Abstract
The 7-nitro-2,1,3-nitrobenzoxadiazole (NBD) derivatives are a series of compounds containing the NBD scaffold that are not glutathione (GSH) peptidomimetics, and result in a strong inhibition of glutathione S-transferases (GSTs). Growing evidences highlight their pivotal roles and outstanding anticancer activity in different tumor models. In particular, 6-(7-nitro-2,1,3-benzoxadiazol-4-ylthio) hexanol (NBDHEX) is extensively studied, which is a very efficient inhibitor of GSTP1-1. It triggers apoptosis in several tumor cell lines and this cytotoxic activity is observed at micro and submicromolar concentrations. Importantly, studies have shown that NBDHEX acts as an anticancer drug by inhibiting GSTs catalytic activity, avoiding inconvenience of the inhibitor extrusion from the cell by specific pumps and disrupting the interaction between the GSTP1-1 and key signaling effectors. Additionally, some researchers also have discovered that NBDHEX can act as late-phase autophagy inhibitor, which opens new opportunities to fully exploit its therapeutic potential. In this review, we summarize the advantages, anticancer mechanisms, and analogs of this compound, which will establish the basis on the usage of NBDHEX in clinical applications in future.
Keywords: anticancer; GSTP1-1 (Glutathione S-transferase Pi); JNK (c-Jun N-Terminal Kinase); NBDHEX (6- (7-nitro-2,1,3-benzoxadiazol-4-ylthio) hexanol)
Introduction
The 7-nitro-2,1,3-benzoxadiazole (NBD) derivatives are a class of non-glutathione (GSH) peptidomimetic compounds, which are synthesized and characterized as very efficient inhibitors of glutathione S-transferases (GSTs), a family of enzymes involved in xenobiotic detoxification, catalyzing the conjugation of GSH with carcinogens, drugs, toxins, as well as products of oxidative stress [1–5]. The dominant member of GSTs is the GSTP1-1 isoenzyme, that is frequently overexpressed in tumor cells and protects them from apoptosis [1,6,7]. More and more evidence have suggested that NBD derivatives exhibit a remarkable cytotoxicity in several cancer cells at low concentrations, and exert significant therapeutic activity in vivo [8–12]. Outstandingly, no treatment-related signs of toxicity are observed in in vivo studies on tumor types xenografted in mice [10,11,13]. Amongst the abundant NBD derivatives, 6-(7-nitro-2,1,3-benzoxadiazol-4-ylthio) hexanol (NBDHEX) [2] (Figure 1) has recently emerged as a considerable anticancer compound in multiple malignancies, including leukemia, osteosarcoma, Ewing’s sarcoma, melanoma, rhabdomyosarcoma, mesothelioma, and small cell lung cancer (SCLC), either alone or in combination with antitumor drugs such as cisplatin, doxorubicin, methotrexate, vincristine, and temozolomide [8,10,13–18]. Specifically, NBDHEX inhibits GST’s catalytic activity and is not a substrate of export pumps [9,15,16]. Additionally, it shows activities against cancer cells through disrupting the interaction between the GSTP1-1 and key signaling effectors, which are crucial factors for apoptosis and cell cycle [16,19]. Besides, its ability to weaken the capacity of tumor cells to endure stress conditions via autophagy is recognized [20]. Moreover, in vivo studies demonstrate that NBDHEX is effective in reducing both cancer growth and metastatic spread and is well tolerated on various tumor type xenografts in mice [10,13,17]. All these indicate that NBDHEX can display anticancer functions in different cancers.
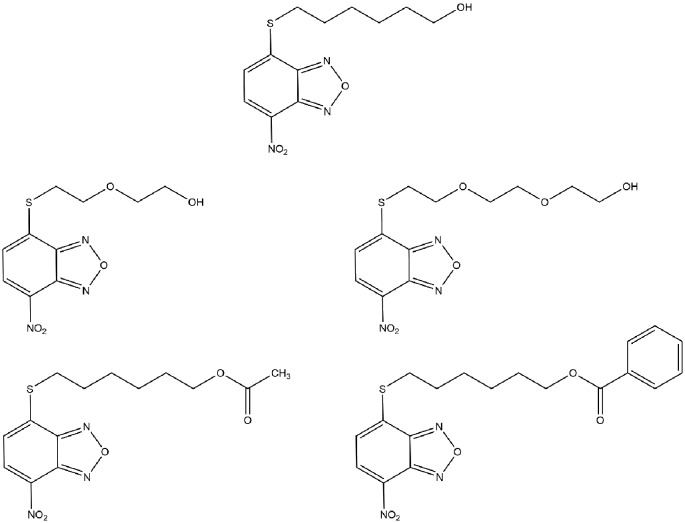
Figure 1. Structures of NBDHEX and its analogs.
Here, we will focus on advantages and mechanisms which are associated with the functions of NBDHEX in the cancer progression and treatment. Consequently, given the encouraging results obtained with NBDHEX, we believe that it will become a new potent antitumor agent.
Advantages of NBDHEX
NBDHEX is a representative molecule of NBD derivatives, a new class of strong and selective inhibitors of GSTs [2]. GST isoenzymes such as GSTP1-1 are overexpressed in many cancer cell lines and can induce drug resistance [6,7,21,22]. Therefore, docking studies aim to design efficient compounds which may modulate their biological activity [2,3,23–25]. However, compounds including ethacrynic acid and GSH derivatives lack class specificity, have scarce affinity, and are often actively extruded from the cell by specific export pumps [3,23,24]. Fortunately, NBDHEX has been shown to overcome these limits, to inhibit GST isoforms at micromolar or submicromolar amounts and to induce cell death in several tumor cell lines [8,10,13]. Although it is not a GSH peptidomimetic compound which has strong specificity of the transferase GSH-binding site (G-site), NBDHEX was conjugated with GSH leading to a stable σ complex in the hydrophobic portion (H-site) of GSTs at the C-4 of the benzoxadiazole ring. H-site is a hydrophobic cavity and as GSTs interact with different hydrophobic toxic species, the H-site normally displays moderate affinity for these compounds [25]. NBDHEX binds efficiently to GSTs and displays lipophilic properties suitable for crossing the plasma membrane [2]. It induces apoptosis and suppresses survival pathways either alone or in combination with conventional anticancer drugs [10,14,15,19] (Table 1). Noteworthy, drug–drug interactions’ analysis considered the combination of NBDHEX with the novel drugs produced synergistic or addictive effects in a lot of cancer cell lines [8,13,18] (Table 2).
Table 1. The involvement of NBDHEX in biology process in different cancers.
| Cancer type | References | In vitro or in vivo | Effect |
|---|---|---|---|
| Acute T-lymphoblastoid leukemia | [14,15] | In vitro | Apoptosis |
| Chronic myeloid leukemia | [2,14] | In vitro | Apoptosis |
| Acute myeloid leukemia | [9] | In vitro | Apoptosis and necrosis |
| SCLC | [2,16] | In vitro | Apoptosis and necrosis |
| Hepatic carcinoma | [14] | In vitro | Apoptosis |
| Osteosarcoma | [8] | In vitro | Apoptosis |
| [13] | In vitro | Proliferation blockage | |
| In vivo | Against metastatization | ||
| [19] | In vitro | Apoptosis and cell cycle arrest | |
| [20] | In vitro | Autophagy inhibition | |
| Ewing’s sarcoma | [13] | In vitro | Cell cycle retardation |
| In vivo | Cytostatic effects | ||
| [11] | In vitro | Antiproliferation | |
| In vivo | Tumor growth inhibition | ||
| Rhabdomyosarcoma | [13] | In vitro | Proliferation blockage |
| Melanoma | [10] | In vitro | Apoptosis and cell cycle arrest |
| In vivo | Apoptosis and antiproliferation | ||
| [17] | In vitro | Apoptosis | |
| In vivo | Tumor growth inhibition | ||
| [12] | In vitro | Apoptosis and antiproliferation | |
| Mesothelioma | [18] | In vitro | Apoptosis |
Table 2. Effects of the in vitro administration of NBDHEX and conventional anticancer drugs in different cell lines.
| Cancer type | Reference | Drug | Treatment schedule | Drug–drug interactions |
|---|---|---|---|---|
| Osteosarcoma | [8] | CDDP | NBDHEX + CDDP | Mostly add |
| NBDHEX → CDDP | Add and syn | |||
| CDDP → NBDHEX | Add | |||
| [13] | DX | NBDHEX + DX | Mostly syn | |
| NBDHEX → DX | Mostly syn | |||
| DX→ NBDHEX | Mostly syn | |||
| MTX | NBDHEX + MTX | Ant | ||
| NBDHEX → MTX | Ant and syn | |||
| MTX→ NBDHEX | Mostly ant | |||
| Ewing’s sarcoma | [13] | DX | NBDHEX + DX | Ant |
| NBDHEX → DX | Mostly syn | |||
| DX→ NBDHEX | Syn | |||
| VCR | NBDHEX + DX | Add, syn, and ant | ||
| NBDHEX → DX | Mostly syn | |||
| DX→ NBDHEX | Syn | |||
| [11] | ETO | NBDHEX + ETO | Syn | |
| Rhabdomyosarcoma | [13] | DX | NBDHEX + DX | Add |
| VCR | NBDHEX + DX | Syn | ||
| Melanoma | [17] | TMZ | NBDHEX + TMZ | Syn |
| Mesothelioma | [18] | CDDP | NBDHEX + CDDP | Syn |
| NBDHEX → CDDP | Add and syn |
Abbreviations: Add, additive (0.90 ≤ combination index ≤ 1.10); ant, antagonistic (combination index > 1.10); CDDP, cisplatin; DX, doxorubicin; ETO, etoposide; MTX, methotrexate; Syn, synergistic (combination index < 0.90); TMZ, temozolomide; VCR, vincristine.
Inhibiting GSTs’ catalytic activity
The most widely investigated function of GSTs is the conjugation reaction of several electrophilic compounds, including endogenous and xenobiotic compounds to reduced GSH. Many anticancer drugs are substrates for the GST and thus their conjugation with the GSH can be catalyzed efficiently and extruded from the cell by specific export pumps [26–28]. It is still widely accepted that the detoxifying activity of GSTs exerts a significant part in drug resistance in some tumor cell types via the activation of the GST/GSH cellular system [26,29]. Interestingly, in some cancer cell lines, the enhanced GSTP1 enzymatic activity is very often associated with an increase in GSTP1 gene expression, increased GSTP1-1 protein level, or both of them. Additionally, the increase in both intracellular levels and enzymatic activity of GSTP1-1 seems to be closely related with the degree of cisplatin resistance [8]. According to these evidences, GSTP1-1 emerges as a potential drug target and NBDHEX acts as strong inhibitors of GST catalytic activity [2,13,17]. However, it needs more evidence to show that NBDHEX can hinder the GST-mediated conjugation of electrophilic anticancer drugs to GSH, and thus may increase intracellular accumulation of the drugs.
Disrupting the interaction between the GSTP1-1 and key signaling effectors
TRAF2 is one of the most ubiquitously expressed TNF receptor-associated factors, a family of proteins interact with a wide range of TNF receptor superfamily members. It directly or indirectly mediates the signal transduction of the receptors involved in the regulation of various cellular responses [30–33]. Of note, it is required for the activation of the apoptosis signal-regulating kinase (ASK1), a mitogen-activated protein kinase kinase kinase (MAP3K). ASK1 can activate both mitogen-activated protein kinase kinase (MKK)4/7–C-jun NH2-terminal kinase (JNK) and MKK3/4/6–p38 signaling pathways [34,35]. De Luca et al. showed experimental evidence that clarified the interaction between GSTP1-1 and TRAF2 and demonstrated the ability of NBDHEX to dissociate the GSTP1-1–TRAF2 complex, further increased the activation of JNK [36]. They also suggested that, in human cisplatin-sensitive and -resistant osteosarcoma cells, GSTP1-1 was able to interfere with the mitogen-activated protein kinase (MAPK) pathway not only at the TRAF2 level, but also at the JNK level [19]. JNK is serine/threonine protein kinase which is at the end of the MAPKs signaling pathway. Cellular processes such as cell growth and apoptosis are closely related to the activation of JNK phosphorylation [37,38]. GSTs can act as JNK regulators through direct association with the JNK, resulting in inhibition of JNK-mediated c-Jun phosphorylation [39,40];
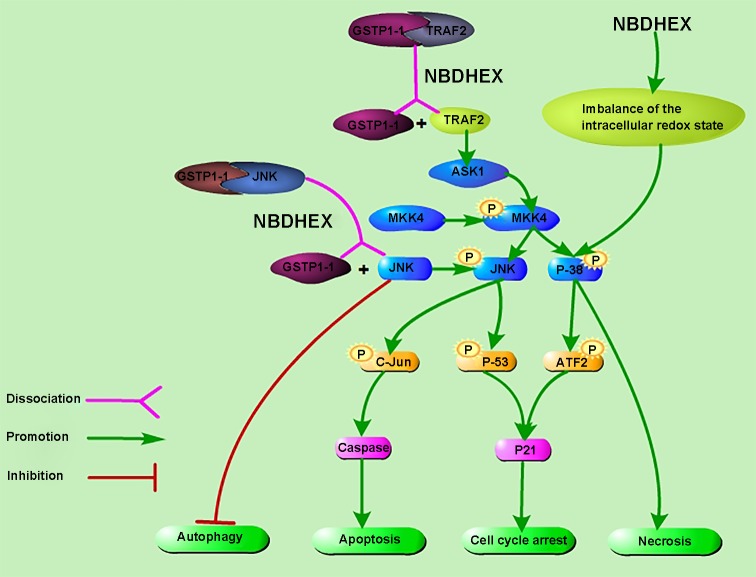
Plenty of studies found that NBDHEX triggered the release of GSTP1-1 from the GSTP1-1–TRAF2 or GSTP1-1–JNK complex, so that it activated the JNK-mediated pathway [15,17,36] (Figure 2). It was reported that in CCRF-CEM and K562 cell lines, the dissociation of the JNK–GSTP1-1 complex was induced by the interaction between NBDHEX and the JNK-linked GSTP1-1and it remained the main pathway of the NBDHEX-triggered apoptosis [14,15]. Also, in melanoma cell lines, NBDHEX activated the JNK pathway through a selective GSTP1-1 targetting and induced apoptosis and cell cycle arrest via the phospho-activation of JNK and p38 and their downstream targets c-Jun, ATF2, and p53 [10,12,17]. Luca et al. [39] showed that GSTP1-1 was unable to inhibit JNK when the active site of the enzyme was occupied by the σ-complex between GSH and NBDHEX. Similar results were obtained in SCLC and mesothelioma cells that NBDHEX activated the JNK signaling pathway [16,18]. Furthermore, NBDHEX promoted a caspase-dependent apoptosis which was unusual in the P-glycoprotein (P-gp) overexpressing cells, and the apoptotic pathway was a direct consequence of dissociation of GSTP1-1from the complex with JNK [15]. It is worth noting that the possibility that NBDHEX directly activated p38 through the imbalance of the intracellular redox state cannot be excluded, in which case, cells died by necrosis (Figure 2). All these results showed that this compound proved to be a promising new strategy by activating vital pathway.
Figure 2. Proposed action mechanism of NBDHEX.
NBDHEX causes the dissociation of both the GSTP1-1–TRAF2 and the GSTP1-1–JNK complexes and triggers the activation of the MAPK signaling pathway. It induces apoptosis and cell cycle arrest via the phospho-activation of JNK and p38 and their downstream targets including c-Jun, ATF2, and p53. Besides, JNK can participate in impairing autophagy. In addition, the possibility that NBDHEX directly activates p38 through the imbalance of the intracellular redox state cannot be excluded, in which case, cells died by necrosis.
Overcoming MDR-mediated cancer cell chemoresistance
Multidrug resistance (MDR), a common phenomenon, is a complex clinical problem in oncology and limits the therapeutic effect of anticancer drugs [41,42]. ATP-binding cassette (ABC) efflux pumps including MDR1–P-gp and the MDR protein 1 (MRP1) are one of the well-known MDR mechanisms [43–46]. Both of them are associated with poor treatment outcomes in various kinds of cancers and contribute to the chemoresistance to doxorubicin, vinblastine, etoposide, as well as some other anticancer drugs [47–50]. Unlike most agents, NBDHEX is not a substrate of these export pumps, so that it accumulates in tumor cells and overcomes chemoresistance in cells which are in the presence of ABC transporters conferring the MDR phenotype [9,15,16]. Turella et al. demonstrated that NBDHEX efficiently killed human acute T lymphoblastoid leukemia cell, human osteosarcoma cell, and their selected P-gp variants [15]. Interestingly, their P-gp variants appeared to be more efficiently committed to death by NBDHEX, compared with the parental cell line [15]. Furthermore, they reported that adriamycin-selected, multidrug-resistant human SCLC cell overexpressing MRP1 transporter did not counteract the cytotoxicity of NBDHEX. Actually, NBDHEX induced apoptosis which might be promoted by the very low level of Bcl-2, an antiapoptotic protein found in the multidrug-resistant SCLC cell. Moreover, the decrease in Bcl-2 appeared to be linked to the MDR phenotype [16]. It was also clarified that NBDHEX efflux could not be mediated by either MDR1–P-gp or MRP1 in MDR variants of human acute myeloid leukemia cell [9]. All these findings indicate that NBDHEX may be a very effective compound in killing tumor cells characterized by high levels of MDR1–P-gp or MRP1.
Inhibiting JNK-mediated last-phase autophagy
The 2016 Nobel Prize in Physiology or Medicine was awarded to the researcher for his discovery of the autophagy machinery [51]. Autophagy is an intracellular catabolic pathway by which cellular macromolecules and dysregulated organelles are engulfed by autophagosome vacuoles, leading to their degradation and breakdown after fusion with autolysosomes [52–55]. Autophagy plays a major role in the progression of established neoplasms by promoting the survival of transformed cells under stress conditions with inadequate supply of oxygen, nutrients, and growth factors [55,56]. Cancer cells live under such microenvironment so that they are predicted to be more susceptible to the suppression of autophagy and tend to activate autophagy constitutively via metabolic reprogramming [54,55,57–60]. Notably, recent investigations indicate constitutive activation of autophagy in tumors is a challenge because it can lead to cancer drug resistance and refractory cancer [61–63]. Such evidences enable us to develop new treatments that inhibit protective autophagy. NBDHEX, acts as autophagy inhibitor, has been reported to impair autophagosome clearance through increasing both LC3-II and the autophagy selective substrate p62. Furthermore, the results were observed in a panel of tumor cell lines of different origins, suggesting that the effects of NBDHEX on autophagy inhibition are general rather than cell type-specific [20]. Interestingly, they also provided evidence that JNK activity was required for autophagy impairment by NBDHEX [20] (Figure 2). In fact, it has been demonstrated previously that suppression of JNK signaling could induce autophagy [64]. By contrast, it could promote autophagy in response to different types of stress signals as well [65,66]. Therefore, improving our understanding of the mechanisms and relationships between NBDHEX, JNK signaling and autophagy may be a significant topic in cancer research.
Analogs of NBDHEX
However, NBDHEX suffers from relatively low target selectivity because of its high affinity toward GSTM2-2, which is widely expressed in many non-cancerous tissues [2,67]. Also, its poor water solubility limits its oral bioavailability. These findings pointed out the need to search for novel NBDHEX analogs with an improved pharmacological profile. Rotili et al. [68] described a series of 40 NBDHEX analogs bearing phenyl-containing moieties as well as substituted alkyl chains, which replaced the hydroxyhexyl portion at the C4-sulphur atom. Most of the new compounds displayed increased water solubility and higher GSTP1-1 selectivity. Nevertheless, amongst these compounds, some alkyl derivatives possessed cytotoxicity comparable or higher than NBDHEX while the presence of a phenyl ring with polar substituents might result in low cytotoxicity in osteosarcoma U-2OS cells [68]. The lower cytotoxicity might be the consequence of their higher propensity to spontaneously react with the abundant intracellular nucleophile GSH, which was another critical aspect of NBD derivatives. As a further development of their studies, the research team then reported two additional NBDHEX analogs, MC3165 and MC3181 (Figure 1) [69], both of which were designed with the aim of increasing the hydrophilicity and to avoid any significant drop of cytotoxic potency. MC3165 is characterized by bearing one oxygen atom within the hydroxy-containing alkyl chain at the C4 position of the NBD scaffold while MC3181 is characterized by the presence of two. As a result, both the compounds improved hydrophilicity compared with NBDHEX while minimizing the changes into the NBD nucleus and MC3181 indicated more promise in consideration of the water solubility, selectivity toward GSTP1-1, and spontaneous reactivity with GSH [69]. In addition, MC3181 displayed a greater selectivity toward GSTP1-1, high cytotoxicity toward osteosarcoma cells, as well as a panel of different human melanoma cell lines, and exhibited a remarkable therapeutic activity against BRAF-V600E-mutant xenografts [12,20,69]. Similar to NBDHEX, no treatment-related toxicity was observed in xenograft models [12,69]. More recently, two other analogs were designed and synthesized, namely MC2753 and MC2752, the benzoic acid ester and the acetic acid ester of NBDHEX, respectively (Figure 1) [70]. MC2752 did not demonstrate the superiority but it is noteworthy that the presence of a hydrophobic moiety in the side chain strongly affects the mode of interaction between MC2753 with the GSTP1-1, and it did not require GSH to trigger the dissociation of the complex between GSTP1-1 and TRAF2. Therefore, MC2753 would not be affected in its anticancer action by fluctuations of GSH levels [70]. However, MC2753 inhibited only 50% of the enzyme activity and showed lower aqueous solubility compared with NBDHEX, so it may only serve as a lead compound for the development of GSTP1-1 inhibitors not affected by GSH levels. All these results lay the basis for future studies with these analogs of NBDHEX.
Conclusion and prospects
In this review, we focussed on the pleiotropic roles of NBDHEX in different kinds of cancers. NBDHEX inhibits GSTs catalytic activity, overcomes the MRP1 or P-gp-mediated efflux and disrupts the interaction between the GSTP1-1 and key signaling effectors. Consequently, it induces cell cycle arrest and apoptosis and inhibits autophagy, alone or in combination with novel anticancer drugs. Additionally, NBDHEX analogs are endowed with higher water solubility and increased GSTP1-1 selectivity. Some of them possess cytotoxicity comparable or higher than NBDHEX. Collectively, we suggest that NBDHEX and its analogs may represent a new therapeutic opportunity and open interesting perspectives for cancer therapy in future.
Abbreviations
- ABC
ATP-binding cassette
- ASK1
apoptosis signal-regulating kinase
- GSH
glutathione
- GST
glutathione S-transferase
- JNK
C-jun NH2-terminal kinase
- MAPK
mitogen-activated protein kinase
- MDR
multidrug resistance
- MKK
mitogen-activated protein kinase kinase
- MRP1
MDR protein 1
- NBD
nitrobenzoxadiazole
- NBDHEX
6-(7-nitro-2,1,3-benzoxadiazol-4-ylthio) hexanol
- P-gp
P-glycoprotein
- SCLC
small cell lung cancer
- TRAF2
TNF receptor-associated factor 2
Funding
This work was supported by the Jiangsu Provincial Medical Youth Talent [grant number QNRC2016653]; the National Nature Science Foundation of Jiangsu Provience [grant number BK20151016]; the National Natural Science Foundation of China [grant number 81602441]; and the Jiangsu Provincial Key Research Development Program [grant number BE2016794].
Competing interests
The authors declare that there are no competing interests associated with the manuscript.
References
- 1.Tew K.D., et al. (1996) Glutathione-associated enzymes in the human cell lines of the National Cancer Institute Drug Screening Program. Mol. Pharmacol. 50, 149–159 [PubMed] [Google Scholar]
- 2.Ricci G., et al. (2005) 7-Nitro-2,1,3-benzoxadiazole derivatives, a new class of suicide inhibitors for glutathione S-transferases. Mechanism of action of potential anticancer drugs. J. Biol. Chem. 280, 26397–405 10.1074/jbc.M503295200 [DOI] [PubMed] [Google Scholar]
- 3.Tew K.D., Dutta S. and Schultz M. (1997) Inhibitors of glutathione S-transferases as therapeutic agents. Adv. Drug. Deliv. Rev. 26, 91–104 10.1016/S0169-409X(97)00029-X [DOI] [PubMed] [Google Scholar]
- 4.Townsend D. and Tew K. (2003) Cancer drugs, genetic variation and the glutathione-S-transferase gene family. Am. J. Pharmacogenomics 3, 157–172 10.2165/00129785-200303030-00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lalle M., et al. (2015) The FAD-dependent glycerol-3-phosphate dehydrogenase of Giardia duodenalis: an unconventional enzyme that interacts with the g14-3-3 and it is a target of the antitumoral compound NBDHEX. Front. Microbiol. 6, 544 10.3389/fmicb.2015.00544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruiz-Gomez M.J., et al. (2000) P-glycoprotein, glutathione S-transferase increase in a colon carcinoma cell line by colchicine. J. Physiol. Biochem. 56, 307–312 10.1007/BF03179798 [DOI] [PubMed] [Google Scholar]
- 7.Inoue T., et al. (1995) Glutathione S transferase Pi is a powerful indicator in chemotherapy of human lung squamous-cell carcinoma. Respiration 62, 223–227 10.1159/000196451 [DOI] [PubMed] [Google Scholar]
- 8.Pasello M., et al. (2008) Overcoming glutathione S-transferase P1-related cisplatin resistance in osteosarcoma. Cancer Res. 68, 6661–6668 10.1158/0008-5472.CAN-07-5840 [DOI] [PubMed] [Google Scholar]
- 9.Ascione A., et al. (2009) The glutathione S-transferase inhibitor 6-(7-nitro-2,1,3-benzoxadiazol-4-ylthio)hexanol overcomes the MDR1-P-glycoprotein and MRP1-mediated multidrug resistance in acute myeloid leukemia cells. Cancer Chemother. Pharmacol. 64, 419–424 10.1007/s00280-009-0960-6 [DOI] [PubMed] [Google Scholar]
- 10.Pellizzari Tregno F., et al. (2009) In vitro in vivo efficacy of 6-(7-nitro-2,1,3-benzoxadiazol-4-ylthio)hexanol (NBDHEX) on human melanoma. Eur. J. Cancer 45, 2606–2617 10.1016/j.ejca.2009.06.033 [DOI] [PubMed] [Google Scholar]
- 11.Zhuo R., et al. (2014) Targeting glutathione S-transferase M4 in Ewing sarcoma. Front. Pediatr. 2, 83 10.3389/fped.2014.00083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graziani G., et al. (2015) A new water soluble MAPK activator exerts antitumor activity in melanoma cells resistant to the BRAF inhibitor vemurafenib. Biochem. Pharmacol. 95, 16–27 10.1016/j.bcp.2015.03.004 [DOI] [PubMed] [Google Scholar]
- 13.Pasello M., et al. (2011) Targeting glutathione-S transferase enzymes in musculoskeletal sarcomas: a promising therapeutic strategy. Anal. Cell Pathol. (Amst.) 34, 131–145 10.1155/2011/414985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turella P., et al. (2005) Proapoptotic activity of new glutathione S-transferase inhibitors. Cancer Res. 65, 3751–3761 10.1158/0008-5472.CAN-04-3903 [DOI] [PubMed] [Google Scholar]
- 15.Turella P., et al. (2006) A strong glutathione S-transferase inhibitor overcomes the P-glycoprotein-mediated resistance in tumor cells. 6-(7-Nitro-2,1,3-benzoxadiazol-4-ylthio)hexanol (NBDHEX) triggers a caspase-dependent apoptosis in MDR1-expressing leukemia cells. J. Biol. Chem. 281, 23725–23732 10.1074/jbc.M604372200 [DOI] [PubMed] [Google Scholar]
- 16.Filomeni G., et al. (2008) 6-(7-Nitro-2,1,3-benzoxadiazol-4-ylthio)hexanol, a specific glutathione S-transferase inhibitor, overcomes the multidrug resistance (MDR)-associated protein 1-mediated MDR in small cell lung cancer. Mol. Cancer Ther. 7, 371–379 10.1158/1535-7163.MCT-07-0487 [DOI] [PubMed] [Google Scholar]
- 17.Tentori L., et al. (2011) The glutathione transferase inhibitor 6-(7-nitro-2,1,3-benzoxadiazol-4-ylthio)hexanol (NBDHEX) increases temozolomide efficacy against malignant melanoma. Eur. J. Cancer 47, 1219–1230 10.1016/j.ejca.2010.12.008 [DOI] [PubMed] [Google Scholar]
- 18.De Luca A., et al. (2013) Glutathione S-transferase P1-1 as a target for mesothelioma treatment. Cancer Sci. 104, 223–230 10.1111/cas.12061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sau A., et al. (2012) Targeting GSTP1-1 induces JNK activationleads to apoptosis in cisplatin-sensitive and -resistant human osteosarcoma cell lines. Mol. Biosyst. 8, 994–1006 10.1039/C1MB05295K [DOI] [PubMed] [Google Scholar]
- 20.Palumbo C., et al. (2016) c-Jun N-terminal kinase activation by nitrobenzoxadiazoles leads to late-stage autophagy inhibition. J. Transl. Med. 14, 37 10.1186/s12967-016-0796-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okuyama T., et al. (1994) Expression of glutathione S-transferase-pisensitivity of human gastric cancer cells to cisplatin. Cancer 74, 1230–1236 10.1002/1097-0142(19940815)74:4%3c1230::AID-CNCR2820740409%3e3.0.CO;2-0 [DOI] [PubMed] [Google Scholar]
- 22.Huang G., Mills L. and Worth L.L. (2007) Expression of human glutathione S-transferase P1 mediates the chemosensitivity of osteosarcoma cells. Mol. Cancer Ther. 6, 1610–1619 10.1158/1535-7163.MCT-06-0580 [DOI] [PubMed] [Google Scholar]
- 23.Zaman G.J., et al. (1996) Transport of the glutathione conjugate of ethacrynic acid by the human multidrug resistance protein MRP. FEBS Lett. 391, 126–130 10.1016/0014-5793(96)00718-1 [DOI] [PubMed] [Google Scholar]
- 24.Lyttle M.H., et al. (1994) Isozyme-specific glutathione-S-transferase inhibitors: design and synthesis. J. Med. Chem. 37, 189–194 10.1021/jm00027a024 [DOI] [PubMed] [Google Scholar]
- 25.Federici L., et al. (2009) Structural basis for the binding of the anticancer compound 6-(7-nitro-2,1,3-benzoxadiazol-4-ylthio)hexanol to human glutathione s-transferases. Cancer Res. 69, 8025–8034 10.1158/0008-5472.CAN-09-1314 [DOI] [PubMed] [Google Scholar]
- 26.Ruzza P., et al. (2009) Glutathione transferases as targets for cancer therapy. Anticancer Agents Med. Chem. 9, 763–777 10.2174/187152009789056895 [DOI] [PubMed] [Google Scholar]
- 27.Tew K.D. and Townsend D.M. (2012) Glutathione-s-transferases as determinants of cell survival and death. Antioxid. Redox Signal. 17, 1728–1737 10.1089/ars.2012.4640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh S. (2015) Cytoprotectiveregulatory functions of glutathione S-transferases in cancer cell proliferation and cell death. Cancer Chemother. Pharmacol. 75, 1–15 10.1007/s00280-014-2566-x [DOI] [PubMed] [Google Scholar]
- 29.Sau A., et al. (2010) Glutathione transferasesdevelopment of new principles to overcome drug resistance. Arch. Biochem. Biophys. 500, 116–122 10.1016/j.abb.2010.05.012 [DOI] [PubMed] [Google Scholar]
- 30.Bradley J.R. and Pober J.S. (2001) Tumor necrosis factor receptor-associated factors (TRAFs). Oncogene 20, 6482–6491 10.1038/sj.onc.1204788 [DOI] [PubMed] [Google Scholar]
- 31.Chung J.Y., et al. (2002) All TRAFs are not created equal: common and distinct molecular mechanisms of TRAF-mediated signal transduction. J. Cell Sci. 115, 679–688 [DOI] [PubMed] [Google Scholar]
- 32.Xie P. (2013) TRAF molecules in cell signalingin human diseases. J. Mol. Signal. 8, 10.1186/1750-2187-8-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Locksley R.M., Killeen N. and Lenardo M.J. (2001) The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell 104, 487–501 10.1016/S0092-8674(01)00237-9 [DOI] [PubMed] [Google Scholar]
- 34.Ichijo H., et al. (1997) Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNKp38 signaling pathways. Science 275, 90–94 10.1126/science.275.5296.90 [DOI] [PubMed] [Google Scholar]
- 35.Wu Y., et al. (2006) Human glutathione S-transferase P1-1 interacts with TRAF2regulates TRAF2-ASK1 signals. Oncogene 25, 5787–5800 10.1038/sj.onc.1209576 [DOI] [PubMed] [Google Scholar]
- 36.De Luca A., et al. (2014) The fine-tuning of TRAF2-GSTP1-1 interaction: effect of ligand binding and in situ detection of the complex. Cell Death Dis. 5, e1015 10.1038/cddis.2013.529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barr R.K. and Bogoyevitch M.A. (2001) The c-Jun N-terminal protein kinase family of mitogen-activated protein kinases (JNK MAPKs). Int. J. Biochem. Cell Biol. 33, 1047–1063 10.1016/S1357-2725(01)00093-0 [DOI] [PubMed] [Google Scholar]
- 38.Seger R. and Krebs E.G. (1995) The MAPK signaling cascade. FASEB J. 9, 726–735 10.1096/fasebj.9.9.7601337 [DOI] [PubMed] [Google Scholar]
- 39.De Luca A., et al. (2012) New insights into the mechanism of JNK1 inhibition by glutathione transferase P1-1. Biochemistry 51, 7304–7312 10.1021/bi300559m [DOI] [PubMed] [Google Scholar]
- 40.Adler V., et al. (1999) Regulation of JNK signaling by GSTp. EMBO J. 18, 1321–1334, 10.1093/emboj/18.5.1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gottesman M.M., Fojo T. and Bates S.E. (2002) Multidrug resistance in cancer: role of ATP-dependent transporters. Nat. Rev. Cancer 2, 48–58 10.1038/nrc706 [DOI] [PubMed] [Google Scholar]
- 42.Ling V. (1997) Multidrug resistance: molecular mechanisms and clinical relevance. Cancer Chemother. Pharmacol., (40 Suppl.), S3–S8 10.1007/s002800051053 [DOI] [PubMed] [Google Scholar]
- 43.Borst P. (1997) Multidrug resistant proteins. Semin. Cancer Biol. 8, 131–134 10.1006/scbi.1997.0072 [DOI] [PubMed] [Google Scholar]
- 44.Higgins C.F. (1992) ABC transporters: from microorganisms to man. Annu. Rev. Cell Biol. 8, 67–113 10.1146/annurev.cb.08.110192.000435 [DOI] [PubMed] [Google Scholar]
- 45.Gottesman M.M., Pastan I. and Ambudkar S.V. (1996) P-glycoprotein and multidrug resistance. Curr. Opin. Genet. Dev. 6, 610–617 10.1016/S0959-437X(96)80091-8 [DOI] [PubMed] [Google Scholar]
- 46.Cole S.P. and Deeley R.G. (1998) Multidrug resistance mediated by the ATP-binding cassette transporter protein MRP. Bioessays 20, 931–940 10.1002/(SICI)1521-1878(199811)20:11%3c931::AID-BIES8%3e3.0.CO;2-J [DOI] [PubMed] [Google Scholar]
- 47.van der Kolk D.M., et al. (2002) The role of drug efflux pumps in acute myeloid leukemia. Leuk. Lymphoma 43, 685–701 10.1080/10428190290016773 [DOI] [PubMed] [Google Scholar]
- 48.David-Beabes G.L., et al. (2000) Doxorubicin-resistant variants of human prostate cancer cell lines DU 145, PC-3, PPC-1,TSU-PR1: characterization of biochemical determinants of antineoplastic drug sensitivity. Int. J. Oncol. 17, 1077–1086 [DOI] [PubMed] [Google Scholar]
- 49.Rappa G., et al. (1997) Overexpression of the multidrug resistance genes mdr1, mdr3, mrp in L1210 leukemia cells resistant to inhibitors of ribonucleotide reductase. Biochem. Pharmacol. 54, 649–655 10.1016/S0006-2952(97)00210-4 [DOI] [PubMed] [Google Scholar]
- 50.Gosland M.P., et al. (1996) Reversal of doxorubicin, etoposide, vinblastine, taxol resistance in multidrug resistant human sarcoma cells by a polymer of spermine. Cancer Chemother. Pharmacol. 37, 593–600 10.1007/s002800050434 [DOI] [PubMed] [Google Scholar]
- 51.Van Noorden R. and Ledford H. (2016) Medicine Nobel for research on how cells ‘eat themselves’. Nature 538, 18–19 10.1038/nature.2016.20721 [DOI] [PubMed] [Google Scholar]
- 52.Glick D., Barth S. and Macleod K.F. (2010) Autophagy: cellular and molecular mechanisms. J. Pathol. 221, 3–12 10.1002/path.2697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Das G., Shravage B.V. and Baehrecke E.H. (2012) Regulation and function of autophagy during cell survival and cell death. Cold Spring Harb. Perspect. Biol. 4, 10.1101/cshperspect.a008813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Galluzzi L., et al. (2015) Autophagy in malignant transformation cancer progression. EMBO J. 34, 856–880 10.15252/embj.201490784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.White E. (2015) The role for autophagy in cancer. J. Clin. Invest. 125, 42–46 10.1172/JCI73941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Panda P.K., et al. (2015) Mechanism of autophagic regulation in carcinogenesis cancer therapeutics. Semin. Cell Dev. Biol. 39, 43–55 10.1016/j.semcdb.2015.02.013 [DOI] [PubMed] [Google Scholar]
- 57.Battisti S., et al. (2012) Nutritional stressarginine auxotrophy confer high sensitivity to chloroquine toxicity in mesothelioma cells. Am. J. Respir. Cell Mol. Biol. 46, 498–506 10.1165/rcmb.2011-0195OC [DOI] [PubMed] [Google Scholar]
- 58.Phan L.M., Yeung S.C. and Lee M.H. (2014) Cancer metabolic reprogramming: importance, main features, and potentials for precise targeted anti-cancer therapies. Cancer Biol. Med. 11, 1–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ward P.S. and Thompson C.B. (2012) Metabolic reprogramming: a cancer hallmark even warburg did not anticipate. Cancer Cell 21, 297–308 10.1016/j.ccr.2012.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yoshida G.J. (2015) Metabolic reprogramming: the emerging concept and associated therapeutic strategies. J. Exp. Clin. Cancer Res. 34, 111 10.1186/s13046-015-0221-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sui X., et al. (2013) Autophagychemotherapy resistance: a promising therapeutic target for cancer treatment. Cell Death Dis. 4, e838 10.1038/cddis.2013.350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang Z.J., et al. (2011) The role of autophagy in cancer: therapeutic implications. Mol. Cancer Ther. 10, 1533–1541 10.1158/1535-7163.MCT-11-0047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Buchser W.J., et al. (2012) Cell-mediated autophagy promotes cancer cell survival. Cancer Res. 72, 2970–2979 10.1158/0008-5472.CAN-11-3396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Basu S., et al. (2014) Suppression of MAPK/JNK-MTORC1 signaling leads to premature loss of organellesnuclei by autophagy during terminal differentiation of lens fiber cells. Autophagy 10, 1193–1211 10.4161/auto.28768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lorin S., et al. (2010) Evidence for the interplay between JNKp53-DRAM signalling pathways in the regulation of autophagy. Autophagy 6, 153–154 10.4161/auto.6.1.10537 [DOI] [PubMed] [Google Scholar]
- 66.Sui X., et al. (2014) p38JNK MAPK pathways control the balance of apoptosis and autophagy in response to chemotherapeutic agents. Cancer Lett. 344, 174–179 10.1016/j.canlet.2013.11.019 [DOI] [PubMed] [Google Scholar]
- 67.Awasthi Y.C., Sharma R. and Singhal S.S. (1994) Human glutathione S-transferases. Int. J. Biochem. 26, 295–308 10.1016/0020-711X(94)90050-7 [DOI] [PubMed] [Google Scholar]
- 68.Rotili D., et al. (2015) Synthesisstructure–activity relationship of new cytotoxic agents targeting human glutathione-S-transferases. Eur. J. Med. Chem. 89, 156–171 10.1016/j.ejmech.2014.10.033 [DOI] [PubMed] [Google Scholar]
- 69.De Luca A., et al. (2015) A novel orally active water-soluble inhibitor of human glutathione transferase exerts a potentselective antitumor activity against human melanoma xenografts. Oncotarget 6, 4126–4143 10.18632/oncotarget.2798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fulci C., et al. (2017) A new nitrobenzoxadiazole-based GSTP1-1 inhibitor with a previously unheard of mechanism of actionhigh stability. J. Enzyme Inhib. Med. Chem. 32, 240–247 10.1080/14756366.2016.1247059 [DOI] [PMC free article] [PubMed] [Google Scholar]