Abstract
Platelet-derived growth factor receptor α (PDGFRα), a tyrosine kinase receptor, is up-regulated in hepatic stellate cells (HSCs) during chronic liver injury. HSCs mediate hepatic fibrosis through their activation from a quiescent state partially in response to profibrotic growth factors. HSC activation entails enhanced expression of profibrotic genes, increase in proliferation, and increase in motility, which facilitates migration within the hepatic lobule. We show colocalization of PDGFRα in murine carbon tetrachloride, bile duct ligation, and 0.1% 3,5-diethoxycarbonyl-1,4-dihydrocollidine models of chronic liver injury, and investigate the role of PDGFRα on proliferation, profibrotic gene expression, and migration in primary human HSCs (HHSteCs) using the PDGFRα-specific inhibitory monoclonal antibody olaratumab. Although lacking any effects on HHSteC transdifferentiation assessed by gene expression of ACTA2, TGFB1, COL1A1, SYP1, and FN1, olaratumab specifically reduced HHSteC proliferation (AlamarBlue assay) and cell migration (transwell migration assays). Using phospho-specific antibodies, we show that olaratumab attenuates PDGFRα activation in response to PDGF-BB, and reduced phosphorylation of extracellular signal–regulated kinase 1 and 2, Elk-1, p38, Akt, focal adhesion kinase, mechanistic target of rapamycin, C10 regulator of kinase II, and C10 regulator of kinase-like, suggesting that PDGFRα contributes to mitogenesis and actin reorganization through diverse downstream effectors. Our findings support a distinct contribution of PDGFRα signaling to HSC proliferation and migration and provide evidence that inhibition of PDGFRα signaling could alter the pathogenesis of hepatic fibrosis.
Hepatic fibrosis is a manifestation of wound healing in chronic liver disease, resulting from dysregulated and excessive collagen deposition by activated hepatic stellate cells (HSCs). This process is mediated by the release of paracrine and autocrine growth factors, and inflammatory chemokines from injured hepatocytes, infiltrating inflammatory cells, and HSCs themselves.1 During the process of liver injury, HSCs undergo a process of transdifferentiation toward a myofibroblast phenotype characterized by increased proliferation as well as altered gene transcription.2 Also, HSCs become motile and migrate within the hepatic lobule to the sites of injury and inflammation guided by chemotactic stimuli, such as platelet-derived growth factors (PDGFs), transforming growth factor-β1 (TGF-β1), and type I collagen.3, 4 In the setting of chronic liver injury, HSCs continue to migrate to the areas of injury and facilitate the progression of hepatic fibrosis.5
PDGFs form five known dimers (AA, AB, BB, CC, and DD), which bind with varying specificity to PDGF receptors (PDGFRs) that exist as α or β monomers.6 On PDGF binding, they form αα, αβ, and ββ dimers and trigger reciprocal tyrosine phosphorylation of specific residues of each receptor.7 Phosphorylation of tyrosine residues in the kinase domain increases catalytic efficiency and serves as binding sites for signaling molecules, including other kinases as well as nonenzymatic adaptor molecules. Both PDGFRβ1, 8, 9 and PDGFRα10, 11, 12, 13 are expressed in HSCs and play important profibrotic roles in chronic liver injury.
A diverse set of downstream effectors mediate an array of PDGF-induced cellular activities. These include mitogen-activated protein kinases, such as extracellular signal–regulated kinase 1 and 2 (Erk1/2) and p38, which are central in the activation, proliferation, and migration of HSCs.14, 15, 16, 17 Factors, such as Akt, are also common targets of PDGF signaling and can function downstream of focal adhesion kinase (FAK), which plays a dual role in cell adhesion and PDGF-induced HSC proliferation.18 Mechanistic target of rapamycin (mTOR) activation can contribute to cellular processes associated with HSC proliferation19 and migration.20 Adaptor proteins also play an important function in PDGF signaling. C10 regulator of kinase II (CrkII) and Crk-like (CrkL) form a stable complex with PDGFRα and are one of the few identified distinctions between downstream effectors of PDGFRα and PDGFRβ.7, 21, 22
Although the cellular function and downstream signaling mediators of PDGFRβ signaling in HSCs have been extensively studied,8, 9, 23 the precise role of PDGFRα in these cells remains unclear.24 Olaratumab (Eli-Lilly & Co., New York, NY) is a humanized monoclonal antibody that targets PDGFRα with high potency and specificity and has been used in both preclinical and clinical studies.25, 26, 27 In the liver, PDGFRα was shown to be up-regulated in a subset of hepatocellular cancers and olaratumab treatment showed a notable decrease in hepatoma cell proliferation and survival.28 The availability of olaratumab provides a unique opportunity to investigate the specific role of PDGFRα in HSCs as well. In the current study, we first show enhanced expression of PDGFRα in HSCs and myofibroblasts in carbon tetrachloride, bile duct ligation, and 3,5-diethoxycarbonyl-1,4-dihydrocollidine models of chronic liver injury. We then examine the role of PDGFRα in human HSCs and show that selective inhibition of PDGFRα with olaratumab inhibits HSC proliferation and migration in vitro, but does not affect profibrotic gene expression. We also show that these cell functions are differentially mediated via multiple downstream signaling pathways, including Erk1/2, p38, Elk-1, Akt, mTOR, FAK, and CrkII/CrkL. Finally, we discuss the implications of using a clinically relevant PDGFRα inhibitor in the treatment of hepatic fibrosis.
Materials and Methods
Mice
Two-month-old C57BL/6 mice were obtained from Jackson Laboratory (Bar Harbor, ME). All animal experiments and procedures were performed under the strict guidelines of the NIH's Guide for the Care and Use of Laboratory Animals29 and after approval by the Institutional Animal Use and Care Committees at the University of Pittsburgh (Pittsburgh, PA).
Liver Fibrosis and Injury Models
As a model of toxic liver injury, animals were injected twice weekly for 8 weeks i.p. with 1:3 carbon tetrachloride diluted in corn oil at a volume of 0.5 μL carbon tetrachloride/g mouse body weight. Controls were injected with corn oil only. Bile duct ligation was performed under anesthesia as a model of cholestatic liver injury by opening the peritoneal cavity to expose the common bile duct, which is cut between two 5-0 silk ligatures. Blood and livers were collected for analysis at 14 days. For a secondary model of cholestatic liver injury and atypical ductular proliferation, mice were fed a special diet containing 0.1% 3,5-diethoxycarbonyl-1,4-dihydrocollidine (Bioserve, Frenchtown, NJ) for 16 days.
Immunofluorescence
Liver sections (6 μm thick) were stained after detergent permeabilization and 37°C incubation, followed by blocking in 2% bovine serum albumin in phosphate-buffered saline (PBS). Serial confocal images (1 μm thick) were obtained using an Olympus FluoView 1000 microscope (Olympus, Center Valley, PA) and FV1000 ASW software version 4.2 (Olympus).
Cell Culture
Human HSCs (HHSteCs; catalog number 5300, lot number 10326) were purchased on two occasions from ScienCell Research Laboratories (Carlsbad, CA) and were cultured according to the company's provided protocol. Cells were used before the sixth passage and serum starved 12 hours before treatment by washing cells twice with sterile, cold PBS, followed by serum-free Stellate Cell Medium (ScienCell Research Laboratories). LX-2 cells were kindly provided by Dr. Scott Friedman (Mount Sinai Hospital, New York, NY) and cultured according to a protocol from the commercial provider (Millipore, Billerica, MA). Cells were cultured/expanded in Techno Plastic Product–treated T75 tissue culture flasks (Sigma-Aldrich, St. Louis, MO). Recombinant human PDGF-AA (R&D Systems, Minneapolis, MN) or PDGF-BB (Sigma-Aldrich) and TGF-β1 (R&D Systems) were diluted with serum-free media and exposed to cells for the indicated time periods after two washes with sterile, cold PBS. For PDGFRα inhibition, PDGFRα-specific human monoclonal antibody inhibitor olaratumab (LY3012207, IMC-3G3) or human IgG (Equitech, Kerrville, TX) was diluted in serum-free media and used at the indicated concentrations after two washes with sterile, cold PBS.
Protein-Binding Assays
PDGFRα and PDGFRβ extracellular domains were purchased from R&D Systems. IgGs were evaluated for binding to PDGFRs in a direct binding assay, as previously described.25 Antibody 2C5 serves as a positive control anti-PDGFRβ antibody.30
Protein Extraction and Western Blotting
Western blots were performed on cell culture lysates homogenized in radioimmunoprecipitation assay buffer with protease/phosphatase inhibitors (Thermo Scientific, Waltham, MA). Protein was quantified using bicinchoninic acid protein assay, and Western blotting was performed as previously described using commercially available primary antibodies from Santa Cruz Biotechnology (Dallas, TX) and Cell Signaling Technology (Danvers, MA) (Table 1).28 Western blots from pooled samples from three technical replicates from each batch of stellate cells were performed at least twice. Representative Western blots from three pooled technical replicates for each time point are shown. Densitometry was performed on these representative Western blots. For detection of PDGFs in HHSteC media, media were concentrated after 24 or 48 hours of serum starvation using Amicon Ultra-15 Centrifugal Filters (Millipore) and resuspended in sample buffer to perform western blot analysis, as described earlier in this paragraph.
Table 1.
List of Primary Antibodies Used in the Study
| Primary antibody | Species | Dilution | Block | Company | Catalog no. |
|---|---|---|---|---|---|
| Akt | Rabbit | 1:1000 | 5% BSA | CST (Danvers, MA) | 4685 |
| P-S473-Akt | Rabbit | 1:1000 | 5% BSA | CST | 4060 |
| c-Abl | Rabbit | 1:1000 | 5% BSA | CST | 2862 |
| P-Y89-c-Abl | Rabbit | 1:1000 | 5% BSA | CST | 3098 |
| P-c-Abl Y412 | Rabbit | 1:1000 | 5% BSA | CST | 2865 |
| CrkII | Rabbit | 1:1000 | 5% BSA | CST | 3492 |
| P-Y221-CrkII | Mouse | 1:1000 | 5% Milk | CST | 3491 |
| CrkL | Rabbit | 1:1000 | 5% BSA | CST | 3182 |
| P-Y207-CrkL | Rabbit | 1:1000 | 5% BSA | CST | 3181 |
| Elk-1 | Rabbit | 1:1000 | 5% BSA | CST | 9182 |
| P-S383 Elk-1 | Rabbit | 1:1000 | 5% BSA | CST | 9181 |
| Erk1/2 | Rabbit | 1:1000 | 5% BSA | CST | 4695 |
| P-T202/Y204-Erk1/2 | Rabbit | 1:1000 | 5% BSA | CST | 4370 |
| FAK | Rabbit | 1:1000 | 5% BSA | CST | 13009 |
| P-Y397-FAK | Rabbit | 1:1000 | 5% BSA | CST | 8556 |
| P-Y576/577-FAK | Rabbit | 1:1000 | 5% BSA | CST | 3281 |
| P-Y925-FAK | Rabbit | 1:1000 | 5% BSA | CST | 3284 |
| P-S2448-mTOR | Rabbit | 1:500 | 5% BSA | CST | 2971 |
| P-S2481-mTOR | Rabbit | 1:1000 | 5% BSA | CST | 2974 |
| p38 | Rabbit | 1:1000 | 5% BSA | CST | 8690 |
| P-T180/Y182-p38 | Rabbit | 1:200 | 5% Milk | CST | 4511 |
| PDGFA | Mouse | 1:200 | 5% Milk | SCBT (Dallas, TX) | sc-9974 |
| PDGFB | Rabbit | 1:1000 | 5% Milk | Abcam (Cambridge, UK) | ab23914 |
| PDGFC | Goat | 1:200 | 3% BSA | R&D Systems (Minneapolis, MN) | AF1560 |
| PDGFD | Goat | 1:1000 | 5% BSA | R&D Systems | AF1159 |
| PDGFRα | Rabbit | 1:300 | 5% Milk | SCBT | sc-338 |
| PDGFRβ | Rabbit | 1:1000 | 5% BSA | SCBT | sc-432 |
| P-Y572/574-PDGFRα/β | Rabbit | 1:1000 | 5% BSA | Invitrogen (Carlsbad, CA) | 44-1000G |
| P-Y742-PDGFRα | Rabbit | 1:1000 | 5% BSA | Invitrogen | 44-1006 |
| P-Y762-PDGFRα | Rabbit | 1:1000 | 5% BSA | CST | 24188 |
| P-Y849/βY857-PDGFRα | Rabbit | 1:1000 | 5% BSA | CST | 3170 |
| P-Y1018-PDGFRα | Rabbit | 1:1000 | 5% BSA | CST | 4547 |
| Pan-phospho-PKC | Rabbit | 1:1000 | 5% BSA | CST | 9371 |
BSA, bovine serum albumin; Crk, C10 regulator of kinase; CrkL, Crk-like; CST, Cell Signaling Technology; Erk1/2, extracellular signal–regulated kinase 1 and 2; FAK, focal adhesion kinase; mTOR, mechanistic target of rapamycin; PDGF, platelet-derived growth factor; PDGFR, PDGF receptor; PKC, protein kinase C; SCBT, Santa Cruz Biotechnology.
Immunoprecipitation
Coprecipitation studies were performed with 500 μg protein lysate in radioimmunoprecipitation assay buffer with protease/phosphatase inhibitors. Mouse monoclonal anti-CrkII (Santa Cruz Biotechnology) primary antibody (2 μg) was incubated with sample for 3 hours, followed by overnight conjugation to Protein G PLUS-Agarose beads (Santa Cruz Biotechnology; sc-2002). Total and phospho-PDGFRα were detected using the same antibodies indicated in Table 1.
RT-PCR
RT-PCR was performed on RNA isolated using TRIzol (Thermo Scientific) RNA extraction from adherent cell cultures. RNA extraction was performed using Direct-zol RNA MiniPrep Plus kit (Zymo Research, Irvine, CA) and following the manufacturer's protocol. Real-time PCR was performed using Power SYBR Green (Thermo Scientific), 100 ng of cDNA, and 0.2 μmol/L of forward and reverse primers. Primers used in this study are listed in Table 2. Data were analyzed using the comparative ΔΔCt method or PCR product was run on 0.8% agarose gel with ethidium bromide and detected by UV lamp. Statistical analysis and graphs were performed in Prism version 7.0a (GraphPad Software, San Diego, CA) using one-way analysis of variance.
Table 2.
RT-PCR Primers Used in this Study
| Gene | Primer sequence | Amplicon, bp |
|---|---|---|
| ACTA2 | ||
| Forward | 5′-TTCATCGGGATGGAGTCTGCTGG-3′ | 141 |
| Reverse | 5′-TCGGTCGGCAATGCCAGGGT-3′ | |
| COL1A1 | ||
| Forward | 5′-TCGTCACAGATCACGTCATCG-3′ | 120 |
| Reverse | 5′-AATCACCTGCGTACAGAACGG-3′ | |
| PDGFA | ||
| Forward | 5′-CACACCTCCTCGCTGTAGTATTTA-3′ | 220 |
| Reverse | 5′-GTTATCGGTGTAAATGTCATCCAA-3′ | |
| PDGFB | ||
| Forward | 5′-ACTCGATCCGCTCCTTTGATGA-3′ | 111 |
| Reverse | 5′-GCTCGCCTCCAGAGTGGG-3′ | |
| PDGFC | ||
| Forward | 5′-TCACAGCCCAAGGTTTCCTC-3′ | 100 |
| Reverse | 5′-CCACACCAGCGCCCTAATAT-3′ | |
| PDGFD | ||
| Forward | 5′-GAACAGCTACCCCAGGAACC-3′ | 100 |
| Reverse | 5′-CTTGTGTCCACACCATCGTC-3′ | |
| FN1 | ||
| Forward | 5′-GGCTGACAGAGAAGATTCCCGAGAG-3′ | 87 |
| Reverse | 5′-CCAGTTTAGATGGATCTTGGCAGAGAGAC-3′ | |
| SYP | ||
| Forward | 5′-GCAATGGGTCTTCGCCATCT-3′ | 134 |
| Reverse | 5′-GCCTGAAGGGGTACTCGAAC-3′ | |
| TGFB1 | ||
| Forward | 5′-CCCTGGACACCAACTATTGC-3′ | 75 |
| Reverse | 5′-TGCGGAAGTCAATGTACAGC-3′ | |
| GAPDH | ||
| Forward | 5′-GAAGGTGAAGGTCGGAGTC-3′ | 226 |
| Reverse | 5′-GAAGATGGTGATGGGATTTC-3′ | |
Proliferation Assays
For proliferation assays, HHSteCs were plated on 48-well plates (BD Falcon; BD Biosciences, Bedford, MA) at an initial plating density of 50,000 cells/well. Cells were serum starved the following day by gentle washing with chilled PBS, followed by culturing in serum-free media overnight. Cells were treated the next day with serum-free media [nontreated (NT)], PDGF-AA, PDGF-BB, olaratumab, or IgG at the indicated concentrations for 24 hours. Next, 20 μL of AlamarBlue Cell Viability Reagent (Thermo Scientific) was added to each well and incubated at 37°C for 1 hour before measuring fluorescence readings at 590-nm wavelength. Statistical analysis and graphs were performed in Prism version 7.0a using one- and two-way analysis of variance.
Transwell Migration Assays
All cells were serum starved for 12 hours before use in migration assays. Polycarbonate filters (8-μm pore; Costar; Fisher Scientific, Waltham, MA) were precoated with 100 μL collagen I (Sigma-Aldrich) and washed two times with sterile PBS before adding cell suspension. Immediately before addition of cell suspension, olaratumab, control IgG, or PDGF-BB was added to the lower chamber in serum-free culture medium. HHSteCs were suspended in serum-free media and plated at 2000 cells in 100 μL/well. After incubation at 37°C for 3 hours, well inserts were fixed with 4% paraformaldehyde and stained by crystal violet. The number of migrated cells was determined by counting the number of cells per representative ×10 field by light microscopy. Statistical analysis and graphs were performed in Prism Version 7.0a (GraphPad Software, Inc., La Jolla, CA) using nonparametric t-tests.
Results
PDGFRα Expression in Murine and Human Hepatic Stellate Cells
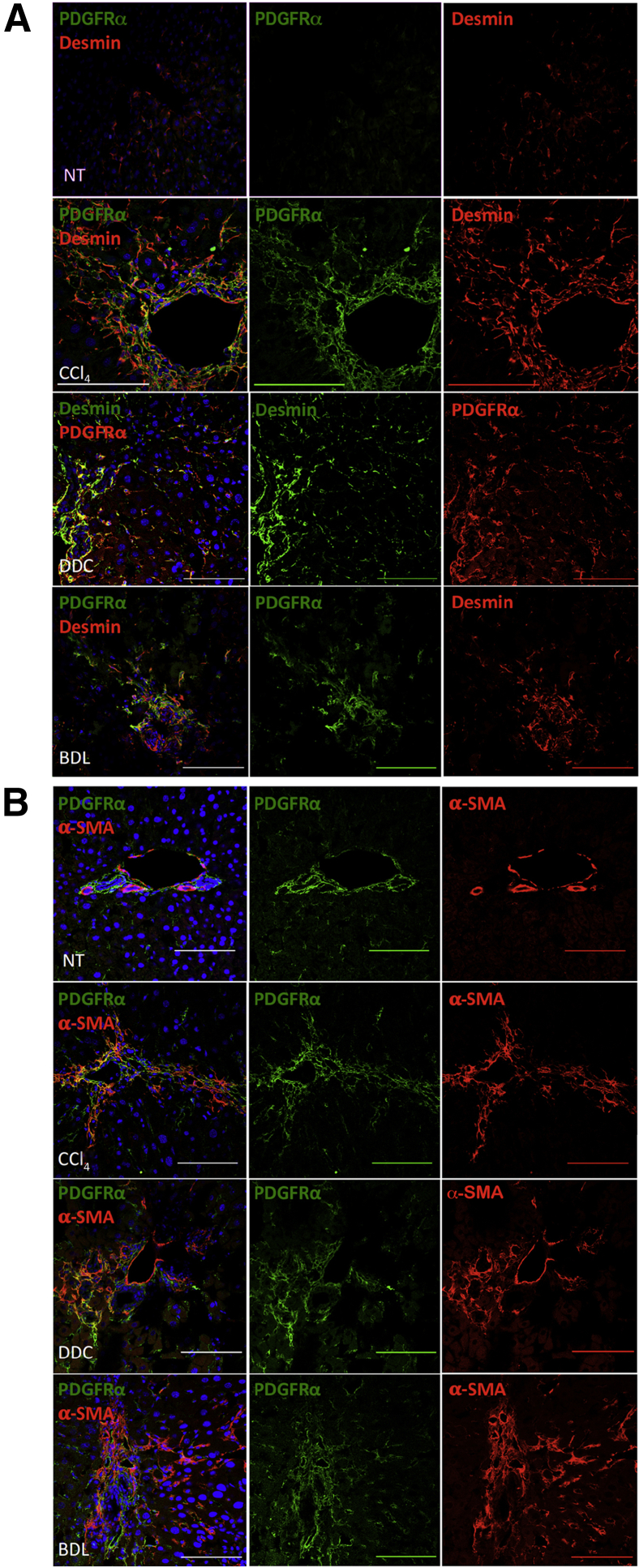
To confirm the expression and pathological relevance of PDGFRα in vivo, we examined PDGFRα expression in livers of C56BL/6 mice at baseline as well as after chronic toxic (8 weeks of carbon tetrachloride administration) and cholestatic (14-day bile duct ligation or 16-day 3,5-diethoxycarbonyl-1,4-dihydrocollidine) liver injury. Using confocal microscopy, we observed close colocalization of PDGFRα in desmin-positive and α-smooth muscle actin–positive cells, representing HSC and activated myofibroblast populations, respectively (Figure 1).
Figure 1.
PDGFRα expression in activated hepatic stellate cells in rodent models of liver fibrosis. A: Confocal immunofluorescence images of murine livers after 8 weeks of carbon tetrachloride (CCl4), 16 days of 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC), or 14 days of bile duct ligation (BDL) show PDGFRα expression colocalizing with desmin. B: Confocal immunofluorescence images of murine livers after 8 weeks of CCl4, 16 days of DDC, or 14 days of BDL show PDGFRα expression colocalizing with α-smooth muscle actin (α-SMA). Nontreated (NT) livers are shown for comparison. Scale bars = 100 μm (A and B).
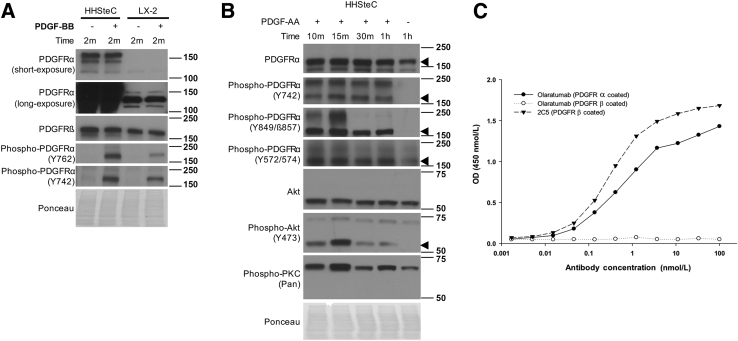
Next, to identify suitable HSCs for studies of PDGFRα, we examined its expression and activation in primary HHSteCs and LX-2, an immortalized HSC line. Although PDGFRβ was present in both cell types, expression of PDGFRα was more profound in HHSteCs (Figure 2A). Furthermore, increased PDGFRα phosphorylation in response to PDGF-BB stimulation was also evident (Figure 2A). LX-2 cells also showed PDGFRα phosphorylation in response to PDGF-BB, albeit to a lesser extent (Figure 2A). Therefore, because of greater basal PDGFRα expression in HHSteCs and activation in response to the highly mitogenic and well-characterized profibrotic ligand PDGF-BB, we opt to use these cells as a representative model of PDGFRα-expressing HSCs for further study.
Figure 2.
PDGFRα expression and activity in hepatic stellate cells. A: HHSteC and LX-2 cells show differential expression of PDGFRα at baseline and a corresponding increase in phosphorylated receptor after 10 ng/mL PDGF-BB treatment for 2 minutes (m). B: HHSteCs stimulated with 10 ng/mL PDGF-AA for the indicated time periods show increased PDGFRα phosphorylation at Y742, Y849, and Y572/574. Associated downstream signaling mediators Akt and protein kinase C (PKC) also showed increased phosphorylation in response to PDGF-AA treatment. Arrowheads indicate the correct molecular weight band. C: Direct binding of human antibodies to immobilized (coated) human PDGFRα or PDGFRβ in an enzyme-linked immunosorbent assay. Antibody 2C5 serves as a positive control anti-PDGFRβ antibody. Ponceau staining is included as a loading control. h, hours.
To assess if PDGFRα is functional in HHSteCs, these cells were treated with PDGFRα-specific ligand PDGF-AA. This led to phosphorylation of PDGFRα at key tyrosine signaling residues (Figure 2B). Furthermore, PDGF-AA treatment induced phosphorylation of downstream Akt and protein kinase C (Figure 2B).
To block PDGFRα, we used the fully human anti-PDGFRα antibody olaratumab.31, 32 To validate the efficacy and specificity of olaratumab, an enzyme-linked immunosorbent assay–based cell-free binding assay was performed. Olaratumab showed a dose-dependent binding to immobilized PDGFRα extracellular domain (Figure 2C). Furthermore, the antibody concentration required for 50% maximum binding to PDGFRα extracellular domain was calculated to be approximately 0.06 nmol/L, as also reported previously.25 Olaratumab did not cross-react with a human PDGFRβ extracellular domain (Figure 2C).
PDGF Treatment Induces PDGFRα-Dependent HHSteC Proliferation, but Not Profibrotic Gene Expression
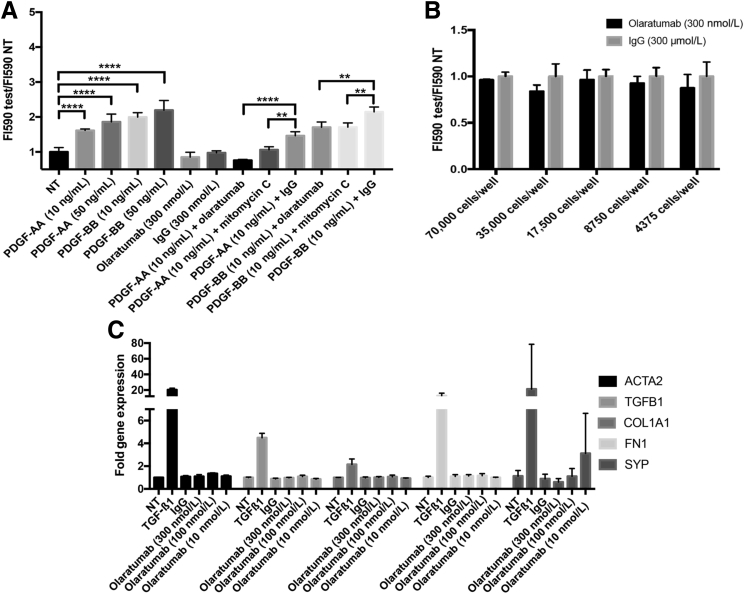
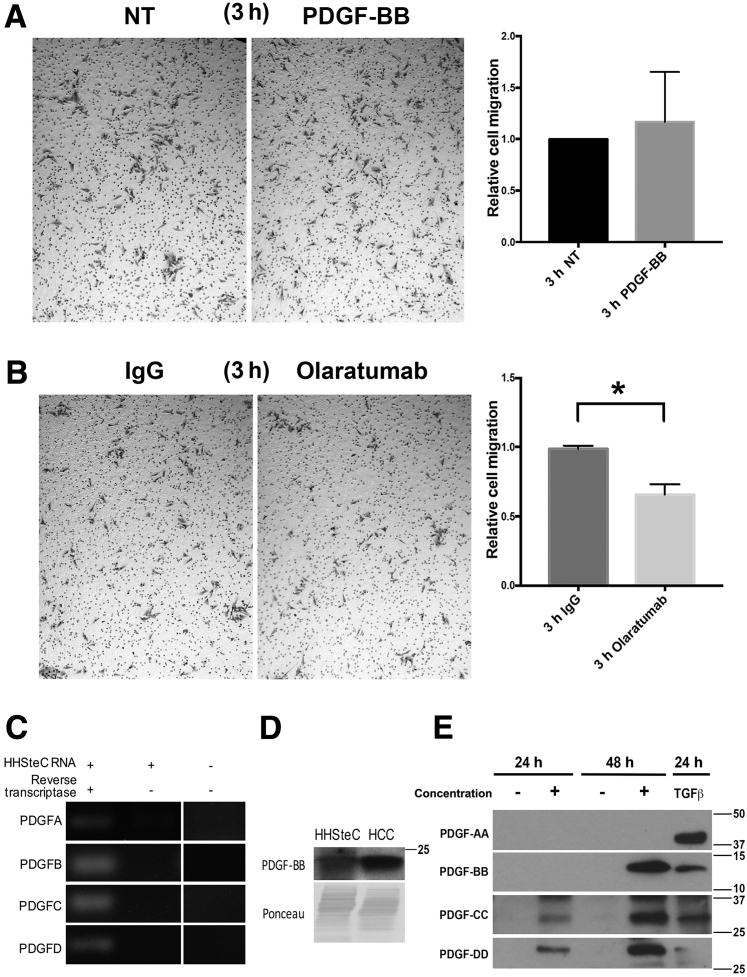
PDGF-BB signaling through PDGFRβ is well-known to play a central role in HSC activation, including proliferation and transdifferentiation to an activated myofibroblast state. However, the specific contribution of PDGFRα in these processes is unknown. To test unambiguously the contribution of PDGFRα to HSC proliferation, we used the AlamarBlue cell viability assay after 24-hour exposure to PDGF-AA and PDGF-BB in the presence of PDGFRα-specific blocker olaratumab or control IgG (Figure 3A). We observed an increase in HHSteC proliferation in response to PDGF-AA and PDGF-BB when compared to NT controls. Although olaratumab alone was insufficient to affect HHSteC proliferation (Figure 3, A and B), incubation of these cells with olaratumab in the presence of either exogenous PDGF-AA or PDGF-BB resulted in a significant decrease in proliferation (Figure 3A).
Figure 3.
PDGFRα signaling contributes to HHSteC proliferation, but not activation, in the presence of exogenous PDGF stimulation. A: Proliferation of HHSteCs was measured using AlamarBlue dye after a 24-hour incubation with PDGF-AA, PDGF-BB, olaratumab, or IgG at the indicated concentrations. PDGF-AA and PDGF-BB induced significant proliferation of HHSteCs. HHSteCs were also exposed simultaneously to PDGF and olaratumab, PDGF and IgG, or PDGF and mitomycin C (DNA synthesis inhibitor). Both olaratumab and mitomycin C cause a reduction in PDGF-induced proliferation compared to IgG control. B: PDGFRα inhibition using olaratumab tested over 24 hours at various plating densities shows no significant change in proliferation compared to IgG-treated controls. C: RT-PCR shows no significant changes in expression of ACTA2, TGFB1, COL1A1, FN1, or SYP in HHSteCs after olaratumab treatment at multiple concentrations. TGF-β1 (2 ng/mL) treatment was included as a positive control. All assays were performed in triplicate, and results are normalized to their respective nontreatment (NT) conditions. ∗∗P < 0.01, ∗∗∗∗P < 0.0001.
Next, we examined any effect of olaratumab on profibrotic gene expression. HSC activation is associated with increased expression of genes like collagen (COL1A1), TGFB1, α-smooth muscle actin (ACTA2), fibronectin (FN1), and synaptophysin (SYP). Olaratumab treatment for 24 hours led to no significant change in expression of any of these genes (Figure 3C). Also, treatment of HHSteCs with either PDGF-AA or PDGF-BB led to no change in the profibrotic gene expression (data not shown).
Olaratumab Inhibits PDGF-BB–Mediated HSC Proliferation through Blockade of PDGFRα and Downstream Signaling
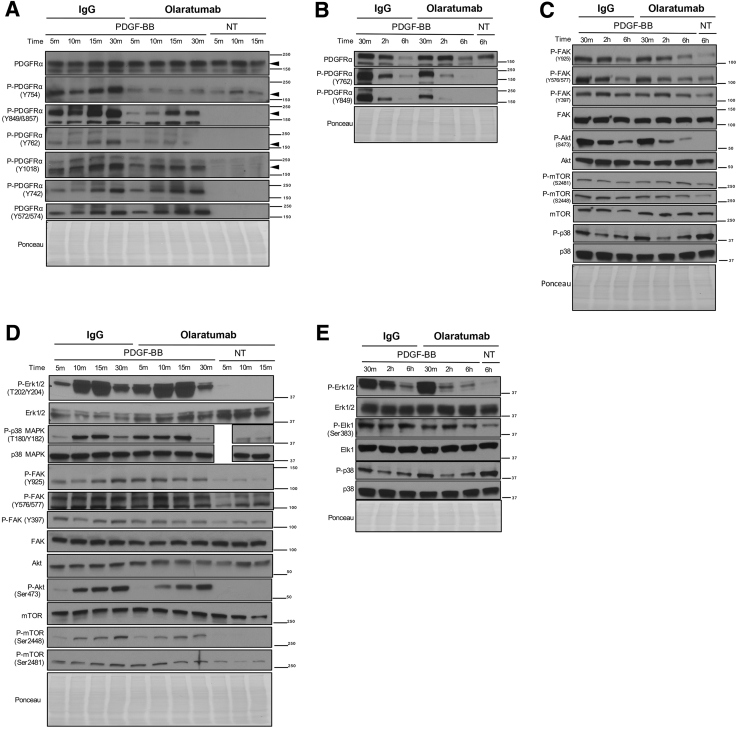
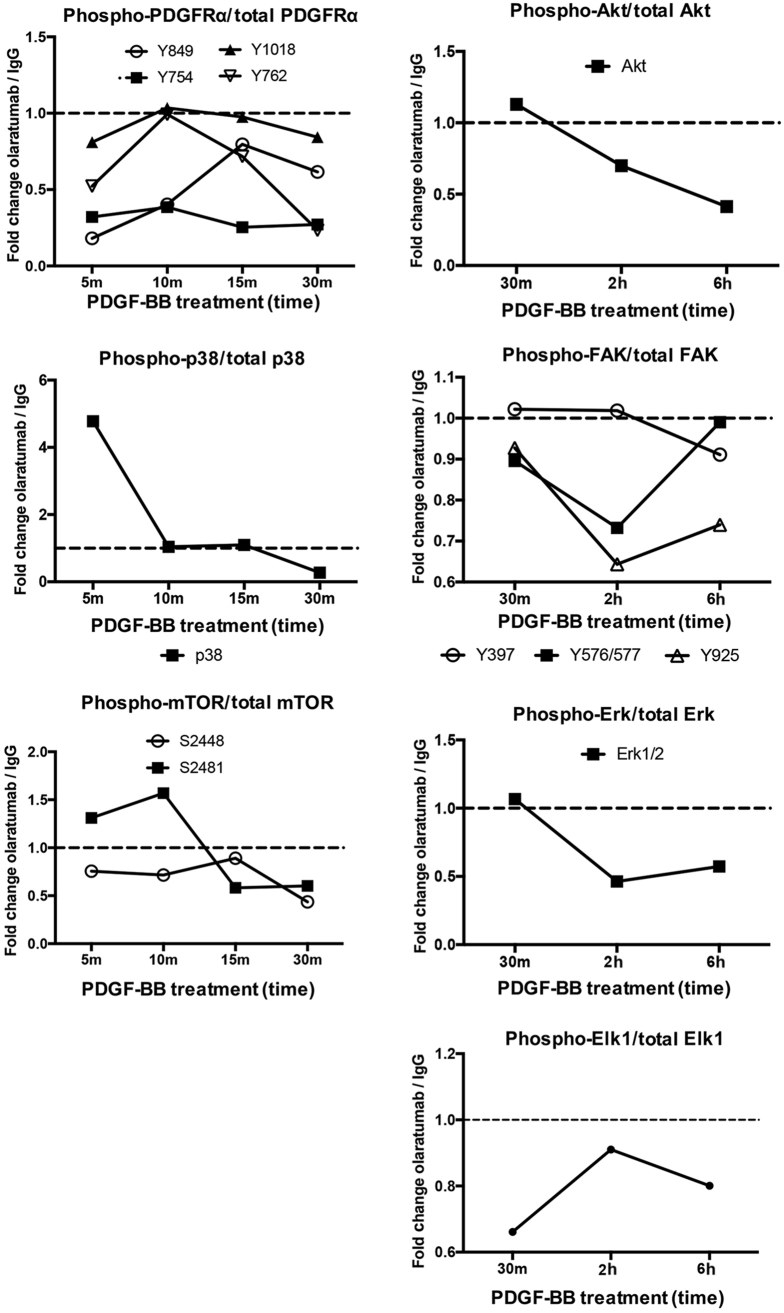
We next sought to determine the signaling pathways downstream of PDGFRα affecting HSC proliferation. To assess the most physiologically relevant signaling pathways involved, we tested the effect of olaratumab in the presence of PDGF-BB (a nonselective isoform of PDGF with a central role in HSC mitogenesis, chemotaxis, and activation during hepatic fibrosis).1, 33 HHSteCs were pretreated with 300 nmol/L olaratumab or 300 nmol/L human IgG for 30 minutes before exposure to PDGF-BB for various times (range, 5 minutes to 6 hours).
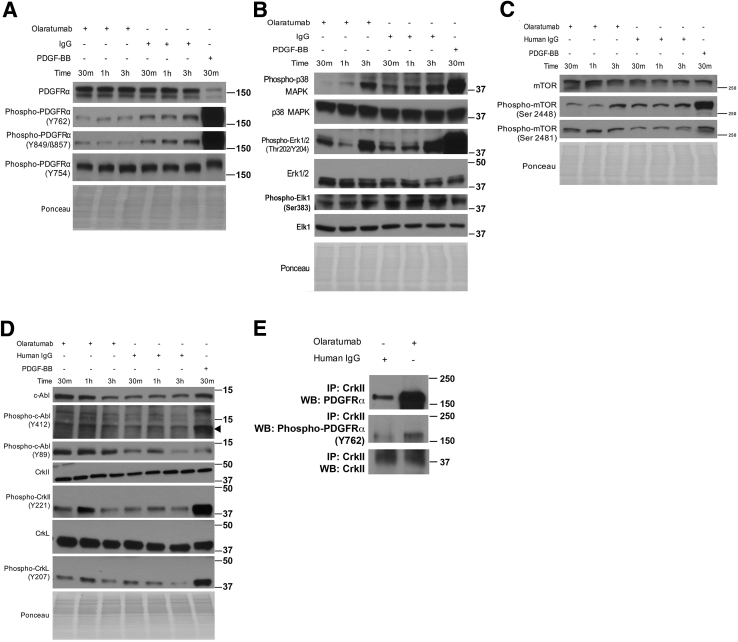
PDGFRα phosphorylation at all sites tested increased within 5 minutes, peaked at 30 minutes, and decreased at 2 and 6 hours of PDGF-BB treatment (Figure 4, A and B). Pretreatment with olaratumab decreased PDGFRα phosphorylation at Y754, Y849, Y762, and Y1018 but not at Y742 and Y572/574 (Figures 4, A and B, and 5).
Figure 4.
Olaratumab inhibits PDGFRα activation and downstream proliferative signaling mediators in HHSteCs in the presence of exogenous PDGF stimulation. A and B: HHSteCs were pretreated with either 300 nmol/L olaratumab or 300 nmol/L human IgG for 30 minutes (m) before 10 ng/mL PDGF-BB exposure for the indicated time periods. Representative Western blots using pooled samples from each time point from three technical replicates show decrease in phosphorylation of tyrosine residues in PDGFRα in cells pretreated with olaratumab, including Y754, Y762, Y849, and Y1018, but not Y742 and Y572/574. Arrowheads indicate the correct molecular weight band. C and D: Representative Western blots using pooled samples from each time point from three technical replicates also show decreased phosphorylation of p38 MAPK (T180/Y182), FAK (Y397, Y576/Y577, Y925), Akt (Ser473), and mTOR (Ser2448, Ser2481) after PDGFRα blockade with 300 nmol/L olaratumab before 10 ng/mL PDGF-BB treatment. E: Decreased phosphorylation of Erk1/2 (T202/Y204) and downstream transcription factor Elk1 (Ser383) was also observed in similar representative Western blots. Ponceau staining is included as a loading control. All Western blots were repeated twice on the same pooled lysates from three technical replicates and performed for the two stellate cell batches. h, hour; NT, nontreated.
Figure 5.
Densitometric quantification of olaratumab-mediated changes in protein phosphorylation of HHSteCs in the presence of exogenous PDGF stimulation. Densitometry of key phosphorylated protein changes from representative Western blots using pooled samples from three technical replicates for each time point shown in Figure 4. Values are normalized to total protein levels and shown as the ratio of the signal of olaratumab-treated HHSteCs/IgG-treated HHSteCs. Dashed lines represent the value at which the relative phosphorylated protein signals of olaratumab-treated HHSteCs and IgG-treated HHSteCs are equal. h, hours; m, minutes.
We next examined downstream effectors of PDGFRα associated with PDGF-induced proliferation, such as Erk1/2,16 p38,14 FAK,18 Akt, and mTOR,34 using phospho-specific antibodies, including T202/Y204 for Erk1/2, T180/Y182 for p38 mitogen-activated protein kinase (MAPK), Y397/Y576/Y577/Y925 for FAK, S473 for Akt, and S2448/S2481 for mTOR. PDGF-BB treatment increased phosphorylation of Erk1/2, p38 MAPK, Akt, mTOR, and FAK as compared to nontreated cells. The peak phosphorylation of Erk and p38 MAPK occurred at 15 minutes, followed by a gradual decrease up to 6 hours after PDGF-BB (Figure 4, C–E). Although earlier time points were unchanged, a notable decrease in Erk1/2 phosphorylation was evident at 2 to 6 hours in the olaratumab group (Figures 4, C–E, and 5). Decreased phosphorylation of p38 MAPK was evident at only 30 minutes in the olaratumab group (Figures 4, C–E, and 5). Consistent with decreased phosphorylation of Erk1/2 and p38, we observed decreased phosphorylation of Elk-1, a known downstream target of Erk1/2 and p38,35 in olaratumab pretreated cells from 30 minutes to 6 hours (Figure 4E and Figure 5). PDGF-BB treatment also led to increased FAK phosphorylation that peaked at 30 minutes to 2 hours. Olaratumab pretreatment modestly affected FAK phosphorylation at Y397 at 15 to 30 minutes and Y925 and Y576/577 at 2 hours (Figures 4, C and D, and 5). PDGF-BB treatment led to a sustained mTOR phosphorylation at both S2481 and S2448, which was reduced by olaratumab for up to 30 minutes (Figures 4, C and D, and 5). Akt phosphorylation after PDGF-BB treatment was also sustained but peaked at 30 minutes to 2 hours (Figure 4, C and D). Olaratumab decreased Akt phosphorylation at all time points, with maximal effect at 2 to 6 hours (Figures 4, C and D, and 5).
Given olaratumab's selective inhibition of PDGFRα, as well as the universal binding of PDGF-BB to both PDGFRα and PDGFRβ receptors, our results provide evidence of an important and independent contribution of PDGFRα in regulating multiple proliferative signaling pathways in human HSCs.
PDGFRα Blockade by Olaratumab Inhibits HHSteC Migration, Even in the Absence of Exogenous PDGF Ligand, because of Autocrine Signaling
PDGF is a potent chemoattractant for HSCs.16, 36 To examine whether PDGFRα signaling contributes to HSC migration, we performed transwell migration assays on HHSteCs, which were exposed to olaratumab, PDGF-BB, human IgG, or serum-free medium (NT). No significant change in HHSteC migration was observed after PDGF-BB treatment (Figure 6A). However, olaratumab treatment alone significantly reduced HHSteC migration (Figure 6B). One possibility for the lack of migratory response to exogenous PDGF-BB but inhibition of migration to PDGFRα blockade could be basal PDGFRα activity in HHSteCs. We qualitatively assessed basal gene expression of PDGFA, PDGFB, PDGFC, and PDGFD in HHSteCs. RT-PCR analysis showed mRNA expression of all PDGFs in NT HHSteCs (Figure 6C). The presence of PDGF-BB protein in cell lysates of NT HHSteCs was also observed (Figure 6D). Secretion of PDGF-BB, PDGF-CC, and PDGF-DD was also confirmed by Western blot analysis of the concentrated cell culture media collected from the NT HHSteCs cultured for 24 or 48 hours (Figure 6E). Taken together, these data indicate a role of autocrine PDGFRα signaling in HHSteC migration.
Figure 6.
Olaratumab inhibits migration of HHSteCs in the absence of exogenous PDGF ligand, because of autocrine baseline signaling. A: Representative images from HHSteC transwell migration assays and quantification shows insignificant difference between no treatment (NT) and PDGF-BB treatment. B: Decreased HHSteC migration after olaratumab versus IgG-treated controls after 3 hours relative to IgG treatment and quantified. C: RT-PCR of cDNA derived from nontreated HHSteCs (left lane) shows baseline expression of PDGFA, PDGFB, PDGFC, and PDGFD compared to HHSteC RNA in the absence of reverse transcriptase (middle lane) or primer only control (right lane) (separated because of noninclusion of technical replicates). D: Representative Western blot shows PDGF-BB expression in nontreated HHSteC whole cell lysates and hepatocellular carcinoma (HCC) sample as a positive control. Ponceau staining is included as a loading control. E: Representative Western blots of PDGF ligands detected in concentrated baseline HHSteC media after 24 or 48 hours of serum starvation indicates autocrine secretion of PDGFB, PDGFC, and PDGFD, but not PDGFA. Concentrated supernatant from TGF-β1–activated HHSteCs is shown for comparison. ∗P < 0.05. Original magnification, ×50 (A and B).
Olaratumab Inhibits Baseline PDGFRα Activation in HHSteCs and Downstream Cell Motility Signaling Pathways
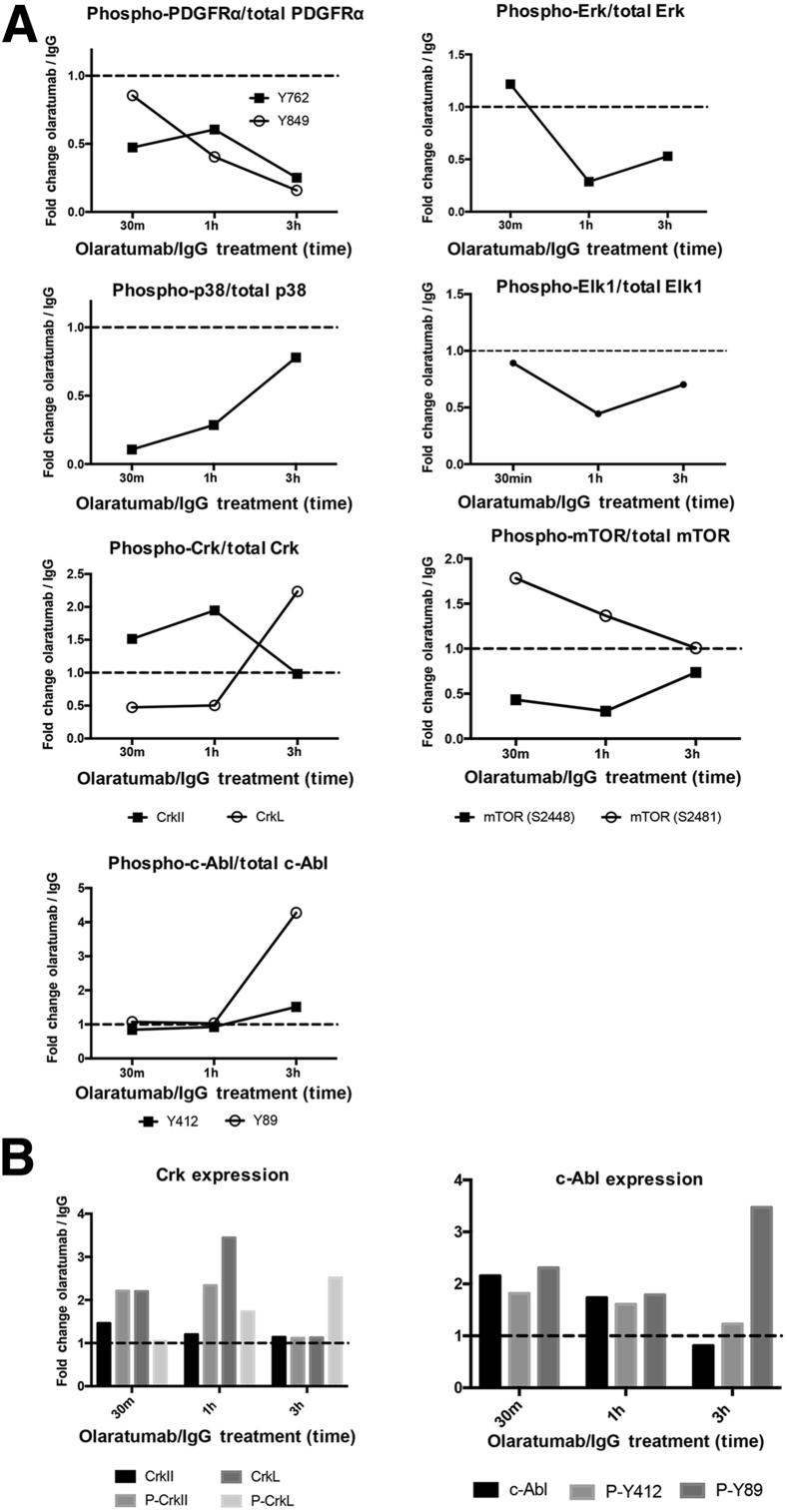
Next, downstream signaling affected by olaratumab in the absence of PDGF ligand was assessed to address the mechanism of PDGFRα-mediated HSC migration. Whole cell lysates from HHSteCs treated with olaratumab or IgG control for 30 minutes, 1 hour, and 3 hours were examined for levels of phosphorylated PDGFRα and its downstream effectors.
A notable decrease in PDGFRα phosphorylation at only Y762 and Y849 was observed, whereas other residues, like Y754, remained unaffected after olaratumab treatment (Figures 7A and 8). Relative decreases in phosphorylation of Erk1/2 at 1 and 3 hours, as well as p38 MAPK at all time points, were observed after olaratumab treatment (Figures 7B and 8). Reduced phosphorylation of Elk-1 is consistent with these findings as Elk-1 is a known downstream target of both Erk1/2 and p38 MAPK (Figure 7B).35 Olaratumab treatment decreased mTOR phosphorylation at serine 2448 at 30 minutes and 1 hour (Figures 7C and 8). Intriguingly, olaratumab treatment increased serine 2481–mTOR at 30 minutes and 1 hour (Figures 7C and 8).
Figure 7.
Olaratumab inhibits baseline PDGFRα signaling in HHSteCs along with downstream effectors. A: Representative Western blots from HHSteC treatment with 300 nmol/L olaratumab using pooled lysates from three technical replicates for each time point showing decreased PDGFRα phosphorylation at Y762 and Y849 compared to IgG-treated controls. PDGF-BB treatment included as a positive control. B: Similar representative Western blots showing decreased Erk and Elk-1 phosphorylation compared to IgG-treated controls. PDGF-BB treatment serves as a positive control. C: Representative Western blots show olaratumab treatment decreases phosphorylation of mTOR at Ser2448 and increases phosphorylation at Ser2481. Olaratumab also decreases p38 phosphorylation. D: Similar representative Western blots showing olaratumab treatment increasing Abl expression and phosphorylation at Y412 and Y89 and increasing phosphorylation at inhibitory tyrosine residues of CrkII (Y221) and CrkL (Y207). Arrowhead indicates the correct molecular weight band. E: Immunoprecipitation of HHSteC lysates using anti-CrkII shows increased binding of CrkII to both total PDGFRα and phospho-PDGFRα Y762 after olaratumab treatment. Ponceau staining is included as a loading control. All Western blots were repeated twice on the same pooled lysates from three technical replicates and performed for the two stellate cell batches. h, hours; IP, immunoprecipitation; m, minutes; WB, Western blotting.
Figure 8.
Densitometric quantification of olaratumab-mediated changes in protein phosphorylation in HHSteCs at baseline. A: Densitometry of key phosphorylated protein changes from representative Western blots using pooled samples from three technical replicates for each time point shown in Figure 7. Values for each phosphorylated protein signal are normalized to total protein levels and shown as the ratio of the signal of olaratumab-treated HHSteCs/IgG-treated HHSteCs. B: c-Abl–phosphorylated and total proteins are represented separately to highlight the increase in both total and phosphorylated proteins. The dashed line represents the value at which the relative phosphorylated protein signals of olaratumab-treated HHSteCs and IgG-treated HHSteCs are equal. h, hours; m, minutes.
Olaratumab's effect on CrkII and its isoform CrkL, which are adaptor proteins with a role in actin reorganization and cell motility, was examined next.37 Phosphorylation of CrkII (Y221) and CrkL (Y207) by Abl kinase results in sequestration of SH2 and SH3 binding domains of Crk proteins, leading to inhibition of downstream signal activation.38 Olaratumab treatment of HHSteCs led to increased phosphorylation of both CrkII and CrkL (Figures 7D and 8). Consistent with inhibition of Crk signaling, we observe activation of Abl kinase, indicated by increased phosphorylation at Y412-Abl39 and Y8940, 41 (Figures 7D and 8). The inhibition of Crk signaling downstream of PDGFRα inhibition is also consistent with the specific inhibition of PDGFRα phosphorylation at Y762 (Figures 7A and 8), necessary for PDGF-dependent Crk signaling.22 We also noted olaratumab to strongly induce CrkII protein interaction to both total and Y762-PDGFRα (Figure 7E).
Thus, the overall basis of PDGFRα blockade on human HSC migration appears to be through a cumulative effect on modulation of Erk, p38 MAPK, mTOR complex (mTORC) 1, and CrkII/CrkL.
Discussion
The development of tyrosine kinase inhibitors has been an important avenue for the research of new treatments for fibrosis and advanced liver diseases. Many of the successful inhibitors to date have cotargeted PDGFRα and PDGFRβ receptors and have shown promise in preclinical studies of hepatic fibrosis42 as well as clinical benefit in the management of diseases like hepatocellular carcinoma.43 However, the development of specific and potent inhibitors, such as olaratumab, that are already in clinical use may present therapeutic and safety advantages.31, 32 For instance, olaratumab shows approximately 100-fold increased effect on PDGF-mediated cell proliferation compared to the multityrosine kinase inhibitor imatinib.27
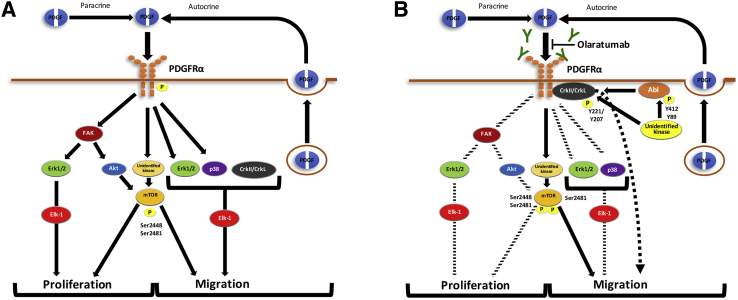
The availability of olaratumab offers a unique opportunity to study exclusive PDGFRα function in human HSCs. After confirming the expression of PDGFRα colocalized to HSCs and activated myofibroblasts in multiple models of murine chronic liver injury, we show that human HSCs respond to exogenous or autocrine PDGFs in part through PDGFRα-specific downstream signaling to contribute to proliferation and migration, but not profibrotic gene expression (Figure 9). Baseline blockade of PDGFRα signaling in HHSteCs affected migration but not proliferation or profibrotic gene expression. Exogenous PDGF-BB did not induce migration or profibrotic gene expression but induced HHSteC proliferation, which was suppressed by PDGFRα blockade. These findings point to distinct biological outcomes of autocrine versus paracrine PDGF-BB/PDGFRα signaling axis. A possible explanation is a distinct cellular response to dose of ligand available at baseline versus during exogenous treatment. Suffice to say that both distinct and common signaling events were associated with the two biological outcomes of PDGFRα blockade. The major translational significance of the current study is the use of a clinically relevant biological agent in human HSCs and its effectiveness in suppressing two key processes of HSCs for treatment of hepatic fibrosis, which remains a major unmet clinical need.
Figure 9.
Proposed effect of PDGFRα blockade on HSC migration and proliferation through inhibition of specific downstream signaling. A: In response to PDGFRα activation by autocrine and/or exogenous PDGF, HSC proliferation and migration are induced through activation of FAK, Erk1/2, Elk-1, p38, Akt, mTOR, and CrkII/CrkL signaling. B: Blockade of PDGFRα by olaratumab decreases the above-mentioned signaling mediators, with FAK, Erk1/2, Elk-1, mTOR, and Akt affecting proliferation and Erk1/2, Elk-1, p38, CrkII/CrkL, decreased mTORC1, and increased mTORC2 regulating migration. Decreases in downstream signaling are represented by dashed arrows.
We found that both PDGF-AA and PDGF-BB promote HHSteC proliferation, which was inhibited by olaratumab. PDGFRβ plays an important role in HSC proliferation. Studies in rat HSCs have shown PDGFRβ to have higher mitogenic activity than PDGFRα.44, 45 Because PDGF-BB is the predominant mitogen for HSCs during hepatic fibrosis, we wanted to address the relative contribution of PDGFRα toward HSC proliferation. In our study, olaratumab blockade before PDGF-BB treatment of HSCs led to decreased HSC proliferation that was associated with inhibition of several phosphorylation sites in PDGFRα and of multiple downstream effectors, including Erk1/2, p38, Elk-1, FAK, mTOR, and Akt. In particular, Erk1/2, Elk-1, FAK, and Akt showed sustained inhibition after olaratumab-mediated PDGFRα inhibition. Erk1/2 is known for its role in HSC proliferation,16 whereas its downstream target Elk-1 promotes gene expression associated with proliferation46 and migration.35 FAK has been shown to serve as a sensor for the detection of integrin-mediated binding to ECM and simultaneously acts as a signaling node for PDGF-induced proliferation in HSCs via the FAK/phosphatidylinositol 3-kinase/Akt pathway.18 FAK also promotes Erk-mediated cell proliferation through phosphorylation at Y925 and Y397, two of the tyrosine residues affected by olaratumab.47 Akt affects cell proliferation through diverse sets of mechanisms.48, 49 PDGF-BB treatment led to an increase in phosphorylation of PDGFRα at Y754, which was decreased by olaratumab. Y754 is an indicator of formation of the PDGFRαβ heterodimer,50 suggesting that olaratumab mediates part of its effect on HSC proliferation through the inhibition of heterodimer formation. However, because olaratumab inhibits phosphorylation of other signaling residues of PDGFR (which are more specific for PDGFRα homodimer) in the presence of exogenous PDGF-BB, we conclude that there is a definite and unique contribution of PDGFRα to HSC proliferation through Erk1/2, Elk-1, FAK, and Akt (Figure 9). A small increase in Erk1/2, Akt, mTOR, and FAK after initial olaratumab treatment may be because of its potential transient and partial agonistic activity.
It was intriguing to note that expression of fibrosis-associated genes did not show a response to olaratumab or PDGF-AA treatment in HHSteCs, suggesting that PDGFRα may be dispensable in HSC profibrotic gene induction. Thus, our study indicates that PDGFRα has a more narrowly defined function than PDGFRβ in HSCs. Indeed, this is consistent with earlier investigations showing less potent activation of HSCs as a result of PDGFRα signaling.23, 51 Our studies do not, however, preclude a ligand-independent contribution of PDGFRα signaling to HSC activation. This is particularly relevant in light of studies showing that PDGFRα is necessary for TGF-β1–mediated SMAD activation after interaction and internalization of a PDGFRα monomer/TGF-β receptor II complex.52
Migration of HSCs is an important mechanism of perpetuation of hepatic fibrosis. PDGF ligands, specifically PDGF-BB, are known chemotactic stimuli for HSCs.33 We did not observe a significant increase in migration of HHSteCs after PDGF-BB treatment. However, HHSteCs expressed and secreted PDGF ligands at baseline, indicating potential autocrine PDGFRα signaling. Indeed, activation of HSCs as a result of cell culture is well-known in both primary53, 54 and immortalized HSCs.55 Olaratumab blocked HHSteC migration, which was associated with decreased PDGFRα phosphorylation at Y762 and Y849. Among residues not affected was Y754, ruling out the role of the PDGFRαβ heterodimer in HSC migration. Previous studies have shown that PDGF-AA specific for the PDGFRαα homodimer is not chemoattractive for HSCs, in contrast to other PDGF ligand dimers.3, 56 However, our study clearly shows a reproducible effect of PDGFRα inhibition on HHSteC migration, which was associated with notable decreases in phosphorylation of well-known mediators of HSC migration, like Erk1/2 and p38 MAPK,57 and others, like mTOR (Y2448) and Crk.
Phosphorylation of PDGFRα at Y762 is necessary for direct binding to and phosphorylation of Crk adaptor proteins.22 The prolonged binding of PDGFRα to Crk proteins is considered a major difference in signaling function between PDGFRα and PDGFRβ.7 On phosphorylation of Y221 on CrkII, and Y207 on CrkL, their SH2 domain is sequestered, leading to inhibition of Crk activity.58 During its effect on cell migration, olaratumab reduced phosphorylation of Y762-PDGFRα, which was associated with increased phosphorylation of CrkII and CrkL. Olaratumab treatment increased c-Abl activation, as indicated by increased phosphorylation, which, in turn, is known to phosphorylate and inactivate CrkII/CrkL.38 The relatively stable interaction of CrkII/CrkL and PDGFRα, in contrast to rapid phosphorylation by PDGFRβ, has been speculated to lead to prolonged activity of CrkII/CrkL, leading us to hypothesize that inhibition of Y762 phosphorylation by olaratumab may dissociate the Crk/PDGFRα complex and lead to early phosphorylation of CrkII/CrkL by another kinase, like c-Abl.21 Surprisingly, olaratumab resulted in a dramatic increase in binding of CrkII to both total and Y762-phosphorylated PDGFRα. It is likely that olaratumab-mediated enhanced phosphorylation and, hence, inhibition of Crk activity occurs despite enhanced PDGFRα-CrkII association. Further studies will be critical to uncover the precise mechanism by which olaratumab alters CrkII/CrkL phosphorylation.
Olaratumab treatment of HHSteCs in the setting of both exogenous PDGF and autocrine signaling shared a reduction in Erk1/2 and mTOR. However, in contrast to mTOR phosphorylation changes in the presence of exogenous PDGF, we observed differential changes in mTOR phosphorylation, suggestive of a shift away from mTORC1 signaling to mTORC2 signaling.59 Because of the association of autocrine PDGFRα activation with HHSteC migration, this implies that mTORC1 is a predominant downstream effector of PDGFRα in HSC chemotaxis. This finding is consistent with studies showing that mTOR inhibitors that primarily affect the rapamycin-sensitive mTORC1 reduce experimental hepatic fibrosis, in part through reduced migration of HSCs.60, 61 Of particular note, rapamycin has been demonstrated to reduce PDGF-induced migration of HSCs in vitro.34
Overall, our study concludes that PDGFRα contributes to human HSC proliferation and migration independent of profibrotic gene expression. These findings suggest that olaratumab, alone or in combination, may have therapeutic activity in the pathogenesis of hepatic fibrosis. However, future research of potential therapeutic approaches aimed at inhibiting the PDGFRα pathway in in vivo models, including potential combinations, is unpredictable and would need to be performed before clinical investigation.
Acknowledgments
A.K. and S.M. conceived all experiments, analyzed the data, and drafted the manuscript; A.K. performed experiments; T.P.-S., S.N., and N.L. provided technical assistance for in vitro experiments using olaratumab and edited the manuscript; all authors reviewed the results and approved the final version of the manuscript.
Footnotes
Supported by NIH grants 4R01DK095498 (S.P.M.) and 5F30DK107129 (A.K.). Imaging was supported by the Center for Biologic Imaging, Pittsburgh Liver Research Center, University of Pittsburgh.
Disclosures: S.P.M. obtained olaratumab from Elli Lilly under a material transfer agreement. N.L. is an employee of Elli Lilly. S.P.M. has corporate research agreements with Abbvie and Dicerna Pharmaceuticals.
References
- 1.Friedman S.L. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88:125–172. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kisseleva T., Brenner D.A. Anti-fibrogenic strategies and the regression of fibrosis. Best Pract Res Clin Gastroenterol. 2011;25:305–317. doi: 10.1016/j.bpg.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hashmi A.Z., Hakim W., Kruglov E.A., Watanabe A., Watkins W., Dranoff J.A., Mehal W.Z. Adenosine inhibits cytosolic calcium signals and chemotaxis in hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2007;292:G395–G401. doi: 10.1152/ajpgi.00208.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang C., Zeisberg M., Mosterman B., Sudhakar A., Yerramalla U., Holthaus K., Xu L., Eng F., Afdhal N., Kalluri R. Liver fibrosis: insights into migration of hepatic stellate cells in response to extracellular matrix and growth factors. Gastroenterology. 2003;124:147–159. doi: 10.1053/gast.2003.50012. [DOI] [PubMed] [Google Scholar]
- 5.Ogawa K., Suzuki J., Mukai H., Mori M. Sequential changes of extracellular matrix and proliferation of Ito cells with enhanced expression of desmin and actin in focal hepatic injury. Am J Pathol. 1986;125:611–619. [PMC free article] [PubMed] [Google Scholar]
- 6.Andrae J., Gallini R., Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008;22:1276–1312. doi: 10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tallquist M., Kazlauskas A. PDGF signaling in cells and mice. Cytokine Growth Factor Rev. 2004;15:205–213. doi: 10.1016/j.cytogfr.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Bonner J.C. Regulation of PDGF and its receptors in fibrotic diseases. Cytokine Growth Factor Rev. 2004;15:255–273. doi: 10.1016/j.cytogfr.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 9.Kocabayoglu P., Lade A., Lee Y.A., Dragomir A.C., Sun X., Fiel M.I., Thung S., Aloman C., Soriano P., Hoshida Y., Friedman S.L. beta-PDGF receptor expressed by hepatic stellate cells regulates fibrosis in murine liver injury, but not carcinogenesis. J Hepatol. 2015;63:141–147. doi: 10.1016/j.jhep.2015.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borkham-Kamphorst E., Kovalenko E., van Roeyen C.R., Gassler N., Bomble M., Ostendorf T., Floege J., Gressner A.M., Weiskirchen R. Platelet-derived growth factor isoform expression in carbon tetrachloride-induced chronic liver injury. Lab Invest. 2008;88:1090–1100. doi: 10.1038/labinvest.2008.71. [DOI] [PubMed] [Google Scholar]
- 11.Hayes B.J., Riehle K.J., Shimizu-Albergine M., Bauer R.L., Hudkins K.L., Johansson F., Yeh M.M., Mahoney W.M., Jr., Yeung R.S., Campbell J.S. Activation of platelet-derived growth factor receptor alpha contributes to liver fibrosis. PLoS One. 2014;9:e92925. doi: 10.1371/journal.pone.0092925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin I.V., Borkham-Kamphorst E., Zok S., van Roeyen C.R., Eriksson U., Boor P., Hittatiya K., Fischer H.P., Wasmuth H.E., Weiskirchen R., Eitner F., Floege J., Ostendorf T. Platelet-derived growth factor (PDGF)-C neutralization reveals differential roles of PDGF receptors in liver and kidney fibrosis. Am J Pathol. 2013;182:107–117. doi: 10.1016/j.ajpath.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Pinzani M., Milani S., Herbst H., DeFranco R., Grappone C., Gentilini A., Caligiuri A., Pellegrini G., Ngo D.V., Romanelli R.G., Gentilini P. Expression of platelet-derived growth factor and its receptors in normal human liver and during active hepatic fibrogenesis. Am J Pathol. 1996;148:785–800. [PMC free article] [PubMed] [Google Scholar]
- 14.Cui X., Zhang X., Yin Q., Meng A., Su S., Jing X., Li H., Guan X., Li X., Liu S., Cheng M. Factin cytoskeleton reorganization is associated with hepatic stellate cell activation. Mol Med Rep. 2014;9:1641–1647. doi: 10.3892/mmr.2014.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parsons C.J., Takashima M., Rippe R.A. Molecular mechanisms of hepatic fibrogenesis. J Gastroenterol Hepatol. 2007;22(Suppl 1):S79–S84. doi: 10.1111/j.1440-1746.2006.04659.x. [DOI] [PubMed] [Google Scholar]
- 16.Pinzani M. PDGF and signal transduction in hepatic stellate cells. Front Biosci. 2002;7:d1720–d1726. doi: 10.2741/A875. [DOI] [PubMed] [Google Scholar]
- 17.Zhang F., Ni C., Kong D., Zhang X., Zhu X., Chen L., Lu Y., Zheng S. Ligustrazine attenuates oxidative stress-induced activation of hepatic stellate cells by interrupting platelet-derived growth factor-beta receptor-mediated ERK and p38 pathways. Toxicol Appl Pharmacol. 2012;265:51–60. doi: 10.1016/j.taap.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 18.Reif S., Lang A., Lindquist J.N., Yata Y., Gabele E., Scanga A., Brenner D.A., Rippe R.A. The role of focal adhesion kinase-phosphatidylinositol 3-kinase-akt signaling in hepatic stellate cell proliferation and type I collagen expression. J Biol Chem. 2003;278:8083–8090. doi: 10.1074/jbc.M212927200. [DOI] [PubMed] [Google Scholar]
- 19.Mori S., Nada S., Kimura H., Tajima S., Takahashi Y., Kitamura A., Oneyama C., Okada M. The mTOR pathway controls cell proliferation by regulating the FoxO3a transcription factor via SGK1 kinase. PLoS One. 2014;9:e88891. doi: 10.1371/journal.pone.0088891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu L., Parent C.A. Review series: TOR kinase complexes and cell migration. J Cell Biol. 2011;194:815–824. doi: 10.1083/jcb.201102090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsumoto T., Yokote K., Take A., Takemoto M., Asaumi S., Hashimoto Y., Matsuda M., Saito Y., Mori S. Differential interaction of CrkII adaptor protein with platelet-derived growth factor alpha- and beta-receptors is determined by its internal tyrosine phosphorylation. Biochem Biophys Res Commun. 2000;270:28–33. doi: 10.1006/bbrc.2000.2374. [DOI] [PubMed] [Google Scholar]
- 22.Yokote K., Hellman U., Ekman S., Saito Y., Ronnstrand L., Saito Y., Heldin C.H., Mori S. Identification of Tyr-762 in the platelet-derived growth factor alpha-receptor as the binding site for Crk proteins. Oncogene. 1998;16:1229–1239. doi: 10.1038/sj.onc.1201641. [DOI] [PubMed] [Google Scholar]
- 23.Wong L., Yamasaki G., Johnson R.J., Friedman S.L. Induction of beta-platelet-derived growth factor receptor in rat hepatic lipocytes during cellular activation in vivo and in culture. J Clin Invest. 1994;94:1563–1569. doi: 10.1172/JCI117497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kikuchi A., Monga S.P. PDGFRalpha in liver pathophysiology: emerging roles in development, regeneration, fibrosis, and cancer. Gene Expr. 2015;16:109–127. doi: 10.3727/105221615X14181438356210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loizos N., Xu Y., Huber J., Liu M., Lu D., Finnerty B., Rolser R., Malikzay A., Persaud A., Corcoran E., Deevi D.S., Balderes P., Bassi R., Jimenez X., Joynes C.J., Mangalampalli V.R., Steiner P., Tonra J.R., Wu Y., Pereira D.S., Zhu Z., Ludwig D.L., Hicklin D.J., Bohlen P., Witte L., Kussie P. Targeting the platelet-derived growth factor receptor alpha with a neutralizing human monoclonal antibody inhibits the growth of tumor xenografts: implications as a potential therapeutic target. Mol Cancer Ther. 2005;4:369–379. doi: 10.1158/1535-7163.MCT-04-0114. [DOI] [PubMed] [Google Scholar]
- 26.Roh J.W., Huang J., Hu W., Yang X., Jennings N.B., Sehgal V., Sohn B.H., Han H.D., Lee S.J., Thanapprapasr D., Bottsford-Miller J., Zand B., Dalton H.J., Previs R.A., Davis A.N., Matsuo K., Lee J.S., Ram P., Coleman R.L., Sood A.K. Biologic effects of platelet-derived growth factor receptor alpha blockade in uterine cancer. Clin Cancer Res. 2014;20:2740–2750. doi: 10.1158/1078-0432.CCR-13-2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shah G.D., Loizos N., Youssoufian H., Schwartz J.D., Rowinsky E.K. Rationale for the development of IMC-3G3, a fully human immunoglobulin G subclass 1 monoclonal antibody targeting the platelet-derived growth factor receptor alpha. Cancer. 2010;116:1018–1026. doi: 10.1002/cncr.24788. [DOI] [PubMed] [Google Scholar]
- 28.Stock P., Monga D., Tan X., Micsenyi A., Loizos N., Monga S.P. Platelet-derived growth factor receptor-alpha: a novel therapeutic target in human hepatocellular cancer. Mol Cancer Ther. 2007;6:1932–1941. doi: 10.1158/1535-7163.MCT-06-0720. [DOI] [PubMed] [Google Scholar]
- 29.Committee for the Update of the Guide for the Care and Use of Laboratory Animals; National Research Council: Guide for the Care and Use of Laboratory Animals: Eighth Edition. Washington, DC, National Academies Press, 2011.
- 30.Shen J., Vil M.D., Prewett M., Damoci C., Zhang H., Li H., Jimenez X., Deevi D.S., Iacolina M., Kayas A., Bassi R., Persaud K., Rohoza-Asandi A., Balderes P., Loizos N., Ludwig D.L., Tonra J., Witte L., Zhu Z. Development of a fully human anti-PDGFRbeta antibody that suppresses growth of human tumor xenografts and enhances antitumor activity of an anti-VEGFR2 antibody. Neoplasia. 2009;11:594–604. doi: 10.1593/neo.09278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shirley M. Olaratumab: first global approval. Drugs. 2017;77:107–112. doi: 10.1007/s40265-016-0680-2. [DOI] [PubMed] [Google Scholar]
- 32.Tap W.D., Jones R.L., Van Tine B.A., Chmielowski B., Elias A.D., Adkins D., Agulnik M., Cooney M.M., Livingston M.B., Pennock G., Hameed M.R., Shah G.D., Qin A., Shahir A., Cronier D.M., Ilaria R., Jr., Conti I., Cosaert J., Schwartz G.K. Olaratumab and doxorubicin versus doxorubicin alone for treatment of soft-tissue sarcoma: an open-label phase 1b and randomised phase 2 trial. Lancet. 2016;388:488–497. doi: 10.1016/S0140-6736(16)30587-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ikeda K., Wakahara T., Wang Y.Q., Kadoya H., Kawada N., Kaneda K. In vitro migratory potential of rat quiescent hepatic stellate cells and its augmentation by cell activation. Hepatology. 1999;29:1760–1767. doi: 10.1002/hep.510290640. [DOI] [PubMed] [Google Scholar]
- 34.Aleffi S., Navari N., Delogu W., Galastri S., Novo E., Rombouts K., Pinzani M., Parola M., Marra F. Mammalian target of rapamycin mediates the angiogenic effects of leptin in human hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2011;301:G210–G219. doi: 10.1152/ajpgi.00047.2010. [DOI] [PubMed] [Google Scholar]
- 35.Kasza A. Signal-dependent Elk-1 target genes involved in transcript processing and cell migration. Biochim Biophys Acta. 2013;1829:1026–1033. doi: 10.1016/j.bbagrm.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 36.Carloni V., Pinzani M., Giusti S., Romanelli R.G., Parola M., Bellomo G., Failli P., Hamilton A.D., Sebti S.M., Laffi G., Gentilini P. Tyrosine phosphorylation of focal adhesion kinase by PDGF is dependent on ras in human hepatic stellate cells. Hepatology. 2000;31:131–140. doi: 10.1002/hep.510310121. [DOI] [PubMed] [Google Scholar]
- 37.Antoku S., Mayer B.J. Distinct roles for Crk adaptor isoforms in actin reorganization induced by extracellular signals. J Cell Sci. 2009;122:4228–4238. doi: 10.1242/jcs.054627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Birge R.B., Kalodimos C., Inagaki F., Tanaka S. Crk and CrkL adaptor proteins: networks for physiological and pathological signaling. Cell Commun Signal. 2009;7:13. doi: 10.1186/1478-811X-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pluk H., Dorey K., Superti-Furga G. Autoinhibition of c-Abl. Cell. 2002;108:247–259. doi: 10.1016/s0092-8674(02)00623-2. [DOI] [PubMed] [Google Scholar]
- 40.Chen S., O'Reilly L.P., Smithgall T.E., Engen J.R. Tyrosine phosphorylation in the SH3 domain disrupts negative regulatory interactions within the c-Abl kinase core. J Mol Biol. 2008;383:414–423. doi: 10.1016/j.jmb.2008.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meyn M.A., 3rd, Wilson M.B., Abdi F.A., Fahey N., Schiavone A.P., Wu J., Hochrein J.M., Engen J.R., Smithgall T.E. Src family kinases phosphorylate the Bcr-Abl SH3-SH2 region and modulate Bcr-Abl transforming activity. J Biol Chem. 2006;281:30907–30916. doi: 10.1074/jbc.M605902200. [DOI] [PubMed] [Google Scholar]
- 42.Hong F., Chou H., Fiel M.I., Friedman S.L. Antifibrotic activity of sorafenib in experimental hepatic fibrosis: refinement of inhibitory targets, dosing, and window of efficacy in vivo. Dig Dis Sci. 2013;58:257–264. doi: 10.1007/s10620-012-2325-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Llovet J.M., Ricci S., Mazzaferro V., Hilgard P., Gane E., Blanc J.F., de Oliveira A.C., Santoro A., Raoul J.L., Forner A., Schwartz M., Porta C., Zeuzem S., Bolondi L., Greten T.F., Galle P.R., Seitz J.F., Borbath I., Haussinger D., Giannaris T., Shan M., Moscovici M., Voliotis D., Bruix J., SHARP Investigators Study Group Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 44.Borkham-Kamphorst E., van Roeyen C.R., Ostendorf T., Floege J., Gressner A.M., Weiskirchen R. Pro-fibrogenic potential of PDGF-D in liver fibrosis. J Hepatol. 2007;46:1064–1074. doi: 10.1016/j.jhep.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 45.Pinzani M., Knauss T.C., Pierce G.F., Hsieh P., Kenney W., Dubyak G.R., Abboud H.E. Mitogenic signals for platelet-derived growth factor isoforms in liver fat-storing cells. Am J Physiol. 1991;260:C485–C491. doi: 10.1152/ajpcell.1991.260.3.C485. [DOI] [PubMed] [Google Scholar]
- 46.Vickers E.R., Kasza A., Kurnaz I.A., Seifert A., Zeef L.A., O'Donnell A., Hayes A., Sharrocks A.D. Ternary complex factor-serum response factor complex-regulated gene activity is required for cellular proliferation and inhibition of apoptotic cell death. Mol Cell Biol. 2004;24:10340–10351. doi: 10.1128/MCB.24.23.10340-10351.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Provenzano P.P., Inman D.R., Eliceiri K.W., Keely P.J. Matrix density-induced mechanoregulation of breast cell phenotype, signaling and gene expression through a FAK-ERK linkage. Oncogene. 2009;28:4326–4343. doi: 10.1038/onc.2009.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Manning B.D., Cantley L.C. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Son M.K., Ryu Y.L., Jung K.H., Lee H., Lee H.S., Yan H.H., Park H.J., Ryu J.K., Suh J.K., Hong S., Hong S.S. HS-173, a novel PI3K inhibitor, attenuates the activation of hepatic stellate cells in liver fibrosis. Sci Rep. 2013;3:3470. doi: 10.1038/srep03470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rupp E., Siegbahn A., Ronnstrand L., Wernstedt C., Claesson-Welsh L., Heldin C.H. A unique autophosphorylation site in the platelet-derived growth factor alpha receptor from a heterodimeric receptor complex. Eur J Biochem. 1994;225:29–41. doi: 10.1111/j.1432-1033.1994.00029.x. [DOI] [PubMed] [Google Scholar]
- 51.Pinzani M., Gentilini A., Caligiuri A., De Franco R., Pellegrini G., Milani S., Marra F., Gentilini P. Transforming growth factor-beta 1 regulates platelet-derived growth factor receptor beta subunit in human liver fat-storing cells. Hepatology. 1995;21:232–239. [PubMed] [Google Scholar]
- 52.Liu C., Li J., Xiang X., Guo L., Tu K., Liu Q., Shah V.H., Kang N. PDGF receptor-alpha promotes TGF-beta signaling in hepatic stellate cells via transcriptional and posttranscriptional regulation of TGF-beta receptors. Am J Physiol Gastrointest Liver Physiol. 2014;307:G749–G759. doi: 10.1152/ajpgi.00138.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.De Minicis S., Seki E., Uchinami H., Kluwe J., Zhang Y., Brenner D.A., Schwabe R.F. Gene expression profiles during hepatic stellate cell activation in culture and in vivo. Gastroenterology. 2007;132:1937–1946. doi: 10.1053/j.gastro.2007.02.033. [DOI] [PubMed] [Google Scholar]
- 54.Friedman S.L., Roll F.J., Boyles J., Arenson D.M., Bissell D.M. Maintenance of differentiated phenotype of cultured rat hepatic lipocytes by basement membrane matrix. J Biol Chem. 1989;264:10756–10762. [PubMed] [Google Scholar]
- 55.Herrmann J., Gressner A.M., Weiskirchen R. Immortal hepatic stellate cell lines: useful tools to study hepatic stellate cell biology and function? J Cell Mol Med. 2007;11:704–722. doi: 10.1111/j.1582-4934.2007.00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pinzani M., Marra F. Cytokine receptors and signaling in hepatic stellate cells. Semin Liver Dis. 2001;21:397–416. doi: 10.1055/s-2001-17554. [DOI] [PubMed] [Google Scholar]
- 57.Mann D.A., Marra F. Fibrogenic signalling in hepatic stellate cells. J Hepatol. 2010;52:949–950. doi: 10.1016/j.jhep.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 58.Kobashigawa Y., Sakai M., Naito M., Yokochi M., Kumeta H., Makino Y., Ogura K., Tanaka S., Inagaki F. Structural basis for the transforming activity of human cancer-related signaling adaptor protein CRK. Nat Struct Mol Biol. 2007;14:503–510. doi: 10.1038/nsmb1241. [DOI] [PubMed] [Google Scholar]
- 59.Copp J., Manning G., Hunter T. TORC-specific phosphorylation of mammalian target of rapamycin (mTOR): phospho-Ser2481 is a marker for intact mTOR signaling complex 2. Cancer Res. 2009;69:1821–1827. doi: 10.1158/0008-5472.CAN-08-3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Piguet A.C., Majumder S., Maheshwari U., Manjunathan R., Saran U., Chatterjee S., Dufour J.F. Everolimus is a potent inhibitor of activated hepatic stellate cell functions in vitro and in vivo, while demonstrating anti-angiogenic activities. Clin Sci (Lond) 2014;126:775–784. doi: 10.1042/CS20130081. [DOI] [PubMed] [Google Scholar]
- 61.Wang W., Yan J., Wang H., Shi M., Zhang M., Yang W., Peng C., Li H. Rapamycin ameliorates inflammation and fibrosis in the early phase of cirrhotic portal hypertension in rats through inhibition of mTORC1 but not mTORC2. PLoS One. 2014;9:e83908. doi: 10.1371/journal.pone.0083908. [DOI] [PMC free article] [PubMed] [Google Scholar]