Abstract
Biogas production from anaerobic co-digestion of fallen teak leaves (Tectona grandis) and microalgae (Chlorella vulgaris) were investigated. In this study, teak leaves and algae mixtures with or without pretreatment were used as the substrates and digested in 1-L of anaerobic fermenter, then optimal conditions were performed in 6-L fermenter. Pretreatment was performed using sodium hydroxide (NaOH) solution (w/v) at different conditions (0, 2, 3 and 4%), with different total solid (TS) ratios (10, 15 and 20%). The digesters were placed in an incubator at 34–36 °C for 45 days. The results showed that the co-digestion of pretreated (10% TS with 2% NaOH) of teak leaves and algae was significantly higher in terms of biodegradability of TS, VS, COD along with biogas yield, methane potential and highest yield was achieved 71.90% than those obtained by mono-digestion. Thus, results demonstrated that anaerobic fermentation of teak leaves and microalgae in digester system could get as high methane yield.
Keywords: Fallen teak leaves, Microalgae, Biogas production, Co-digestion
Introduction
The increasing population size, industrialization, and motorization resulting with an increase consumption of energy alongside with the limited and gradually decreasing supply of conventional fossil fuels have led international communities to seek an alternative, renewable, sustainable, efficient, and cost-effective energy resources (Ramaraj et al. 2015a; Unpaprom et al. 2015a). At the present, renewable energy becomes a popular solution to this energy crisis. Renewable energy promises a sustainable and clean energy. There are several kinds of renewable energy such as solar, wind, tide, geothermal, biofuel, etc. Currently, biofuel is gaining a worldwide attention due to its potential to be a substitute for petroleum-derived transportation fuels (Ramaraj et al. 2015c).
Biofuel is a type of renewable energy made from biomass. Biomass is a biological material derived from living organisms. It is often used as a plant-based material for biogas production. Biogas is a combustible mixture of gases which consists mainly of methane (CH4) and carbon dioxide (CO2); it can be formed from the anaerobic bacterial decomposition of organic compounds. Biogas production through anaerobic digestion (AD) has emerged as one of the effective choice for renewable energy production (Unpaprom et al. 2015b; Pantawong et al. 2015). Co-digestion has been defined as the anaerobic treatment of a mixture of at least two different substrates with the aim of improving the efficiency of the anaerobic digestion process (Dussadee et al. 2014). Nowadays, there are an increasing number of full-scale co-digestion plants treating manure and industrial organic wastes. Co-digestion of mixed substrates offers many advantages including ecological, technological, and economic benefits as compared to single substrate digestion (Dussadee et al. 2017).
Teak (Tectona grandis) is a type of biomass and is considered one of the world’s premier hardwoods. It occurs naturally in specific areas such as the Indian peninsula and some eastern parts of Asia include Burma, Laos, and Thailand (Pandey and Brown 2000). Teak leaves are widely available biomass material in Thailand and other tropical/subtropical countries. Chemical composition of teak leaves has been reported and the result shows 39.45% dry matter (carbohydrate and fat), 10.13% crude protein, 10.97% crude fiber, other secondary metabolites and minerals (Devadiga et al. 2015; Oke and Ogunjimi 2016). Fallen teak leaves biomass was found to be a potentially valuable fermentation substrate that can produce 7.467 L (0.007 m3) methane gas (Wannapokin et al. 2017). In our previous study, the potential of fallen teak leaves biomass as a feedstock for biogas production has been theoretically estimated (Wannapokin et al. 2017). The total biogas yield achieved through theoretical estimation was 1.0740 m3/kg or 1073.99 L/kg; and total methane yield was reached 0.5964 m3. Wannapokin et al. (2017) preliminary screen study was suggested that it is possible to achieve stable operation using fallen teak leaves, as a substrate for biogas production in laboratory studies to ensure the potential of fallen leaves. On the other hand, the leaves contain highly nutritious rich organic substances that are suitable for sustaining microbial life in an anaerobic fermentation process, and transform substrates into biogas.
Another type of biomass that’s drawing an interest from researchers around the world is microalgae. Microalgae are small organisms resembling bacteria; they can make their own food through the process of photosynthesis. Moreover, microalgae contain huge amount of nutritious components such as protein, lipid, and carbohydrates (Ramaraj et al. 2016); these substances can add support in fermentation process. Therefore, the main objectives of this research work were to investigate the potential improvement of fallen teak leaves with co-digestion with microalgae as a substrate for estimation and production of biogas.
Materials and methods
Feedstock and inoculums
The dried fallen teak leaves (T. grandis) were collected from Sansai (18°56′14″N; 99°3′38″E), Chiang Mai, Thailand. Teak leaves were crushed by machine into small particles. The crushed leaves were dried in an oven at 40 °C for 48 h to achieved a moisture content of < 10% then reduce to a particle size of 0.5–1 mm using a blender (OTTO BE-127 blender). The dried powder was stored and sealed in a desiccator under ambient temperature for further usage.
The inoculum was collected from a swine farm at the Faculty of Animal Science and Technology, Maejo University, Chiang Mai, Thailand. It was kept in air-tight buckets at 4 °C in a walk-in cooler. Prior to use, the inoculum was acclimated and degassed at 35 °C for 3 weeks to minimize the effect of methane production from inoculum.
Algae culture (Chlorella vulgaris)
A total of 5 L of cultured microalgae were transferred to an open-type cement pond (total volume = 200 L) for the production of a large amount of biomass through Rameshprabu medium. The medium was prepared with rice fertilizer (100 g), rice bran (400 g), fish meal (100 g), lime (50 g), and urea (200 g). The cement pond has the dimensional of width and height was 80 and 40 cm, respectively. The pond was filled with 150 L of water and medium, which reached a height of 25 cm with a total of 10 L of water and mixed ingredients were to a pond which was connected with an air pump. The pond was left for one night to release ammonia and to allow the medium to dissolve in the water properly. The stock algae were transferred in the following day to the triplicate cement ponds. Algal growth was measured and recorded everyday. Additionally, the pond was stirred every day to prevent precipitation of the algae.
Pretreatment
Pretreatment is an important tool for practical cellulose conversion processes and is the subject of this article. Pretreatment is required to alter the structure of cellulosic biomass to make cellulose more accessible to the enzymes that convert the carbohydrate polymers into fermentable sugars. In this study, fallen teak leaves and algae were pretreated with 10, 15, and 20% of NaOH at room temperature for 1 week. The samples were prepared for fermentation. The effects of pretreatment and no pretreatment were determined through analysis of the main sugar composition of the dry biomass of fallen teak leaves.
Analytical methods
The samples were analyzed for total solids (TS), volatile solids (VS), chemical oxidation demand (COD), and pH by standard methods (APHA-AWWA-WPCE 2005). Carbon and nitrogen contents in the samples were determined with the help of a C–N–H–O–S analyzer using an element analyzer (2400 II CHNS/O Elemental Analyzer, Perkin-Elmer, USA). The samples were characterized according to ASTM E870-82, ASTM E1755-01, and ASTM E 872-82 for the determination of moisture (M), ash (A), and volatile matter (V), and the fixed carbon and were calculated by difference. Subsequently, the amount of fixed carbon (FC) was determined from the formula:
where A, V, and FC were calculated on a dry weight basis (db) and M was calculated on a wet basis (wb).
Total sugar determination using the phenol–sulfuric method standard curve of sugar was carried using a glucose concentration (0–250 µg/mL) in distilled water. A total of 500 µL of 50% phenol solution was prepared. The mixtures were shaken and then 2.5 mL of concentrated sulfuric acid was added. All mixtures were homogenized by vortex and subsequently left to stand for 10 min. The absorbance of the reaction mixture was measured at 490 nm by spectrophotometer (Spectronic Genesys 20, Thermo Fisher Scientific). Finally, the relation between A490 and glucose concentration was plotted. The total sugar was determined by the method described above. The reaction mixture was composed of 500 µL of sample solution, 500 µL of 5% phenol solution, and 2.5 mL of concentrated sulfuric acid solution.
Experimental design and procedure
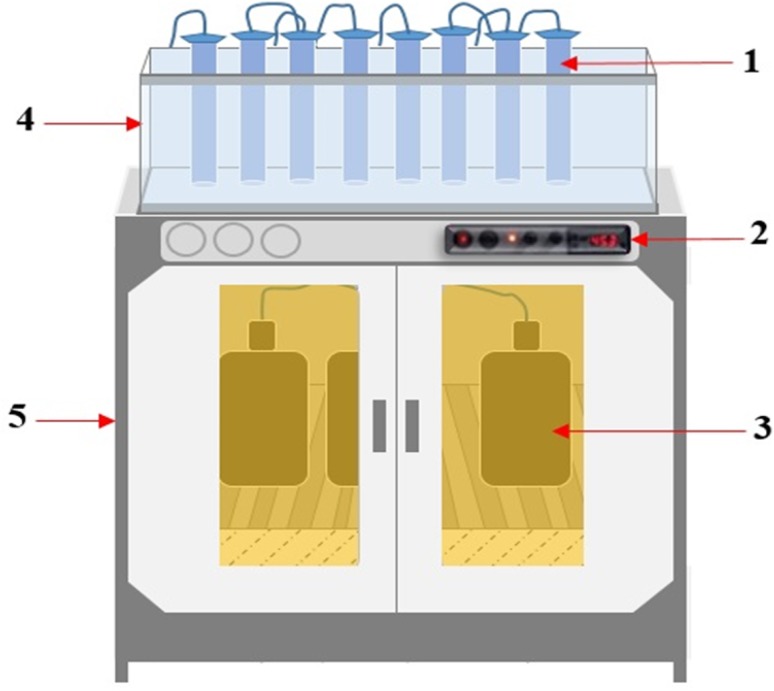
The experimental study was divided into three parts: first, the theoretical estimation, second, the experimental biogas production, and third, scale-up used 5000 mL working volume (i.e., volume size increased to make sure for future large-scale applications). In the experimental biogas production, the digester used in this study was a 1-L Duran bottle with a working volume of 800 mL and a sampling outlet with a gas sampling port and a feed inlet. It was sealed using a rubber stopper in which there was a pipe to extract biogas. The digester was connected to a gas-collection system consisting of a displacement container and a storage container. Prior to operation, the reactors were purged with nitrogen gas for 5 min to ensure anaerobic conditions. Thereafter, the digesters were placed in an incubator (schematic shown in Fig. 1) at 34–36 °C for 45 days. Each digester was mixed manually twice a day.
Fig. 1.
Schematic view of the experimental set up during anaerobic digestion of teak leaves. (1) gas measuring cylinder, (2) temperature controller, (3) digester, (4) water bath and (5) incubator box
Powder of fallen teak leaves was pretreated with sodium hydroxide solution (w/v) at different concentrations (0, 2, 3, and 4%) and different ratios of TS (10, 15, and 20%) for start-up 2 days before by adding effluent from a stable biogas digester. The sample was fed into a wide-mouthed glass bottle with a capacity of 1 L on a batch basis for 45 days at ambient temperature. The volume of the substrate in the glass bottle was 800 mL. It was inoculated with sludge collected from the Energy Research Center, Maejo University, Chiang Mai, Thailand. After pretreatment, anaerobic reactors containing different proportions of substrate and sodium hydroxide solution were set up. The total gas production was measured by the water displacement method at intervals of 24 h. The fermenter was mixed thrice a day. The contents of the fermenter were mixed manually after every gas measurement. Daily gas production was recorded.
In the 3rd experiment study, a digester consisting of a 6-L plastic container with a working volume of 5000 mL was used. It consisted of a sampling outlet, a gas sampling port, and a feed inlet. It was sealed using a faucet that could be used as a valve in which there was a pipe to extract biogas. The digester was connected to a gas-collection system consisting of a displacement container and a storage container. Prior to operation, the reactors were purged with nitrogen gas for 5 min to ensure anaerobic conditions. Thereafter, the digesters were placed in an incubator at 34–36 °C for 45 days. Fallen teak leaves were pretreated in a concentration of with 2% of sodium hydroxide solution (w/v), and a 10% TS ratio was mix with algae for start-up 7 days by adding effluent from the stable biogas digester; and the digester was mixed manually thrice a day.
Biogas estimation
Biogas potential production was calculated according to Von Sperling and Chemicharo (2005). Theoretical methane potential was calculated by Bushwell’s formula, which is derived by stoichiometric conversion of the compound to CH4, CO2, and NH3 (Buswell and Boruff 1932). Another way of estimating the biogas yield is based on the chemical oxygen demand (COD) content of the material. Since COD is a measure of the organic matter in the residues, the biogas yield can be estimated stoichiometrically from the COD measurement, where 1 g of COD has a maximum methane potential of 0.35 L of CH4 under standard conditions (Angelidaki and Ellegaard 2003; Ramaraj et al. 2015b). The biogas from the experimental system, including CH4, CO2, H2, H2S, and O2, was measured using an automated gas analyzer according to Brettschneidera et al. (2004).
Statistical analysis
All the values or readings are the result of the mean of three replicates. Data are reported as the mean ± standard deviation (SD). Statistical analyses were performed using Microsoft Excel.
Results and discussion
Comparative physico-chemical properties of teak leaves and other plant leaves
The results of the physical–chemical characterization of the studied samples are reported in Table 1. In our study, the proximate analysis of the teak leaves powder biomass was carried out; the average values of moisture, ash, volatile matter, and fixed carbon were found to be 2.83, 11.33, 83.44 and 2.4%, respectively. Ultimate analysis of the teak leaves powder biomass was carried out; the average values of carbon, hydrogen, oxygen, and nitrogen were found to be 48.88, 5.83, 30.04 and 0.55%, respectively.
Table 1.
Physical, chemical and composition of teak leaves
| Parameters | Teak leaves |
|---|---|
| Proximate analysis (%) | |
| Moisture | 2.83 |
| Ash | 11.33 |
| Volatile matter | 83.44 |
| Fixed carbon | 2.4 |
| Ultimate analysis (%) | |
| Carbon (%) | 48.88 |
| Hydrogen (%) | 5.83 |
| Oxygen (%) | 30.04 |
| Nitrogen (%) | 0.55 |
| Composition and others | |
| TS (mg/kg) | 982,151.93 |
| VS (mg/kg) | 819,412.60 |
| COD (mg/L) | 21,333.33 |
| pH | 5.38 |
The TS and VS contents in the teak leaf powder biomass were measured and the average values were found to be 982,151.9252 and 819,412.6 mg/L, respectively. The average pH was 5.38 and the average COD was 21,333.33 mL/L. Methane formation takes place within a relatively narrow pH interval from about 6.5–8.5 with the optimum interval between 7.0 and 8.0. The process is severely inhibited if the pH decreases below 6.0 or rises above 8.5. When the C, H, O, and N contents of wastewater or substrate were known, the stoichiometric relationship reported by Buswell and Boruff (1932) and Angelidaki and Sanders (2004) were used to estimate the theoretical gas composition on a percentage molar basis. In this equation, the organic matter was stoichiometrically converted to methane, carbon dioxide, and ammonia.
Proximate analysis provides the moisture, ash, volatile, and FC content, which impacts the drying, ignition, and ash. The proximate analysis of all of the different plant leaf samples was carried out using the standard procedure. The contents of moisture (M), volatile matter (VM), and ash (A) of all the samples were shown in Table 1. It may be observed from the table that the bigtooth maple and Gambel oak samples have the highest moisture contents (87.50%) and chamise has the lowest (80.00%). From this, the arrived conclusion was bigtooth maple and Gambel oak samples have a lower calorific value due to a were longer heating time and VM is a substance that can be vaporized or turned from solid to gas. The VM content of teak leaves was high (83.44%) compared to badam (47.3%) or Senna alexandrina (25.5%) but was not different from those of Gambel oak (83.50%) or bigtooth maple (83.90%). Based on the results, high VM content has the possibility to produce a high biogas yield after the fermentation process.
The ultimate analysis of different plant leaves data is illustrated in Table 2. Ultimate analysis provides the elemental composition of the fuel, which determines the gas products of thermal conversion. The heating value was also very important for the design of incinerators. Thermogravimetric analysis (TGA) is one of the most common techniques used to investigate the thermal behavior of small fuel samples and has no limitations on the heat and mass transfer at low heating rates (Chen et al. 2014). The obtained results can be used to determine the reactivity, which includes the pyrolysis rate (mass loss per time unit) and mass loss kinetics of the fuels. Meanwhile, the results of TGA can be obtained easily and usually have very good repeatability. The composition of teak leaves showed high potential for biogas production with the carbon, hydrogen, oxygen, and nitrogen contents were 48.88, 05.83, 30.04, and 0.55%, respectively.
Table 2.
Proximate and ultimate analysis of dry leaves (% wt dry basis)
| Parameters Plant leaf material |
Proximate analysis (%) | References | |||
|---|---|---|---|---|---|
| FC | VM | M | Ash | ||
| Musa sapientum Linn | 14.00 | 75.30 | 07.17 | 10.70 | Maia et al. (2014) |
| Zea mays Linn | 05.66 | 79.08 | 07.44 | 07.82 | Danishac et al. (2015) |
| E. polybractea | 21.30 | 74.80 | 18.50 | 03.90 | Burton and Wu (2016) |
| Prunus dulcis | 18.70 | 47.30 | 18.20 | 15.80 | Raju et al. (2014) |
| Eucalyptus | 10.30 | 79.20 | 04.40 | 10.50 | Mishra et al. (2010) and Liu et al. (2013) |
| Cynara cardunculus | 10.90 | 59.50 | – | 29.60 | Damartzis et al. (2010) |
| Senna alexandrina | 25.50 | 57.20 | – | 17.30 | Grover et al. (2002) |
| Saccharum | 14.90 | 77.40 | – | 07.70 | |
| Casuarina equisetifolia | 16.46 | 73.50 | – | 03.93 | Sugumaran and Seshadri (2009) |
| Lantana camara | 11.83 | 70.46 | – | 07.26 | |
| Phoenix dactylifera | 05.20 | 78.10 | 05.00 | 11.70 | Sait et al. (2012) |
| Pinus sabiniana | 18.70 | 80.00 | 09.14 | 01.30 | García et al. (2014) |
| F. simplex | 16.84 | 75.21 | – | 07.95 | Xiao et al. (2011) |
| Populus nigra | 15.57 | 68.74 | – | 15.69 | Zhou et al. (2013) |
| Smilax china L | 21.03 | 69.74 | – | 09.23 | |
| Ginkgo biloba | 15.19 | 73.19 | – | 11.62 | Zhou et al. (2015) |
| Arecaceae | 11.92 | 66.76 | 09.00 | 12.32 | Abnisa et al. (2013) |
| Bambusoideae | 18.70 | 70.30 | – | 11.00 | Huang et al. (2011) |
| Arctostaphylos glandulosa | – | 76.90 | 75.00 | 02.20 | Fletcher et al. (2007) |
| Ceanothus crassifolius | – | 75.80 | 70.00 | 03.20 | |
| Chamise | – | 76.90 | 80.00 | 02.80 | |
| Scrub oak | – | 74.50 | 70.00 | 05.10 | |
| Gambel oak | – | 83.50 | 87.50 | 02.90 | |
| Bigtooth maple | – | 83.90 | 87.50 | 03.50 | |
| Utah juniper | – | 84.80 | 55.00 | 04.00 | |
| Big sagebrush | – | 85.20 | 57.50 | 03.90 | |
| Teak leaves | 02.40 | 83.44 | 02.83 | 11.33 | This study |
FC fixed carbon, VM volatile matter, M moisture
Hydrogen sulfide is an extremely toxic gas. The inhalation of relatively large quantities leads to internal suffocation, as with hydrogen cyanide. Air containing just 350 ppm of H2S has a life-threatening effect following lengthy exposure, and hydrogen sulfide can no longer be perceived as an odor at concentrations above 500 ppm. The effect of concentrations above 1000 ppm is lethal within just a few seconds. In the same way as hydrogen sulfide has a cytotoxic effect on the human nervous system, concentrations above 50 mg/L of dissolved hydrogen sulfide in the fermentation substrate have a toxic effect and inhibit the methane-forming bacteria. In addition, high hydrogen sulfide concentrations can lead to a situation where certain microorganisms reduce sulfur energetically more efficiently than methane-forming bacteria can reduce CO2 to CH4. A dissolved hydrogen sulfide content above approximately 2% results in a decline in the methane concentration in the biogas.
It was found that teak leaves do not contain sulfur which eliminates the production of sulfur gas in fermentation. It can be observed from that the sample teak leaves have no sulfur compared with Musa sapientum Linn, Zea mays Linn, Eucalyptus polybractea, Prunus dulcis, eucalyptus, Cynara cardunculus, Phoenix dactylifera, Pinus sabiniana, Firmiana simplex, Populus nigra, Smilax china L, Ginkgo biloba, and Bambusoideae leaves, respectively.
Theoretical analysis of biogas and biochemical methane production from teak leaves
The first step of the present study was the characterization of the considered leaf biomass to obtain its composition. In fact, the maximum theoretical biogas production and the amount of methane fraction may be predicted based on the elemental composition of the organic matter. The carbon (C), hydrogen, nitrogen (N), oxygen (O), and hydrogen sulfide (H2S) contents were tested in this study (Table 2). Ultimate analysis and HHV (High heating value) data were obtained from Fletcher et al. (2007), Sugumaran and Seshadri (2009), Grover et al. (2002), Mishra et al. (2010), Damartzis et al. (2010), Huang et al. (2011), Xiao et al. (2011), Sait et al. (2012), Abnisa et al. (2013), Liu et al. (2013), Zhou et al. (2013), García et al. (2014), Raju et al. (2014), Zhou et al. (2015), Burton and Wu (2016) for compared with this study teak leaves.The stoichiometric relationship reported by Buswell and Boruff (1932) and Pavlostathis and Giralo-gamez (1991) were used to estimate the theoretical gas composition on a percentage molar basis (Table 3), experimental data stated in the later section by concentration i.e., percentage of concentration. The calculation of the elemental composition was expressed by Eq. (1). In this equation, the organic matter was stoichiometrically converted to CH4, CO2, and NH3 (Ramaraj et al. 2015a, c; Ramaraj and Dussadee 2015). The specific methane yield expressed in liters of CH4 per gram of VS can thus be calculated as:
| 1 |
Table 3.
Ultimate analysis of dry leaves (% wt dry basis)
| Parameters Plant leaf material |
Ultimate analysis (%) | References | |||||
|---|---|---|---|---|---|---|---|
| C | H | O | N | S | HHV (MJ/kg) | ||
| Musa sapientum Linn | 44.28 | 6.23 | 37.90 | 0.80 | 0.30 | 17.70 | Mata-Alvarez et al. (2014) |
| Zea mays Linn | 47.04 | 5.41 | 46.82 | 0.68 | 0.05 | 17.37 | Danishac et al. (2015) |
| E. polybractea | 52.19 | 6.55 | 39.19 | 1.35 | 0.72 | – | Burton and Wu (2016) |
| Prunus dulcis | 42.50 | 3.80 | 31.40 | 1.10 | 0.35 | – | Raju et al. (2014) |
| Eucalyptus | 46.96 | 6.22 | 44.82 | 1.25 | 0.77 | 18.9 | Mishra et al. (2010) and Liu et al. (2013) |
| Cynara cardunculus | 34.10 | 4.90 | 29.80 | 1.40 | 0.20 | 17.90 | Damartzis et al. (2010) |
| Senna alexandrina | 36.20 | 4.72 | 37.49 | 4.29 | – | 18.13 | Grover et al. (2002) |
| Saccharum | 39.75 | 5.55 | 46.82 | 0.17 | – | 17.40 | |
| Casuarina equisetifolia | 46.12 | 6.90 | 42.64 | 1.18 | – | 18.48 | Sugumaran and Seshadri (2009) |
| Lantana camara | 45.01 | 6.68 | 43.79 | 2.02 | – | – | |
| Phoenix dactylifera | 49.40 | 5.80 | 42.30 | 1.20 | 1.30 | Sait et al. (2012) | |
| Pinus sabiniana | 47.65 | 5.43 | 46.21 | 0.27 | 0.44 | 18.70 | García et al. (2014) |
| F. simplex | 48.02 | 4.99 | 36.77 | 1.15 | 1.13 | – | Xiao et al. (2011) |
| Populus nigra | 41.77 | 4.42 | 36.75 | 1.11 | 0.26 | 16.85 | Zhou et al. (2013) |
| Smilax china L | 48.06 | 4.43 | 37.06 | 0.92 | 0.30 | 19.12 | |
| Ginkgo biloba | 41.35 | 5.54 | 50.88 | 1.36 | 0.87 | 15.28 | Zhou et al. (2015) |
| Arecaceae | 40.40 | 5.58 | 52.09 | 1.94 | – | – | Abnisa et al. (2013) |
| Bambusoideae | 40.50 | 5.80 | 52.80 | 0.70 | 0.20 | – | Huang et al. (2011) |
| Arctostaphylos glandulosa | 52.77 | 6.32 | 40.13 | 0.78 | – | – | Fletcher et al. (2007) |
| Ceanothus crassifolius | 52.94 | 6.30 | 01.08 | 39.67 | – | – | |
| Chamise | 51.48 | 6.61 | 01.31 | 40.60 | – | – | |
| Scrub oak | 51.47 | 6.5 | 01.99 | 40.03 | – | – | |
| Gambel oak | 49.15 | 6.23 | 42.10 | 2.52 | – | – | |
| Bigtooth maple | 45.93 | 6.14 | 45.82 | 2.11 | – | – | |
| Utah juniper | 49.92 | 6.88 | 41.87 | 1.33 | – | – | |
| Big sagebrush | 48.52 | 6.46 | 42.77 | 2.25 | – | – | |
| Teak leaves | 48.88 | 5.83 | 30.04 | 0.55 | – | – | This study |
HHV higher heating value
Equation (1) is a theoretical approach that allows estimation of the maximum potential yields. Using Eq. (1), it is possible to compute a theoretical specific methane yield. The data are presented in Table 4.
Table 4.
Therotical biogas composition and production of fallen teak leaves
| Yield | |
|---|---|
| Biogas composition | |
| CH4% | 55.47 |
| CO2% | 43.57 |
| NH3% | 0.96 |
| Biogas production | |
| CH4 | 0.5964 m3 |
| CO2 | 0.4675 m3 |
| NH3 | 0.0101 m3 |
| Biogas | 1.0740 m3/kg or 1073.99 L/kg |
The details of the yield of methane and biogas production from teak leaves were presented in Table 4 (dry weight basis). The carbon dioxide (43.57%) and methane (55.47%) contents of the biogas were estimated. The teak leaves showed distinct differences in their chemical composition; carbon, hydrogen, nitrogen, oxygen, and hydrogen sulfide contents in the teak leaves were 46.88, 5.83, 30.04, 0.55, and < 1%, respectively.
The amount of substrates that are readily obtainable for biomethanation, furthermore, the biogas potential presented here is a theoretical, yet conservative estimate. On the other hand, since literature data about the AD of fallen leaf waste are limited, it appears to be useful to estimate the theoretical biogas and methane production to evaluate the technical and economic feasibility of the process for the successive laboratory-scale and pilot-scale digestion tests. The theoretical biogas composition, total biogas production, and theoretical methane production biogas yield of different plant leaves are presented in Table 5.
Table 5.
Biogas composition, total biogas production and theoretical biogas yield of different plant leaves
| Parameter Plant leaf material |
Gas composition (%) | Total gas production (m3) | Total theoretical amount of gas | |||||
|---|---|---|---|---|---|---|---|---|
| CH4 | CO2 | NH3 | CH4 | CO2 | NH3 | m3/kg | L/kg | |
| Musa sapientum Linn | 53.64 | 44.83 | 1.52 | 0.50 | 0.42 | 0.01 | 0.94 | 935.85 |
| Zea mays Linn | 47.54 | 51.24 | 1.22 | 0.42 | 0.45 | 0.01 | 0.88 | 884.73 |
| E. polybractea | 52.74 | 45.09 | 2.17 | 0.53 | 0.45 | 0.02 | 0.99 | 997.83 |
| Prunus dulcis | 47.67 | 50.16 | 2.17 | 0.49 | 0.51 | 0.02 | 1.02 | 1023.63 |
| Eucalyptus | 49.98 | 47.79 | 2.23 | 0.45 | 0.43 | 0.02 | 0.90 | 898.61 |
| Cynara cardunculus | 52.02 | 44.58 | 3.40 | 0.49 | 0.42 | 0.03 | 0.93 | 933.69 |
| Senna alexandrina | 42.06 | 48.72 | 9.22 | 0.38 | 0.44 | 0.08 | 0.89 | 894.75 |
| Saccharum | 48.54 | 51.09 | 0.37 | 0.39 | 0.41 | 0.00 | 0.80 | 802.76 |
| Casuarina equisetifolia | 53.12 | 44.74 | 2.15 | 0.48 | 0.40 | 0.02 | 0.90 | 903.80 |
| Lantana camara | 50.63 | 45.67 | 3.70 | 0.45 | 0.41 | 0.03 | 0.89 | 890.01 |
| Phoenix dactylifera | 49.74 | 48.22 | 2.04 | 0.47 | 0.46 | 0.02 | 0.95 | 948.72 |
| Pinus sabiniana | 48.49 | 51.02 | 0.48 | 0.43 | 0.46 | 0.00 | 0.89 | 893.09 |
| F. simplex | 49.45 | 48.54 | 2.01 | 0.50 | 0.49 | 0.02 | 1.00 | 1000.73 |
| Populus nigra | 47.44 | 50.33 | 2.22 | 0.45 | 0.47 | 0.02 | 0.94 | 943.75 |
| Smilax china L | 47.97 | 50.42 | 1.61 | 0.48 | 0.50 | 0.02 | 1.00 | 1002.6 |
| Ginkgo biloba | 44.71 | 52.55 | 2.74 | 0.36 | 0.42 | 0.02 | 0.80 | 796.24 |
| Arecaceae | 43.22 | 52.83 | 3.95 | 0.34 | 0.41 | 0.03 | 0.78 | 780.74 |
| Bambusoideae | 45.81 | 52.74 | 1.46 | 0.35 | 0.40 | 0.01 | 0.76 | 764.65 |
| Arctostaphylos glandulosa | 52.56 | 46.18 | 1.25 | 0.52 | 0.46 | 0.01 | 0.99 | 992.41 |
| C. crassifolius | 52.23 | 46.05 | 1.72 | 0.52 | 0.46 | 0.02 | 1.00 | 1000.40 |
| Chamise scrub oak | 52.50 | 45.36 | 2.13 | 0.51 | 0.44 | 0.02 | 0.98 | 976.83 |
| Gambel oak | 51.41 | 45.38 | 3.21 | 0.51 | 0.45 | 0.03 | 0.99 | 987.47 |
| Bigtooth maple | 49.14 | 46.65 | 4.2.1 | 0.47 | 0.44 | 0.04 | 0.95 | 952.60 |
| Utah juniper | 47.98 | 48.23 | 3.79 | 0.43 | 0.43 | 0.03 | 0.89 | 886.30 |
| Big sagebrush | 52.88 | 44.88 | 2.23 | 0.50 | 0.43 | 0.02 | 0.95 | 948.18 |
| Teak leaves | 55.47 | 43.57 | 0.96 | 0.60 | 0.47 | 0.01 | 1.07 | 1073.99 |
The methane content, total methane production, and theoretical biogas yield of different plant leaves are shown in Table 5. High methane contents were found in teak leaves (55.47%), Musa sapientum Linn (53.64%), and E. polybractea (52.74%). The highest total methane production was obtained from teak leaves and was 0.60 m3, while the values of total methane production from E. polybractea, Arctostaphylos glandulosa, and Ceanothus crassifolius were 0.53 and 0.52 m3, respectively. The total theoretical amount of gas from teak leaves was the highest as well, at 1.07 m3/kg or 1073.99 L/kg.
Potential production of methane from teak leaves and banana plants was found to be higher than that from other plant leaf materials. The results of methane and biogas yields differed between leaves of different species. The comparison data clearly demonstrated the high methane yield and biogas potential production of fallen teak leaves. Consequently, teak leaves have plenty of nutritious substance for the biogas production process and were suitable use as energy crops for biogas production.
Efficiency of pretreatment and biogas production from mono-digestion of fallen teak leaves
Generally, chemical pretreatment is used to achieve the destruction of the organic compounds by means of strong acids, alkalis, or oxidants. During the pretreatment process the compact structure of lignocellulosic should be disrupted and cellulose fiber is exposed. Pretreatment of the lignocellulosic material is carried out to overcome recalcitrance through the combination of chemical and structural changes to the lignin and carbohydrates. Previous studies have reported different methods of pretreatment. However, according to a study, these traditional methods of pretreatment are cost intensive, as additional chemicals or energy is required. The basic understanding of each step in the process with regard to subsequent commercial viability and operation is required for commercial success in transforming biomass into energy (Amin et al. 2017). In this study, pretreatment was performed using a sodium hydroxide solution (w/v) at different concentrations as above (2, 3, and 4%), with a total solids content of 10%. The substrate degradability efficiency is displayed in Tables 6, 7, 8, 9, 10, and 11. All the pretreatment results confirmed that the degradability efficiency was much higher compare to the substrate that has not undergone pretreatment. Furthermore, the sample pretreated with 2% NaOH presented a higher degradability efficiency than that of the 3 and 4% NaOH. Therefore, from the study results it can be recommended that pretreatment with 2% NaOH is suitable for further scale-up with 5000 mL working volume of the biogas production process.
Table 6.
Degradability efficiency for TS, VS and COD of 10% teak leaves powder with different conditions 2, 3 and 4%
| Sample | TS (mg/L) | VS (mg/L) | COD (mg/L) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Start | End | Degradability efficiency (%) | Start | End | Degradability efficiency (%) | Start | End | Degradability efficiency (%) | |
| 10% teak powder + 0% NaOH + inoculum | 40,701 | 28,233 | 30.6c | 32,000 | 16,000 | 50.0c | 7876 | 2907 | 50.0b |
| 10% teak powder + 2% NaOH + inoculum | 61,327 | 20,775 | 66.1a | 47,562 | 6417 | 86.5a | 74,666 | 21,333 | 71.4a |
| 10% teak powder + 3% NaOH + inoculum | 67,681 | 26,201 | 61.3ab | 52,116 | 12,488 | 76.0a | 90,667 | 48,000 | 47.1cb |
| 10% teak powder + 4% NaOH + inoculum | 55,483 | 28,808 | 48.1b | 38,414 | 20,773 | 45.9b | 1,013,333 | 64,000 | 36.8c |
Data in the table: mean ± SD
Mean with different superscript letters (a, b, c) are significantly different (P < 0.05)
Table 7.
Degradability efficiency for TS, VS and COD of 15% teak leaves powder with different conditions 2, 3 and 4%
| Sample | TS (mg/L) | VS (mg/L) | COD (mg/L) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Start | End | Degradability efficiency (%) | Start | End | Degradability efficiency (%) | Start | End | Degradability efficiency (%) | |
| 15% teak powder + 0% NaOH + inoculum | 56,889 | 32,246 | 43.3a | 32,368 | 11,517 | 64.4a | 37,333 | 21,333 | 42.9a |
| 15% teak powder + 2% NaOH + inoculum | 84,118 | 27,991 | 66.7c | 81,789 | 11,063 | 86.5a | 106,667 | 48,000 | 55.0a |
| 15% teak powder + 3% NaOH + inoculum | 105,632 | 41,728 | 60.5a | 76,248 | 27,863 | 63.5a | 101,333 | 74,667 | 26.3a |
| 15% teak powder + 4% NaOH + inoculum | 118,459 | 58,500 | 50.6a | 74,087 | 30,918 | 58.3a | 128,000 | 96,000 | 25.0a |
Data in the table: mean ± SD
Mean with different superscript letters (a, b, c) are significantly different (P < 0.05)
Table 8.
Degradability efficiency for TS, VS and COD of 20% teak leaves powder with different conditions 2, 3 and 4%
| Sample | TS (mg/L) | VS (mg/L) | COD (mg/L) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Start | End | Degradability efficiency (%) | Start | End | Degradability efficiency (%) | Start | End | Degradability efficiency (%) | |
| 20% teak powder + 0% NaOH + inoculum | 125,916 | 78,172 | 37.9a | 100,940 | 64,950 | 35.7b | 42,667 | 26,667 | 37.5a |
| 20% teak powder + 2% NaOH + inoculum | 264,390 | 177,117 | 33.0a | 222,263 | 133,612 | 39.9ab | 144,000 | 85,333 | 40.7a |
| 20% teak powder + 3% NaOH + inoculum | 255,213 | 147,497 | 42.2a | 213,453 | 81,103 | 62.0a | 133,333 | 96,000 | 28.0a |
| 20% teak powder + 4% NaOH + inoculum | 229,580 | 155,107 | 32.4a | 202,249 | 114,025 | 43.6ab | 133,333 | 101,333 | 24.0a |
Data in the table: mean ± SD
Mean with different superscript letters (a, b, c) are significantly different (P < 0.05)
Table 9.
Degradability efficiency for total sugar, alkalinity, VFA and pH of 10% teak leaves powder with different conditions 2, 3 and 4%
| Sample | Total sugar (µg/L) | Alk mg/L-CaCO3 | VFA mg/g | pH | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Start | End | Degradability efficiency (%) | Start | End | Degradability efficiency (%) | Start | End | Degradability efficiency (%) | Start | End | |
| 10% teak powder + non-pretreatment | 7876 | 2907 | 63.1b | 3503 | 3457 | 1.3c | 7480 | 5260 | 29.7c | 7.5 | 7.4 |
| 10% teak powder + 2% NaOH + inoculum | 11,821 | 2488 | 79.0a | 5390 | 4330 | 19.7a | 9273 | 5792 | 37.5a | 7.4 | 7.0 |
| 10% teak powder + 3% NaOH + inoculum | 12,771 | 2549 | 80.0a | 5950 | 5770 | 3.0b | 10,727 | 7065 | 34.1b | 7.5 | 7.1 |
| 10% teak powder + 4% NaOH + inoculum | 12,728 | 2346 | 81.6a | 7400 | 6127 | 17.2b | 12,052 | 8519 | 29.3c | 7.4 | 7.1 |
Data in the table: mean ± SD
Mean with different superscript letters (a, b, c) are significantly different (P < 0.05)
Table 10.
Degradability efficiency for total sugar, alkalinity, VFA and pH of 15% teak leaves powder with different conditions 2, 3 and 4%
| Sample | Total sugar (µg/L) | Alk mg/L-CaCO3 | VFA mg/g | pH | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Start | End | Degradability efficiency (%) | Start | End | Degradability efficiency (%) | Start | End | Degradability efficiency (%) | Start | End | |
| 15% teak powder + no pretreatment | 15,167 | 4148 | 72.6b | 4877 | 4133 | 15.2a | 8234 | 5403 | 34.4a | 7.5 | 7.0 |
| 15% teak powder + 2% NaOH + inoculum | 20,216 | 4698 | 76.8a | 7787 | 6337 | 18.6b | 17,584 | 11,667 | 33.7a | 7.4 | 6.9 |
| 15% teak powder + 3% NaOH + inoculum | 25,451 | 5506 | 78.4a | 9423 | 8057 | 14.5c | 18,078 | 13,229 | 26.8b | 7.4 | 6.9 |
| 15% teak powder + 4% NaOH + inoculum | 28,364 | 6327 | 77.7a | 12,153 | 8407 | 30.8a | 22,597 | 15,584 | 31.0ab | 7.3 | 6.9 |
Data in the table: mean ± SD
Mean with different superscript letters (a, b, c) are significantly different (P < 0.05)
Table 11.
Degradability efficiency for total sugar, alkalinity, VFA and pH of 20% teak leaves powder with different conditions 2, 3 and 4%
| Sample | Total sugar (µg/L) | Alk mg/L-CaCO3 | VFA mg/g | pH | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Start | End | Degradability efficiency (%) | Start | End | Degradability efficiency (%) | Start | End | Degradability efficiency (%) | Start | End | |
| 20% teak powder + non-pretreatment | 15,074 | 5296 | 64.9b | 6473 | 6210 | 4.1c | 12,701 | 9584 | 24.5b | 7.4 | 7.0 |
| 20% teak powder + 2% NaOH + inoculum | 21,772 | 6056 | 72.2a | 9420 | 7913 | 16.0a | 17,766 | 12,338 | 30.6a | 7.4 | 6.9 |
| 20% teak powder + 3% NaOH + inoculum | 30,895 | 7710 | 75.0a | 9820 | 8327 | 15.2a | 22,909 | 17,143 | 25.2b | 7.4 | 7.0 |
| 20% teak powder + 4% NaOH + inoculum | 26,623 | 13,895 | 47.8c | 10,483 | 9933 | 5.2b | 23,143 | 18,511 | 20.0c | 7.4 | 7.0 |
Data in the table: mean ± SD
Mean with different superscript letters (a, b, c) are significantly different (P < 0.05)
The results of TS, VS, and COD degradability efficiency in 10% teak leaf powder with different concentrations of 2, 3, and 4% are reported in Table 6. The degradability efficiency of TS and VS in 10% teak powder + 2% NaOH + inoculum was highest, with values of 66.1 and 86.5%, respectively. For the same treatment, the COD degradability efficiency was maximal at 71.4% with 10% TS + 3% NaOH + inoculum; the results showed that the average values of TS, VS, and COD degradability efficiency were 61.3, 86.5, and 47.1%, respectively. The treatment with 10% teak powder + 0% NaOH + inoculum gave values of TS, VS, and COD degradability of 30.6, 50.0, and 50.0%, respectively. This was not different from the treatment with 10% teak powder + 4% NaOH + inoculum. However, the results showed that the values of TS, VS, and COD degradability efficiency obtained with 2% NaOH pretreatment were higher than those obtained without pretreatment or with treatment of 3 and 4% NaOH. NaOH pretreatment can change the composition and structure of teak leaves, even at a mesophilic temperature. The lignin was removed effectively by the NaOH pretreatment. Compared with no pretreatment, NaOH pretreatment exhibits good performance in enhancing enzymatic hydrolysis.
It is also with 10% of teak leaves results better than 15 and 20% because the results of TS, VS and COD degradability efficiency of 15% teak leaves powder with different conditions 2, 3, and 4% are reported in Table 7. The TS and VS contents obtained with 15% teak powder + 2% NaOH + inoculum were 66.7 and 63.5%, respectively, and these values were higher than the values obtained with other treatments. Treatments of 15% teak powder + 3% NaOH + inoculum and 15% teak powder + 4% NaOH + inoculum showed the lowest values of VS and COD degradability efficiency. This may be due to the high NaOH concentration. The results obtained from the present work for individual leaves are comparable with values obtained by Viswanath et al. (1992), who reported that banana and tomato contain 29.5 and 11.8% TS, respectively. The reduction of the COD value means the reduction of pollution load from any substrate by the treatment method. The COD of the slurry was considerably reduced by the anaerobic process. The results for the TS, VS, and COD degradability efficiency obtained with 20% teak leaf powder and different NaOH concentrations of 2, 3, and 4% are reported in Table 8. The TS and VS contents obtained with 20% teak powder + 3% NaOH + inoculum were 42.2 and 62.0%, respectively, which were higher than those obtained in other treatments. Treatments of 20% teak powder + 2% NaOH + inoculum and 20% teak powder + 4% NaOH + inoculum gave the lowest VS and COD degradability efficiencies, which may be due to the high NaOH concentration.
The results for the degradability efficiency of the total sugar, alkalinity, volatile fatty acids (VFA), and pH obtained with 10% teak leaf powder with different concentrations of 2, 3, and 4% are reported in Table 9. The content of total sugar obtained with 10% teak powder + 4% NaOH + inoculum was 81.6%, which was similar to the content obtained with 10% teak powder + 3% NaOH + inoculum. The alkalinity and VFA obtained with 10% teak powder + 2% NaOH + inoculum was 19.7 and 37.5%, respectively, which were higher than the values obtained with other treatments. Treatments of 10% teak powder + 0% NaOH + inoculum and 10% teak powder + 2% NaOH + inoculum showed the lowest total sugar, alkalinity, and VFA degradability efficiencies. This may be due to the high NaOH concentration. The average pH ranges from 7.0 to 7.5 (Table 10).
In general, the total quantities of VFAs increased with the amount of leaves in the Duran bottles. The concentrations were similar in the biochemical methane potential (BMP) tests carried out with the same of teak leaves contents. This suggests that the hydrolysis and acidogenesis processes were efficient whatever the organic matter (Table 11). This is confirmed by except for propionate produced by MU leaves, the maximum concentration of each VFA measured in the different BMP tests was proportional to the initial substrate concentration and similar trends were recorded for MU and MI leaves. The MU or MI leaves (1.7 and 6.7 g/L) were converted to biogas. Therefore, these results show that the low yields and conversion rates of MI leaves to methane are especially due to the concentrations and the synergism of their bioactive compounds (Chen et al. 2008) and probably due to carbon conversion for as above biomass formation. The results for the degradability efficiency of total sugar, alkalinity, VFA, and pH obtained with 15% teak leaves powder with different concentrations of 2, 3, and 4% are reported in Table 13. The content of total sugar obtained with 15% teak powder + 3% NaOH + inoculum was 78.4%, and similar results were obtained with 15% teak powder + 2% NaOH + inoculum and 15% teak powder + 4% NaOH + inoculum. The alkalinity obtained with 10% teak powder + 2% NaOH + inoculum was 30.8 mg/L-CaCO3, which was higher than that obtained with the other treatments. The VFA obtained with 10% teak powder + no pretreatment + inoculum was similar to that obtained with 15% teak powder + 2% NaOH + inoculum, at 34.4%, while treatments with 10% teak powder + 3% NaOH + inoculum showed the lowest alkalinity and VFA degradability efficiency. This may be due to the high NaOH concentration. The pH of teak leaves in each digester ranged from 7 to 7.5, which was comparable with the optimum range of pH for the production of biogas. This result showed that the microorganisms in the anaerobic digesters were not affected by the pH of the slurry in the digester. Therefore, there was no inhibition of biogas production from teak leaves mixed with pig manure due to the effect of pH. The temperature in all digesters ranged from 26 to 32 °C, which happens to be in the mesophilic range of 25–45 °C, which is suitable for the production of biogas.
Table 13.
Degradability efficiency for TS, VS and COD (in scale up with 5000 mL working volume study)
| Sample | TS (mg/L) | VS (mg/L) | COD (mg/L) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Start | End | Degradability efficiency (%) | Start | End | Degradability efficiency (%) | Start | End | Degradability efficiency (%) | |
| 10% teak leaves + inoculum non-pretreatment | 153,173 | 78,521 | 45.8b | 136,637 | 65,930 | 48.5b | 471,467 | 144,000 | 69.5b |
| 10% teak leaves + inoculum + 2% NaoH | 143,617 | 56,001 | 57.0b | 137,165 | 42,759 | 65.2b | 506,133 | 101,333 | 80.0a |
| 10% teak leaves + inoculum + 2% NaoH + algae | 72,786 | 6644 | 90.9a | 57,896 | 3988 | 93.3a | 474,667 | 58,667 | 87.6a |
Data in the table: mean ± SD
Mean with different superscript letters (a, b, c) are significantly different (P < 0.05)
The results for the degradability efficiency of total sugar, alkalinity, VFA, and pH obtained with 20% teak leaf powder with different concentrations of 2, 3, and 4% are reported in Table 14. The total sugar content obtained with 20% teak powder + 3% NaOH + inoculum was 75.0%, which was similar to that obtained with 20% teak powder + 2% NaOH + inoculum. The alkalinity and VFA of TS 20% + 2% NaOH + inoculum was 16.0 and 30.6%, which were higher than those obtained with other treatments. Treatments of 20% teak powder + no pretreatment + inoculum and 20% teak powder + 4% NaOH + inoculum showed the lowest alkalinity and VFA degradability efficiencies. This may be due to the high NaOH concentration. The average pH ranges from 7.0 to 7.5.
Table 14.
Degradability efficiency for total sugar, alkalinity, VFA and pH (in scale up with 5000 mL working volume study)
| Sample | Total sugar (µg/L) | Alk mg/L-CaCO3 | VFA mg/g | pH | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Start | End | Degradability efficiency (%) | Start | End | Degradability efficiency (%) | Start | End | Degradability efficiency (%) | Start | End | |
| 10% teak leaves + inoculum non-pretreatment | 28,091 | 2623 | 90.7a | 6258 | 4495 | 28.0b | 10,987 | 8208 | 25.2a | 7.5 | 7.0 |
| 10% teak leaves + inoculum + 2% NaoH | 35,500 | 2582 | 92.5a | 11,050 | 6440 | 41.6a | 17,091 | 12,208 | 28.5a | 7.4 | 7.1 |
| 10% teak leaves + inoculum + 2% NaoH + algae | 38,409 | 2259 | 94.1a | 8433 | 3793 | 55.0a | 14,961 | 9444 | 36.9a | 7.5 | 7.1 |
Data in the table: mean ± SD
Mean with different superscript letters (a, b, c) are significantly different (P < 0.05)
Biogas production from mono-digestion of fallen teak leaves
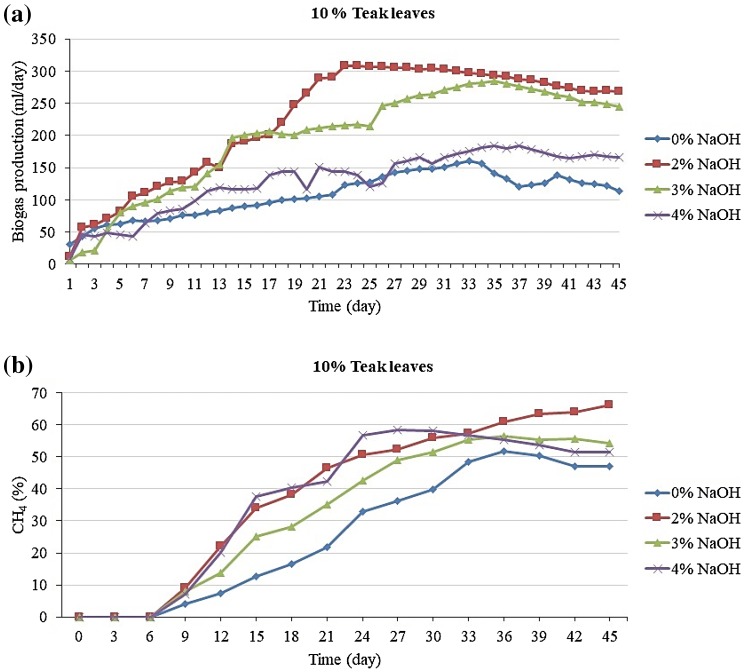
The efficiency of the pretreatment is highly dependent on the process conditions, such as the NaOH concentration, the operating temperature, and the treatment time. Depending on the NaOH concentration, the pretreatment can be performed at low (0.5–4 wt%) or high (5–8 wt%) NaOH concentrations. Usually, when a low NaOH concentration is used, the aim of pretreatment is to remove lignin and hemicellulose from the lignocellulosic materials (Mancini et al. 2016). Accordingly, in these experimental studies, NaOH solution (w/v) was used at different concentrations (0, 2, 3, and 4%), and the resulting biogas production is presented in Fig. 2a while the methane production is illustrated in Fig. 2b. The values of total biogas production obtained through pretreatments with 0, 2, 3, and 4% NaOH were 4849, 10,121, 8894, and 5829 mL, respectively; and highest methane contents obtained with 0, 2, 3 and 4% NaOH were 51.83, 66.22, 56.54, and 58.42%, respectively.
Fig. 2.
Potential production of biogas (a) and methane (b) from 10% fallen teak leaves with different pretreatment conditions
The results proved that NaOH pretreatment was an efficient approach to enhance biogas production from fallen teak leaves. The increase in biogas yield was attributed to the improved biodegradability of teak leaves after NaOH pretreatment, which made more substrate available to be digested by anaerobic microorganisms. Therefore, research needs to be conducted to explore the mechanism of improvement resulting from NaOH pretreatment. Thus, the highest total biogas yield of 9931 mL and highest methane content of 66.22% were obtained at a TS ratio of 10% with 2% NaOH, which was significantly higher the amounts obtained without pretreatment or with pretreatment with 3 and 4% NaOH solution. The biogas production results are presented in Fig. 2a while the methane production is illustrated in Fig. 2b. The values of total biogas production obtained through pretreatments with 0, 2, 3 and 4% NaOH were 4241, 9812, 4812, and 5513 mL, respectively. Simultaneously, the highest methane contents obtained with 0, 2, 3 and 4% NaOH were 42.66, 47.56, 52.78, and 51.59%, respectively.
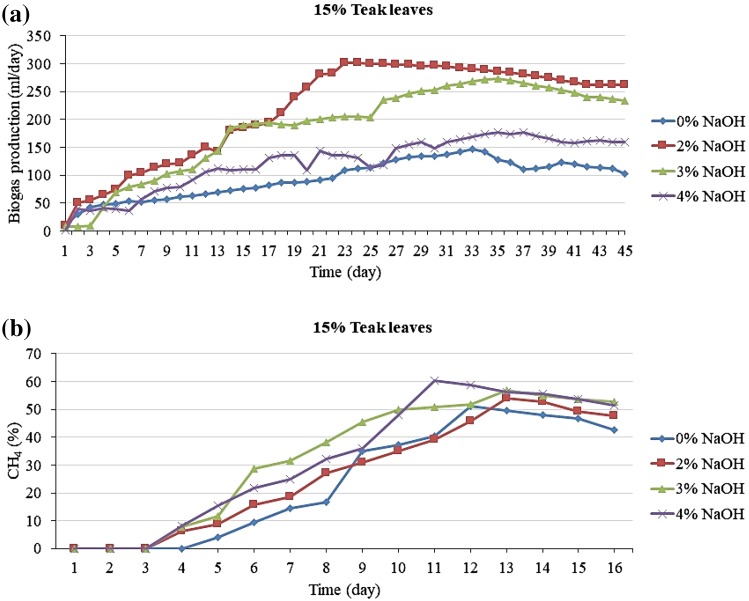
Thus, the highest total biogas yield of 9812 mL and the highest methane content of 52.78% were obtained at a TS ratio of 15% with 2 and 3% NaOH, respectively, which were significantly higher than those obtained without pretreatment or with pretreatment with 4% NaOH. The biogas production results are presented in Fig. 3a, b. The values of total biogas production obtained with pretreatment using 0, 2, 3, and 4% NaOH were 4849, 10,121, 8894, and 5828 mL, respectively. Simultaneously, the highest values of methane content of 0, 2, 3, and 4% NaOH were 51.83, 66.22, 56.54, and 58.42%, respectively. Therefore, research needs to be conducted to explore the mechanism of this improvement resulting from NaOH pretreatment. The highest total biogas yield of 9931 mL and highest methane content of 66.22% were obtained at a TS ratio of 20% with 2% NaOH, which were significantly higher than those obtained without pretreatment or with treatment with 3 and 4% NaOH solution.
Fig. 3.
Potential production of biogas (a) and methane (b) from 15% fallen teak leaves with different pretreatment conditions
Chen and Oswald (1998) pointed out that pretreatment techniques are a necessary step for microalgae cell disruption and biogas production. The effectiveness of pretreatment methods in biogas production depends on the characteristics of the algae, that is, the toughness and structure of the cell wall and the macromolecular composition of cells. Pretreatment methods can be divided into four categories: thermal, mechanical, chemical, and biological processes. Chemical pretreatments have been proven to be successful; among them, mostly alkali pretreatments have been applied to microalgae, often combined with heat.
The results for total biogas yield and methane content obtained from 10, 15, and 20% teak leaf powder with different concentrations of 2, 3, and 4% are reported in Table 12. The highest total biogas yield of 10,121 mL and highest methane content of 66.22% were obtained at a TS ratio of 10% with 2% NaOH, which were significantly higher than the amounts obtained without pretreatment or with TS ratios of 15 and 20% and NaOH concentrations of 3 and 4%. This result suggested that a TS ratio of 10% along with an NaOH concentration of 2% are good conditions to achieve stable operation using fallen leaves as a substrate for biogas production by co-digestion in the next experiment.
Table 12.
Total biogas yield and methane content of fallen teak leaves with different pretreatment
| Teak leaves (%) | Concentration of NaOH % | Total biogas yield (mL) | Methane content (%) |
|---|---|---|---|
| 10% teak leaves | Non-pretreatment | 4849c | 51.83a |
| 2% | 10,121a | 66.22a | |
| 3% | 8894b | 56.54a | |
| 4% | 5828c | 58.42a | |
| 15% teak leaves | Non-pretreatment | 4241b | 42.66a |
| 2% | 9812a | 47.56a | |
| 3% | 8412a | 52.78a | |
| 4% | 5514b | 51.59a | |
| 20% teak leaves | Non-pretreatment | 4416b | 49.61b |
| 2% | 9931a | 47.55b | |
| 3% | 8371a | 66.71a | |
| 4% | 5637b | 60.22a |
Data in the table: mean ± SD
Mean with different superscript letters (a, b, c) are significantly different (P < 0.05)
Evaluation of biogas production from fallen teak leaves with co-digestion of microalgae
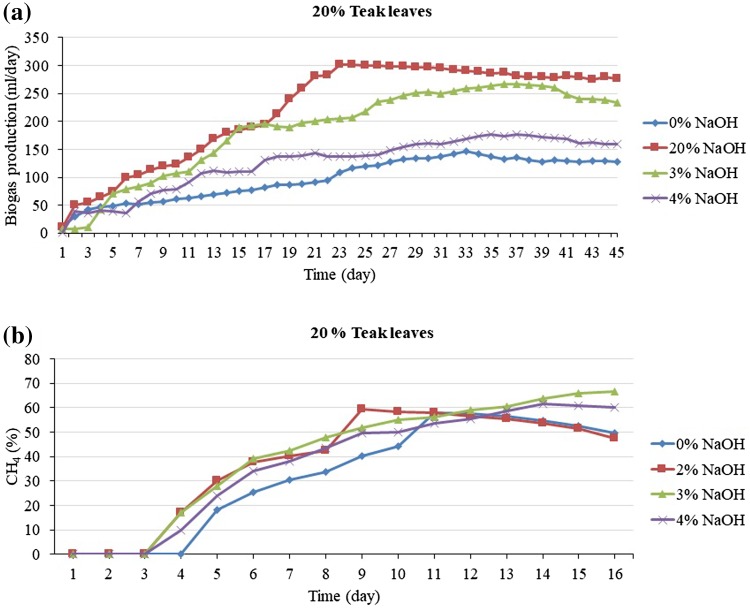
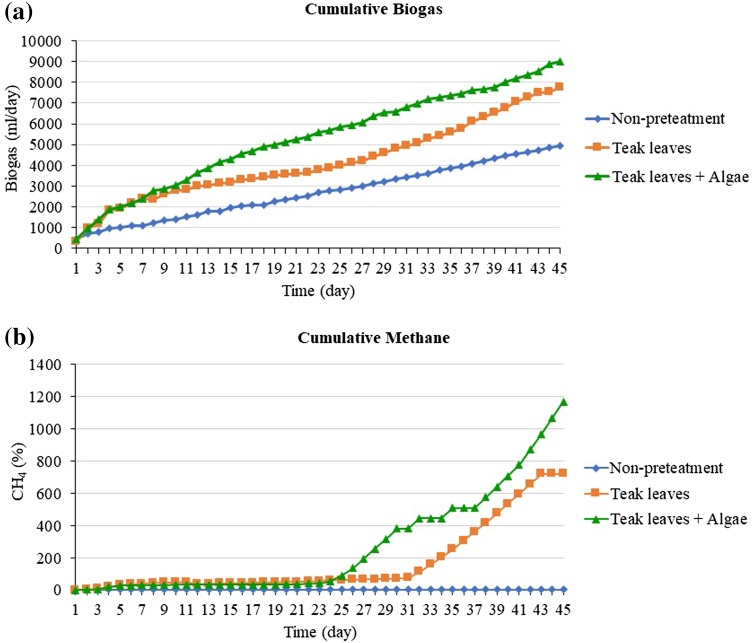
Anaerobic co-digestion, the simultaneous anaerobic digestion of two or more substrates, improves economic viability of AD plants due its potential to yield higher methane production compare to digestion of single substrates (Mata-Alvarez et al. 2014). The increase in methane production from anaerobic co-digestion is mainly a result of increased organic loading rate; however, synergism can further enhance the methane production. According to Mata-Alvarez et al. (2014) statement that the beyond the implementation and operation expenses, onsite cultivation of algae presents some advantages over the use of other co-substrates. Such advantages include: reduced or nullified co-substrate transport cost, which is one of the most important co-substrate selection criteria; minimizing the effect of seasonality of some agro-industrial co-substrates, where supply can be variable or cease; and providing a co-substrate in regional areas where co-substrates are otherwise utilized or are not available. Taking into account these facts, algal biomass appears as a potential co-substrate for animal manure digester located in rural/remote areas (Astals et al. 2015). Furthermore, algae contain also a significant amount of mineral salts and carbohydrates (30–50%), which make up the bulk of their dry matter (approx. 60%); proteins represent approx. 7–15% of algae dry matter. Experimental results, cumulative gas production in a fermenter is shown in Fig. 4a. Due to the crushing of leaves, the particle size decreased many times and the surface area was increased. As a result, gas production was increased compared to the condition with uncrushed teak leaves. It was observed that the lag phase prevailed for up to 5–6 days during the digestion period. After the lag period, the cumulative volume of gas increased up to 42–45 days of fermentation, after which the rate of generation decreased and this declination continued. All of the reactors were taken for further study of pH effect, VS destruction, COD reduction, and so on (Fig. 5).
Fig. 4.
Potential production of biogas (a) and methane (b) from 20% fallen teak leaves with different pretreatment conditions
Fig. 5.
Cumulative gas production (a) and average methane (b) yields of non-pretreatment teak leaves and teak leaves with algae
The methanogenic phase, which occurs after the acid phase in the biodigestion process, is characterized by a methane concentration at a level of 50–60%, with a decrease in the concentration of carboxylic acids and consequent increase in the pH of the environment (Barlaz et al. 1989). From Fig. 4b it can be seen that, as the methanogenic phase advances, the methane concentration increases, while the carbon dioxide decreases, basically in the same proportion. It is also observed that, reached 71.9% in the methane concentration in the teak leaves whereas, for the teak leaves, the biogas reaches the 62.9% higher methane concentration more than non-pretreatment. The steady state of anaerobic digesters in this investigation occurs after 15 days of the start-up process. In the steady state, the degradability efficiency of the average TS, VS, and COD in 10% teak leaves under different co-digestion conditions of no pretreatment, 2% NaOH, and 2% NaOH + algae are reported in Table 13. In our study, the TS and VS contents obtained with 10% teak leaves + inoculum + 2% NaOH + algae were measured; the average results were higher, at 90.9 and 93.3%, respectively. The average COD was 87.6%. Subsequently, 10% teak leaves + inoculum + 2% NaOH were measured; the results were average as 57.0%, 65.2, and average COD 80.0%, respectively. The degradability efficiency obtained with 10% teak leaves + inoculum non-pretreatment was measured; the results were average as 45.8%, 48.5% and an average COD of 69.5%, respectively. These values are comparable with the VS reductions reported in the literature for various substrates (Rouf et al. 2010; Thangamani et al. 2010). The concentration of VS in the slurry decreased with increasing digestion period. A reduction of VS in the test reactors was observed and was in the range of 35.33 and 34% when using 6 and 4% pretreated ground leaves, respectively (Rouf et al. 2015).
The results for the degradability efficiency of total sugar, alkalinity, VFA, and pH obtained with 10% teak leaves with co-digestion under different conditions of no pretreatment, 2% NaOH, and 2% NaOH + algae are reported in Table 14. In our study, the total sugar content, alkalinity, VFA, and pH obtained with 10% teak leaves + inoculum + 2% NaOH + algae were measured; the results were higher on average, at 94.1, 55.0, and 36.9%, respectively. The average pH ranged from 7.5 to 7.1. Subsequently, 10% teak leaves + inoculum + 2% NaOH were measured; the results were high average as 92.5, 41.6 and 28.5%, respectively. The average pH ranged from 7.4 to 7.1. The degradability efficiency of 10% teak leaves + inoculum with no pretreatment was measured; the results were average as 90.7, 28.0, and 25.2%, respectively, and the average pH ranged from 7.5 to 7.0. Chemical pretreatments have been used commonly less than thermal and mechanical ones (Yan 2015). Among the chemical methods, mostly alkali pretreatments have been applied to microalgae. Alkali reagents are commonly used to solubilize polymers, favoring the availability of organic compounds for enzymatic attacks (Bohutskyi and Bouwer 2013). The small amount of residual alkali remaining in pretreated biomass may be helpful to prevent pH reduction during the subsequent acidogenesis step. Co-digestion of microalgae with carbon-rich feedstock has been proposed as a cost-effective and efficient approach to avoid ammonia inhibition. This has mainly been investigated in batch anaerobic digestion tests in which significant increases in methane production up to 62% have been recorded the co-digestion mix is widely regarded as optimal for a balanced nutrient supply (Herrmann et al. 2016). In this experimental study, results showed that the methane yield was increased by 71.90% with the co-digestion, and much higher compared to mono-digestion of microalgae and fallen teak leaves. These results were verified the robustness of co-digestion through mixing with fallen teak leaves in harvesting methane from microalgae.
Conclusions
The co-digestion experimental results confirm that algae could potentially be useful. The methane content was 71.90% higher than mono fermentation substrate of fallen teak leaves. Therefore, this co-digestion approach is feasible for application to farm-scale digesters. Additionally, these results indicate that fallen teak leaves along with microalgae can be successfully converted using AD, and while further investigation into the techno-economics is required, this process is expected to be economical and further scalable. Consequently, the results of this study suggest that it is possible to achieve stable operation using fallen leaves as a substrate and co-substrate for biogas production in pilot or large-scale biogas plants in the future.
Acknowledgements
We thank Dr. Kanda Whangchai and Dr. Niwooti Whangchai for guidance, technical assistance, equipments and laboratory facilities.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Abnisa F, Niya AA, Daud WMA, Sahu JN, Noor IM. Utilization of oil palm tree residues to produce bio-oil and bio-char via pyrolysis. Energy Convers Manag. 2013;76:1073–1082. doi: 10.1016/j.enconman.2013.08.038. [DOI] [Google Scholar]
- Amin FR, Khalid H, Zhang H, Rahman SU, Zhang R, Liu G, Chen C. Pretreatment methods of lignocellulosic biomass for anaerobic digestion. AMB Express. 2017;7:72. doi: 10.1186/s13568-017-0375-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelidaki I, Ellegaard L. Codigestion of manure and organic wastes in centralized biogas plants: status and future trends. Appl Biochem Biotechnol. 2003;109:95–105. doi: 10.1385/ABAB:109:1-3:95. [DOI] [PubMed] [Google Scholar]
- Angelidaki I, Sanders W. Assessment of the anaerobic biodegradability of macropollutants. Rev Environ Sci Biotechnol. 2004;3:117–129. doi: 10.1007/s11157-004-2502-3. [DOI] [Google Scholar]
- APHA-AWWA-WPCE . Standards methods for the examination of water and wastewater. 21. Washington, DC: APHA-AWWA-WPCF; 2005. [Google Scholar]
- Astals S, Musenze RS, Bai X, Tannock S, Tait S, Pratt S, Jensen PD. Anaerobic co-digestion of pig manure and algae: impact of intracellular algal products recovery on co-digestion performance. Bioresour Technol. 2015;181:97–104. doi: 10.1016/j.biortech.2015.01.039. [DOI] [PubMed] [Google Scholar]
- Barlaz MA, Ham RK, Schaefer DM. Massbalance analysis of anaerobically decomposed refuse. J Environ Eng ASCE. 1989;115:1088–1102. doi: 10.1061/(ASCE)0733-9372(1989)115:6(1088). [DOI] [Google Scholar]
- Bohutskyi P, Bouwer E. Biogas production from algae and cyanobacteria through anaerobic digestion: a review, analysis, and research needs. In: Lee JW, editor. Advanced biofuels and bioproducts. New York: Springer; 2013. pp. 873–975. [Google Scholar]
- Brettschneidera O, Thielea R, Fabera R, Thielertb H, Woznya G. Experimental investigation and simulation of the chemical absorption in a packed column for the system NH3–CO2–H2S–NaOH–H2O. Sep Purif Technol. 2004;39:139–159. doi: 10.1016/S1383-5866(03)00165-5. [DOI] [Google Scholar]
- Burton A, Wu H. Bed agglomeration during the drying of mallee leaf in fluidized bed. Ind Eng Chem Res. 2016;55:1796–1800. doi: 10.1021/acs.iecr.5b04479. [DOI] [Google Scholar]
- Buswell AM, Boruff CS. The relationship between chemical composition of organic matter and the quality and quantity of gas produced during sludge digestion. Sew Work J. 1932;4:454–460. [Google Scholar]
- Chen PH, Oswald WJ. Thermochemical treatment for algal fermentation. Environ Int. 1998;24:889–897. doi: 10.1016/S0160-4120(98)00080-4. [DOI] [Google Scholar]
- Chen Y, Cheng JJ, Kurt S. Creamer inhibition of anaerobic digestion process: a review. Bioresour Technol. 2008;99:4044–4064. doi: 10.1016/j.biortech.2007.01.057. [DOI] [PubMed] [Google Scholar]
- Chen T, Wu J, Zhang J, Wu J, Sun L. Gasification kinetic analysis of the three pseudocomponents of biomass-cellulose, semicellulose and lignin. Bioresour Technol. 2014;153:223–229. doi: 10.1016/j.biortech.2013.12.021. [DOI] [PubMed] [Google Scholar]
- Damartzis T, Vamvuka D, Sfakiotakis S, Zabaniotou A. Thermal degradation studies and kinetic modeling of cardoon (Cynara cardunculus) pyrolysis using thermogravimetric analysis (TGA) Bioresour Technol. 2010;102:6230–6238. doi: 10.1016/j.biortech.2011.02.060. [DOI] [PubMed] [Google Scholar]
- Danishac M, Naqvib M, Farooqc U, Naqvid S. Characterization of South Asian agricultural residues for potential utilization in future energy mix. Energy Procedia. 2015;75:2974–2980. doi: 10.1016/j.egypro.2015.07.604. [DOI] [Google Scholar]
- Devadiga A, Shetty KV, Saidutta MB. Timber industry waste-teak (Tectona grandis Linn.) leaf extract mediated synthesis of antibacterial silver nanoparticles. Int Nano Lett. 2015;5:205–214. doi: 10.1007/s40089-015-0157-4. [DOI] [Google Scholar]
- Dussadee N, Reansuwan K, Ramaraj R. Potential development of compressed bio-methane gas production from pig farms and elephant grass silage for transportation in Thailand. Bioresour Technol. 2014;155:438–441. doi: 10.1016/j.biortech.2013.12.126. [DOI] [PubMed] [Google Scholar]
- Dussadee N, Reansuwan K, Ramaraj R. Biotechnological application of sustainable biogas production through dry anaerobic digestion of Napier grass. 3 Biotech. 2017;7(1):47. doi: 10.1007/s13205-017-0646-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher TH, Pickett BM, Smith SG, Spittle GS, Woodhouse MM, Haake E. Effects of moisture on ignition behavior of moist California chaparral and Utah leaves. Combust Sci Technol. 2007;79:1183–1203. doi: 10.1080/00102200601015574. [DOI] [Google Scholar]
- García R, Pizarro C, Bueno AG. Spanish biofuels heating value estimation. Part II: proximate analysis data. Fuel. 2014;117:1139–1147. doi: 10.1016/j.fuel.2013.08.049. [DOI] [Google Scholar]
- Grover PD, Iyer PVR, Rao TR. Biomass-thermochemical characterization. 3. New Delhi: IIT-Delhi and MNES; 2002. [Google Scholar]
- Herrmann C, Kalita N, Wall D, Xia A, Murphy JD. Optimised biogas production from microalgae through co-digestion with carbon-rich co-substrates. Bioresour Technol. 2016;214:328–337. doi: 10.1016/j.biortech.2016.04.119. [DOI] [PubMed] [Google Scholar]
- Huang YF, Kuan WH, Chiueh PT, Lo SL. Pyrolysis of biomass by thermal analysis–mass spectrometry (TA–MS) Bioresour Technol. 2011;102:3527–3534. doi: 10.1016/j.biortech.2010.11.049. [DOI] [PubMed] [Google Scholar]
- Liu Z, Quek A, Hoekman SK, Balasubramanian R. Production of solid biochar fuel from waste biomass by hydrothermal carbonization. Fuel. 2013;103:943–949. doi: 10.1016/j.fuel.2012.07.069. [DOI] [Google Scholar]
- Maia BGDO, Souzab O, Marangonib C, Hotzaa D, Oliveiraa APND, Sellin N. Production and characterization of fuel briquettes from banana leaves waste. Chem Eng Trans. 2014;37:439–444. [Google Scholar]
- Mancini G, Papirio S, Lens PNL, Esposito G. Solvent pretreatments of lignocellulosic materials to enhance biogas production: a review. Energy Fuels. 2016;30:1892–1903. doi: 10.1021/acs.energyfuels.5b02711. [DOI] [Google Scholar]
- Mata-Alvarez J, Dosta J, Romero-Güiza MS, Fonoll X, Peces M, Astals S. A critical review on anaerobic co-digestion achievements between 2010 and 2013. Renew Sust Energy Rev. 2014;36:412–427. doi: 10.1016/j.rser.2014.04.039. [DOI] [Google Scholar]
- Mishra V, Balomajumder C, Agarwal VK. Zn(II) ion biosorption onto surface of eucalyptus leaf biomass: isotherm, kinetic, and mechanistic modeling. Clean Soil Air Water. 2010;38:1062–1073. doi: 10.1002/clen.201000030. [DOI] [Google Scholar]
- Oke DG, Ogunjimi BE. Comparative analysis of medicinal properties of the bark and leaves of Tectona grandis. Chem Sci Rev Lett. 2016;5:27–30. [Google Scholar]
- Pandey D, Brown C. An overview of global teak resources and issues affecting future outlook. Teak: a global overview. Rome: Forest Resources Development Service, FAO; 2000. pp. 3–12. [Google Scholar]
- Pantawong R, Chuanchai A, Thipbunrat P, Unpaprom Y, Ramaraj R. Experimental investigation of biogas production from water lettuce, Pistia stratiotes L. Emergent Life Sci Res. 2015;1:41–46. [Google Scholar]
- Pavlostathis SG, Giralo-Gomez E. Kinetics of anaerobic treatment: a critical review. Crit Rev Environ Control. 1991;21:411–490. doi: 10.1080/10643389109388424. [DOI] [Google Scholar]
- Raju CAI, Jyothi KR, Satya M, Praveena U. Studies on development of fuel briquettes for household and industrial purpose. IJRET. 2014;2:2321–7308. [Google Scholar]
- Ramaraj R, Dussadee N. Biological purification processes for biogas using algae cultures: a review. IJSGE. 2015;4:20–32. [Google Scholar]
- Ramaraj R, Dussadee N, Whangchai N, Unpaprom Y. Microalgae biomass as an alternative substrate in biogas production. IJSGE. 2015;4:13–19. [Google Scholar]
- Ramaraj R, Kawaree R, Unpaprom Y. A newly isolated green alga, Pediastrum duplex Meyen, from Thailand with efficient hydrogen production. IJSGE. 2015;4:7–12. [Google Scholar]
- Ramaraj R, Unpaprom Y, Whangchai N, Dussadee N. Culture of macroalgae spirogyra ellipsospora for long-term experiments, stock maintenance and biogas production. Emergent Life Sci Res. 2015;1:38–45. [Google Scholar]
- Ramaraj R, Unpaprom Y, Dussadee N. Cultivation of green microalga, Chlorella vulgaris, for biogas purification. IJNTR. 2016;2:117–122. [Google Scholar]
- Rouf MA, Bajpai PK, Jotshi CK. Optimization of biogas generation from press mud in batch reactor. Bangladesh J Sci Ind Res. 2010;45:371–376. [Google Scholar]
- Rouf MA, Islam MS, Rabeya T, Mondal AK. Anaerobic digestion of mixed dried fallen leaves by mixing with cow dung. Bangladesh J Sci Ind Res. 2015;50:163–168. doi: 10.3329/bjsir.v50i3.25579. [DOI] [Google Scholar]
- Sait HH, Hussain A, Salema AA, Ani FN. Pyrolysis and combustion kinetics of date palm biomass using thermogravimetric analysis. Bioresour Technol. 2012;118:382–389. doi: 10.1016/j.biortech.2012.04.081. [DOI] [PubMed] [Google Scholar]
- Sugumaran P, Seshadri S. Evaluation of selected biomass for charcoal production. J Sci Ind Res. 2009;68:719–723. [Google Scholar]
- Thangamani A, Rajakumar S, Ramanujam RA. Anaerobic co-digestion of hazardous tannery solid waste and primary sludge: biodegradation kinetics and metabolite analysis. Clean Technol Environ Policy. 2010;12:517–524. doi: 10.1007/s10098-009-0256-x. [DOI] [Google Scholar]
- Unpaprom Y, Intasaen O, Yongphet P, Ramaraj R. Cultivation of microalga Botryococcus braunii using red Nile tilapia effluent medium for biogas production. J Ecol Environ Sci. 2015;3:58–65. [Google Scholar]
- Unpaprom Y, Tipnee S, Ramaraj R. Biodiesel from green algae Scenedesmus acuminatus. IJSGE. 2015;4:1–6. [Google Scholar]
- Viswanath P, Sumithra S, Nand K. Anaerobic digestion of fruit and vegetable processing wastes for biogas production. Bioresour Technol. 1992;40:43–48. doi: 10.1016/0960-8524(92)90117-G. [DOI] [Google Scholar]
- Von Sperling M, Chemicharo CAL. Biological wastewater treatment in warm climate regions. London: IWA Publishing London; 2005. [Google Scholar]
- Wannapokin A, Ramaraj R, Unpaprom Y. An investigation of biogas production potential from fallen teak leaves (Tectona grandis) Emer Life Sci Res. 2017;3:1–10. [Google Scholar]
- Xiao R, Chen X, Wang F, Yu G. The physicochemical properties of different biomass ashes at different ashing temperature. Renew Energy. 2011;36:244–249. doi: 10.1016/j.renene.2010.06.027. [DOI] [Google Scholar]
- Yan J (2015) Handbook of clean energy systems, vol. 6. Sustainability of energy systems. Wiley
- Zhou H, Long Y, Meng A, Li Q, Zhang Y. The pyrolysis simulation of five biomass species by hemi-cellulose, cellulose and lignin based on thermogravimetric curves. Thermochim Acta. 2013;566:36–43. doi: 10.1016/j.tca.2013.04.040. [DOI] [Google Scholar]
- Zhou H, Long Y, Meng A, Li Q, Zhang Y. Classification of municipal solid waste components for thermal conversion in waste-to-energy research. Fuel. 2015;145:151–157. doi: 10.1016/j.fuel.2014.12.015. [DOI] [Google Scholar]