B., Geletka L., Martinez-Santibanez G., Kaciroti N., Lumeng C.N., O'Rourke R.W. Adipose tissue fibrosis, hypertrophy, and hyperplasia: correlations with diabetes in human obesity. Obesity (Silver Spring) 2016;24:597–605. doi: 10.1002/oby.21377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Godfrey C., Escolar D., Brockington M., Clement E.M., Mein R., Jimenez-Mallebrera C., Torelli S., Feng L., Brown S.C., Sewry C.A., Rutherford M., Shapira Y., Abbs S., Muntoni F. Fukutin gene mutations in steroid-responsive limb girdle muscular dystrophy. Ann Neurol. 2006;60:603–610. doi: 10.1002/ana.21006. [DOI] [PubMed] [Google Scholar]
- 52.Hussein M.R., Hamed S.A., Mostafa M.G., Abu-Dief E.E., Kamel N.F., Kandil M.R. The effects of glucocorticoid therapy on the inflammatory and dendritic cells in muscular dystrophies. Int J Exp Pathol. 2006;87:451–461. doi: 10.1111/j.1365-2613.2006.00470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matthews E., Brassington R., Kuntzer T., Jichi F., Manzur A.Y. Corticosteroids for the treatment of Duchenne muscular dystrophy. Cochrane Database Syst Rev. 2016;5:CD003725. doi: 10.1002/14651858.CD003725.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saxon M.E., Filippov A.K., Porotikov U.I. The possible role of phospholipase A2 in cardiac membrane destabilization under calcium overload conditions. Basic Res Cardiol. 1984;79:668–678. doi: 10.1007/BF01908384. [DOI] [PubMed] [Google Scholar]
- 55.Gerritsen M.E., Schwarz S.M., Medow M.S. Glucocorticoid-mediated alterations in fluidity of rabbit cardiac muscle microvessel endothelial cell membranes: influences on eicosanoid release. Biochim Biophys Acta. 1991;1065:63–68. doi: 10.1016/0005-2736(91)90011-v. [DOI] [PubMed] [Google Scholar]
- 56.Accornero F., Kanisicak O., Tjondrokoesoemo A., Attia A.C., McNally E.M., Molkentin J.D. Myofiber-specific inhibition of TGFbeta signaling protects skeletal muscle from injury and dystrophic disease in mice. Hum Mol Genet. 2014;23:6903–6915. doi: 10.1093/hmg/ddu413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Quattrocelli M., Spencer M.J., McNally E.M. Outside in: the matrix as a modifier of muscular dystrophy. Biochim Biophys Acta. 2017;1864:572–579. doi: 10.1016/j.bbamcr.2016.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee Y.S., Lehar A., Sebald S., Liu M., Swaggart K.A., Talbot C.C., Jr., Pytel P., Barton E.R., McNally E.M., Lee S.J. Muscle hypertrophy induced by myostatin inhibition accelerates degeneration in dysferlinopathy. Hum Mol Genet. 2015;24:5711–5719. doi: 10.1093/hmg/ddv288. [DOI] [PMC free article] [PubMed] [Google Scholar]